Abstract
Objectives
To evaluate the effect of maternal administration of antenatal steroids (ANS) on cord blood cytokine levels at birth in preterm infants.
Methods
Cord blood cytokine concentrations were measured for pro-inflammatory cytokines (IL-1β, IL-6, IL-8); anti-inflammatory cytokines (IL-4, IL-10 and TGF β); and neurotrophic cytokines (BDNF, NT-3, and NT-4) in two hundred preterm infants. Data were analyzed using multivariable linear regression to model the independent and joint effects of ANS and inflammation on mean log cord blood cytokine concentrations adjusted for gestational age and Apgar scores.
Results
Exposure to ANS had no significant effect on the cord blood concentrations of cytokines measured in this study. All three pro-inflammatory cytokines levels and levels of IL-10 were significantly increased and cord blood levels of TGF-β and NT-3 were significantly decreased in infants with placental inflammation.
Conclusion
Although exposure to ANS did not have any significant effect on cord blood levels of cytokines, there was a trend toward the attenuation of inflammatory response and higher levels of neurotrophic cytokines in infants born to mothers with placental inflammation and exposure to ANS compared to infants born to mothers with placental inflammation and no ANS exposure.
Introduction
Maternal administration of antenatal steroids (ANS) has been shown to significantly reduce the morbidity and mortality in preterm infants born between 24 and 34 weeks gestation. (1, 2) The long-term follow up studies of infants have shown a lower incidence of cerebral palsy and overall improved outcomes in infants in the ANS treated group. (3, 4) Though the pulmonary benefits of ANS treatment are known to be secondary to its effect on lung maturation and increased production of surfactant by type II pneumocytes, the underlying mechanisms for other beneficial effects of ANS are not as well understood. (2) In-vivo animal studies and in-vitro studies suggest that some of the beneficial effects of ANS may be mediated by modulation of cytokine levels in the fetus. (5-7)
A growing body of literature suggests an association between cytokine levels in the perinatal period and neonatal outcomes. (8-13) Elevated cord blood IL-6 levels have been associated with an increased risk for periventricular leukomalacia, intraventricular hemorrhage, and necrotizing enterocolitis in infants born at less than 32 weeks gestation. (8-10, 13) Prenatal exposure to maternal infection has been shown to affect the levels of pro-inflammatory and neurotrophic cytokines in the fetus. (14, 15) Other factors such as gestational age, perinatal asphyxia, and IUGR have also been reported to affect fetal cytokine levels. (16-20) However, the effects of maternal administration of ANS on fetal cytokine levels have not been well studied. We hypothesized that some of the beneficial effects of ANS may be mediated by the effects of ANS on cytokine levels in the fetus; and ANS modulates infection/inflammation mediated changes in fetal cytokine concentrations. Thus the primary objective of this study was to evaluate the effect of maternal administration of ANS on fetal cytokine levels measured in cord blood at birth. A secondary objective was to evaluate whether maternal administration of ANS modulates cord blood cytokine levels in the presence of placental inflammation.
Methods
Study population
The population for this analysis was drawn from a larger case-control study evaluating environmental and genetic determinants of preterm delivery and low birth weight. (21) Eligible cases were live singleton preterm infants born between 24 and 34 weeks gestation. Multiple pregnancies, births that resulted from maternal trauma and newborns with major birth defects were excluded. Cord blood was collected from all births and placentas were sent for pathology. The study was approved by the Institutional Review Boards at Boston University Medical Center, Children’s Hospital Boston, Beth Israel Deaconess Medical Center at Boston, and Children’s Memorial Hospital Chicago. An informed consent was obtained from all study participants.
Clinical and demographic information
Maternal interviews were conducted using a structured questionnaire that included demographic characteristics as well as medical and reproductive histories. In addition, medical record reviews were conducted using a standardized abstraction form that included data on prenatal care, clinical presentation, intrapartum management, pregnancy complications, and birth outcomes. The clinical protocol during the study period was to give two 12 mg doses of betamethasone 24 hours apart to all mothers in preterm labor before 34 weeks of gestational age. Gestational age was assessed with an algorithm based upon last menstrual period and early ultrasound before 20 weeks gestation. (21)
Cord blood cytokine assays
Cord blood was obtained by trained nursing staff. Samples were kept on ice and subsequently centrifuged for 10 minutes in a tabletop refrigerated centrifuge at 2500 rpm. Plasma was removed from the cell pellet by pipette. Each subject’s plasma sample was then split into 3 aliquots and stored in a -80°C freezer. Simultaneous measurement of the cytokines in cord blood plasma was performed by immunoassay using flowmetric Luminex xMAP technology (Luminex Corp, Austin, TX). Antibodies specific for cytokines, chemokines, and neurotrophins (R&D Systems, Minneapolis, MN; BD Biosciences Pharmingen, San Diego, CA; MBL [Medical Biological Laboratories], Woburn, MA; BioSource, Nivelles, Belgium) were coupled to carboxylated beads (Luminex Corp). Biomarkers were quantified by sandwich immunoassays using bead-coupled capture antibodies, biotinylated detection antibodies, and phycoerythrin-labeled streptavidin according to the techniques described by Skogstrand, et al. (22) Measurements were performed on a pool of human serum, and both intra- and interassay coefficients of variance were determined. Working range was defined as the range of concentrations for which the coefficients of variance (standard deviation/mean ×100) was <20%. Cytokine values were measured for: a) Pro-inflammatory cytokines – IL-1β, IL-6, IL-8; b) Anti-inflammatory cytokines – IL-4, IL-10 and TGF-β; and c) Neurotrophic cytokines – BDNF, NT-3, and NT-4.
