Abstract
The critical role of IFNα in the pathogenesis of human systemic lupus erythematosus (SLE) has been highlighted in recent years. Exposure of young lupus-prone NZB/W F1 mice to IFNα in vivo leads to an accelerated lupus phenotype that is dependent on T cells and is associated with elevated serum levels of BAFF, IL-6 and TNFα, increased splenic expression of IL-6 and IL-21, formation of large germinal centers and the generation of large numbers of short-lived plasma cells that produce IgG2a and IgG3 autoantibodies. Here we show that both IgG2a and IgG3 autoantibodies are pathogenic in IFNα accelerated lupus and their production can be dissociated by using low dose CTLA4Ig. Only high dose CTLA4Ig attenuates both IgG2a and IgG3 autoantibody production and significantly delays death from lupus nephritis. In contrast, BAFF/APRIL blockade has no effect on germinal centers or the production of IgG anti-dsDNA antibodies but, if given at the time of IFNα challenge, delays the progression of lupus by attenuating systemic and renal inflammation. Temporary remission of nephritis induced by combination therapy with cyclophosphamide (CTX), anti-CD40L antibody and CTLA4Ig is associated with abrogation of germinal centers and depletion of short-lived plasma cells but relapse occurs more rapidly than in conventional NZB/W F1 mice. Our study demonstrates that IFNα renders NZB/W F1 relatively resistant to therapeutic intervention and suggests that the IFN signature should be taken into account when randomizing patients into and analyzing the results of human clinical trials in SLE.
INTRODUCTION
Systemic lupus erythematosus (SLE) is an autoimmune disease characterized by the loss of tolerance to nucleic acids and their binding proteins and the production of autoantibodies that induce tissue damage (1). Nucleic acid containing immune complexes are internalized into TLR containing intracellular compartments in B cells and plasmacytoid dendritic cells and amplify disease by enhancing cell activation and by inducing the production of Type I IFNs (2). IFNα induces maturation of myeloid DCs that provide costimulation for naïve CD4+ T cells and produce both IL-6 and B cell-activating factor of the TNF Family (BAFF), a cytokine that enhances selection, survival and class switching of autoreactive B cells (3–5).
In young lupus prone NZB/W mice, but not in BALB/c mice, administration of adenovirus expressing IFNα (Ad-IFNα) rapidly induces T cell activation and extensive germinal center (GC) formation with the generation of large numbers of short-lived plasma cells producing IgG2a and IgG3 autoantibodies that cause glomerulonephritis (6, 7). CD4 T cells are absolutely required for the production of pathogenic autoantibodies and the initiation of Ad-IFNα induced disease (6). In addition, serum BAFF, IL-6 and TNFα are elevated in Ad-IFNα treated mice and B cells in these mice express high levels of TLR7 (6).
Therapeutic agents that target T cell costimulatory pathways or that target BAFF and its homolog APRIL are being developed for the treatment of SLE. CTLA4Ig, a drug that inhibits CD28-B7 costimulation prevents SLE onset in NZB/W mice but does not induce remission when used as a single agent (8). Remission of nephritis can be induced in NZB/W F1 mice by combination therapy with cyclophosphamide (CTX) and CTLA4Ig (8) or with CTX, CTLA4Ig and anti-CD40L (triple therapy)(9); a clinical trial of abatacept (human CTLA4Ig) in combination with CTX for SLE nephritis is currently in progress (Clinicaltrials.gov identifier NCT00774852). Inhibition of BAFF can also prevent SLE onset in murine models and reverses disease in some of these models (10–14). An anti-BAFF antibody, belimumab, has shown efficacy in two recent Phase III trials of moderately active SLE (15).
In the present study, we show that both the B7-CD28 antagonist CTLA4Ig and the BAFF/APRIL inhibitor TACI-Ig delay disease onset in IFNα induced SLE but a higher dose of CTLA4Ig is required than in conventional NZB/W mice. Neither drug reverses or delays disease once high titer autoantibodies are present in the serum. Triple therapy depletes autoantibody producing plasma cells and induces remission in IFNα accelerated disease mice with a similar efficacy as it does in conventional NZB/W F1 mice. However, IFNα accelerates relapse in a dose dependent manner. We further show that the clinical effects of CTLA4Ig and TACI-Ig are achieved by different mechanisms. High dose CTLA4Ig attenuates both IgG3 and IgG2a autoantibody production and significantly decreases nephritis-associated mortality. In contrast, TACI-Ig treatment does not alter T cell activation or the production of pathogenic anti-dsDNA antibodies, but attenuates the renal inflammatory response to immune complex deposition.
MATERIALS AND METHODS
Prevention studies
NZB/W F1 females were purchased from Jackson Laboratory (Bar Harbor, ME) and were housed in a pathogen free facility. Groups of mice were treated at 12 weeks of age with a single i.v. injection of Ad-IFNα [3.3 × 108 viral particles as previously described (6)], and either received fully murine TACI-Ig (12) (500ug, 3 times/week) or CTLA4Ig (16) (100ug or 200ug, 3 times/week), or no treatment starting on the day of virus injection or 21 days thereafter. Mice were bled every other week, and urine was tested for proteinuria by dipstick (Multistick; Fisher Scientific, Pittsburg, PA) weekly. Some mice were sacrificed and analyzed after five weeks and some were followed until death.
Remission induction in IFNα adenovirus (Ad-IFNα) treated NZB/W F1 mice
NZB/W mice were treated at 12 weeks of age with a single i.v. injection of Ad-IFNα (3.3 × 108 viral particles), a dose optimized to induce proteinuria starting at 22–25 days (6). Once fixed proteinuria of > 300mg/dl was detected on 2 occasions 24 hours apart, the mice were randomized to treatment with a single i.p. injection of CTX (Cytoxan; Bristol-Myers Squibb, New York, NY) 50mg/kg together with CTLA4Ig (100ug) and anti-CD40L antibody (250ug) 6 doses each over two weeks (triple therapy), or with CTX together with 6 doses of CTLA4Ig over two weeks (double therapy), or with single agents alone (9). Controls received no treatment. Sera were collected and proteinuria was measured twice weekly. Six to fourteen mice per group were sacrificed and analyzed four weeks after treatment initiation. In a separate experiment, NZB/W F1 mice injected with Ad-IFNα (3.3 × 108 (n=15) or 1.0 × 108 viral particles (n=15)) were treated with triple therapy at the onset of fixed proteinuria, and the survival rate of these mice was compared to that of untreated controls.
All experiments using animals were carried out according to protocols that were reviewed and approved by the Institutional Animal Care and Use Committee of the Feinstein Medical Research Institute.
Serum immunoglobulin levels and anti-DNA antibody levels
These were performed by ELISA as previously described (12, 16). Standard curves for immunoglobulin were established using serial dilutions of purified murine IgM, IgG2a, or IgG3 (Sigma-Aldrich, St. Louis, MA) and data expressed in ug/ml. Standard curves for dsDNA binding were obtained using sera from a high-titer mouse assigned an arbitrary level of 512 U and run in serial dilution on each plate.
ELISpot assay
ELISpot assays for total immunoglobulin-secreting cells and for anti-dsDNA–secreting cells were performed as previously described (16).
Flow cytometry analysis of spleen and peripheral blood
Spleen and PBMCs were analyzed for cell surface markers as previously described (17). In brief, B cells were gated using anti-CD19. T1 cells were CD23lo/IgMhi/CD21−, T2 cells were CD23hi/IgMhi/CD21+, marginal zone cells were CD23lo/IgMhi/CD21hi, follicular cells were IgDhi/IgMint and class switched cells were IgDneg/IgMneg. Plasma cells were B220int/CD138hi. CD4+ T cells were classified as memory (CD44hi/CD62Llo) or naïve (CD44lo/CD62Lhi). TFH cells were CD4+/CXCR5hi/PD-1hi/PSGL1lo as previously described (18)
Immunohistochemistry and immunofluorescence
H and E sections were scored for glomerular damage and interstitial inflammation as previously described (12). Cryosections (5 μm) of kidney and spleen were stained (17) with the following antibodies: FITC-conjugated anti-mouse IgG2a, IgG3 (Southern Biotech, Birmingham, Alabama), peanut agglutinin (PNA - Vector Laboratories, Inc. Burlingame, CA), or PE-conjugated anti-mouse IgD (BD Pharmingen, San Diego, CA). Images were captured using a Zeiss Axiocam digital camera connected to a Zeiss Axioplan2 microscope.
