Short abstract
Cataract surgery has advanced a lot over the years. This review describes the latest techniques and how they are used to customise the surgery to the needs of the individual patient
Cataract is an important cause of visual impairment worldwide. In the United Kingdom, 30% of people aged over 65 have visually impairing cataract (that is, Snellen visual acuity of less than 6/12 attributable to a lens opacity) in one eye or both eyes.1 (See table A on bmj.com for risk factors.) A visual acuity of 6/12 is below the legal vision requirement to drive in the United Kingdom, which approximates to a visual acuity of 6/10, and evidence indicates that cataract surgery may even decrease the incidence of road traffic crashes among people over 65.2 The NHS does approximately 200 000 cataract operations annually, making this one of the most common surgical procedures in the country.3 Despite this, 88% of people with treatable visual impairment from cataract are not in contact with any eye healthcare services, which represents a very large potential healthcare need.1
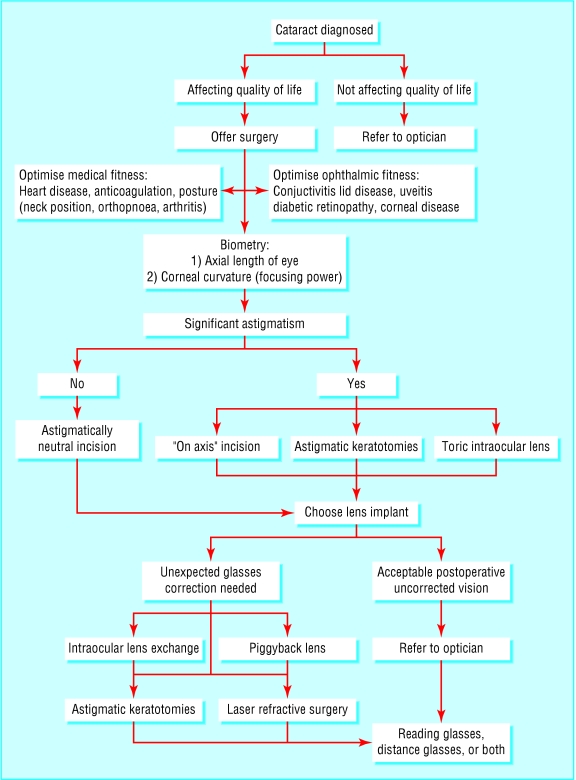
The fundamental aim of cataract surgery, the removal of the opacified natural lens to improve vision, has remained the same for hundreds of years. However, the way in which this is achieved and the expectations of the people having the surgery have altered drastically (table 1). Here we give an overview of some of the advances that allow cataract surgery to be customised for each patient. We discuss the process that patients go through from their first meeting with an ophthalmologist, through the preoperative assessment, to the actual surgery itself and beyond to the appraisal of the results of that surgery. The visual potential of the patient can be realised in many different ways, and when looked at in this context very few cataract operations are the same.
Table 1.
Historical perspective of cataract surgery
| Date | Technique |
|---|---|
| 800 BC |
Couching—dislodgment of lens into posterior of eye with a needle without removal and without replacement of lens |
| 1752 |
Large incision cataract extraction (intracapsular and extracapsular) |
| 1949 |
Intraocular lens |
| 1967 |
Small incision cataract extraction (phacoemulsification) |
| 1990s | Refractive cataract surgery |
Sources and selection criteria
Most of the information used in this review came from our day to day experience as surgeons doing cataract extractions. We identified the papers used in the review by a computerised literature search of the PubMed database with the keywords phacoemulsification, extracapsular cataract extraction, intraocular lens, and biometry. We gathered other information from lens manufacturers' data sheets.
