Abstract
Background
Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme of folate and methionine metabolism, making it crucial for DNA synthesis and methylation. The objective of this study was to analyze MTHFR gene 677C>T polymorphism in infertile male individuals from North India, followed by a meta-analysis on our data and published studies.
Methodology/Principal Findings
We undertook genotyping on a total of 837 individuals including well characterized infertile (N = 522) and confirmed fertile (N = 315) individuals. The SNP was typed by direct DNA sequencing. Chi square test was done for statistical analysis. Published studies were searched using appropriate keywords. Source of data collection for meta-analysis included ‘Pubmed’, ‘Ovid’ and ‘Google Scholar’. Those studies analyzing 677C>T polymorphism in male infertility and presenting all relevant data were included in meta-analysis. The genotype data for infertile subjects and fertile controls was extracted from each study. Chi square test was done to obtain odds ratio (OR) and p-value. Meta-analysis was performed using Comprehensive Meta-analysis software (Version 2). The frequency of mutant (T) allele (p = 0.0025) and genotypes (CT+TT) (p = 0.0187) was significantly higher in infertile individuals in comparison to fertile controls in our case-control study. The overall summary estimate (OR) for allele and genotype meta-analysis were 1.304 (p = 0.000), 1.310 (p = 0.000), respectively, establishing significant association of 677C>T polymorphism with male infertility.
Conclusions/Significance
677C>T substitution associated strongly with male infertility in Indian population. Allele and genotype meta-analysis also supported its strong correlation with male infertility, thus establishing it as a risk factor.
Introduction
Folic acid metabolism is important for stability and integrity of the genome due to its role in maintaining a balance between deoxyribonucleotides (dNTPs) for error free DNA synthesis, DNA methylation pattern and repair [1]. Therefore, deficiency in folate intake or polymorphism(s) in the enzymes of folate pathway may result in aberrant DNA synthesis and methylation. Methylenetetrahydrofolate reductase (MTHFR) is an important enzyme of folate and methionine metabolism, making it crucial for DNA synthesis and methylation. MTHFR reduces methylenetetrahydrofolate to methyltetrahydrofolate which then donates methyl group to homocysteine to form methionine. Methionine is ultimately converted to S-adenosyl methionine which acts as a ‘methyl’ donor for DNA methylation [2]. Also, methylenetetrahydrofolate participates in DNA synthesis by converting uracil to thymine [3]. MTHFR activity is higher in adult testis than other organs in mouse, indicating its critical role in spermatogenesis [2], [4]–[5]. Recent research has identified epigenetic regulation of several genes playing important role in spermatogenesis and fertility [6]. Therefore, aberrations in the MTHFR gene could compromise the process of spermatogenesis and predispose the carriers to infertility [7]–[8].
Four SNPs (203G>A, 677C>T, 1286A>C and 1793G>A) in the MTHFR gene have been described to affect activity of this enzyme [1], [9]. Of these, 677C>T resulting in the replacement of alanine with valine (A222V, rs1801133), has been studied most often [1]–[4], [9]–[20]. This substitution results in reduced MTHFR specific activity and increased thermolability [21]. Homozygous 677TT variant has ∼30% of residual activity and heterozygous 677CT variant has ∼70% of residual activity when compared to 677CC variant [2], [4], [11], [21]–[22]. This substitution also results in enhanced level of homocysteine and low plasma folate level [10], [20]–[21]. The frequency of 677C>T polymorphism varies with geographical location and the association status with infertility may vary due to ethnic differences [1]–[4], [ 9]–[19]. It has been suggested that low level of folate associated with MTHFR polymorphism could be the cause of infertility due to alteration in the synthesis of DNA and RNA molecules [4], [15]. Increase in sperm concentration upon folic acid and zinc sulfate supplementation further suggests the importance of this pathway in spermatogenesis [14]. This is also supported by induction of hypo-methylation by 5-aza deoxy cytidine, which inhibits the differentiation of spermatogonia into spermatocytes in murine model [23].
Most of the studies till date have analyzed this polymorphism in small sample size, giving way to over-estimation of association. We have, therefore, analyzed 677C>T polymorphism in a large sample size (N = 837) to elucidate the correlation between this polymorphic variant and male infertility in Indian population. We also performed a meta-analysis on all eligible published case-control studies, including our data, which established MTHFR 677C>T substitution to be a strong risk factor for male infertility.
Results
Case-control study
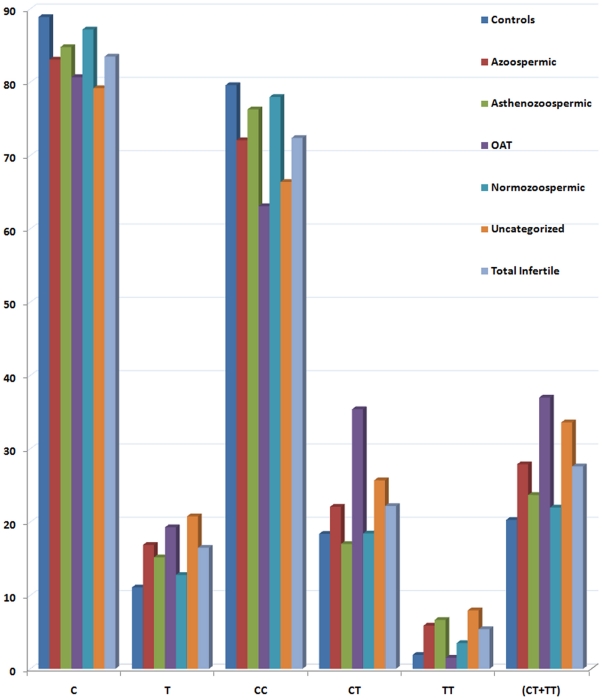
Genotyping by direct sequencing on the DNA samples of all infertile and fertile controls individuals was undertaken. Genotype data for control population fitted well in the Hardy Weinberg Equilibrium equation [p (Exact test) = 0.246620]. Distribution of mutant allele and genotypes was significantly different between infertile individuals and controls (Figure 1).
Figure 1. Allele and genotype distribution.
Bar diagram showing distribution of allele and genotypes in control and different groups of infertile individuals in our population.
Mutant allele (T) was more frequent in infertile men (16.5%) in comparison to fertile controls (11.1%) (Table 1) and the difference was statistically significant (p = 0.0025) with an odds ratio of 1.58 (CI, 1.17–2.13) (Table 1). The frequency of ‘T’ allele in each infertile sub-group was significantly higher in comparison to fertile controls (11.1%) and was almost double (19.3%) in oligoasthenoteratozoospermic (OAT) group, making the difference statistically significant (p = 0.01). Comparison of allele frequency between cases and controls suggested strong association of mutant allele with infertility and with OAT (Table 1).