Real-time PCR analysis of sorted splenic B cells, total spleen cells and kidney cells
Real-time PCR was performed as previously described (6, 19). Data was first normalized to β-actin expression and then to the mean of naive controls which was given an arbitrary value of 1.
Serum cytokine levels
Serum IFNα levels were measured by commercial ELISA (PBL, Piscataway, NJ) at Day 3 and Day 14 after adenovirus injection in groups of 4–5 mice. Serum levels of IL-6, IL-17, IL-21, BAFF, IFNγ and TNFα were measured in groups of 4–8 mice using a commercial multiplex assay (Assaygate, Inc. Ijamsville, MD). Experiments were repeated once.
Statistics
Survival data were analyzed using Kaplan-Meier curves and log-rank test. Comparisons in the other figures and tables were performed using Mann-Whitney test. p values ≤ 0.05 were considered significant.
RESULTS
Disease induction
We have previously reported that Ad-IFNα but not control Ad-LacZ induces the onset of SLE in NZB/W F1 mice starting 3–4 weeks after virus administration and that this is associated with formation of large germinal centers, production of autoantibodies and activation of CD4 T cells and dendritic cells (6). In contrast, no immune activation occurs in Ad-LacZ treated mice and they do not develop accelerated disease (6). Increased serum levels of IFNα were detected at both Day 3 (78 +/− 52 pg/ml) and Day 14 (212 +/− 110 pg/ml), after Ad-IFNα administration compared with undetectable levels (<25pg/ml) in naïve or Ad-LacZ injected controls (either 3.3 × 108 or 3 × 109 particles) and markedly increased IFNα mRNA expression was detected in the spleens of Ad-IFNα treated mice for 2 weeks followed by a slow decline over the subsequent 4 weeks. This was accompanied by increased expression of IFN inducible genes in the Ad-IFNα treated mice but not in Ad-LacZ treated controls (Supplementary Figure 1). Due to the low dose administered, neither virus induced an acute cytokine response (20, 21) at 1 hr (data not shown).
TACI-Ig treatment delays disease onset in Ad-IFNα treated NZB/W F1 mice
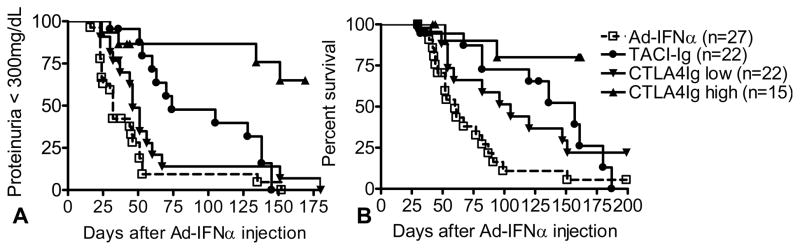
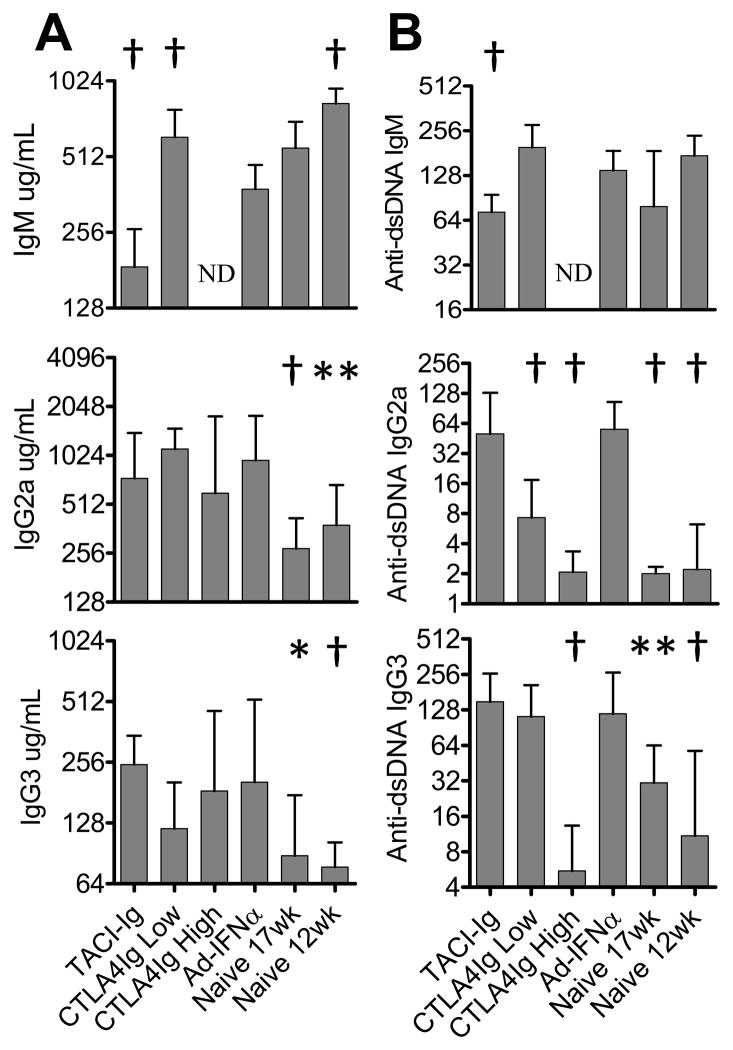
Serum levels of BAFF increased in IFNα treated mice starting 2 weeks after Ad-IFNα injection (6). To determine whether BAFF contributes to the accelerated disease in Ad-IFNα treated mice, we administered continuous TACI-Ig treatment 500ug three times weekly starting on the same day as Ad-IFNα injection or 3 weeks later. TACI-Ig treatment at Day 0, but not at Day 21 (Supplementary Figure 2) delayed the onset of proteinuria (p = 0.0005, Figure 1A) and death (p = 0.0077, Figure 1B) in Ad-IFNα treated mice. TACI-Ig treatment significantly decreased the numbers of transitional 2 (T2), marginal zone, and follicular B cells in the spleen (Table I). Despite the depletion of B cells, CD138+ plasma cells/plasmablasts were found in comparable numbers in the spleens of TACI-Ig treated and untreated mice (Table I). TACI-Ig treatment significantly lowered serum levels of both total IgM and IgM anti-dsDNA antibodies but it had no effect on IgG antibodies (Figure 2). ELISpot assays confirmed that TACI-Ig treated mice had significantly fewer total IgM secreting cells in the spleens (Figure 3A) but no decrease in IgG secreting cells. Immunofluorescence staining of spleens and kidneys indicated that TACI-Ig treatment had no effect on the formation of GCs, the generation of IgG producing cells in spleen, or glomerular IgG deposition (Figure 3B and 4B). Despite the presence of renal IgG deposits, TACI-Ig treated mice showed significantly less glomerular and interstitial damage than untreated controls (Figure 4A).
Figure 1.
Effects of treatments on proteinuria onset and survival. Proteinuria (A) and survival (B) of Ad-IFNα treated NZB/W F1 mice. p values against Ad-IFNα treated controls: p = 0.0005 (proteinuria) and p = 0.0077 (survival), TACI-Ig treated mice; p = 0.0555 (proteinuria) and p = 0.0483 (survival), low dose CTLA4Ig treated mice; p<0.0001 (proteinuria and survival), high dose CTLA4Ig treated mice. Data are pooled from two representative experiments with a total of 15–27 mice per group.
Table I.
