Abstract
Purpose
A controversy of current PSA-based prostate cancer screening is the overdetection of potentially insignificant prostate cancer. Because PSA kinetics have previously been linked to prostate cancer-specific mortality, our objective was to determine whether PSA velocity (PSAV) was associated with clinically significant prostate cancer.
Materials and Methods
From 1992 to 2008, 1073 men underwent radical prostatectomy with data on PSA velocity and tumor volume. “Insignificant” cancer was defined by the Ohori criteria (organ-confined, tumor volume ≤0.5 cc, no primary or secondary Gleason pattern 4 or 5). We calculated the proportion of men with pathologically “insignificant” prostate cancer, stratified by PSAV.
Results
A preoperative PSAV >0.4 ng/ml was significantly associated with high-grade disease (p=0.008), positive surgical margins (p=0.003), and seminal vesicle invasion (p=0.007) at radical prostatectomy. The median tumor volume was also significantly higher among men with a preoperative PSAV >0.4 ng/ml/year (3.1 vs. 2.4 cc, p=0.0001). Overall, 69 (6%) met the Ohori criteria for “insignificant” cancer. Patients with a preoperative PSAV >0.4 ng/ml/year were 50% less likely to have “insignificant” disease (10% vs. 5%, respectively, p=0.003).
Conclusions
A PSAV threshold of 0.4 ng/ml/year distinguished between men who did or did not meet published pathology criteria for potentially “insignificant” prostate cancer. These results suggest that PSAV may be a useful adjunct in prostate cancer screening to increase the specificity for identifying patients with clinically significant disease.
Keywords: prostate cancer, insignificant, screening, PSA, PSA velocity
INTRODUCTION
Currently there is considerable controversy over PSA-based screening. The European Randomized Study of Screening for Prostate Cancer demonstrated a 20% reduction in cancer-specific mortality in the screening arm.1 However, they reported that 1410 men would need to be screened and 48 treated to prevent one prostate cancer death.
Correspondingly, there is considerable concern regarding the overdiagnosis of potentially insignificant prostate cancer. Nevertheless, current clinical staging modalities are limited in their ability to accurately predict tumor biology. This has fueled an ongoing search for more specific markers for clinically significant prostate cancer.
One promising marker is PSA velocity.2 Our research group has previously demonstrated a relationship between PSA velocity and Gleason grade in the radical prostatectomy specimen.3 Specifically, the median preoperative PSAV was 0.84, 0.97, and 1.39 ng/mL/year in patients with a prostatectomy Gleason score of 6, 7, and 8-10, respectively (p=0.05). Other studies have demonstrated a relationship between pretreatment PSA velocity with the risk of prostate cancer-specific mortality.4-6 Taken together, these findings suggest that PSAV (or a rapidly rising PSA) may represent a surrogate marker for prostate cancer aggressiveness. Conversely, a low PSAV (small PSA slope over time) might indicate a greater likelihood of indolent disease.
The 2007 National Comprehensive Cancer Network Guidelines and others have suggested the use of a PSAV threshold of ~0.35 to 0.4 ng/ml/year in prostate cancer screening protocols.7, 8 We tested whether this PSA velocity threshold was associated with the pathology features and likelihood of finding histologically “insignificant” prostate cancer at radical prostatectomy.
METHODS
From 1992 to 2002, approximately 26,000 men participated in a prostate cancer screening study, as previously described.9 PSA and digital rectal examination (DRE) were performed at 6 to 12 month intervals, and biopsy was recommended for a PSA level >4.0 (before 1995) or >2.5 ng/ml (after 1995), or findings suspicious for cancer (induration or irregularity) on DRE. From this population, 1374 underwent radical prostatectomy by a variety of different surgeons. Of these men, 556 met the following inclusion criteria for the present study: tumor volume data and multiple preoperative PSA measurements to enable a PSAV calculation. Included men from the screening study were slightly older (64 vs. 63, p=0.02) and a greater proportion were Caucasian (92% vs. 89%, p=0.09).
