Abstract
Earthworms are key components of temperate soil ecosystems but key aspects of their ecology remain unexamined. Here we elucidate the role of olfactory cues in earthworm attraction to food sources and document specific chemical cues that attract Eisenia fetida to the soil fungi Geotrichum candidum. Fungi and other microorganisms are major sources of volatile emissions in soil ecosystems as well as primary food sources for earthworms, suggesting the likelihood that earthworms might profitably use olfactory cues to guide foraging behavior. Moreover, previous studies have documented earthworm movement toward microbial food sources. But, the specific olfactory cues responsible for earthworm attraction have not previously been identified. Using olfactometer assays combined with chemical analyses (GC-MS), we documented the attraction of E. fetida individuals to filtrate derived from G. candidum colonies and to two individual compounds tested in isolation: ethyl pentanoate and ethyl hexanoate. Attraction at a distance was observed when barriers prevented the worms from reaching the target stimuli, confirming the role of volatile cues. These findings enhance our understanding of the mechanisms underlying key trophic interactions in soil ecosystems and have potential implications for the extraction and collection of earthworms in vermiculture and other applied activities.
Introduction
Olfaction is a key sensory modality by which animals, and many other organisms, acquire information about the surrounding world [1], [2]. In addition to perceiving semiochemicals (i.e., pheromones and kairomones), which play key roles in many interactions within and between species [3], many organisms also detect and respond to general odorant cues deriving from biotic and abiotic features of their environments. Indeed, most organisms have specialized sensory, information processing, and behavioral mechanisms dedicated to detecting and reacting to chemicals present in the external environment [4], [5]. Among terrestrial invertebrates, the perception and use of olfactory cues by insects, and some other arthropods, has been extensively studied [2], [6], [7], [8] as has the ecological role of olfactory cues in interactions among insects and plants (e.g., [9], [10], [11], [12]) Previous work has also explored the detection of olfactory cues by nematodes which entails the activation of papilla and setae on the body surface that are connected to chemosensory neurons known as the AWA cells [1], [13] and the response of nematodes to various olfactory cues [14], [15], [16]. In contrast, olfaction by annelids remains poorly studied. Chemoreceptors have been identified on the prostonium and the buccal epithelium of earthworms [17] and have been shown to detect sucrose, glucose and quinine [18]. Recently, olfaction by earthworms has been suggested to be involved in the coordination of collective movement [19].
Previous studies exploring the feeding strategies of various earthworm species suggest that these animals exhibit orientation and movement toward particular food sources, including specific species of protozoa, bacteria, fungi and plants [20], [21], [22], [23]. Microorganisms are both major components of earthworm diets [20] and principle sources of volatile organic compound emissions in soil ecosystems [24], suggesting that olfaction may play a key role in earthworm foraging. Moreover, Bonkowski and Schaefer [25] reported that Aporrectodae caliginosa actively moved toward foraging sites exhibiting higher densities of protozoa and naked amobae. Soil fungi are particularly important food sources for earthworms, especially for epigeic species that consume litter typically colonized by fungi [26], [27], including Geotrichum candidum, Mucor sp., and Aspergillus flavus [28], [29]. Bonkowski et al. [28] conducted feeding choice assays to document the preferences of five earthworm species for a variety of soil fungi and reported a general pattern in which worms exhibited a preference for early successional species (e.g., Fusarium nivale and Cladosporium cladosporioides) that are presumably indicative of relatively new and nutrient rich organic resources. The factors underlying the observed preferences were not determined, though the investigators postulated that differences in the nutritional value of the fungi, or the presence of antibiotic compounds or other metabolites in or around the mycelia might be important.
Because soil fungi release volatile and non-volatile chemicals, as well as influencing the release of plant-derived compounds [30], [31], olfactory cues associated with the presence of fungi may be expected to play an important role in earthworm foraging for fungal food sources. However, previous studies have not explicitly addressed the role of olfaction or documented the specific cues responsible for orientation and attraction. Therefore, we explored the role of olfaction in the foraging of E. fetida, an epigeic earthworm species with economic significance for various industrial processes, on the soil fungus G. candidum, which is an important food source for this worm [29].
