Abstract
1. Objectives
Relative vasopressin deficiency, a contributor to vasodilatory septic shock may also be a cause of the vasodilatory state in liver disease. This study assesses endogenous vasopressin levels in patients with liver disease and their hemodynamic response to exogenous vasopressin.
2. Design
Prospective, observational study
3. Setting
Single center, tertiary hospital
4. Participants
Human subjects undergoing liver transplantation or major surgery
5. Interventions
Vasopressin levels were measured in 28 patients with liver disease undergoing liver transplantation and 7 control patients with normal liver function. Additionally intravenous vasopressin was given to 20 liver transplant recipients and the hemodynamic response was observed.
6. Measurements and Main Results
Patients with liver disease had significantly lower baseline vasopressin levels than controls (19.3 +/− 27.1 pg/mL versus 50.9 +/− 36.7 pg/mL, p=0.015). Patients with low vasopressin levels (• 20 pg/mL) were more likely to have low baseline mean blood pressure (• 80 mm Hg) than patients with high vasopressin levels (11 of 16 vs. 0 of 4, p=0.013). Systemic vascular resistance increased by 33% three minutes after intravenous vasopressin. Thirteen of 16 patients with low vasopressin levels compared to one of four patients with high vasopressin levels responded to exogenous vasopressin with an increase of mean blood pressure by more than 20% (p=0.028).
7. Conclusions
Patients with liver disease have lower vasopressin levels than controls and respond with a brisk vasoconstrictor response to exogenous vasopressin. Relative endogenous vasopressin deficiency may therefore contribute to vasodilatory shock in liver disease similar to what has been observed in septic shock
Keywords: hepatic cirrhosis, vasopressor, liver transplantation, vasopressin deficiency, vasoconstrictors, vasodilation
Introduction
End-stage liver disease causes arterial vasodilation despite high levels of endogenous catecholamines and angiotensin resulting in mal-distribution of blood flow and low perfusion pressure (1–4). This hemodynamic condition is similar to what has previously been observed during prolonged septic shock. Vasodilation in septic shock is exacerbated by relative vasopressin deficiency: endogenous levels of vasopressin are low in septic shock (5) and a low dose infusion of exogenous vasopressin that would otherwise have no effect on blood pressure in healthy subjects can restore pressure tone in these patients (6). In liver failure vasopressin analogues (terlipressin or ornipressin) are able to reverse hepatorenal syndrome and restore renal function (7, 8) similar to the effect of vasopressin in septic shock (9). Methylene blue has also been suggested as treatment for vasopressor resistant vasoplegia syndrome in liver transplantation (10) but should probably be reserved only for situations where other vasopressors failed to maintain adequate perfusion pressure.
Our study aims to evaluate the role of endogenous vasopressin in the vasodilatory state in liver disease. We tested the hypothesis that patients with end stage liver disease have lower baseline vasopressin levels when compared to patients with normal liver function. We further hypothesized that patients with end stage liver disease are more likely to be relative vasopressin deficient defined as low to normal baseline vasopressin levels (under 20 pg/mL) combined with low baseline blood pressure (mean arterial blood pressure < 80 mmHg) and a pronounced sensitivity to exogenous vasopressin (increase of mean arterial blood pressure by more than 20% as a response to an intravenous bolus of 3 units arginine-vasopressin) compared to patients with normal liver function.
Methods
Patients
The Institutional Review Board of Columbia University approved this study. We obtained informed signed consent on all patients who participated in this study.
All adult patients undergoing liver transplantation (cadaveric or living related) at Columbia-University Medical Center were eligible for inclusion. Adult patients undergoing Whipple operation or partial hepatectomies were included as controls since these patients also underwent major surgery comparable to liver transplantation but had normal preoperative liver function. We excluded patients with liver disease, abnormal liver function tests or abnormal coagulation tests from the control group.
Measurements
In all liver transplant patients a pulmonary artery catheter (with continuous cardiac output and mixed venous oxygen saturation measurement, Edwards Lifesciences, Irvine, CA, USA) was inserted per routine prior to surgery. Additionally an 18F gastric tonometry tube (Datex-Ohmeda, Madison, WI, USA) was inserted into the stomach to measure gastric mucosal carbon dioxide partial pressure (pCO2). None of the control patients received a pulmonary artery catheter or a gastric tonometry tube.