From 2003 to 2008, an additional 517 men underwent radical prostatectomy by a single surgeon at Northwestern University with data on tumor volume and PSA velocity. Accordingly, the combined overall study population included 1073 men. Compared to included men from the screening study, the 517 men from Northwestern were significantly younger (p<0.0001), but the racial distribution was similar (p=0.86).
Clinical and pathological features were recorded in a prospective database. Tumor volume was primarily determined by visual estimation, which has been shown to correlate well with the grid morphometric method.10 Insignificant prostate cancer was defined according to the previously published Ohori criteria (organ-confined, tumor volume ≤0.5 cc, no Gleason pattern 4 or 5).11 PSA velocity was calculated by regression analysis of PSA measurements within the year prior to prostate cancer diagnosis, as previously described.4
The t-test, chi-square, and Wilcoxon rank sum tests were used to compare clinico-pathological features based on PSA velocity. Logistic regression was used for multivariate analysis to predict insignificant prostate cancer. Receiver operating characteristic (ROC) analysis was performed using MedCalc. Subgroup analysis was also performed for the 464 men with a preoperative PSA level <4 ng/ml, to examine the ability of PSAV to distinguish insignificant prostate cancer at lower PSA levels. SAS was used for all statistical analysis.
RESULTS
Table 1 shows the demographic characteristics of the study population. The mean age was 62 years, and most men were Caucasian. The median PSA at diagnosis was 4.3 ng/ml, and the majority of patients had clinical stage T1c prostate cancer with a biopsy Gleason score of 6.
Table 1.
Clinical characteristics of the study population.
| Overall population | PSAV < 0.4 ng/ml/year | PSAV>0.4 ng/ml/year | p-value | |
|---|---|---|---|---|
| Mean Age | 62 (41-78) | 62 (43-78) | 62 (41-77) | 0.42 |
|
| ||||
| Race (%black) | 50 (5) | 20 (5) | 30 (4) | 0.74 |
|
| ||||
| Family history | 223/740 (30) | 65 (25) | 158 (33) | 0.05 |
|
| ||||
| Median PSA | 4.3 (0.2-63.3) | 4.0 (0.2-28.8) | 4.5 (1.1-63.3) | <0.0001 |
|
| ||||
| Clinical stage | ||||
| T1 | 842 (79) | 270 (73) | 572 (82) | |
| ≥T2 | 228 (21) | 102 (27) | 126 (18) | 0.0005 |
|
| ||||
| Biopsy Gleason score | ||||
| 6 | 848 (79) | 304 (82) | 544 (78) | |
| ≥ 7 | 219 (21) | 66 (18) | 153 (22) | 0.13 |
Men with a PSAV >0.4 ng/ml/year had a higher PSA level at diagnosis (4.5 vs. 4.0 ng/ml, p<0.0001) and a significantly lower proportion with clinical stage ≥T2 disease (18% vs. 27%, p=0.0005) compared to those with a PSAV <0.4 ng/ml/year (Table 1). Age, race and the biopsy Gleason score distribution were similar between the groups.
At radical prostatectomy, 858 (80%) men had organ-confined disease (Table 2). Positive surgical margins were significantly more likely among men with a PSAV >0.4 ng/ml/year (19% vs. 12%, p=0.003), as was seminal vesicle invasion (4% vs. 1%, p=0.007). Similarly, patients with a PSAV >0.4 ng/ml/year were significantly more likely to have a Gleason score ≥7 (p=0.008) and had a significantly higher tumor volume (p=0.0001) compared to those with a PSAV <0.4 ng/ml/year.
Table 2.