Summary points
Cataract extraction is one of the most common elective procedures in the United Kingdom
Around 30% of people aged over 65 have visually impairing cataract
Every patient's eye is unique, and surgery must be tailored to the individual
Surgery can now be customised to reduce dependence on glasses for both distance vision and reading
Discussion of expectations and risks
Having a cataract is not in itself an indication for surgery. In general, if people are still able to do all the things they enjoy with their vision then surgery is not needed. Informed consent is vital to allow patients to assess the risk for themselves. One particular indication is achieving the legal vision requirement to drive.
Preoperative assessment
Most cataract extractions can be done under local anaesthetic, injected or applied topically as drops, and as a day case. However, attention still needs to be paid to the patient's general medical condition, and this requires multidisciplinary cooperation.
Cardiovascular considerations
Uncontrolled hypertension presents an increased risk of orbital haemorrhage during injection of local anaesthetic and potentially of peroperative suprachoroidal expulsive haemorrhage (a catastrophic complication). Another risk factor for suprachoroidal expulsive haemorrhage is a pulse rate greater than 85 beats/min, so special consideration must be given to highly anxious patients (perhaps sedation) or those with poorly controlled atrial fibrillation.4
Glossary of terms
Astigmatism
A condition in which the surface of the cornea is not spherical but is irregularly shaped like the back of a spoon. An astigmatic cornea prevents light images from being focused to a single point, causing image distortion
Extracapsular cataract extraction (ECCE)
A surgical procedure that removes the cataractous lens of the eye but leaves the posterior lens capsule intact, enabling an intraocular lens to be positioned in its place. Technically this term also applies to phacoemulsification, but ECCE is now widely used in ophthalmic circles to mean a large incision cataract extraction in which the nucleus is expressed whole
Intracapsular cataract extraction (ICCE)
A surgical procedure in which the cataractous lens is removed completely within its capsule, leaving no support structures for an intraocular lens posterior to the pupil. In this procedure intraocular lenses were either sutured to the iris in the plane of the pupil or placed in the anterior chamber of the eye (anterior to the iris)
Optical aberrations
Chromatic—Failure of a lens to bring light of different wavelengths to a common focus. This can be compensated for by using an achromatic lens
Spherical—Failure of a lens system to image the central and peripheral rays at the same focal point
Coma—Blurring in the image plane (surface) of off-axis object points, caused by different parts of spherical lens surfaces exhibiting different degrees of magnification. An off-axis object point is not a sharp image point but appears as a characteristic comet-like flare
Phacoemulsification
A surgical procedure that uses an ultrasonic vibration to shatter and break up a cataractous lens, allowing it to be removed through a small sutureless incision
Anticoagulation
Anticoagulant levels must be checked before surgery, as the risk of orbital haemorrhage during injection of local anaesthetic is increased. However, for most cataract surgery anticoagulant levels within the recommended therapeutic ratio are acceptable.
Posture
Cataract surgery is done with patients supine. The patient needs to be able to lie flat and still for 20-30 minutes. This can be difficult for people with orthopnoea or kyphoscoliosis. Positioning of the patient can therefore increase the surgical difficulty and hence the risks of the procedure.
Ocular considerations
The eyes need to have been assessed for concurrent ocular conditions that may increase the risk of the surgery (for example, lid disease or conjunctivitis) or that may prevent the patient from getting a useful visual improvement (for example, age related macular degeneration or diabetic retinopathy).
Ophthalmologists are under ever increasing pressure to achieve “20/20” vision for their patients. Achieving this requires careful preoperative measurements and the use of the appropriate formula to calculate the power of the replacement intraocular lens. In order to decide this we need two basic pieces of information: the length of the eye and the degree of focusing power afforded by the cornea (box 1).
