Abstract
Background
Matrix metalloproteinases (MMPs) are known to modulate left ventricular (LV) remodeling after a myocardial infarction (MI). However, the temporal and spatial variation of MMP activation and their relationship to mechanical dysfunction post MI remains undefined.
Methods and Results
MI was surgically induced in pigs (n=23) and cine MR and dual isotope hybrid SPECT/CT imaging obtained using thallium-201 (201Tl) and a technetium-99m labeled MMP targeted tracer (99mTc-RP805) at 1, 2 and 4 weeks post MI along with controls (n=5). Regional myocardial strain was computed from MR images and related to MMP zymography and ex vivo myocardial 99mTc-RP805 retention. MMP activation as assessed by in vivo and ex vivo 99mTc-RP805 imaging/retention studies was increased nearly 5-fold within the infarct region at 1 week post-MI and remained elevated up to 1 month post-MI. The post-MI change in LV end-diastolic volumes was correlated with MMP activity (y=31.34e0.48x, p=0.04). MMP activity was increased within the border and remote regions early post-MI, but declined over 1 month. There was a high concordance between regional 99mTc-RP805 uptake and ex vivo MMP-2 activity.
Conclusions
A novel, multimodality non-invasive hybrid SPECT/CT imaging approach was validated and applied for in vivo evaluation of MMP activation in combination with cine MR analysis of LV deformation. Increased 99mTc-RP805 retention was seen throughout the heart early post-MI and was not purely a reciprocal of 201Tl perfusion. 99mTc-RP805 SPECT/CT imaging may provide unique information regarding regional myocardial MMP activation and predict late post-MI LV remodeling.
Keywords: MMP, LV remodeling, spatiotemporal imaging
Left ventricular (LV) remodeling following myocardia l infarction (MI) is characterized by infarct expansion, progressive LV dilation, hypertrophy of non-infarct zones, and overall global ventricular remodeling 1-4. Clinical and animal studies have demonstrated an association between the development of adverse LV remodeling after MI and poor outcomes 1, 2, 5, 6. The changes in anatomic structure or function associated with post-MI LV remodeling are routinely evaluated using imaging modalities including echocardiography, magnetic resonance (MR) imaging, cine x-ray computed tomography (CT), or even conventional nuclear based approaches, like single photon emission computed tomography (SPECT). Although changes in LV size and shape appear to be a direct measurement of remodeling, these indices do not provide information on the underlying molecular processes, which may then be used as surrogate markers of LV remodeling 1.
A cause-effect relationship has been established between LV remodeling and activation of the matrix metalloproteinases (MMPs) 7-12. However, defining the spatial and temporal variation of MMP activation post MI in relationship to LV remodeling has been difficult because of the lack of a direct in vivo approach to assess MMP activity. Past studies demonstrated the feasibility of targeted non-invasive imaging with a 99mTc-labeled MMP-targeted radiotracer (99mTc-RP805) for the in vivo detection of regional myocardial MMP activation following MI 13, 14. The current study extended these initial findings to a relevant pre-clinical post MI porcine model in order to test the central hypothesis that enhanced MMP activation occurs following MI, and is associated with altered regional myocardial deformation. The rationale for these studies is that defining the relationship between regional MMP activity and LV geometry and mechanics will be critical to better understand the mechanism underlying LV remodeling post-MI. Accordingly, the aims of the current study were two-fold: First, validate an MMP-targeted radiotracer approach for in vivo evaluation of MMP activation post-MI, comparing radiotracer derived indices of regional MMP activation with quantitative MMP zymography, and second, employ hybrid SPECT/CT imaging of MMP activation in combination with MR image derived indices of regional myocardial deformation to relate temporal changes in regional MMP activation with changes in regional myocardial deformation.
Methods
Surgical Preparation
MI was surgically induced in Yorkshire pigs (n = 23; male, 25kg) as previously described 9, 10. Briefly, MI was induced through the ligation of the first two obtuse marginal branches of the circumflex artery, which results in a uniform MI size involving ~22% of the LV in a region positioned on the posterior-lateral wall9, 10. Following a 7-10 day recovery period, pigs were randomly assigned to undergo cine 3-dimensional cardiac MR imaging and in vivo SPECT/CT imaging with euthanasia at 1 week (n= 9), 2 weeks (n = 6) or 4 weeks (n = 8) after MI. An additional group of non-operated controls (n=5) were used for reference imaging and biochemical studies. All experimental protocols were approved by Institutional Animal Care and Use Committees at the Medical University of South Carolina and the Yale University School of Medicine. Studies were performed according to the NIH guidelines for Care and Use of Laboratory Animals (1996).
Imaging Protocol
MR Imaging
Images were acquired on a Siemens Sonata 1.5T magnet using a multiple phase ECG triggered TrueFISP sequence (TR = 47.2ms, TE = 1.58ms, field of view = 340 mm, slice thickness = 5 mm, interslice gap = 1 mm, flip angle = 60°) 15. After orienting the acquisition plane along both long axes of the heart, contiguous 5 mm thick short axis images were obtained with an end-expiratory breath hold, achieved by transient disconnection of ventilator. The final image resolution was 1.5mm × 1.5mm × 5mm. The raw images were then converted to a 4D DICOM format.
MR Image Analysis
The MR images were semi-automatically segmented from below the membranous septum down to the apex from end diastole to end systole using a modified version of ITK-SNAP 16 and Bioimagesuite 17. Local shape properties of the endocardial and epicardial surfaces were used to derive 3-dimensional trajectories for a dense field of points on the surface of the heart over the cardiac cycle. These trajectories were then used to deform a mesh that represented the LV myocardial volume using a continuum bio-mechanics model. This integrated shape-based approach for tracking regional myocardial deformation has been previously validated in both 2- and 3-dimensions 17.
In vivo SPECT/CT Imaging and reconstruction
SPECT/CT images were acquired (GE Infinia, Hawkeye) ungated with a 360 degree acquisition orbit (32 stops/head, 20 seconds/stop), zoom factor was 1.3, and 128x128 matrix size. In vivo SPECT/CT imaging was performed at 112±14 minutes after intravenous injection of 99mTc-RP805 (25 mCi), a macrocyclic compound that binds to the catalytic domain on MMPs 13, thus being able to provide for localization of regions where MMPs are activated. This was followed by dual isotope imaging 15 minutes after intravenous injection of 201Tl (3 mCi). Images were obtained using 15% windows centered on the photopeaks of 201Tl (75±5% keV) and 99mTc (140±5% keV).
SPECT/CT transaxial images were reconstructed as described previously 14. The 201Tl images were used for reorientation of the heart into standard short and long axis views. 201Tl and 99mTc-RP805 images were batch reconstructed to assure exact orientation.