Methods
Eisenia fetida rearing
Earthworms (Eisenia fetida) provided by Ouroboros s.a. (Belgium) were reared in PVC boxes (42 cm long×30 cm wide×10 cm high) filled with universal compost DCM ® (De Ceuster Meststoffen s.a.,Grobbendonk, Belgium) composed of a mixture of brown peat, white peat, and lava. The compost was changed every two months and cocoons and hatchling earthworms were placed in new boxes with fresh compost. Boxes were maintained at 23±1°C. Only mature earthworms (with a clitellum) were used for our experiments.
Culture of Geotrichum candidum
Geotrichum candidum, isolated from compost mixed with milky fermented product, was cultured in 100 ml of liquid medium 863 (2 g glucose; 1 g yeast extract; 1 g peptone) at 27±3°C during 42 h, and the culture was filtered with Pall Supor® (Whatman Ltd, England) - 450 membrane 47 mm–0.45 µm filter.
Bioassays
Earthworm response to cues associated with Geotrichum candidum
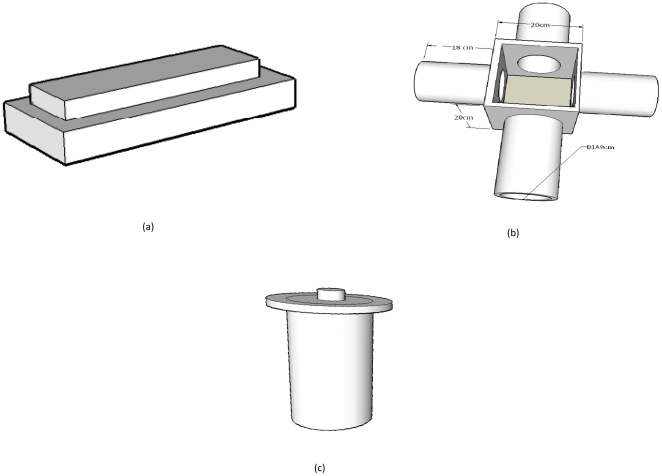
A PVC box (Box #1: 56 cm×36 cm×8 cm) was filled with moist compost (76% humidity content; obtained by drying a 25 g sample of moist compost at 105°C for 48 h), and 200 earthworms (100 matures and 100 immatures) were placed randomly within it. A second box (Box #2: 37 cm×26 cm×9 cm) was placed on top of Box 1 (Fig. 1). Box 2 had 5 slots (30 cm long×0.5 cm wide) in its bottom and was also filled with moist compost (prepared as above) Filtrate (275 ml) from the G. candidum culture was then poured evenly across the surface of Box 2. Pairs of control boxes were similarly placed but received tap water instead of the fungal filtrate. After 120 h, the number of earthworms in each box was determined. Six repetitions were conducted with the G. candidum filtrate and three for the controls.
Figure 1. Experimental set-up.
(a) Dual-box earthworm sampling device, (b) four-arm olfactometer (A = central chamber, B = (identical) arms), (c) vertical olfactometer.
Four-arm olfactometer experiments
Earthworm behavior was more precisely observed in a below-ground olfactometer consisting of a central PVC chamber (20 cm×20 cm×10 cm) connected to four equidistantly space side arms (9 cm in diameter, 18 cm long) (Fig. 1b). For each experiment, the entire system was filled with moist compost (as above). Target stimuli (i.e., G. candidum filtrate or filter paper treated with individual compounds) were placed at the far end of one arm (selected randomly), while the three remaining arms acted as controls. Between repetitions, each piece of the olfactometer was cleaned with tap water and then with norvanol before being dried overnight at 70°C. For each stimulus tested, groups of earthworms varying in number/density from 5 to 160 individuals were introduced in the central chamber and allowed to make choices (the specific numbers used for each assay are listed in table 1). One day after release, the olfactometer was disassembled, the compost in each arm was placed in a separate container, and the number of earthworms was recorded. The specific stimuli assayed are presented in table 1. When testing effects of volatile cues on earthworm behavior, a circular metal screen was placed in the middle of each arm to prevent physical contact with the target. Four different doses of ethyl pentanoate and ethyl hexanoate (1 µl, 10 µl, 100 µl and 1000 µl) were evaluated (sample purity was 97% and 98%, respectively for the two esters). At least 10 replicates were conducted for each trial. A control with only compost in the four arms of the olfactometer was also employed in 3 replicates.