Placental histopathology
All placentas were reviewed by a designated perinatal pathologist, and a subset was independently reviewed by a second pathologist to confirm reliability. According to our previously published protocol the presence and location of inflammation was reported using well-established algorithms. (23) Using a standardized abstraction form, the histologic examination status was classified as placental inflammation if either the maternal or fetal compartment had signs of inflammation consistent with infection. (23)
Statistical methods
We compared demographic and clinical characteristics of mother-infant pairs with and without antenatal steroid treatment as well as those with and without placental inflammation using t-tests (for continuous measures), Wilcoxin-Mann-Whitney test for medians (for Apgar score) and chi-square tests (for categorical variables). We used a square root transformation of gestational age to normalize it prior to conducting the t-tests. We next used multivariable linear regression to model the mean cord blood concentration of each cytokine by steroid exposure with and without adjustment for one minute Apgar score ≤3, gestational age, and placental inflammation. We used the logarithmic transform of each cytokine in order to meet modeling assumption of normally distributed residuals. We ensured that the residuals of each model were homoskedastic by visually inspecting plots of residuals by predicted values. Using the same methods, we modeled the mean log cord concentration of each cytokine by placental inflammation with and without adjustment for one minute Apgar score ≤3, gestational age, and steroid exposure. We presented figures for mean values of cytokines within the four groups defined by ANS (yes/no) and placental inflammation (yes/no) normalized for one minute Apgar score ≤3 and gestational age using least squares means in a general linear model using SAS procedure GENMOD.
Results
200 preterm infants (100 with exposure to ANS and 100 controls) born between 24 and 34 completed weeks gestation were included in this study. Table 1 summarizes the important demographic characteristics of the infants. The mean gestational age and birth weight were lower but the incidence of PPROM and intrauterine infection were higher among infants born after exposure to antenatal steroids (Table 1). Sixty seven (33%) infants had histological evidence of placental inflammation; these infants had lower mean gestational age and birth weight as compared to infants with no histological evidence of placental inflammation (Table 2). The following results are presented using the cytokine concentrations after adjusting for gestational age and one minute Apgar scores.
Table 1.
Characteristics of 200 mother-infant pairs with birth ≤34 weeks’ gestation by exposure to antenatal steroids.
| Antenatal Steroids | |||
|---|---|---|---|
| Yes (n=100) | No (n=100) | p value * | |
| Gestational age - mean ± SD | 31 ± 2.9 | 33.1 ± 2.1 | <0.001 |
| Birth weight - mean ± SD | 1663 ± 601 | 2105 ± 570 | <0.001 |
| Male gender - n (%) | 53(53) | 56(56) | 0.776 |
| Delivery type - n (%) | 0.257 | ||
| Vaginal | 58(58) | 49(49) | |
| C/section | 42(42) | 51(51) | |
| 1 minute Apgar score - median (range) | 7(1 – 9) | 7(1 – 9) | 0.725 |
| 1 minute Apgar score ≤ 3 - n (%) | 12(12) | 8(8) | 0.480 |
| Race - n (%) | 0.957 | ||
| Black | 65(65) | 63(63) | |
| White | 12(12) | 12(12) | |
| Hispanic | 17(17) | 17(17) | |
| Other | 6(6) | 8(8) | |
| Preterm delivery subtype - n (%) | 0.043 | ||
| Spontaneous | 44(44) | 38(38) | |
| PPROM | 32(32) | 22(22) | |
| Medically indicated | 24(24) | 40(40) | |
| Intrauterine infection - n (%) | |||
| Clinical diagnosis | 10(10) | 1(1) | 0.013 |
| Histopathological diagnosis | 50(50) | 17(17) | <0.0001 |
t test for continuous measures, Wilcoxin-Mann-Whitney test for medians, and X2 test for categorical or binary measures. Square root of gestational age used for t-test.