Pathological features in the study population, stratified by PSA velocity.
| Overall population | < 0.4 ng/ml/year | >0.4 ng/ml/year | p-value | |
|---|---|---|---|---|
| Organ-confined | 858 (80) | 309 (83) | 549 (79) | 0.13 |
|
| ||||
| Positive surgical margins | 173 (16) | 43 (12) | 130 (19) | 0.003 |
|
| ||||
| Extracapsular extension only | 140 (13) | 50 (13) | 90 (13) | 0.85 |
|
| ||||
| Seminal vesicle invasion | 31 (3) | 4 (1) | 27 (4) | 0.007 |
|
| ||||
| Lymph node metastases | 5 (0.5) | 2 (0.6) | 3 (0.4) | 0.99 |
|
| ||||
| Prostatectomy Gleason score | ||||
| 6 | 688 (64) | 260 (70) | 428 (61) | 0.008 |
| ≥ 7 | 385 (36) | 114 (30) | 271 (39) | |
|
| ||||
| Median Tumor volume | 2.9 (0.0-71.2) | 2.4 (0.0-37.8) | 3.1 (0.0-71.2) | 0.0001 |
Median PSAV was 0.79, 0.92 and 2.78 ng/ml/year in patients with a prostatectomy Gleason score of 6 of less, 7 and 8-10, respectively (p<0.0001). On ROC analysis, PSAV had an AUC of 0.70 to predict a prostatectomy Gleason score of 8-10.
Overall, 69 (6%) men had pathologically insignificant cancer according to the criteria by Ohori et al.11 Insignificant disease was significantly more likely among men with a preoperative PSAV <0.4 ng/ml/year (10% vs. 5%, p=0.003). The median PSAV was 0.36 ng/ml/year in men with insignificant disease versus 0.89 ng/ml/year in those who did not meet the criteria for insignificant disease (p=0.005). Of the 1055 men with PSA <15 ng/ml, insignificant disease was reported in 10% vs. 5% of those with PSAV <0.4 vs. >0.4 ng/ml/year (p=0.005).
On univariate analysis age (OR 1.0, 95% CI 1.0-1.1, p=0.04), Gleason score (OR 9.3, 95% CI 2.3-38.3, p=0.02), PSA (OR 1.4, 95% CI 1.2-1.7, p<0.0001) and PSAV >0.4 ng/ml/year (OR 2.0, 95% CI 1.2-3.3, p=0.004) were significantly associated with significant prostate cancer. Figure 1 shows the ROC curve to predict significant prostate cancer using PSAV alone.
Figure 1.
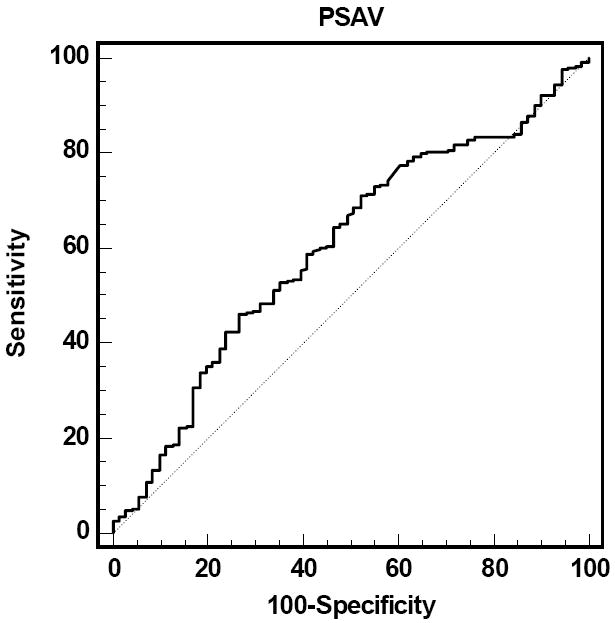
ROC analysis of significant prostate cancer prediction by PSAV
On multivariate analysis (Table 3a), PSAV was an independent predictor of significant prostate cancer (OR 1.8, 95% CI 1.1-3.0, p=0.02) after controlling for age, clinical stage, and biopsy Gleason score. After additional adjustment for PSA (Table 3b), PSAV was associated with a nonsignificant increased likelihood of significant disease. On ROC analysis, a model including age, clinical stage, biopsy Gleason score and PSA had an area under the curve (AUC) of 0.73 which increased to 0.74 with the addition of PSAV.