Table 1. Treatments employed in the four-arm olfactometer bioassays.
| Experiment | Number of earthworms | Tested substances | Quantity of tested substance | Repetitions |
| Influence of G. candidum | 20 | G. candidum filtrate | 25 ml | 18 |
| Influence of earthworms density | 5 | G. candidum filtrate | 25 ml | 18 |
| 10 | G. candidum filtrate | 25 ml | 18 | |
| 40 | G. candidum filtrate | 25 ml | 18 | |
| 80 | G. candidum filtrate | 25 ml | 18 | |
| 160 | G. candidum filtrate | 25 ml | 18 | |
| Influence of identified volatile compounds | 20 | Ethyl acetate | 1 ml | 10 |
| 20 | Ethyl propionate | 1 ml | 10 | |
| 20 | Ethyl pentanoate | 1 ml | 10 | |
| 20 | Ethyl hexanoate | 1 ml | 10 | |
| 20 | 3-octanone | 1 ml | 10 | |
| 20 | 2-methylbutan-1-ol | 1 ml | 10 | |
| 20 | 3-methylbutna-1-ol | 1 ml | 10 | |
| 20 | 2-methylpropan-1-ol | 1 ml | 10 |
Vertical olfactometer experiments
A second, vertical olfactometer (Fig. 1c) was also employed to study earthworm responses to chemical cues over greater distances and to test the feasibility of using chemical cues to attract earthworms to the soil surface, as in vermicomposting. This olfactometer constituted PVC tubes 9 cm in diameter and either 25 cm, 40 cm or 105 cm long that were filled with moist compost (as above). Ten earthworms were placed at the bottom of the olfactometer and 25 ml of G. candidum culture filtrate was introduced at the top. After 24 h (for olfactometers having heights of 25 and 40 cm) and 65 h (for the olfactometer having a height of 105 cm), earthworms present in the top five centimeters of the olfactometer were counted. As a control, similar trials were conducted without filtrate for each olfactometer length. Each experiment was replicated 18 times.
Sampling fungal volatiles
A 5 ml sample of G. candidum filtrate was placed in a glass vial with a septum (opening) in the lid. A 75 µm carboxen-polydiméthylsiloxane (CAR/PDMS) solid-phase micro-extraction (SPME) (Supelco) fiber was inserted through the septum and exposed for 30 min at 40°C. The compounds adsorbed on the fiber were analyzed by gas chromatography-mass spectrometry (GC-MS). The GC–MS system comprised a GC (5890 Serie II Plus, Hewlett Packard) linked to a quadrupole type mass selective detector (5989A, Hewlett Packard). The fiber was inserted manually into the injector port (240°C), desorbed, and the sample chromatographed on an apolar column (Factor four VF-5 ms, 30 m, 0.25 mm internal diameter, 0.25 µm film thickness, Varian). Helium at a constant pressure of 55 kPa was used for carrier gas flow. After fiber insertion, the column temperature started at 40°C during 30 sec, increased to 180°C at 5°C/min then to 240°C at 15°C/min followed by a final stage of 2 min at 240°C. Electron impact mass spectra were recorded over the range 30–350 m/z (Electron energy: 70 eV). Identifications were performed by Wiley 275 library searches and by comparison with the retention time of external standards. Three replicate samples were analyzed. Volatiles of filtrated culture medium (medium 863) were collected and analyzed as controls.
Statistical analyses
A Chi-square Goodness-of-fit test (Minitab® v15.0, State College, Pennsylvania USA - α = 5%, 3 degree of freedom) was used to compare earthworms' distribution in each arm of the four-arm olfactometer to a theoretical distribution based on random preferences for each of the olfactometer arms. A one-way ANOVA (Minitab® v15.0, State College, Pennsylvania USA - α = 5%) was used to compare the numbers of earthworms in the top five centimeters of the vertical olfactometer in presence or absence of filtrate.
