Abstract
Chemokine-receptor signaling plays an important role in the inflammatory response often associated with tumor growth and metastasis. The NF-κB pathway, essential for transcription of chemokines/chemokine receptors and other key inflammatory modulators, has emerged as a potential target for tumor therapy. Here we describe an efficient approach to monitor drugs that target the NF-κB signaling as related to tumor growth and metastasis in vivo. For bioluminescence imaging, the firefly luciferase (Fluc) reporter has the advantage of stable signaling, while Gaussia luciferase (Gluc) provides very sensitive signaling based on secretion of Gluc. We introduce the use of monitoring intratumoral Gluc, which rapidly diffuses into the blood circulation and urine. The peripheral Gluc assay may complement bioluminescence imaging and provide a kinetic, noninvasive, real-time read-out of NF-κB activity by directly determining Gluc reporter activity in blood or urine samples from tumor-bearing mice.
1. INTRODUCTION
Nuclear factor-κB (NF-κB), a key signal transduction pathway in chemokine–chemokine receptor expression, inflammation, and cancer, is important target for drug discovery and development. It has been increasingly important to develop noninvasive, high-resolution, in vivo imaging to elucidate mechanisms that identify and validate drugs that target the NF-κB pathway. Recent advances in technology allow visualization of signal transduction as related to biological processes in vivo (Phair and Misteli, 2001). Use of fluorescent proteins revolutionizes static microscopy images by providing the ability to make dynamic recordings of protein–protein interaction in living animals. More recently, a Gaussia luciferase that possesses a natural secretory signal, allowing secretion into the cell microenvironment, offers great promise for real-time ex vivo monitoring of NF-κB signaling in tumor development and progression in conscious animals.
2. DEVELOPMENT OF NF-κB REPORTER MODEL FOR TUMORS
To monitor NF-κB signaling in tumorigenesis, we constructed a luciferase reporter vector that expresses a transcription factor–mediated reporter protein. To generate the NF-κB–mediated transcription, firefly-luciferase reporter vector, we inserted a commercial vector with a transcription blocker that is composed of adjacent polyadenylation and transcription pause sites for reducing background transcription. An NF-κB consensus sequence was fused to a TATA-like promoter, followed by a firefly- or Gaussia-luciferase reporter gene for monitoring NF-κB signaling (Yang et al., 2007). These vectors are designated as NF-κB-Fluc or NF-κB-Gluc, respectively. This system enables signal amplification of a transcription factor–mediated signal.
To create NF-κB–promoter reporter cells, human melanoma cells (Hs294T) were transfected with the linearized NF-κB-Gluc vector and stably transfected cells were selected with 2 mg/ml G418. Gaussia luciferase in the cultured medium or cell lysate was characterized based on the catalytic reaction with its substrate, coelenterazine. We subcutaneously inoculated nude mice with these melanoma NF-κB-Gluc reporter cells and allowed tumor xenografts to grow over 14 days. The following approaches are used to monitor NF-κB signaling changes in real time during tumor progression and in response to molecular targeting therapy.
