Summary
Background
Photodynamic therapy (PDT) has been shown to be effective in the treatment of malignancies of a variety of organ systems, including the lungs, bladder, gastrointestinal tract and skin. Cutaneous lesions serve as ideal targets of PDT because of the accessibility of the skin to light. To achieve optimum results, the photosensitizer must be delivered effectively into the target layers of the skin within a practical timeframe, via noninvasive methods.
Aim
To determine whether topical application of a second-generation photosensitizer, silicon phthalocyanine (Pc) 4 [SiPc(OSi(CH3)2(CH2)3N(CH3)2)(OH)], results in effective penetration of the skin barrier.
Methods
Penetration of Pc 4 was evaluated using standard Franz-type vertical diffusion cell experiments on surrogate materials (silicone membranes) and laser-scanning confocal microscopy of normal skin biopsy samples from human volunteers.
Results
The Franz diffusion data indicate that Pc 4 formulated in an ethanol/propylene glycol solution (70/30%, v/v) can penetrate the membrane at a flux that is appreciable and relatively invariant. Using the same formulation, Pc 4 uptake could be detected in human skin via laser-scanning confocal microscopy.
Conclusion
After topical application, Pc 4 is absorbed into the epidermis in as little as 1h, and the absorption increased with increasing time and dose. Pc 4 can be effectively delivered into human skin via topical application. The data also suggest that the degree of penetration is time- and dose-dependent.
Introduction
Photodynamic therapy (PDT) is a rapidly advancing treatment for various clinical conditions. PDT has been shown to be effective in the treatment of cancers of several organ systems, including the lungs, bladder, gastrointestinal tract, skin and other tissues,1,2 and of inflammatory dermatoses, such as acne.3,4 This therapy involves administering a photosensitizing drug either systemically or locally, and delivering light of an appropriate wavelength, fluence and fluence rate to the pathological tissue, such as a nodule of cutaneous basal cell carcinoma (BCC) or a focus of oesophageal epithelial dysplasia. In the presence of molecular oxygen in tissue, the interaction of photosensitizer and light results in a cascade of events, leading to the generation of singlet oxygen and other forms of reactive oxygen to exert cytotoxic effects on organelles such as mitochondria, and eventually resulting in apoptosis induction and tumour cell destruction.5-7
The first US Food and Drug Administration (FDA)-approved porphyrin-derivative photosensitizer, porfimer sodium (Photofrin®) is given intravenously before irradiation of the tumour or pathological tissue with 633-nm light. Because the drug remains present in tissues for up to several weeks, patients are advised to avoid natural sunlight or intense artificial light to minimize the likelihood of developing widespread photosensitivity reactions.8,9 This precautionary measure considerably affects patient lifestyle and is considered a major disadvantage of porfimer-PDT.
Local or topical delivery of photosensitizers offers a more targeted approach, particularly in treating cutaneous lesions, because of the accessibility of the skin. Aminolaevulinic acid (ALA) and its ester, methyl-ALA (MAL), meet this criterion, and represent another group of PDT drugs that have US FDA approval. ALA-PDT and MAL-PDT have shown efficacy in treating actinic keratosis (AKs), Bowen disease (BD), BCCs, acne, photoageing, psoriasis and cutaneous T-cell lymphoma.4,10-12 However, because both ALA and MAL are pro-drugs that need to undergo conversion to the active photosensitizer, protoporphyrin IX, an interval range from 3 to 14 h is often required between drug administration and light delivery, which can complicate the treatment schedule. Moreover, ALA-PDT is commonly accompanied by considerable pain that may not be sufficiently alleviated by the use of local anaesthetics.13 A recent study found that reducing the fluence rate (irradiance) during light delivery could reduce the pain during treatment of BCCs by ALA-PDT at the cost of a modest increase in the time required to deliver the treatment light.14 Whether this method will make the treatment more acceptable for all cutaneous lesions is not yet clear. These complications have led to the development of a second generation of photosensitizing agents that are designed to ameliorate the disadvantages of their predecessors.
