Abstract
The key to understanding centromere identity is likely to lie in the chromatin containing the histone H3 variant, CENP-A. CENP-A is the prime candidate to carry the epigenetic information that specifies the chromosomal location of the centromere in nearly all eukaryotic species, raising questions fundamental to understanding chromosome inheritance: How is the epigenetic centromere mark propagated? What physical properties of CENP-A-containing complexes are important for epigenetically marking centromeres? What are the molecules that recognize centromeric chromatin and serve as the foundation for the mitotic kinetochore? We discuss recent advances from our research groups that have yielded substantial insight into these questions and present our current understanding of the centromere. Future work promises an understanding of the molecular processes that confer fidelity to genome transmission at cell division.
Chromosomes, the functional unit of inheritance, must segregate with high fidelity every time a cell divides, and both prokaryotes and eukaryotes have evolved elaborate mechanisms to achieve accurate chromosome delivery (Hayes and Barilla 2006; Santaguida and Musacchio 2009). For eukaryotes, a common mechanism in mitosis is employed, where sister chromatids are physically attached to each other and bidirectionally oriented towards poles of the microtubule-based spindle that physically move complete sets of chromosomes to each daughter cell. This bidirectionally orientated attachment is mediated by a proteinaceous structure, the kinetochore, that forms during mitosis at the microtubule/chromosome interface. The site of kinetochore formation is defined by a region of the chromosome, the centromere. Without functional centromeres, chromosomes are mis-segregated at cell division, leading to aneuploidy in the daughter cells.
In budding yeast, classic experiments defined the centromere as a small (~125 bp) sequence-specified region of DNA (Clarke and Carbon 1980; Fitzgerald-Hayes et al. 1982). This region is comprised of three conserved elements (CDEI, II, and III) and recruits sequence-specific centromeric DNA binding proteins (such as members of the well-studied CBF3 complex which is recruited to CDEIII)(Lechner and Carbon 1991). This simple and elegant system for marking centromeres is not conserved, however, in other eukaryotes, except for in a subset of related yeasts. For most eukaryotes, the centromere is much larger and is not defined by a particular DNA sequence. For both simple and more complex centromeres there is a “core” centromeric chromatin at the foundation of the kinetochore as well as a surrounding specialized chromatin domain (this is defined by highly phased nucleosomes in budding yeast and is a distinct “heterochromatin” domain in flies, mammals, etc.) required for sister-chromatid cohesion. Both of these regions of the centromere are essential for successful chromosome transmission at cell division.
Evidence for an Epigenetic Mechanism for Centromere Identity
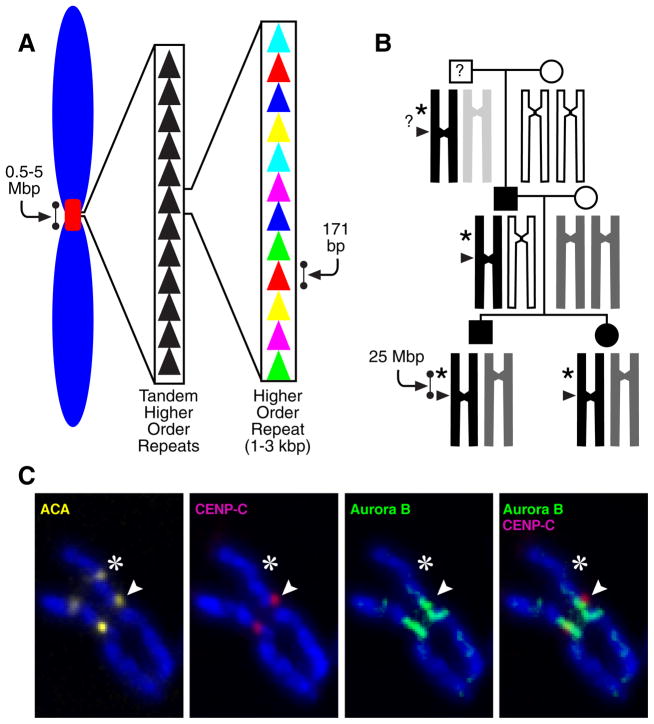
The centromere is typically located within a region of repetitive satellite DNA in diverse plant and animal phyla (Henikoff et al. 2001; Jiang et al. 2003). In humans, the predominant centromeric satellite, α-type I, consists of repeats of 171 bp monomers that extend for several megabases at most centromeres(Fig. 1A)(Manuelidis and Wu 1978; Willard 1985; Willard and Waye 1987). Despite the strong correlation between centromere location and the presence of these satellites, chromosomal rearrangements in humans have revealed instances in which a centromere has been silenced (in the case of rearrangements that would have produced a dicentric chromosome if one of the two centromeres had not been inactivated)(Earnshaw and Migeon 1985; Sullivan and Schwartz 1995) or generated de novo at a chromosome arm locus lacking detectable α satellite DNA (such new centromeres are referred to as neocentromeres)(Depinet et al. 1997; du Sart et al. 1997; Warburton et al. 1997; Choo 2001). Two human cases have described instances where a centromere relocated within an intact chromosome 3 or 4, respectively, from the original location to a new location on the chromosome arm (Amor et al. 2004; Ventura et al. 2004). A remarkable finding was that this new location persists in multiple family members for at least two generations(Fig. 1B) (Amor et al. 2004). The ability to permanently silence an existing centromere with no rearrangement or deletion of centromeric repeat DNA sequences, create a neocentromere at a non-centromeric region of the chromosome that lacks α-satellite DNA, or both (Fig. 1B,C), provides substantial support for the notion that human centromeres are not defined by a particular DNA sequence. Such evidence strongly argues that centromere identity is primarily or exclusively specified epigenetically.
Figure 1.
Epigenetic centromere specification. (A) DNA at normal human centromeres is repetitive with a monomer length of ~171 bp multimerized for megabase stretches. While this general theme in centromere organization is seen in most eukaryotes, centromeric DNA sequences are amongst some of the most rapidly evolving sequences in the genome. (B) Stable inheritance of a human neocentromere after centromere relocation along an intact human chromosome 4 (Amor et al. 2004). At left is the family pedigree, showing the generational inheritance of a neocentromere containing variant chromosome 4 (‘PD-NC4’; black bar). The chromosomal allele containing the neocentromere was inherited from the paternal grandfather of the brother and sister who initially were found to carry PD-NC4. The neocentromere is carried by their father, and the grandfather was not available for study. (C) Relocation of both centromere-specifying chromatin and inner centromere components to the PD-NC4 neocentromere(Bassett et al. 2010). Anti-centromere antisera (ACA) recognizes both CENP-A at the neocentromere (arrowhead) and CENP-B at the silenced centromere at the original location (asterisk). Kinetochore-forming components, represented by the CENP-A-binding protein, CENP-C, vacate the original site and relocate to the neocentromere (Amor et al. 2004). Inner centromere components, represented by the Aurora B kinase also vacate the original site and relocate to chromosome arm positions proximal to the neocentromere(Bassett et al. 2010).
In evolutionary terms, the movement of centromere location has emerged as an attractive candidate to participate in the mechanism of speciation (Ventura et al. 2001; Amor et al. 2004). While speciation events in mammals maintain a high degree of synteny at most sites, centromere location can vary greatly between even closely related species (Carbone et al. 2006). Centromere “re-positioning” is correlated to the high evolutionary rate of chromosomal break-points that are preferentially found in or near centromeres (Murphy et al. 2005). Interestingly, while satellite DNA is typically found at mammalian centromeres, the sequence of the repeat unit is not well conserved (Henikoff et al. 2001).
A Centromere-specific H3 Variant that Marks Centromeres
The most attractive candidate for an epigenetic mark that specifies the centromere is the histone H3 variant CENP-A (Earnshaw and Rothfield 1985; Palmer and Margolis 1985; Sullivan et al. 1994). Together with canonical histones H2A, H2B, and H4 it forms nucleosomes at active centromeres, and CENP-A relatives have an essential role at centromeres in diverse eukaryotic species (Stoler et al. 1995; Buchwitz et al. 1999; Howman et al. 2000; Takahashi et al. 2000; Regnier et al. 2005). CENP-A is found at all active centromeres in a manner that appears to be independent of DNA sequence, including human neocentromeres lacking detectable α-satellites (Fig. 1)(Warburton et al. 1997; Amor et al. 2004). Conversely, despite the retention of αI satellite arrays, CENP-A is absent when centromeres are silenced (Warburton et al. 1997; Amor et al. 2004; Han et al. 2006). In order to distinguish the centromere from chromatin found at all other chromosome locations, CENP-A must somehow physically distinguish the nucleosomes into which it assembles. Contributions to physically marking the centromere could potentially come from its N-terminal ‘tail’ that lacks conservation among CENP-A orthologues but is uniformly lacking in any sequence identity with conventional histone H3. Alternatively, physical divergence could come from its histone fold domain that is relatively conserved among CENP-A orthologues but diverges by 30–45% from H3. Despite this divergence within the histone fold domain, CENP-A spontaneously forms a heterotetramer upon co-expression with its partner histone H4 (Black et al. 2004). While identical in stoichiometry to the sub-nucleosomal (H3:H4)2 heterotetramer, (CENP-A:H4)2 is dynamically and structurally divergent from its conventional counterpart. The first evidence of such divergence—coming from mass spectrometry-based hydrogen/deuterium exchange experiments that dynamically measure the conformational flexibility of the polypeptide backbone—was the finding that the predicted interface between CENP-A and H4 is >10-fold more rigid than the corresponding portion of the (H3:H4)2 heterotetramer (Black et al. 2004).
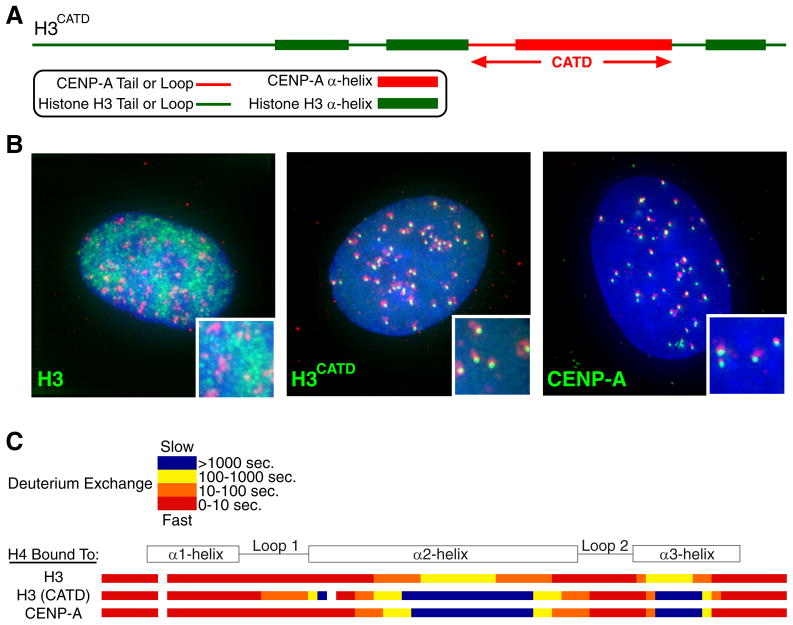
The portion of CENP-A, its α2-helix, that contacts histone H4 and generates the rigid interface is of high interest because it, along with the preceding loop (L1), is the only region of CENP-A where mutation results in defective centromeric targeting (Shelby et al. 1997). Indeed, a chimeric histone H3 carrying 22 amino acid substitutions from CENP-A in the L1 and α2-helix (Fig. 2A), H3CATD (CENP-A Targeting Domain), targets efficiently to centromeres (Fig. 2B)(Black et al. 2004). Importantly, the chimeric H3CATD forms a tetramer with H4 that has a nearly identical hydrogen/deuterium exchange profile as bona fide (CENP-A:H4)2 heterotetramers (Fig. 2C), and restricted flexibility relative to canonical nucleosomes is maintained after assembly of CENP-A into nucleosomes (Black et al. 2007a). After arriving at centromeres, the H3CATD chimera can substitute for CENP-A protein in centromere specifying nucleosomes, rescuing lethality in cell culture following siRNA-mediated depletion of endogenous CENP-A (Black et al. 2007b).