Number of spleen cell subsets a
| No. of cells per spleen (mean ± SD) | TACI-Ig (n=8) | CTLA4-Ig low (n=7) | CTLA4-Ig high (n=5) | Untreated (n=7) | Naïve (n=6) |
|---|---|---|---|---|---|
| Total cell no. × 107 | 9.5 ± 2.5 d | 11.1 ± 2.2 | 11.8 ± 4.7 | 13.0 ± 3.1 | 8.1 ± 1.5 d |
| CD19 × 107 | 1.9 ± 0.8 b | 4.2 ± 1.1 | 3.4 ± 1.5 | 5.8 ± 1.6 | 3.6 ± 1.1 c |
| CD19/CD69 × 106 | 2.4 ± 1.5 b | 1.6 ± 0.7 d | 1.1 ± 0.6 b | 4.0 ± 2.3 | 1.2 ± 0.5 d |
| Follicular × 107 | 0.6 ± 0.3 d | 2.5 ± 0.9 | ND | 3.0 ± 0.7 | 2.1 ± 0.8 |
| T1 × 106 | 2.2 ± 1.1 d | 2.7 ± 0.8 | ND | 5.7 ± 2.1 | 2.2 ± 0.4 d |
| T2 × 106 | 1.3 ± 0.8 b | 7.8 ± 3.5 | ND | 7.7 ± 2.4 | 5.2 ± 2.1 |
| T1/T2 | 2.1 ± 1.0 b | 0.4 ± 0.2 | ND | 0.8 ± 0.3 | 0.4 ± 0.1 b |
| MZ × 106 | 0.7 ± 0.5 b | 4.9 ± 1.7 | ND | 6.7 ± 1.9 | 3.7 ± 0.9 d |
| IgM−/IgD−x 106 (switched) | 5.8 ± 3.3 | 2.0 ± 1.2 b | 2.0 ± 1.0 b | 8.2 ± 5.1 | 1.7 ± 0.8 d |
| CD138+ × 106 | 1.5 ± 1.2 | 0.9 ± 0.4 b | 0.5 ± 0.3 b | 3.0 ± 2.3 | 0.7 ± 0.3 d |
| CD4 × 107 | 3.8 ± 1.0 | 3.6 ± 0.8 | 3.1 ± 1.3 | 3.7 ± 0.8 | 2.4 ± 0.5 d |
| CD4/CD69 × 107 | 6.2 ± 2.1 | 2.4 ± 2.6 d | 1.0 ± 0.6 b | 5.4 ± 2.8 | 1.5 ± 0.8 d |
| CD4/CD44+/CD62L− (memory) × 107 | 1.0 ± 0.4 | 0.6 ± 0.3 c | 0.4 ± 0.2 b | 1.1 ± 0.4 | 0.6 ± 0.1 d |
| CD4/CD44−/CD62L+ (naive) × 107 | 2.4 ± 0.7 | 2.7 ± 0.4 b | 2.5 ± 1.1 | 2.2 ± 0.7 | 1.6 ± 0.3 b |
| CD8 × 107 | 1.9 ± 0.7 | 2.0 ± 0.3 d | 1.6 ± 0.6 | 1.5 ± 0.5 | 1.2 ± 0.2 |
| CD11b/CD11c × 106 | 0.6 ± 0.3 b | 0.6 ± 0.4 b | 0.6 ± 0.3 | 1.0 ± 0.3 | 0.3 ± 0.1 d |
p values are compared with untreated control Ad-IFNα mice. Only significant p values are shown.;
p < 0.01;
p < 0.02;
p < 0.05.
Figure 2.
Effects of treatments on serum Ig levels. Serum Ig (A) and anti-dsDNA Ig (B) levels in different groups of mice were quantitated by ELISA. p values are compared with Ad-IFNα treated controls. Median + interquartile range shown *: p < 0.05, **: p < 0.02, †: p < 0.01. Data are representative of 2 experiments of 5–15 mice per group. ND: not done
Figure 3.
Effects of treatments on germinal centers and plasma cells in the spleen. A: the numbers of IgG or IgM plasma cells per spleen from different groups of mice were determined by ELISpot assay. p values are compared with Ad-IFNα treated controls. Median + interquartile range shown *: p < 0.05, **: p < 0.02, †: p < 0.01. Data are representative of 2 experiments of 5–10 mice per group. B: immunofluorescence staining of spleens (5X magnification) with anti-IgD (red), peanut agglutinin (green; upper), and anti-IgG2a (green; lower). Data are representative of two experiments of 5–8 mice per group. Results are similar between the spleens from the mice treated with high dose or low dose CTLA4Ig. IgG3 producing B cells were present even in naïve mice and their numbers were variable both in treated mice and Ad-IFNα controls (not shown).
Figure 4.
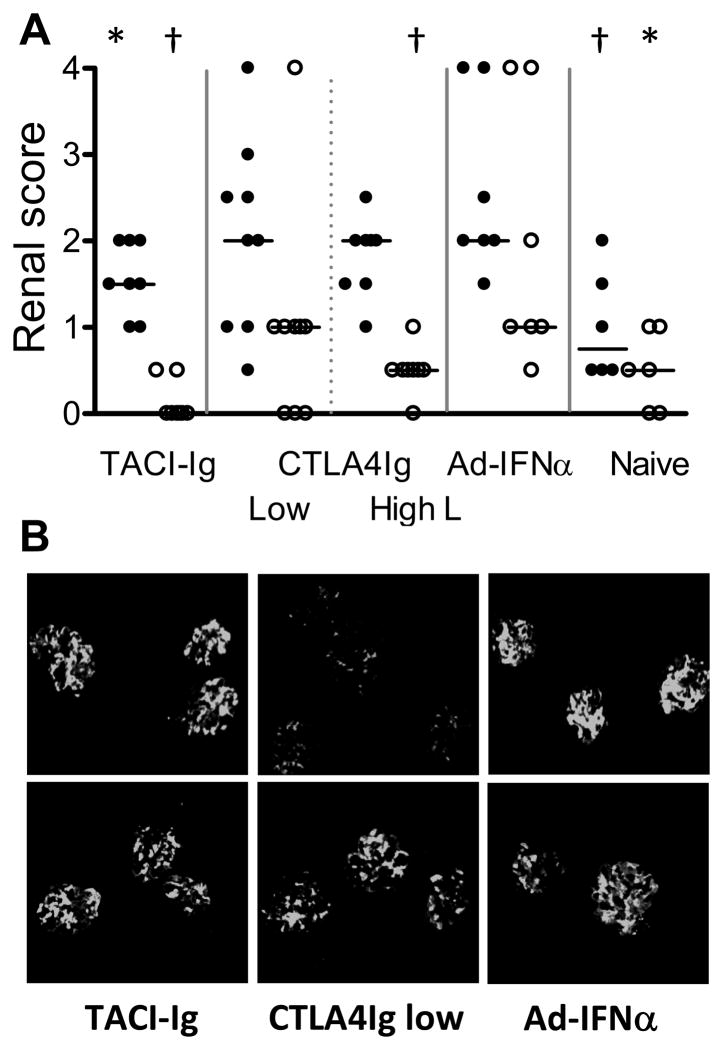
Effects of treatments on renal IgG deposition and damage. A: renal glomerular damage (filled circles) and interstitial inflammation (open circles) in different groups of mice. The mice with >300mg/dl proteinuria that died before tissue could be harvested were assigned a histological score of 4 for statistical analysis. p values are compared with Ad-IFNα treated controls. Horizontal bars represent median score. *: p < 0.05, †: p < 0.01. B: renal immunofluorescence staining with anti-IgG2a (upper) and anti-IgG3 (lower), 10X magnification. Results of high dose CTLA4Ig treated mice are variable and not shown (see text). Data are representative of two experiments with 4–5 mice per group.
High dose but not low dose CTLA4Ig delays disease onset in Ad-IFNα treated NZB/W F1 mice
Ad-IFNα treated mice were treated continuously with CTLA4Ig starting on the day of Ad-IFNα injection or 21 days later. CTLA4Ig treatment at Day 0 decreased the number of activated T cells and B cells as well as class switched B cells (Table I). There were significantly fewer CD138+ plasma cells in the spleens of CTLA4Ig treated mice than in those of the untreated controls (p = 0.035, vs. untreated controls; Table I). Surprisingly however, CTLA4Ig given at a dose of 100ug 3 times per week, sufficient to prevent disease in conventional NZB/W mice [(22) and our unpublished data], had only a modest effect on the onset of proteinuria (p = 0.0555, Figure 1A) and death of the mice (p = 0.0483, Figure 1B). We therefore repeated this experiment with a higher dose of CTLA4Ig (200ug 3 times per week) and found a significant improvement in clinical outcome (p<0.0001, both proteinuria and survival; Figure 1). Interestingly, 6/10 mice developed proteinuria during the 6–8 week window when IFNα derived from the exogenous adenovirus was expressed in the serum. By day 160 however proteinuria reversed in 2 mice and only 2/10 had died.
Serological studies showed that neither dose of CTLA4Ig treatment affected serum levels of total IgG2a or IgG3 (Figure 2A), although it inhibited GC formation in the spleen (Figure 3B). Low dose CTLA4Ig treatment resulted in a significant decrease in serum IgG2a anti-dsDNA antibody levels (Figure 2B) and in the number of anti-dsDNA IgG secreting cells in the spleen (Figure 3A) but it had no effect on the serum levels of anti-dsDNA IgG3 antibodies (Figure 2B). High dose CTLA4Ig attenuated the production of both IgG2a and IgG3 autoantibodies in the serum initially (Figure 2B) as well as the number of IgG anti-dsDNA secreting cells in the spleen (Figure 3A) but 6/8 mice that survived long-term developed either IgG2a or IgG3 anti-dsDNA antibodies over time despite continuous therapy (data not shown).