The silicon phthalocyanine (Pc) 4 (Fig. 1) represents one of these second-generation agents, and has excellent potential for treating skin lesions.15 The phthalocyanine ring system absorbs light optimally at 675 nm, a wavelength that penetrates tissue well. Pharmacokinetic data in mice indicate that when delivered systemically, Pc 4 is much more rapidly cleared than porfimer sodium, thereby minimizing the possibility of prolonged generalized photosensitivity.16
Figure 1.
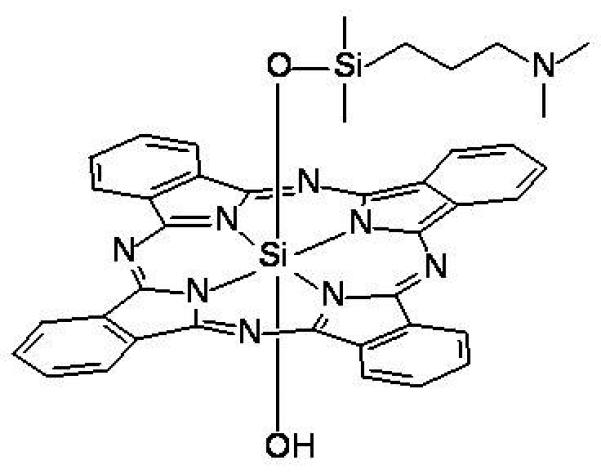
Structure of Pc 4.
A prerequisite for effective topical treatment with a photosensitizer is effective delivery into the target layers of the skin within a practical timeframe to achieve optimum lesion response upon photoillumination. In this study, we aimed to determine whether topical application of Pc 4 allows effective penetration of the skin barrier, using standard Franz-type vertical diffusion cell experiments on surrogate materials such as silicone membranes, and laser-scanning confocal microscopy after ex vivo application of Pc 4 to normal human skin.
Methods
The study was approved by the institutional review board of University Hospitals of Cleveland, and written informed consent was obtained from all participants.
Formulation of Pc 4
Pc 4 was dissolved in absolute ethanol to 1 mg/mL and stored protected from light. The optical density (OD) was determined by spectrophotometry and used to calculate the concentration of the stock Pc 4 solution (extinction coefficient at 668 nm = 2.3 × 105/cm/mol). The Pc 4 stock solution was then formulated in a vehicle of 70% ethanol/30% propylene glycol (v/v) to the desired final concentrations: 0.01, 0.05 and 0.1 mg/mL.
Vertical diffusion cell method: the Franz apparatus
The penetration apparatus was a manual, six-cell test system (Manual Start-up System; Hanson Research, Chatsworth, CA, USA). The constant temperature bath of the apparatus was set at 32.5 °C. The receptor chambers, with a volume of 4.3 mL, were rinsed and filled with the receptor medium (75% phosphate-buffered saline/25% ethanol, v/v). The membrane, with an exposed area of 0.64 cm2, was positioned above a stirring bar that maintained a constant speed of 0.45 g during the procedure. The penetrant (3.2 mg of Pc 4 in ethanol/propylene glycol solution) was added to the donor chambers (400 μL each), and the cells were capped. Portions of fresh medium (300 μL) were injected into the receptor chambers of the cells at hourly intervals. The first 150 μL fractions of Pc 4-bearing expelled medium were rejected, and the remaining fractions were used for absorbance measurements. From the data accumulated, the flux of Pc 4 through the membrane was calculated by taking into account the amount of Pc 4 expelled in the sampling process. The lag time was determined by extrapolation of the cumulative penetration line to the time axis.
Spectrometry in the visible range
Amounts of Pc 4 in the receptor fluid samples were measured at 670 nm with spectrometer (DU800; Beckman Coulter, Fullerton, CA, USA) controlled by a personal computer (Dimension; Dell, Round Rock, TX, USA). The samples were held in fused quartz cuvettes (10 mm, 150 μL; Beckman Coulter).