Figure 2.
Initial evidence linking the targeting of CENP-A to centromeres with physical divergence from conventional histone H3 (Black et al. 2004). (A) Diagram of a histone H3 chimera containing the CENP-A targeting domain (CATD). (B) Centromere targeting of H3CATD. (C) Hydrogen/deuterium exchange profile of histone H4 bound to conventional H3, the H3CATD chimera, and CENP-A. The nearly identically localized blue regions on histone H4 within (CENP-A:H4)2 and (H3CATD:H4)2 heterotetramers are >10-fold slower to exchange amide protons with deuterons in heavy water than from any portion within conventional (H3:H4)2, indicating substantial rigidity imparted by the CATD.
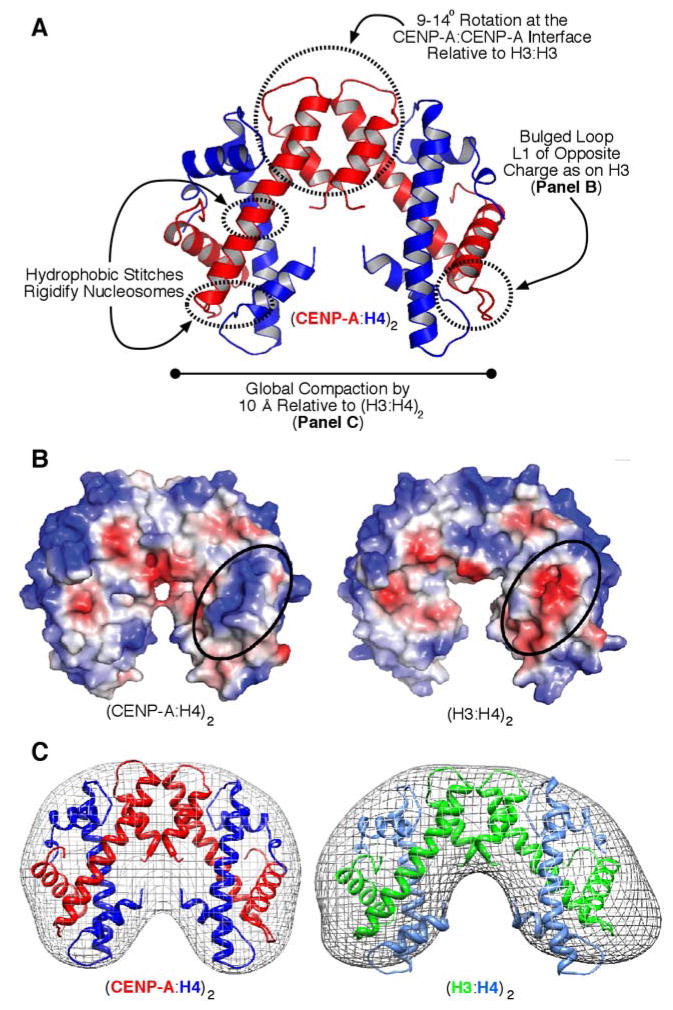
The molecular nature of (CENP-A:H4)2 was revealed in atomic detail with high resolution (2.1–2.5 Å) crystal structures and lower resolution solution studies (small angle x-ray scattering; SAXS)(Sekulic et al. 2010)(Fig. 3). The structural basis for the rigidified interface with H4 resides in hydrophobic ‘stitches’ between CENP-A-specific side-chains and H4 that restrict locally polypeptide backbone flexibility (Fig. 3A). In addition, the hydrogen bonding between L1 of CENP-A and L2 of H4, generates a bulge of the opposite charge as is found on conventional (H3:H4)2 heterotetramers (Fig. 3A,B)(Sekulic et al. 2010) at a location that remains exposed even after assembly into conventional nucleosomes (Luger et al. 1997). Most strikingly, the crystal and SAXS studies revealed a compaction of the entire tetramer by ~10 Å that corresponds to a rotation at the CENP-A/CENP-A interface (Fig. 3A,C)(Sekulic et al. 2010). All three distinguishing structural features (hydrophobic stitches, positively charged L1 bulge, and the compaction emanating from its rotated CENP-A:CENP-A interface) are encoded by the CENP-A-specific amino acid changes within the CATD. Together with the studies of CENP-A hydrogen/deuterium exchange behavior (Black et al. 2004; Black et al. 2007a) and functional analysis of the H3CATD chimera (Black et al. 2007b), the crystal and solution structural studies (Sekulic et al. 2010) lead to a working model wherein CENP-A marks centromeric chromatin by altering nucleosome structure from within its folded histone core.
Figure 3.
(CENP-A/H4)2 heterotetramers structurally deviate from their conventional counterparts. (A) Crystal structure of the (CENP-A:H4)2 heterotetramer (PDB ID 3NQJ)(Sekulic et al. 2010) highlighting features that distinguish it from conventional (H3:H4)2. (B) Surface alterations encoded by the CATD of CENP-A include a basic charged protrusion (circled) that is bulged further away from the helical core of the complex and of the opposite charge as on the counterpart (H3:H4)2 heterotetramer. (C) Solution measurements of (CENP-A:H4)2 indicate that it is compacted by ~10 Å relative to its conventional counterpart, as predicted by the compact structure of (CENP-A:H4)2 in crystal form. The mesh indicates the molecular envelope corresponding to the rotational state that best matches the SAXS data collected for each type of heterotetramer.
Requirements for Inheriting the Centromere Mark Through Cell Cycles
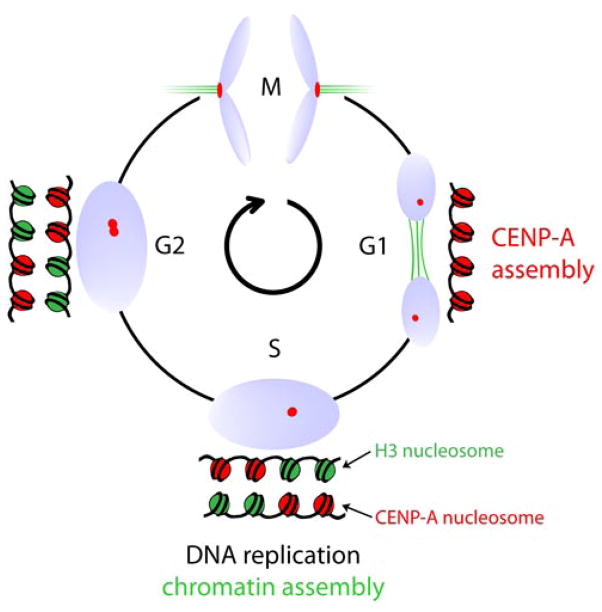
The structurally divergent CENP-A nucleosome is a prime candidate for the epigenetic propagation of centromere identity. The current view of cell cycle-coupled centromere inheritance posits that a critical arrangement of CENP-A nucleosomesis sufficient to trigger and propagate a functional centromere. At least three important criteria are likely to be required for epigenetic centromere inheritance. First, the mark must be stable enough to survive through key cell cycle steps, including DNA replication and mitotic passage. This property is central to centromere inheritance, but the underlying mechanisms are largely unidentified for any proposed epigenetic mark. Second, centromeric chromatin duplication must be self-templating (e.g., by pre-existing chromatin-bound CENP-A molecules). Third, replication of the centromeric mark must be tightly coupled to the cell cycle. We address below recent advances on all three of these aspects of epigenetic centromere propagation.
Centromeric Chromatin is Stable Across Mitotic Divisions
Expression shut-off experiments have shown that the total cellular CENP-A pool is turned over at a rate of ~50% per cell division suggesting CENP-A to be stably maintained across mitotic division (Shelby et al. 2000; Regnier et al. 2005). To examine stability of CENP-A after loading into centromeric chromatin, we have employed tagging of CENP-A with the ~30 kDa SNAP-tag, a modified variant of the suicide enzyme O6-alkylguanine-DNA alkyltransferase, whose normal function is in DNA repair. This protein has been extensively engineered to covalently and irreversibly modify (and inactivate) itself through acceptance of the cell permeable guanine derivative O6-benzylguanine (or fluorescent derivatives thereof). This allows for irreversible fluorescent pulse labeling of SNAP fusion proteins at will in vivo (Keppler et al. 2003; Keppler et al. 2004). It is worth noting the specific virtues of the SNAP-tag-based pulse labeling strategy. It stands out from other cell biological tools to determine protein dynamics, such as fluorescence recovery after photobleaching (FRAP) experiments, in that it allows the determination of protein turnover on a much longer time scale (days rather than minutes)and is therefore well suited for proteins with long half lives.
We applied this methodology to determine CENP-A turnover specifically at centromeres. We demonstrated nearly all centromeric CENP-A remains centromere associated during centromeric DNA replication and the subsequent mitosis (Jansen et al. 2007). This extreme stability is consistent with a role for CENP-A as an epigenetic mark maintaining centromere identity. Indeed, following centromeric DNA replication in S phase and chromosome segregation in mitosis, the preexisting “old” (i.e., fluorescently tagged) centromere bound CENP-A levels were reduced at each daughter centromere to half the level of the unreplicated centromere prior to DNA replication, strongly suggesting “old” CENP-A nucleosomes are redistributed during S phase, with half deposited/re-deposited onto each of the replicated DNA copies as the DNA replication fork passes.
Cell Cycle Coupling of Centromeric Chromatin Replication
The two fold dilution of CENP-A nucleosomes during DNA replication indicates that half the CENP-A pool requires replenishment every cell division. Canonical histone expression (including the replication dependent histone H3 variant, H3.1) is tightly cell cycle regulated and restricted to S phase. Indeed, assembly of histones is directly coupled to the DNA replication machinery ensuring formation of nascent nucleosomes in the wake of the DNA replication fork (Annunziato 2005; Xu et al. 2010). Given the resemblance of CENP-A to H3, the question arose how CENP-A assembly is regulated and how this variant is discriminated from canonical H3 in view of the high levels of H3.1 expression during S phase. Initial models suggested differences in timing of replication of centromeric DNA versus the genome overall as a means to provide a temporal window permissive for CENP-A loading(O’Keefe et al. 1992; Csink and Henikoff 1998). However, subsequent work showed this not to be the case, as replication of centromeric DNA is not restricted to a specific time during S-phase(Shelby et al. 2000).
The attractive alternative would be if CENP-A loading was temporally separated from assembly of canonical histones altogether, with CENP-A assembly outside S-phase. Indeed, CENP-A mRNA and protein levels peak only after S phase during late G2 and assembly does not occur coincident with DNA replication consistent with a disconnect between the timing of CENP-A and H3 assembly(Shelby et al. 1997; Shelby et al. 2000). With our use of SNAP-tagged CENP-A to fluorescently mark only newly synthesized CENP-A, direct evidence in human cells was obtained for replication of centromeric chromatin solely at the exit from mitosis and in early G1 (Jansen et al. 2007), that is, half a cell cycle after centromeric DNA replication (Figs. 4 & 5). Subsequent photo bleaching experiments in human cells confirmed this (Hemmerich et al. 2008). A similar conclusion was also reached in Drosophila, where GFP-CENP-ACID photo bleaching experiments in rapidly cycling Drosophila syncytial embryos showed fluorescence recovery during a brief window following mitotic exit concurrent with an increase of overall GFP-CENP-ACID levels at the centromere (Schuh et al. 2007). Dissociation of centromeric DNA replication from that of centromeric chromatin is apparently wide spread: in fission yeast cells CENP-ACnp1 is assembled in early S phase which commences immediately following mitotic exit, suggesting a temporal control similar to that found in higher eukaryotes. However, unlike in fly embryo and human cultured cell examples, there is a second wave of assembly in the fission yeast G2 phase(Takahashi et al. 2005).