Table 3.
Multivariate analyses to predict significant prostate cancer in the (a, b) overall population, and (c) in the subset with a PSA <4 ng/ml and biopsy Gleason score of 6.
| (a) | ||
|---|---|---|
| OR (95% CI) | p-value | |
| Age decade | 1.04 (0.99-1.07) | 0.07 |
| Clinical stage | 1.0 (0.6-1.9) | 0.93 |
| Biopsy Gleason score | 8.9 (2.2-36.7) | 0.003 |
| PSAV >0.4 ng/ml/year | 1.8 (1.1-3.0) | 0.02 |
| (b) | ||
| OR (95% CI) | p-value | |
| Age decade | 1.04 (0.99-1.08) | 0.07 |
| Clinical stage | 1.5 (0.8-3.0) | 0.20 |
| Gleason score | 7.0 (1.7-28.9) | 0.007 |
| PSA | 1.4 (1.2-1.6) | 0.0002 |
| PSAV >0.4 ng/ml/year | 1.4 (0.8-2.4) | 0.18 |
| (c) | ||
| OR (95% CI) | p-value | |
| Age decade | 1.06 (1.01-1.11) | 0.02 |
| Clinical stage | 1.3 (0.6-2.7) | 0.55 |
| PSAV >0.4 ng/ml/year | 2.1 (1.1-4.1) | 0.03 |
Finally, in the subset of 464 men with total PSA levels <4 ng/ml, 48 (10%) met the Ohori criteria for insignificant prostate cancer. Similar to the overall population, men with a PSAV <0.4 ng/ml were twice as likely to have pathologically insignificant prostate cancer, compared to those with a PSAV >0.4 ng/ml/year (14% vs. 7%, p=0.02). A PSAV >0.4 ng/ml/year was also associated with a significantly higher tumor volume (2.3 vs. 2.1, p=0.03) and greater proportion of Gleason ≥7 tumors at prostatectomy (36% vs. 22%, p=0.002). Among “low-risk” men with PSA levels <4 and a biopsy Gleason score of 6, a PSAV >0.4 ng/ml/year was associated with a 2.1-fold increased odds (p=0.03) of significant cancer on multivariate analysis with age and clinical stage (Table 3b).
DISCUSSION
PSA has been criticized as a marker due to its limited specificity for prostate cancer. As a result, PSA kinetics measurements such as PSA velocity (PSAV) have recently gained considerable attention.
The initial clinical report by Carter et al. in 1992 compared longitudinal changes in PSA between 20 men with BPH, 18 men with prostate cancer and 16 controls from the Baltimore Longitudinal Study on Aging.12 In this non-screening population with PSA levels between 4 and 10 ng/ml, a PSAV greater than 0.75 ng/ml/year was useful to distinguish prostate cancer from benign conditions (p<0.01).
Subsequently, Smith and Catalona tested these results in 9,492 men with serial PSA measurements from a formal prostate cancer screening study.13 Of these men, 982 underwent a prostate biopsy. Prostate cancer was detected in 47% of men with a PSAV > 0.75 ng/ml/year, compared to only 11% of men with a lower PSAV.
