Abstract
Objective To assess the risk of lung cancer in smokers of medium tar filter cigarettes compared with smokers of low tar and very low tar filter cigarettes.
Design Analysis of the association between the tar rating of the brand of cigarette smoked in 1982 and mortality from lung cancer over the next six years. Multivariate proportional hazards analyses used to assess hazard ratios, with adjustment for age at enrolment, race, educational level, marital status, blue collar employment, occupational exposure to asbestos, intake of vegetables, citrus fruits, and vitamins, and, in analyses of current and former smokers, for age when they started to smoke and number of cigarettes smoked per day.
Setting Cancer prevention study II (CPS-II).
Participants 364 239 men and 576 535 women, aged ≥ 30 years, who had either never smoked, were former smokers, or were currently smoking a specific brand of cigarette when they were enrolled in the cancer prevention study.
Main outcome measure Death from primary cancer of the lung among participants who had never smoked, former smokers, smokers of very low tar (≤ 7 mg tar/cigarette) filter, low tar (8-14 mg) filter, high tar (≥ 22 mg) non-filter brands and medium tar conventional filter brands (15-21 mg).
Results Irrespective of the tar level of their current brand, all current smokers had a far greater risk of lung cancer than people who had stopped smoking or had never smoked. Compared with smokers of medium tar (15-21 mg) filter cigarettes, risk was higher among men and women who smoked high tar (≥ 22 mg) non-filter brands (hazard ratio 1.44, 95% confidence interval 1.20 to 1.73, and 1.64, 1.26 to 2.15, respectively). There was no difference in risk among men who smoked brands rated as very low tar (1.17, 0.95 to 1.45) or low tar (1.02, 0.90 to 1.16) compared with those who smoked medium tar brands. The same was seen for women (0.98, 0.80 to 1.21, and 0.95, 0.82 to 1.11, respectively).
Conclusion The increase in lung cancer risk is similar in people who smoke medium tar cigarettes (15-21 mg), low tar cigarettes (8-14 mg), or very low tar cigarettes (≤ 7 mg). Men and women who smoke non-filtered cigarettes with tar ratings ≥ 22 mg have an even higher risk of lung cancer.
Introduction
During the past 50 years, changes in the design and manufacture of cigarettes have markedly reduced their machine measured “tar” yields.1,2 The introduction of cellulose acetate filters in the 1950s, and subsequently more porous cigarette papers, reduced the average tar rating per cigarette in the United States from about 37 mg in 1950 to 22 mg in 1967.1 The introduction of air ventilation holes in the filter tip in the late 1960s and expanded tobacco in the 1970s permitted manufacturers to market low tar (generally in the range of 8-14 mg per cigarette) and very low tar cigarettes (≤ 7 mg per cigarette). Concomitantly, the US average tar level per cigarette, as rated by the US Federal Trade Commission (FTC), declined to about 13 mg by 1990.1 Similar trends in standardised tar yields have been reported in the United Kingdom3,4 and other countries.
While many case-control and cohort studies have examined risk of lung cancer in relation to type of cigarette smoked,5-53 nearly all have compared the risks of smoking high tar non-filter brands with smoking medium tar filter brands,5,14-44 or to the corresponding ranges of tar yield.6-13,45-53 The three case-control studies that have included participants who smoked low tar brands11-13 yielded negative or equivocal results, but the observation periods for these studies ended in 1980-1, when the combined market share of low tar and very low tar cigarettes in the United States had exceeded 10% for only five or six years.54 In most epidemiological studies,7,8,10-13,14-29,34,45-50,52 the observation period ended before 1986, when the market share in the United States had exceeded 10% for only a decade.54 Thus no large, long term prospective study has specifically compared the risk of lung cancer in smokers of medium tar filter cigarettes with that in smokers of low tar and very low tar filter cigarettes.
We analysed the relation between the tar rating of the brand of cigarette smoked in 1982 and mortality from lung cancer over six years among men and women in the cancer prevention study II (CPS-II), a nationwide prospective cohort of over one million US adults aged 30 years or older. We specifically compared the risk of lung cancer among smokers of very low tar (≤ 7 mg) filter, low tar (8-14 mg) filter, or high tar (≥ 22 mg) non-filter brands with the risk among those who smoke conventional medium tar (15-21 mg) filter brands.
Methods
Details of the cancer prevention study, initiated by the American Cancer Society (ACS) in 1982, have been published elsewhere.55-58 From the cohort of 508 318 men and 676 270 women, we excluded those who reported a history of cancer other than non-melanoma skin cancer; men who ever smoked pipes or cigars or chewed tobacco; and men and women whose current smoking status could not be ascertained. The resulting cohort comprised 364 239 men and 576 535 women. The outcome measure was death from cancer of the trachea, bronchus, or lung as the underlying cause, coded from the death certificate. During the six year follow up, 2622 men and 1406 women died from these cancers.
On the basis of brand name reported by each current smoker at enrolment, as well as the size (regular, king size, 100 mm, 120 mm), presence or absence of menthol and of a filter, we assigned a tar rating from the Federal Trade Commission tables for December 1981.55,59 We then grouped current brand tar ratings into very low tar (≤ 7 mg), low tar (8-14 mg), medium tar (15-21 mg), and high tar (≥ 22 mg). Unspecified current brands, as well as those current brands that could not otherwise be classified, were considered as a separate category. All brands in the very low and low tar ranges, as well as 99% of brands in the medium range, were filter cigarettes. Those in the high tar range were exclusively non-filter cigarettes.
The American Cancer Society did not collect information on changes in smoking behaviour during follow up of the entire cancer prevention study-II cohort. We therefore restricted our mortality follow up to six years (1982-8) to reduce possible mis-classification of exposure due to quitting or brand switching during longer follow up. However, we were able to assess changes in the smoking status of 14 523 men and 15 509 women who reported current smoking at enrolment in the initial CPS-II cohort in 1982 and were also enrolled in the subsequent CPS-II nutrition cohort in 1992.56 For this subgroup, we computed the proportions of current smokers in each tar category in 1982 who had quit smoking 10 years later.
We used Cox proportional hazards methods60 to estimate hazard ratios and 95% confidence intervals of mortality from lung cancer in people who had never smoked, former smokers, and current smokers of very low, low, and high tar brands, relative to smokers of brands with tar ratings of 15-21 mg (medium tar). Former smokers were stratified into those who had quit aged ≤ 35 years, aged 35-54 years, and aged ≥ 55 years. All statistical analyses were performed separately for men and women.
In our proportional hazards analyses of mortality from lung cancer among current, former, and never smokers we adjusted for multiple covariates that reflected possible differences in participants' demographics, dietary practices, occupational exposures, or medical histories. Demographic covariates included exact age at enrolment, race, education, and marital status. Dietary covariates included intake of vegetables, citrus fruits, and vitamins A, C, and E. Occupational covariates included whether the most recent job was blue collar (such as car mechanics and construction workers) and whether the participant had been employed in an occupation with high asbestos exposure (such as pipe fitters and shipyard workers) for ≥ 10 years. Other indicator variables were a history of chronic bronchitis, emphysema, heart disease, stroke, and diabetes and self report of being currently sick, taking heart drugs, or pain in the legs during walking that went away with rest. All covariates except exact age at enrolment were modeled as categorical variables, where missing values were coded as separate categories.