Figure 4.
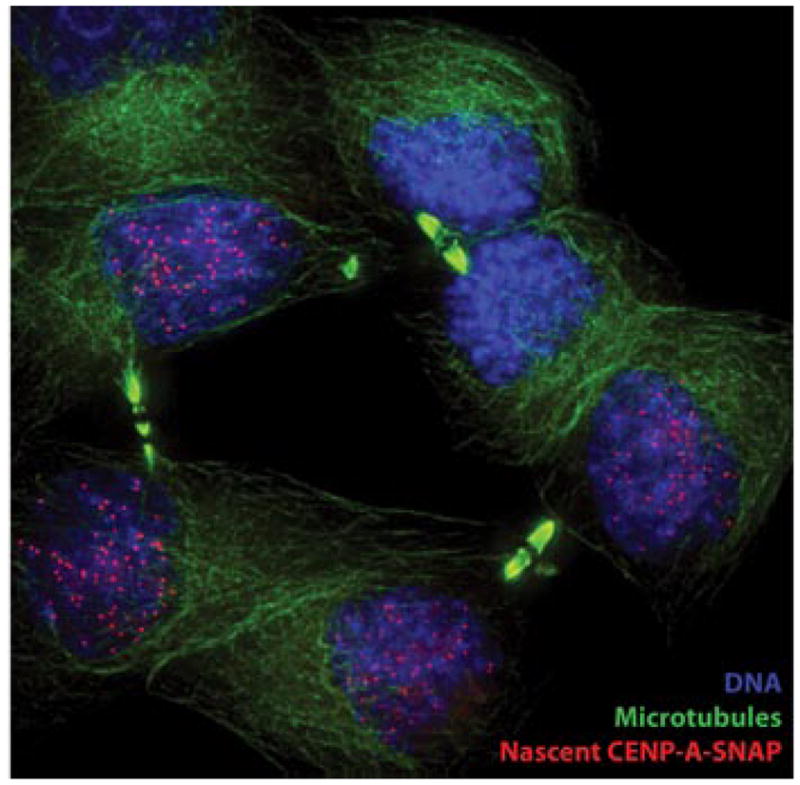
A pool of CENP-A-SNAP, synthesized during S phase, was fluorescently pulse labeled in G2 phase. Cells were then cycled through mitosis and fixed. Accumulation of nascent CENP-A-SNAP (pulse labeled SNAP, red) at centromeres is evident in cells in late telophase, marked by reformed nuclei (DNA, Blue) and mid-bodies identifying daughter cells (microtubules, green).
Figure 5.
A schematic representation of the temporal uncoupling of DNA replication and canonical chromatin assembly in S phase from centromeric nucleosome replication in G1.
Factors Directing CENP-A Assembly in a Temporally Controlled Manner
The restricted cell cycle window during which CENP-A assembly occurs predicts that factors uniquely involved in the delivery and assembly of nascent CENP-A localize to the centromere within the same timeframe. Indeed, several components have now been identified that meet this requirement. The most striking member of this group of proteins is Mis18, first identified in fission yeast and shown to be required for CENP-ACnp1 localization to the centromere(Hayashi et al. 2004). Mis18 is absent from centromeres during mitosis but rapidly relocalizes to centromeres following mitotic exit. This temporal localization pattern is preserved in a set of human proteins that include the Mis18 homologues, hMis18α and hMis18β, and the associated protein M18BP1/HsKNL2 that was independently identified as the human homolog of the C. elegans KNL2 protein(Fujita et al. 2007; Maddox et al. 2007). Depletion of any of these proteins severely impacts CENP-A localization at the centromere. In this mammalian context, CENP-A recruitment to telophase centromeres closely follows the appearance of Mis18 at the centromere in anaphase (Fujita et al. 2007; Jansen et al. 2007; Maddox et al. 2007; Silva and Jansen 2009). Recent quantitative measurements of CENP-A centromere levels during mitotic exit indicate CENP-A assembly can initiate as early as 10 minutes after anaphase onset suggesting the assembly process may be nearly simultaneous with Mis18 recruitment (Lagana et al. 2010). Despite their suggestive localization pattern and role in CENP-A assembly none of the Mis18 homologs nor M18BP1/HsKNL2 appear to bind to CENP-A directly(Hayashi et al. 2004; Foltz et al. 2006; Fujita et al. 2007; Lagana et al. 2010). In addition, they have not been found in proteomic screens for CENP-A nucleosome or prenucleosome binding factors described below.
Recruitment of the Constitutive Centromere
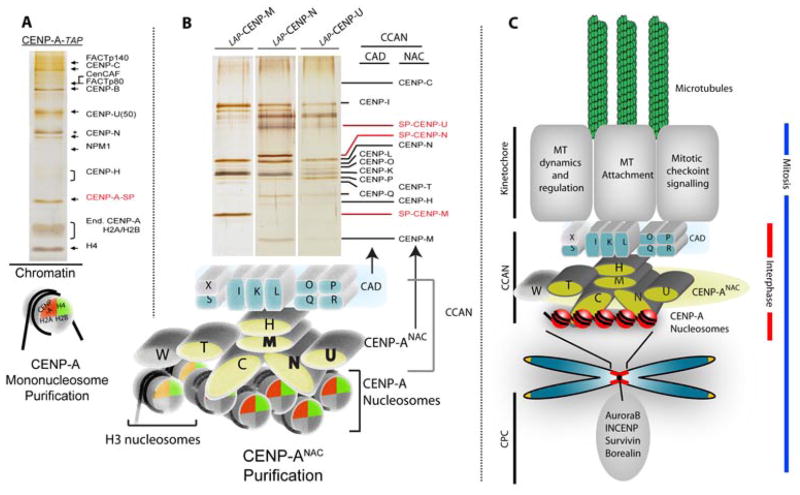
The CENP-A nucleosome forms the basis for a large centromere complex associated with the centromere throughout the cell cycle. The earliest examples of constitutive centromere proteins were identified by human autosera which in addition to CENP-A recognized constitutive centromere proteins CENP-B and CENP-C (Earnshaw et al. 1986). Affinity purification of intact CENP-A nucleosomes greatly expanded the knowledge of the protein complement and architecture of the human centromere components most directly bound to CENP-A chromatin (Foltz et al. 2006). Affinity purifications of CENP-A nucleosomes identified a set of known and novel CENP-A nucleosome associated proteins (CENP-ANAC) (Fig. 6) which included CENP-C, M, N, U and T. Subsequent affinity purification of the new proteins within the CENP-ANAC identified an additional set of constitutive centromere proteins that were associated with the centromere, but not themselves associated with the CENP-A nucleosome, called CENP-A distal (CAD) proteins(Foltz et al. 2006; Okada et al. 2006). The CENP-ANAC and CENP-ACAD have since been collectively termed the Constitutive Centromere Associated Network (CCAN) and can be further subdivided into partially overlapping subcomplexes(Cheeseman et al. 2008; Hori et al. 2008b; Amano et al. 2009). While recruitment of the CENP-ANAC is dependent on CENP-A nucleosomes, the assembly of the CENP-ANAC is also dependent on itself, as elimination of CENP-N, M or T results in the loss of the remaining CENP-ANAC proteins from the centromere.
Figure 6.
Identification of centromere constituents. (A) Tandem affinity purification was used to identify the set of centromere proteins most closely associated with the CENP-A nucleosome (CENP-ANAC) by affinity purification of intact CENP-A nucleosomes derived from an MNase digested chromatin fraction. (B) Serial affinity purification of newly identified CENP-ANAC proteins was used to identify the more distal constitutive components of the centromere that were not in close proximity to the CENP-A nucleosome. (C) Cartoon model of centromere organization from CENP-A nucleosomes to outer kinetochore formation (Foltz et al. 2006).
Further affinity purification by Fukagawa and colleagues of components bound to CENP-T identified an additional CENP-T binding partner, CENP-W(Hori et al. 2008a). While CENP-T is clearly associated with, and dependent on the CENP-ANAC for its recruitment, the CENP-T/W complex also interacts with H3-containing chromatin (Foltz et al. 2006; Hori et al. 2008a; Carroll et al. 2010). These data suggest that the CENP-ANAC in addition to establishing the constitutive centromere at the CENP-A containing locus may be capable of organizing the surrounding histone H3 containing chromatin. Two components identified within the CENP-ANAC, CENP-N and CENP-C, act as recognition factors which couple the recruitment of the CCAN to the CENP-A nucleosome. CENP-N recognizes the CENP-A nucleosome by virtue of the CATD within CENP-A (Carroll et al. 2009). In contrast, CENP-C is recruited to CENP-A containing chromatin through an interaction with the extreme C-terminus of CENP-A (Carroll et al. 2010). Since CENP-C and CENP-N recognize distinct domains, they may be able to recognize the same CENP-A nucleosome, although it is unclear whether this occurs in vivo. It is also unclear whether these recognition factors work cooperatively to recruit the CCAN or whether they represent different modes of the CCAN binding to the CENP-A nucleosome that may be utilized at different times in the cell cycle.
Two other sets set of proteins were co-purified with intact CENP-A nucleosomes which were not observed to directly associate with other components of the CCAN, the FACT complex (Obuse et al. 2004; Foltz et al. 2006)and HJURP (previously termed hFLEG1). As we document below, it is now highly likely that the HJURP protein co-purifying with CENP-A nucleosomes represents the fraction of CENP-A containing chromatin that is actively undergoing assembly (see below). The FACT complex has been previously implicated in transcription through chromatinized DNA (Orphanides et al. 1998). FACT activity appears to be important for CENP-A deposition (Okada et al. 2009) and may act to allow access of centromeric assembly factors to the already chromatinized centromere. Recently, by virtue of a human artificial chromosome, it was shown that the centromere has low but detectable levels of active transcription and displays a set of histone modifications that facilitates transcription (Bergmann et al. 2011). Specific removal of one such mark, histone H3 dimethylated at lysine 4 (H3K4me2) from an engineered centromere severely impacts centromeric transcription and interferes with recruitment of nascent CENP-A with the concomitant loss of centromeric chromatin structure (Bergmann et al. 2011). Consistent with the presence of FACT, these results suggest that either transcription itself or the chromatin environment it generates helps maintain CENP-A nucleosomes.
Identification of a CENP-A Specific Histone Chaperone
The stable propagation of the centromere requires assembly of new CENP-A chromatin/nucleosomes each cell cycle to avoid the loss of CENP-A nucleosomes through their successive dilution. Epigenetic maintenance of the centromere is therefore dependent on the restricted and targeted assembly of CENP-A into chromatin at the pre-existing centromere. In general, nucleosome deposition is regulated by histone chaperone proteins. The histone H3.1 and H3.3 variants utilize partially overlapping chaperone complexes to achieve distinct temporal and spatial distributions within the genome. Prenucleosomal histones H3.1 and H4 associate with the chromatin assembly factor-1 (CAF-1) complex, consisting of CAF-1 p150, CAF-1 p60, and CAF-1 p46/48 and as a dimer with the anti-silencing factor 1 chaperone (ASF1) (English et al. 2005; English et al. 2006; Groth et al. 2007a; Natsume et al. 2007). In contrast, while the histone H3.3 variant also interacts with ASF1 outside of S phase, it is incorporated into chromatin independent of DNA synthesis through the action of a distinct prenucleosomal complex that includes HIRA and CAF-1 p48, but is devoid of CAF-1 p150 and CAF-1 p60(Ahmad and Henikoff 2002b; Tagami et al. 2004).