Table 1. Statistical analysis of mutant allele/genotype in our case-control study.
| Group (N) | Parameter | C | T | CC | CT | TT | CT+TT |
| Controls (315) | N (%) | 560 (88.9) | 70 (11.1) | 251 (79.6) | 58 (18.4) | 6 (1.9) | 64 (20.31) |
| Azoo$ (68) | N (%) | 113 (83.1) | 23 (16.9) | 49 (72.1) | 15 (22.1) | 4 (5.9) | 19 (27.9) |
| OR/95% CI | 1.63 (0.98–2.72)# | 1.33 (0.70–2.53) | 3.42 (0.93–12.55) | 1.52 (0.84–2.76) | |||
| p-value/Chi-square | 0.06/.53 | 0.39/0.73 | 0.07/- | 0.17/1.91 | |||
| Astheno (135) | N (%) | 229 (84.8) | 41 (15.2) | 103 (76.3) | 23 (17.03) | 9 (6.67) | 32 (23.7) |
| OR/95% CI | 1.43 (0.95–2.17) | 0.97 (0.57–1.65) | 3.66 (1.27–10.53) | 1.22 (0.75–1.97) | |||
| p-value/Chi-square | 0.09/2.9 | 0.89/0.02 | 0.02*/- | 0.42/0.65 | |||
| Normo (141) | N (%) | 246 (87.2) | 36 (12.8) | 110 (78) | 26 (18.45) | 5 (3.5) | 31 (22) |
| OR/95% CI | 1.17 (0.76–1.80) | 1.02 (0.61–1.71) | 1.90 (0.57–6.36) | 1.11 (0.68–1.79) | |||
| p-value/Chi-square | 0.47/0.52 | 0.92/0.01 | 0.33/- | 0.69/0.16 | |||
| OAT (65) | N (%) | 105 (80.7) | 25 (19.3) | 41 (63.1) | 23 (35.4) | 1 (1.5) | 24 (37) |
| OR/95% CI | 1.90 (1.15–3.15) | 2.43 (1.35–4.36) | 1.02 (0.12–8.70) | 2.30 (1.29–4.07) | |||
| p-value/Chi-square | 0.01*/6.5 | 0.002*/9.19 | 1.00/- | 0.00385*/8.35 | |||
| Uncateg (113) | N (%) | 179 (79.2) | 47 (20.8) | 75 (66.4) | 29 (25.7) | 9 (7.96) | 38 (33.6) |
| OR/95% CI | 2.10 (1.40–3.15) | 1.67 (1.00–2.80) | 5.02 (1.73–14.56) | 1.99 (1.23–3.20) | |||
| p-value/Chi-square | 0.0002*/13.22 | 0.0485*/3.89 | 0.003*/- | 0.004*/8.12 | |||
| Total (522) | N (%) | 872 (83.5) | 172 (16.5) | 378 (72.4) | 116 (22.2) | 28 (5.4) | 144(27.6) |
| OR/95% CI | 1.578 (1.17–2.13) | 1.33 (0.93–1.89) | 3.10 (1.27–7.60) | 1.49 (1.07–2.09) | |||
| p-value/Chi-square | 0.0025*/9.14 | 0.12/2.49 | 0.0095*/6.73 | 0.0187*/5.56 |
The calculations for mutant allele have been done with reference to allele ‘C’; for mutant genotypes with reference to ‘CC’ between cases and controls.
Azoo-azoospermia; Astheno-asthenozoospermia; Normo-normozoospermia; OAT-oligoasthenoteratozoospermia; Uncateg: uncategorized.
*Indicates statistically significant value.
Similarly, the frequency of homozygous mutant genotype (TT) in infertile individuals was almost three times (5.4%) to that of controls (1.9%), and the difference was statistically significant (p = 0.009 at 99% level of confidence). The frequency of heterozygous genotype was also higher in infertile group (22.2%) in comparison to control samples (18.4%); however, the difference was not statistically significant (Table 1). In sub-group analysis, homozygous mutant genotype showed strong association with asthenozoospermia (p = 0.02) and uncategorized infertile (p = 0.003) groups. Overall, the presence of all mutant genotypes (CT+TT) was higher in infertile individuals in comparison to controls, and the difference was statistically significant (p = 0.0187) (Table 1).
Meta analysis
Literature assessment
Twenty nine studies were retrieved by our literature search strategy. Out of these, only 15 had analyzed 677C>T substitution in correlation with male infertility. Two studies were excluded from the analysis as one did not provide detailed information required for meta-analysis [19] and the other was not directly relating the genotypes with infertility [18]. Hence, only 13 studies qualifying our strict selection criteria were included in the analysis. Along with the present study from India, this meta-analysis included data on 3094 cases and 2877 controls. Allele and genotypes data for all these studies were tabulated (Table 2).
Table 2. Data extracted from published studies included in the meta-analysis.
| Study | Population | Group | Cases | Controls | ||||||||||
| Total | CC | CT | TT | C | T | Total | CC | CT | TT | C | T | |||
| Bezold et al, 2001 | No Info | Total | 255 | 114 | 93 | 48 | 321 | 189 | 200 | 92 | 89 | 19 | 273 | 127 |
| Stuppia et al, 2003 | Italian | Total | 93 | 37 | 37 | 19 | 111 | 75 | 105 | 33 | 43 | 29 | 109 | 101 |
| Ebisch et al, 2003 | Dutch | Total | 77 | 42 | 28 | 7 | 112 | 42 | 113 | 50 | 48 | 15 | 148 | 78 |
| Singh et al, 2005 | Indian | Total | 151 | 105 | 40 | 6 | 250 | 52 | 200 | 163 | 37 | 0 | 363 | 37 |
| Park et al, 2005 | Korean | Total | 373 | 105 | 205 | 63 | 415 | 331 | 396 | 145 | 200 | 51 | 490 | 302 |
| Paracchini et al, 2006 | Italian | Total | 59 | 11 | 32 | 16 | 54 | 64 | 46 | 18 | 21 | 7 | 57 | 35 |
| Lee et al, 2006 | Korean | Azoo$ | 174 | 44 | 100 | 30 | 188 | 160 | 325 | 118 | 166 | 41 | 402 | 248 |
| OAT | 186 | 71 | 81 | 34 | 223 | 149 | ||||||||
| Total | 360 | 115 | 181 | 64 | 411 | 309 | ||||||||
| A et al, 2007 | Chinese | Azoo | 228 | 83 | 97 | 48 | 263 | 193 | 252 | 128 | 95 | 29 | 351 | 153 |
| Oligo | 127 | 47 | 63 | 17 | 157 | 97 | ||||||||
| Total | 355 | 130 | 160 | 65 | 420 | 290 | ||||||||
| Dhillon et al, 2007 | Indian | OAT | 179 | 81 | 77 | 21 | 259 | 119 | 200 | 70 | 100 | 30 | 240 | 160 |
| Tetik A et al, 2008 | Turkish | Azoo | 50 | 23 | 22 | 5 | 68 | 32 | 50 | 30 | 20 | 0 | 80 | 20 |
| Oligo | 50 | 21 | 22 | 7 | 64 | 36 | ||||||||
| Total | 100 | 44 | 44 | 12 | 132 | 68 | ||||||||
| Ravel et al, 2009 | French | Azoo | 70 | 33 | 31 | 6 | 97 | 43 | 114 | 49 | 52 | 13 | 150 | 78 |
| Oligo | 180 | 85 | 70 | 25 | 240 | 120 | ||||||||
| Total | 250 | 118 | 101 | 31 | 337 | 163 | ||||||||
| Safarinejad et al, 2011 | Asian | OAT | 164 | 58 | 80 | 26 | 196 | 132 | 328 | 144 | 148 | 36 | 436 | 220 |
| Gava et al, 2011 | Brazilian | Azoo | 49 | 27 | 15 | 7 | 69 | 29 | 233 | 167 | 53 | 13 | 387 | 79 |
| Oligo | 107 | 54 | 45 | 8 | 153 | 61 | ||||||||
| Total | 156 | 81 | 60 | 15 | 222 | 90 | ||||||||
Azoo-azoospermia; OAT-oligoasthenoteratozoospermia; oligo-oligozoospermia.