Excluding participants who had never smoked, we further performed multivariate proportional hazards analyses of current and former smokers that adjusted not only for demographic, dietary, occupational, and medical history covariates but also for age when they began smoking and the average number of cigarettes smoked a day.
Finally, in a series of sensitivity analyses of current smokers only, we restricted our analysis to people who had smoked their current brand for a minimum of 5 or 10 years; excluded smokers with a history of emphysema; excluded participants who reported any smoking related condition (emphysema, chronic bronchitis, heart disease, use of heart drugs, stroke, diabetes, claudication, currently sick); varied the definition of the tar categories to include 8 mg tar brands in the very low tar category and 15 mg brands in the low tar category; and estimated hazard ratios without controlling for the average number of cigarettes smoked a day. The latter analysis examined the view61 that a study of risk of lung cancer in relation to type of cigarette smoked should exclude number of cigarettes smoked a day as a covariate because smokers of lower tar and nicotine brands may compensate by smoking more cigarettes a day.
Results
Tables 1 and 2 show descriptive characteristics of the cohorts of 100 868 men and 124 270 women who were current smokers at enrolment. Tables 3 and 4 show the corresponding data for the cohorts of 263 371 men and 452 265 women who never smoked or who had quit smoking at enrolment. Smokers of brands with medium or high tar ratings were more likely to be African American; more likely to have attained no more than a high school education; more likely to have a recent blue collar job or a history of potential occupational asbestos exposure; and less likely to report use of vitamins A, C, and E than participants who smoked lower tar brands, who never smoked, or who quit smoking before age 35 years. Current smokers of very low tar cigarettes (especially men) tended to smoke more cigarettes a day. Moreover, among the subset of current smokers who were re-enrolled in the CPS-II nutrition cohort, those men and women who had smoked very low tar and low tar cigarettes in 1982 were more likely to have quit smoking by 1992.
Table 1.
Characteristics of men who currently smoked, according to tar level of cigarettes smoked in 1982
|
Tar level (mg)
|
|||||
|---|---|---|---|---|---|
| 0-7 | 8-14 | 15-21 | ≥22 | Unclassified | |
| No of participants | 6243 | 27 044 | 38 527 | 6439 | 22 615 |
| White (%) | 96.0 | 94.5 | 90.8 | 91.9 | 89.9 |
| Educated to ≤high school (%) | 28.1 | 38.5 | 45.7 | 51.4 | 41.8 |
| Married (%) | 93.0 | 92.8 | 91.5 | 91.7 | 90.9 |
| Most recent/current job—blue collar (%) | 21.3 | 27.5 | 32.8 | 36.3 | 28.8 |
| Vitamin use: | |||||
| A (%) | 9.4 | 6.4 | 5.7 | 4.7 | 6.8 |
| E (%) | 18.4 | 14.0 | 12.2 | 10.1 | 13.6 |
| C (%) | 26.2 | 20.3 | 17.1 | 13.9 | 19.0 |
| Mean servings of vegetables and citrus fruit a week | 26.5 | 26.0 | 25.7 | 25.0 | 25.1 |
| Occupational exposure to asbestos (%) | 8.8 | 10.9 | 13.1 | 15.9 | 11.7 |
| Mean years of smoking current brand | 4.5 | 7.9 | 14.1 | 25.5 | 12.4 |
| Cigarettes smoked a day (%): | |||||
| 1-9 | 6.3 | 7.8 | 8.7 | 6.2 | 12.7 |
| 10-19 | 12.0 | 13.4 | 14.4 | 13.4 | 15.7 |
| 20 | 23.2 | 27.6 | 30.5 | 32.7 | 28.0 |
| 21-39 | 25.8 | 25.1 | 23.0 | 23.8 | 19.8 |
| 40 | 20.7 | 18.2 | 16.2 | 16.7 | 14.9 |
| >41 | 10.7 | 6.7 | 5.5 | 5.6 | 5.1 |
| Mean | 28.7 | 26.2 | 25.0 | 25.7 | 23.6 |
| Age (years) when started smoking (%): | |||||
| <16 | 22.9 | 25.1 | 28.1 | 32.0 | 24.5 |
| 16-17 | 24.8 | 25.4 | 25.5 | 27.0 | 23.7 |
| 18-20 | 31.0 | 29.5 | 27.7 | 25.7 | 27.9 |
| >21 | 19.6 | 17.8 | 16.0 | 12.3 | 19.0 |
| Mean | 18.1 | 17.8 | 17.5 | 16.8 | 18.0 |
| CPS-II nutrition cohort: | |||||
| No of participants | 1031 | 4 119 | 5 468 | 944 | 2 961 |
| Quit smoking by 1992 (%)* | 57.9 | 59.7 | 55.3 | 50.0 | 59.0 |
For those former smokers in CPS-II nutrition cohort who had reported the age at which they quit, their mean computed year of smoking cessation was 1987 irrespective of the tar level of the brand smoked in 1982.
Table 2.
Characteristics of women who currently smoked, according to tar level of cigarettes smoked in 1982
|
Tar level (mg)
|
|||||
|---|---|---|---|---|---|
| 0-7 | 8-14 | 15-21 | ≥22 | Unclassified | |
| No of participants | 15 524 | 48 821 | 44 124 | 4079 | 11 722 |
| White (%) | 96.1 | 92.6 | 90.7 | 91.5 | 87.7 |
| Educated to ≤high school (%) | 35.8 | 43.1 | 48.4 | 54.6 | 46.9 |
| Married (%) | 75.6 | 73.9 | 72.6 | 70.1 | 70.0 |
| Most recent/current job—blue collar (%) | 7.1 | 9.2 | 10.5 | 11.8 | 10.5 |
| Vitamin use: | |||||
| A (%) | 9.9 | 8.1 | 7.3 | 6.2 | 8.4 |
| E (%) | 22.6 | 18.3 | 15.9 | 13.3 | 17.9 |
| C (%) | 31.6 | 25.9 | 22.4 | 18.9 | 24.4 |
| Mean servings of vegetables and citrus fruit a week | 29.8 | 29.1 | 28.9 | 28.4 | 28.4 |
| Occupational exposure to asbestos (%) | 1.3 | 1.6 | 2.0 | 2.7 | 1.7 |
| Mean years of smoking current brand | 4.2 | 6.8 | 10.8 | 23.3 | 7.1 |
| Cigarettes smoked a day (%): | |||||
| 1-9 | 10.9 | 14.8 | 15 | 9.9 | 19.6 |
| 10-19 | 20.2 | 22.9 | 23 | 23.2 | 20.7 |
| 20 | 31.5 | 32.1 | 33 | 36.5 | 26.8 |
| 21-39 | 19.2 | 15.8 | 14.7 | 15.5 | 10.9 |
| 40 | 11.5 | 8.4 | 8.3 | 7.8 | 6.3 |
| ≥41 | 2.7 | 1.7 | 1.6 | 1.4 | 1.5 |
| Mean | 21.9 | 19.7 | 19.5 | 20.3 | 17.8 |
| Age (years) when started smoking (%): | |||||
| <16 | 9.4 | 10.9 | 12.4 | 17.6 | 9.5 |
| 16-17 | 19.0 | 19.5 | 20.5 | 23.9 | 16.7 |
| 18-20 | 36.8 | 35.8 | 34.4 | 32.9 | 31.9 |
| ≥21 | 33.2 | 32.1 | 30.9 | 23.7 | 33.6 |
| Mean | 20.7 | 20.6 | 20.4 | 19.0 | 21.3 |
| CPS-II nutrition cohort: | |||||
| No of participants | 2 226 | 6 295 | 5 330 | 435 | 1 223 |
| Quit smoking by 1992 (%)* | 56.9 | 53.5 | 51.2 | 34.9 | 55.6 |
For those former smokers in CPS-II nutrition cohort who had reported the age at which they quit, their mean computed year of smoking cessation was 1987 irrespective of the tar level of the brand smoked in 1982.