Ex vivo Imaging and Sampling
Immediately after in vivo SPECT/CT imaging, pigs were euthanized and the heart extracted to measure 201Tl and 99mTc radioactivity as previously described 14. Briefly, the hearts were filled with an alginate material and then cut into 5mm thick short axis slices. One slice containing the infarct region was divided into 8 epicardial and endocardial radial sectors and flash frozen in liquid nitrogen for subsequent MMP zymography. The remaining LV slices were placed on the collimator of a gamma camera (GE Millenium VG) and planar images acquired using the same energy peaks of 201Tl and 99mTc. These LV slices were also divided into 8 epicardial and endocardial radial sectors (16 total sectors) for gamma well counting. All reported values for regional myocardial 201Tl and 99mTc-RP805 retention were derived from gamma well counting of tissue samples. Absolute myocardial uptake was computed as a percentage of the injected dose (ID) per gram (%ID/gm) tissue after correcting for background activity and radioactive decay 14.The radial segments from the slice used for zymographic analysis were compared with the corresponding segments above and below those analyzed for radiotracer activity.
MMP Zymography, Immunoblotting, and Activity Assay
The radial sections were maintained at −80°C to allow for radioactive decay (~30 days), and then LV extracts prepared for MMP zymographic analysis 9, 10, 13, 18. Briefly, LV homogenates (2 μg protein) were subjected to electrophoresis using gels containing denatured collagen, and the proteolytic bands corresponding to proMMP-9 (92 kDa), proMMP-2 (72 kDa) and the active form of MMP-2 (64 kDa) were digitized. MMP-7 (primary antibody: Ab38996, Abcam) and MT1-MMP (Open Bio systems) levels were det ermined by immunoblotting 9, 18.
A specific global MMP fluorogenic substrate (Enzo Life Sciences BML-P126; excitation 330 nm; emission 405 nm) was utilized to measure MMP activity within the tissue homogenates 19, 20. Briefly, 30 μM of the fluorogenic MMP substrate was incubated with myocardial tissue extract (25 μg) and fluorescence of the cleaved substrate recorded (FLUOstar, BMG Labtech, Durham, NC). The fluorescence signal was converted to MMP activity (ng/g wet weight) based on a calibration curve determined using active MMP-2 catalytic domain (BML-SE237, Enzo Life Sciences) and the initial wet tissue weight of the homogenate.
Data Analysis
End-diastolic volumes were indexed to body mass and values at each post-MI time point were compared using analysis of variance (ANOVA) using time as the independent variable and adjusted for multiple comparisons using Dunnett’s test with the non-MI pigs being designated as the comparison control group. Selected mid-ventricular MR images were manually aligned and registered with the postmortem slices using structural features. The LV radial segments analyzed by zymography were aligned by sector with adjacent segments from the well counting. In order to develop a relationship between perfusion, MMP tracer uptake and MMP zymography, a 2-dimensional histogram was constructed. For each LV section, the MMP tracer values and zymographic values were normalized on a scale from 0-100 and these values assigned to each of the 16 sectors, and displayed in a concentric circle format for the endocardial and epicardial regions using a colorimetric registration. Regional 201Tl and 99mTc-RP805 uptake and MMP expression/activity were subjected to 2-way ANOVA, using time post-MI and LV region as independent factors. Given that the myocardial samples for each region were obtained from the same pigs, an adjustment for a within group effect was performed by including each pig as a random effect variable within the ANOVA design. Post-hoc separation of means was performed using Bonferroni-adjusted pairwise comparison of means (module prcomp, STATA). Measured 99mTc-RP805 activity was correlated with quantitative estimates of total MMP activity for each MMP subspecies derived from the zymography using standard curve fitting.
Average radial strain was the computed for the infarct, border and remote regions for each animal and compared between groups using ANOVA, which included animal as a random effect variable. Means for each region were analyzed using Welch’s t-test. Statistical analyses were performed using STATA (v8.0, College Station, TX) or BMDP (Statistical Solutions, Saugas, MA). Values are presented as Mean ± SEM. Values of p<0.05 were considered to be statistically significant.
Results
Post-MI Model
Animals demonstrated comparable hemodynamics during the serial MR and SPECT imaging. LV end-diastolic volumes indexed for body mass in the control pigs was 1.43±0.10 mL/kg. Consistent with LV remodeling post-MI 9, 10, LV end-diastolic volume index was increased by 59±8% over control values by 1 week post-MI (2.17±0.12 mL/kg, p<0.05), remained elevated over control values at 2 weeks post-MI (2.09±0.14 mL/kg, p<0.05), and was further increased by 4 weeks post-MI (2.24±0.11 mL/kg, p<0.05 vs. controls).
Dual-Isotope In Vivo SPECT/CT Imaging and Ex Vivo Planar Imaging
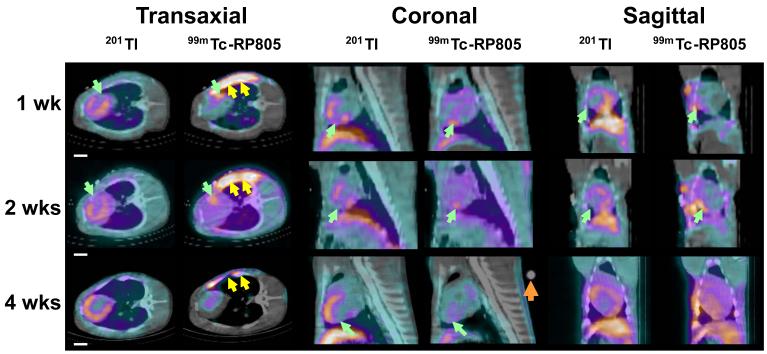
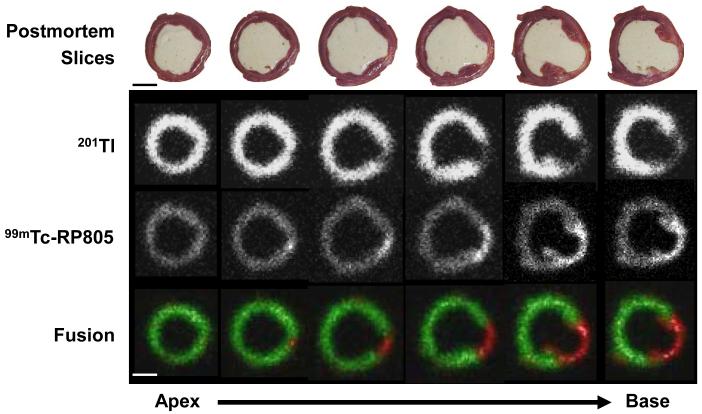
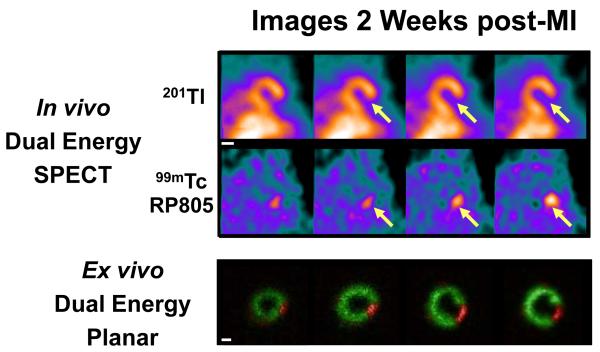
In vivo dual isotope (99mTc-RP805 and 201Tl) SPECT-CT images from representative MI pigs at one week, two weeks and four weeks post MI show a 201Tl perfusion defect in the posterolateral wall with corresponding focal 99mTc-RP805 uptake within the perfusion defect (Figure 1A). The maximal 99mTc-RP805 uptake was observed at 2 week post-MI. Ex vivo planar dual energy imaging of LV slices confirmed the co-localization of the perfusion defect and the region of maximal increase in MMP tracer uptake (Figure 1B), although 99mTc-RP805 uptake was also seen in remote regions at 1 and 2 weeks post-MI. There was an excellent correspondence between the in vivo and ex vivo images (Figure 1C).