The extinction coefficient spectra were also measured at 670 nm with a spectrometer (Lambda 25, Perkin Elmer, Shelton, CT, USA) controlled by computer (Dimension; Dell). The samples were held in fused quartz cuvettes (1.00 cm, 4 mL, Fisher Scientific, Pittsburgh, PA). Absorbance of Pc 4 in the receptor medium was measured at 670 nm at concentrations of 5.49 × 10−8 to 2.78 × 10−7 mol/L. With Beer's law, these data gave an ε value of 2.12 × 105 for Pc 4 in the receptor medium.
Pc 4 application on skin biopsies
Skin was harvested from five healthy volunteers (two men, three women; mean ± SD age 31.0 ± 3.4 years, range 20–40), with Fitzpatrick skin types I to III). Local anaesthesia was injected into normal buttock skin and biopsy samples (0.4 mm) were obtained using a handheld, nonmotororized keratome device (model C 0459; Aesculap AG, Melsungen, Germany) for obtaining a horizontal skin biopsy. Biopsies were immediately placed in normal saline. In a sterile 60-mm dish, a 1 × 1 cm2 biopsy section was laid epidermis side down on a bed of two layers of 2 × 2 cm2 sterile gauze. A 1-mL sample of the desired concentration of Pc 4 solution was applied to the corners of the gauze, so that it thoroughly saturated the gauze through capillary action and allowed an even distribution of the Pc 4 solution to be exposed to the outermost skin layer (stratum corneum; SC). To confirm that this method allowed the Pc 4 to penetrate only through the top of the skin and that there was no contaminating flow over the edges of the skin sample, a similar biopsy sample was placed upside-down on the gauze (i.e. dermis side down) and the Pc 4 solution applied as above. In addition, vehicle or phosphate-buffered saline (1 × PBS) alone was applied to a biopsy sample (epidermis side down) to assess vehicle and tissue autofluorescence.
Culture dishes were covered, sealed with plastic wrap (Parafilm®; Pechiney Plastic Packaging Company, Chicago, IL, USA) and placed in the dark at 37 °C. After 1–4 h of Pc 4 exposure, skin samples were removed from the gauze and washed three times with 1 × PBS to remove excess Pc 4.
For imaging of Pc 4 fluorescence, each biopsy sample was transferred flat to a 35 mm diameter glass-bottomed microwell dish (MatTek Inc, Ashland, MA, USA) in 150 μL of 1 × PBS, with the tissue side that had been in contact with the gauze now facing the glass. Biopsies were then removed and placed onto fresh saline gauze for sectioning.
To further ensure that Pc 4 penetration into the biopsy proceeded from the epidermal surface only, additional experiments were conducted. The same type of glass-bottomed microwell dish (MatTek) was used; from these, the glass bottom was carefully removed before the experiment. The biopsy sample was then placed over the resulting hole in the 35-mm plate without gauze, and the microwell plate was placed inside a larger 60-mm culture dish. Pc 4 solution with the desired concentration was placed inside the large dish and allowed to penetrate the epidermal layer of the biopsy sample by capillary action.
Measurement of Pc 4 uptake by confocal imaging
Confocal images of Pc 4 fluorescence were acquired using a confocal imaging system (LSM 510; Zeiss, Thornwood, NY, USA) with a water immersion objective lens [Achroplan 20 x numerical aperture (NA) 0.5 lens; Zeiss], using a 633-nm excitation light from a helium/neon laser, a 633-nm dichroic mirror and a 650-nm long pass filter. Stacked images were three-dimensionally reconstructed (LSM 510 software; Zeiss) and then exported and quantitatively analysed [MetaMorph Premier (Molecular Devices Corp., Sunnyvale, CA, USA) and Adobe Photoshop (Adobe Systems Inc., San Jose, CA, USA]. For each sample, the intensities of at least 10 regions of interest were averaged, and then plotted as a function of Pc 4 dose and time of application.