3. BIOLUMINESCENT IMAGING OF INTRATUMOR SIGNALING OF ANESTHETIZED MICE
3.1. Firefly luciferase reporter
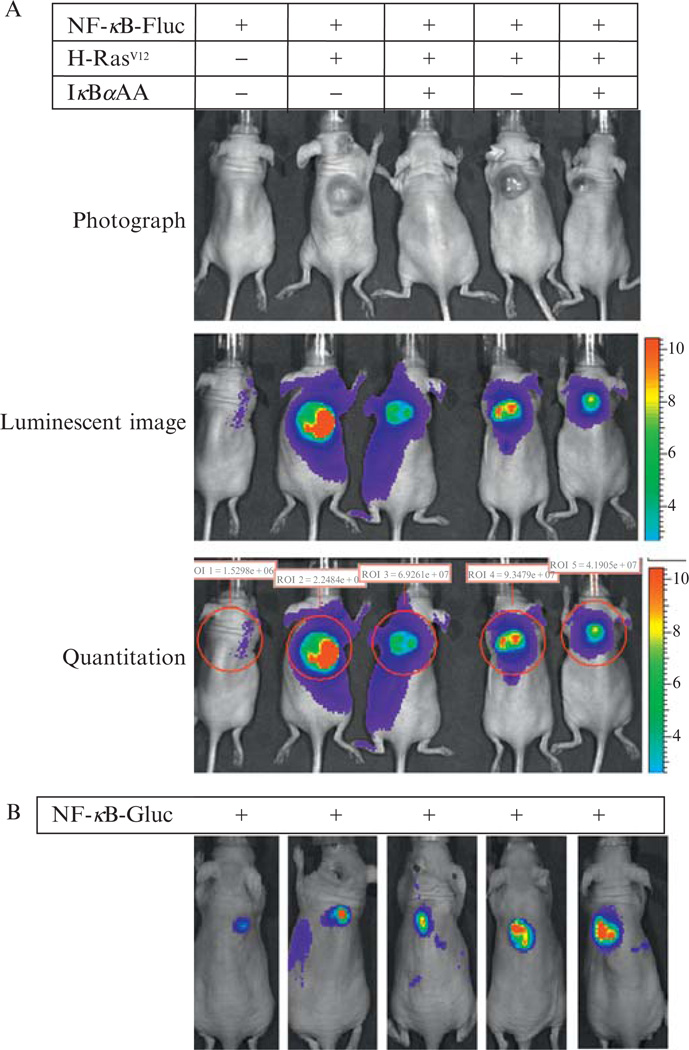
Bioluminescence images can be taken using the IVIS 200 Imaging System (Xenogen Imaging Technologies). The system is composed of an imaging chamber, gas anesthesia system, and a highly sensitive charge-coupled device (CCD) camera. The CCD camera is cryogenically cooled for highly efficiency photon detection and displays a wide-range signal image. It takes approximately 3 min to anesthetize small mammals such as mice using the gas anesthesia system that is connected to an oxygen cylinder and isoflurane tank with flow rates set at scale. Fresh luciferin solution is prepared by dissolving luciferin powder (15 mg/ml) in phosphate-buffered saline (PBS) (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, and adjusted to a final pH of 7.4). Mice are injected intraperitoneally with 150 µg of luciferin per gram body weight and transferred into the image chamber. The image field is set according to the number of mice to be imaged. To acquire the live images, the focus is adjusted to 1.5cm for a subcutaneous (SC) tumor (Fig. 17.1), or to 1 cm for deep organ tumors such as a tumor metastatic to the liver (Fig. 17.2). The exposure time is usually 1 min for SC tumors and 3 min for tumors metastatic to the liver. The peak time of image intensity is around 10 min postinjection of substrate (luciferin), although this time is variable depending on the anatomy of the tumor location. The image file is saved as TIF format and the image intensity is quantitated using the Living Image software 3.0 from Xenogen Imaging Technologies (Fig. 17.1).
Figure 17.1.
Intratumoral NF-κB reporter models. (A) NF-κB-Fluc reporter model. Mouse melanocytes null for INK4A/ARF were genetically engineered with the NF-κB-Fluc reporter (lanes 1to 5),CMVpromoter^driven oncogenic H-RASV12 expression (lanes 2 to 5), and/or a Tet-On inducible IkBa(S32A/S36A) superrepressor expression vector (lanes 3 and 5).These cellswere inoculated into nudemice to develop xenografts. Tumors were photographed (upper panels) and intratumoral NF-κB activities were determined by quantitative luminescent imaging (lower panels), illustrating that H-RASV12 inducedNF-κBactivation in vivowas inhibitedwith the IκBα superrepressor. (B) NF-κB-Gluc reporter model. NF-κB-Gluc was stably expressed in human melanoma Hs294Tcells (1 × 106) and these cells were subcutaneously inoculated into nude mice. The individual bioluminescent image was taken immediately following intravenously injection of 100 µg native coelenterazine per mouse.
Figure 17.2.
Melanoma metastasis to the liver. (A) NF-κB-Fluc reporter model of metastasis. 2 × 105 of mouse melanocytes (NF-κB-Fluc+, H-RASV12+, INK4A/ARF−/−) were intravenously injected into each nude mouse. Sixty days after injection, the bioluminescent images were obtained (upper panel), mice were euthanized and (B) histological analysis (H&E staining) of organs were performed, indicating melanoma metastasis to the liver. (C) 2 × 105 of GFP-tagged melanocytes (H-RASV12+, INK4A/ARF−/−)were intravenously injected into nude mice. Thirty days after cell injection, frozen sections were examined under the fluorescent microscope (10× magnification). H, hepatocytes; M, melanoma cells; RBC, red blood cells.