Table 2.
Characteristics of 200 mother-infant pairs with birth ≤34 weeks’ gestation by placental inflammation
| Placental inflammation | |||
|---|---|---|---|
| Yes (n=67) | No (n=133) | p value * | |
| Gestational age - mean ± SD | 30.3 ± 3.2 | 32.9 ± 2.0 | <0.001 |
| Birth weight - mean ± SD | 1538 ± 574 | 2058 ± 577 | <0.001 |
| Male gender - n (%) | 33(49.3) | 76(57.1) | 0.364 |
| Delivery type - n (%) | 0.003 | ||
| Vaginal | 46(68.7) | 61(45.9) | |
| C/section | 21(31.3) | 72(54.1) | |
| 1 minute Apgar score - median (range) | 7(1 – 9) | 8(1 – 9) | 0.036 |
| 1 minute Apgar score ≤ 3 - n (%) | 9(13.4) | 11(8.3) | 0.369 |
| Race - n (%) | 0.645 | ||
| Black | 45(67.2) | 83(62.4) | |
| White | 7(10.4) | 17(12.8) | |
| Hispanic | 9(13.4) | 25(18.8) | |
| Other | 6(9) | 8(6) | |
| Preterm delivery subtype - n (%) | <0.0001 | ||
| Spontaneous | 29(43.3) | 53(39.8) | |
| PPROM | 33(49.3) | 21(15.8) | |
| Medically indicated | 5(7.5) | 59(44.4) | |
Pro-inflammatory cytokines
Exposure to ANS had no significant effect on the cord blood concentrations of pro-inflammatory cytokines (Table 3). The concentrations of all three pro-inflammatory cytokines were significantly increased in infants with placental inflammation (Table 4). The cord blood concentrations of all pro-inflammatory cytokines were lower in infants with placental inflammation and ANS exposure compared to the infants with placental inflammation and no ANS, but these differences were not statistically significant (Figure 1).
Table 3.
Mean cord blood cytokine concentrations in preterm births with and without exposure to antenatal steroids
| n | Mean ± SD ** | Crude
|
Adjusted * |
|||
|---|---|---|---|---|---|---|
| ß (SE) | p | ß (SE) | p | |||
| Log(IL1β) | ||||||
| No Steroids | 100 | 61.7 ± 164.1 | Ref | Ref | ||
| Steroids | 100 | 83.3 ± 190.1 | 0.215(0.142) | 0.130 | -0.029(0.151) | 0.848 |
| Log(IL6) | ||||||
| No Steroids | 100 | 385.9 ± 1077.4 | Ref | Ref | ||
| Steroids | 100 | 670.8 ± 1354.7 | 0.502(0.273) | 0.066 | -0.263(0.266) | 0.324 |
| Log(IL8) | ||||||
| No Steroids | 100 | 193.3 ± 652.8 | Ref | Ref | ||
| Steroids | 100 | 336.2 ± 819.1 | 0.592(0.241) | 0.014 | -0.161(0.224) | 0.473 |
| Log(IL4) | ||||||
| No Steroids | 100 | 7.1 ± 5.4 | Ref | Ref | ||
| Steroids | 100 | 7.6 ± 9.9 | -0.093(0.099) | 0.350 | -0.037(0.109) | 0.732 |
| Log(IL10) | ||||||
| No Steroids | 100 | 491.0 ± 393.1 | Ref | Ref | ||
| Steroids | 100 | 567.6 ± 620.2 | -0.124(0.264) | 0.640 | -0.230(0.286) | 0.422 |
| Log(TGFβ) | ||||||
| No Steroids | 100 | 157.0 ± 119.3 | Ref | Ref | ||
| Steroids | 100 | 141.7 ± 114.1 | -0.1(0.129) | 0.436 | 0.025(0.141) | 0.858 |
| Log(BDNF) | ||||||
| No Steroids | 100 | 2271.7 ± 1918.0 | Ref | Ref | ||
| Steroids | 100 | 2324.8 ± 2633.4 | -0.218(0.141) | 0.122 | -0.043(0.153) | 0.779 |
| Log(NT3) | ||||||
| No Steroids | 100 | 133.3 ± 173.0 | Ref | Ref | ||
| Steroids | 100 | 145.3 ± 261.8 | -0.04(0.227) | 0.860 | 0.291(0.242) | 0.229 |
| Log(NT4) | ||||||
| No Steroids | 100 | 25.9 ± 15.6 | Ref | Ref | ||
| Steroids | 100 | 24.7 ± 22.7 | -0.066(0.106) | 0.535 | -0.035(0.117) | 0.763 |
Adjusted for 1 minute Apgar ≤ 3, gestational age and placental inflammation.