Results
Pc 4 in ethanol/propylene glycol penetrates silicone membranes consistently
A typical visible spectrum of the samples analysed is shown in Fig. 2; the Q-band of Pc 4 is sharp, and is suitable for the spectrophotometric analytical procedure used. Cumulative penetration data for Pc 4 through a silicone membrane over the course of 32 h in two separate runs are shown in Table 1. The slope of the linear portion in each provides the average flux value (mg/cm2/h) at a steady state, which was found to have very little variation (Table 2; Fig. 3a,b). Our data indicate that the Pc 4 formulated in an ethanol–propylene glycol solution (70/30%, v/v) can penetrate the silicone membrane model at an appreciable flux with little variation detected by the Franz-type vertical diffusion cell apparatus, providing a strong basis to test the permeability of this Pc 4 formulation on human skin.
Figure 2.
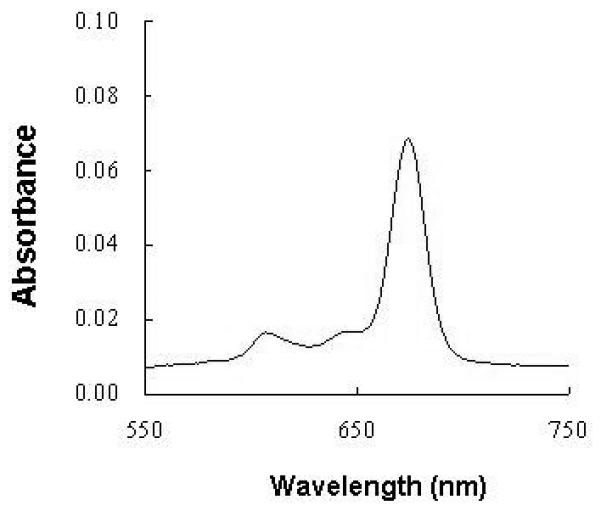
A typical visible spectrum of the sample analysed for the Franz-type vertical diffusion apparatus.
Table 1.
Cumulative penetration of Pc 4 at selected time points.
| Cumulative penetration, μg/cm2 | Lag time, hours |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Run | Cell | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | |
| 1 | 1 | 0.22 | 0.28 | 0.4 | 0.42 | 0.42 | 0.54 | 0.60 | 0.70 | 0.76 | 0.82 | 0.89 | 18 | |
| 2 | 0.29 | 0.45 | 0.44 | 0.51 | 0.59 | 0.70 | 0.78 | 0.83 | 0.89 | 0.98 | 0.96 | 17 | ||
| 4 | 0.29 | 0.31 | 0.31 | 0.32 | 0.37 | 0.46 | 0.51 | 0.58 | 0.66 | 0.74 | 0.81 | 19 | ||
| 5 | 0.22 | 0.28 | 0.27 | 0.36 | 0.43 | 0.51 | 0.54 | 0.66 | 0.7 | 0.8 | 0.22 | 18 | ||
| Mean | 0.25 | 0.33 | 0.35 | 0.41 | 0.45 | 0.55 | 0.61 | 0.69 | 0.75 | 0.84 | 0.89 | 18 | ||
| 2 | 1 | 0.14 | 0.15 | 0.14 | 0.22 | 0.26 | 0.30 | 0.34 | 0.41 | 0.52 | 0.55 | 0.63 | 0.71 | 21 |
| 2 | 0.17 | 0.16 | 0.24 | 0.28 | 0.34 | 0.46 | 0.49 | 0.54 | 0.64 | 0.70 | 0.76 | 0.82 | 20 | |
| 4 | 0.17 | 0.16 | 0.17 | 0.25 | 0.31 | 0.36 | 0.38 | 0.44 | 0.54 | 0.57 | 0.65 | 0.7 | 20 | |
| 5 | 0.19 | 0.25 | 0.22 | 0.28 | 0.35 | 0.37 | 0.41 | 0.48 | 0.56 | 0.61 | 0.67 | 0.77 | 19 | |
| 6 | 0.19 | 0.21 | 0.22 | 0.26 | 0.37 | 0.39 | 0.44 | 0.51 | 0.58 | 0.69 | 0.75 | 0.86 | 20 | |
| Mean | 0.17 | 0.19 | 0.2 | 0.26 | 0.33 | 0.37 | 0.41 | 0.48 | 0.57 | 0.62 | 0.69 | 0.77 | 20 | |
Table 2.