Anesthetic management
Anesthesia for all patients (liver transplants and controls) was induced with propofol or etomidate and succhinylcholine and maintained with fentanyl and sevoflurane. Muscle relaxation was achieved by using cisatracurium. There was no significant difference in the groups (responders and non-responders) with regard to the anesthetic technique or the amount of anesthetic drugs or gases given to the patient by the time we started the vasopressin bolus and infusion.
Vasopressin bolus and infusion
In 20 patients undergoing liver transplantation an intravenous bolus of 3 units vasopressin (8-arginine-vasopressin, Monarch Pharmaceuticals Inc., Bristol, TN, USA) was administered followed by a continuous intravenous infusion of 3 units/hour for 20 minutes at the end of the dissection phase. It is our clinical practice at Columbia University Medical Center to start a vasopressor infusion at this time-point in order to prepare the patient for the anhepatic phase and caval crossclamping. Eight patients undergoing liver transplantation did not receive vasopressin because they required vaoconstrictors for hypotension prior to the measurements (3 patients received vasopressin together with norepinephrine) or for logistical reasons (5 patients: the investigators were not available prior to the anhepatic phase of the liver transplant). None of the control patients received vasopressin.
Vasopressin levels (Radio immune assay - RIA)
Three ml arterial blood was drawn after induction of anesthesia prior to surgery. The blood was immediately spun at 2000g for 20 minutes at 4°C and the plasma was frozen at − 80 °C. Vasopressin levels were determined using a commercially available radio-immune assay kit (Alpco, Salem, NH, USA) according to the manufacturer’s instructions. The intra-assay precision of this test is 6.0%, interassay precision 9.9 % with an analytical sensitivity of 0.75 pg/mL and specificity of 1.3 pg/mL. The upper level of detection (undiluted) was 80 pg/ml (11). We defined any level above 80 pg/mL as 80 pg/mL for the purpose of statistical calculations.
Statistics
Values are presented as mean ± SD. Comparisons of paired variables were made either using a paired t-test for variables with normal distribution or Wilcoxon matched pairs test for variables without Gaussian distribution. Gaussian distribution was determined using the Kolmogorov-Smirnov test. Pearson’s correlation was used when evaluating the correlation of two continuous variables. To evaluate the hemodynamic response to vasopressin repeated measure one-way analysis of variance (ANOVA) and a post-hoc test for linear trend were used. P values were 2 tailed and p < 0.05 was considered significant.
We based our sample size analysis on our first hypotheses that that patients with end stage liver disease have lower baseline vasopressin levels when compared to patients with normal liver function. For the purpose of estimating the sample size we assumed that patients with end stage liver disease have a baseline vasopressin level of 10 pg/mL and patients with normal liver function a baseline vasopressin level of 20 pg/mL with a common standard deviation of 5 pg/mL. Setting an alpha = 0.05 we would require 4 patients in each group to achieve a power (1-beta) = 0.8. We enrolled 7 controls to compensate for potential problems with the measurements and 20 subjects to adequately address hypothesis 2. SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and Graphpad Prism 4.0 (San Diego, CA, USA) software was used for the statistical analysis.
Results
After obtaining informed, signed consent 28 patients undergoing liver transplantations and 7 control patients (2 Whipple operations and 5 partial hepatectomies) were enrolled. The demographic information is listed in Table 1.
Table 1.