3.2. Gaussian luciferase reporter
To produce a bioluminescence image reporter for Gaussia luciferase activity, native coelenterazine (50 to 100 µg/mouse) injected intravenously gives an accurate read-out of NF-κB activity (unpublished). Coelenterazine is easily dissolved into either ethanol or methanol. To make up a stock of native coelenterazine, 4 mg coelenterazine is dissolved in 1 ml methanol. This stock solution can be stored at −70 °C for 2 weeks. However, for the most accurate reproducible comparative data, freshly mixed coelenterazine is recommended. The coelenterazine stock should be diluted 1:20 (v/v) with a buffer (150 mmol/l NaCl, 2 mmol/l KCl, 1 mmol/l MgCl2, 10 mmol/l Na2HPO4, 2 mmol/l KH2PO4, pH 7.4) prior to intravenous injection. The injected mouse will be immediately subjected to bioluminescence imaging for the reason that the Gaussia luciferase signal rapidly fades relative to firefly luciferase. Thus, Gaussia luciferase imaging allows analysis of only one mouse at a time, while firefly luciferase imaging can be performed on a maximum 5 mice for one time point (Fig. 17.1).
4. CELL-BASED ASSAYS FOR KINASE AND TRANSCRIPTIONAL ACTIVITY IN VITRO
In a nonstimulated cell, IκBα is tightly complexed with NF-κB to hold NF-κB in the cytoplasm and keep it in a biologically inactive form. In this manner, IκBα serves as the brake for the NF-κB signal transduction cascade. When ligand binding activates receptors at the cell surface, the signal cascade is triggered through activation of IκB kinase (IKK). The activated IKK phosphorylates IκBα protein, which enables ubiquitination followed by degradation of IκBα and the eventual nuclear translocation of NF-κB (RelA/p50). The classical method for determining IKK activity has relied on an in vitro kinase assay where the IKK complex is immunoprecipitated from cell lysate and the activated IKK catalyzes the transfer of radiolabeled phosphate to a purified IκBα protein. In contrast, the newly developed IκB-Gluc reporter plasmid or NF-κB-Gluc reporter plasmid reflects cellular IκBα levels or transcriptional NF-κB activity in intact cells without interruption. When cells are transfected to stably express the reporter plasmid, Gaussia luciferase is naturally secreted into the culture medium. Addition of the IKK inhibitor, BMS-345541 inhibits NF-kB-Gluc and increase IkB-Gluc activity. Gaussia luciferase as a reporter accurately reflects NF-κB activity based on luminescence imaging (Fig. 17.3), or by measuring 10-s photon counts using a luminometer following a reaction of 10 µl of medium with the substrate (100 micromoles/l coelenterazine) in buffer containing 500 mmol/l NaCl, 2 mmol/l KCl, 10 mmol/l MgCl2, 10 mmol/l Na2HPO4, 2 mmol/l KH2PO4, 1 mmol/l EDTA and adjusted to a pH of 7.8.
Figure 17.3.
Luminescent imaging of cultured dish. Hs294TIκB-Gluc reporter cells or Hs294T NF-κB-Gluc reporter cells were treated with the IKK inhibitor, BMS-345541 (0 to 10 mM) for 36 h. The plate containing the cultured cells and medium was subjected to bioluminescent imaging immediately following addition of 100 mM of coelenterazine. Two independent experiments were performed and similar results were achieved. Data showan increase in the level of IκBα (upper panels) and reducedNF-κB transcriptional activity (lower panels) upon treatment with BMS-345541.