Because all of these early studies were based upon men with PSA levels greater than 4 ng/ml, their generalizability to men with lower total PSA levels is unclear. Nevertheless, the vast majority of men aged 40 and older have PSA levels less than 4 ng/ml. According to data from the National Health and Nutrition Examination Survey (2001-2002), the overall estimated mean and median PSA levels were 1.56 (95% CI, 1.37-1.74) and 0.9 (25th-75th percentile: 0.5-1.4) ng/ml, respectively, for U.S. men aged 40 to 84 years.14
Interestingly, Carter et al. demonstrated that prostate cancer-specific mortality was significantly higher among men with a PSAV >0.35 ng/ml/year more than a decade prior to diagnosis, at a time when total PSA levels were low.6 Correspondingly, some groups are now recommending the use of PSA velocity thresholds in the range of 0.3 to 0.5 ng/ml/year.7, 15 The 2007 National Comprehensive Cancer Network Guidelines recommend considering a prostate biopsy for men with a PSA <2.5 ng/ml with a PSAV >0.35 ng/ml/year.8
It is unclear whether the use of these lower PSA velocity thresholds will merely increase the detection of insignificant prostate cancer, or, conversely, enhance the specificity of PSA-based screening for identifying clinically significant disease. The objective of this study was therefore to compare the tumor features and proportion of pathologically “insignificant” tumors between men with a pretreatment PSA velocity <0.4 versus >0.4 ng/ml/year.
Indeed, we found that men with a PSAV >0.4 ng/ml/year were significantly more likely to have adverse pathological features at radical prostatectomy, including positive surgical margins, seminal vesicle invasion, Gleason score ≥7, and a higher tumor volume. Moreover, the PSAV distribution was significantly different between men who did and did not meet the Ohori11 criteria for insignificant prostate cancer. Specifically, pathologically insignificant disease was 50% less likely among men with a preoperative PSAV >0.4 ng/ml/year. After controlling for age, clinical stage, and Gleason score, a PSAV >0.4 ng/ml/year was associated with approximately a 2-fold increased odds of pathologically significant prostate cancer. Although this association was slightly attenuated with the addition of PSA to the model, this is likely related to the strong interaction between the variables and requires further exploration. Interestingly, in a subset analysis of low-risk men with PSA levels <4 ng/ml and a biopsy Gleason score of 6, a PSAV >0.4 ng/ml/year was similarly associated with a 2-fold increased odds of significant disease in a multivariate model. These results suggest that PSAV might be useful to distinguish clinically significant prostate cancer among otherwise low-risk patients.
Limitations of our study include the small sample size with “insignificant” disease reducing our statistical power. In addition, both the optimal method for PSA velocity calculation and the definition for pathologically insignificant11 prostate cancer are subject to debate. There are insufficient data to prove that individuals who meet pathological criteria for insignificant prostate cancer will not experience metastasis or prostate cancer death in the long term. For this reason, we also provided data on the relationship between PSA velocity with specific pathological tumor features as additional support for its role as a potential marker for prostate cancer aggressiveness. Finally, we selected the threshold of 0.4 ng/ml/year for analysis based on prior literature and National Comprehensive Cancer Network guidelines. However, this may not represent the optimal cutoff and this issue requires further prospective study.
CONCLUSIONS
Patients with a preoperative PSAV >0.4 ng/ml/year were significantly less likely to have pathologically “insignificant” prostate cancer and had a significantly higher tumor volume at radical prostatectomy. On multivariate analysis with age, clinical stage and Gleason score, PSAV was significantly associated with the risk of clinically significant prostate cancer. Similarly, among men with a PSA <4 ng/ml and biopsy Gleason score of 6, a PSAV >0.4 ng/ml was associated with a 50% reduced odds of findings pathologically insignificant prostate cancer. These results suggest that PSAV may be useful in conjunction with other variables to help enhance the specificity of prostate cancer screening for the detection of clinically significant prostate cancer.
Acknowledgments
Supported in part by the Urological Research Foundation, Prostate SPORE grant (P50 CA90386-05S2) and the Robert H. Lurie Comprehensive Cancer Center grant (P30 CA60553).