Mean ± SD for original cytokine concentration prior to log transformation for modeling.
Table 4.
Mean cord blood cytokine concentrations in preterm births with and without placental inflammation
| n | Mean ± SD ** | Crude
|
Adjusted * |
|||
|---|---|---|---|---|---|---|
| ß (SE) | p | ß (SE) | p | |||
| Log(IL1β) | ||||||
| No Inflammation | 133 | 41.5 ± 29.9 | Ref | Ref | ||
| Inflammation | 67 | 134 ± 295.6 | 0.634(0.144) | <0.0001 | 0.571(0.162) | 0.0004 |
| Log(IL6) | ||||||
| No Inflammation | 133 | 143.3 ± 590.6 | Ref | Ref | ||
| Inflammation | 67 | 1292.8 ± 1722.2 | 1.887(0.259) | <0.0001 | 1.687(0.286) | <0.0001 |
| Log(IL8) | ||||||
| No Inflammation | 133 | 46.3 ± 79.3 | Ref | Ref | ||
| Inflammation | 67 | 698.4 ± 1166.8 | 1.905(0.221) | <0.0001 | 1.699(0.241) | <0.0001 |
| Log(IL4) | ||||||
| No Inflammation | 133 | 7 ± 5.2 | Ref | Ref | ||
| Inflammation | 67 | 8.1 ± 11.7 | -0.097(0.105) | 0.355 | -0.033(0.118) | 0.777 |
| Log(IL10) | ||||||
| No Inflammation | 133 | 501.7 ± 401.4 | Ref | Ref | ||
| Inflammation | 67 | 584.3 ± 697.4 | -0.318(0.279) | 0.254 | -0.68(0.308) | 0.027 |
| Log(TGFβ) | ||||||
| No Inflammation | 133 | 160 ± 115.7 | Ref | Ref | ||
| Inflammation | 67 | 128.2 ± 116.7 | -0.331(0.134) | 0.014 | -0.307(0.151) | 0.042 |
| Log(BDNF) | ||||||
| No Inflammation | 133 | 2433.9 ± 2268.3 | Ref | Ref | ||
| Inflammation | 67 | 2029 ± 2349.8 | -0.366(0.148) | 0.013 | -0.234(0.165) | 0.155 |
| Log(NT3) | ||||||
| No Inflammation | 133 | 164.2 ± 253.7 | Ref | Ref | ||
| Inflammation | 67 | 89.9 ± 123.8 | -0.866(0.232) | 0.0002 | -0.884(0.26) | 0.0007 |
| Log(NT4) | ||||||
| No Inflammation | 133 | 24.5 ± 14 | Ref | Ref | ||
| Inflammation | 67 | 27 ± 27.2 | -0.046(0.112) | 0.682 | 0.001(0.126) | 0.995 |
Adjusted for 1 minute Apgar ≤ 3, gestational age and steroid exposure.
Mean ± SD for original cytokine concentration prior to log transformation for modeling.
Figure 1.
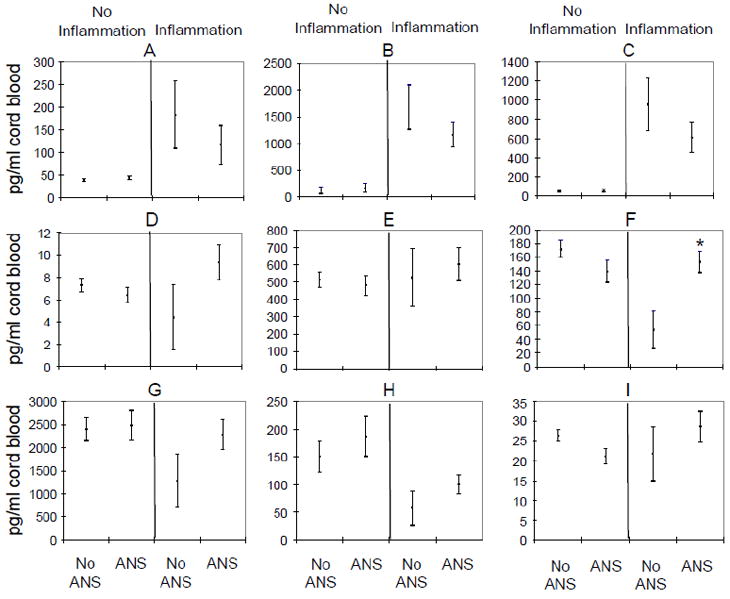
Effect of antenatal steroid exposure on cord blood cytokine concentrations in infants with and without evidence of placental inflammation.