Heterogeneity test and Sensitivity analysis
A true heterogeneity existed between studies for allele (Pheterogeneity = 0.00, Q = 39.66, df(Q) = 13, I2 = 67.221, var = 0.001, τ2 = 0.050, SE = 0.031,τ = 0.224) and genotype (Pheterogeneity = 0.00, Q = 44.44, df(Q) = 13, I2 = 70.75, var = 0.004, τ2 = 0.109, SE = 0.064, τ = 0.330) comparisons. The ‘I2’ value of more than 50% for between studies comparison in both allele and genotype analysis shows high level of true heterogeneity.
In allele meta-analysis, sensitivity analysis performed by exclusion of the studies involving small sample size [13]–[15], decreased heterogeneity (Pheterogeneity = 0.016, I2 = 54.07, Q = 21.77, df(Q) = 10, var = 0.000, τ2 = 0.025, SE = 0.021, τ = 0.158) to a small extent but exclusion of studies with very high p values [1], [4], [13]–[14] decreased heterogeneity to a large extent (Pheterogeneity = 0.136, I2 = 33.98, Q = 13.63, df(Q) = 9, var = 0.000, τ2 = 0.012, SE = 0.016, τ = 0.108). Here, τ2 defines between studies variance and is used to asses heterogeneity; however, this is not commonly used for this purpose as it depends on the particular effect metric used in the analysis. Var denotes variance and SE denotes standard error for heterogeneity test. Similarly, in genotype analysis exclusion of the studies with small sample size [13]–[15] decreased heterogeneity to a small extent (Pheterogeneity = 0.001, I2 = 68.07, Q = 31.32, df(Q) = 10, var = 0.003, τ2 = 0.082, SE = 0.056, τ = 0.287). As a result of this exclusion, there was no significant difference in the results of fixed effect (OR = 1.361, p = 0.000) and random effect (OR = 1.363, p = 0.004) models. After exclusion of studies with high p values [1], [3], [10], [13]–[14], heterogeneity decreased to a lesser extent (Pheterogeneity = 0.002, I2 = 67.13, Q = 24.343, df(Q) = 8, var = 0.005, τ2 = 0.091, SE = 0.071, τ = 0.301) and the results in random effect model (OR = 1.571, p = 0.000) were almost identical to that of fixed effect model (OR = 1.528, p = 0.000). Sensitivity analysis thus showed that differences in the outcome between the two models (fixed and random effect) could be attributed to a few studies with very small sample size or very high p values. Under the conditions of heterogeneity, random-effects model is more appropriate. However, since there was no change in the inference adopting either model, we present results of both the models. There is no difference in the overall inference except in genotype sub-group analysis for oligozoospermic cases, where we use fixed effect model for drawing inference since random effect model gives more weight to studies with smaller sample size.
Meta-analysis using allele frequency
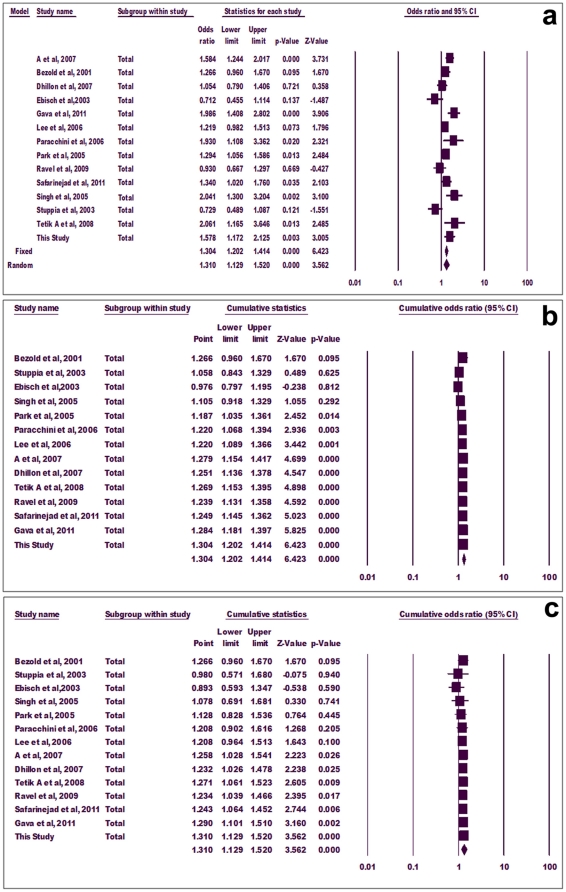
Mutant allele showed significant association with infertility in both fixed effect (p = 0.000, OR = 1.304, 95% CI = 1.202-1.414) and random effect (p = 0.000, OR = 1.310, 95% CI = 1.129-1.520) models (Figure 2a). In cumulative analysis using fixed and random effect models, the association of mutant ‘T’ allele with infertility turned statistically significant with the addition of study of Park et al (2005) and A et al (2007), respectively, and stayed significant thereafter (Figure 2b and c).
Figure 2. Forest plot.
Meta-analysis using allele frequency (a), cumulative allele meta-analysis using fixed effect (b) and random effect models (c).
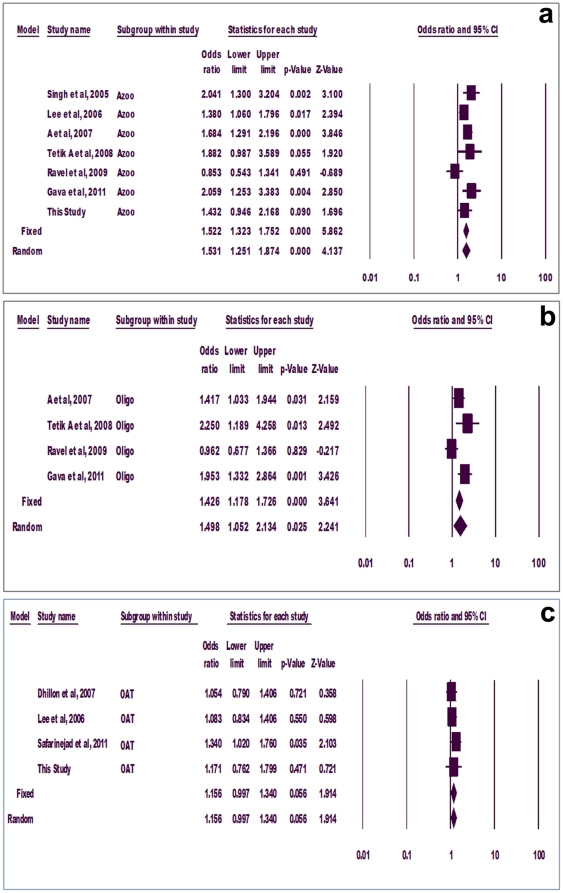
Sub-group analyses were done for azoospermia, oligozoospermia and OAT groups. Few of the previous studies did not categorize infertile subjects into sub-groups; therefore, such studies had to be excluded from sub-group analysis. Significant association of mutant allele with azoospermia was observed using both fixed (p = 0.000, OR = 1.522, 95% CI = 1.32-1.175) and random (p = 0.000, OR = 1.531, 95%CI = 1.25-1.87) effect models (Figure 3a). Similarly, mutant allele showed positive correlation with oligozoospermia adopting both fixed (p = 0.000, OR = 1.426, 95% CI = 1.178-1.726) and random (p = 0.025, OR = 1.498, 95% CI = 1.052-2.134) effect models (Figure 3b). However, mutant allele did not show association with OAT using either model (p = 0.056) (Figure 3c).