References
- 1.Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Ciatto S, Nelen V, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Sutcliffe P, Hummel S, Simpson E, Young T, Rees A, Wilkinson A, et al. Use of classical and novel biomarkers as prognostic risk factors for localised prostate cancer: a systematic review. Health Technol Assess. 2009;13:iii. doi: 10.3310/hta13050. [DOI] [PubMed] [Google Scholar]
- 3.Loeb S, Sutherland DE, D’Amico AV, Roehl KA, Catalona WJ. PSA velocity is associated with gleason score in radical prostatectomy specimen: marker for prostate cancer aggressiveness. Urology. 2008;72:1116. doi: 10.1016/j.urology.2008.01.082. [DOI] [PubMed] [Google Scholar]
- 4.D’Amico AV, Chen MH, Roehl KA, Catalona WJ. Preoperative PSA velocity and the risk of death from prostate cancer after radical prostatectomy. N Engl J Med. 2004;351:125. doi: 10.1056/NEJMoa032975. [DOI] [PubMed] [Google Scholar]
- 5.D’Amico AV, Renshaw AA, Sussman B, Chen MH. Pretreatment PSA velocity and risk of death from prostate cancer following external beam radiation therapy. Jama. 2005;294:440. doi: 10.1001/jama.294.4.440. [DOI] [PubMed] [Google Scholar]
- 6.Carter HB, Ferrucci L, Kettermann A, Landis P, Wright EJ, Epstein JI, et al. Detection of life-threatening prostate cancer with prostate-specific antigen velocity during a window of curability. J Natl Cancer Inst. 2006;98:1521. doi: 10.1093/jnci/djj410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moul JW, Sun L, Hotaling JM, Fitzsimons NJ, Polascik TJ, Robertson CN, et al. Age adjusted prostate specific antigen and prostate specific antigen velocity cut points in prostate cancer screening. J Urol. 2007;177:499. doi: 10.1016/j.juro.2006.09.063. [DOI] [PubMed] [Google Scholar]
- 8.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology. Prostate Cancer Early Detection. [May 2, 2009]; doi: 10.6004/jnccn.2010.0016. http://www.nccn.org/professionals/physician_gls/PDF/prostate_detection.pdf. [DOI] [PubMed]
- 9.Smith DS, Catalona WJ. The nature of prostate cancer detected through prostate specific antigen based screening. J Urol. 1994;152:1732. doi: 10.1016/s0022-5347(17)32372-8. [DOI] [PubMed] [Google Scholar]
- 10.Humphrey PA, Vollmer RT. Percentage carcinoma as a measure of prostatic tumor size in radical prostatectomy tissues. Mod Pathol. 1997;10:326. [PubMed] [Google Scholar]
- 11.Ohori M, Wheeler TM, Dunn JK, Stamey TA, Scardino PT. The pathological features and prognosis of prostate cancer detectable with current diagnostic tests. J Urol. 1994;152:1714. doi: 10.1016/s0022-5347(17)32369-8. [DOI] [PubMed] [Google Scholar]
- 12.Carter HB, Pearson JD, Metter EJ, Brant LJ, Chan DW, Andres R, et al. Longitudinal evaluation of prostate-specific antigen levels in men with and without prostate disease. Jama. 1992;267:2215. [PMC free article] [PubMed] [Google Scholar]
- 13.Smith DS, Catalona WJ. Rate of change in serum prostate specific antigen levels as a method for prostate cancer detection. J Urol. 1994;152:1163. doi: 10.1016/s0022-5347(17)32528-4. [DOI] [PubMed] [Google Scholar]
- 14.Porter MP, Stanford JL, Lange PH. The distribution of serum prostate-specific antigen levels among American men: implications for prostate cancer prevalence and screening. Prostate. 2006;66:1044. doi: 10.1002/pros.20417. [DOI] [PubMed] [Google Scholar]
- 15.Loeb S, Roehl KA, Nadler RB, Yu X, Catalona WJ. Prostate specific antigen velocity in men with total prostate specific antigen less than 4 ng/ml. J Urol. 2007;178:2348. doi: 10.1016/j.juro.2007.08.016. [DOI] [PubMed] [Google Scholar]