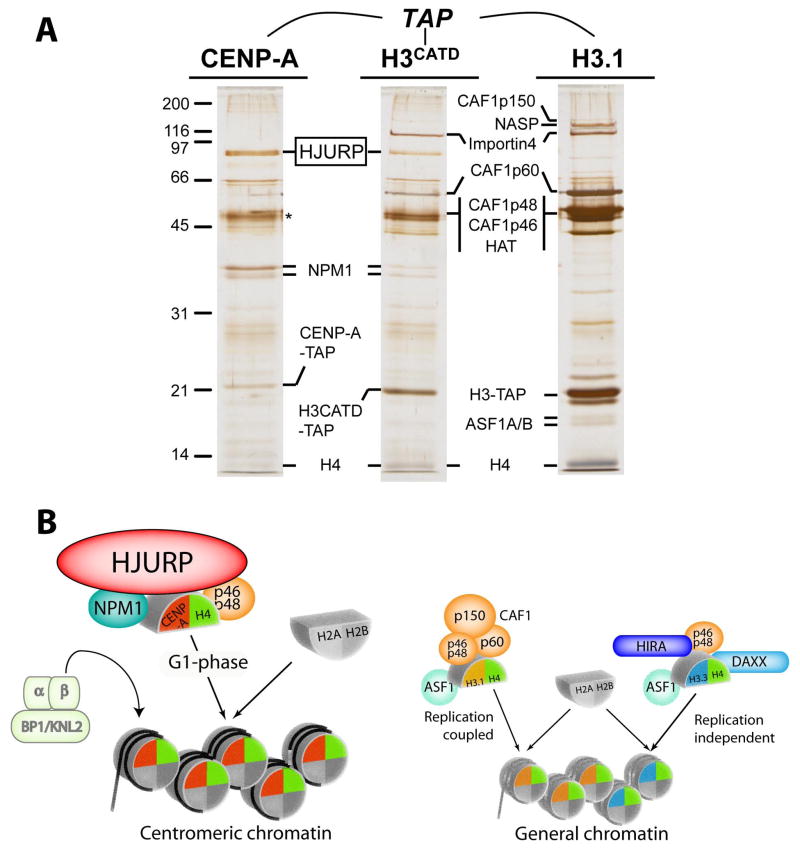
These preceding examples with other histone H3 variants provided the initial support for a distinct chaperone to achieve centromere specific deposition of CENP-A. We therefore conducted tandem affinity purification from chromatin-free extracts to identify a prenucleosomal CENP-A complex(es)(Fig. 7). Consistent with a stepwise nucleosome assembly where the (CENP-A:H4)2 tetramer is brought to the DNA separately from the H2A:H2B dimer, similar to the assembly steps culminating in conventional nucleosome assembly (Smith and Stillman 1989; Jackson 1990; Smith and Stillman 1991), we purified a complex that contained CENP-A and histone H4, but was devoid of H2A and H2B. The major unique non-histone protein associated with prenucleosomal CENP-A was the 83 kDa protein HJURP (Holliday Junction Recognition Protein) (Kato et al. 2007; Dunleavy et al. 2009; Foltz et al. 2009). Note that although HJURP was initially named for its ability to interact with a synthetic Holliday junction in vitro, there is no indication as to whether this property has any relevance to its role in its function as a CENP-A specific histone chaperone. HJURP is, however, specifically recruited to centromeres in early G1, at the time when new CENP-A nucleosomes are assembled. Further, SNAP labeling experiments clearly demonstrated that the deposition of new CENP-A nucleosomes is specifically eliminated when HJURP is depleted using siRNA. The interaction between HJURP and CENP-A is mediated by the CENP-A CATD, since swapping of the CATD region into histone H3 was sufficient for recognition and binding by HJURP (Kato et al. 2007; Dunleavy et al. 2009; Foltz et al. 2009).
Figure 7.
Isolation of pre-nucleosomal histone complexes. (A) Affinity purifications of CENP-A, H3CATD and histone H3.1 were conducted from chromatin-free extracts to identify CENP-A specific preassembly complexes (Foltz et al. 2009). Prenucleosomal CENP-A is uniquely associated with HJURP and NPM1 compared with histone H3.1. The CATD domain of CENP-A mediates the recruitment of HJURP as the H3CATD chimeric protein, which is recruited to centromeres, interacts with HJURP. (B) Model of the distinct histone chaperone complexes utilized by the histone H3 variants.
The CENP-A prenucleosomal complex also includes two additional proteins, Nucleophosmin1 and RbAp48 (Dunleavy et al. 2009; Foltz et al. 2009). Both proteins were subsequently identified in affinity purifications of HJURP (Shuaib et al. 2010), suggesting that NPM1, RbAp48, HJURP, and CENP-A exist in a common complex. Both RbAp46/48 and NPM1 have proposed roles in assembly of general chromatin. It is likely that HJURP provides the specific assembly of CENP-A into chromatin, and NPM1 and RbAp46/48 may play roles in providing chromatin remodeling properties of this complex that are required for the deposition of CENP-A nucleosomes into centromeric chromatin that is likely (but not definitively demonstrated) to already contain H3 histones in the period between centromere DNA replication in S and activation of CENP-A loading at mitotic exit(Fig. 5).
Interestingly, both the epigenetically defined regional centromere of S. pombe and the genetically encoded point centromere of S. cerevisiae require the HJURP homolog Scm3 for the assembly of CENP-A onto centromeres(Mizuguchi et al. 2007; Camahort et al. 2009; Pidoux et al. 2009; Williams et al. 2009). The timing of centromeric association differs: in fission yeast, Scm3 is present at the yeast centromere during the majority of the cell cycle but clearly absent during mitosis (Pidoux et al. 2009; Williams et al. 2009), a much more expanded time frame than that observed in vertebrate cells where HJURP recruitment is restricted to early G1 (Fig. 5). For budding yeast the situation is highly controversial. One hypothesis is for an unusual CENP-ACse4 hexomeric “nucleosome” in which Scm3 replaces H2A and H2B (Mizuguchi et al. 2007). Another proposes a Scm3-containing trisome of CENP-ACse4, H4, and Scm3 with right-handed DNA wrapping (Furuyama and Henikoff 2009), one final proposal is for more conventional octameric nucleosomes(Camahort et al. 2009). As we argue elsewhere (Black and Cleveland, submitted), a plausible, partial resolution of these conflicting claims could be that one or more of these represent CENP-ACse4 chromatin at different points in the budding yeast cell cycle.
Assembly of histone H3.1 nucleosomes is coupled to replication. This is achieved through interaction between histone H3.1 and the MCM replicative helicase and between CAF1p150 and PCNA (Shibahara and Stillman 1999; Moggs et al. 2000; Groth et al. 2007b). Both PCNA and CAF1p150 are major components of the replication machinery, such that new histone H3.1 nucleosome assembly occurs in close proximity to DNA synthesis. Exactly how CENP-A assembly is coupled to existing centromeresis not yet understood. One hypothesis is pre-existing CENP-A nucleosomes direct the incorporation of new CENP-A nucleosomes either directly or through the recruitment of intermediate factors. These factors could include the covalent modification of surrounding centromeric chromatin or one or more members of the CCAN could play this role. In turn, HJURP must recognize either the existing CENP-A nucleosome or the intermediate factors or modifications they induce in order to direct the assembly of new CENP-A nucleosomes.
Purifications of prenucleosomal or nucleosomal CENP-A and HJURP have failed to identify a mammalian Mis18 homologue, and likewise HJURP and CENP-A are not evident in purifications of the members of the Mis18 complex, as mentioned above. This finding has led to the hypothesis that Mis18 complex is required for priming the centromere in preparation for HJURP mediated CENP-A nucleosome assembly(Fujita et al. 2007; Silva and Jansen 2009). Other factors with roles in CENP-A assembly may include Rsf-1 and SNF2h, both of which are part of the remodeling and spacing factor (RSF) complex. This chromatin targeted ATPase has been implicated in CENP-A assembly and suggested to participate in the nucleosome incorporation step (Perpelescu et al. 2009). In addition, the small GTPase activating protein MgcRacGAP, previously implicated in cyctokinesis has now been identified as a component involved in CENP-A assembly (Lagana et al. 2010). Strikingly, Rsf-1 and MgcRacGAP along with one of its targets, Cdc42, in human cells localize to centromeres during mid and late G1 respectively(Perpelescu et al. 2009; Lagana et al. 2010). While it is unclear to what extent these processes are temporally distinct and to what aspect of centromeric CENP-A metabolism they contribute, it appears likely that the incorporation of new CENP-A into centromeric chromatin is a multistep process that may span several hours. In agreement with this notion, quantitative fluorescent measurements of centromeric CENP-A levels indicate that, although CENP-A assembly initiates in early G1, accumulation of CENP-A can continue for up to 10 hours in human HeLa cells (Lagana et al. 2010).
Alternate Forms of Centromeric Nucleosomes: The Verdict is Still Out
While several studies have supported the notion that CENP-A is present at the centromere inoctameric nucleosomes ([CENP-A:H4:H2A:H2B]2 + DNA) in which it replaces both copies of H3 (Shelby et al. 1997; Foltz et al. 2006; Camahort et al. 2009), several other proposals (some introduced above) have been put forward (Dalal et al. 2007; Mizuguchi et al. 2007; Furuyama and Henikoff 2009; Lavelle et al. 2009; Williams et al. 2009). Of these proposals, some are likely to represent G1 assembly intermediates in the assembly/maturation of centromere specifying nucleosomes, involving either the absence (Williams et al. 2009) or replacement of H2A/H2B dimers with the putative chromatin assembly protein Scm3/HJURP (Mizuguchi et al. 2007).
A much more unconventional challenge to an octameric configuration has come from Henikoff and colleagues with the initial proposal that CENP-A exists at centromeres in a hemisomal configuration with one copy each CENP-A, H4, H2A, and H2B(Dalal et al. 2007)and extended more recently to include a model of right handed DNA wrapping (Furuyama and Henikoff 2009), opposite of the left handed wrapping that induces negative supercoils upon conventional nucleosome assembly into closed, circular DNA. Initial findings interpreted to support the hemisome proposal emerged from atomic force microscopy (AFM) evidence that CENP-ACid chromatin purified from Drosophila cells (Dalal et al. 2007)(and recently extended to CENP-A containing material derived from mammalian (HeLa) cells (Dimitriadis et al. 2010)) indicate that immunopurified CENP-A nucleosomes are half the height of the major nucleosome conformation observed in unpurified bulk chromatin.
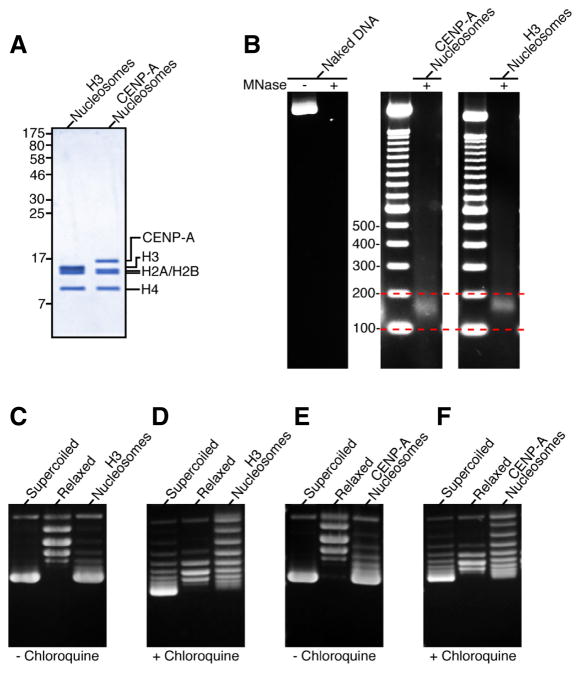
It is important to note that the hemisome model conflicts with data from structural and functional studies that indicate that the CENP-A/CENP-A interface, completely absent in a hemisome model, is an essential feature of a CENP-A-containing nucleosome (Camahort et al. 2009; Sekulic et al. 2010), even conferring specificity for a homotypic (two copies of CENP-A) versus a heterotypic (one copy each, CENP-A and H3) nucleosome (in the case of budding yeast (Kingston et al. 2011)). Indeed, human CENP-A self-assembles with histones H2A, H2B, and H4 into octameric nucleosomes that protect ~150 bp of DNA from nuclease digestion and wrap DNA with the conventional left-handedness, inducing negative supercoils into DNA (Fig. 8)(Sekulic et al. 2010).