Results
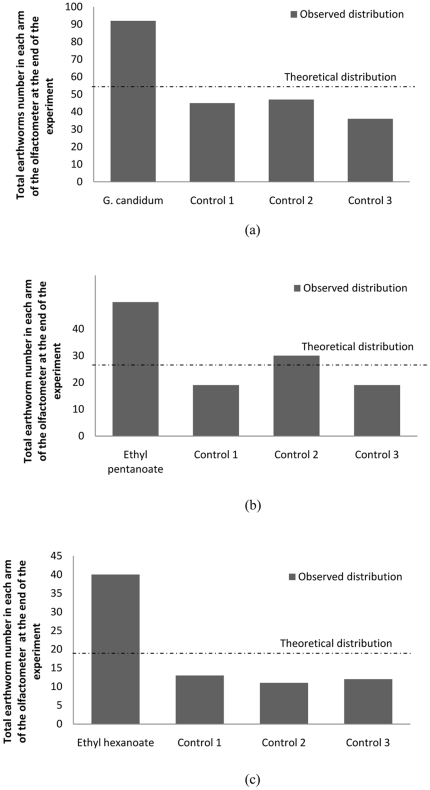
To determine whether E. fetida respond to olfactory cues associated with G. candidum, responses to fungal filtrate and specific compounds were examined in semi-natural conditions using pairs of stacked boxes. Significantly more earthworms were collected in target box when G. candidum filtrate was applied, 179±6.26 (mean ± SD) vs 87±23.31 (mean ± SD) when filtrate was absent (One-way ANOVA, p<0.001). Because of the long exposure time employed in this assay (120 h), it is possible that some fluid components of the filtrate, in addition to olfactory cues, may have percolated through box two and arrive in box one. Subsequent experiments, described below, more effectively test attraction to volatile cues alone. Initial experiments conducted with a four-arm olfactometer produced similar results, as significantly more earthworms were recovered from the olfactometer arms treated with G. candidum filtrate (Fig. 2a; Chi-square Goodness-of-fit test, χ2 3 = 34.44, p<0.001). There was no apparent bias in the experimental set-up, as a fairly uniform distribution of earthworms across the 4 olfactometer arms was observed on controls where filtrate was not introduced. (Arm1: 7; arm2: 7, arm3: 3, arm4: 5; Chi-square Goodness-of-fit test, χ2 3 = 2, p = 0.572). Similar earthworm attraction was observed across a range of population densities (Table 2). Moreover, in experiments conducted with three vertical olfactometers (25, 40 and 105 cm) earthworms reached the soil surface only when G. candidum filtrate was present (Table 3), demonstrating vertical attraction to olfactory cues over significant distances and thus the feasibility of collecting earthworms at soil surface with G. candidum filtrate.
Figure 2. Earthworm behavior in four arm olfactometer.
Observed and theoretical (i.e. random) distributions of earthworms in each arm of the four-arm olfactometer when one arm is treated with (a) G. candidum filtrate, (b) ethyl pentanoate (100% v/v), or (c) ethyl hexanoate (100% v/v). The distributions are compared by a Chi-square Goodness-of-fit test.
Table 2. Influence of earthworm density on earthworm attraction in the four-arm olfactometer.
| Earthworms density | ||||||
| 5 earthworms | 10 earthworms | 20 earthworms | 40 earthworms | 80 earthworms | 160 earthworms | |
| G. candidum | 49 | 83 | 92 | 326 | 528 | 1536 |
| Control 1 | 5 | 19 | 45 | 64 | 156 | 202 |
| Control 2 | 11 | 18 | 47 | 60 | 127 | 169 |
| Control 3 | 3 | 15 | 36 | 55 | 126 | 138 |
| χ2 | 82.35 | 96.08 | 34.44 | 421.7 | 493.63 | 2742.69 |
| p-value | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Total earthworm number across all replicates is given in the table.
Table 3. Earthworm behavior in vertical olfactometer.
| Olfactometer height | |||
| 25 cm | 40 cm | 105 cm | |
| With G. candidum filtrate | 8.3±0.3 | 6.9±0.2 | 6.1±0.4 |
| Without G. candidum filtrate | 1.3±0.3 | 1.3±0.3 | 0.4±0.1 |
| p-value | <0.001 | <0.001 | <0.001 |
Numbers of earthworms (mean ± SD) collected in the top 5 cm of the vertical olfactometer in presence and in absence of G. candidum filtrate for each olfactometer arm length.