Patient Characteristics
| OLT n = 28 |
Control n = 7 |
p value | |
|---|---|---|---|
| Preoperative | |||
| Female - n (%) | 10 (35.7 %) | 5 (71.4 %) | ns |
| Age – mean ± SD [years] | 52.9 +/− 12.5 | 52.0 +/− 8.0 | ns |
| MELD – mean ± SD | 17.8 +/− 9.0 | 8.7 +/− 3.3 | < 0.05 |
| BMI– mean ± SD | 27.1 +/− 5.8 | 25.9 +/− 4.9 | ns |
| Creatinine – mean ± SD [mg/dL] | 1.19 +/− 1.19 | 0.87 +/− 0.45 | ns |
| Total Bilirubin – mean ± SD [mg/dL] | 6.6 +/− 9.3 | 0.9 +/− 0.8 | < 0.005 |
| INR | 3.9 +/− 11.9 | 1.04 +/− 0.14 | ns |
| Albumin | 3.94 +/− 5.15 | 4.14 +/− 0.49 | ns |
| Ascites - n (%) | 13 (46.4 %) | 0 | ns |
| LRLT - n (%) | 6 (21.4 %) | - | ns |
| Indication for Surgery | |||
| Hepatitis C -n (%) | 16 (57.1 %) | 0 | |
| ETOH -n (%) | 3 (10.7 %) | 0 | |
| PSC –n (%) | 4 (14.3 %) | 0 | |
| HCC -n (%) | 9 (32.1 %) | 1 (14.3 %) | |
| Others -n (%) | 5 (17.9 %) | 2 (28.6 %) | |
| Pancreatic Ca -n (%) | - | 3 (42.9 %) | |
| Living liver donor -n (%) | - | 1 (14.3 %) | |
| Intraoperative | |||
| Length of anesthesia – mean ± SD [hours] | 11.2 +/− 2.5 | 9.2 +/− 3.4 | ns |
| Re-operation - n (%) | 2 (7.1 %) | 0 | ns |
| PRBC – mean ± SD [Units] | 14.1 +/− 4.8 | 4.4 +/− 5.6 | ns |
| FFP – mean ± SD [Units] | 15.4 +/− 14.7 | 2.9 +/− 4.2 | < 0.05 |
MELD- Model for End-stage Liver Disease score
BMI - Body mass index
INR - International normalized ratio
LRLT – Living related liver transplant
ETOH – alcohol induced hepatic cirrhosis
PSC – Primary sclerosing cholangitis
HCC - Hepato-cellular carcinoma
PRBC - number of packed red blood cells used intraoperatively
FFP – number of fresh frozen plasma used intraoperatively
Baseline vasopressin levels
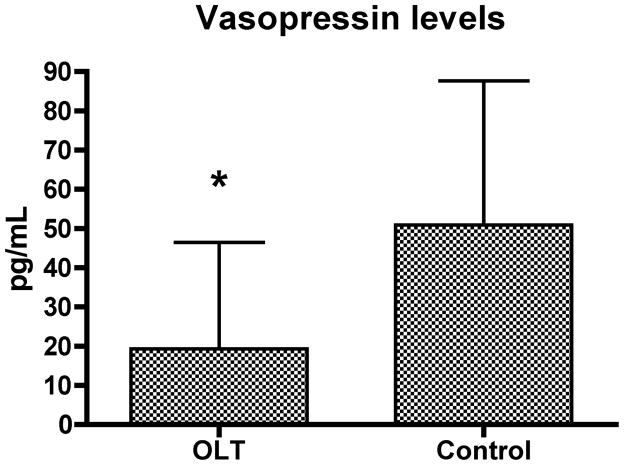
Eight patients had vasopressin levels above the level of detection of 80 pg/mL [4 of 28 liver transplant recipients (14.3 %) and 4 of 7 controls (57.1 %), p=0.01]. Patients receiving liver transplants had significantly lower vasopressin levels than control patients: (19.3 +/− 27.1 pg/mL versus 50.9 +/− 36.7 pg/mL, p=0.015; Figure 1).
Figure 1.
Baseline vasopressin levels in patients with liver disease undergoing liver transplantations (OLT, n=28) and control patients with normal liver function undergoing hepatectomies or Whipple procedures (n=7), (mean +/− SD, * − p< 0.05). Four OLT and 4 control patients had baseline vasopressin levels above the detection limit of 80 pg/mL and their levels were defined as 80 pg/mL.
Hemodynamics before and after vasopressin
Eight liver transplant patients did not receive vasopressin as per protocol because they either required a vasopressor (norepinephrine and vasopressin infusion) prior to initiation of the protocol (3 subjects) or for logistical reasons (5 subjects). None of the control patients received vasopressin because the responsible anesthesiologists did not consider the administration of vasopressin to be clinically indicated.
Baseline hemodynamics
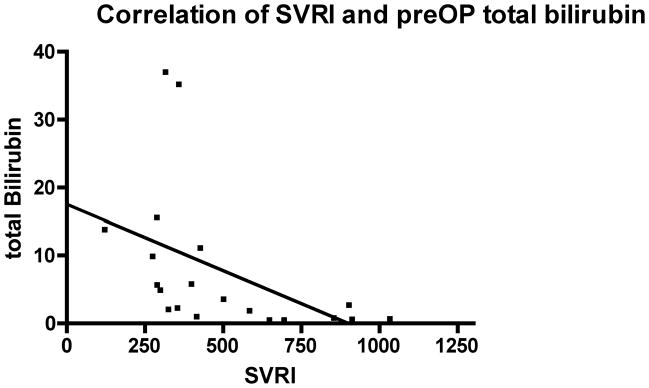
Baseline hemodynamic variables were recorded 3 minutes prior to initiation of vasopressin. The mean blood pressure, heart rate, pulmonary artery pressure, central venous pressure, cardiac output and systemic vascular resistance are depicted in Table 2. Systemic vascular resistance index were inversely correlated with preoperative MELD score (Pearson r = − 0.567, p < 0.01) and with preoperative total bilirubin (Pearson r = − 0.469, p < 0.05); Figures 2 and 3.