Figure 8.
CENP-A assembles into octameric nucleosomes with conventional handedness of DNA wrapping (Sekulic et al. 2010). (A) Histone content of assembled H3- and CENP-A-containing nucleosomes. (B) Digestion of nucleosome arrays with micrococcal nuclease reveals that both H3-and CENP-A-containing nucleosomes protect ~150 bp of DNA. (C–F) Topological analysis of H3-(C and D) and CENP-A-containing (E and F) nucleosomes. Analysis by gel electrophoresis in the absence (C and E) or presence (D and F) of chloroquine reveals that both H3- and CENP-A-containing nucleosomes wrap DNA in a left-handed manner.
Beyond the structural evidence against the hemisome model, there are also plausible alternative interpretations of the primary evidence (intranucleosomal chemical crosslinking (Dalal et al. 2007), atomic force microscopy (Dalal et al. 2007)and apparent handedness of DNA supercoiling (Furuyama and Henikoff 2009)) that support the hemisome model (Black and Bassett 2008; Lavelle et al. 2009).
The crosslinking experiments were done with dimethyl suberimidate, something used early on in nucleosome studies to define the oligomeric state of purified sub-nucleosomal (H3:H4)2 heterotetramers and H2A:H2B heterodimers (Kornberg and Thomas 1974). This linker has an 11 Å spacer arm and interacts with primary amines. Within conventional nucleosomes most of the inter-histone crosslinks are between H2B and the other histones (H2A, H3, and H4) within ‘half’ of the nucleosomes, with crosslinks at the interface between H3:H3 less efficient(Suda and Iwai 1979). The H3:H3 crosslinks very likely come from lysines at position 115 and 122 (K115 and K115′ are ~8 Å apart, K115 and K122′ are ~5 Å apart, and K122 and K122′ are ~15 Å apart, with side-chains facing each other in each case; (Luger et al. 1997)). The Drosophila CENP-ACID used in the crosslinking studies lacks these lysines, and the nearest residue with a crosslinkable side-chain is a lysine at the position corresponding to I112 in H3. For this pair of lysines at the putative CID:CID interface the lysines are predicted to be ~20 Å apart and oriented so their side chains are in opposite directions (Luger et al. 1997). Less efficient crosslinking of an octameric species, in the case of CID nucleosomes, is therefore the expected result, even if they exist in an octameric form with two copies of CID.
Interpreting the “height” of DNA complexes with AFM is not as straightforward as it initially seems. Well-established AFM distortions measure the height of double stranded DNA at 0.5–0.8 nm, instead of its native 2.0–2.5 nm diameter (Dalal et al. 2007; Klinov et al. 2009). Further, since there is no additional height reported from co-purifying centromere components (e.g. CENP-B, CENP-C, and presumably other CCAN components), the ~1.7 nm CENP-A nucleosome height and the ~3.5 nm bulk chromatin nucleosome height (Dalal et al. 2007; Dimitriadis et al. 2010) are each likely primarily contributed by the number DNA wraps/crosses, not the protein constituents. While height difference has been interpreted as evidence for a hemisome, it is also consistent with changes in DNA wrapping within an octameric nucleosome occurring during CENP-A nucleosome immuno-purification (Dalal et al. 2007; Dimitriadis et al. 2010), either as a consequence of proposed changes of nucleosome shape (Sekulic et al. 2010)or as a function of actual differences in DNA wrapping in native chromatin. To the latter point, reconstituted CENP-A nucleosomes do not favor the conventional crossed DNA at the entry/exit site and likewise also disfavor linker histone binding (Conde e Silva et al. 2007). The altered DNA crossing arises from weakening of the interaction between the αN helix of human CENP-A and the entry/exit DNA that fails to clamp as well as the corresponding αN helix of H3 the final turn of nucleosomal DNA(Conde e Silva et al. 2007). Similar loss of tight binding at the DNA entry/exit site is also the case for budding yeast CENP-A which forms octameric nucleosomes that wrap only ~125 bp of DNA (i.e. ~one turn of DNA missing at each DNA entry/exit site)(Kingston et al. 2011).
For the in vitro supercoiling experiments that were interpreted to indicate unconventional right handed DNA wrapping of CENP-ACid-containing nucleosomes, Prunell and colleagues (Lavelle et al. 2009) have argued that there are equally plausible, alternative interpretations. Included here are unconventional intra- and inter-histone particle interactions of one type or a mixture of tetrasomes (CENP-A:H4)2, hexasomes (CENP-A:H4)2(H2A:H2B), or octameric nucleosomes (CENP-A:H4:H2A:H2B)2.
Perhaps the most persuasive of the evidence for right handed DNA wrapping in centromeric chromatin was the loss, when functional centromere sequences were included, of two negative supercoils on a budding yeast minichromosome of a size that could accommodate nine conventional nucleosomes yeast (Furuyama and Henikoff 2009). Rather than positive supercoiling at the centromere, the reduction in negative supercoiling could also indicate that the inclusion of a centromere and the centromere proteins and cohesins recruited to it sterically blocks assembly of more than one adjacent conventional nucleosome (and the negative supercoils they impart). This latter interpretation is firmly supported by the altered DNAase sensitivity known to flank yeast centromeres (Bloom and Carbon 1982)and the major higher order conformational alterations that yeast centromeres impart to such minichromosomes(Surcel et al. 2008).
In sum, the issue of whether CENP-A marks centromeres by conferring distinguishing physical properties to octameric nucleosomes into which it assembles (Black et al. 2007a; Sekulic et al. 2010), or whether (and if so how) it somehow overcomes its intrinsic spontaneous assembly into octameric nucleosomes in favor of a hemisome (Dalal et al. 2007; Furuyama and Henikoff 2009), awaits future definitive experimentation. In this vein, it important to remember that hemisomes/nucleosomes were debated in the 1970s for conventional nucleosomes (for example, see (Weintraub et al. 1976)) and many complementary approaches with native chromatin and purified components were required to form our current view of the relevant major nucleosomal form in chromatin.
Closure of the Epigenetic Feedback Loop: A Model for CENP-A Targeting to the Centromere
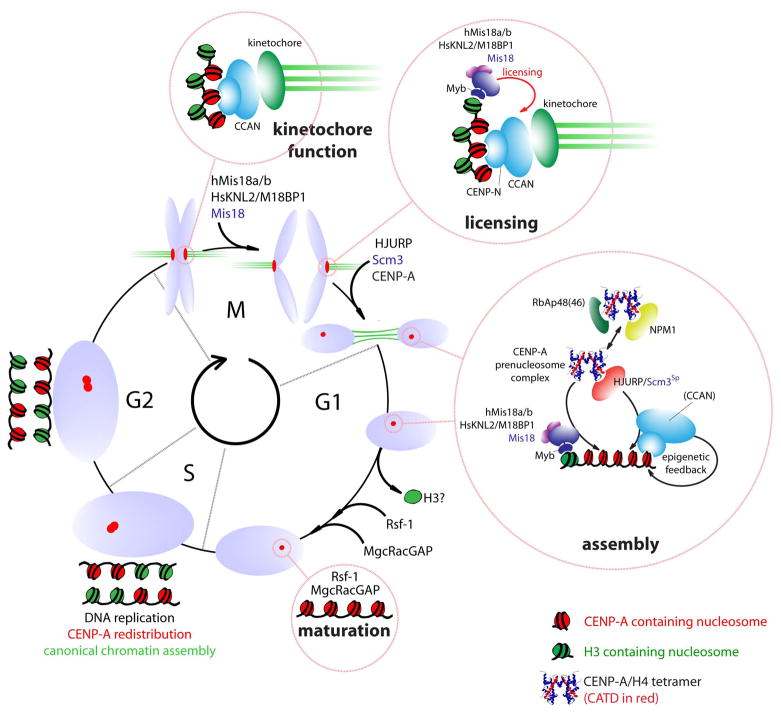
CENP-A assembly at centromeres in human cells is affected not only by the G1 phase-specific residents described above (Mis18 complex (Fujita et al. 2007; Maddox et al. 2007) and HJURP (Dunleavy et al. 2009; Foltz et al. 2009)) but also by members of the constitutive centromere complex (CCAN) and the Mis12 complex (Kline et al. 2006; Okada et al. 2006; Carroll et al. 2010). Merging all of these findings supports a model where the CENP-A prenucleosome complex is targeted to centromeres by HJURP through an interaction either with constitutive centromere components or by binding the Mis18:KNL2 proteins(Fig. 9). There is some support for the latter possibility in fission yeast. Scm3Sp interacts with Mis18 in pull downs as well as in vitro assays, potentially providing a molecular link between CENP-A and the Mis18 proteins (Pidoux et al. 2009).
Figure 9.
Model representing the centromeric chromatin cycle. CENP-A-containing nucleosomes are redistributed during DNA replication in S phase. The resulting mixed CENP-A/H3 chromatin supports kinetochore formation during mitotis. An unknown mitotic signal triggers “licensing” of centromeres by Mis18:KNL2 proteins in anaphase for subsequence assembly of CENP-A. The molecular nature of the licensing step is unknown but is likely precedes recruitment of CENP-A as a separate step. Assembly of new CENP-A is mediated by the HJURP-containing prenucleosomal complex throughout the first hours of G1. Targeting of the prenucleosomal complex may involve the Mis18:KNL2 proteins or CCAN members. Assembly of CENP-A is predicted to involve exchange of H3 from centromeric chromatin and may require additional activities provided by the RSF complex and the small GTPase activating protein MgcRacGAP in late G1 (provisionally termed ‘maturation’).
How then are the Mis18:KNL2 proteins targeted to the centromeres? The human Mis18: KNL2 proteins arrives at the centromere in a manner that is largely unaffected by RNAi-mediated knockdown of CENP-A protein(Hayashi et al. 2004; Fujita et al. 2007). Consistent with this, M18BP1/KNL2 protein harbors a divergent Myb/SANT domain(Boyer et al. 2004; Maddox et al. 2007), suggesting the complex may be targeted directly to DNA or histone tails. Perhaps the Mis18:KNL2 proteins may target to centromeres somewhat distal from CENP-A nucleosomes and “license” the centromere for recruitment of new CENP-A nucleosomes. In human cells, the consequences of hMis18α depletion apparently can be partially alleviated by experimentally increasing global acetylation levels(Fujita et al. 2007), consistent with acetylation of an as of yet unknown target as a central step involved in centromere licensing for CENP-A assembly.
How the HJURP:CENP-A:H4 complex targets to the centromere in human G1 cells is unknown. Members of the CCAN would be potential candidates. In light of this, it is noteworthy that the constitutive centromere protein CENP-N not only binds to CENP-A nucleosomes directly but also affects CENP-A assembly (Carroll et al. 2010). This may represent an epigenetic feedback loop where the reader (CENP-N) of the epigenetic mark (the CENP-A nucleosome) contributes to the propagation of that mark. This may explain, in part, how epigenetic centromere identity provided by the CENP-A nucleosome is transferred to the next generation of nucleosomes in subsequent cell divisions(Fig. 9).