5. PERIPHERAL SPYING OF INTRATUMORAL SIGNALING OF CONSCIOUS MICE
Bioluminescence imaging studies have shown that Gaussia luciferase as a reporter gives a 200-fold brighter signal than that of firefly luciferase (Tannous et al., 2005). However, the Gaussia luciferase signal is less stable during bioluminescent process than the firefly luciferase luminescence imaging. Thus, Gaussia luciferase is not an ideal reporter for luminescence imaging. Rather, Gluc is a small monomer enzyme that is rapidly secreted from cells (Verhaegent and Christopoulos, 2002) and much more sensitive than the other established secretory marker, alkaline phosphatase (Wurdinger et al., 2008). We demonstrate that Gaussia luciferase expressed in melanoma cells is secreted into culture medium in vitro; in vivo Gluc diffuses into blood circulation and subsequently is excreted into the urine in melanoma-bearing mice. This has been confirmed by reconstitution of Gluc diffusion through the vascular system (Fig. 17.4). Thus, Gluc may serve as an ideal reporter of intratumoral NF-κB activity by following the release of Gluc into the blood and/or urine of tumor-bearing animals. During the course of exposure to drug treatment, the NF-κB activity in cultured tumor cells or in mouse tumor xenografts can be continuously monitored by measuring the Gaussia luciferase activity in cultured medium or in blood and/or urine samples. Consequently, Gluc can play an important role toward predicting cancer drug efficacy in vitro and in vivo. This system has been validated by well-established IKK inhibitors and IKK stimuli, suggesting that this approach is feasible for screening the effect of cancer drugs on the NF-κB pathway.
Figure 17.4.
Reconstitution of Gluc diffusion via vascular system. Conditioned medium containing the secreted Gluc (2 × 108 RLU) was intraperitoneally injected into adult mice. The Gluc activity in the blood or the urine was detected over the course of 4 h. The average value of each time point shown was from six animals. Experiments were repeated twice with similar results. The peak time for Gluc diffusion from the intraperitoneal compartment into blood is 1h and into urine is 1.5 h.
To prepare blood samples, 5 µl blood were withdrawn using pipette tip from a small incision at the tail tip of conscious mice. The 5 µl of blood was mixed into 10 µl buffer (500 mmol/l NaCl, 2 mmol/l KCl, 10 mmol/l MgCl2, 10 mmol/l Na2HPO4, 2 mmol/l KH2PO4, 1 mmol/l EDTA, pH 7.8) and stored at 4 °C for measuring Gluc activity within 3 weeks. Sampling of urine is easily done since the mouse readily offers approximately 100 µl of urine triggered by the fear response to being “caught” by the gentle hand of the investigator. Of notice, the daily average mouse voiding frequency is 16 times and the urine volume per void is approximately 160 µl; thus, urine samples were also collected from a clean glass jar where mouse was temporally kept. The urine Gluc activity was determined between 0 and 24 h without loss of Gluc activity. Gluc activity was measured using the Monolight TM 3010 luminometer (BD Biosciences Pharmingen, San Diego, CA). Ten microliters of sample were quickly mixed well with 20 µl of 100 µM coelenterazine in the buffer above and 10-s photon counts were acquired.
In conclusion, bioluminescence imaging of intratumoral NF-kB signaling in living animals can provide useful information about the molecular basis of tumorigenesis and molecular response to therapeutic agents. Firefly luciferase is superior to Gaussia luciferase as a reporter for quantitative molecular imaging. Advantages of Gaussia luciferase are that it is naturally secreted and extremely sensitive, suggesting it is extremely useful as a peripheral marker for real-time monitoring of intratumoral molecular signaling in conscious mice.
ACKNOWLEDGMENTS
We thank colleagues at the Richmond laboratory for insightful discussions and Richard Baheza of Vanderbilt University Institute of Imaging Science for excellent technical assistance. Work from the authors’ program was supported through funding by the Department of Veterans Affairs through a VA Merit Award (AR) and a VA Senior Research Career Scientist Award (AR), National Institutes of Health grants (CA 098807) (A.R.), the Vanderbilt Ingram Cancer Center support grant (CA 68485), and the Skin Disease Research Center grant (SP30 AR 41943).
REFERENCES
- Phair RD, Misteli T. Kinetic modelling approaches to in vivo imaging. Nat. Rev. Mol. Cell. Biol. 2001;2:898–907. doi: 10.1038/35103000. [DOI] [PubMed] [Google Scholar]
- Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Verhaegent M, Christopoulos TK. Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 2002;74:4378–4385. doi: 10.1021/ac025742k. [DOI] [PubMed] [Google Scholar]
- Wurdinger T, Badr C, Pike L, de Kleine R, Weissleder R, Breakefield XO, Tannous BA. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat. Methods. 2008;5:171–173. doi: 10.1038/nmeth.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Pan WH, Clawson GA, Richmond A. Systemic targeting inhibitor of kappaB kinase inhibits melanoma tumor growth. Cancer Res. 2007;67:3127–3134. doi: 10.1158/0008-5472.CAN-06-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]