Table 2.
Hemodynamic variables before and after vasopressin (3 units bolus followed by 3 units/hour) in all patients (responders and non-responders) with end-stage liver disease undergoing liver transplantation who received vasopressin (p-value: compared to baseline values)
| Baseline | 0 min | 1 min | 3 min | 5 min | 10 min | 15 min | 20 min | ANOVA Post-test | |
|---|---|---|---|---|---|---|---|---|---|
| Pulse -/min | 76 +/− 18 | 75 +/− 16 ns |
75 +/− 16 ns |
71 +/− 14 p=0.002 |
71 +/− 14 p=0.001 |
73 +/− 15 p=0.03 |
75 +/− 16 ns |
75 +/− 16 ns |
p<0.0001 ns |
| Syst. BP - mmHg | 111 +/− 18 | 113 +/− 17 ns |
129 +/− 23 p=0.0001 |
141 +/− 27 p=0.0001 |
134 +/− 24 p=0.0001 |
124 +/− 21 p=0.0007 |
122 +/− 22 p=0.008 |
119 +/− 23 ns |
p<0.0001 p=0.03 |
| Mean BP – mmHg | 80 +/− 14 | 81 +/− 13 ns |
94 +/− 17 p=0.0001 |
102 +/− 19 p=0.0001 |
97 +/− 17 p=0.0001 |
88 +/− 15 p=0.003 |
88 +/− 14 p=0.01 |
87 +/− 15 ns |
p<0.0001 p=0.01 |
| Diast. BP – mmHg | 59 +/− 12 | 60 +/− 12 ns |
70 +/− 16 p=0.0005 |
76 +/− 16 p=0.0001 |
71 +/− 14 p=0.0001 |
66 +/− 12 p=0.004 |
65 +/− 11 p=0.01 |
65 +/− 12 p=0.03 |
p<0.0001 p=0.03 |
| Syst. PAP – mmHg | 28 +/− 6 | 29 +/− 6 p=0.03 |
31 +/− 7 p=0.002 |
35 +/− 9 p=0.0001 |
34 +/− 8 p=0.0001 |
32 +/− 8 p=0.007 |
31 +/− 9 ns |
29 +/− 9 ns |
p<0.0001 ns |
| Mean PAP – mmHg | 20 +/− 5 | 21 +/− 6 ns |
22 +/− 7 p=0.001 |
26 +/− 7 p=0.0001 |
26 +/− 6 p=0.0001 |
23 +/− 7 p=0.0001 |
22 +/− 7 ns |
21 +/− 8 ns |
p<0.0001 p=0.01 |
| Diast PAP – mmHg | 14 +/− 5 | 15 +/− 5 ns |
16 +/− 6 p=0.01 |
19 +/− 5 p=0.0001 |
19 +/− 6 p=0.0001 |
17 +/− 5 p=0.0008 |
16 +/− 6 ns |
15 +/− 6 ns |
p<0.0001 p=0.005 |
| CVP – mmHg | 9.6 +/− 4.3 | 10.2 +/− 4.5 ns |
12.2 +/− 3.5 p=0.002 |
12.9 +/− 4.5 p=0.0001 |
13.3 +/− 4.9 p=0.0001 |
12.3 +/− 4.6 p=0.0001 |
11.3 +/− 4.7 p=0.01 |
10.9 +/− 5.5 p=0.03 |
p<0.0001 p<0.0001 |
| CI - L/min/m2 | 3.55 +/− 1.27 | 3.2 +/− 1.2 ns |
3.6 +/− 1.4 ns |
3.5 +/− 1.4 ns |
3.4 +/− 1.3 ns |
3.4 +/− 1.4 ns |
3.6 +/− 1.6 ns |
3.5 +/− 1.5 ns |
ns |
| SVRI - dyn·s/cm5/m2 | 499 +/− 257 | 552 +/− 273 ns |
591 +/− 347 p=0.002 |
685 +/− 350 p=0.0005 |
668 +/− 363 p=0.001 |
615 +/− 379 p=0.02 |
588 +/− 417 ns |
622 +/− 412 p=0.048 |
p<0.0001 p<0.0001 |
| MAP/mPAP ratio | 4.4 +/− 1.5 | 4.4 +/− 1.2 ns |
4.2 +/− 1.1 ns |
4.0 +/− 1.1 ns |
4.1 +/− 1.2 ns |
4.2 +/− 1.4 ns |
4.5 +/− 1.5 ns |
4.9 +/− 2.0 ns |
p=0.016 ns |
| SvO2 - % | 84.1 +/− 8.4 | 84.1 +/− 6.9 ns |