Figure 3. Forest plot.
Group-wise allele meta-analysis for azoospermia (a), oligozoospermia (b) and oligoasthenoteratozoospermia (c).
Meta-analysis using genotype frequency
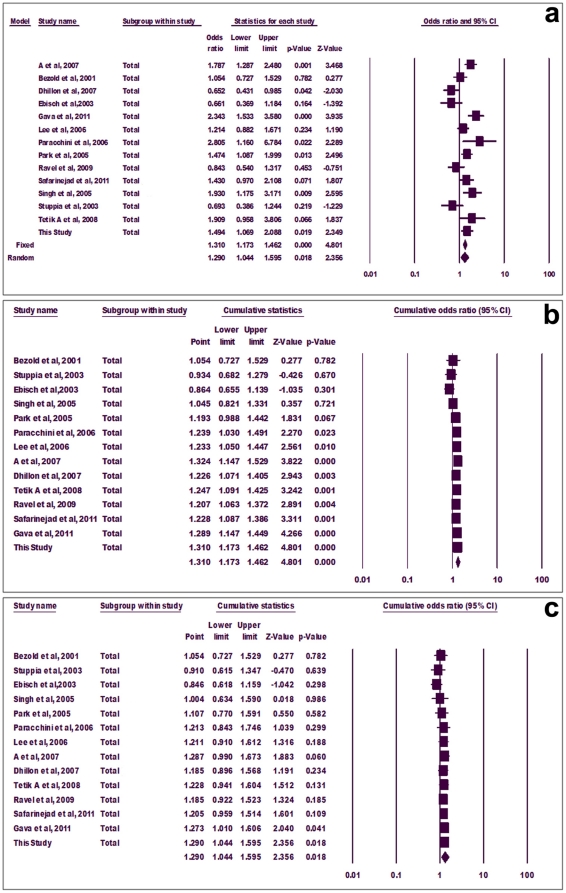
Similar to allele meta-analysis, pooled odds ratio for mutant genotypes (CT+TT) showed statistically significant association with infertility adopting both fixed (p = 0.000, OR = 1.310, 95% CI = 1.173-1.462) and random (p = 0.018, OR = 1.290, 95% CI = 1.044-1.595) effect models (Figure 4a). The fixed effect model cumulative analysis showed that addition of the study of Paracchini et al (2006) turned the overall association significant (p = 0.023) (Figure 4b). With introduction of another two studies, p value became highly significant (p = 0.00, significant at 99% level of confidence) and stayed significant thereafter (Figure 4b). However, with random effect model, the overall association turned significant only after inclusion of the study of Gava et al, 2011 (p = 0.041) and further introduction of our study supported the inference (p = 0.018) (Figure 4c).
Figure 4. Forest plot.
Meta-analysis using genotype frequency (a), cumulative genotype meta-analysis using fixed effect (b) and random effect models (c).
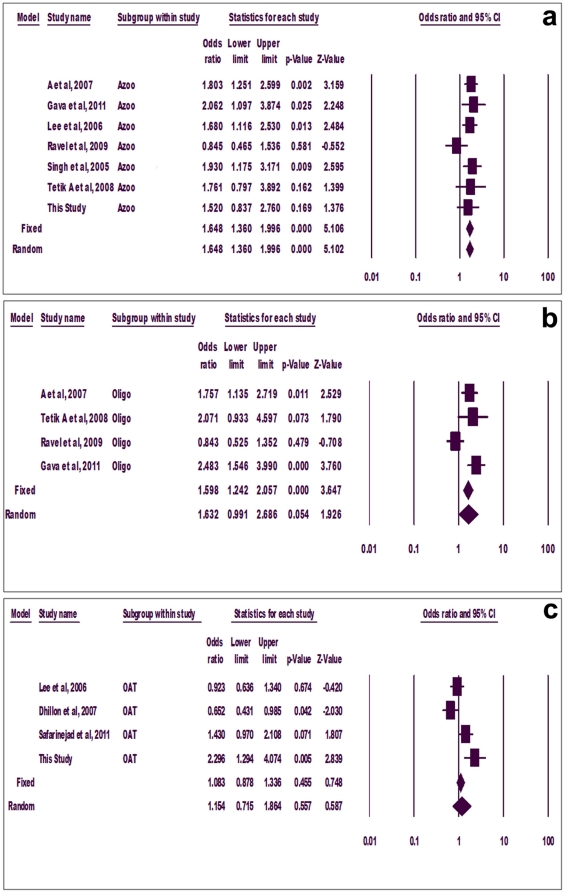
Sub-group analysis showed significant association of mutant genotypes with azoospermia using both fixed and random effect models (p = 0.000, OR = 1.648, 95% CI = 1.360-1.996) (Figure 5a). Mutant genotypes also associated with oligozoospermia using fixed (p = 0.000) but not random (p = 0.054) effect models (Figure 5b). However, mutant genotypes did not associate with OAT in any case (fixed model, p = 0.455, random model, p = 0.557) (Figure 5c).
Figure 5. Forest plot.
Group-wise genotype meta-analysis for azoospermia (a), oligozoospermia (b) and oligoasthenoteratozoospermia (c).