Penetration of Pc 4 (per hr) at steady state.
| Run | Cell | Penetration (μg/cm2/h) |
|---|---|---|
| 1 | 1 | 0.07 |
| 2 | 0.07 | |
| 4 | 0.07 | |
| 5 | 0.07 | |
| Mean ± SD | 0.07 ± 0.01 | |
| 2 | 1 | 0.062 |
| 2 | 0.07 | |
| 4 | 0.06 | |
| 5 | 0.06 | |
| 6 | 0.07 | |
| Mean ± SD | 0.06 ± 0.01 | |
Figure 3.
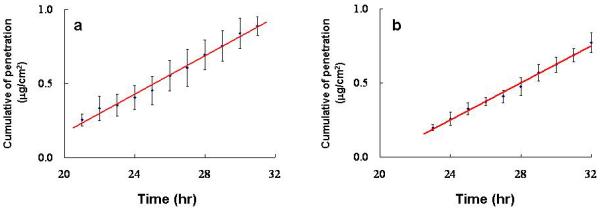
Cumulative penetration of Pc 4 through the silicone membrane over 32 h on (a) four and (b) five runs. The slope of the linear portion in each plot provides the average flux value (mg/cm2/h) at a steady state, and the results are expressed as means ± SD.
Effective penetration of Pc 4 through intact human stratum corneum is achieved within 1 h
Using laser confocal microscopy, we found that after 1 h of contact with Pc 4 solution at a concentration as low as 0.01 mg/mL, Pc 4 has penetrated through the SC, seen as fluorescence in the cells of human skin biopsy samples. Figure 4a shows bright pseudo-green Pc 4 fluorescence in all layers of the epidermis and some fluorescent areas in the upper dermis of a serial section of a skin biopsy. Haematoxylin and eosin staining of the next serial section (Fig. 4b) of the same skin sample illustrates the tissue architecture and provides a guide for assessing the level of Pc 4 penetration. Incubation of skin in 1 × PBS or ethanol/propylene glycol solution alone did not result in any fluorescence being detectable with precisely the same confocal image acquisition settings (Fig. 4c), indicating that the fluorescence in the epidermal cells seen in Fig. 4a resulted from Pc 4 penetration from the surface of the SC.
Figure 4.
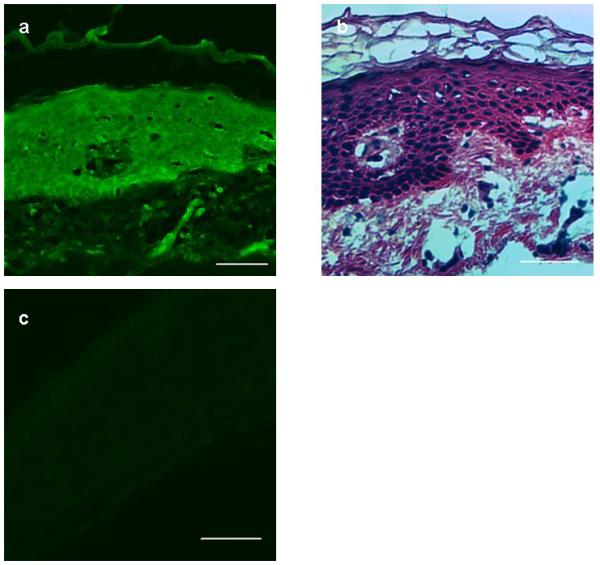
(a) After 0.01 mg/mL Pc 4 applied for 1 h to the stratum corneum, there was demonstrable fluorescence (pseudo green) throughout the epidermis and portions of the superficial dermis, using laser confocal microscopy. (b) Corresponding histological staining of adjacent skin section panel confirmed the depth of penetration and distribution (haematoxylin and eosin). (c) Incubation of skin in ethanol/propylene glycol solution alone did not result in any fluorescence on confocal imaging. Scale Bar 50 μm.