86.0 +/− 6.6 ns |
85.2 +/− 6.9 ns |
84.3 +/− 8.3 ns |
84.1 +/− 8.3 ns |
84.5 +/− 8.8 ns |
82.6 +/− 10.1 ns |
ns |
| g-pCO2 - mmHg | 43.8 +/− 8.4 | 43.1 +/− 8.6 ns |
41.9 +/− 8.1 ns |
43.2 +/− 7.7 ns |
44.1 +/− 8.1 ns |
46.1 +/− 9.8 p=0.003 |
45.8 +/− 10.2 p=0.006 |
46.8 +/− 10.0 p=0.005 |
p<0.0001 p<0.0001 |
Abbreviations:
Min - minutes
BP – blood pressure
Syst. – systolic blood pressure
Diast. – diastolic blood pressure
CVP – central venous pressure
CO – cardiac output
CI – cardiac index
SVR – systemic vascular resistance
SVRI - systemic vascular resistance index
MAP/mPAP ratio – ratio of mean arterial pressure and mean pulmonary artery pressure
SvO2 – mixed venous oxygen saturation
g-pCO2 –gastric mucosal carbon dioxide partial pressure
ANOVA –repeated measure analysis of variance
Post test - post-hoc test for linear trend
Figure 2.
Correlation of baseline systemic vascular resistance index (SVRI) with preoperative (preOP) Model of Endstage Liver Disease (MELD) score; Pearson r = − 0.567, p < 0.01
Figure 3.
Correlation of baseline systemic vascular resistance index (SVRI) with preoperative (preOP) total bilirubin; Pearson r = − 0.469, p < 0.05
Hemodynamic response to vasopressin
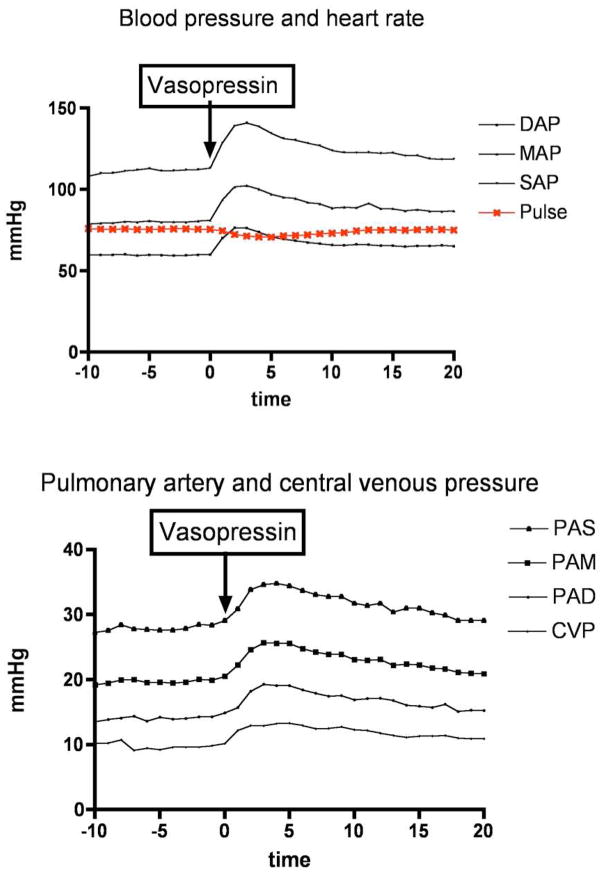
Vasopressin was given as per protocol at the end of the dissection phase 4.6 +/− 1.2 hours (mean +/− SD) after induction of anesthesia. One minute after the vasopressin bolus and start of the vasopressin infusion blood pressure, central venous pressure and pulmonary artery pressure increased significantly compared to baseline values and peaked 3 minutes after initiation of vasopressin. Systemic vascular resistance increased three minutes after infusion of vasopressin (peak increase of 33%). Blood, central venous and pulmonary artery pressures remained elevated until approximately 15 minutes later. Mean arterial pressure to mean pulmonary artery pressure did not change significantly when using t-tests but the repeated measure ANOVA demonstrated a significant difference (p = 0.16) of this ratio (but no significant linear trend). Mixed venous oxygen saturation and cardiac output did not change during the 20 minutes observation period. Gastric mucosal pCO2 increased significantly 10 and 20 minutes after vasopressin bolus and infusion (from 44 +/− 9 mm Hg to 46 +/− 10 mm Hg, p<0.005 and 47 +/− 10 mm Hg, p<0.005 respectively). Table 2 and figure 4.