Publication bias
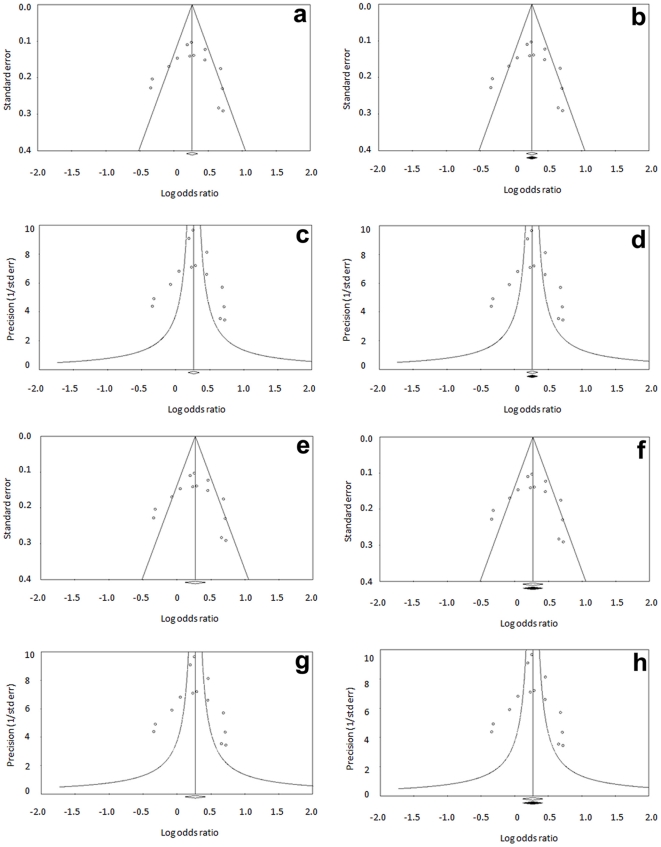
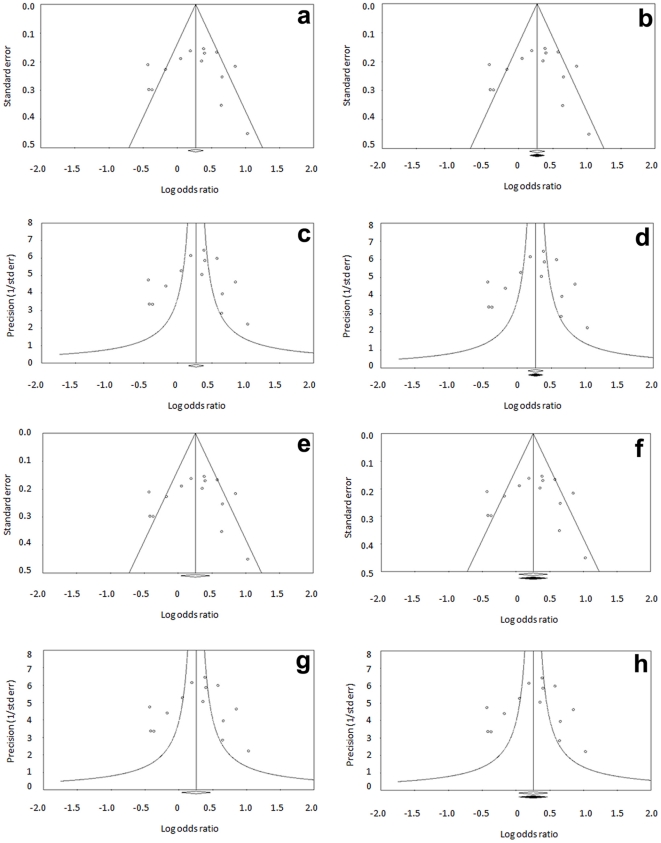
We generated funnel plots using standard error and precision values for allele (Figure 6a–h) and genotypes (Figure 7a–h) using both fixed and random effect models. Apart from observed sets of studies, the plots were also drawn after imputation. Symmetrical distribution of studies in the funnel plots suggests absence of publication bias. This is also supported by other tests described below.
Figure 6. Publication bias (Allele meta-analysis).
Funnel plot of standard error by log odds ratio using fixed model for observed set of studies (a) and after imputation (b). Funnel plot of precision by log odds ratio using fixed model for observed set of studies (c) and after imputation (d). Funnel plot of standard error by log odds ratio using random effect model for observed set of studies (e) and after imputation (f). Funnel plot of precision by log odds ratio using random effect model for observed set of studies (g) and after imputation (h).
Figure 7. Publication bias (Genotype meta-analysis).
Funnel plot of standard error by log odds ratio using fixed model for observed set of studies (a) and after imputation (b). Funnel plot of precision by log odds ratio using fixed model for observed set of studies (c) and after imputation (d). Funnel plot of standard error by log odds ratio using random effect model for observed set of studies (e) and after imputation (f). Funnel plot of precision by log odds ratio using random effect model for observed set of studies (g) and after imputation (h).
In allele analysis, classic fail-safe ‘N’ value of 130 (p = 0.000, Z = 6.28) suggests that 130 null studies would have to be included to nullify the effect or convert the combined ‘p’ to a non-significant (>0.050) value. Orwin's fail-safe ‘N’ to bring observed odds ratio of 1.30 to 1.1 is 25, indicating that at least 25 null studies would be required to bring the effect size to null. Begg and Mazumdar Rank correlation test also showed no evidence of publication bias. Egger's intercept (B0) was 0.422 with t value of 0.253 (one tailed p value = 0.40, two tailed p value = 0.81), further suggesting no publication bias. No change in overall summary estimate (odds ratio) after ‘trim and fill’ procedure suggests absence of publication bias (Figure 6 b,d,f,h).
Similarly, in genotype analysis, no evidence of publication bias was seen. Classic fail safe ‘N’ was 60 (p = 0.00001, z = 4.51), indicating that additional 60 null studies would be required to bring the p value in non-significant range. In the same way, Orwin's fail safe ‘N’ to bring observed odds ratio of 1.31 to 1.1 was 26, indicating that additional 26 null studies would be required to bring effect size to null. Similar to the allele analysis, Begg and Mazumdar Rank correlation test showed no evidence of publication bias. Egger's intercept (B0) was -0.49 with t value of 0.25 (one tailed p = 0.40, two tailed p = 0.80), suggesting no publication bias. No changes in the pooled odds ratio after ‘trim and fill’ procedure (Figure 7 b,d,f,h), confirmed absence of publication bias. Symmetry of the funnel plots and all statistical tests described above showed no trace of publication bias. Therefore, we are confident that there is no over-estimate of the infertility risk associated with this SNP.
Discussion
Mutant allele and genotypes at 677C>T were strongly associated with male infertility in our population. This was also supported by meta-analysis on previously published studies including our results. The findings of our study become particularly interesting since two previous studies on Indian populations had reported contrasting observations to each other [4], [11]. Singh et al (2005) showed significant association of mutant genotypes with infertility while Dhillon et al (2007) showed no statistically significant difference of 677C>T variants between infertile and fertile males. Dhillon et al, tried to explain the variation in results on the basis that their study included majority of OAT patients in comparison to the majority of azoospermic cases in the study by Singh et al. In the present study, we included both azoospermic and OAT individuals, and had contrasting observations. Our analysis has shown significant association of mutant allele and genotypes with infertility and with OAT in sub-group analysis. Presence of mutant genotypes did not associate with azoospermia in our samples. To the best of our knowledge, the three Indian populations studied so far are not ethnically different. The differences in the results could therefore be attributed to other factors such as variations in recruitment of subjects.
677C>T substitution has been studied in different populations for its possible association with male infertility [1]–[4], [ 9]–[19]. Bezold et al (2001) showed for the first time association of homozygous mutant genotype with male infertility. Several studies later on showed significant association of homozygous mutant genotype with infertility in different populations [2]–[3], [ 11]–[12], [ 15]–[17]. Two studies conducted on Korean population [2], [12] showed significant increase of homozygous mutant genotype in infertile males than fertile controls. Park et al showed that OAT and non obstructive azoospermic patients in unexplained infertile group had higher frequency of homozygous mutant genotype in comparison to explained infertile males. Similarly, a study on Turkish population also inferred homozygous mutant genotype to be a risk factor for infertility characterized by oligozoospermia and non-obstructive azoospermia [16]. Other studies provided evidence for association of this polymorphism with male infertility in the Chinese [2] and Brazilian [17] populations.
In contrast to the above, a study on Dutch population revealed that mutant genotype was not a risk factor for male infertility [14]. Two studies on Italian populations reported contrasting observations [13], [15], one showing no significant association of heterozygous or homozygous mutant genotypes with male sub-fertility [13] and the second reporting increased risk of infertility in individuals with homozygous mutant genotype in comparison to heterozygous and wild type genotypes [15]. Similarly, in a French population, no statistically significant correlation of mutant genotype with male sub-fertility was observed [1]. We found true heterogeneity between studies in both allele and genotype meta-analysis, which is also evident from slight variations in the results of fixed and random effect models. The presence of heterogeneity would force us to use random effect model for further analysis. However, after doing sensitivity analysis we chose to use fixed effect model on the basis that there were few small studies affecting the results of meta-analysis. Since there was no significant difference in the results using either model, we present results of both; however, the inference is based on fixed effect model.