The effective concentration of Pc 4 in normal skin changes as a function of contact time and dose
Pc 4 was clearly present in skin cells after 1 h of topical application (Fig. 4a), and the amount of Pc 4 penetration appeared to increase with the length of incubation. Three biopsy samples were topically treated with 0.1 mg/mL, but with different incubation times (1, 2 and 4 h). At each time point, 20 scanned images were selected and analysed, then, 10 regions of interest were selected per time point and their averaged intensities were plotted. Pc 4 fluorescence was relatively higher for the 2-h and 4-h treatments, suggesting greater Pc 4 absorption (Fig. 5a) as contact time increases. In addition, Pc 4 fluorescence also increased with increasing dose (Fig. 5b). Different doses of Pc 4 (0.01, 0.05and 0.1 mg/mL) were applied to the skin biopsies. After 4 h of topical application, Pc 4 fluorescence was measured by confocal microscopy. The greater the Pc 4 dose applied, the higher the detected Pc 4 fluorescence intensity, suggesting greater Pc 4 uptake by cutaneous cells.
Figure 5.
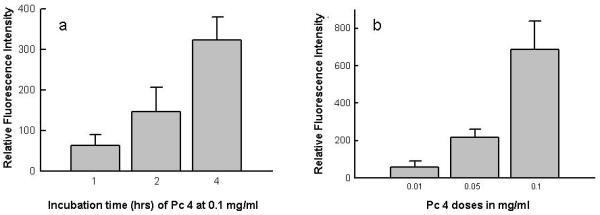
(a) Increasing concentration of Pc 4 solution from 0.01 to 0.1 mg/mL applied for 4 h to skin biopsy samples. (b) Using 0.1 mg/mL Pc 4 solution applied to the stratum corneum, the average increase Pc 4 fluorescence intensity correlated with increasing incubation time.
Subcellular distribution of Pc 4 in the basal epidermis confirms prior in vitro data on Pc 4 binding with cytosolic membranes
Initially, at the basal layers of the epidermis, the intracellular Pc 4 fluorescence had a diffuse pattern, which was not very specific. However, this may be due to the low magnification and low NA of the 20x objective used for all the experiments. In high-resolution single cell Pc 4 fluorescence images, Pc 4 has been shown to bind with membranous portions of the mitochondria, Golgi apparatus and endoplasmic reticulum (ER).6 Additional experiments using an objective with a higher NA and counterstaining with chloromethyl-X-rosamine (MitoTracker Red; Molecular Probes Inc., Eugene, OR, USA) indicated that the intracellular Pc 4 fluorescence seems to have a punctate pattern (unpublished data).
Discussion
To our knowledge, this is the first demonstration of effective penetration through the SC of a novel topical silicon phthalocyanine compound, and represents a major breakthrough in the development of Pc 4 for topical PDT of cutaneous lesions.
In this study, we found that Pc 4 was present in significant concentrations in normal skin as early as 1 h postapplication. In addition, the amount of Pc 4 penetration, which was mainly evident in the epidermis, with a small amount also detected in the superficial dermis, was time- and dose-dependent.
A major advantage of this drug over other topical photosensitizers is that it does not require a prolonged interval between drug application and the application of light, as Pc 4 is already an active photosensitizer. Although Pc 4 offers a practical time frame for outpatient treatment, any topical photosensitizer must also be delivered effectively into target layers of the skin to achieve the optimum PDT results. Although Pc 4 is a relatively large molecule, there has been success with using other large molecular weight compounds as topical agents, including tacrolimus and pimecrolimus, an ascomycin derivative.17 Our Pc 4 stock solution was formulated in a vehicle of 70% ethanol/30% propylene glycol (v/v), and then diluted to the desired final concentrations. Our data indicate that this formulation can penetrate a silicone membrane model at an appreciable flux, with little variation as measured by the Franz-type vertical diffusion cell apparatus.