Figure 4.
Hemodynamic response after vasopressin (3 units bolus followed by 3 units/hour) in patients with liver disease undergoing liver transplantation (DAP - diastolic arterial pressure, MAP mean arterial pressure, SAP – systolic arterial pressure, PAS – pulmonary artery systolic pressure, PAM – pulmonary artery mean pressure, PAD – pulmonary artery diastolic pressure, CVP – central venous pressure)
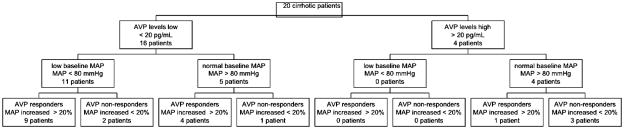
Eleven of 16 liver transplant patients with low vasopressin levels (≤ 20 pg/mL) had a low baseline mean arterial blood pressure (≤ 80 mm Hg) compared to none of the four patients with high vasopressin levels (p=0.013). Thirteen of 16 patients with low vasopressin levels (≤ 20 pg/mL) compared to only one of four patients with high vasopressin levels (> 20 pg/mL) responded to exogenous vasopressin with an increase of mean arterial pressure by more than 20% after 3 minutes (p=0.028). (Figure 5)
Figure 5.
Distribution of patients with and without low baseline vasopressin (AVP) level (< 20 pg/mL), comparing low versus normal baseline mean arterial blood pressure (MAP) and if patients responded with an increase of mean arterial blood pressure by more than 20% three minutes after exogenous vasopressin was given (AVP responders versus AVP non responders)
Discussion
This study demonstrated that patients with liver disease have low endogenous vasopressin levels; the degree of liver injury (MELD score and total bilirubin) correlated with the extent of systemic vasodilation. We further showed that exogenous intravenous vasopressin increased blood pressure through an increase of systemic vascular resistance while pulmonary artery pressure increased concomitant with an increase of central venous pressure. We could not conclusively demonstrate if this increase of pulmonary artery pressures is due to (non significant) increases of cardiac output or due to direct pulmonary vasoconstriction since we did not measure pulmonary capillary wedge pressures and calculate pulmonary vascular resistance. We did not want to expose coagulopathic patients to increased risk of pulmonary artery injury associated with measuring pulmonary capillary wedge pressures. However there was no change in the ratio of mean arterial pressure to mean pulmonary artery pressure when comparing the values with baseline using student t-tests and no linear trend when using a post-hoc test of the ANOVA.
Patients with low baseline vasopressin levels were more likely to have a low baseline blood pressure and were more likely to respond pressure to exogenous vasopressin with an increase of mean arterial than patients with high baseline vasopressin levels. Low blood pressure may therefore be a clinical indicator for vasopressin deficiency and warrant a trial of exogenous vasopressin in patients with liver disease.
In septic shock vasopressin plasma levels are lower than what would be considered appropriate considering the vasodilatory state (5, 12). As a consequence of low vasopressin levels in septic shock patients develop a profound sensitivity to low dose exogenous vasopressin (and decreased catecholamine sensitivity) and respond with restoration of the vasomotor tone through stimulation of V-1 vasopressor receptors (9). Furthermore KATP channels in vascular smooth muscle that open in vasoplegic shock and cause hyperpolarization of the smooth muscle cell are inactivated by vasopressin. Vasopresin also blunts nitric oxide induced increases in cyclic guanosine monophosphate (cGMP) (13). These mechanisms cause a restoration of catecholamine sensitivity and arterial vascular tone in vasoplegic shock. Normal volunteers with regular vasomotor tone on the other hand do not exhibit an increase of blood pressure when given low dose vasopressin (14–16).
Our study indicates a similar vasopressin deficiency in liver disease. Baseline vasopressin levels were low in cirrhotic patients and these patients responded with a substantial increase in systemic vascular resistance to exogenous vasopressin. Patients with low vasopressin levels had lower baseline mean arterial pressures and were more likely to respond with an increase of mean arterial pressure to exogenous vasopressin.