Meta-analysis showed significant association of mutant allele and mutant genotypes (CT+TT) with infertility. Introduction of the studies of Park et al, 2005 and Paracchini et al, 2006 in the allele and genotype cumulative analysis, respectively, provided enough data to conclude that the mutant allele and genotypes are risk factors for infertility. Addition of more studies later on strengthened the conclusion and now this substitution has been established as a risk factor. The strength of our conclusion is also indicated by a very narrow confidence interval for odds ratio. Subgroup analysis has shown significant association of mutant allele and genotypes (CT+TT) with infertility characterized by azoospermia and oligozoospermia but not OAT. Analysis according to geographical distribution of populations could not be undertaken due to relatively lesser number of studies. An earlier meta-analysis on this SNP included eight studies and reported a highly significant association between mutant genotypes and infertility (p = 0.002; OR- 1.23; 95% CI- 1.08-1.41) [24]. A recent meta-analysis on this polymorphism included 10 studies and concluded no overall association between this polymorphism and male infertility [25]. Several observations in the latter study, such as association of this mutation with azoospermia and no association with OAT, are similar to us. However, overall inference in Wu et al., [25] differs from ours as well as from Tuttlemann et al. [24]. Cumulative analysis in our study clearly states less likelihood of finding no overall association as reported by Wu et al. However, inclusion in our meta-analysis of few recent studies and data from our case-control study could be responsible for differences in the overall inference.
It is apparent from our analysis that 677C>T substitution associates strongly with infertility in Indian population and meta-analysis establishes it as a risk factor for male infertility. High fail safe ‘N’ and symmetrical distribution of studies in funnel plots ascertains absence of publication bias, further strengthening our conclusion. The penetration of this SNP could be affected by dietary folate intake and geographical factors such that overall phenotype is an outcome of interaction between these factors. Low dietary intake of folic acid could cause several health problems including but not limited to neural tube defects in developing embryos [26], homocysteine accumulation [27], and impaired DNA synthesis and repair [27]. Low folate level in the Indian and African populations in comparison to the Western and European populations, makes them more susceptible to infertility [11] and other health problems listed above. Inadequate folic acid intake [28] and prevalence of vegetarian diet in India, in coupling with more than 10% overall frequency of this polymorphism, could justify folic acid supplementation for both men and women.
Though exact mechanism by which 677C>T substitution affects fertility is not yet clear, some possible mechanisms have been put forward. Induction of hypo-methylation by 5-aza deoxy cytidine inhibited the differentiation of spermatogonia into spermatocytes in murine model [29], which could explain the association between this SNP and infertility. Since several genes participating in spermatogenesis are regulated by DNA methylation [8], individuals with 677T allele are associated with decreased global genomic methylation [14]. Further, low levels of folate may lead to hyperhomocysteinemia, resulting in oxidative stress. Oxidative stress is well known to cause damage to sperm plasma membrane, and mitochondrial and nuclear DNA [4]. Hyperhomocysteinemia leads to precocious atherosclerosis which results in lower blood flow in testicular arteries, resulting in alteration in spermatogenesis [2]. All the above taken into account partially explains the association between this polymorphism and male infertility. In nutshell, it appears, we have now enough data to conclude that 677 C>T is a risk factor for infertility in general and azoospermia in particular.
Materials and Methods
Case-control study
Sample Collection
The study was approved by the Institutional Human Ethics Committee of the Ajanta Hospitals and IVF Centre, Lucknow. Before enrolment in the study, each subject's informed written consent was obtained in response to a fully written and verbal explanation of the nature of study. In the case-control study, a total of 837 individuals from north India including 522 infertile and 315 fertile controls of Indo-Aryan ethnicity were recruited. The patients and controls were recruited at the Ajanta Hospitals and IVF Centre Pvt. Ltd. 765, ABC complex, Alambagh, Lucknow. The inclusion criteria of the cases included infertility persisting longer than one year and absence of any obvious fertility problem in the partner. Clinical observations on the female partners including menstruation and ovulation ruled out any problem on female side. Patients having infection of accessory glands, diabetes, hypertension, arthritis, tuberculosis, human immunodeficiency virus, and those on drugs known to influence fertility were excluded. The patients were further categorized in sub-groups as per WHO 1999 criteria [30]. Normozoospermic infertile men (N = 141) had normal semen profile (defined as in the control group except fertility) and infertility of unknown etiology. Asthenozoospermic infertile men (N = 135) had a sperm count >20×106/mL, motility <50%, and > = 30% normal morphology; OAT (N = 65) had a sperm count <20×106/mL, motility <50% and <30% normal morphology; non-obstructive azoospermic infertile men (N = 68) with no sperm in the ejaculate and 113 infertile patients who were not categorized due to lack of one or the other semen parameter. The controls were recruited following the criteria of normal semen profile (WHO 1999) with confirmed paternity.
Genomic DNA Isolation and Sequencing
Genomic DNA was extracted from the peripheral blood of patient and control samples using phenol-chloroform-isoamyl method [31]. The point mutation (677C>T) in the MTHFR gene was typed using direct DNA sequencing technique. Briefly, primers around the polymorphic site were designed with the help of GENETOOL software. PCR was carried out in a total reaction volume of 10 µl each in thin walled tubes consisting of 1.0 µl of PCR buffer (10X), 1.0 µl of MgCl2 (25 mM), 1.0 µl of dNTPs (10 mM), 2.0 pM of each of the forward (5′ CATCCCTATTGGCAGGTTACCC 3′) and reverse (5′ GGGAAGAACTCAGCGAACTCAG 3′) primers, 1.0 unit of Taq DNA polymerase enzyme (Applied Biosystems) and 40 ng of genomic DNA. PCR cycling was carried out in ABI Veriti thermal cycler (Applied Biosystems, USA). PCR amplification conditions included denaturation at 95 °C for 10 minutes followed by 35 cycles of denaturation at 95 °C for 30 seconds, annealing at 63°C for 30 seconds and polymerization at 72°C for 40 sec, and a final stage of polymerization at 72°C for 7 minutes. The amplified products were directly sequenced using BigDye™ chain termination chemistry on ABI 3730 DNA analyzer (Applied Biosystems, USA) [32]. Multiple alignment and sequence analysis was done using Auto Assembler Software (Applied Biosystems, USA).
Statistical Analysis
Genotype data for control population was analyzed for fitness in the Hardy Weinberg Equilibrium. For this purpose, data was analyzed using calculator available at http://ihg.gsf.de/cgi-bin/hw/hwa1.pl. Chi square analysis was used to compare the allele and genotype data between cases and controls. In addition to the comparison of all the patients with the controls, each sub-group of infertile individuals was compared with controls. Data was analyzed using the Vassar Stats Online Calculator (http://faculty.vassar.edu/lowry/VassarStats.html). P-value of less than 0.05 was considered to be statistically significant.
Meta-analysis
677C>T has been explored in several studies in different ethnic groups, making it important to conduct meta-analysis. We had used Comprehensive Meta-analysis Version 2 software for this purpose.
Identification of studies
A systematic search was done on published literature using the keywords ‘MTHFR and male infertility’, ‘folate metabolism and male infertility’, ‘MTHFR 677C>T polymorphism and male infertility’ through ‘Pubmed’ ‘Ovid’ and ‘Google Scholar’ up to march 2011. Detailed information for each study on 677C>T polymorphism in male infertility such as the purpose and design of the study, presentation of the data, genotyping method used, inclusion and exclusion criteria of the cases and controls was collected. Detailed information, wherever not available, was collected by contacting authors.