Table 3.
Characteristics of men who had never smoked or were former smokers, according to age when they quit smoking
|
Age at quitting (years)
|
|||||
|---|---|---|---|---|---|
| Never smoked | <35 | 35-54 | ≥55 | Unknown | |
| No of participants | 121 784 | 36 768 | 79 543 | 24 025 | 1251 |
| White (%) | 93.5 | 95.4 | 95.5 | 95.8 | 87.7 |
| Educated to ≤high school (%) | 30.7 | 29.9 | 35.6 | 48.6 | 50.4 |
| Married (%) | 93.0 | 95.3 | 95.9 | 93.9 | 91.6 |
| Most recent/current job—blue collar (%) | 25.8 | 24.8 | 25.9 | 29.7 | 34.9 |
| Vitamin use: | |||||
| A (%) | 8.6 | 8.5 | 7.6 | 6.5 | 6.9 |
| E (%) | 16.8 | 16.9 | 16.9 | 15.9 | 14.3 |
| C (%) | 24.8 | 24.9 | 22.9 | 19.9 | 18.1 |
| Mean servings of vegetables and citrus fruit a week | 26.3 | 26.9 | 27.0 | 26.7 | 25.3 |
| Occupational exposure to asbestos (%) | 8.3 | 9.2 | 10.2 | 12.9 | 13.3 |
| Cigarettes smoked a day (%): | |||||
| 1-9 | 12.8 | 6.6 | 5.7 | 14.8 | |
| 10-19 | 20.2 | 13.6 | 12.5 | 11.0 | |
| 20 | 34.5 | 32.2 | 32.5 | 26.1 | |
| 21-39 | 12.5 | 16.8 | 16.5 | 7.0 | |
| 40 | 9.8 | 16.0 | 16.7 | 8.9 | |
| ≥41 | 5.2 | 11.2 | 10.4 | 4.5 | |
| Mean | 21.5 | 27.2 | 27.4 | 21.3 | |
| Age (years) when started smoking (%): | |||||
| <16 | 19.9 | 21.2 | 23.3 | 11.0 | |
| 16-17 | 25.5 | 23.7 | 22.6 | 12.2 | |
| 18-20 | 38.0 | 33.7 | 29.3 | 13.8 | |
| ≥21 | 15.8 | 20.5 | 22.9 | 11.9 | |
| Mean | 17.7 | 18.2 | 18.5 | 20.6 | |
Table 4.
Characteristics of women who had never smoked or were former smokers, according to age when they quit smoking
|
Age at quitting (years)
|
|||||
|---|---|---|---|---|---|
| Never smoked | <35 | 35-54 | ≥55 | Unknown | |
| No of participants | 326 826 | 47 067 | 59 804 | 16 815 | 1753 |
| White (%) | 92.7 | 95.4 | 94.8 | 95.6 | 88.7 |
| Educated to ≤high school (%) | 46.1 | 28.3 | 35.5 | 45.2 | 43.5 |
| Married (%) | 75.9 | 85.8 | 79.8 | 60.5 | 69.9 |
| Most recent/current job—blue collar (%) | 9.4 | 6.8 | 7.0 | 8.0 | 10.2 |
| Vitamin use: | |||||
| A (%) | 9.0 | 10.8 | 10.5 | 8.4 | 10.9 |
| E (%) | 19.4 | 22.0 | 23.8 | 20.6 | 20.8 |
| C (%) | 26.3 | 31.2 | 31.4 | 27.4 | 28.3 |
| Mean servings of vegetables and citrus fruit a week | 28.9 | 29.9 | 30.6 | 30.2 | 29.4 |
| Occupational exposure to asbestos (%) | 1.8 | 0.9 | 1.3 | 2.1 | 2.4 |
| Cigarettes smoked a day (%): | |||||
| 1-9 | 38.5 | 23.9 | 18.6 | 35.5 | |
| 10-19 | 22.0 | 22.6 | 21.3 | 11.4 | |
| 20 | 18.8 | 25.2 | 27.5 | 10.5 | |
| 21-39 | 5.0 | 9.3 | 9.0 | 2.1 | |
| 40 | 3.8 | 7.2 | 8.1 | 1.9 | |
| ≥41 | 1.5 | 3.4 | 3.2 | 1.9 | |
| Mean | 13.1 | 17.8 | 19.0 | 11.7 | |
| Age (years) when started smoking (%): | |||||
| <16 | 10.1 | 9.4 | 7.6 | 5.0 | |
| 16-17 | 22.3 | 19.5 | 16.1 | 9.9 | |
| 18-20 | 46.5 | 37.2 | 28.3 | 20.5 | |
| ≥21 | 20.1 | 32.5 | 44.5 | 22.0 | |
| Mean | 18.7 | 20.6 | 23.2 | 22.1 | |
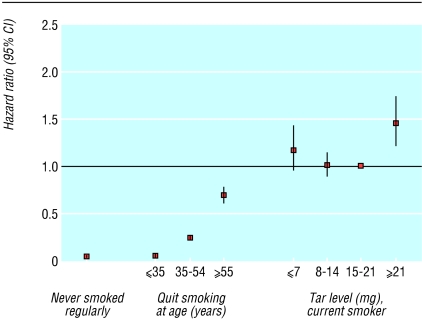
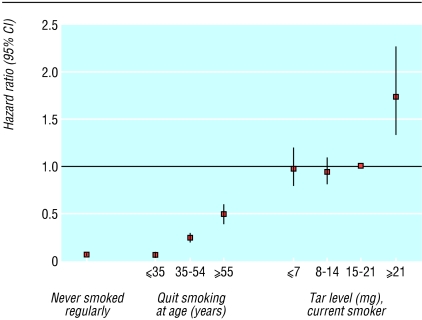
Figures 1 and 2 show multivariate adjusted hazard ratios and 95% confidence intervals for never smokers, for former smokers who had quit at various ages, and for current smokers of brands with various tar ratings, relative to current smokers of brands with 15-21 mg tar. Men and women who smoked very low tar (≤ 7 mg) and low tar (8-14 mg) brands had risks of lung cancer indistinguishable from those who smoked medium tar (15-21 mg) brands (Wald test for homogeneity of strata62 P = 0.27 for men, P = 0.80 for women). The risk was higher in those who smoked non-filter cigarettes and substantially lower in men (fig 1) and women (fig 2) who quit smoking. People who quit smoking before age 35 years had risks of lung cancer approaching those of people who had never smoked. Further adjustment for age when people started to smoke and number of cigarettes smoked a day showed nearly identical patterns in current and former smokers to those shown in figures 1 and 2. For men the adjusted figures were 0.06 (0.04 to 0.09) for those who quit aged ≤ 35 years, 0.23 (0.20 to 0.27) for those who quit aged 35-54, and 0.63 (0.55 to 0.71) for those who quit aged ≥ 55. For women the corresponding figures were 0.09 (0.05 to 0.14), 0.26 (0.21 to 0.32), and 0.47 (0.38 to 0.59).