Immunohistochemical analysis of kidney sections revealed significantly less IgG2a deposition in the glomeruli of low dose CTLA4Ig treated mice than in untreated controls (Figure 4B). In contrast, comparable levels of renal IgG3 deposition were detected in low dose CTLA4Ig treated and untreated mice (Figure 4B) and the mice demonstrated similar levels of glomerular and interstitial damage compared with the untreated controls (Figure 4A). Interestingly, some of the mice in the high dose CTLA4Ig group had glomerular antibody deposition in the absence of a serum anti-dsDNA response, indicating that non-DNA binding autoantibodies had arisen (data not shown). Nevertheless survival was markedly prolonged in this group of mice (Figure 1B) and they had a significantly lower interstitial renal score when they were sacrificed at 35 wks of age than IFNα controls sacrificed at 19 wks (Figure 4A).
Delayed low dose CTLA4Ig treatment had a similar effect on proteinuria and survival as did treatment beginning at Day 0 (Supplementary Figure 2).
Effects of TACI-Ig and CTLA4Ig treatment on inflammatory mediators in Ad-IFNα treated NZB/W F1 mice
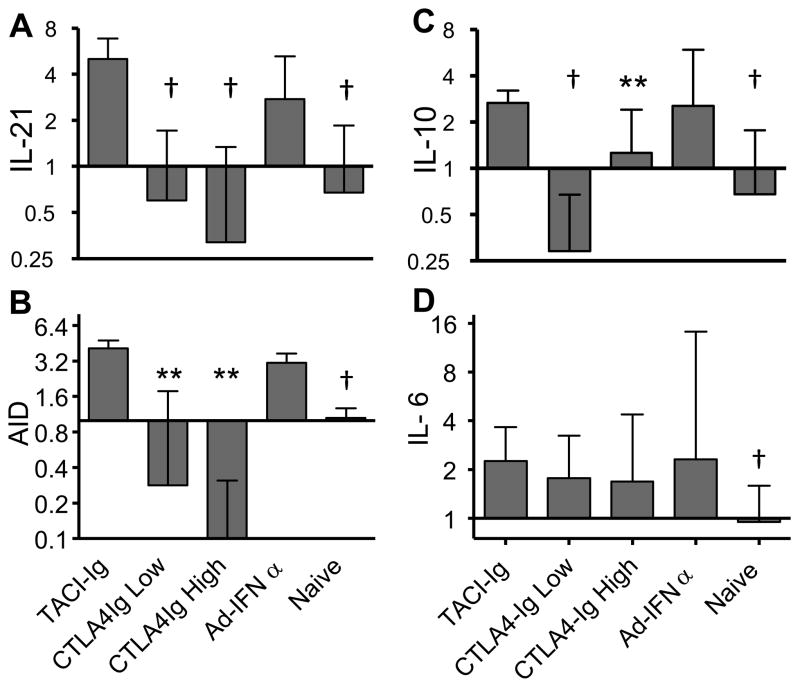
Using real-time PCR, we detected elevated levels of IL-6, IL-10, AID and IL-21 transcripts in the spleen cells of Ad-IFNα treated mice. Upregulation of AID, IL-21 and IL-10, but not of IL-6, was inhibited by both low and high dose CTLA4Ig (Figure 5). The expression of AID and IL-21 remained low in the spleen of mice treated with high dose CTLA4Ig treatment even after 23 weeks of treatment (data not shown) indicating that differentiation of follicular T helper cells was inhibited. In contrast, TACI-Ig treatment did not significantly affect the expression of any of these molecules in the spleen (Figure 5). However, TACI-Ig treatment resulted in significantly decreased serum levels of TNF-α (TACI-Ig treated (n=4) vs. Ad-IFNα controls (n=5), mean ± STD, 5.3 ± 4.7 vs. 25.9 ± 14.1 pg/mL, p = 0.0159). No changes were observed in the serum levels of the other cytokines measured (data not shown) and neither treatment altered the splenic expression of IFNα inducible genes (Supplementary Figure 1).
Figure 5.
Effects of treatments on the expression of selected genes in the spleen. Expression of IL-21(A), AID (B), IL-10 (C), and IL-6 (D) in spleen of different groups of mice. Bars represent fold expression, median + interquartile range shown. p values are compared with Ad-IFNα treated controls. Data are representative of two experiments of 4–8 mice per group.
We have previously shown that the kidneys of NZB/W mice express a number of inflammatory mediators at the onset of proteinuria and that some of these decrease to pre-nephritic levels after remission induction, suggesting that they are biomarkers for active renal inflammation (19). Some of these mediators are elaborated by renal mononuclear phagocytes that become activated at the onset of disease (19, 23) and a subset of these was tested by real-time PCR. We detected elevated expression of matrix metalloproteinase (MMP)-14, CCL2, CCL5, osteopontin, CXCL1, ITGAM, CXCL13, IKBKE, CCL20, LCN2, and VCAM-1 by real-time PCR in the kidneys of Ad-IFNα treated mice compared with age matched naïve controls. Of these, TACI-Ig treatment significantly inhibited the upregulation of MMP-14, CCL5, IKBKE, CCL20, and LCN2 with a trend towards downregulation of CXCL13. Similarly, in long term survivors of high dose CTLA4Ig treatment we observed decreased renal expression of ITGAM, LCN2, IKBKE and MMP14 after 23 weeks of treatment compared with IFNα treated mice harvested at the age of 19 weeks (Supplementary Figure 3). These findings show a decrease in renal inflammation consistent with the lower renal histologic scores.
Triple therapy induces remission in Ad-IFNα treated NZB/W F1 mice
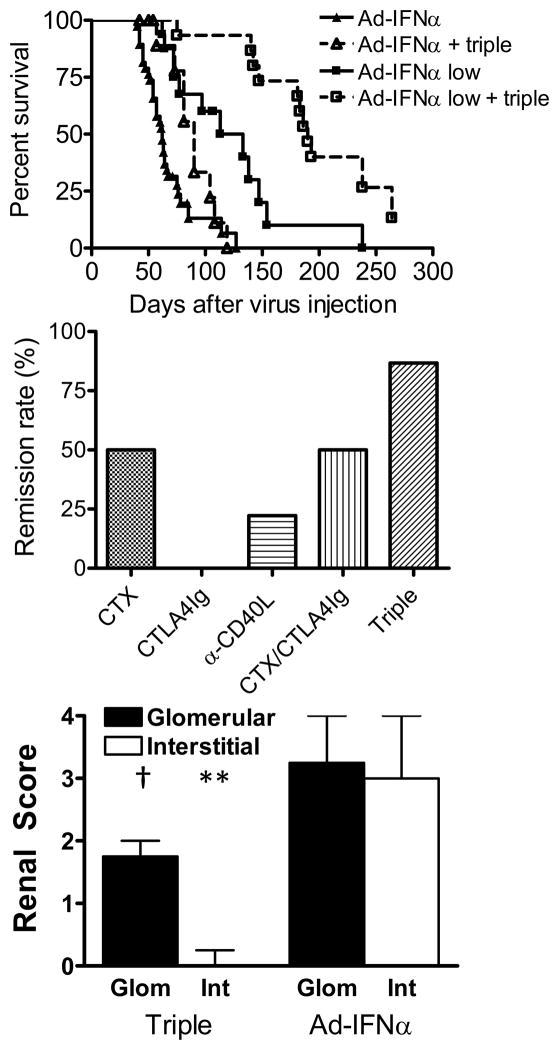
We have previously shown that therapy with a single dose of CTX, together with a 2 week course of CTLA4Ig and anti-CD40L antibodies induces prolonged remission of established kidney disease in NZB/W F1 mice (9). In Ad-IFNα treated mice triple therapy induced complete remission of proteinuria (defined as proteinuria ≤ 30mg/dL measured at least twice, one week apart) in 86.7% of mice treated with 3.3 × 108 viral particles and in 66.7% of mice treated with 1.0 × 108 viral particles. Triple therapy significantly prolonged the life span of Ad-IFNα treated mice (Figure 6A) but the mice that had received the higher dose of Ad-IFNα mice relapsed more rapidly than their low dose treated counterparts (mean remission duration of high dose Ad-IFNα treated mice vs. low dose Ad-IFNα treated mice = 15.9 ± 14.0 days vs. 73.4 ± 52.5 days; p = 0.0097). Control studies were performed in mice receiving the higher virus dose. CTLA4Ig alone did not induce remission in these mice whereas single therapy with either CTX or anti-CD40L achieved 50% and 22.2% remission rates respectively (Figure 6B). Combined therapy with CTX and CTLA4Ig induced remission in half of the mice (Figure 6B). Even when this combination was started at Day 21, prior to the onset of proteinuria, the benefit was no different to that of CTX alone (Supplementary Figure 2). Mice treated with triple therapy showed significantly less glomerular (p = 0.0033) and interstitial damage (p = 0.0129) than the untreated controls (Figure 6C) whereas mice that received control single or double therapy showed similar levels of renal damage compared to the untreated mice (data not shown).