Inclusion and exclusion criteria
The following inclusion criteria were set for the meta-analysis: (i) each trial is an independent case-control study; (ii) the purpose of all the studies and statistical methods is similar; (iii) it supplied enough information to calculate the odds ratio; (iv) SNP typing was done at high resolution level and (v) inclusion of the patients was done according to the standard diagnosis parameter. The exclusion criteria included: i) study not providing enough information (incomplete raw data) and ii) not well-described.
Data Extraction and Statistical Approach
Genotype data for MTHFR 677C>T polymorphism related to male infertility was gathered. Information regarding the first author, year of publication, ethnicity of study population, number of cases and controls and allele and genotype frequency was collected.
Statistical analysis
Chi-square analysis was performed and the odds ratio with 95% confidence interval was calculated using Vassar Stats online statistical calculators (http://faculty.vassar.edu/lowry/VassarStats.html) for all the possible genotypes. Association analysis was undertaken to compare the frequency of mutant allele and mutant genotypes between cases and controls.
For meta-analysis, major consideration is the type of ‘effect size’ chosen for statistical analysis. In the present study, computed effect size in the form of ‘odds ratio’ and ‘confidence interval’ was chosen. A Chi square based ‘Q’ test defined by Cochran was used to assess the heterogeneity (between study variability) in the meta-analysis. A significance level of P<0.10 instead of traditional P<0.05 was used because of its low power and to avoid type II errors for statistical test of heterogeneity [33]. Since the ‘Q’ statistics is only useful for testing the existence of heterogeneity qualitatively but not quantitatively, another index ‘I2’, calculated as the percentage of the total variability in a set of effect sizes due to true heterogeneity, was used to quantify the degree of heterogeneity. A tentative classification of ‘I2’ values proposed by Higgins and Thompson has been used to interpret the magnitude; viz. 25%, 50% and 75% which corresponds to low, medium and high heterogeneity, respectively [34]. In the absence of significant heterogeneity determined by the results of Q test, the Mantel-Haenszel fixed effect model (Peto method) was used for the combination of data, while in the presence of significant heterogeneity, the Dersimonian Laird random effect model (DL method) was used for combining the data [34]-[36]. Sensitivity analysis was also done to validate the assumptions and decisions made, and for the robustness of the method used in the analysis.
A comparison of results based on fixed and random effects models before and after exclusion of outlier studies or studies involving small sample size was used as a method for sensitivity analysis [33]. High resolution plots (forest plots) were generated to estimate the pooled odds ratio corresponding to 95% confidence interval and the p value. Both fixed effect and random effect models were used to analyze the data. Cumulative meta-analysis was also done to observe the effect of subsequent addition of each study. Subgroup analysis according to infertility phenotype (azoospermia, oligozoospermia and OAT) was also carried out to estimate the specific odds ratio for a particular sub-group.
Publication bias was investigated by using the funnel plots; viz. funnel plot of standard error by log odds ratio and funnel plot of precision by log odds ratio. Different statistical tests such as Begg and Mazumdar rank correlation, Egger's regression intercept, Duvall and Tweedie's trim and fill procedure and Fail-safe ‘N’ were adopted to assess and quantify the publication bias and its impact on the analysis. The classic fail-Safe N and the Orwin fail-safe ‘N’ assess if the entire observed effect is an artifact of bias. Rank correlation and regression procedures are used for testing the presence of bias. Duvall and Tweedie's trim and fill procedure tests how the effect size will shift, if the apparent bias were to be removed.
Footnotes
Competing Interests: SG, AD, GK and AK are employed by Ajanta Hospitals and IVF Centre Pvt. Ltd. The authors' employment does not alter their adherence to all the PLoS ONE policies on sharing data and materials.
Funding: The study was financially supported by the Ministry of Health and Family Welfare, Government of India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Ravel C, Chantot-Bastaraud S, Chalmey C, Barreiro L, Aknin-Seifer I, et al. Lack of association between genetic polymorphisms in enzymes associated with folate metabolism and unexplained reduced sperm counts. Plos One. 2009;4:e6540. doi: 10.1371/journal.pone.0006540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.A ZC, Yang Y, Zhang SZ, Li N, Zhang W. Single nucleotide polymorphism 677C>T in the methylenetetrahydrofolate reductase gene might be a genetic risk factor for infertility for Chinese men with azoospermia or severe oligozoospermia. Asian J Androl. 2007;9:57–62. doi: 10.1111/j.1745-7262.2007.00225.x. [DOI] [PubMed] [Google Scholar]
- 3.Lee HC, Jeong YM, Lee SH, Cha KY, Song SH, et al. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Hum Reprod. 2006;21:3162–3170. doi: 10.1093/humrep/del280. [DOI] [PubMed] [Google Scholar]
- 4.Dhillon VS, Shahid M, Husain SA. Associations of MTHFR DNMT3b 4977bp deletion in mtDNA and GSTM1 deletion, and aberrant CpG island hypermethylation of GSTM1 in non-obstructive Infertility in Indian men. Mol Hum Reprod. 2007;13:213–222. doi: 10.1093/molehr/gal118. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Karaplis AC, Ackerman SL, Pogribny IP, Melnyk S, et al. Mice deficient in methylenetethrahydrofolate reductase exhibit hyperhomocysteinemia and decreased methylation capacity, with neuropathology and aortic lipid deposition. Hum Mol Genet. 2001;10:433–443. doi: 10.1093/hmg/10.5.433. [DOI] [PubMed] [Google Scholar]
- 6.Minocherhomji S, Madon PF, Parikh FR. Obstet Gynecol Int pii.; 2010. Epigenetic regulatory mechanisms associated with infertility.198709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Khazamipour N, Noruzinia M, Fatehmanesh P, Keyhanee M, Pujol P. MTHFR promoter hypermethylation in testicular biopsies of patients with non-obstructive azoospermia: the role of epigenetics in male infertility. Hum Reprod. 2009;24:2361–2364. doi: 10.1093/humrep/dep194. [DOI] [PubMed] [Google Scholar]
- 8.Singh K, Singh SK, Raman R. MTHFR A1298C polymorphism and idiopathic male infertility. J Postgrad Med. 2010;56:267–269. doi: 10.4103/0022-3859.70935. [DOI] [PubMed] [Google Scholar]