Fig 1.
Hazard ratios for lung cancer in men, 1982-8, by smoking status and tar yield of brand smoked, relative to current smokers of brands with tar ratings 15-21 mg
Fig 2.
Hazard ratios for lung cancer in women, 1982-8, by smoking status and tar yield of brand smoked, relative to current smokers of brands with tar ratings 15-21 mg
Among men who never smoked, 93 died from lung cancer. Among men who quit smoking, 23 who quit aged ≤ 35 years, 344 who quit aged 35-54 years, and 540 who quit aged ≥ 55 years died from lung cancer. Among women who never smoked, 211 died from lung cancer. Among women who quit smoking, 16 who quit aged ≤ 35 years, 122 who quit aged 35-54 years, and 131 who quit aged ≥ 55 years died from lung cancer.
Tables 5 and 6 show multivariate sensitivity analyses in current smokers that examine whether varying the exclusion criteria or the boundaries of the very low tar and low tar categories materially alter the results. In both men and women, the findings were essentially unchanged when people with emphysema and other diseases attributable to smoking were excluded, when the analyses were restricted to people who had smoked their current brand for a minimum of 5 or 10 years, or when the boundaries of the very low or low tar categories were altered slightly.
Table 5.
Multivariate analyses of mortality from lung cancer of men who were current smokers, according to tar level of cigarette smoked in 1982*
|
Hazard ratio (95% CI)
|
||||
|---|---|---|---|---|
| No of participants | No of deaths | Without cigarettes/day as covariate | With cigarettes/day as covariate | |
| Entire cohort | ||||
| 0-7 mg | 6 243 | 103 | 1.17 (0.95 to 1.45) | 1.11 (0.90 to 1.37) |
| 8-14 mg | 27 044 | 378 | 1.02 (0.90 to 1.16) | 1.00 (0.88 to 1.14) |
| 15-21 mg | 38 527 | 563 | 1.0 | 1.0 |
| ≥22 mg | 6 439 | 150 | 1.44 (1.20 to 1.73) | 1.43 (1.19 to 1.71) |
| Unclassifiable | 22 615 | 410 | 1.10 (0.97 to 1.25) | 1.12 (0.98 to 1.27) |
| Excluding history of emphysema | ||||
| 0-7 mg | 5 896 | 84 | 1.11 (0.88 to 1.40) | 1.05 (0.83 to 1.32) |
| 8-14 mg | 25 835 | 322 | 0.99 (0.86 to 1.14) | 0.97 (0.85 to 1.12) |
| 15-21 mg | 36 832 | 495 | 1.0 | 1.0 |
| ≥22 mg | 6 089 | 135 | 1.48 (1.22 to 1.79) | 1.46 (1.21 to 1.77) |
| Unclassifiable | 21 579 | 373 | 1.13 (0.99 to 1.30) | 1.16 (1.01 to 1.32) |
| Excluding prevalent smoking related diseases† | ||||
| 0-7 mg | 4 026 | 47 | 1.04 (0.76 to 1.42) | 0.97 (0.71 to 1.32) |
| 8-14 mg | 17 527 | 181 | 0.95 (0.78 to 1.14) | 0.93 (0.77 to 1.12) |
| 15-21 mg | 24 653 | 282 | 1.0 | 1.0 |
| ≥22 mg | 3 967 | 69 | 1.38 (1.06 to 1.79) | 1.36 (1.05 to 1.77) |
| Unclassifiable | 14 624 | 214 | 1.12 (0.93 to 1.34) | 1.15 (0.96 to 1.38) |
| Smoked current brand for ≥5 years | ||||
| 0-7 mg | 2 328 | 42 | 1.18 (0.86 to 1.63) | 1.09 (0.79 to 1.50) |
| 8-14 mg | 16 362 | 238 | 1.04 (0.88 to 1.21) | 1.02 (0.87 to 1.19) |
| 15-21 mg | 29 796 | 426 | 1.0 | 1.0 |
| ≥22 mg | 5 777 | 131 | 1.47 (1.20 to 1.78) | 1.45 (1.19 to 1.77) |
| Unclassifiable | 13 405 | 230 | 1.11 (0.94 to 1.30) | 1.12 (0.95 to 1.31) |
| Smoked current brand for ≥10 years | ||||
| 0-7 mg | 817 | 15 | 1.16 (0.69 to 1.96) | 1.11 (0.66 to 1.86) |
| 8-14 mg | 9 313 | 145 | 1.08 (0.89 to 1.32) | 1.06 (0.88 to 1.29) |
| 15-21 mg | 23 775 | 339 | 1.0 | 1.0 |
| ≥22 mg | 5 513 | 128 | 1.53 (1.25 to 1.88) | 1.52 (1.24 to 1.87) |
| Unclassifiable | 9 881 | 173 | 1.13 (0.94 to 1.35) | 1.14 (0.95 to 1.37) |
| 8 mg in very low tar category | ||||
| 0-8 mg | 11 441 | 173 | 1.11 (0.93 to 1.32) | 1.07 (0.90 to 1.27) |
| 9-14 mg | 21 846 | 308 | 1.02 (0.89 to 1.17) | 1.00 (0.87 to 1.15) |
| 15-21 mg | 38 527 | 563 | 1.0 | 1.0 |
| ≥22 mg | 6 439 | 150 | 1.44 (1.20 to 1.73) | 1.43 (1.19 to 1.71) |
| Unclassifiable | 22 615 | 410 | 1.10 (0.97 to 1.25) | 1.12 (0.98 to 1.27) |
| 15 mg in low tar category | ||||
| 0-7 mg | 6 243 | 103 | 1.17 (0.94 to 1.46) | 1.11 (0.89 to 1.38) |
| 8-15 mg | 38 436 | 549 | 1.01 (0.89 to 1.15) | 1.00 (0.88 to 1.14) |
| 16-21 mg | 27 135 | 392 | 1.0 | 1.0 |
| ≥22 mg | 6 439 | 150 | 1.44 (1.19 to 1.74) | 1.43 (1.18 to 1.72) |
| Unclassifiable | 22 615 | 410 | 1.09 (0.95 to 1.26) | 1.12 (0.97 to 1.28) |
Covariates included age when started smoking (see tables 1, 2, 3, 4); education (≤high school graduate, some college or vocational school, college graduate or higher, missing); race (white, non-white, missing); marital status (married, not married, missing); current or most recent job (blue collar, not blue collar, missing); weekly intake of vegetables and citrus fruits (categorised by fifths, and missing); use of vitamin A, E, and C (<15 times/month, ≥15 times/month, missing); occupational asbestos exposure (exposed <10 years; ≥10 years); and, in right column, cigarettes smoked/day (see tables 1, 2, 3, 4).