Figure 1.
Dual-isotope in vivo SPECT/CT imaging and ex vivo planar imaging. (A) In vivo 201Tl and 99mTc-RP805 SPECT-CT images of pigs at 1 week, 2 weeks and 4 weeks post MI are shown in transaxial, coronal and sagittal views. Note the perfusion defect in the lateral wall and the time dependent changes in the intensity of 99mTc-RP805 retention in the same regions (green arrows). The yellow double arrows point to 99mTc-RP805 activity in the surgical sternal wound. A known point source (orange arrow) can be used to quantify hotspot uptake. Scale bars: 2cm. (B) Postmortem short axis myocardial slices filled with alginate (top row) are shown from a representative pig at one week post-MI. Slices are oriented with the anterior wall on top. Below are the corresponding ex vivo short axis 201Tl perfusion images (second row), and 99mTc-RP805 images (third row), and color coded fused images (201Tl – green; 99mTc-RP805 – red). The infarct can be seen as a thinned fibrotic area in the lateral wall of the four most basal slices. The fusion image clearly demonstrates maximal uptake of 99mTc-RP805 in the infarct region. Scale bars: 2cm. (C) In vivo SPECT short axis images and ex vivo dual isotope planar slice images from a pig at 2 weeks post MI. There is excellent correlation between the 201Tl perfusion defect and the 99mTc-RP805 retention, which is seen on both in vivo and ex vivo images. Scale bars: 2cm.
Gamma Well Counting of 201Tl and 99mTc-RP805 Radioactivity
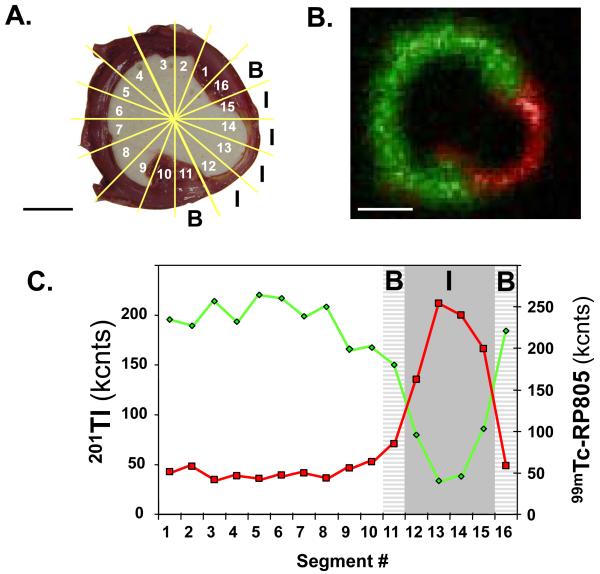
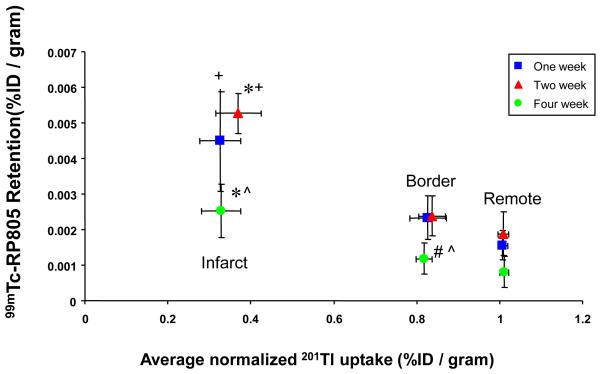
Myocardial segments were segregated into infarct, border, and remote regions based on the regional quantitative 201Tl activity from well counting as illustrated in Figure 2. Specifically, myocardial segments in which 201Tl uptake was lower than 60% of the remote region were designated as the infarct region and the two myocardial segments immediately adjacent to the infarct region (on either side of the infarct region) were designated as the border region. Time-dependent changes in 201Tl and 99mTc-RP805 retention are summarized in Table 1 and the relationship between 201Tl and 99mTc-RP805 activity in the infarct, border, and remote regions is shown in Figure 3. At one and two weeks post-MI, there was increased in 99mTc-RP805 retention in all myocardial regions, with over a 4-fold increase in 99mTc-RP805 activity within the infarct region compared to control pigs. By four weeks post MI, 99mTc-RP805 retention returned to control levels in the remote region, but remained higher than control levels in the infarct and border regions.
Figure 2.
Analysis of 201Tl and 99mTc-RP805 radioactivity. (A) Short axis slice through the infarct region of a pig at 1 week post MI showing sections (numbered) used for determination of 201Tl and 99mTc-RP805 uptake. B- border region, I – infarct region. (B) Ex vivo planar image of the same slice shows increased 99mTc-RP805 (red) retention in the 201Tl perfusion (green) defect in the infarct region located in the thinned inferolateral wall. Scale bar: 2cm. (C) Circumferential count profiles of corresponding well counting data shows increased 99mTc-RP805 activity in the region of diminished 201Tl activity. B- border region (light gray), I – infarct region (dark gray).
Table 1.