To determine the olfactory cues responsible for the observed attraction, volatiles from G. candidum filtrate were collected by SPME and analyzed by GC-MS. These analyses identified 18 molecules, of which 16 were specifically identified from filtrate of G. candidum: ethyl acetate, 2-methyl-1-propanol, ethyl propionate, 3-methyl-1-butanol, 2-methyl-1-butanol, ethyl 2-methylpropanoate, ethyl butanoate, ethyl but-2-enoate, ethyl 2-methylbutanoate, ethyl 3-methylbutanoate, ethyl pentanoate, ethyl 3-methylbut-2-enoate, ethyl 2-methylbut-2-enoate, 3-octanone, ethyl hexanoate, ethyl hex-2-enoate. Authentic standards of eight molecules that were commercially available were tested separately in the four-arm olfactometer. Two esters exhibited significant attraction of E. fetida: ethyl pentanoate (Chi-square Goodness-of-fit test, χ2 3 = 0.3105, p<0.001) and ethyl hexanoate (Chi-square Goodness-of-fit test, χ2 3 = 0.2173, p<0.001) (Fig. 2b and 2c).
The poor solubility of these molecules suggests that volatile cues, diffusing in compost, are likely attractants.
To confirm that volatile cues were responsible for attraction, attraction to the two esters was measured in the four-arm olfactometer using a metallic mesh to prevent the earthworms from contacting the odor source (thus ruling out contact cues). Significant attraction was observed for ethyl pentanoate at quantities above 10 µl and for ethyl hexanoate at quantities above 100 µl, and weak attraction was observed for both compounds even at levels as low as 1 µl (Table 4).
Table 4. Quantities of ethyl pentanoate and ethyl hexanoate tested and E. fetida responses to each.
| Molecules | Quantity | Attraction | p-value |
| Ethyl pentanoate | 1 µl | ◊ | 0.032 |
| 10 µl | ◊ | 0.006 | |
| 100 µl | ◊ | <0.001 | |
| 1000 µl | ◊ | <0.001 | |
| Ethyl hexanoate | 1 µl | ◊ | 0.030 |
| 10 µl | - | 0.67 | |
| 100 µl | ◊ | <0.001 | |
| 1000 µl | ◊ | <0.001 |
- = no attraction, ◊ = earthworm attraction.
Note: Although p-values at 1 µl for both esters are significant (and in each case earthworms were overrepresented in the treatment arm relative to expectations based on a random distribution) attraction to the treatment arm was not significantly different than to at least one adjacent control arm. Instead earthworms were significantly underrepresented in the most distant control arm. This result is consistent with weak attraction to this low concentration of the target compound.
Discussion
Our results clearly demonstrate that E. fetida are attracted by olfactory cues associated with G candidum, and thus complement previous reports that earthworms are able to actively search for food sources [20]. We furthermore identified two specific compounds from the filtrate of G. candidum colonies that exhibit significant attraction for E. fetida, the esters ethyl pentanoate and ethyl hexanoate. To the best of our knowledge, no previous studies have identified specific olfactory cues used by earthworms. In nematodes, attraction has been shown for unidentified olfactory cues deriving from insect larvae [32], and for several specific chemical compounds, including diacetyl, (E)-ß-caryophyllene, isobutanol [14], [15]. In C. elegans, chemiotaxis to volatiles were observed for at least 50 compounds, and specific neurons and genes involved for these responses have been described [1], [33]. The perception of volatile odorants by E. fetida may also involve some specific corporal receptors associated with neurons. Indeed, earthworms are known to have chemoreceptors, principally on the prostonium or on the buccal epithelium that are associated with the nervous system and more particularly with axons and dendrites [18]. The foraging strategy of E. fetida may also bear similarity to social strains of C. elegans, as these worms have been observed to aggregate in areas where bacteria are numerous [34] and there is some evidence for coordinated movement in E. fetida [19].
The two esters we found to be attractive to E. fetida have previously been shown to function as cues for insects in other systems. Ethyl pentanoate has an attractant activity for the dung beetle, Pachylomerus femoralis [35], and ethyl hexanoate, in combination with 1,8-cineole and hexanol, attracts the Mexican fruit fly, Anastrepha ludens, to fermenting, immature fruit of yellow chapote [36]. The latter compound also stimulates upwind flight of the lepidopteran, Ectomyelois ceratoniae [37].
Among the molecules we identified from G. candidum filtrate, ethyl propionate, ethyl acetate, 3-methylbutan-1-ol, 2-methylbutan-1-ol and 2-methylpropanol have previously been found in the volatile profile of G. candidum and other microorganisms [38], [39]. The formation of 2-methylpropanol, 2-methylbutanol, and 3-methylbutanol by G. candidum almost certainly involves deamination of glutamic and aspartic acids and of leucine, phenylalanine and methionine, which are commonly found in fungi [40], [41]. Two other molecules emitted by G. candidum filtrate, hexanoic acid ethyl ester and 3-octanone, were previously identified as volatiles from the fungi Aspergillus candidus [39]. Different strains of lactic acid bacteria are able to synthesize ethyl ester from 2 to 10 carbon atoms, mainly ethyl hexanoate [42]. The hydrolysis products of G. candidum lipases may be the precursors of various volatile compounds such as alcohols, methyl ketones and esters [40].