Box 1: Optimising results of biometry
Axial length—measurement by laser
Topography—keratometry
Formulas—SRK-T or Holladay-II, depending on axial length
Axial length—An ultrasound scan is routinely used to measure the axial length of the eye (generally about 24 mm). However, 54% of the errors in predicted postoperative correction by glasses after intraocular lens implantation are caused by errors in measurement of length.5 A 200 μm error in axial length measurement drops the visual acuity by one line on a Snellen chart. More recently, measurement of axial length by laser has started to come into use and has been shown to yield substantially better prediction of the intraocular lens power in cataract surgery than has biometry using ultrasonography.6
Corneal curvature—The refractive or focusing power of the cornea can be measured with a keratometer. Most corneas are not perfectly spherical but tend to have a steep axis and a flatter axis. The difference between the focusing powers of these two axes, measured in dioptres, is the astigmatism. An average of these two readings is used in working out the power of intraocular lens to be used.
Lens power formulas—After the eye's length and corneal curvature have been measured, the information is used to predict (calculate) the power of the intraocular lens implant that will be used to replace the patient's cataractous lens. Many different formulas have been developed to calculate this, but considerable variation can still occur in intraocular lens power predicted with different formulas (table B on bmj.com). The various regression formulas of Sanders, Retzlaff, and Kraff have become very popular because they are simple to derive and use.7-10 However, some of the other formulas are more accurate in patients with unusually long or short eyes—for example, Holladay-II.
Operative considerations
Correcting astigmatism
Astigmatism is a condition in which the surface of the cornea is not spherical but is irregularly shaped like the back of a spoon. This prevents light images from being focused clearly, causing a blurred image. An incision in the cornea causes a change in its shape. It relaxes the curvature, making the cornea flatter. In cataract surgery the smaller the corneal wound the less flattening (astigmatic change) is produced by the incision. Large incisions heal with added tissue, which leads to considerable flattening of the cornea in that axis, causing substantial astigmatism.11 With the advent of phacoemulsification and foldable intraocular lenses, small clear corneal incisions became practicable. Incisions of less than 3 mm, if prepared correctly, can be astigmatically neutral (that is, not flatten the natural corneal curvature).12
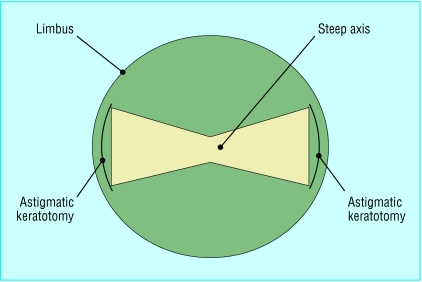
The surgeon has several options for reducing or even attempting to eradicate a person's pre-existing astigmatism. The position and size of the corneal incision can be varied to counteract the patient's preoperative astigmatism. Alternatively, the shape of the cornea can be deliberately changed by making partial thickness relaxing incisions (cuts to allow the cornea to gape slightly), called astigmatic keratotomies, in it (fig 1).
Fig 1.
Schematic diagram of astigmatic keratotomies
Lens implantation
The first intraocular lenses needed a relatively large incision (about 6 mm) to get them into the eye. Modern lens technology has advanced to the development of foldable silicone and acrylic lenses that can be placed in the eye through very small incisions that are self sealing and do not even need suturing (fig 2).
Fig 2.