Based on the observed depth, pattern and intensity of Pc 4 uptake after topical application into human skin (Fig. 4a) in this study, we hypothesize that Pc 4 solution could be even more effectively used for PDT of superficial premalignant and malignant lesions such as AKs, BD, patch-stage mycosis fungoides, superficial BCCs, and inflammatory lesions. It has been known that some cutaneous pathologies (e.g. eczema) are often associated with increased skin permeability, which may further optimize Pc 4 absorption.18,19 Preclinical studies on Pc 4 have shown that the drug localizes to cytosolic membranes including those in the mitochondria, Golgi apparatus and ER.6 Further analyses of Pc 4 localization in vitro have confirmed the binding site to be in close proximity to the mitochondrial pore, where the antiapoptotic protein Bcl-2 is efficiently targeted.20,21
In the present study, using human tissues, the pattern of Pc 4 fluorescence, at least near the basal layers of the epidermis, confirmed previous data obtained from cells in vitro.
Conclusion
The novel photosensitizer Pc 4 can be effectively delivered into human skin by topical application. Its degree of penetration may be dose- and time dependent. Pc 4 is therefore suitable for PDT of cutaneous lesions after topical application to human skin. It is possible that deeper tissue penetration of Pc 4 could occur in cutaneous lesions when the epidermal barrier is perturbed. Methods of optimizing Pc 4 delivery for thick skin lesions are under way.
Acknowledgements
We thank Dr S. J. Howell for his assistance with the image data analysis with MetaMorph software. This study was supported by NIH Grants: 5P30AR39750 (NIAMS Skin Diseases Research Center Grant) and P30 CA43703.
Footnotes
Conflict of interest: TSM, MEK, NLO, KDC, and EDB, are inventors on patents concerning Pc 4-PDT. TSM, MEK, NLO, and KDC are associated with Fluence Therapeutics, Inc., a company developing photodynamic therapy with the photosensitizer Pc 4
References
- 1.Sibata C, Colussi V, Oleinick N, Kinsella T. Photodynamic therapy in oncology. Expert Opin Pharmacother. 2001;2:917–27. doi: 10.1517/14656566.2.6.917. [DOI] [PubMed] [Google Scholar]
- 2.Dougherty T. An update on photodynamic therapy applications. J Clin Laser Med Surg. 2001;20:3–7. doi: 10.1089/104454702753474931. [DOI] [PubMed] [Google Scholar]
- 3.Bissonnette R, Tremblay J, Juzenas P, et al. Systemic photodynamic therapy with aminolevulinic acid induces apoptosis in lesional T lymphocytes of psoriatic plaques. J Invest Dermatol. 2002;119:77–83. doi: 10.1046/j.1523-1747.2002.01827.x. [DOI] [PubMed] [Google Scholar]
- 4.Ibbotson S. Topical 5-aminolevulinic acid photodynamic therapy for the treatment of skin conditions other than non-melanoma skin cancer. Br J Dermatol. 2002;146:178–88. doi: 10.1046/j.0007-0963.2001.04689.x. [DOI] [PubMed] [Google Scholar]
- 5.Oleinick N, Morris R, Belichenko I. Apoptosis in response to photodynamic therapy: what, where, why and how. Photochem Photobiol Sci. 2002;1:1–21. doi: 10.1039/b108586g. [DOI] [PubMed] [Google Scholar]
- 6.Oleinick N, Evans H. The photobiology of photodynamic therapy: cellular targets and mechanisms. Radiat Res. 1998;150:S146–56. [PubMed] [Google Scholar]
- 7.Dougherty T, Gomer C, Henderson B, et al. Photodynamic therapy: review. J Natl Cancer Inst. 1998;90:889–902. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dougherty T, Cooper M, Mang T. Cutaneous phototoxic occurrences in patients receiving photofrin. Lasers Surg Med. 1990;10:485–8. doi: 10.1002/lsm.1900100514. [DOI] [PubMed] [Google Scholar]