Implications of G1 CENP-A Assembly for Centromere Function and Inheritance
The abrupt onset of CENP-A assembly into centromeric chromatin exclusively after reentry into G1, but not in mitosis, has important implications for epigenetic centromere inheritance. First, the temporal disconnect between CENP-A assembly in G1 and histone H3 loading in bulk chromatin in S-phase may contribute to providing specificity in the assembly of these related histone H3 family members. Second, loading of new CENP-A following mitosis dictates that centromeres and the kinetochores assembled on them proceed through mitosis with only half the complement of CENP-A. During S phase, CENP-A protein is diluted among sister centromeres, leaving CENP-A missing from half the DNA sequences occupied by it prior to DNA replication. What protein complexes are loaded onto these sites has not been established, although it seems most likely that they are occupied by typical histone H3.1-containing nucleosomes, which are available in excess during DNA replication.
In agreement with this view, histone H3-containing nucleosomes have been detected on mitotic centromeres interspersed with CENP-A-containing nucleosomes and have been shown to occupy centromeric chromatin when CENP-A levels are depleted (Blower et al. 2002; Sullivan and Karpen 2004). G1 assembly of CENP-A directly suggests that H3 found interspersed with CENP-A on mitotic chromosomes may represent the pool that acts as a placeholder for exchange with CENP-A later. In this way, H3 represents an integral part of mitotic centromeric chromatin that may help promote kinetochore formation during mitosis. Indeed, while the constitutive centromere proteins CENP-T and CENP-W depend on CENP-A for their association with centromeres, they also make direct contacts with H3 containing nucleosomes(Hori et al. 2008a; Ribeiro et al. 2010).
Unresolved: What Activates Centromeric Chromatin Replication at Exit from Mitosis?
The onset of CENP-A assembly at centromeres at the end of mitosis firmly supports a model in which loading of CENP-A requires inactivation of one or more inhibitors at mitotic exit or activation of one or more key components by entry into early G1. An early proposal hypothesized a role for microtubule-mediated tension generated across centromeric chromatin in signaling CENP-A assembly (Ahmad and Henikoff 2002a; Mellone and Allshire 2003; Allshire and Karpen 2008). This possibility has been falsified, at least in a strict since, as exit from mitosis in which all kinetochore microtubule attachment was blocked still triggered comparable CENP-A loading at centromeres in the subsequent G1(Jansen et al. 2007; Schuh et al. 2007). A role for a putative diffusible, cytoplasmic factor sufficient to trigger CENP-A assembly has also been eliminated by cell-cell fusion experiments in which G2 phase cells were fused to G1 phase cells. Under those conditions the G1 derived nucleus was proficient in assembly of new CENP-A while the G2 derived nucleus was not, despite bearing a nascent, unloaded pool of CENP-A and sharing the same cytoplasm with the actively loading G1 nucleus (Jansen et al. 2007).
A highly selective screen for factors that affect CENP-A levels at the centromere in Drosophila tissue culture cells identified four components, two of which are cell cycle regulators, Cyclin A and RCA1 (known as Emi1 in mammalian cells)(Erhardt et al. 2008). Both of these factors negatively regulate the anaphase promoting complex (APC) activity prior to mitosis (Zachariae et al. 1998; Di Fiore and Pines 2007). Although an S/G2 arrest and concurrent endoreduplication (which results from depletion of either of these factors)was ruled out as the cause for CENP-ACID delocalization, it is not at all obvious how inhibition of APC activity would positively influence CENP-A assembly. Nevertheless, a report of a proportion of cyclin A bound at centromeres has implicated this factor in centromere maintenance in a direct manner (Erhardt et al. 2008), albeit this too is perplexing as cyclin A is destroyed midway through mitosis, yet CENP-A loading is delayed until earliest G1.
In sum, the critical signal to initiate post-mitotic centromeric chromatin replication remains to be identified. Possibilities include that CENP-A assembly and/or the recruitment of assembly factors at the centromere is dependent on 1) conditioning of chromatin during mitosis (Mellone and Allshire 2003; Jansen et al. 2007), 2) nuclear envelope breakdown, thereby allowing access to chromatin of a specific assembly factor, 3) kinetochore assembly and/or disassembly(Jansen et al. 2007; Allshire and Karpen 2008), or 4) mitotic modification of CENP-A itself. Any of these could create an environment that is permissive for subsequent CENP-A loading. An additional possibility is that components of the greater centromere/kinetochore (and which are therefore recruited in a CENP-A-dependent manner) may in turn affect CENP-A loading or stabilization after loading. Indeed, defects in structural centromere proteins have been shown to affect CENP-A levels which includes proteins that assemble during mitosis (Kline et al. 2006; Okada et al. 2006; Carroll et al. 2010).
Outlook
The centromere is the chromosomal element whose job is to insure faithful Mendelian inheritance of chromosomes, yet the molecular basis for specifying centromere identity and its maintenance through generations are just now being uncovered. While substantial progress has been made in determining how CENP-A physically marks the chromatin into which it assembles, defining when newly expressed CENP-A is deposited into centromeric chromatin each cell cycle, and identifying the proteins recruited to centromeric nucleosomes and those that chaperone newly made CENP-A to the centromeres, major issues remain to be resolved for understanding of how centromeres are epigenetically replicated every cell cycle (Fig. 9). Issues of immediate attention are resolving what are the major oligomeric form(s)of CENP-A nucleosomes through the cell cycle, characterizing the regulatory mechanisms that restrict newly expressed CENP-A centromere deposition to G1, and understanding the precise role of the HJURP chaperone in the assembly reaction that culminates in deposition of CENP-A into centromeric chromatin.
Acknowledgments
This work was supported by grants from the National Institutes of Health to B.E.B. (GM82989) and D.W.C. (GM74150), the American Cancer Society to D.R.F. (RSG-09-165-01), and the Fundação Calouste Gulbenkian, the Fundação para a Ciência e a Tecnologia (BIA-BCM/100557/2008& BIA-PRO/100537/2008), the European Commission FP7 programme, and an EMBO installation grant to L.E.T.J. B.E.B. is also supported by a Career Award in the Biomedical Sciences from the Burroughs Wellcome Fund and a Rita Allen Foundation Scholar Award. D.W.C. receives salary support from the Ludwig Institute for Cancer Research. We thank E. Bassett, N. Sekulic, and S. Wood from the Black laboratory for providing data figures.
References
- Ahmad K, Henikoff S. Histone H3 variants specify modes of chromatin assembly. Proc Natl Acad Sci U S A. 2002a;99(Suppl 4):16477–16484. doi: 10.1073/pnas.172403699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad K, Henikoff S. The histone variant H3.3 marks active chromatin by replication-independent nucleosome assembly. Mol Cell. 2002b;9(6):1191–1200. doi: 10.1016/s1097-2765(02)00542-7. [DOI] [PubMed] [Google Scholar]
- Allshire RC, Karpen GH. Epigenetic regulation of centromeric chromatin: old dogs, new tricks? Nat Rev Genet. 2008;9(12):923–937. doi: 10.1038/nrg2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano M, Suzuki A, Hori T, Backer C, Okawa K, Cheeseman IM, Fukagawa T. The CENP-S complex is essential for the stable assembly of outer kinetochore structure. J Cell Biol. 2009;186(2):173–182. doi: 10.1083/jcb.200903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor DJ, Bentley K, Ryan J, Perry J, Wong L, Slater H, Choo KH. Human centromere repositioning “in progress”. Proc Natl Acad Sci U S A. 2004;101(17):6542–6547. doi: 10.1073/pnas.0308637101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziato AT. Split decision: what happens to nucleosomes during DNA replication? J Biol Chem. 2005;280(13):12065–12068. doi: 10.1074/jbc.R400039200. [DOI] [PubMed] [Google Scholar]
- Bassett EA, Wood S, Salimian KJ, Ajith S, Foltz DR, Black BE. Epigenetic centromere specification directs Aurora B accumulation but is insufficient for correcting mitotic errors. J Cell Biol. 2010;190(2):177–185. doi: 10.1083/jcb.201001035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann JH, Rodriguez MG, Martins NM, Kimura H, Kelly DA, Masumoto H, Larionov V, Jansen LE, Earnshaw WC. Epigenetic engineering shows H3K4me2 is required for HJURP targeting and CENP-A assembly on a synthetic human kinetochore. Embo J. 2011 doi: 10.1038/emboj.2010.329. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Bassett EA. The histone variant CENP-A and centromere specification. Curr Opin Cell Biol. 2008;20(1):91–100. doi: 10.1016/j.ceb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Black BE, Brock MA, Bédard S, Woods VL, Cleveland DW. An epigenetic mark generated by the incorporation of CENP-A into centromeric nucleosomes. Proc Natl Acad Sci U S A. 2007a;104:5008–5013. doi: 10.1073/pnas.0700390104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BE, Foltz DR, Chakravarthy S, Luger K, Woods VL, Cleveland DW. Structural determinants for generating centromeric chromatin. Nature. 2004;430(6999):578–582. doi: 10.1038/nature02766. [DOI] [PubMed] [Google Scholar]
- Black BE, Jansen LET, Maddox PS, Foltz DR, Desai AB, Shah JV, Cleveland DW. Centromere identity maintained by nucleosomes assembled with histone H3 containing the CENP-A targeting domain. Mol Cell. 2007b;25(2):309–322. doi: 10.1016/j.molcel.2006.12.018. [DOI] [PubMed] [Google Scholar]
- Bloom KS, Carbon J. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell. 1982;29(2):305–317. doi: 10.1016/0092-8674(82)90147-7. [DOI] [PubMed] [Google Scholar]
- Blower MD, Sullivan BA, Karpen GH. Conserved organization of centromeric chromatin in flies and humans. Dev Cell. 2002;2(3):319–330. doi: 10.1016/s1534-5807(02)00135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer LA, Latek RR, Peterson CL. The SANT domain: a unique histone-tail-binding module? Nat Rev Mol Cell Biol. 2004;5(2):158–163. doi: 10.1038/nrm1314. [DOI] [PubMed] [Google Scholar]
- Buchwitz BJ, Ahmad K, Moore LL, Roth MB, Henikoff S. A histone-H3-like protein in C. elegans. Nature. 1999;401(6753):547–548. doi: 10.1038/44062. [DOI] [PubMed] [Google Scholar]
- Camahort R, Shivaraju M, Mattingly M, Li B, Nakanishi S, Zhu D, Shilatifard A, Workman JL, Gerton JL. Cse4 is part of an octameric nucleosome in budding yeast. Mol Cell. 2009;35(6):794–805. doi: 10.1016/j.molcel.2009.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone L, Nergadze SG, Magnani E, Misceo D, Francesca Cardone M, Roberto R, Bertoni L, Attolini C, Francesca Piras M, de Jong P, et al. Evolutionary movement of centromeres in horse, donkey, and zebra. Genomics. 2006;87(6):777–782. doi: 10.1016/j.ygeno.2005.11.012. [DOI] [PubMed] [Google Scholar]