In a previous study we demonstrated that vasopressin directly decreases portal vein flow and pressure in patients undergoing liver transplantation, a consequence of splanchnic vasoconstriction (17). In that study we did not observe a statistically significant increase of blood pressure after vasopressin but we then did not give a bolus of vasopressin but only a continuous infusion. This may not be sufficient dose in cirrhotic patients with an altered volume of distribution to achieve adequate plasma levels of vasopressin and increase blood pressure, higher doses may be required to cause sustained increases of blood pressure similar to what we now observed in our current study shortly after a bolus. The difference of baseline levels of vasopressin in the two studies is likely due to the different assays used to measure vasopressin levels (ELISA in the previous study with a mean +/− SD baseline vasopressin = 8.3 +/− 10.5 pg/mL compared RIA in the current study with a mean vasopressin level = 19.3 +/− 27.1 pg/mL).
We observed an increase of gastric mucosal pCO2 as evidence of decreased splanchnic perfusion caused by vasopressin. It would however take much longer than the 20 minutes observation period to observe changes in pH, base excess or lactic acid production. Further clinical studies are required to evaluate if decreasing splanchnic perfusion to counteract the splanchnic hyperemia associated with liver disease (18, 19) is beneficial in patients with liver disease. Future studies will also need to compare the hemodynamic response and effect on splanchnic perfusion of vasopressin with other vasopressors such as phenylephrine or norepinephrine.
We did not give vasopressin to the control patients with normal liver function because we could not justify giving vasopressin to these subjects without any potential benefit and potential harm. However previous studies have shown that vasopressin has no effect on blood pressure in healthy subjects who are not vasodilated (20, 21) and we must assume that our control subject would have likely not responded with an increase of blood pressure to exogenous vasopressin.
In summary this study indicates that relative vasopressin deficiency and increased vasopressin sensitivity may be a cause for systemic vasodilation in liver disease. Further research will be required to elucidate the role of endogenous vasopressin deficiency in liver disease and the potential effect of vasopressin therapy on outcome in hepatic cirrhosis.
Acknowledgments
Funding:
This work was funded by the intramural grant support from the Department of Anesthesiology, Columbia University College of Physicians and Surgeons, New York, NY, USA
The project described was supported by Grant Number UL1 RR024156 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Information on NCRR is available at NCRR Website. Information on Re-engineering the Clinical Research Enterprise can be obtained from NIH Roadmap website.
Footnotes
Disclosures:
None of the authors have conflicts of interest to disclose
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Gebhard Wagener, Email: gw72@columbia.edu.
Galina Kovalevskaya, Email: gk49@columbia.edu.
Moury Minhaz, Email: mm3597@columbia.edu.
Fallon Mattis, Email: fam2103@columbia.edu.
Jean C. Emond, Email: je111@columbia.edu.
Donald W. Landry, Email: dwl1@columbia.edu.
References
- 1.Henriksen JH, Moller S. Liver cirrhosis and arterial hypertension. World J Gastroenterol. 2006;12:678–685. doi: 10.3748/wjg.v12.i5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrier RW, Arroyo V, Bernardi M, Epstein M, Henriksen JH, Rodes J. Peripheral arterial vasodilation hypothesis: a proposal for the initiation of renal sodium and water retention in cirrhosis. Hepatology. 1988;8:1151–1157. doi: 10.1002/hep.1840080532. [DOI] [PubMed] [Google Scholar]
- 3.Knotek M, Rogachev B, Schrier RW. Update on peripheral arterial vasodilation, ascites and hepatorenal syndrome in cirrhosis. Can J Gastroenterol. 2000;14 (Suppl D):112D–121D. doi: 10.1155/2000/340128. [DOI] [PubMed] [Google Scholar]