Regional and time-dependent changes in gamma well counting following myocardial infarction (MI). All data corrected for tissue weight (mg)
| Control | 1 Week MI | 2 Week MI | 4 Week MI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Remote | Border | MI | Remote | Border | MI | Remote | Border | MI | ||
| Perfusion (201TI, ×10−2) | ||||||||||
| ENDO | 107.3±2.9 | 110.0 ±1.8 | 100.7±2.1R | 46.9±4.8* RB | 109.4±1.9 | 94.0±5.2* R | 42.6±4.7* RB | 108.8±3.0 | 93.5±2.2* R | 41.9±3.4* RB |
| EPI | 95.7±2.1 | 88.9±2.2 | 83.2±3.1* | 40.0±3.3* RB | 86.9±2.8 | 94.6±6.9# | 46.7±6.3* RB | 94.6±2.4 | 83.7±2.7* R | 38.9±4.4* RB |
| TOTAL | 101.5±2.5 | 100.0 ±1.4 | 91.8±2.3* R | 43.2±2.3* RB | 98.2±2.2 | 92.3±3.2* | 47.2±5.4* RB | 101.7±1.4 | 90.9±1.1* R | 40.7±3.9* RB |
| MMP Tracer (99mTc-RP805, ×10−4) | ||||||||||
| ENDO | 11.3±2.5 | 13.7±1.3 | 16.6±2.1 | 40.0 ±5.3* RB | 15.6±1.9 | 17.9±2.4 | 52.8±8.5*# RB | 6.8±1.5 | 10.1±1.9 | 26.1±3.7*#$ RB |
| EPI | 11.6±2.1 | 14.9±1.6 | 19.4±2.2 | 38.6±4.7* RB | 17.7±2.1 | 21.3±1.7 | 42.5±7.7* RB | 7.6±1.5$ | 10.4±1.8#$ | 24.5±3.2*#$ RB |
| TOTAL | 11.5±2.3 | 13.8 ±1.5 | 18.3±2.0 | 39.7±4.8* RB | 16.7±1.9 | 21.3±1.7 | 42.5±7.8* RB | 7.2±1.5$ | 9.8±1.8#$ | 24.5±2.7*#$ RB |
| Sample Size (n) | 5 | 9 | 9 | 9 | 6 | 6 | 6 | 8 | 8 | 8 |
Values presented as Mean ± SEM.
p<0.05 vs.Control,
p<0.05 vs. 1 Week Post MI (Same region),
p<0.05 vs. 2 Week Post MI (Same region),
p<0.05 vs. Remote(same week post-MI),
p<0.05 vs. Border (same week post-MI)
Figure 3.
Gamma well counting of 201Tl and 99mTc-RP805 radioactivity. Correlation of the average relative myocardial 201Tl activity and 99mTc-RP805 activity for infarct, border and remote myocardial regions in pigs at 1, 2 and 4 weeks post-MI as determined by gamma well counting. Notice that the relative 201Tl perfusion deficit does not change over time, although there are dramatic time dependent changes in 99mTc-RP805 activity. These data suggest that 99mTc-RP805 imaging may provide unique information following myocardial infarction. *p<0.05 vs. remote. +p<0.05 vs. Border region. #p<0.05 vs. 1 week post-MI. ^p<0.05 vs. 2 week post-MI.
Comparison of 201Tl and 99mTc-RP805 Activity with MMP Zymography
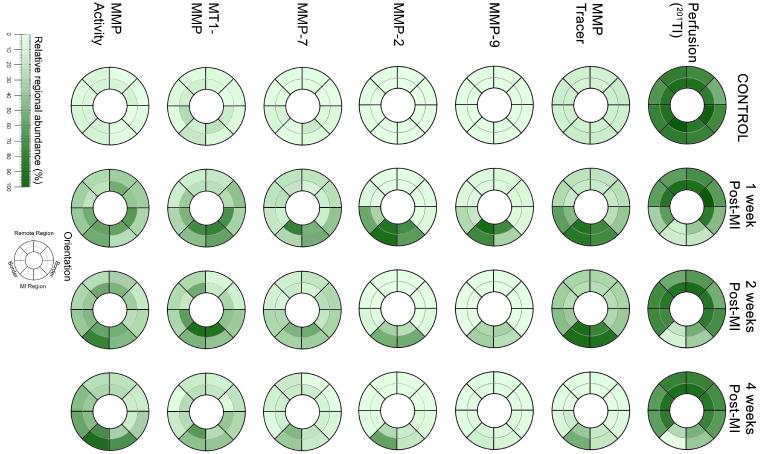
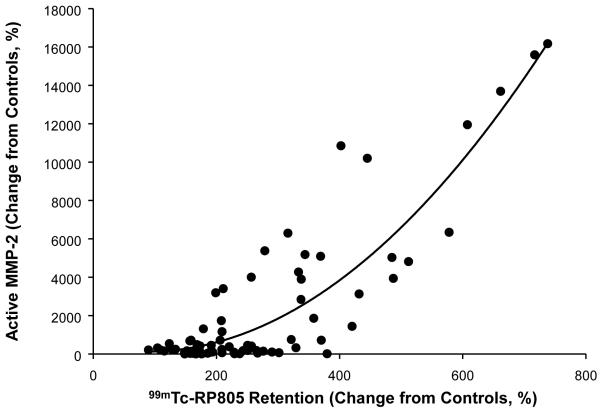
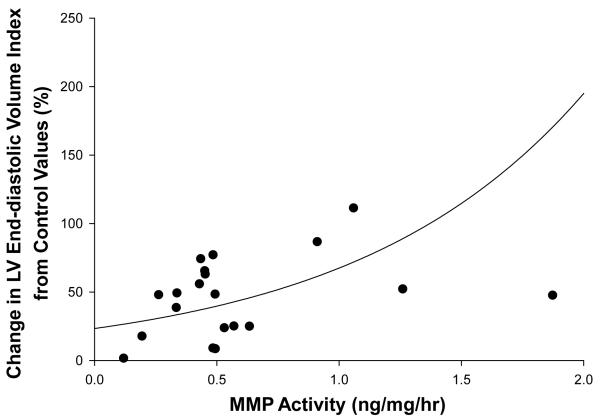
Changes in the levels of MMP2, MMP-7, MMP-9, and MT1-MMP and MMP activity are summarized in Table 2. There were distinct spatial and temporal patterns in the expression of the various MMP types in the post-MI period. For example, MMP-9 levels within the MI region were elevated in the earlier post-MI time points and then were lower than one week post-MI values at 4 weeks post-MI. MMP-7 and MT1-MMP levels within the border region were elevated at one week post-MI but were similar to control levels by 4 weeks post-MI. MMP activity, which was measured as a function of cleavage of a MMP substrate 20, was increased within the MI region at 1 week post-MI and was further elevated at 4 weeks post-MI. In order to provide a representation of the spatial-temporal changes in MMP targeted radiotracer retention and MMP zymographic levels, the normalized distribution of average values were computed for the endocardial and epicardial regions for each LV sector and are pictorially depicted in Figure 4. A clear spatial and temporal concordance was observed between the perfusion defect, 99mTc-RP805 retention, and MMP levels, particularly for the active form of MMP-2 (Figure 5). The segment by segment correlation coefficient between the active form of MMP-2 and absolute 99mTc-RP805 retention was excellent at one week post-MI (r = 0.78, p<0.01), although this relationship continued to declined between two weeks (r = 0.72) and 4 weeks (r = 0.59) post MI. There was an exponential relationship between the post-MI change in LV end-diastolic volume and MMP activity (y=31.34e0.48x, r: 0.38, p=0.04; Figure 6).
Table 2.