- 9.Moriwaki S, Misawa J, Yoshinari Y, et al. Analysis of photosensitivity in Japanese cancer-bearing patients receiving photodynamic therapy with porfimer sodium (Photofrin) Photoderm Photoimmunol Photomed. 2001;17:241–3. doi: 10.1034/j.1600-0781.2001.170507.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang I, Bendsoe N, Klinteberg C, et al. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol. 2001;144:832–40. doi: 10.1046/j.1365-2133.2001.04141.x. [DOI] [PubMed] [Google Scholar]
- 11.Coors E, Driesch P. v.d. Topical photodynamic therapy for patients with therapy-resistant lesions of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2004;50:363–7. doi: 10.1016/s0190-9622(03)00868-5. [DOI] [PubMed] [Google Scholar]
- 12.Szeimies R, Landthaler M, Karrer S. Non-oncologic indications for ALA-PDT. J Dermatol Treat. 2002;13:S13–18. doi: 10.1080/095466302317414654. [DOI] [PubMed] [Google Scholar]
- 13.Holmes M, Dawe R, Ferguson J, Ibbotson S. A randomized, double-blind, placebo-controlled study of the efficacy of tetracaine gel (Ametop) for pain relief during topical photodynamic therapy. Br J Dermatol. 2004;150:337–40. doi: 10.1111/j.1365-2133.2004.05652.x. [DOI] [PubMed] [Google Scholar]
- 14.Wiegell SR, Skiveren J, Phillipsen PA, Wulf HC. Pain during photodynamic therapy is associated with protoporphyrin IX fluorescence and fluence rate. Br J Dermatol. 2008;158:727–33. doi: 10.1111/j.1365-2133.2008.08451.x. [DOI] [PubMed] [Google Scholar]
- 15.Oleinick N, Antunez A, Clay M, et al. New phthalocyanine photosensitizers for photodynamic therapy. Photochem Photobiol. 1993;57:242–7. doi: 10.1111/j.1751-1097.1993.tb02282.x. [DOI] [PubMed] [Google Scholar]
- 16.Egorin M, Zuhowski E, Sentz D, et al. Plasma pharmacokinetics and tissue distribution in CD2F1 mice of Pc 4 (NSC 676418), a silicone phthalocyanine photodynamic sensitizing agent. Cancer Chemother Pharmacol. 1999;44:283–94. doi: 10.1007/s002800050979. [DOI] [PubMed] [Google Scholar]
- 17.Meingassner JG, Aschauer H, Stuetz A, Billich A. Pimecrolimus permeates less than tacrolimus through normal, inflamed, or corticosteroid-pretreated skin. Experl Derm. 2005;14:752–7. doi: 10.1111/j.1600-0625.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- 18.Madision KC. Barrier function of the skin. ‘la raison d'être’ of the epidermis. J Invest Derm. 2003;121:231–41. doi: 10.1046/j.1523-1747.2003.12359.x. [DOI] [PubMed] [Google Scholar]
- 19.Fartasch M. Epidermal barrier in disorders of the skin. Microsc Res Tech. 1997;38:361–72. doi: 10.1002/(SICI)1097-0029(19970815)38:4<361::AID-JEMT4>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 20.Usuda J, Chiu S, Murphy E, et al. Domain-dependent photodamage to Bcl-2. J Biol Chem. 2003;278:2021–9. doi: 10.1074/jbc.M205219200. [DOI] [PubMed] [Google Scholar]
- 21.Morris R, Azizuddin K, Lam M, et al. Fluorescence resonance energy transfer reveals a binding site of a photosensitizer for photodynamic therapy. Cancer Res. 2003;63:5194–7. [PubMed] [Google Scholar]
- 22.Lam M, Oleinick NL, Nieminen AL. Photodynamic therapy-induced apoptosis in epidermoid carcinoma cells. Reactive oxygen species and mitochondrial inner membrane permeabilization. J Biol Chem. 2001;276:47379–86. doi: 10.1074/jbc.M107678200. [DOI] [PubMed] [Google Scholar]