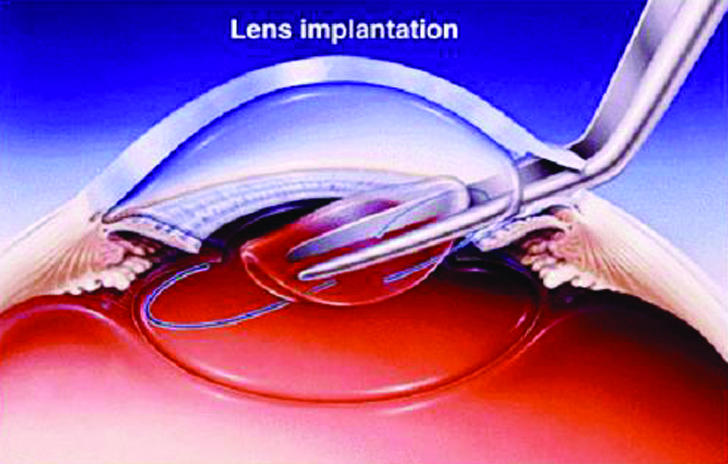
Implantation of the lens
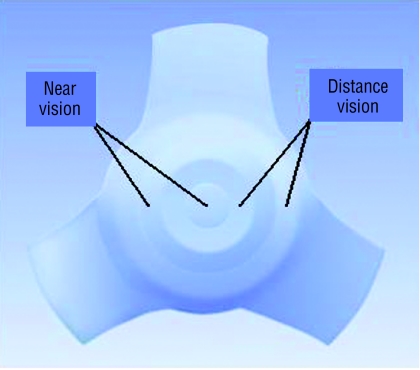
Standard intraocular lenses are monofocal—that is, focused in one position (usually distance)—but patients still need glasses for near and intermediate distances. The development of multifocal intraocular lenses offers a further option for patients (fig 3). The lenses are made up of multiple concentric rings that focus for either near or distance vision. This allows the eye to be in focus for near and far simultaneously, and the brain decides which image it will ignore. The use of this sort of lens needs extremely accurate biometry for the patient to be able to benefit from unaided near and distance vision. As the whole lens is not devoted to the generation of each image, the quality of the vision is inevitably somewhat reduced compared with a standard monofocal lens, and the risk of halos and glare at night also exists.
Fig 3.
Multifocal intraocular lens (courtesy of IOLTech, France)
An advance that overcomes these problems is the accommodating intraocular lens, which can move in the same way as the natural crystalline lens does before it hardens with age. Another type of intraocular lens available is the toric lens, which is shaped in such away as to overcome astigmatism without the need to resort to surgical manipulation of the cornea (box 2).
Box 2: Types of lens
Monofocal
-
Multifocal:
- near dominant
- far dominant
Accommodating
Toric
Position of lens
Depending on the complexity of the case, several different options exist for where to place the intraocular lens within the eye (box 3). The posterior chamber is the ideal position for the lens in terms of both optical performance and potential complications.
Box 3: Position of the lens in eye
Anterior chamber (anterior to the iris)
Sulcus (between posterior iris and lens capsule)
Posterior chamber (natural position): “in the bag” (in original lens's capsule) sutured
Postoperative assessment
Patients often notice a useful improvement in their vision the day after phacoemulsification, compared with several weeks in those having large incision surgery. However, before the final outcome can be ascertained the eye must be allowed to settle down for three to four weeks. An optometrist in the community usually obtains the final correction with glasses, but this information is vital to monitor outcomes. The postoperative assessment is also vital for the diagnosis and management of potential complications resulting from the surgery (table C on bmj.com).
Box 4: Fine tuning results
Intraocular lens exchange
-
Piggyback intraocular lens:
- “in the bag”
- in the sulcus
- anterior chamber
Laser refractive surgery
Astigmatic keratotomies
Fine tuning
The cataract surgery is not necessarily the end of the story. The surgery itself may go very well and the patient may be capable of seeing very clearly but only with an appropriate correction by glasses. If a patient gets an unexpected postoperative glasses error, many things can be done to improve matters (box 4). If the error is very large, usually as a result of inaccurate biometry, the best option may be to proceed straight to a lens exchange or to implant a second “piggyback” lens.
Corneal refractive surgery provides another option. Photorefractive keratectomy, laser assisted epithelial keratomileusis, and laser in situ keratomileusis are different techniques that use an excimer (excited dimer) laser to change the shape of the central cornea and induce a change in the glasses correction of the eye. Astigmatic keratotomies may also be of use after the operation in situations in which unexpected or unacceptable postoperative corneal astigmatism occurs. All of the above treatments are available on the NHS, although postoperative laser refractive surgery is available only at a few specialist centres owing to lack of availability of equipment and experience of surgeons.