Regional and time-dependent changes in levels of matrix metalloproteinases (MMPs) following myocardial infarction (MI). All data corrected for tissue weight (mg)
| Control | 1 Week MI | 2 Week MI | 4 Week MI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Remote | Border | MI | Remote | Border | MI | Remote | Border | MI | ||
| MMP-9 | ||||||||||
| ENDO | 90±17 | 222 ±107 | 510 ±135 | 3543 ±1130* RB | 41±11 | 68±31 | 2210±854* RB | 142±78 | 344±148 | 750±465# |
| EPI | 98±40 | 326±154 | 574±203 | 2097±505* RB | 42±17 | 62±18 | 1704±1111* RB | 108±51 | 208±79 | 657±238# |
| TOTAL | 94±28 | 318±144 | 539±144 | 2596±596* RB | 42±13 | 200±86 | 1604±789*# RB | 125±63 | 213±93 | 617±271#$ |
| MMP-2 | ||||||||||
| ENDO | 43±9 | 107±13 | 462±129 | 3190±629* RB | 73±30 | 150±48 | 2541±651* RB | 209±124 | 735±284 | 1743±419*# RB |
| EPI | 63±25 | 132±23 | 571±217 | 3387±717* RB | 71±9 | 385±168 | 2674±1200* RB | 131±67 | 367±100 | 2550±626* RB |
| TOTAL | 53±16 | 119±15 | 535±145 | 3146±454* RB | 72±14 | 362±99 | 2275±918* RB | 170±95 | 434±126 | 2157±389*# RB |
| MMP-7 (×10-1) | ||||||||||
| ENDO | 1.5±0.2 | 8.1±3.0* | 15.9±9.6*R | 12.5±6.8* | 6.1±2.5* | 9.8±5.1* | 8.2±7.4* | 3.0±0.9 | 4.6±1.3 | 23.5±11.0RB |
| EPI | 1.7±0.2 | 4.1±2.2 | 4.5±1.4 | 3.2±1.3 | 3.0±1.3 | 3.9±1.0 | 5.3±2.4* | 2.2±0.7 | 1.2±0.7# | 14.2±8.6*#$ RB |
| TOTAL | 1.6±0.2 | 5.1±2.9* | 9.2±3.7* | 6.5±4.5* | 4.6±1.9 | 9.9±3.6* | 5.9±3.1* | 2.6±0.7 | 3.0±0.5 | 14.2±9.1*$ RB |
| MT1-MMP | ||||||||||
| ENDO | 11.1±1.2 | 22.5±4.0* | 27.1±5.3* | 34.0±6.4*R | 20.8±5.3* | 36.5±7.7*R | 36.2±8.5*R | 14.0±2.5 | 16.2±2.8 | 30.9±10.2*RB |
| EPI | 8.6±0.7 | 14.0±1.5* | 15.0±2.8* | 15.7±4.4* | 13.6±2.1 | 17.3±3.2* | 25.8±4.7*#R | 11.3±1.2 | 11.1±2.6 | 12.0±3.5$ |
| TOTAL | 9.9±0.7 | 18.0±2.1* | 21.5±3.1* | 26.2±4.2*R | 17.2±2.9* | 28.7±6.1* | 30.9±5.3*R | 12.6±1.4 | 13.7±1.9 | 19.5±5.0*R |
| MMP Activity (ng/mg/hr, ×10−1) | ||||||||||
| ENDO | 2.3±0.4 | 5.5 ±0.7 | 4.9 ±0.9 | 6.3±0.8* | 4.8±0.5 | 5.1±0.5 | 6.2±1.1 | 3.3±0.5 | 4.7±1.0 | 10.4±2.0*#$ RB |
| EPI | 2.0±0.3 | 4.2±0.2 | 3.9±0.4 | 5.2±0.6 | 3.4±0.4 | 4.3±0.5 | 7.4±1.3* R | 2.9±0.3 | 3.4±0.4 | 8.2±1.8*#$ RB |
| TOTAL | 2.2±0.3 | 4.8±0.4* | 4.3±0.6 | 5.6±0.6* | 4.1±0.3 | 4.8±0.4 | 6.6±1.0* | 3.1±0.2 | 3.9±0.6 | 9.3±1.7*#$ RB |
| Sample Size (n) | 5 | 9 | 9 | 9 | 6 | 6 | 6 | 8 | 8 | 8 |
Values presented as Mean ± SEM.
p<0.05 vs.Control,
p<0.05 vs. 1 Week Post MI (Same region),
p<0.05 vs. 2 Week Post MI (Same region),
p<0.05 vs. Remote(same week post-MI),
p<0.05 vs. Border (same week post-MI)
Figure 4.
Comparison of 201Tl and 99mTc-RP805 Activity with MMP Zymography. Shown are color coded spatial maps of relative myocardial 201Tl activity (top row), 99mTc-RP805 activity (second row), MMP-9 activity assessed by zymography (third row), total MMP-2 activity assessed by zymography (fourth row), MMP-7 levels (fifth row), MT1-MMP levels (sixth row), and MMP activity determined as a function of cleavage of a fluorescent substrate (seventh row). Images are oriented with the lateral wall on the right. Note the time dependent changes in regional 99mTc-RP805 activity correlate with spatial and temporal changes in MMP levels/activity as assessed by zymography. Reference color bar and an orientation grid are at the bottom.
Figure 5.
Relationship between in vivo and in vitro determination of MMP activity. In vivo MMP activity, determined as retention of 99mTc-RP805, was significantly related to in vitro changes in levels of active MMP-2 (y = 1687e0.033x, r=0.89, p<0.05).
Figure 6.
There was a significant relationship between the change in body mass indexed LV end-diastolic volume relative to control values and MMP activity within the MI region (y=31.34e0.48x, r=0.38, p=0.04).
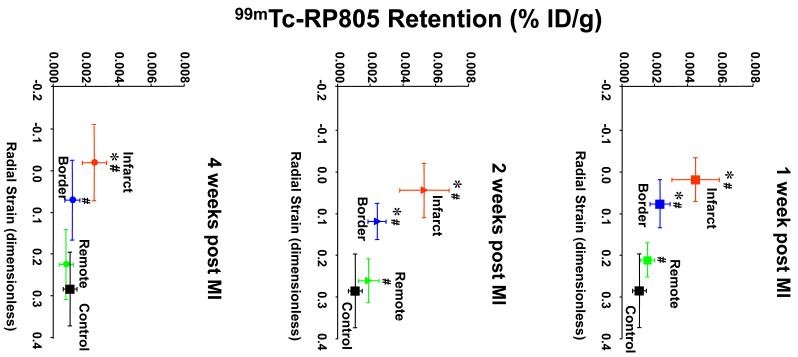
Comparison of 99mTc-RP805 Retention with Regional Myocardial Strain
The interaction between altered LV mechanics and MMP activation was assessed by comparing regional myocardial 99mTc-RP805 retention with regional myocardial thickening (radial strain) at different time points post-MI (Figure 7). At one week post MI, there was approximately a 4-fold increase in 99mTc-RP805 retention in the dyskinetic infarct region compared to referent control values. Although the border region initially demonstrated a similar degree of dysfunction to the infarct region with near zero radial strain, 99mTc-RP805 retention was much lower than in the central infarct region. There was also increased 99mTc-RP805 retention in the remote regions of the heart suggesting global activation of MMPs early post-MI associated with the global remodeling process. At two weeks post-MI, the infarct and border regions remained dysfunctional, and again a significant increase in 99mTc-RP805 retention was seen in all three regions. By four weeks post-MI, regional 99mTc-RP805 retention remained increased only in the infarct region which remained akinetic/dyskinetic.