Prevalent smoking related diseases included emphysema, chronic bronchitis, heart disease or use of heart medication, stroke, diabetes, pain in legs, or sick at time of survey.
Table 6.
Multivariate analyses of mortality from lung cancer of women who were current smokers, according to tar level of cigarette smoked in 1982*
|
Hazard ratio (95% CI)
|
||||
|---|---|---|---|---|
| No of participants | No of deaths | Without cigarettes/day as covariate | With cigarettes/day as covariate | |
| Entire cohort | ||||
| 0-7 mg | 15 524 | 120 | 0.98 (0.80 to 1.21) | 0.89 (0.72 to 1.10) |
| 8-14 mg | 48 821 | 336 | 0.95 (0.82 to 1.11) | 0.94 (0.81 to 1.10) |
| 15-21 mg | 44 124 | 329 | 1.0 | 1.0 |
| ≥22 mg | 4 079 | 64 | 1.64 (1.26 to 2.15) | 1.60 (1.22 to 2.09) |
| Unclassifiable | 11 722 | 73 | 0.72 (0.56 to 0.93) | 0.74 (0.57 to 0.96) |
| Excluding history of emphysema | ||||
| 0-7 mg | 15 176 | 114 | 1.00 (0.80 to 1.24) | 0.91 (0.73 to 1.13) |
| 8-14 mg | 48 034 | 319 | 0.96 (0.82 to 1.12) | 0.95 (0.81 to 1.11) |
| 15-21 mg | 43 396 | 310 | 1.0 | 1.0 |
| ≥22 mg | 3 966 | 58 | 1.60 (1.21 to 2.12) | 1.56 (1.18 to 2.07) |
| Unclassifiable | 11 489 | 68 | 0.71 (0.55 to 0.93) | 0.73 (0.56 to 0.96) |
| Excluding prevalent smoking related diseases† | ||||
| 0-7 mg | 10 546 | 73 | 0.99 (0.76 to 1.30) | 0.90 (0.68 to 1.18) |
| 8-14 mg | 32 773 | 187 | 0.88 (0.72 to 1.08) | 0.87 (0.71 to 1.07) |
| 15-21 mg | 28 909 | 195 | 1.0 | 1.0 |
| ≥22 mg | 2 511 | 33 | 1.50 (1.04 to 2.18) | 1.49 (1.02 to 2.15) |
| Unclassifiable | 7 650 | 36 | 0.61 (0.42 to 0.87) | 0.63 (0.44 to 0.90) |
| Smoked current brand for ≥5 years | ||||
| 0-7 mg | 5 136 | 45 | 1.04 (0.75 to 1.43) | 0.92 (0.66 to 1.27) |
| 8-14 mg | 23 678 | 186 | 1.06 (0.87 to 1.29) | 1.06 (0.87 to 1.28) |
| 15-21 mg | 29 117 | 219 | 1.0 | 1.0 |
| ≥22 mg | 3 464 | 57 | 1.74 (1.30 to 2.33) | 1.70 (1.27 to 2.28) |
| Unclassifiable | 4 185 | 31 | 0.92 (0.63 to 1.34) | 0.92 (0.63 to 1.34) |
| Smoked current brand for ≥10 years | ||||
| 0-7 mg | 1 613 | 16 | 1.16 (0.69 to 1.94) | 1.07 (0.64 to 1.80) |
| 8-14 mg | 12 626 | 111 | 1.14 (0.89 to 1.45) | 1.16 (0.91 to 1.48) |
| 15-21 mg | 21 070 | 161 | 1.0 | 1.0 |
| ≥22 mg | 3 268 | 55 | 1.81 (1.33 to 2.47) | 1.79 (1.32 to 2.44) |
| Unclassifiable | 2 195 | 16 | 0.89 (0.53 to 1.50) | 0.87 (0.52 to 1.47) |
| 8 mg in very low tar category | ||||
| 0-8 mg | 18 123 | 131 | 0.91 (0.74 to 1.12) | 0.83 (0.68 to 1.02) |
| 9-14 mg | 46 222 | 325 | 0.98 (0.84 to 1.14) | 0.97 (0.83 to 1.13) |
| 15-21 mg | 44 124 | 329 | 1.0 | 1.0 |
| ≥22 mg | 4 079 | 64 | 1.64 (1.26 to 2.15) | 1.60 (1.22 to 2.09) |
| Unclassifiable | 11 722 | 73 | 0.72 (0.56 to 0.93) | 0.74 (0.57 to 0.96) |
| 15 mg in low tar category | ||||
| 0-7 mg | 15 524 | 120 | 0.93 (0.74 to 1.17) | 0.86 (0.69 to 1.08) |
| 8-15 mg | 66 734 | 461 | 0.89 (0.76 to 1.05) | 0.91 (0.77 to 1.07) |
| 16-21 mg | 26 211 | 204 | 1.0 | 1.0 |
| ≥22 mg | 4 079 | 64 | 1.56 (1.18 to 2.07) | 1.54 (1.16 to 2.04) |
| Unclassifiable | 11 722 | 73 | 0.68 (0.52 to 0.89) | 0.72 (0.54 to 0.94) |
Covariates included age when started smoking (see tables 1, 2, 3, 4); education (≤high school graduate, some college or vocational school, college graduate or higher, missing); race (white, non-white, missing); marital status (married, not married, missing); current or most recent job (blue collar, not blue collar, missing); weekly intake of vegetables and citrus fruits (categorised by fifths, and missing); use of vitamin A, E, and C (<15 times/month, ≥15 times/month, missing); occupational asbestos exposure (exposed <10 years; ≥10 years); and, in right column, cigarettes smoked/day (see tables 1, 2, 3, 4).
Prevalent smoking related diseases included emphysema, chronic bronchitis, heart disease or use of heart medication, stroke, diabetes, pain in legs, or sick at time of survey.
Discussion
While smokers of non-filter high tar cigarettes with tar ratings ≥ 22 mg experienced the highest risk of lung cancer, we detected no difference in risk among people who smoked medium tar cigarettes (15-21 mg), low tar cigarettes (8-14 mg), or very low tar cigarettes (≤ 7 mg). This pattern persisted after we adjusted for demographic characteristics, dietary habits, and occupational and medical histories. Moreover, our results were robust in sensitivity analyses that were restricted to people who had smoked their current brand for a minimum of 10 years or excluded smokers with emphysema and other smoking related diseases. Similarly, the findings were essentially unchanged by minor variations in the boundaries of the low tar and very low tar categories or by omission of the number of cigarettes smoked a day as a covariate.