Earthworm attraction to chemical cues associated with food has potential application for the development of techniques for the extraction and sampling of earthworms, for example in vermicomposting. Other behavioral techniques have previously been employed for such purposes, including heat extraction, electrical extraction, and mechanical vibration [18], [43], [44], and chemical extraction methods using natural repellents or irritants, like formalin, mustard extract, exotic-plant extracts have been reported [45], [46], [47], [48], [49]. Because they are based on attraction rather than repulsion, the esters presented above may have advantages over the existing chemical methods (e.g., efficacy when applied at low concentrations and on restricted spatial scales) but confirming this will require further study.
In conclusion, this study provides the first documentation of specific olfactory cues involved in annelid foraging. Microbiota are key producers of volatile compounds in soil ecosystems [24] as well as major components of earthworm diets [20]. Thus, further elucidation of the mechanisms by which earthworms perceive and respond to olfactory cues will enhance our understanding of the ecology of soil ecosystems, in which earthworms play a tremendously important role in temperate regions. Furthermore, exploration of earthworm olfaction will help us to understand how these animals orient themselves and coordinate their behavior. For example, it has previously been suggested that chemical cues are involved in earthworms [18], [50], and there is some evidence that L. terrestris follows mucus trails to find its partner [51], but the role of volatile perception in such interactions remains to be documented. Finally, as noted above, such work has potential implications for the development of techniques for the extraction and sampling of earthworms in vermicomposting and other applied settings.
Acknowledgments
We thank the members of the group of E. Haubruge for their continuous support; D. Conoir for his technical assistance; and Ouroboros s.a. society for providing E. fetida earthworms.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: LZ was financially supported by a PhD grant from the Fonds pour la formation à la Recherche dans l'Industrie et l'Agriculture (FRIA), Belgium. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bargmann CI, Horvitz HR. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell. 1993;74:515–527. doi: 10.1016/0092-8674(93)80053-h. [DOI] [PubMed] [Google Scholar]
- 2.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 3.Wyatt TD. Pheromones and Animal behaviour: Communication by smell and taste. Cambridge University Press; 2003. 391 [Google Scholar]
- 4.de Bruyne M, Baker TC. Odor detection in insects: Volatile codes. J Chem Ecol. 2008;34:882–897. doi: 10.1007/s10886-008-9485-4. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrand JG. Analysis of chemical signals by nervous systems. Proc Natl Acad Sci USA. 1995;92:67–74. doi: 10.1073/pnas.92.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dacks AM, Guerenstein PG, Reisenman CE, Martin JP, Lei H, et al. Olfaction in Invertebrates: Manduca. Encyclopedia of Neuroscience. 2009:49–57. [Google Scholar]
- 7.Hansson BS. Insect olfaction. Germany: Springer; 1999. 457 [Google Scholar]
- 8.Verheggen FJ, Haubruge E, Mescher M. Litwack G, editor. Alarm pheromones: Chemical signaling in response to danger. Pheromones. 2010. pp. 215–240. [DOI] [PubMed]
- 9.De Moraes CM, Mescher MC, Tumlinson JH. Caterpillar-induced nocturnal plant volatiles repel conspecific females. Nature. 2001;410:577–580. doi: 10.1038/35069058. [DOI] [PubMed] [Google Scholar]
- 10.Randlkofer B, Obermaier E, Hilker M, Meiners T. Vegetation complexity - The influence of plant species diversity and plant structures on plant chemical complexity and arthropods. Basic Appl Ecol. 2010;11:383–395. [Google Scholar]
- 11.Runyon JB, Mescher MC, De Moraes CM. Volatile chemical cues guide host location and selection by parasitic plants. Science. 2006;313:1964–1967. doi: 10.1126/science.1131371. [DOI] [PubMed] [Google Scholar]
- 12.Turlings TCJ, Tumlinson JH, Lewis WJ. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science. 1990;250:1251–1253. doi: 10.1126/science.250.4985.1251. [DOI] [PubMed] [Google Scholar]
- 13.Grassé P-P. Traité de Zoologie: anatomie, systématique, biologie. 1965. 731 Némathelminthes: Masson et compagnie.