Cord blood concentrations of IL-1β (A), IL-6 (B), IL-8 (C), IL-4 (D), IL-10 (E), TGFß (F), BDNF (G), NT-3 (H), and NT-4 (I) are presented as mean ± standard deviations and normalized for 1 minute Apgar score ≤3 and gestational age. 133 infants had no evidence of placental inflammation, 83 of these 133 infants had no ANS and 50 infants had ANS. 67 infants had evidence of placental inflammation and 17 of these infants had no ANS and 50 had ANS. Cytokine concentrations were compared for infants with ANS exposure and without ANS exposure in each subgroup of infants with and without evidence of placental inflammation. * = p value <0.05, (Using linear regression and adjustment for 1 minute Apgar ≤ 3 and gestational age at birth, TGF-β levels were significantly lower in infants with placental inflammation and no ANS compared to infants with placental inflammation and ANS)
Anti-inflammatory cytokines
Exposure to ANS had no significant effect on the cord blood concentrations of anti-inflammatory cytokines (Table 3). There was a modest increase in the cord blood level of IL-10, no significant change in level of IL-4, and a decrease in level of TGF-β in presence of placental inflammation (Table 4). In the absence of antenatal steroid exposure, presence of placental inflammation was associated with a significant decrease in TGF-β level compared to TGF-β level in infants with no placental inflammation (p value = 0.003), (Figure 1). TGF-β level in infants with placental inflammation and exposure to ANS was similar to the level observed in infants without any placental inflammation (Figure 1). Similar but not statistically significant change was observed for IL-4 (Figure 1).
Neurotrophic cytokines
The cord blood concentrations of neurotrophic cytokines were also not affected by exposure to ANS (Table 3). The level of NT-3 was significantly decreased in infants with placental inflammation but cord blood levels of other two neurotrophic cytokines, BDNF and NT-4, remained unchanged irrespective of the placental inflammation (Table 4). Infants born to mothers with placental inflammation and exposure to ANS had a trend toward higher cord blood concentrations of all three neurotrophic factors compared to infants with placental inflammation and no ANS, but these differences were not statistically significant (Figure 1).
Discussion
The exposure to ANS did not have a significant effect on cord blood levels of cytokines measured in this study but the results of this study do confirm previous observations that cord blood levels of pro-inflammatory cytokines such as IL-6, IL-8 and IL-1β significantly increase in response to intrauterine infection/inflammation in human fetus. There was a trend toward the attenuation of inflammatory response and higher levels of neurotrophic cytokines in infants born to mothers with placental inflammation and exposure to ANS compared to infants born to mothers with placental inflammation and no ANS but these differences were not statistically significant.
ANS and inflammatory cytokines
Several investigators have demonstrated that intrauterine infection in the preterm human fetus leads to increased levels of pro-inflammatory cytokines. (8, 10, 11) However, the effect of intrauterine infection/inflammation on levels of anti-inflammatory cytokines has not been studied so far. Similar to the previous reports, we observed an increase in cord blood levels of all pro-inflammatory cytokines in pregnancies with placental inflammation but the changes in the levels of anti-inflammatory cytokines were variable. We observed an increase in IL-10 levels and a decrease in TGF-β levels in the presence of placental inflammation but levels of IL-4 were unchanged. The modulatory effect of ANS on fetal pro- and anti-inflammatory cytokines production has been contradictory. Some animal in-vivo and in-vitro studies suggest that ANS treatment temporarily suppresses fetal pulmonary inflammation. (5, 6) However, a study on the kinetics of pro- and anti-inflammatory cytokines in an ex vivo cord blood culture has shown that ANS exposure had no significant effects on pro-inflammatory cytokine levels. (17) Another study reported similar lack of effect of antenatal glucocorticoid treatment on fetal monocytes’ capacity to produce IL-6 after ex vivo stimulation with LPS. (24) The effect of ANS treatment on pro-inflammatory cytokine levels in human fetus is even less clear. Goepfert et al measured IL-6 levels in 309 preterm infants and reported that the median cord IL-6 levels were higher among women who did not receive steroids before delivery compared with those receiving at least 1 dose. (8) However, the difference was not statistically significant (P value 0.048). (8) In our cohort of 200 preterm infants, the cord blood concentrations of all pro-inflammatory cytokines were lower in infants with placental inflammation and ANS exposure compared to the infants with placental inflammation and no ANS. Although, these differences did not achieve a statistical significance, this would suggest that exposure to ANS may attenuate fetal response to placental inflammation. The use of ANS had no significant effect on the levels of two of the three anti-inflammatory cytokines, IL-4 and IL-10, but placental inflammation associated decrease in TGF-β level was attenuated by exposure to ANS.