We observed the smoking habits of all participants only at enrolment in 1982. However, based on a 13% subsample of participants who were re-enrolled in the CPS-II nutrition cohort, we found that men and women who smoked very low tar and low tar cigarettes in 1982 were more likely to have quit smoking by 1992. Differential cessation during the six year follow up would thus result in underestimation rather than overestimation of the actual risk of lung cancer associated with smoking very low tar and low tar cigarettes.
In keeping with previous reports,63 the rate of deaths from lung cancer in former smokers who had stopped smoking by age 35 approached that in those who had never smoked, and even the rate for those who stopped smoking by age 55 was substantially below that of continuing smokers. All current smokers, regardless of the tar level of their current brand, had substantially greater risks of lung cancer than people who had never smoked or who had stopped by age of 35.
Non-linear relation between tar levels and risk of lung cancer
Our findings challenge the assumption that the association between tar rating and lung cancer risk is necessarily linear. As the data points for current smokers in figures 1 and 2 show, extrapolations based on comparisons of only the highest and lowest tar groups,10,13,64 or parametric models in which tar yield is a continuous linear variable,6-9 can obscure a non-linear relation between tar levels and risk of disease. Indices of lifetime cumulative tar exposure9,65 are especially problematic because they confound tar yield with the number of cigarettes smoked a day and the number of years of smoking.
By the end of follow up in 1988, low tar and very low tar cigarettes had been on the US market for about two decades. Participants in the cancer prevention study who smoked very low tar cigarettes had used their current brand for an average of four to five years, while those who smoked low tar cigarettes had smoked their current brand for an average of seven to eight years. We did not attempt to analyse the tar levels of brands that participants recalled smoking in the past. Because the median age at entry was 53-54 years, most current smokers in the cohort could not have smoked low tar or very low tar brands exclusively over their lives. Accordingly, we could not evaluate the effect of the exclusive use of low and very low tar cigarettes from adolescence onward.
Findings consistent with other evidence
Our finding that there was no difference in the risk of lung cancer between people who smoked medium tar filter, low tar filter, and very low tar filter cigarettes is consistent with evidence of compensatory smoking. Addicted smokers who switch from a higher to lower tar cigarette can maintain their nicotine intake by blocking ventilation holes, increasing the puff volume or the time during which the smoke is retained in the lungs, and smoking more cigarettes.66 As a result, the actual dose of toxicants to the smoker may be much higher than is predicted by machine measured yields. Changes in inhalation patterns induced by lower tar cigarettes may increase the surface area of the lung exposed to carcinogens in smoke and thus result in greater deposition of submicron sized particles deeper into the airways.61 An increase in the depth of inhalation may have contributed to the marked increase among smokers in the incidence of adenocarcinoma of the lung (a cancer that arises in the more peripheral tissues of the lung) in the United States and other countries.5 In fact, adenocarcinoma of the lung was found to be more strongly associated with cigarette smoking in the second cancer prevention study (1982-8) than in the first (1960-72).5 Finally, changes in tobacco curing and blending have increased the delivery of carcinogenic tobacco specific nitrosamines (TSNA)1,67 even as average tar yields have declined. Tar yield is a relatively weak predictor of a brand's delivery of carcinogenic TSNA.68
While our finding that smokers of high tar non-filter cigarettes had higher risks of lung cancer may reflect unmeasured differences between smokers of non-filter and filter cigarettes,61 it is none the less consistent with many other case-control and cohort studies.14,15,18-21,23,25,27,28,30-33,35-37,40,42-44,69 Reducing the use of high tar non-filter cigarettes may thus provide limited public health benefits in those countries where such products are commonly used. While non-filter cigarettes currently represent no more than 1% of cigarette sales in the United States and the United Kingdom,70,71 they still comprise about 20% of cigarettes sold in China,72 15% in France, and 6-20% in Eastern Europe as late as 1996.70
What is already known on this topic
Nearly all previous epidemiological studies of risk of lung cancer in relation to the type of cigarette smoked have compared smokers of high tar non-filter cigarettes (≥ 22 mg tar) with those of medium tar filter cigarettes (15-21 mg)
No large, long term prospective epidemiological study has specifically compared the risk of lung cancer in smokers of medium tar filter brands with the risk in smokers of low tar (8-14 mg) and very low tar (≤ 7 mg) filter brands
What this paper adds
The risk of lung cancer was no different in people who smoked medium tar cigarettes, low tar cigarettes, or very low tar cigarettes
Men and women who smoked non-filtered cigarettes with tar ratings ≥ 22 mg had even higher risks of lung cancer
All current smokers, regardless of the tar level of their current brand, had substantially greater risks of lung cancer than those people who had never smoked or who had quit smoking
Contributors: JEH and MJT contributed to the conception and design of the study, analysis and interpretation of the data, and drafting the manuscript. AMM performed the data tabulations and statistical analyses. EEC directed data collection and analysis of the prospective cohorts followed by the American Cancer Society, on which this study was based. All contributors reviewed the manuscript before submission and publication. MJT is guarantor.
Funding: No specific funding.
Competing interests: JEH has testified as an expert witnesses on behalf of plaintiffs in tobacco-related litigation. MJT has testified as an uncompensated expert on behalf of plaintiffs and the American Cancer Society in tobacco-related cases.
Ethical approval: Approved by the institutional review board at Emory University.
References
- 1.Hoffmann D, Djordjevic M, Brunnemann K. Changes in cigarette design and composition over time and how they influence the yields of smoke constituents. In: Shopland D, ed. The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of US cigarettes: report of the NCI expert committee. NCI smoking and tobacco control monograph No 7. Bethesda MD: US National Institutes of Health, National Cancer Institute, 1996: 15-37. (NIH Publication No 96-4028.)
- 2.Kozlowski LT, O'Connor RJ, Sweeney CT. Cigarette design. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: US National Institutes of Health, National Cancer Institute, NCI Smoking and Tobacco Control, 2001: 13-37. (Monograph No 13.)
- 3.Wald N, Doll R, Copeland G. Trends in tar, nicotine, and carbon monoxide yields of UK cigarettes manufactured since 1934. BMJ 1981;282: 763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiryluk S, Wald N. Trends in cigarette smoking habits in the United Kingdom, 1905-1985. In: Wald N, Froggatt P, eds. Nicotine, smoking and the low tar programme. Oxford: Oxford University Press, 1989.