Figure 7.
Comparison of 99mTc-RP805 retention with regional myocardial strain. The correlation of changes in regional 99mTc-RP805 activity and MR-derived regional radial strain for infarct, border, and remote regions for pigs at 1, 2, and 4 weeks post-MI is illustrated. There were complex time dependent changes in regional myocardial strain and MMP activation. Regional MMP activation appears to be dissociated from regional strain. *p< 0.05 strain vs. control and remote. #p < 0.05 RP805 vs. control. #p < 0.05 RP805 vs. 1 wk infarct.
Discussion
Animal models have demonstrated a clear cause and effect relationship between matrix metalloproteinase (MMP) activation and LV remodeling post MI 7-10, 12. In addition, clear changes in plasma MMP profiles occur in patients post-MI, which were related to indices of post-MI outcomes21-23. However, plasma profiling is a surrogate marker, and a direct relationship to myocardial MMP activation post-MI and to the LV remodeling process remains to be established. Thus, a critical translational research development would be to develop an approach for noninvasively detecting and quantifying in vivo MMP activity following MI. Accordingly, this study employed a combined molecular-mechanical approach to define the relationship between regional changes in MMP activity and regional mechanics in a well established and clinically relevant porcine model of post-MI remodeling. The significant and unique findings of this study were 2-fold. First, a validated MMP targeted radiotracer was utilized for in vivo evaluation of MMP activation, and allowed for comparing quantitative radiotracer-based indices of regional MMP activation with quantitative MMP zymography. Second, a hybrid SPECT/CT imaging approach for quantifying MMP activation was utilized in combination with MR derived myocardial strains to quantify the temporal and spatial variation of MMP activation with regional myocardial strain during the initial four weeks following MI. This non-invasive multi-modality approach demonstrated that early global activation of MMPs was associated with severe infarct and peri-infarct mechanical dysfunction and significant post-MI remodeling within the first month post-MI. Moreover, this study demonstrated that clinically applicable radiotracer-based imaging can be used to visualize and quantify myocardial MMP activation in the post-MI context.
Spatial and Temporal changes in 99mTc-RP805 retention in relationship to regional MMP activation
A past study from this laboratory using a rodent model of MI provided proof-of-concept that 99mTc-RP805 could be used to provide in vivo localization of myocardial MMP activation in the setting of LV remodeling post-MI 13. However, it must be recognized that significant differences exist with respect to processes that contribute to LV remodeling post-MI in rodents compared to humans 8, 11, 24. First, the portfolio of MMPs expressed in mice is notably different from that in larger mammalian species, including humans 24. Second, MI sizes in mice are often considerably larger than that in other larger mammalian species 7, 8, 12, which may activate a different set of MMPs during the progression of LV remodeling post-MI. Finally, given the high intrinsic heart rate in mice, it is technically difficult to determine relationships bet ween regional MMP act ivation and mechanical fu nction post-MI. Accordingly, the findings of the present study build upon those of the past rodent study by demonstrating that 99mTcRP805 may be effectively used to localize MMP activation in a clinically-relevant, large animal MI model, using a conventional clinical SPECT imaging system.
Changes in the myocardial levels of several MMP types occurs with distinct temporal trajectories in the post-MI period 24, 25. For example, MMP-9 levels achieve peak values early in the post-MI period and decline over longer durations 25. In contrast, the increase in MMP-2 levels is sustained over longer post-MI periods 25. Consistent with these past findings, levels of both MMP-2 and MMP-9 were increased within the infarct region at all post-MI time points. However, while the increase in MMP-2 levels was sustained through 4 weeks post-MI, there was a time-dependent decline in MMP-9 levels at the later post-MI time points. This observation is in agreement with a previous study that demonstrated temporal and spatial changes of MMP-2, MMP-8, MMP-13 and MT1-MMP levels in border, remote and infarct regions 18. In the present study, the greatest retention of the MMP-targeted radiotracer, 99mTc-RP805, was observed within the infarct region at one week post MI, which was correlated to changes in the levels of active MMP-2, MMP-7, MMP-9, and MT1-MMP at this early post-MI time point. Myocardial 99mTc-RP805 retention remained elevated within the infarct and border regions at two and four weeks post-MI and correlated with MMP-2 zymographic levels. The significance of these findings is two-fold: First, gamma well counting of 99mTc-RP805 demonstrates a complex spatial and temporal variation of MMP activity post MI. Second, the correlation between in vivo 99mTc-RP805 retention and ex vivo measurements of MMP levels provides validation for using targeted radiotracers as a means to longitudinally track fundamental molecular processes that contribute of LV remodeling post-MI.
Relationship between regional deformation and MMP activation
Mechanical dysfunction post MI has been hypothesized to cause increased MMP activation, potentially leading to further infarct expansion and exacerbation of LV dilation post-MI 2, 9. Some in vitro models have shown that fibroblasts grown on mechanically relaxed collagen I produced high levels of MMP-2 and MT1-MMP 26, while others have shown that cyclic stretching of myofibroblasts causes increased MT-MMP activity 27. Altering the mechanical activation pattern of a region of the LV free wall in vivo increased MMP-9 levels, interstitial MMP activity, and increased collagen degradation 19, 28. Rohde et. al. reported that increased wall stress in the border zones was associated with increased MMP-9 activity 8. The present study builds upon these previous investigations by directly correlating regional LV strain with MMP activation at selected time points post MI. Specifically, at one week post MI there was a significant correlation between MMP activation and mechanical dysfunction in the infarct and border regions, although there was also MMP activation in remote regions with normal radial strains. In addition, MMP activation became dissociated from radial strain in the border region over time. The effects of mechanical dysfunction on MMP activation post MI are likely multifactorial, although our analysis of the relationship between changes in regional MMP activation and mechanical functioning post-MI was restricted to systolic strain. One may hypothesize that other mechanical events including localized shear forces and diastolic dysfunction may contribute more to MMP activation and LV remodeling. Nevertheless, our results support a complex relationship between regional LV mechanics and MMP activation.