Figure 6.
Effects of triple therapy on the survival and proteinuria of nephritic Ad-IFNα treated NZB/W F1 mice. A: Survival of the mice treated with a regular or low dose of Ad-IFNα alone, or together with triple therapy. Triple therapy was started upon proteinuria onset. p = 0.0308, regular dose Ad-IFNα treated mice with vs. without triple therapy; p =0.0014, low dose Ad-IFNα treated mice with vs. without triple therapy. B: Percentage of mice in which complete remission was induced by triple therapy. Complete remission is defined as proteinuria ≤ 30mg/dL for at least one week. Data are pooled from three representative experiments with a total of 15–34 mice per group. C: Renal glomerular damage and interstitial inflammation in triple therapy treated mice and Ad-IFNα only controls. Median + interquartile range shown. The mice that died before tissue could be harvested were assigned a histological score of 4 for statistical analysis. p values are compared with Ad-IFNα only controls. **: p < 0.02, †: p < 0.01.
Spleens were harvested for phenotypic analysis 3–4 weeks after treatment initiation (Table II). Triple therapy reduced the number of follicular (p = 0.0008, vs. untreated controls), activated (p = 0.0429) and class switched B cells (p = 0.0012) but not MZ B cells in the spleens. We have previously shown that the IFNα induced expansion of MZ B cells in the spleen is T cell independent. The total number of splenic CD4 T cells was not affected by triple therapy. However, fewer CD69+ activated (p = 0.0012) and CD44+/CD62L− effector memory CD4 T cells (p = 0.0012) were found in spleens of triple therapy treated mice compared to those of untreated controls. Splenocytes from mice treated with double therapy showed a similar phenotype to that of the triple therapy treated mice. Mice treated with anti-CD40L antibody showed fewer class switched B cells and effector memory T cells in the spleens than the untreated controls. Only a decrease in follicular B cells was observed 4 weeks after a single dose of CTX. CTLA4Ig treatment alone had no effect on the phenotype of splenocytes in Ad-IFNα treated mice, consistent with its lack of therapeutic effect (Table II).
Table II.
Number of spleen cell subsetsa
| No. of cells per spleen (mean ± SD) | CTLA4Ig (n=5) | CTX (n=5) | α-CD40L ab (n=5) | CTX/CTLA4Ig (n=5) | Triple therapy (n=6) | IFNα (n=8) |
|---|---|---|---|---|---|---|
| Total cell no. × 107 | 15.5 ± 3.6 | 13.4 ± 3.9 | 12.1 ± 1.9 | 10.2 ± 2.5 | 11.0 ± 2.7 | 16.3 ± 5.7 |
| CD19 × 107 | 6.5 ± 1.4 | 5.7 ± 1.6 | 5.3 ± 0.9 | 5.4 ± 1.5 | 3.8 ± 1.0 b | 8.7 ± 2.8 |
| CD19/CD69 × 106 | 4.4 ± 2.0 | 3.2 ± 2.1 | 2.8 ± 0.4 | 0.9 ± 0.3 b | 1.8 ± 1.2 c | 4.9 ± 2.7 |
| Follicular × 107 | 4.1 ± 0.9 | 2.5 ± 0.4 c | 2.9 ± 0.2 | 2.9 ± 1.1 d | 1.6 ± 0.5 b | 4.8 ± 1.8 |
| MZ × 106 | 3.8 ± 1.4 | 8.3 ± 3.2 | 4.5 ± 1.1 | 10.8 ± 3.8 | 9.1 ± 3.1 | 9.4 ± 2.7 |
| IgM−/IgD− × 106 (switched) | 6.3 ± 1.5 | 7.7 ± 3.6 | 3.9 ± 2.5 d | 2.6 ± 1.1 b | 0.7 ± 0.3 b | 8.2 ± 2.5 |
| CD4 × 107 | 4.9 ± 0.6 | 4.1 ± 1.2 | 3.9 ± 0.5 | 2.4 ± 0.5 | 3.2 ± 0.5 | 4.6 ± 2.4 |
| CD4/CD69 × 107 | 1.0 ± 0.5 | 0.8 ± 0.2 | 0.5 ± 0.2 | 0.2 ± 0.1b | 0.2 ± 0.1 b | 1.1 ± 0.6 |
| CD4/CD44+/CD62L− (memory) × 107 | 2.2 ± 1.0 | 1.7 ± 0.4 | 1.3 ± 0.5 c | 1.2 ± 0.3 b | 0.9 ± 0.3 b | 3.0 ± 1.8 |
| CD4/CD44−/CD62L+ (naive) × 107 | 2.0 ± 1.3 | 1.7 ± 1.0 | 2.1 ± 0.5 | 0.8 ± 0.3 | 1.8 ± 0.9 | 0.9 ± 1.1 |
| CD8 × 107 | 1.5 ± 1.1 | 1.8 ± 0.8 | 1.6 ± 0.2 | 1.1 ± 0.5 | 2.0 ± 0.8 | 1.3 ± 0.5 |
p values are compared with untreated Ad-IFNα pretreated controls. Only significant p values are shown.
p < 0.01;
p < 0.02;
p < 0.05.
Triple therapy eliminates GCs and reduces the numbers of antibody-secreting cells (ASCs) in the spleens of Ad-IFNα treated NZB/W F1 mice
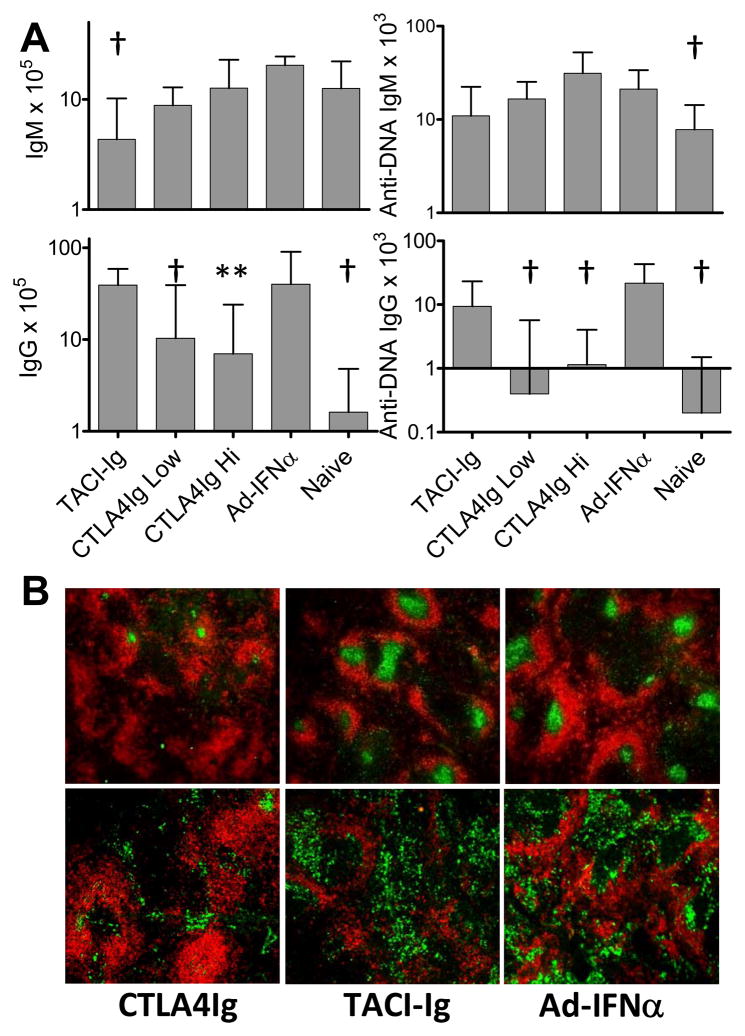
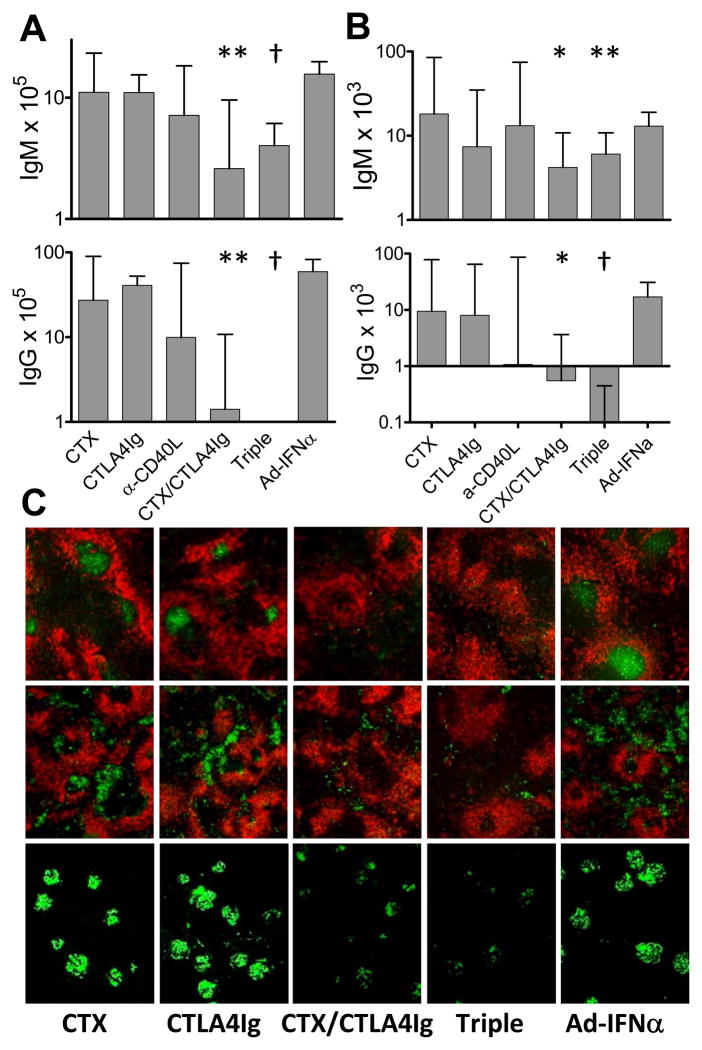
By ELISpot assay, triple therapy treated mice had a significant reduction in the number of total and anti-dsDNA IgM and IgG secreting cells in the spleens (Figures 7A, 7B). Similar effects were observed in the mice receiving double therapy (Figure 7A, 7B). The number of antibody secreting cells in the spleens of Ad-IFNα treated mice treated with anti-CD40L, CTLA4Ig, or CTX single therapy was not altered by the time spleens were harvested 4 weeks after therapy (Figure 7A, 7B).
Figure 7.
Effects of triple therapy on germinal centers and autoantibody production. The numbers of total (A) or anti-dsDNA (B) IgG or IgM producing plasma cells per spleen from different groups of mice were determined by ELISpot assay. p values are compared with Ad-IFNα only controls. Median + interquartile range shown *: p < 0.05, **: p < 0.02, †: p < 0.01. Data are pooled from three representative experiments with a total of 15–34 mice per group. C: immunofluorescence staining of spleens (upper and middle - 5X magnification) or kidneys (lower–10X magnification) with anti-IgD (red) and peanut agglutinin (green; upper), or anti-IgG2a (green; middle and lower). Results of anti-CD40L treated mice are variable and not shown. Data are representative of 5–6 mice per group.