- 5.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW Jr. Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst 1997;89: 1580-6. [DOI] [PubMed] [Google Scholar]
- 6.Garfinkel L, Stellman SD. Smoking and lung cancer in women: findings in a prospective study. Cancer Res 1988;48: 6951-5. [PubMed] [Google Scholar]
- 7.Ockene JK, Kuller LH, Svendsen KH, Meilahn E. The relationship of smoking cessation to coronary heart disease and lung cancer in the multiple risk factor intervention trial (MRFIT). Am J Public Health 1990;80: 954-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuller LH, Ockene JK, Meilahn E, Wentworth DN, Svendsen KH, Neaton JD. Cigarette smoking and mortality. MRFIT research group. Prev Med 1991;20: 638-54. [DOI] [PubMed] [Google Scholar]
- 9.Zang EA, Wynder EL. Cumulative tar exposure. A new index for estimating lung cancer risk among cigarette smokers. Cancer 1992;70: 69-76. [DOI] [PubMed] [Google Scholar]
- 10.Tang JL, Morris JK, Wald NJ, Hole D, Shipley M, Tunstall-Pedoe H. Mortality in relation to tar yield of cigarettes: a prospective study of four cohorts. BMJ 1995;311: 1530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vutuc C, Kunze M. Lung cancer risk in women in relation to tar yields of cigarettes. Prev Med 1982;11: 713-6. [DOI] [PubMed] [Google Scholar]
- 12.Vutuc C, Kunze M. Tar yields of cigarettes and male lung cancer risk. J Natl Cancer Inst 1983;71: 435-7. [PubMed] [Google Scholar]
- 13.Wilcox HB, Schoenberg JB, Mason TJ, Bill JS, Stemhagen A. Smoking and lung cancer: risk as a function of cigarette tar content. Prev Med 1988;17: 263-72. [DOI] [PubMed] [Google Scholar]
- 14.Bross ID, Gibson R. Risks of lung cancer in smokers who switch to filter cigarettes. Am J Public Health Nations Health 1968;58: 1396-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wynder EL, Mabuchi K, Beattie EJ Jr. The epidemiology of lung cancer. Recent trends. JAMA 1970;213: 2221-8. [PubMed] [Google Scholar]
- 16.Hawthorne VM, Fry JS. Smoking and health: the association between smoking behaviour, total mortality, and cardiorespiratory disease in west central Scotland. J Epidemiol Community Health 1978;32: 260-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todd GF, Hunt BM, Lambert PM. Four cardiorespiratory symptoms as predictors of mortality. J Epidemiol Community Health 1978;32: 267-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wynder EL, Stellman SD. Impact of long-term filter cigarette usage on lung and larynx cancer risk: a case-control study. J Natl Cancer Inst 1979;62: 471-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee PN, Garfinkel L. Mortality and type of cigarette smoked. J Epidemiol Community Health 1981;35: 16-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimington J. The effect of filters on the incidence of lung cancer in cigarette smokers. Environ Res 1981;24: 162-6. [DOI] [PubMed] [Google Scholar]
- 21.Lubin JH, Blot WJ, Berrino F, Flamant R, Gillis CR, Kunze M, et al. Modifying risk of developing lung cancer by changing habits of cigarette smoking. BMJ 1984;288: 1953-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buffler PA, Pickle LW, Mason TP, Contant C. The causes of lung cancer in Texas. In: Mizell M, Correa P, eds. Lung cancer causes and prevention: New York, NY: Verlag Cheim Int, 1984: 83-99.
- 23.Benhamou S, Benhamou E, Tirmarche M, Flamant R. Lung cancer and use of cigarettes: a French case-control study. J Natl Cancer Inst 1985;74: 1169-75. [PubMed] [Google Scholar]
- 24.Alderson MR, Lee PN, Wang R. Risks of lung cancer, chronic bronchitis, ischaemic heart disease, and stroke in relation to type of cigarette smoked. J Epidemiol Community Health 1985;39: 286-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pathak DR, Samet JM, Humble CG, Skipper BJ. Determinants of lung cancer risk in cigarette smokers in New Mexico. J Natl Cancer Inst 1986;76: 597-604. [DOI] [PubMed] [Google Scholar]
- 26.Benhamou E, Benhamou S, Flamant R. Lung cancer and women: results of a French case-control study. Br J Cancer 1987;55: 91-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynder EL, Kabat GC. The effect of low-yield cigarette smoking on lung cancer risk. Cancer 1988;62: 1223-30. [DOI] [PubMed] [Google Scholar]
- 28.Benhamou E, Benhamou S, Auquier A, Flamant R. Changes in patterns of cigarette smoking and lung cancer risk: results of a case-control study. Br J Cancer 1989;60: 601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Augustine A, Harris RE, Wynder EL. Compensation as a risk factor for lung cancer in smokers who switch from nonfilter to filter cigarettes. Am J Public Health 1989;79: 188-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Relationship of the type of tobacco and inhalation pattern to pulmonary and total mortality. Eur Respir J 1992;5: 1111-7. [PubMed] [Google Scholar]
- 31.Jockel KH, Ahrens W, Wichmann HE, Becher H, Bolm-Audorff U, Jahn I, et al. Occupational and environmental hazards associated with lung cancer. Int J Epidemiol 1992;21: 202-13. [DOI] [PubMed] [Google Scholar]
- 32.Benhamou E, Benhamou S. Black (air-cured) and blond (flue-cured) tobacco and cancer risk. VI: Lung cancer. Eur J Cancer 1993;29A: 1778-80. [DOI] [PubMed] [Google Scholar]
- 33.Pezzotto SM, Mahuad R, Bay ML, Morini JC, Poletto L. Variation in smoking-related lung cancer risk factors by cell type among men in Argentina: a case-control study. Cancer Causes Control 1993;4: 231-7. [DOI] [PubMed] [Google Scholar]
- 34.Benhamou S, Benhamou E, Auquier A, Flamant R. Differential effects of tar content, type of tobacco and use of a filter on lung cancer risk in male cigarette smokers. Int J Epidemiol 1994;23: 437-43. [DOI] [PubMed] [Google Scholar]
- 35.Agudo A, Barnadas A, Pallares C, Martinez I, Fabregat X, Rosello J, et al. Lung cancer and cigarette smoking in women: a case-control study in Barcelona (Spain). Int J Cancer 1994;59: 165-9. [DOI] [PubMed] [Google Scholar]
- 36.Kabat GC. Aspects of the epidemiology of lung cancer in smokers and nonsmokers in the United States. Lung Cancer 1996;15: 1-20. [DOI] [PubMed] [Google Scholar]
- 37.Engeland A, Haldorsen T, Andersen A, Tretli S. The impact of smoking habits on lung cancer risk: 28 years' observation of 26,000 Norwegian men and women. Cancer Causes Control 1996;7: 366-76. [DOI] [PubMed] [Google Scholar]
- 38.De Stefani E, Fierro L, Correa P, Fontham E, Ronco A, Larrinaga M, et al. Mate drinking and risk of lung cancer in males: a case-control study from Uruguay. Cancer Epidemiol Biomarkers Prev 1996;5: 515-9. [PubMed] [Google Scholar]