Prediction of LV remodeling
LV remodeling following MI can vary on a patient-to-patient basis in terms of LV dilation and changes in pump function 2. Past studies have provided evidence that several factors including, but not limited to, infarct size, the transmurality of injury, extent of myocardial viability, and post MI wall stress are correlated to the degree of LV dilation post-MI 2-4. Targeted imaging of MMPs, which are central to LV remodeling, provides an attractive approach to follow the process of LV remodeling in vivo, and a potential means to predict late outcome following MI. Our earlier work demonstrated the feasibility of 99mTc-RP805 for target imaging of MMP activation in vivo in a mouse model of MI 13. The present study extends those observations to a more clinically relevant porcine model of transmural infarction and defines the regional spatial and temporal changes in 99mTc-RP805 retention in relation to changes in myocardial strain and global LV geometry. These dynamic changes in 99mTc-RP805 retention were not directly associated with specific regional changes in 201Tl perfusion or myocardial strain, suggesting the 99mTc-RP805 imaging might provide unique information about the LV remodeling process following MI. The retention of 99mTc-RP805 was not purely a reciprocal of 201Tl activity, and may provide unique information about regional MMP activation that can be derived in vivo with noninvasive SPECT imaging. Perhaps more importantly, a significant increase in 99mTc-RP805 retention was observed in the remote regions, which may provide a better predictor of late global remodeling than other estimates of MI size or molecular events within the infarct region.
Experimental limitations
In the current study, the MI was created surgically via a left lateral thoracotomy that resulted in significant chest wall inflammation and increased focal retention of 99mTc-RP805 in the chest wall immediately over the injured region of the heart, complicating the evaluation of myocardial radiotracer retention. Therefore, clinical 99mTc-RP805 SPECT/CT images are likely to be of higher quality than in the pigs. In our correlation of 99mTc-RP805 retention with MMP activity, we were restricted to analyzing radial segments from a single short axis slice of the heart with segments from immediately adjacent slices that were used for gamma well counting of myocardial 99mTc-RP805 activity. Additional analyses at even earlier time points (one day post MI and three days post MI) or in the setting of ischemia/reperfusion and non-transmural infarction may further help elucidate the factors involved in post infarct LV remodeling. In the present study, changes in LV geometry and MMP activation were determined during terminal examination at each of the designated post-MI time points. Therefore, longitudinal post-MI changes in LV geometry and MMP activation could not be determined. Finally, the dosage of 201Tl and 99mTc-RP805 that were employed were selected based on standard clinical dosing for imaging with our clinical SPECT/CT camera, and ratios of the two radiotracers w selected to optimize in vivo dual isotope imaging and facilitate reliable gamma well counting. However, this dosage would represent approximately 3 times the standard clinical dose used (based on subject weight). These dosages per subject weight could be easily reduced for clinical translation, particularly with application of newer solid state multi-detector imaging technology that provides ~5 times the sensitivity of a conventional camera.
Conclusions
A novel multimodality non-invasive hybrid SPECT/CT imaging approach was validated and applied for in vivo evaluation of MMP activation in combination with cine MR analysis of LV deformation in a clinically relevant porcine model to quantify the temporal and spatial variation of MMP activation and regional myocardial strain during the initial four weeks following MI. Past studies have provided evidence of cause-effect relationships between MMP activation and LV remodeling post-MI 7-9. Moreover, plasma profiling of MMPs has been suggested to hold prognostic value in terms of predicting LV remodeling post-MI 21-23. However, plasma levels of MMP can represent spillover from multiple compartments and may not directly reflect net myocardial MMP activity. The present study provides proof-of-concept that direct high quality in vivo imaging of myocardial MMP activation is feasible on a standard clinical hybrid SPECT/CT system and may provide a clinically applicable means to determine the effects of MMP activation within the context of LV remodeling post-MI.
POTENTIAL CLINICAL IMPACT.
Left ventricular (LV) remodeling following myocardial infarction (MI) remains an important cause of morbidity and mortality. While changes in LV geometry can be clinically assessed using a variety of imaging modalities, these images do not address fundamental underlying mechanisms that contribute to LV dilation post-MI. A number of animal studies have demonstrated a causal relationship between LV dilation post-MI and activation of the matrix metalloproteinases (MMPs). In the present study, a technetium-99m labeled compound (99mTc-RP805) designed to bind to the catalytic domain of a number of MMPs was used to image in vivo MMP activation post-MI. The myocardial spatial distribution and specificity of this MMP targeted compound was validated through direct comparisons with ex vivo immunoblotting and zymography for the determination of regional MMP levels/activity. Regional in vivo MMP activation was also correlated with temporal changes in regional myocardial strain and post-MI remodeling. The post-MI change in LV end-diastolic volumes correlated with MMP activity. High quality in vivo images of 99mTc-RP805 retention were obtained on a standard clinical hybrid SPECT/CT system, suggesting that this approach holds potential for extension to imaging MMP activation in patients following MI with potential value for risk stratification and directing therapy. Thus, 99mTc-RP805 SPECT/CT imaging may provide unique information regarding regional myocardial MMP activation and predict late post-MI LV remodeling.
Acknowledgements
We are very grateful to the many people at both Yale University and Medical University of South Carolina without whose diligence and help the experiments presented in this paper would not have been possible. In particular, we thank Patti Cavaliere, Christi Hawley, Jennifer Hu, Grace Chung, Donna Dione and Christopher Weyman from Yale University, and G. Patricia Escobar, Stuart M. Saunders, and Julie E. McLean, from Medical University of South Carolina.
Sources of Funding This work was supported by NIH grants HL078650, HL078825, HL057952, HL059165 and HL095608, and the Research Service of the Department of Veterans Affairs. The 99mTc-RP805 used in this study was provided in kind by Lantheus Medical Imaging.