ANS and neurotrophic cytokines
Neurotrophins (NTs) play an important role in the growth and development of nervous system and also provide neuroprotection by reducing apoptosis and promoting survival and maintenance of neurons in the peripheral and central nervous system. (25-27) Elevated plasma levels of NTs have been reported in inflammatory, autoimmune, and allergic diseases. (28) It has been suggested that the beneficial effects of ANS and the adverse effects of infection on the neurological outcome of preterm infants are in part mediated by the effect of ANS and infections on the levels of NTs. (16, 28, 29)
The effects of antenatal steroids on neurotrophic factor levels in animals have been contradictory. Bruschettini et al reported that a clinically equivalent dose of ANS decreased neurotrophic factor concentrations in serum and hippocampus in rats while half of this dose reduced serum concentrations but did not affect neurotrophin concentrations in hippocampus. (30) In contrast, Roskoden et al observed an increase in mRNA expression of both neurotrophins and their receptors with early postnatal corticosteroid treatment in rats. (31) Two human studies have reported higher cord blood levels of BDNF after a course of ANS and the authors speculated that the improved neurodevelopmental outcome in infants exposed to ANS is probably mediated by the effect of ANS on BDNF levels. (16, 29) However, both these studies have small total sample sizes of 60 and 33 patients respectively, and the number of mothers who received a course of ANS was only 16 and 18 respectively in these studies. It is also unclear if these differences were adjusted for variables with potential to affect the levels of BDNF such as gestational age, asphyxia and infection. (32-34) After adjusting for these potentially confounding variables, we did not observe any effect of ANS on cord blood levels of neurotrophic cytokines including BDNF in our study.
The effect of intrauterine infection on cord blood levels of neurotrophic cytokines in humans has also not been studied well. Marx et al measured NGF, BDNF, and NT-3 in amniotic fluid and reported that patients with evidence of infection had significantly lower NGF and BDNF levels but levels of NT-3 did not change with infection. (34) To the best of our knowledge, effect of infection on cord blood levels of neurotrophic cytokines has not been reported so far. In this study, we observed significantly lower levels of NT3 in the presence of placental inflammation, but the levels of BDNF and NT-4 were similar in the presence and absence of placental inflammation.
In summary, exposure to ANS did not have any significant effect on cord blood levels of pro-inflammatory, anti-inflammatory and neurotrophic cytokines measured in this study. However, subgroup analyses demonstrate trends suggesting that the exposure to ANS may modify and attenuate fetal cytokine responses associated with placental inflammation. The results of our study suggest that further studies are needed to determine if some of the beneficial effects of ANS in preterm infants are mediated by modulation of fetal cytokine responses particularly in pregnancies complicated by intrauterine inflammation. However, the results of this study should be viewed in the light of the following limitations. First, although this study is among the largest of this kind, the statistical power may still be limited. The observed beneficial but non-significant trends after maternal ANS administration in presence of intrauterine infection/inflammation could be statistically significant in a study with larger sample size. Second, this study only examines a single time point (at birth) after a variable interval between ANS exposure and delivery. Therefore, it is possible that the effect of ANS on fetal cytokine levels is for a short period of time and not sustained as shown in animal studies. (5) There could be other pathways or unknown confounding variables which were not included in our regression analysis model and could have affected the results of this analysis. These limitations emphasize the need for additional large, prospective studies to fully understand the impact of ANS on fetal cytokine levels during multiple time points from ANS administration to delivery. Such information may in turn guide clinical practice on the use of ANS in a most effective way and improve neonatal and long-term outcomes.