The decrease of IgG secreting cells in the spleens from the mice treated with triple or double therapy was confirmed by immunofluorescence staining and was associated with abolished development of germinal centers in the spleens. In contrast, the mice receiving single therapy with CTLA4Ig or CTX had comparable GCs and IgG producing cells in their spleens to those of the untreated controls (Figure 7C). The mice treated with anti-CD40L antibody showed variable results that correlated with the low rate of remission induced by this therapy (data not shown).
The decrease in serum autoantibodies and the lack of renal damage in the mice treated with triple therapy was in parallel with greatly diminished glomerular IgG deposition (Figure 7B). Double therapy also reduced glomerular IgG deposits, albeit to a lesser extent (Figure 7B). In contrast, single CTX or CTLA4Ig treatment did not reduce glomerular IgG deposition (Figure 7B). Thus remission induced by triple therapy was due not only to the downstream effects on the kidneys that we have previously described (9) but also to clearance of renal immune complex deposits.
DISCUSSION
IFNα is a key cytokine in the pathogenesis of SLE (24) and its overexpression accelerates disease progression several murine SLE models (7, 25, 26). We have previously shown that IFNα accelerated lupus is accompanied by T and B cell activation and GC formation, elevated serum levels of IgG2a and IgG3 autoantibodies, increased production of BAFF, IL-6 and TNFα and upregulation of TLR7 in splenic B cells. Nevertheless, T cells are absolutely required for initiation of disease in the IFNα accelerated model. In the present study, we assessed the importance of B7-CD28 costimulation and BAFF/APRIL signaling in the pathogenesis of IFNα accelerated lupus using CTLA4Ig and TACI-Ig treatment, respectively. We show here that low dose CTLA4Ig treatment did not prevent or delay the onset of nephritis in Ad-IFNα treated mice despite preventing T and B cell activation, GC formation, and production of pathogenic IgG2a anti-dsDNA antibodies. Resistance to low dose CTLA4Ig was likely due to persistence of pathogenic IgG3 autoantibodies that were only attenuated after administration of high dose CTLA4Ig. Even in mice treated with high dose CTLA4Ig, protection was not complete and kidney deposition of IgG eventually occurred despite continued treatment. Nevertheless, CTLA4Ig treatment markedly delayed proteinuria onset and protected the mice from interstitial inflammation. In contrast, TACI-Ig treatment significantly ameliorated IFNα accelerated lupus without affecting lymphocyte activation, GC formation, production of autoantibodies or deposition of IgG2a and IgG3 in the kidneys.
B7-CD28 costimulation is absolutely required for the break of tolerance in conventional NZB/W F1 mice, as prophylactic CTLA4Ig treatment decreases both class switching and somatic mutation, and prevents the production of pathogenic IgG anti-dsDNA antibodies (16, 22). In Ad-IFNα treated mice, we have evidence that IgG2a autoantibodies derive predominantly from germinal centers whereas IgG3 anti-dsDNA antibodies derive predominantly from extrafollicular sources (6). The pathogenicity of the anti-dsDNA IgG3 antibodies that arise in Ad-IFNα treated mice may be the result of either T cell driven clonal expansion of high affinity B cells or T cell dependent affinity maturation that takes place in extrafollicular foci (27). Recent studies have identified an expanded extrafollicular Th population in SLE prone mice (28–30) that mediates IgG production through IL-21 and CD40L. Our data suggest that T cells supporting the extrafollicular response are less dependent on B7-CD28 costimulation than are germinal center TFH cells.
We have previously shown that the expression of IL-21 and IL-6 is elevated in the spleens of Ad-IFNα treated mice (6). CTLA4Ig treatment inhibited the upregulation of IL-21 but did not prevent the elevated expression of IL-6 in the spleens of Ad-IFNα treated mice. The ability of IL-6 to promote the production of anti-dsDNA antibodies has previously been implicated in the pathogenesis of lupus (31–33). While both IL-6 and elevated serum levels of BAFF may contribute to the relative resistance of IFNα treated mice to CTLA4Ig, these are not sufficient to induce autoantibodies if T cells are completely absent (6).
Ad-IFNα treated NZB/W F1 mice treated with TACI-Ig manifested a delay in onset of proteinuria and prolonged survival despite the robust production of pathogenic autoantibodies. Although TACI-Ig treatment reduced the number of splenic IgM plasma cells, the short-lived IgG plasma cells that produce pathogenic autoantibodies in the IFNα induced model (6, 34) were totally unaffected by TACI-Ig treatment. It is not entirely clear why IgM-producing plasma cells are more sensitive to BAFF/APRIL blockade than are IgG-producing plasma cells. One explanation involves the strength of BCR signaling itself since IgG expressing cells have different rates of BCR clustering (35), an exaggerated calcium flux and different gene expression compared with IgM-bearing cells (36). The resistance of splenic IgG plasma cells to TACI-Ig treatment in IFNα treated NZB/W mice is different from findings previously reported in NZM2410 and MRL/lpr mice (37–39). This discrepancy suggests that support for IgG plasma cell survival after IFNα acceleration is due to extrinsic factors that render these cells independent of BAFF/APRIL signaling for their survival.