- Carroll CW, Milks KJ, Straight AF. Dual recognition of CENP-A nucleosomes is required for centromere assembly. J Cell Biol. 2010;189(7):1143–1155. doi: 10.1083/jcb.201001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll CW, Silva MC, Godek KM, Jansen LE, Straight AF. Centromere assembly requires the direct recognition of CENP-A nucleosomes by CENP-N. Nat Cell Biol. 2009;11(7):896–902. doi: 10.1038/ncb1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheeseman IM, Hori T, Fukagawa T, Desai A. KNL1 and the CENP-H/I/K complex coordinately direct kinetochore assembly in vertebrates. Mol Biol Cell. 2008;19(2):587–594. doi: 10.1091/mbc.E07-10-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo KH. Domain organization at the centromere and neocentromere. Dev Cell. 2001;1(2):165–177. doi: 10.1016/s1534-5807(01)00028-4. [DOI] [PubMed] [Google Scholar]
- Clarke L, Carbon J. Isolation of a yeast centromere and construction of functional small circular chromosomes. Nature. 1980;287(5782):504–509. doi: 10.1038/287504a0. [DOI] [PubMed] [Google Scholar]
- Conde e Silva N, Black BE, Sivolob A, Filipski J, Cleveland DW, Prunell A. CENP-A-containing nucleosomes: easier disassembly versus exclusive centromeric localization. J Mol Biol. 2007;370(3):555–573. doi: 10.1016/j.jmb.2007.04.064. [DOI] [PubMed] [Google Scholar]
- Csink AK, Henikoff S. Something from nothing: the evolution and utility of satellite repeats. Trends Genet. 1998;14(5):200–204. doi: 10.1016/s0168-9525(98)01444-9. [DOI] [PubMed] [Google Scholar]
- Dalal Y, Wang H, Lindsay S, Henikoff S. Tetrameric structure of centromeric nucleosomes in interphase Drosophila cells. PLoS Biol. 2007;5(8):e218. doi: 10.1371/journal.pbio.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depinet TW, Zackowski JL, Earnshaw WC, Kaffe S, Sekhon GS, Stallard R, Sullivan BA, Vance GH, Van Dyke DL, Willard HF, et al. Characterization of neo-centromeres in marker chromosomes lacking detectable alpha-satellite DNA. Hum Mol Genet. 1997;6(8):1195–1204. doi: 10.1093/hmg/6.8.1195. [DOI] [PubMed] [Google Scholar]
- Di Fiore B, Pines J. Emi1 is needed to couple DNA replication with mitosis but does not regulate activation of the mitotic APC/C. J Cell Biol. 2007;177(3):425–437. doi: 10.1083/jcb.200611166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadis EK, Weber C, Gill RK, Diekmann S, Dalal Y. Tetrameric organization of vertebrate centromeric nucleosomes. Proc Natl Acad Sci U S A. 2010;107(47):20317–20322. doi: 10.1073/pnas.1009563107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Sart D, Cancilla MR, Earle E, Mao JI, Saffery R, Tainton KM, Kalitsis P, Martyn J, Barry AE, Choo KH. A functional neo-centromere formed through activation of a latent human centromere and consisting of non-alpha-satellite DNA. Nat Genet. 1997;16(2):144–153. doi: 10.1038/ng0697-144. [DOI] [PubMed] [Google Scholar]
- Dunleavy EM, Roche D, Tagami H, Lacoste N, Ray-Gallet D, Nakamura Y, Daigo Y, Nakatani Y, Almouzni-Pettinotti G. HJURP is a cell-cycle-dependent maintenance and deposition factor of CENP-A at centromeres. Cell. 2009;137(3):485–497. doi: 10.1016/j.cell.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Earnshaw W, Bordwell B, Marino C, Rothfield N. Three human chromosomal autoantigens are recognized by sera from patients with anti-centromere antibodies. J Clin Invest. 1986;77(2):426–430. doi: 10.1172/JCI112320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw WC, Migeon BR. Three related centromere proteins are absent from the inactive centromere of a stable isodicentric chromosome. Chromosoma. 1985;92(4):290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3–4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- English CM, Adkins MW, Carson JJ, Churchill ME, Tyler JK. Structural basis for the histone chaperone activity of Asf1. Cell. 2006;127(3):495–508. doi: 10.1016/j.cell.2006.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English CM, Maluf NK, Tripet B, Churchill ME, Tyler JK. ASF1 binds to a heterodimer of histones H3 and H4: a two-step mechanism for the assembly of the H3–H4 heterotetramer on DNA. Biochemistry. 2005;44(42):13673–13682. doi: 10.1021/bi051333h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erhardt S, Mellone BG, Betts CM, Zhang W, Karpen GH, Straight AF. Genome-wide analysis reveals a cell cycle-dependent mechanism controlling centromere propagation. J Cell Biol. 2008;183(5):805–818. doi: 10.1083/jcb.200806038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald-Hayes M, Clarke L, Carbon J. Nucleotide sequence comparisons and functional analysis of yeast centromere DNAs. Cell. 1982;29(1):235–244. doi: 10.1016/0092-8674(82)90108-8. [DOI] [PubMed] [Google Scholar]
- Foltz DR, Jansen LE, Bailey AO, Yates JR, 3rd, Bassett EA, Wood S, Black BE, Cleveland DW. Centromere-specific assembly of CENP-A nucleosomes is mediated by HJURP. Cell. 2009;137(3):472–484. doi: 10.1016/j.cell.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz DR, Jansen LET, Black BE, Bailey AO, Yates JR, 3rd, Cleveland DW. The human CENP-A centromeric nucleosome-associated complex. Nat Cell Biol. 2006;8:458–469. doi: 10.1038/ncb1397. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Hayashi T, Kiyomitsu T, Toyoda Y, Kokubu A, Obuse C, Yanagida M. Priming of Centromere for CENP-A Recruitment by Human hMis18alpha, hMis18beta, and M18BP1. Dev Cell. 2007;12(1):17–30. doi: 10.1016/j.devcel.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Furuyama T, Henikoff S. Centromeric nucleosomes induce positive DNA supercoils. Cell. 2009;138(1):104–113. doi: 10.1016/j.cell.2009.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groth A, Corpet A, Cook AJ, Roche D, Bartek J, Lukas J, Almouzni G. Regulation of replication fork progression through histone supply and demand. Science. 2007a;318(5858):1928–1931. doi: 10.1126/science.1148992. [DOI] [PubMed] [Google Scholar]
- Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007b;128(4):721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Han F, Lamb JC, Birchler JA. High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize. Proc Natl Acad Sci U S A. 2006;103(9):3238–3243. doi: 10.1073/pnas.0509650103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Fujita Y, Iwasaki O, Adachi Y, Takahashi K, Yanagida M. Mis16 and Mis18 are required for CENP-A loading and histone deacetylation at centromeres. Cell. 2004;118(6):715–729. doi: 10.1016/j.cell.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Hayes F, Barilla D. Assembling the bacterial segrosome. Trends Biochem Sci. 2006;31(5):247–250. doi: 10.1016/j.tibs.2006.03.002. [DOI] [PubMed] [Google Scholar]
- Hemmerich P, Weidtkamp-Peters S, Hoischen C, Schmiedeberg L, Erliandri I, Diekmann S. Dynamics of inner kinetochore assembly and maintenance in living cells. J Cell Biol. 2008;180(6):1101–1114. doi: 10.1083/jcb.200710052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henikoff S, Ahmad K, Malik HS. The centromere paradox: stable inheritance with rapidly evolving DNA. Science. 2001;293(5532):1098–1102. doi: 10.1126/science.1062939. [DOI] [PubMed] [Google Scholar]
- Hori T, Amano M, Suzuki A, Backer CB, Welburn JP, Dong Y, McEwen BF, Shang WH, Suzuki E, Okawa K, et al. CCAN makes multiple contacts with centromeric DNA to provide distinct pathways to the outer kinetochore. Cell. 2008a;135(6):1039–1052. doi: 10.1016/j.cell.2008.10.019. [DOI] [PubMed] [Google Scholar]
- Hori T, Okada M, Maenaka K, Fukagawa T. CENP-O class proteins form a stable complex and are required for proper kinetochore function. Mol Biol Cell. 2008b;19(3):843–854. doi: 10.1091/mbc.E07-06-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howman EV, Fowler KJ, Newson AJ, Redward S, MacDonald AC, Kalitsis P, Choo KH. Early disruption of centromeric chromatin organization in centromere protein A (Cenpa) null mice. Proc Natl Acad Sci U S A. 2000;97(3):1148–1153. doi: 10.1073/pnas.97.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson V. In vivo studies on the dynamics of histone-DNA interaction: evidence for nucleosome dissolution during replication and transcription and a low level of dissolution independent of both. Biochemistry. 1990;29(3):719–731. doi: 10.1021/bi00455a019. [DOI] [PubMed] [Google Scholar]
- Jansen LE, Black BE, Foltz DR, Cleveland DW. Propagation of centromeric chromatin requires exit from mitosis. J Cell Biol. 2007;176(6):795–805. doi: 10.1083/jcb.200701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Birchler JA, Parrott WA, Dawe RK. A molecular view of plant centromeres. Trends Plant Sci. 2003;8(12):570–575. doi: 10.1016/j.tplants.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Kato T, Sato N, Hayama S, Yamabuki T, Ito T, Miyamoto M, Kondo S, Nakamura Y, Daigo Y. Activation of Holliday junction recognizing protein involved in the chromosomal stability and immortality of cancer cells. Cancer Res. 2007;67(18):8544–8553. doi: 10.1158/0008-5472.CAN-07-1307. [DOI] [PubMed] [Google Scholar]
- Keppler A, Gendreizig S, Gronemeyer T, Pick H, Vogel H, Johnsson K. A general method for the covalent labeling of fusion proteins with small molecules in vivo. Nat Biotechnol. 2003;21(1):86–89. doi: 10.1038/nbt765. [DOI] [PubMed] [Google Scholar]
- Keppler A, Pick H, Arrivoli C, Vogel H, Johnsson K. Labeling of fusion proteins with synthetic fluorophores in live cells. Proc Natl Acad Sci U S A. 2004;101(27):9955–9959. doi: 10.1073/pnas.0401923101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston IJ, Yung JS, Singleton MR. Biophysical characterisation of the centromere-specific nucleosome from budding yeast. J Biol Chem. 2011 doi: 10.1074/jbc.M110.189340. Published online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline SL, Cheeseman IM, Hori T, Fukagawa T, Desai A. The human Mis12 complex is required for kinetochore assembly and proper chromosome segregation. J Cell Biol. 2006;173(1):9–17. doi: 10.1083/jcb.200509158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinov DV, Neretina TV, Prokhorov VV, Dobrynina TV, Aldarov KG, Demin VV. High-resolution atomic force microscopy of DNA. Biochemistry (Mosc) 2009;74(10):1150–1154. doi: 10.1134/s0006297909100113. [DOI] [PubMed] [Google Scholar]
- Kornberg RD, Thomas JO. Chromatin structure; oligomers of the histones. Science. 1974;184(139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Lagana A, Dorn JF, De Rop V, Ladouceur AM, Maddox AS, Maddox PS. A small GTPase molecular switch regulates epigenetic centromere maintenance by stabilizing newly incorporated CENP-A. Nat Cell Biol. 2010;12(12):1186–1193. doi: 10.1038/ncb2129. [DOI] [PubMed] [Google Scholar]
- Lavelle C, Recouvreux P, Wong H, Bancaud A, Viovy JL, Prunell A, Victor JM. Right-handed nucleosome: myth or reality? Cell. 2009;139(7):1216–1217. doi: 10.1016/j.cell.2009.12.014. author reply 1217–1218. [DOI] [PubMed] [Google Scholar]
- Lechner J, Carbon J. A 240 kd multisubunit protein complex, CBF3, is a major component of the budding yeast centromere. Cell. 1991;64(4):717–725. doi: 10.1016/0092-8674(91)90501-o. [DOI] [PubMed] [Google Scholar]