Acknowledgments
Financial Support: The parent study received funding from the National Institute of Child Health and Human Development (R01 HD41702), National Institute of Environmental Health Sciences (R21ES11666), and the March of Dimes Birth Defects Foundation (20-FY98-0701 and 20-FY02-56)
Abbreviations
- ANS
antenatal steroids
- NGF
nerve growth factor
- BDNF
brain-derived neurotrophic factor
- NT
neurotrophin
- NT-3
neurotrophin-3
- NT-4
neurotrophin-4
Footnotes
Conflict of interest statement: We have no conflict to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Antenatal Corticosteroid Therapy for Fetal Maturation. ACOG Committee Opinion No. 402. American College of Obstetricians and Gynecologists Committee on Obstetric Practice. Obstet Gynecol. 2008;111:805–7. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- 2.Roberts D, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database of Systematic Reviews. 2006;(3) doi: 10.1002/14651858.CD004454.pub2. Art. No.:CD004454. [DOI] [PubMed] [Google Scholar]
- 3.Doyle LW, Ford GW, Rickards AL, Kelly EA, Davis NM, Callanan C, et al. Antenatal corticosteroids and outcome at 14 years of age in children with birth weight less than 1501 grams. Pediatrics. 2000;106:e2. doi: 10.1542/peds.106.1.e2. [DOI] [PubMed] [Google Scholar]
- 4.Dessens AB, Haas HS, Kope JG. Twenty-year follow-up of antenatal corticosteroid treatment. Pediatrics. 2000;105:e77. doi: 10.1542/peds.105.6.e77. [DOI] [PubMed] [Google Scholar]
- 5.Kramer BW, Ikegami M, Moss TJM, Nitsos I, Newnham JP, Jobe AH. Antenatal betamethasone changes cord blood monocyte responses to endotoxin in preterm lambs. Pediatr Res. 2004;55:764–76. doi: 10.1203/01.PDR.0000120678.72485.19. [DOI] [PubMed] [Google Scholar]
- 6.Schwartz WJ, 3rd, Christensen HD, Carey JC, Rayburn WF, Gonzalez C. Systemic administration of betamethasone delays endotoxin-induced preterm labor in the murine model. Am J Obstet Gynecol. 2003;188:439–43. doi: 10.1067/mob.2003.72. [DOI] [PubMed] [Google Scholar]
- 7.Mann SA, Versmold B, Marx R, Stahlhofen S, Dietzel ID, Heumann R, et al. Corticosteroids reverse cytokine-induced block of survival and differentiation of oligodendrocyte progenitor cells from rats. J Neuroinflammation. 2008;5:39. doi: 10.1186/1742-2094-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goepfert AR, Andrews WW, Carlo W, Ramsey PS, Cliver SP, Goldenberg RL, et al. Umbilical cord plasma interleukin-6 concentrations in preterm infants and risk of neonatal morbidity. Am J Obstet Gynecol. 2004;191:1375–81. doi: 10.1016/j.ajog.2004.06.086. [DOI] [PubMed] [Google Scholar]
- 9.Tauscher MK, Berg D, Brockmann M, Seidenspinner S, Speer CP, Groneck P. Association of histologic chorioamnionitis, increased levels of cord blood cytokines, and intracerebral hemorrhage in preterm neonates. Biol Neonate. 2003;83:166–70. doi: 10.1159/000068924. [DOI] [PubMed] [Google Scholar]
- 10.Weeks JW, Reynolds L, Taylor D, Lewis J, Wan T, Gall SA. Umbilical cord blood interleukin-6 levels and neonatal morbidity. Obstet Gynecol. 1997;90:815–8. doi: 10.1016/S0029-7844(97)00421-3. [DOI] [PubMed] [Google Scholar]
- 11.Ambalavanan N, Carlo WA, D’Angio CT, McDonald SA, Das A, Schendel D, et al. Cytokines associated with bronchopulmonary dysplasia or death in extremely low birth weight infants. Pediatrics. 2009;123:1132–41. doi: 10.1542/peds.2008-0526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paananen R, Husa A, Vuolteenaho R, Herva R, Kaukola T, Hallman M. Blood cytokines during the perinatal period in very preterm infants: relationship of inflammatory response and bronchopulmonary dysplasia. J Pediatr. 2009;154:39–43.e3. doi: 10.1016/j.jpeds.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 13.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG. 2005;112(S1):16–8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- 14.Santana C, Guindeo MC, González G, García-Muñoz F, Saavedra P, Doménech E. Cord blood levels of cytokines as predictors of early neonatal sepsis. Acta Paediatr. 2001;90:1176–81. doi: 10.1080/080352501317061602. [DOI] [PubMed] [Google Scholar]
- 15.Gilmore JH, Jarskog LF, Vadlamudi S. Maternal infection regulates BDNF and NGF expression in fetal and neonatal brain and maternal-fetal unit of the rat. J Neuroimmunol. 2003;138:49–55. doi: 10.1016/s0165-5728(03)00095-x. [DOI] [PubMed] [Google Scholar]
- 16.Chouthai NS, Sampers J, Desai N, Smith GM. Changes in neurotrophin levels in umbilical cord blood from infants with different gestational ages and clinical conditions. Pediatr Res. 2003;53:965–9. doi: 10.1203/01.PDR.0000061588.39652.26. [DOI] [PubMed] [Google Scholar]