- 9.Safarinejad MR, Shafiei N, Safarinejad S. Relationship Between Genetic Polymorphisms of Methylenetetrahydrofolate Reductase (C677T, A1298C, and G1793A) as Risk Factors for Idiopathic Male Infertility. Reprod Sci. 2011;18:304–315. doi: 10.1177/1933719110385135. [DOI] [PubMed] [Google Scholar]
- 10.Bezold G, Lange M, Peter RU. Homozygous methylenetetrahydrofolate reductase 677C>T mutation and male infertility. N Engl J Med. 2001;344:1172–1173. doi: 10.1056/NEJM200104123441517. [DOI] [PubMed] [Google Scholar]
- 11.Singh K, Singh SK, Sah R, Singh I, Raman R. Mutation 677C>T in the methylenetetrahydrofolate reductase gene is associated with male infertility in Indian population. Int J Androl. 2005;28:115–119. doi: 10.1111/j.1365-2605.2004.00513.x. [DOI] [PubMed] [Google Scholar]
- 12.Park JH, Lee HC, Jeong YM, Chung TG, Kim HJ, et al. MTHFR 677C>T polymorphism associates with unexplained infertile male factors. J Assist Reprod Genet. 2005;22:361–368. doi: 10.1007/s10815-005-6795-0. [DOI] [PubMed] [Google Scholar]
- 13.Stuppia L, Gatta V, Scarciolla O, Colosimo A, Guanciali-Franchi P, et al. The methylenetethrahydrofolate reductase (MTHFR) 677C>T polymorphism and male infertility in Italy. J Endocrinol Invest. 2003;26:620–622. doi: 10.1007/BF03347018. [DOI] [PubMed] [Google Scholar]
- 14.Ebisch IMW, van Heerde WL, Thomas CM, van der Put N, Wong WY, et al. 677C>T methylenetetrahydrofolate reductase polymorphism interferes with the effects of folic acid and zinc sulfate on sperm concentration. Fertil Steril. 2003;80:1190–1194. doi: 10.1016/s0015-0282(03)02157-5. [DOI] [PubMed] [Google Scholar]
- 15.Paracchini V, Garte S, Taioli E. MTHFR 677C>T polymorphism, GSTM1 deletion and male infertility: a possible suggestion of a gene/gene interaction? Biomarkers. 2006;11:53–60. doi: 10.1080/13547500500442050. [DOI] [PubMed] [Google Scholar]
- 16.Tetik A, Aliyeva U, Cetintas VB, Semerci B, Topcuoglu N, et al. Influence of methylenetetrahydrofolate reductase (MTHFR) 677C>T and 1298 A>C gene polymorphisms on male infertility in turkish infertile men with azoospermia and oligozoospermia. Eur Urol. 2008;(Suppl 7):92. [Google Scholar]
- 17.Gava MM, de Oliveira Chagas E, Bianco B, Christofolini DM, Pompeo AC, et al. Methylenetetrahydrofolate Reductase Polymorphisms Are Related to Male Infertility in Brazilian Men. Genet Test Mol Biomarkers. 2011;15:153–157. doi: 10.1089/gtmb.2010.0128. [DOI] [PubMed] [Google Scholar]
- 18.Montjean D, Benkhalifa M, Dessolle L, Cohen-Bacrie P, Belloc S, et al. Polymorphisms in MTHFR and MTRR genes associated with blood plasma homocysteine concentration and sperm counts. Fertil Steril. 2011;95:635–640. doi: 10.1016/j.fertnstert.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 19.Lee S, Jeong YM, Lee SK, Cha KY, Chung TG, et al. The 677 C>T polymorphism in methylenetetrahydrofolate reductase (MTHFR) gene associates with unexplained male infertility with severe OAT. Fertil Steril. 2003;80:229. [Google Scholar]
- 20.Heijmans BT, Gussekloo J, Kluft C, Droog S, Lagaay AM, et al. Mortality risk in men is associated with a common mutation in the methylenetetrahydrofolate reductase gene (MTHFR). Eur J Hum Genet. 1999;7:197–204. doi: 10.1038/sj.ejhg.5200283. [DOI] [PubMed] [Google Scholar]
- 21.Frosst P, Blom HJ, Milos R, Goyette P, Sheppard CA, et al. A candidate genetic risk factor for vascular disease: a common mutation in methylenetetrahydrofolate reductase. Nat Genet. 1995;10:111–113. doi: 10.1038/ng0595-111. [DOI] [PubMed] [Google Scholar]
- 22.Van der Put NM, Gabreëls F, Stevens EM, Smeitink JA, Trijbels FJ, et al. A second common mutation in the methylenetetrahydrofolate reductase gene: An additional risk factor for Neural-Tube Defects. Am J Hum Genet. 1998;62:1044–1051. doi: 10.1086/301825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raman R, Narayan G. 5-Aza deoxyCytidine-induced inhibition of differentiation of spermatogonia into spermatocytes in the mouse. Mol Reprod Dev. 1995;42:284–290. doi: 10.1002/mrd.1080420304. [DOI] [PubMed] [Google Scholar]
- 24.Tüttelmann F, Rajpert-De Meyts E, Nieschlag E, Simoni M. Gene polymorphisms and male infertility–a meta-analysis and literature review. Reprod Biomed Online. 2007;15:643–658. doi: 10.1016/s1472-6483(10)60531-7. [DOI] [PubMed] [Google Scholar]
- 25.Wu W, Shen O, Qin Y, Lu J, Niu X, et al. Int J Androl [Epub ahead of print]; 2011. Methylenetetrahydrofolate reductase C677T polymorphism and the risk of male infertility: a meta-analysis. [DOI] [PubMed] [Google Scholar]
- 26.Rasmussen LB, Andersen NL, Andersson G, Lange AP, Rasmussen K, et al. Folate and neural tube defects; Recommendations from a Danish working group. Dan Med Bull. 1998;45:213–217. [PubMed] [Google Scholar]
- 27.Forges T, Monnier-Barbarino P, Alberto JM, Guéant-Rodriguez RM, Daval JL, et al. Impact of folate and homocysteine metabolism on human reproductive health. Hum Reprod Update. 2007;13:225–238. doi: 10.1093/humupd/dml063. [DOI] [PubMed] [Google Scholar]
- 28.Misra A, Vikram NK, Pandey RM, Dwivedi M, Ahmad FU, et al. Hyperhomocysteinemia, and low intakes of folic acid and vitamin B12 in urban North India. Eur J Nutr. 2002;41:68–77. doi: 10.1007/s003940200010. [DOI] [PubMed] [Google Scholar]
- 29.Raman R, Narayan G. 5-Aza deoxyCytidine-induced inhibition of differentiation of spermatogonia into spermatocytes in the mouse. Mol Reprod Dev. 1995;42:284–290. doi: 10.1002/mrd.1080420304. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. 4th ed Cambridge, United Kingdom: Cambridge University Press; 1999. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. [Google Scholar]
- 31.Thangaraj K, Joshi MB, Reddy AG, Gupta NJ, Chakravarty B, et al. CAG repeat expansion in the androgen receptor gene is not associated with male infertility in Indian populations. J Androl. 2002;23:815–818. [PubMed] [Google Scholar]
- 32.Thangaraj K, Singh L, Reddy AG, Rao VR, Sehgal SC, et al. Genetic affinities of the Andaman Islanders, a vanishing human population. Curr Biol. 2003;13:86–93. doi: 10.1016/s0960-9822(02)01336-2. [DOI] [PubMed] [Google Scholar]
- 33.Petitti DB. Approaches to heterogeneity in meta-analysis. Stat Med. 2001;20:3625–3633. doi: 10.1002/sim.1091. [DOI] [PubMed] [Google Scholar]
- 34.Huedo- Medina TB, Sanchez-Meca J, Marin-Martinez F, Botella J. Assessing heterogeneity in Meta- analysis: Q statistic or I2 Index/. Physcol Methods. 2006;11:193–206. doi: 10.1037/1082-989X.11.2.193. [DOI] [PubMed] [Google Scholar]
- 35.Der Simonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 36.Lin ZH, Xin YN, Dong QJ, Wang Q, Jiang XJ, et al. Association between HLA-DRB1 alleles polymorphism and hepatocellular carcinoma: a meta-analysis. BMC Gastroenterol. 2010;10:145. doi: 10.1186/1471-230X-10-145. [DOI] [PMC free article] [PubMed] [Google Scholar]