- Luger K, Mader AW, Richmond RK, Sargent DF, Richmond TJ. Crystal structure of the nucleosome core particle at 2.8 A resolution. Nature. 1997;389(6648):251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- Maddox PS, Hyndman F, Monen J, Oegema K, Desai A. Functional genomics identifies a Myb domain-containing protein family required for assembly of CENP-A chromatin. J Cell Biol. 2007;176(6):757–763. doi: 10.1083/jcb.200701065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L, Wu JC. Homology between human and simian repeated DNA. Nature. 1978;276(5683):92–94. doi: 10.1038/276092a0. [DOI] [PubMed] [Google Scholar]
- Mellone BG, Allshire RC. Stretching it: putting the CEN(P-A) in centromere. Curr Opin Genet Dev. 2003;13(2):191–198. doi: 10.1016/s0959-437x(03)00019-4. [DOI] [PubMed] [Google Scholar]
- Mizuguchi G, Xiao H, Wisniewski J, Smith MM, Wu C. Nonhistone Scm3 and histones CenH3-H4 assemble the core of centromere-specific nucleosomes. Cell. 2007;129(6):1153–1164. doi: 10.1016/j.cell.2007.04.026. [DOI] [PubMed] [Google Scholar]
- Moggs JG, Grandi P, Quivy JP, Jonsson ZO, Hubscher U, Becker PB, Almouzni G. A CAF-1-PCNA-mediated chromatin assembly pathway triggered by sensing DNA damage. Mol Cell Biol. 2000;20(4):1206–1218. doi: 10.1128/mcb.20.4.1206-1218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy WJ, Larkin DM, Everts-van der Wind A, Bourque G, Tesler G, Auvil L, Beever JE, Chowdhary BP, Galibert F, Gatzke L, et al. Dynamics of mammalian chromosome evolution inferred from multispecies comparative maps. Science. 2005;309(5734):613–617. doi: 10.1126/science.1111387. [DOI] [PubMed] [Google Scholar]
- Natsume R, Eitoku M, Akai Y, Sano N, Horikoshi M, Senda T. Structure and function of the histone chaperone CIA/ASF1 complexed with histones H3 and H4. Nature. 2007;446(7133):338–341. doi: 10.1038/nature05613. [DOI] [PubMed] [Google Scholar]
- O’Keefe RT, Henderson SC, Spector DL. Dynamic organization of DNA replication in mammalian cell nuclei: spatially and temporally defined replication of chromosome-specific alpha-satellite DNA sequences. J Cell Biol. 1992;116(5):1095–1110. doi: 10.1083/jcb.116.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obuse C, Yang H, Nozaki N, Goto S, Okazaki T, Yoda K. Proteomics analysis of the centromere complex from HeLa interphase cells: UV-damaged DNA binding protein 1 (DDB-1) is a component of the CEN-complex, while BMI-1 is transiently co-localized with the centromeric region in interphase. Genes Cells. 2004;9(2):105–120. doi: 10.1111/j.1365-2443.2004.00705.x. [DOI] [PubMed] [Google Scholar]
- Okada M, Cheeseman IM, Hori T, Okawa K, McLeod IX, Yates JR, 3rd, Desai A, Fukagawa T. The CENP-H-I complex is required for the efficient incorporation of newly synthesized CENP-A into centromeres. Nat Cell Biol. 2006;8(5):446–457. doi: 10.1038/ncb1396. [DOI] [PubMed] [Google Scholar]
- Okada M, Okawa K, Isobe T, Fukagawa T. CENP-H-containing complex facilitates centromere deposition of CENP-A in cooperation with FACT and CHD1. Mol Biol Cell. 2009;20(18):3986–3995. doi: 10.1091/mbc.E09-01-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orphanides G, LeRoy G, Chang CH, Luse DS, Reinberg D. FACT, a factor that facilitates transcript elongation through nucleosomes. Cell. 1998;92(1):105–116. doi: 10.1016/s0092-8674(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Palmer DK, Margolis RL. Kinetochore components recognized by human autoantibodies are present on mononucleosomes. Mol Cell Biol. 1985;5(1):173–186. doi: 10.1128/mcb.5.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perpelescu M, Nozaki N, Obuse C, Yang H, Yoda K. Active establishment of centromeric CENP-A chromatin by RSF complex. J Cell Biol. 2009;185(3):397–407. doi: 10.1083/jcb.200903088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux AL, Choi ES, Abbott JK, Liu X, Kagansky A, Castillo AG, Hamilton GL, Richardson W, Rappsilber J, He X, et al. Fission yeast Scm3: A CENP-A receptor required for integrity of subkinetochore chromatin. Mol Cell. 2009;33(3):299–311. doi: 10.1016/j.molcel.2009.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnier V, Vagnarelli P, Fukagawa T, Zerjal T, Burns E, Trouche D, Earnshaw W, Brown W. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol Cell Biol. 2005;25(10):3967–3981. doi: 10.1128/MCB.25.10.3967-3981.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro SA, Vagnarelli P, Dong Y, Hori T, McEwen BF, Fukagawa T, Flors C, Earnshaw WC. A super-resolution map of the vertebrate kinetochore. Proc Natl Acad Sci U S A. 2010;107(23):10484–10489. doi: 10.1073/pnas.1002325107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santaguida S, Musacchio A. The life and miracles of kinetochores. Embo J. 2009;28(17):2511–2531. doi: 10.1038/emboj.2009.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh M, Lehner CF, Heidmann S. Incorporation of Drosophila CID/CENP-A and CENP-C into centromeres during early embryonic anaphase. CurrBiol. 2007;17(3):237–243. doi: 10.1016/j.cub.2006.11.051. [DOI] [PubMed] [Google Scholar]
- Sekulic N, Bassett EA, Rogers DJ, Black BE. The structure of (CENP-A-H4)2 reveals physical features that mark centromeres. Nature. 2010;467:347–351. doi: 10.1038/nature09323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby RD, Monier K, Sullivan KF. Chromatin assembly at kinetochores is uncoupled from DNA replication. J Cell Biol. 2000;151(5):1113–1118. doi: 10.1083/jcb.151.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelby RD, Vafa O, Sullivan KF. Assembly of CENP-A into centromeric chromatin requires a cooperative array of nucleosomal DNA contact sites. J Cell Biol. 1997;136(3):501–513. doi: 10.1083/jcb.136.3.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara K, Stillman B. Replication-dependent marking of DNA by PCNA facilitates CAF-1-coupled inheritance of chromatin. Cell. 1999;96(4):575–585. doi: 10.1016/s0092-8674(00)80661-3. [DOI] [PubMed] [Google Scholar]
- Shuaib M, Ouararhni K, Dimitrov S, Hamiche A. HJURP binds CENP-A via a highly conserved N-terminal domain and mediates its deposition at centromeres. Proc Natl Acad Sci U S A. 2010;107(4):1349–1354. doi: 10.1073/pnas.0913709107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MC, Jansen LE. At the right place at the right time: novel CENP-A binding proteins shed light on centromere assembly. Chromosoma. 2009;118(5):567–574. doi: 10.1007/s00412-009-0227-3. [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B. Purification and characterization of CAF-I, a human cell factor required for chromatin assembly during DNA replication in vitro. Cell. 1989;58(1):15–25. doi: 10.1016/0092-8674(89)90398-x. [DOI] [PubMed] [Google Scholar]
- Smith S, Stillman B. Stepwise assembly of chromatin during DNA replication in vitro. Embo J. 1991;10(4):971–980. doi: 10.1002/j.1460-2075.1991.tb08031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoler S, Keith KC, Curnick KE, Fitzgerald-Hayes M. A mutation in CSE4, an essential gene encoding a novel chromatin-associated protein in yeast, causes chromosome nondisjunction and cell cycle arrest at mitosis. Genes Dev. 1995;9(5):573–586. doi: 10.1101/gad.9.5.573. [DOI] [PubMed] [Google Scholar]
- Suda M, Iwai K. Identification of suberimidate cross-linking sites of four histone sequences in H1-depleted chromatin. Histone arrangement in nucleosome core. J Biochem. 1979;86(6):1659–1670. doi: 10.1093/oxfordjournals.jbchem.a132686. [DOI] [PubMed] [Google Scholar]
- Sullivan BA, Karpen GH. Centromeric chromatin exhibits a histone modification pattern that is distinct from both euchromatin and heterochromatin. Nat Struct Mol Biol. 2004;11(11):1076–1083. doi: 10.1038/nsmb845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan BA, Schwartz S. Identification of centromeric antigens in dicentric Robertsonian translocations: CENP-C and CENP-E are necessary components of functional centromeres. Hum Mol Genet. 1995;4(12):2189–2197. doi: 10.1093/hmg/4.12.2189. [DOI] [PubMed] [Google Scholar]
- Sullivan KF, Hechenberger M, Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol. 1994;127(3):581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surcel A, Koshland D, Ma H, Simpson RT. Cohesin interaction with centromeric minichromosomes shows a multi-complex rod-shaped structure. PLoS One. 2008;3(6):e2453. doi: 10.1371/journal.pone.0002453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagami H, Ray-Gallet D, Almouzni G, Nakatani Y. Histone H3.1 and H3.3 complexes mediate nucleosome assembly pathways dependent or independent of DNA synthesis. Cell. 2004;116(1):51–61. doi: 10.1016/s0092-8674(03)01064-x. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Chen ES, Yanagida M. Requirement of Mis6 centromere connector for localizing a CENP-A-like protein in fission yeast. Science. 2000;288(5474):2215–2219. doi: 10.1126/science.288.5474.2215. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Takayama Y, Masuda F, Kobayashi Y, Saitoh S. Two distinct pathways responsible for the loading of CENP-A to centromeres in the fission yeast cell cycle. Philos Trans R Soc Lond B Biol Sci. 2005;360(1455):595–606. doi: 10.1098/rstb.2004.1614. discussion 606-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Archidiacono N, Rocchi M. Centromere emergence in evolution. Genome Res. 2001;11(4):595–599. doi: 10.1101/gr.152101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura M, Weigl S, Carbone L, Cardone MF, Misceo D, Teti M, D’Addabbo P, Wandall A, Bjorck E, de Jong PJ, et al. Recurrent sites for new centromere seeding. Genome Res. 2004;14(9):1696–1703. doi: 10.1101/gr.2608804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton PE, Cooke CA, Bourassa S, Vafa O, Sullivan BA, Stetten G, Gimelli G, Warburton D, Tyler-Smith C, Sullivan KF, et al. Immunolocalization of CENP-A suggests a distinct nucleosome structure at the inner kinetochore plate of active centromeres. Curr Biol. 1997;7(11):901–904. doi: 10.1016/s0960-9822(06)00382-4. [DOI] [PubMed] [Google Scholar]
- Weintraub H, Worcel A, Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Willard HF. Chromosome-specific organization of human alpha satellite DNA. Am J Hum Genet. 1985;37(3):524–532. [PMC free article] [PubMed] [Google Scholar]
- Willard HF, Waye JS. Hierarchical order in chromosome-specific human alpha satellite DNA. Trends Genet. 1987;3(7):192–198. [Google Scholar]
- Williams JS, Hayashi T, Yanagida M, Russell P. Fission yeast Scm3 mediates stable assembly of Cnp1/CENP-A into centromeric chromatin. Mol Cell. 2009;33(3):287–298. doi: 10.1016/j.molcel.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Long C, Chen X, Huang C, Chen S, Zhu B. Partitioning of histone H3–H4 tetramers during DNA replication-dependent chromatin assembly. Science. 2010;328(5974):94–98. doi: 10.1126/science.1178994. [DOI] [PubMed] [Google Scholar]
- Zachariae W, Schwab M, Nasmyth K, Seufert W. Control of cyclin ubiquitination by CDK-regulated binding of Hct1 to the anaphase promoting complex. Science. 1998;282(5394):1721–1724. doi: 10.1126/science.282.5394.1721. [DOI] [PubMed] [Google Scholar]