- 4.Nicholls KM, Shapiro MD, Van Putten VJ, Kluge R, Chung HM, Bichet DG, Schrier RW. Elevated plasma norepinephrine concentrations in decompensated cirrhosis. Association with increased secretion rates, normal clearance rates, and suppressibility by central blood volume expansion. Circ Res. 1985;56:457–461. doi: 10.1161/01.res.56.3.457. [DOI] [PubMed] [Google Scholar]
- 5.Sharshar T, Blanchard A, Paillard M, Raphael JC, Gajdos P, Annane D. Circulating vasopressin levels in septic shock. Crit Care Med. 2003;31:1752–1758. doi: 10.1097/01.CCM.0000063046.82359.4A. [DOI] [PubMed] [Google Scholar]
- 6.Landry DW, Levin HR, Gallant EM, Seo S, D’Alessandro D, Oz MC, Oliver JA. Vasopressin pressor hypersensitivity in vasodilatory septic shock. Crit Care Med. 1997;25:1279–1282. doi: 10.1097/00003246-199708000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Martin-Llahi M, Pepin MN, Guevara M, Diaz F, Torre A, Monescillo A, Soriano G, et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 8.Fabrizi F, Dixit V, Messa P, Martin P. Terlipressin for hepatorenal syndrome: A meta-analysis of randomized trials. Int J Artif Organs. 2009;32:133–140. [PubMed] [Google Scholar]
- 9.Holmes CL, Walley KR, Chittock DR, Lehman T, Russell JA. The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. 2001;27:1416–1421. doi: 10.1007/s001340101014. [DOI] [PubMed] [Google Scholar]
- 10.Fischer GW, Bengtsson Y, Scarola S, Cohen E. Methylene blue for vasopressor-resistant vasoplegia syndrome during liver transplantation. J Cardiothorac Vasc Anesth. 2010;24:463–466. doi: 10.1053/j.jvca.2008.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Smith SW, Howland MA, Hoffman RS, Nelson LS. Acetaminophen overdose with altered acetaminophen pharmacokinetics and hepatotoxicity associated with premature cessation of intravenous N-acetylcysteine therapy. Ann Pharmacother. 2008;42:1333–1339. doi: 10.1345/aph.1K680. [DOI] [PubMed] [Google Scholar]
- 12.Wilson MF, Brackett DJ, Tompkins P, Benjamin B, Archer LT, Hinshaw LB. Elevated plasma vasopressin concentrations during endotoxin and E. coli shock. Adv Shock Res. 1981;6:15–26. [PubMed] [Google Scholar]
- 13.Landry DW, Oliver JA. The pathogenesis of vasodilatory shock. N Engl J Med. 2001;345:588–595. doi: 10.1056/NEJMra002709. [DOI] [PubMed] [Google Scholar]
- 14.Braunwald E, Wagner HN., Jr The pressor effect of the antidiuretic principle of the posterior pituitary in orthostatic hypotension. J Clin Invest. 1956;35:1412–1418. doi: 10.1172/JCI103398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Padfield PL, Brown JJ, Lever AF, Morton JJ, Robertson JI. Blood pressure in acute and chronic vasopressin excess: studies of malignant hypertension and the syndrome of inappropriate antidiuretic hormone secretion. N Engl J Med. 1981;304:1067–1070. doi: 10.1056/NEJM198104303041803. [DOI] [PubMed] [Google Scholar]
- 16.Padfield PL, Brown JJ, Lever AF, Morton JJ, Robertson JI. Changes of vasopressin in hypertension: Cause or effect? Lancet. 1976;1:1255–1257. doi: 10.1016/s0140-6736(76)91734-7. [DOI] [PubMed] [Google Scholar]
- 17.Wagener G, Gubitosa G, Renz J, Kinkhabwala M, Brentjens T, Guarrera JV, Emond J, et al. Vasopressin decreases portal vein pressure and flow in the native liver during liver transplantation. Liver Transpl. 2008;14:1664–1670. doi: 10.1002/lt.21602. [DOI] [PubMed] [Google Scholar]
- 18.Michielsen PP, Pelckmans PA. Haemodynamic changes in portal hypertension: new insights in the pathogenesis and clinical implications. Acta Gastroenterol Belg. 1994;57:194–205. [PubMed] [Google Scholar]
- 19.Gatta A, Bolognesi M, Merkel C. Vasoactive factors and hemodynamic mechanisms in the pathophysiology of portal hypertension in cirrhosis. Mol Aspects Med. 2008;29:119–129. doi: 10.1016/j.mam.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Wall BM, Huch KM, Runyan KR, Williams HH, Gavras H, Cooke CR. Effects of vasopressin V1-receptor blockade during acute and sustained hypovolemic hypotension. Am J Physiol. 1996;270:R356–364. doi: 10.1152/ajpregu.1996.270.2.R356. [DOI] [PubMed] [Google Scholar]
- 21.Huch KM, Wall BM, Mangold TA, Bobal MA, Cooke CR. Hemodynamic response to vasopressin in dehydrated human subjects. J Investig Med. 1998;46:312–318. [PubMed] [Google Scholar]