Audit
Postoperative data must be constantly audited to ensure that appropriate standards are maintained and also to identify areas for potential improvement. Computerised databases can be of great use in this sort of audit, especially when integrated into the day to day running of a unit. For example, we can identify whether the preoperative measurements are continuing to be taken accurately enough and whether our incisions are causing excessive flattening of the corneas.
What is next?
Commonly implanted intraocular lenses block or reduce the transmission of light only in the low frequency ultraviolet spectrum. A new lens is now being evaluated that also blocks the “higher” frequency blue light (up to 450 nm) that may damage the retina and accelerate the development of age related macular degeneration.
Corneal incision sizes are continuing to reduce through a combination of advances in ultrasonic probes and design of intraocular lenses. New phacoemulsification probes and intraocular lens injection devices can be used in 1 mm corneal incisions, reducing the astigmatic impact on the cornea of the incision and the chance of wound leakage.
The material science of intraocular lens technology has also advanced. New lenses are becoming available that can cancel out some of the optical aberrations occurring naturally in the eye (for example, the positive spherical aberration of the cornea) to enhance contrast sensitivity for improved visual function.
Another approach that may come into common practice is two stage cataract surgery. The first stage would involve standard cataract surgery, and a second stage refractive procedure would then use a wavefront guided excimer laser. Wavefront technology now gives us the ability to map the higher optical aberrations of the eye accurately. These aberrations and any residual postoperative glasses errors would then be neutralised by accurately adjusting the shape of the cornea with wavefront guided excimer laser treatment.
Intraocular lenses of which the focusing strength can be adjusted after cataract surgery have also been developed. The lens is composed of a material whose shape can be changed by the application of the appropriate wavelength of light. Variables in the healing process and errors arising from inaccurate biometry or previous refractive procedures can be neutralised by postoperative adjustment of the lens with non-invasive light. Some of these lenses may also allow the eye to focus for near and distance (accommodate), removing the need for glasses.
Additional educational resources
Useful websites
Cochrane Eyes and Vision Group (www.cochraneeyes.org/reviews.asp)—lists systematic reviews related to cataract surgery as well as published protocols
Royal College of Ophthalmologists (www.rcophth.ac.uk/publications/guidelines/cataract_surgery.html)—cataract surgery guidelines
Department of Health action on cataracts (www.doh.gov.uk/cataracts/index.htm)—NHS policy and guidance on cataracts. Full pdf document downloadable from this site
European Cataract Outcome Study Group (www.eurocat.net)—produces data on the provision and outcome of cataract surgery in Europe
British Ophthalmic Anaesthesia Society (www.boas.org)—organisation of anaesthetists, ophthalmologists, and other clinicians, sharing education and information on anaesthetic management during ophthalmic surgery
Nurses Eye Site (www.nurseseyesite.nhs.uk/spec_area_cataract/index.asp)—orientated towards nursing staff specialising in ophthalmology, with information on the assessment and care of patients with cataract as well as details about surgery and audit
Information resources for patients
Moorfields Eye Hospital (www.moorfields.co.uk/EyeHealth/Cataracts)—patient information about cataracts and cataract surgery from the largest eye centre in the British Isles
National Eye Institute (www.nei.nih.gov/health/cataract/cataract_facts.htm)—an extensive site containing a large amount of information on cataracts and cataract surgery from one of the US federal government's National Institutes of Health
American Society of Cataract and Refractive Surgery (www.ascrs.org/eye/ptguide.html)—a guide for patients
Medem medical library (www.medem.com/medlb/articleslb.cfm?sub_cat = 119)—contains a library on multiple eye disorders, including cataract
Conclusion
The treatment of cataracts has progressed enormously since the days when the “couchers” used to roam from town to town dislocating cataractous lenses with needles, and it continues to evolve in the 21st century with an increasing trend towards customisation to the individual patient's needs (fig 4). What is clear, however, is that the provision of cataract surgery customised to the individual patient relies on far more than just the skill of the surgeon removing the cataract. Customised cataract surgery needs a multidisciplinary approach at several levels. Industry has a vital role in the development of new technologies, as do health services in the provision of appropriate eye care to populations. At the level of the individual patient such surgery involves the close cooperation of several professional groups, including physicians, anaesthetists, surgeons, opticians, and nurses, sharing information to carefully plan, carry out, and assess the results of every procedure. The future for people with cataracts is bright.