It has been previously shown that immune complex deposition in the kidneys does not lead to renal pathology unless renal effector cells are also activated (9, 40, 41). In conventional NZB/W F1 mice TACI-Ig mediated B cell depletion results in significantly decreased numbers of activated T cells and dendritic cells in the spleens, which is associated with an overall decrease in circulating inflammatory cytokines and dampened endothelial activation, thus decreasing inflammatory cell infiltration into target organs (12, 13, 17). In Ad-IFNα treated NZB/W F1 mice however, TACI-Ig mediated B cell depletion did not result in significant decreases in spleen size or impairment of T cell activation, nor did it affect the splenic expression of IL-21 and the T cell dependent production of pathogenic IgG anti-dsDNA antibodies. Finally, although B cells have been shown to produce IL-6 in lupus animal models and in SLE patients (42, 43), the expression of IL-6 in the spleens of Ad-IFNα treated mice was not inhibited by TACI-Ig treatment. These observations in sum suggest that TACI-Ig treatment may not protect Ad-IFNα treated mice entirely by depleting B cells.
An alternate explanation is that TACI-Ig treatment directly targets the kidney. Ad-IFNα treated NZB/W F1 mice develop interstitial infiltrates of macrophages at the onset of proteinuria (6). We found that TACI-Ig treatment decreased renal infiltration with macrophages and the infiltrating cells failed to upregulate CD11b, a hallmark of renal inflammation (11, 19). In addition, our study shows that despite substantial renal immune complex deposition, the upregulation of some inflammatory markers (MMP-14, CCL5, CCL20, IKBKE, and LCN2) in the kidneys of Ad-IFNα treated NZB/W F1 mice was significantly inhibited by TACI-Ig treatment. Many of these inflammatory mediators are produced by infiltrating mononuclear phagocytes that have encountered immune complexes (19, 23, 44). Furthermore TACI-Ig prevented the increase of serum levels of TNFα that occurs at nephritis onset. In NZM2410 mice that typically have few renal infiltrating cells, TACI-Ig treatment similarly reduced renal damage, and activation of renal macrophages and endothelial cells (11). These findings suggest that TACI-Ig treatment may exert its protective role by inhibiting the initial activation of intrinsic renal cells upon encountering immune complexes, leading to less production of inflammatory mediators in the kidney. A direct effect of BAFF on dendritic cells has been reported by Lam et. al. who showed that silencing of BAFF in the inflamed synovium decreases local dendritic cell activation (45). Further experiments are required to identify the cells bearing BAFF/APRIL receptors in the inflamed kidneys of Ad-IFNα treated NZB/W F1 mice.
We previously demonstrated that triple therapy with CTX, anti-CD40L and CTLA4Ig induced remission in 84% of NZB/W F1 mice with established nephritis (9). We show here that a similar percentage of Ad-IFNα treated mice entered remission after triple therapy however they relapsed rapidly. Mice treated with high dose IFNα virus relapsed more quickly than mice treated with a lower dose of virus and these in turn relapsed more quickly than conventional NZB/W mice (9). Triple therapy markedly reduced the production of pathogenic anti-dsDNA antibodies in Ad-IFNα treated NZB/W F1 mice and reversed both glomerular and interstitial damage as it does in conventional NZB/W mice (19) but this was only temporary. Thus although the short-lived plasma cells induced by IFNα (6) are susceptible to cytotoxic reagents and costimulatory blockade, they rapidly return following the cessation of treatment. We have previously shown in NZB/W F1 mice that IFNα promotes renal infiltration of activated macrophages that produce matrix metalloproteinases and growth factors, resulting in early fibrosis and glomerular cell proliferation (44). Based on these studies, we hypothesize that IFNα may also promote the return of activated macrophages to the kidneys once immune complexes deposit again, leading to relapse.
Taken together, our findings show that IFNα does not merely accelerate the progress of lupus; it also alters important aspects of the disease and renders mice more resistant to immune modulation. IFNα has similarly been shown to prevent anti-CD40L antibody from establishing tolerance in an animal model of skin transplantation by enhancing the expression of costimulatory molecules on DCs and consequently promoting CD8 T cell priming (46, 47). These findings are clinically relevant as lupus patients with the IFNα signature may have clinical features that are distinct from the general population of SLE patients (48–51) and may respond differently to therapies. This calls for special consideration when designing clinical studies or developing therapeutics for SLE.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants R01 AI083901 and R01 AI059636-01A2 (AD) and Rheuminations (RB).
References
- 1.Bertsias GK, Salmon JE, Boumpas DT. Therapeutic opportunities in systemic lupus erythematosus: state of the art and prospects for the new decade. Ann Rheum Dis. 2010;69:1603–1611. doi: 10.1136/ard.2010.135186. [DOI] [PubMed] [Google Scholar]
- 2.Baccala R, Hoebe K, Kono DH, Beutler B, Theofilopoulos AN. TLR-dependent and TLR-independent pathways of type I interferon induction in systemic autoimmunity. Nat Med. 2007;13:543–551. doi: 10.1038/nm1590. [DOI] [PubMed] [Google Scholar]
- 3.Blanco P, Palucka AK, Gill M, Pascual V, Banchereau J. Induction of dendritic cell differentiation by IFN-alpha in systemic lupus erythematosus. Science (New York, NY. 2001;294:1540–1543. doi: 10.1126/science.1064890. [DOI] [PubMed] [Google Scholar]
- 4.Groom JR, Fletcher CA, Walters SN, Grey ST, Watt SV, Sweet MJ, Smyth MJ, Mackay CR, Mackay F. BAFF and MyD88 signals promote a lupuslike disease independent of T cells. J Exp Med. 2007;204:1959–1971. doi: 10.1084/jem.20062567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jego G, Palucka AK, Blanck JP, Chalouni C, Pascual V, Banchereau J. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 6.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, Davidson A. Interferon-alpha accelerates murine systemic lupus erythematosus in a T cell-dependent manner. Arthritis Rheum. 2011;63:219–229. doi: 10.1002/art.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathian A, Weinberg A, Gallegos M, Banchereau J, Koutouzov S. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black x New Zealand White) F1 but not in BALB/c mice. J Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 8.Daikh DI, Finck BK, Linsley PS, Hollenbaugh D, Wofsy D. Long-term inhibition of murine lupus by brief simultaneous blockade of the B7/CD28 and CD40/gp39 costimulation pathways. J Immunol. 1997;159:3104–3108. [PubMed] [Google Scholar]
- 9.Schiffer L, Sinha J, Wang X, Huang W, von Gersdorff G, Schiffer M, Madaio MP, Davidson A. Short term administration of costimulatory blockade and cyclophosphamide induces remission of systemic lupus erythematosus nephritis in NZB/W F1 mice by a mechanism downstream of renal immune complex deposition. J Immunol. 2003;171:489–497. doi: 10.4049/jimmunol.171.1.489. [DOI] [PubMed] [Google Scholar]
- 10.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-{gamma} establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. The Journal of experimental medicine. 2010 doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Davidson A. Selective blockade of BAFF for the prevention and treatment of systemic lupus erythematosus nephritis in NZM2410 mice. Arthritis Rheum. 2010;62:1457–1468. doi: 10.1002/art.27368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, Porcelli S, Davidson A. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kahn P, Ramanujam M, Bethunaickan R, Huang W, Tao H, Madaio MP, Factor SM, Davidson A. Prevention of murine antiphospholipid syndrome by BAFF blockade. Arthritis Rheum. 2008;58:2824–2834. doi: 10.1002/art.23764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W, Szalai A, Zhao L, Liu D, Martin F, Kimberly RP, Zhou T, Carter RH. Control of spontaneous B lymphocyte autoimmunity with adenovirus-encoded soluble TACI. Arthritis Rheum. 2004;50:1884–1896. doi: 10.1002/art.20290. [DOI] [PubMed] [Google Scholar]