- 14.Hong RL, Sommer RJ. Chemoattraction in Pristionchus nematodes and implications for insect recognition. Curr Biol. 2006;16:2359–2365. doi: 10.1016/j.cub.2006.10.031. [DOI] [PubMed] [Google Scholar]
- 15.Rasmann S, Kollner TG, Degenhardt J, Hiltpold I, Toepfer S, et al. Recruitement of entomopathogenic nematodes by insect-damaged maize roots. Nature. 2005;434:732–737. doi: 10.1038/nature03451. [DOI] [PubMed] [Google Scholar]
- 16.Yamazoe A, Kimura K. The nematode C. elegans responds to the gradient direction of a repulsive odor 2-nonanone. Neurosci Res. 2010;68:278. [Google Scholar]
- 17.Laverack MS. Tactile and chemical perception in earthworms. I. Responses to touch, sodium chloride, quinine and sugars. Comp Biochem Physiol. 1960;1:155–163. [Google Scholar]
- 18.Edwards CA, Bohlen PJ. Biology and Ecology of Earthworms. Chapman and Hall London; 1996. 426 [Google Scholar]
- 19.Zirbes L, Deneubourg JL, Brostaux Y, Haubruge E. A New Case of Consensual Decision: Collective Movement in Earthworms. Ethology. 2010;116:546–553. [Google Scholar]
- 20.Curry JP, Schmidt O. The feeding ecology of earthworms - A review. Pedobiologia. 2007;50:463–477. [Google Scholar]
- 21.Doube BM, Schmidt O, Killham K, Correll R. Influence of mineral soil on the palatability of organic matter for lumbricid earthworms: a simple food preference study. Soil Biol Biochem. 1997;29:569–575. [Google Scholar]
- 22.Neilson R, Boag B. Feeding preferences of some earthworm species common to upland pastures in Scotland. Pedobiologia. 2003;47:1–8. [Google Scholar]
- 23.Satchell JE. Lumbricidae. In: Burgess A, Raw F, editors. Soil Biology. Academic Press NY; 1967. pp. 259–322. [Google Scholar]
- 24.Chen F, Ro D, Petri J, Gershenzon J, Bohlmann J, et al. Characterization of a root-specific Arabidopsis terpene synthase responsible for the formation of the volatile monoterpene 1,8-cineole. Plant Physiol. 2004;135:1956–1966. doi: 10.1104/pp.104.044388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bonkowski M, Schaefer M. Interactions between earthworms and soil protozoa: a trophic component in soil food web. Soil Biol Biochem. 1997;29:499–502. [Google Scholar]
- 26.Doube BM, Brown GG. Edwards CA, editor. Life in a complex community: functional interaction between earthworms, organic matter, microorganisms and plants. 1998. pp. 179–212. Earthworm Ecology: CRC Press, Boca Raton, FL, USA.