- 17.Dembinski J, Behrendt D, Martini R, Heep A, Bartmann P. Modulation of pro- and anti-inflammatory cytokine production in very preterm infants. Cytokine. 2003;21:200–6. doi: 10.1016/s1043-4666(02)00498-2. [DOI] [PubMed] [Google Scholar]
- 18.Xanthou M, Fotopoulos S, Mouchtouri A, Lipsou N, Zika I, Sarafidou J. Inflammatory mediators in perinatal asphyxia and infection. Acta Paediatr Suppl. 2002;91:92–7. doi: 10.1111/j.1651-2227.2002.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 19.Okazaki K, Nishida A, Kato M, Kozawa K, Uga N, Kimura H. Elevation of cytokine concentrations in asphyxiated neonates. Biol Neonate. 2006;89:183–9. doi: 10.1159/000089180. [DOI] [PubMed] [Google Scholar]
- 20.Malamitsi-Puchner A, Nikolaou KE, Economou E, Boutsikou M, Boutsikou T, Kyriakakou M, et al. Intrauterine growth restriction and circulating neurotrophin levels at term. Early Hum Dev. 2007;83:465–9. doi: 10.1016/j.earlhumdev.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 21.Wang X, Zuckerman B, Pearson C, Kaufman G, Chen C, Wang G, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 22.Skogstrand K, Thorsen P, Norgaard-Pedersen B, Schendel DE, Sorensen LC, Hougaard DM. Simultaneous measurement of 25 inflammatory markers and neurotrophins in neonatal dried blood spots by immunoassay with xMAP technology. Clin Chem. 2005;51:1854–66. doi: 10.1373/clinchem.2005.052241. [DOI] [PubMed] [Google Scholar]
- 23.Gupta M, Mestan KK, Martin CR, Pearson C, Ortiz K, Fu L, et al. Impact of clinical and histologic correlates of maternal and fetal inflammatory response on gestational age in preterm births. J Matern Fetal Neonatal Med. 2007;20:39–46. doi: 10.1080/14767050601156861. [DOI] [PubMed] [Google Scholar]
- 24.Kavelaars A, van der Pompe G, Bakker JM, van Hasselt PM, Cats B, Visser GH, et al. Altered immune function in human newborns after prenatal administration of betamethasone: enhanced natural killer cell activity and decreased T cell proliferation in cord blood. Pediatr Res. 1999;45:306–12. doi: 10.1203/00006450-199903000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Arenas EHP. Neurotrophin-3 prevents the death of adult central noradrenergic neurons in vivo. Nature. 1994;367:368–71. doi: 10.1038/367368a0. [DOI] [PubMed] [Google Scholar]
- 26.Tucker KL, Meyer M, Barde YA. Neurotrophins are required for nerve growth during development. Nat Neurosci. 2001;4:29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- 27.ElShamy WM, Ernfors P. Brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 complement and cooperate with each other sequentially during visceral neuron development. J Neurosci. 1997;17:8667–75. doi: 10.1523/JNEUROSCI.17-22-08667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malamitsi-Puchner A, Economou E, Boutsikou T, Nikolaou KE, Vrachnis N. Neurotrophin-3 and FLT3 Tyrosine Kinase Receptor in Perinatal Life. Mediators Inflamm. 2005;(1):53–6. doi: 10.1155/MI.2005.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rao R, Mashburn CB, Mao J, Wadhwa N, Smith GM, Desai NS. Brain-derived neurotrophic factor in infants <32 weeks gestational age: correlation with antenatal factors and postnatal outcomes. Pediatr Res. 2009;65:548–52. doi: 10.1203/PDR.0b013e31819d9ea5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bruschettini M, van den Hove DL, Gazzolo D, Bruschettini P, Blanco CE, Steinbusch HW. A single course of antenatal betamethasone reduces neurotrophic factor S100B concentration in the hippocampus and serum in the neonatal rat. Brain Res Dev Brain Res. 2005;159:113–8. doi: 10.1016/j.devbrainres.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 31.Roskoden T, Otten U, Schwegler H. Early postnatal corticosterone administration regulates neurotrophins and their receptors in septum and hippocampus of the rat. Exp Brain Res. 2004;154:183–91. doi: 10.1007/s00221-003-1656-5. [DOI] [PubMed] [Google Scholar]
- 32.Malamitsi-Puchner A, Economou E, Rigopoulou O, Boutsikou T. Perinatal changes of brain-derived neurotrophic factor in pre- and fullterm neonates. Early Hum Dev. 2004;76:17–22. doi: 10.1016/j.earlhumdev.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 33.Nikolaou KE, Malamitsi-Puchner A, Boutsikou T, Economou E, Boutsikou M, Puchner KP, et al. The varying patterns of neurotrophin changes in the perinatal period. Ann N Y Acad Sci. 2006;1092:426–33. doi: 10.1196/annals.1365.041. [DOI] [PubMed] [Google Scholar]
- 34.Marx CE, Vance BJ, Jarskog LF, Chescheir NC, Gilmore JH. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am J Obstet Gynecol. 1999;181:1225–30. doi: 10.1016/s0002-9378(99)70113-4. [DOI] [PubMed] [Google Scholar]