- 15.Navarra SV, Guzman RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, Leon MG, Tanasescu C, Nasonov E, Lan JL, Pineda L, Zhong ZJ, Freimuth W, Petri MA. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 16.Mihara M, Tan I, Chuzhin Y, Reddy B, Budhai L, Holzer A, Gu Y, Davidson A. CTLA4Ig inhibits T cell-dependent B-cell maturation in murine systemic lupus erythematosus. J Clin Invest. 2000;106:91–101. doi: 10.1172/JCI9244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 18.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. The Journal of experimental medicine. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP, Davidson A. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shayakhmetov DM, Li ZY, Ni S, Lieber A. Interference with the IL-1-signaling pathway improves the toxicity profile of systemically applied adenovirus vectors. J Immunol. 2005;174:7310–7319. doi: 10.4049/jimmunol.174.11.7310. [DOI] [PubMed] [Google Scholar]
- 21.Di Paolo NC, Miao EA, Iwakura Y, Murali-Krishna K, Aderem A, Flavell RA, Papayannopoulou T, Shayakhmetov DM. Virus binding to a plasma membrane receptor triggers interleukin-1 alpha-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Finck BK, Linsley PS, Wofsy D. Treatment of murine lupus with CTLA4Ig. Science. 1994;265:1225–1227. doi: 10.1126/science.7520604. [DOI] [PubMed] [Google Scholar]
- 23.Bethunaickan R, Berthier CC, Ramanujam M, Sahu R, Zhang W, Sun Y, Bottinger EP, Ivashkiv L, Kretzler M, Davidson A. A Unique Hybrid Renal Mononuclear Phagocyte Activation Phenotype in Murine Systemic Lupus Erythematosus Nephritis. J Immunol. 2011;186:4994–5003. doi: 10.4049/jimmunol.1003010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 25.Ramanujam M, Kahn P, Huang W, Tao H, Madaio MP, Factor SM, Davidson A. Interferon-alpha treatment of female (NZW x BXSB)F(1) mice mimics some but not all features associated with the Yaa mutation. Arthritis Rheum. 2009;60:1096–1101. doi: 10.1002/art.24414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairhurst AM, Mathian A, Connolly JE, Wang A, Gray HF, George TA, Boudreaux CD, Zhou XJ, Li QZ, Koutouzov S, Banchereau J, Wakeland EK. Systemic IFN-alpha drives kidney nephritis in B6.Sle123 mice. Eur J Immunol. 2008;38:1948–1960. doi: 10.1002/eji.200837925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herlands RA, William J, Hershberg U, Shlomchik MJ. Anti-chromatin antibodies drive in vivo antigen-specific activation and somatic hypermutation of rheumatoid factor B cells at extrafollicular sites. European journal of immunology. 2007;37:3339–3351. doi: 10.1002/eji.200737752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bubier JA, Sproule TJ, Foreman O, Spolski R, Shaffer DJ, Morse HC, 3rd, Leonard WJ, Roopenian DC. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc Natl Acad Sci U S A. 2009;106:1518–1523. doi: 10.1073/pnas.0807309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol. 2009;183:3139–3149. doi: 10.4049/jimmunol.0901690. [DOI] [PubMed] [Google Scholar]
- 30.Odegard JM, Marks BR, DiPlacido LD, Poholek AC, Kono DH, Dong C, Flavell RA, Craft J. ICOS-dependent extrafollicular helper T cells elicit IgG production via IL-21 in systemic autoimmunity. J Exp Med. 2008;205:2873–2886. doi: 10.1084/jem.20080840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mihara M, Ohsugi Y. Possible role of IL-6 in pathogenesis of immune complex-mediated glomerulonephritis in NZB/W F1 mice: induction of IgG class anti-DNA autoantibody production. International archives of allergy and applied immunology. 1990;93:89–92. doi: 10.1159/000235285. [DOI] [PubMed] [Google Scholar]
- 32.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. The Journal of clinical investigation. 1994;94:585–591. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clinical and experimental immunology. 1998;112:397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mathian A, Gallegos M, Pascual V, Banchereau J, Koutouzov S. Interferon-alpha induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZBxNZW)F1 mice but not in BALB/c mice. Eur J Immunol. 2011;41:863–872. doi: 10.1002/eji.201040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu W, Meckel T, Tolar P, Sohn HW, Pierce SK. Intrinsic properties of immunoglobulin IgG1 isotype-switched B cell receptors promote microclustering and the initiation of signaling. Immunity. 2010;32:778–789. doi: 10.1016/j.immuni.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horikawa K, Martin SW, Pogue SL, Silver K, Peng K, Takatsu K, Goodnow CC. Enhancement and suppression of signaling by the conserved tail of IgG memory-type B cell antigen receptors. The Journal of experimental medicine. 2007;204:759–769. doi: 10.1084/jem.20061923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujimura S, Kuwahara K, Ezaki T, Tomita K, Hirose S, Sakaguchi N. Spontaneous increase of plasma-like cells with high GANP expression in the extrafollicular region of lymphoid organs of autoimmune-prone mice. J Autoimmun. 2003;20:291–301. doi: 10.1016/s0896-8411(03)00041-6. [DOI] [PubMed] [Google Scholar]
- 38.Culton DA, O’Conner BP, Conway KL, Diz R, Rutan J, Vilen BJ, Clarke SH. Early preplasma cells define a tolerance checkpoint for autoreactive B cells. J Immunol. 2006;176:790–802. doi: 10.4049/jimmunol.176.2.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Erickson LD, Lin LL, Duan B, Morel L, Noelle RJ. A genetic lesion that arrests plasma cell homing to the bone marrow. Proc Natl Acad Sci U S A. 2003;100:12905–12910. doi: 10.1073/pnas.2131686100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clynes R, Dumitru C, Ravetch JV. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science. 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 41.Chan OT, Madaio MP, Shlomchik MJ. The central and multiple roles of B cells in lupus pathogenesis. Immunol Rev. 1999;169:107–121. doi: 10.1111/j.1600-065x.1999.tb01310.x. [DOI] [PubMed] [Google Scholar]
- 42.Thibault DL, Graham KL, Lee LY, Balboni I, Hertzog PJ, Utz PJ. Type I interferon receptor controls B-cell expression of nucleic acid-sensing Toll-like receptors and autoantibody production in a murine model of lupus. Arthritis research & therapy. 2009;11:R112. doi: 10.1186/ar2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tackey E, Lipsky PE, Illei GG. Rationale for interleukin-6 blockade in systemic lupus erythematosus. Lupus. 2004;13:339–343. doi: 10.1191/0961203304lu1023oa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Triantafyllopoulou A, Franzke CW, Seshan SV, Perino G, Kalliolias GD, Ramanujam M, van Rooijen N, Davidson A, Ivashkiv LB. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:3012–3017. doi: 10.1073/pnas.0914902107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lai Kwan Lam Q, King Hung Ko O, Zheng BJ, Lu L. Local BAFF gene silencing suppresses Th17-cell generation and ameliorates autoimmune arthritis. Proc Natl Acad Sci U S A. 2008;105:14993–14998. doi: 10.1073/pnas.0806044105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thornley TB, Phillips NE, Beaudette-Zlatanova BC, Markees TG, Bahl K, Brehm MA, Shultz LD, Kurt-Jones EA, Mordes JP, Welsh RM, Rossini AA, Greiner DL. Type 1 IFN mediates cross-talk between innate and adaptive immunity that abrogates transplantation tolerance. J Immunol. 2007;179:6620–6629. doi: 10.4049/jimmunol.179.10.6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller DM, Thornley TB, Pearson T, Kruger AJ, Yamazaki M, Shultz LD, Welsh RM, Brehm MA, Rossini AA, Greiner DL. TLR agonists prevent the establishment of allogeneic hematopoietic chimerism in mice treated with costimulation blockade. J Immunol. 2009;182:5547–5559. doi: 10.4049/jimmunol.0802077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baechler EC, Batliwalla FM, Karypis G, Gaffney PM, Ortmann WA, Espe KJ, Shark KB, Grande WJ, Hughes KM, Kapur V, Gregersen PK, Behrens TW. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. The Journal of experimental medicine. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nikpour M, Dempsey AA, Urowitz MB, Gladman DD, Barnes DA. Association of a gene expression profile from whole blood with disease activity in systemic lupus erythematosus. Ann Rheum Dis. 2007 doi: 10.1136/ard.2007.074765. [DOI] [PubMed] [Google Scholar]
- 51.Bauer JW, Petri M, Batliwalla FM, Koeuth T, Wilson J, Slattery C, Panoskaltsis-Mortari A, Gregersen PK, Behrens TW, Baechler EC. Interferon-regulated chemokines as biomarkers of systemic lupus erythematosus disease activity: A validation study. Arthritis Rheum. 2009;60:3098–3107. doi: 10.1002/art.24803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.