Footnotes
Disclosures None.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 2.St. John Sutton M, Sharpe N. Left ventricular remodeling after myocardial infarction. Pathophysiology and therapy. Circulation. 2000;101:2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 3.Maskali F, Franken PR, Poussier S, Tran N, Vanhove C, Boutley H, Gall H Le, Karcher G, Zannad F, Lacolley P, Marie PY. Initial infarct size predicts subsequent cardiac remodeling in the rat infarct model: an in vivo serial pinhole gated SPECT study. J Nucl Med. 2006;47:337–344. [PubMed] [Google Scholar]
- 4.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, Marra MP, Napodano M, Ramondo A, Iliceto S. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006;98:1033–1040. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Pfeffer M, Braunwald E. Experimental observations and clinical implications. Ventricular remodeling after myocardial infarction. Circulation. 1990;81:1161–1172. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 6.Gaudron P, Kugler I, Hu K, Bauer W, Eilles C, Ertl G. Time course of cardiac structural, functional and electrical changes in asymptomatic patients after myocardial infarction: their inter-relation and prognostic impact. J Am Coll Cardiol. 2001;38:33–40. doi: 10.1016/s0735-1097(01)01319-5. [DOI] [PubMed] [Google Scholar]
- 7.Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rohde LE, Ducharme A, Arroyo LH, Aikawa M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix Metalloproteinase Inhibition Attenuates Early Left Ventricular Enlargement After Experimental Myocardial Infarction in Mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- 9.Mukherjee R, Brinsa TA, Dowdy KB, Scott AA, Baskin JM, Deschamps AM, Lowry AS, Escobar GP, Lucas DG, Yarbrough WM, Zile MR, Spinale FG. Myocardial infarct expansion and matrix metalloproteinase inhibition. Circulation. 2003;107:618–625. doi: 10.1161/01.cir.0000046449.36178.00. [DOI] [PubMed] [Google Scholar]
- 10.Yarbrough WM, Mukherjee R, Escobar GP, Mingoia JT, Sample JA, Hendrick JW, Dowdy KB, McLean JE, Lowry AS, O’Neill TP, Spinale FG. Selective targeting and timing of matrix metalloproteinase inhibition in post-myocardial infarct ion remodeling. Circulation. 2003;108:1753–1759. doi: 10.1161/01.CIR.0000091087.78630.79. [DOI] [PubMed] [Google Scholar]
- 11.Dixon JA, Spinale FG. Large animal models of heart failure: a critical link in the translation of basic science to clinical practice. Circ Heart Fail. 2009;2:262–271. doi: 10.1161/CIRCHEARTFAILURE.108.814459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spinale FG, Escobar GP, Hendrick JW, Clark LL, Camens SS, Mingoia JP, Squires CG, Stroud RE, Ikonomidis JS. Chronic matrix metalloproteinase inhibition following myocardial infarction in mice: differential effects on short and long-term survival. J Pharmacol Exp Ther. 2006;318:966–973. doi: 10.1124/jpet.106.104455. [DOI] [PubMed] [Google Scholar]
- 13.Su H, Spinale FG, Dobrucki LW, Song J, Hua J, Sweterlitsch S, Dione DP, Cavaliere P, Chow C, Bourke BN, Hu X-Y, Azure M, Yalamanchili P, Liu R, Cheesman EH, Robinson S, Edwards DS, Sinusas AJ. Noninvasive Targeted Imaging of Matrix Metalloproteinase Activation in a Murine Model of Postinfarction Remodeling. Circulation. 2005;112:3157–3167. doi: 10.1161/CIRCULATIONAHA.105.583021. [DOI] [PubMed] [Google Scholar]
- 14.Liu YH, Sahul Z, Weyman CA, Dione DP, Dobrucki WL, Mekkaoui C, Brennan MP, Ryder WJ, Sinusas AJ. Accuracy and reproducibility of absolute quantification of myocardial focal tracer uptake from molecularly targeted SPECT/CT: a canine validation. J Nucl Med. 2011;52:453–60. doi: 10.2967/jnumed.110.082214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuchs F, Laub G, Othomo K. TrueFISP--technical considerations and cardiovascular applications. Eur J Radiol. 2003;46:28–32. doi: 10.1016/s0720-048x(02)00330-3. [DOI] [PubMed] [Google Scholar]
- 16.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, Gerig G. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 17.Papademetris X, Sinusas AJ, Dione DP, Constable RT, Duncan JS. Estimation of 3-D left ventricular deformation from medical images using biomechanical models. IEEE Transactions on Medical Imaging. 2002;21:786–800. doi: 10.1109/TMI.2002.801163. [DOI] [PubMed] [Google Scholar]
- 18.Wilson EM, Moainie SL, Baskin JM, Lowry AS, Deschamps AM, Mukherjee R, Guy TS, St John-Sutton MG, Gorman JH, 3rd, Edmunds LH, Jr., Gorman RC, Spinale FG. Region- and type-specific induction of matrix metalloproteinases in post-myocardial infarction remodeling. Circulation. 2003;107:2857–2863. doi: 10.1161/01.CIR.0000068375.40887.FA. [DOI] [PubMed] [Google Scholar]
- 19.Mukherjee R, Zavadzkas JA, Rivers WT, McLean JE, Chang EI, Bouges S, Matthews RG, Koval CN, Stroud RE, Spinale FG. Short-term disruption in regional left ventricular electrical conduction patterns increases interstitial matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2010;299:H217–224. doi: 10.1152/ajpheart.00065.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deschamps AM, Apple KA, Leonardi AH, McLean JE, Yarbrough WM, Stroud RE, Clark LL, Sample JA, Spinale FG. Myocardial interstitial matrix metalloproteinase activity is altered by mechanical changes in LV load: interaction with the angiotensin type 1 receptor. Circ Res. 2005;96:1110–1118. doi: 10.1161/01.RES.0000167830.12010.6b. [DOI] [PubMed] [Google Scholar]
- 21.Webb CS, Bonnema DD, Ahmed SH, Leonardi AH, McClure CD, Clark LL, Stroud RE, Corn WC, Finklea L, Zile MR, Spinale FG. Specific temporal profile of matrix metalloproteinase release occurs in patients after myocardial infarction: relation to left ventricular remodeling. Circulation. 2006;114:1020–1027. doi: 10.1161/CIRCULATIONAHA.105.600353. [DOI] [PubMed] [Google Scholar]
- 22.Schmidt R, Bultmann A, Fischel S, Gillitzer A, Cullen P, Walch A, Jost P, Ungerer M, Tolley ND, Lindemann S, Gawaz M, Schomig A, May AE. Extracellular matrix metalloproteinase inducer (CD147) is a novel receptor on platelets, activates platelets, and augments nuclear factor kappaB-dependent inflammation in monocytes. Circ Res. 2008;102:302–309. doi: 10.1161/CIRCRESAHA.107.157990. [DOI] [PubMed] [Google Scholar]
- 23.Kelly D, Khan SQ, Thompson M, Cockerill G, Ng LL, Samani N, Squire IB. Plasma tissue inhibitor of metalloproteinase -1 and matrix metalloproteinase-9: novel indicators of left ventricular remodelling and prognosis after acute myocardial infarction. Eur Heart J. 2008;29:2116–2124. doi: 10.1093/eurheartj/ehn315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson JT, Li H, Dillon L, Bryant JW. Evolution of matrix metalloprotease and tissue inhibitor expression during heart failure progression in the infarcted rat. Cardiovasc Res. 2000;46:307–315. doi: 10.1016/s0008-6363(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Tung CH, Allport JR, Chen S, Weissleder R, Huang PL. Near-infrared fluorescent imaging of matrix metalloproteinase activity after myocardial infarction. Circulation. 2005;111:1800–1805. doi: 10.1161/01.CIR.0000160936.91849.9F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomasek JJ, Halliday NL, Updike DL, Ahern-Moore JS, Vu TK, Liu RW, Howard EW. Gelatinase A activation is regulated by the organization of the polymerized actin cytoskeleton. J Biol Chem. 1997;272:7482–7487. doi: 10.1074/jbc.272.11.7482. [DOI] [PubMed] [Google Scholar]
- 27.MacKenna D, Summerour SR, Villarreal FJ. Role of mechanical factors in modulating cardiac fibroblast function and extracellular matrix synthesis. Cardiovasc Res. 2000;46:257–263. doi: 10.1016/s0008-6363(00)00030-4. [DOI] [PubMed] [Google Scholar]
- 28.Garcia RA, Brown KL, Pavelec RS, Go KV, Covell JW, Villarreal FJ. Abnormal cardiac wall motion and early matrix metalloproteinase activity. Am J Physiol Heart Circ Physiol. 2005;288:H1080–1087. doi: 10.1152/ajpheart.00860.2004. [DOI] [PubMed] [Google Scholar]