- 27.Edwards CA, Fletcher KE. Interaction between earthworms and microorganisms in organic-matter breakdown. Agricul Ecosyst Environ. 1988;24:235–247. [Google Scholar]
- 28.Bonkowski M, Griffin BS, Ritz K. Food preferences of earthworms for soil fungi. Pedobiologia. 2000;44:666–676. [Google Scholar]
- 29.Parthasarathi K, Ranganathan LS, Anandi V, Zeyer J. Diversity of microflora in the gut and casts of tropical composting earthworms reared on different substrates. J Environ Biol. 2007;28:87–97. [PubMed] [Google Scholar]
- 30.Mendgen K, Wirsel SGR, Jux A, Hoffmann J, Boland W. Volatiles modulate the development of pathogenic rust fungi. Planta. 2006;224:1353–1361. doi: 10.1007/s00425-006-0320-2. [DOI] [PubMed] [Google Scholar]
- 31.Wenke K, Kai M, Piechulla B. Belowground volatiles facilities interactions between plant roots and soil organisms. Planta. 2010;231:499–506. doi: 10.1007/s00425-009-1076-2. [DOI] [PubMed] [Google Scholar]
- 32.Boff MIC, Zoon FC, Smits PH. Orientation of Heterorohabditis megidis to insect hosts and plant roots in a Y-tube sand olfactometer. Entomol Experimen Appl. 2001;98:329–337. [Google Scholar]
- 33.Bargmann CI. Comparative chemosensation from receptors to ecology. Nature. 2006;444:295–301. doi: 10.1038/nature05402. [DOI] [PubMed] [Google Scholar]
- 34.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 35.Burger BV, Petersen WGB, Tribe GD. Semiochemicals of the Scarabaeinae, IV: identification of an attractant for the dung beetle Pachylomerus femoralis in the abdominal secretion of the dung beetle Kheper lamarcki. Z Naturforschung, Biosci. 1995;50:675–680. [Google Scholar]
- 36.Robacker DC, Warfield WC, Flath RA. A four component attractant for the Mexican fruit fly, Anastrepha ludens (Diptera: Tephritidae), from host fruit. J Chem Ecol. 1992;18:1239–1254. doi: 10.1007/BF00980077. [DOI] [PubMed] [Google Scholar]
- 37.Cosse AA, Endris JJ, Millar JG, Baker TC. Identification of volatile compounds from fungus-infected date fruit that stimulate upwind flight in female Ectomyelois ceratoniae. Entomol Experimen Appli. 1994;72:233–238. [Google Scholar]
- 38.Boutrou R, Guéguen M. Interest in Geotrichum candidum for cheese technology. Int J Food Microbiol. 2005;102:1–20. doi: 10.1016/j.ijfoodmicro.2004.12.028. [DOI] [PubMed] [Google Scholar]
- 39.Fischer G, Schwalbe R, Moller M, Ostrowski R, Dott W. Species-specific production of microbial volatile organic compounds (MVOC) by airbone fungi from a compost facility. Chemosphere. 1999;39:795–810. doi: 10.1016/s0045-6535(99)00015-6. [DOI] [PubMed] [Google Scholar]
- 40.Jollivet N, Chataud J, Vayssier Y, Bensoussan M, Belin JM. Production of volatile compounds in model milk and cheese media by eight strains of Geotrichum candidum link. J Dairy Res. 1994;61:241–248. [Google Scholar]
- 41.Latrasse A, Dameron P, Hassani H, Staron T. Production d'un arôme fruité par Geotrichum candidum. Sci aliments. 1987;7:637–645. [Google Scholar]
- 42.Abeijon Mudski MC, Medina RB, Alvarez M, Gonzales SN. Ester synthesis by lactic bacteria isolated from goat's and ewe's milk and cheeses. Food Chem. 2009;117:241–247. [Google Scholar]
- 43.Coleman DC, Crossley DAJ, Hendrix PF. Fundamentals of soil ecology. Elsevier Academic Press, USA; 2004. 386 [Google Scholar]
- 44.Lee KE. Earthworms: Their ecology and relationships with soil and land use. London: Academic Press; 1985. 411 [Google Scholar]
- 45.Chan K-Y, Munro K. Evaluating mustard extracts for earthworm sampling. Pedobiologia. 2001;45:272–278. [Google Scholar]
- 46.Chaudhuri PS, Chaudhuri D, Nanda DK, Achari B, Bhattacharya D, et al. Chemical nature of earthworm repellent factor in the plant (Polygonum hydropiper Linn.) extract. Indian J Exp Biol. 1996;34:277–278. [Google Scholar]
- 47.Grønstøl GB, Solhøy T, Løyning MK. A comparison of mustard, household detergent and formalin as vermifuges for earthworm sampling. Fauna Norv. 2000;20:27–30. [Google Scholar]
- 48.Högger C. Mustard flour instead of formalin for the extraction of earthworms in the field. Bull Bodenk Ges Schweiz. 1993;17:5–8. [Google Scholar]
- 49.Muramoto J, Werner MR. Mustard powder vermifuge: an alternative to formalin expulsion method for earthworm sampling. Edaphologia. 2002;70:7–11. [Google Scholar]
- 50.Olive PJW, Clark RB. Physiology of reproduction. In: Mill PJ, editor. Physiology of annelids. London: Academic Press; 1978. pp. 271–368. [Google Scholar]
- 51.Nuutinen V, Butt KR. The mating behaviour of the earthworm Lumbricus terrestris (Oligochaeta: Lumbricidae). J Zool, London. 1997;242:783–798. [Google Scholar]