- 39.Stellman SD, Muscat JE, Thompson S, Hoffmann D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer 1997;80: 382-8. [DOI] [PubMed] [Google Scholar]
- 40.Khuder SA, Dayal HH, Mutgi AB, Willey JC, Dayal G. Effect of cigarette smoking on major histological types of lung cancer in men. Lung Cancer 1998;22: 15-21. [DOI] [PubMed] [Google Scholar]
- 41.Matos E, Vilensky M, Boffetta P, Kogevinas M. Lung cancer and smoking: a case-control study in Buenos Aires, Argentina. Lung Cancer 1998;21: 155-63. [DOI] [PubMed] [Google Scholar]
- 42.Armadans-Gil L, Vaque-Rafart J, Rossello J, Olona M, Alseda M. Cigarette smoking and male lung cancer risk with special regard to type of tobacco. Int J Epidemiol 1999;28: 614-9. [DOI] [PubMed] [Google Scholar]
- 43.Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, Darby SC, et al. Lung cancer and cigarette smoking in women: a multicenter case-control study in Europe. Int J Cancer 2000;88: 820-7. [DOI] [PubMed] [Google Scholar]
- 44.Simonato L, Agudo A, Ahrens W, Benhamou E, Benhamou S, Boffetta P, et al. Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer 2001;91: 876-87. [DOI] [PubMed] [Google Scholar]
- 45.Hammond EC, Garfinkel L, Seidman H, Lew EA. “Tar” and nicotine content of cigarette smoke in relation to death rates. Environ Res 1976;12: 263-74. [DOI] [PubMed] [Google Scholar]
- 46.Higenbottam T, Shipley MJ, Rose G. Cigarettes, lung cancer, and coronary heart disease: the effects of inhalation and tar yield. J Epidemiol Community Health 1982;36: 113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borland C, Chamberlain A, Higenbottam T, Shipley M, Rose G. Carbon monoxide yield of cigarettes and its relation to cardiorespiratory disease. BMJ 1983;287: 1583-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lubin JH, Blot WJ, Berrino F, Flamant R, Gillis CR, Kunze M, et al. Patterns of lung cancer risk according to type of cigarette smoked. Int J Cancer 1984;33: 569-76. [DOI] [PubMed] [Google Scholar]
- 49.Petitti DB, Friedman GD. Cardiovascular and other diseases in smokers of low yield cigarettes. J Chronic Dis 1985;38: 581-8. [DOI] [PubMed] [Google Scholar]
- 50.Gillis CR, Hole DJ, Boyle P. Cigarette smoking and male lung cancer in an area of very high incidence. I. Report of a case-control study in the West of Scotland. J Epidemiol Community Health 1988;42: 38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman DW, Palmer JR, Rosenberg L, Stolley P, Warshauer E, Shapiro S. Tar content of cigarettes in relation to lung cancer. Am J Epidemiol 1989;129: 703-11. [DOI] [PubMed] [Google Scholar]
- 52.Sidney S, Tekawa IS, Friedman GD. A prospective study of cigarette tar yield and lung cancer. Cancer Causes Control 1993;4: 3-10. [DOI] [PubMed] [Google Scholar]
- 53.Speizer FE, Colditz GA, Hunter DJ, Rosner B, Hennekens C. Prospective study of smoking, antioxidant intake, and lung cancer in middle-aged women (USA). Cancer Causes Control 1999;10: 475-82. [DOI] [PubMed] [Google Scholar]
- 54.US Federal Trade Commission. Domestic market share of cigarettes by tar yield. Federal Trade Commission cigarette report for 2000. Washington, DC: US Federal Trade Commission, 2002.
- 55.Stellman SD, Garfinkel L. Smoking habits and tar levels in a new American Cancer Society prospective study of 1.2 million men and women. J Natl Cancer Inst 1986;76: 1057-63. [PubMed] [Google Scholar]
- 56.Thun MJ, Calle EE, Rodriguez C, Wingo PA. Epidemiological research at the American Cancer Society. Cancer Epidemiol Biomarkers Prev 2000;9: 861-8. [PubMed] [Google Scholar]
- 57.Garfinkel L. Selection, follow-up, and analysis in the American Cancer Society prospective studies. Washington, DC: Government Printing Office, 1985: 49-52. (NIH Publication 85-2713, National Cancer Institute Monograph 67.) [PubMed]
- 58.Calle EE, Terrell DD. Utility of the national death index for ascertainment of mortality among cancer prevention study II participants. Am J Epidemiol 1993;137: 235-41. [DOI] [PubMed] [Google Scholar]
- 59.US Federal Trade Commission. Report of `tar,' nicotine, and carbon monoxide of the smoke of 200 varieties of cigarettes. Washington, DC: US Federal Trade Commission, December 1981.
- 60.Cox DR. Regression models and life-tables. J Royal Stat Soc 1972;34: 187-220. [Google Scholar]
- 61.Burns DR, Major JM, Shanks TG, Thun MJ, Samet JM. Smoking lower yield cigarettes and disease risks. In: Shopland DR, Burns DM, Benowitz NI, Amacher RH, eds. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: US National Institutes of Health, National Cancer Institute, 2001: 65-158. (NCI Smoking and Tobacco Control Monograph No 13.)
- 62.Rothman KJ, Greenland S. Modern epidemiology. 2nd ed. Philadelphia: Lippincott Williams & Wilkins Publishers, 1998.
- 63.Peto R, Darby S, Deo H, Silcocks P, Whitley E, Doll R. Smoking, smoking cessation, and lung cancer in the UK since 1950: combination of national statistics with two case-control studies. BMJ 2000;321: 323-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lee PN. Lung cancer and type of cigarette smoked. Inhal Toxicol 2001;13: 951-76. [DOI] [PubMed] [Google Scholar]
- 65.Kunze M, Vutuc C. Threshold of tar exposure: analysis of smoking history of male lung cancer cases and controls. In: Gori GB, Bock FG, eds. A safe cigarette? Banbury report 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory, 1980: 29-36.
- 66.Benowitz N. Compensatory smoking of low-yield cigarettes. In: Shopland DR, Burns DM, Benowitz NI, Amacher RH, eds. Risks associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Bethesda, MD: US National Institutes of Health, National Cancer Institute, 2001: 39-64. (NCI Smoking and Tobacco Control Monograph No 13.)
- 67.Hoffmann D, Hoffmann I. Tobacco consumption and lung cancer. In: Hansen HH, ed. Lung cancer. Boston: Kluwer Academic Publications, 1994: 1-42.
- 68.Harris JE. Smoke yields of tobacco-specific nitrosamines in relation to FTC tar level and cigarette manufacturer: analysis of the Massachusetts benchmark study. Public Health Rep 2001;116: 336-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Thun MJ, Heath CW, Jr. Changes in mortality from smoking in two American Cancer Society prospective studies since 1959. Prev Med 1997;26: 422-6. [DOI] [PubMed] [Google Scholar]
- 70.Forey B, Hamling J, Lee P, Wald N, eds. International smoking statistics. 2nd ed. London: Oxford University Press, 2002.
- 71.US Department of Agriculture, Economic Research Service. Tobacco outlook, Washington, DC: USDA, April 2002. (TBS-252.)
- 72.Yang G, Fan L, Tan J, Qi G, Zhang Y, Samet JM, et al. Smoking in China: findings of the 1996 national prevalence survey. JAMA 1999;282: 1247-53. [DOI] [PubMed] [Google Scholar]