Fig 4.
Customised cataract surgery
Supplementary Material
 Three extra tables and extra references appear on bmj.com
Three extra tables and extra references appear on bmj.com
Contributors: MW did most of the background research, wrote the text and tables, referenced the paper, and created or sourced the figures. SS had the idea of writing the paper, helped to plan the content, and did much of the editing. RJS provided most of the information on the new developments in cataract surgery, helped to plan the preoperative assessment section, and edited the final paper. MW and SS accept full responsibility for the content of the paper and controlled the decision to publish.
Funding: None.
Competing interests: None declared.
References
- 1.Reidy A, Minassian DC, Vafidis G, Joseph J, Farrow S, Wu J, et al. Prevalence of serious eye disease and visual impairment in a north London population: population based, cross sectional study. BMJ 1998;316: 1643-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owsley C, McGwin G Jr, Sloane M, Wells J, Stalvey BT, Gauthreaux S. Impact of cataract surgery on motor vehicle crash involvement by older adults. JAMA. 2002;288: 841-9. [DOI] [PubMed] [Google Scholar]
- 3.Royal College of Ophthalmologists. Cataract surgery: guidelines, February 2001. London: Royal College of Ophthalmologists, 2001:7. Available at www.rcophth.ac.uk/publications/guidelines/cataract_surgery.html
- 4.Speaker MJ, Guerriero PN, Met JA, Coad CT, Berger A, Marmor M. A case-control study of risk factors for intraoperative suprachoroidal haemorrhage. Ophthalmology 1991;98: 202-10. [DOI] [PubMed] [Google Scholar]
- 5.Olsen T. Sources of error in intraocular lens power calculation. J Cataract Refract Surg 1992;18: 125-9. [DOI] [PubMed] [Google Scholar]
- 6.Findl O, Drexler W, Menapace R, Heinzl H, Hitzenberger CK, Fercher AF. Improved prediction of intraocular lens power using partial coherence interferometry. J Cataract Refract Surg 2001;27: 861-7. [DOI] [PubMed] [Google Scholar]
- 7.Sanders DR, Kraff MC. Improvement of intraocular lens power calculation using empirical data [correction appears in Am Intra-Ocular Implant Soc J 1981;7: 82]. [DOI] [PubMed] [Google Scholar]; Am Intra-Ocular Implant Soc J 1980;6: 263-7. [DOI] [PubMed] [Google Scholar]
- 8.Retzlaff J. A new intraocular lens calculation formula. Am Intra-Ocular Implant Soc J 1980;6: 148-52. [DOI] [PubMed] [Google Scholar]
- 9.Sanders DR, Retzlaff J, Kraff MC. Comparison of the accuracy of the Binkhorst, Colenbrander, and SRK™ implant power prediction formulas. Am Intra-Ocular Implant Soc J 1981;7: 337-40. [DOI] [PubMed] [Google Scholar]
- 10.Sanders DR, Retzlaff J, Kraff MC. Comparison of empirically derived and theoretical aphakic refraction formulas. Arch Ophthalmol 1983;101: 965-7. [DOI] [PubMed] [Google Scholar]
- 11.Ernest P, Tipperman R, Eagle R, Kardasis C, Lavery K, Sensoli A, et al. Is there a difference in incision healing based on location? J Cataract Refract Surg 1998;24: 482-6. [DOI] [PubMed] [Google Scholar]
- 12.Nielsen PJ. Prospective evaluation of surgically induced astigmatism and astigmatic keratotomy effects of various self-sealing small incisions. J Cataract Refract Surg 1995;21: 43-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.