Abstract
MHC class I-restricted CD8+ T-cells play an important role in protective immunity against mycobacteria. Previously, we showed that p113-121, derived from Mycobacterium leprae protein ML1419c, induced significant IFN-γ-production by CD8+ T-cells in 90% of paucibacillary leprosy patients and 80% of multibacillary patients' contacts, demonstrating induction of M. leprae-specific CD8+ T-cell immunity. Here, we studied the in vivo role and functional profile of ML1419c p113-121-induced T-cells in HLA-A*0201-transgenic mice. Immunization with 9- or 30mer covering p113-121 sequence combined with TLR9 agonist CpG induced HLA-A*0201-trestricted, M. leprae-specific CD8+ T-cells as visualized by p113-121/HLA-A*0201 tetramers. Most CD8+ T-cells produced IFN-γ, but distinct IFN-γ+/TNF-α+ populations were detected simultaneously with significant secretion of CXCL10/IP-10, CXCL9/MIG and VEGF. Strikingly, peptide immunization also induced high ML1419c-specific IgG levels, strongly suggesting that peptide-specific CD8+ T-cells provide help to B-cells in vivo as CD4+ T-cells were undetectable. An additional important characteristic of p113-121-specific CD8+ T-cells was their capacity for in vivo killing of p113-121-labeled, HLA-A*0201+ splenocytes. The cytotoxic function of p113-121/ HLA-A*0201 specific CD8+ T-cells extended into direct killing of splenocytes infected with live M. smegmatis expressing ML1419c: both 9- and 30mer induced CD8+ T-cells that reduced the number of ML1419c-expressing mycobacteria with 95% while no reduction occurred using wild-type M. smegmatis.
These data, combined with previous observations in Brazilian cohorts, show that ML1419c p113-121 induces potent CD8+ T-cells that provide protective immunity against M. leprae and B-cell help for induction of specific IgG, suggesting its potential use in diagnostics and as subunit(vaccine) for M. leprae infection.
Keywords: Mycobacterium leprae (M. leprae), diagnostics, vaccines, in vivo cytotoxicity, CD8+ T-cells, HLA-A*0201
Introduction
Host defence activity against mycobacteria is chiefly dependent on cell-mediated immunity in which the adaptive immune response plays a crucial role in inhibiting mycobacterial multiplication. It has long been established that CD4+ T-cells are key mediators of immunity to mycobacteria, notably in the acute phase of infection (1), but it has taken longer to acknowledge the importance of CD8+ T-cells (2). Moreover, the role of CD8+ T-cells, at least in M. tuberculosis infection, seems to be more focussed on control of latent infection (3,4) and can be mediated by production of Th1 cytokines like IFN-γ which activate microbicidal effector functions of infected macrophages, as well as by the release of cytotoxic granules containing perforin, granzyme and granulysin, leading to the killing of infected phagocytes and intracellular mycobacteria (5).
Mycobacterium leprae (M. leprae), the causative agent of leprosy, has a predilection for nerve cells and skin leading to severe nerve damage and subsequent disabilities. Clinical leprosy presents as a spectrum in which interindividual variability in resistance correlates with the hosts' ability to mount effective cell-mediated immunity to the pathogen (6). This is clear from the characteristic immunological and clinical leprosy spectrum, ranging from strong cellular immunity in tuberculoid/ borderline tuberculoid (TT/BT) patients with localized disease to predominantly humoral responses and lack of T-cell immunity in lepromatous (LL) leprosy (7). The strong M. leprae-specific Th1 cell responses (CD4+ and CD8+) present in TT/BT leprosy patients, characterized by production of substantial levels of IFN-γ, are believed to be responsible for bacterial control. Similarly, in animal studies of M. leprae infection, IFN-γ producing T-cells have been reported to control bacterial growth (8). These differences in outcome of infection in leprosy are most likely caused by different host defense mechanisms (9-11), and a recent genome-wide association study showed that susceptibility to leprosy was associated with polymorphisms in seven genes in the innate NOD2-signalling pathway, in addition to HLA (12).
Despite the efforts and successes of WHO to markedly decrease the number of registered leprosy cases worldwide over the last 20 years, the decline in new cases is stagnant demonstrating that transmission of M. leprae is persistent and not affected sufficiently by current control measures (13-15). There are no tools available to identify subclinical M. leprae infection: although the level of anti-M. leprae specific phenolglycolipid (PGL-I) antibodies in serum reflects the bacterial load in individuals exposed to M. leprae, it does not represent a reliable marker for subclinical M. leprae infection progressing to active disease (16).
Deciphering the sequences of various mycobacterial genomes, including those of two M. leprae strains (17) has provided the necessary data for selecting M. leprae-specific antigens as tools to analyse M. leprae-specific immunity, e.g. induction of in vitro IFN-γ production (18-21). Using algorithms for binding to HLA class I molecules an M. leprae-specific nonamer p113-121, derived from the regulatory protein ML1419c, was selected from M. leprae unique candidate proteins (19,21). Following in vitro stimulation of PBMC with this peptide, IFN-γ production was induced in CD8+ T-cells derived from BT leprosy patients and contacts of MB patients, providing higher sensitivity than PGL-I-based tests to detect M. leprae infection in these individuals (21). However, the molecular basis of this epitope's HLA-restriction remains unknown. Moreover, the function of these CD8+ T-cells, in particular their potential inhibitory activity on mycobacterial replication, remain equally unidentified. As mentioned, HLA class I-restricted CD8+ T-cells play a role in immunity against leprosy and tuberculosis (4), but evidence showing that CD8+ T-cells participate in protective immunity to M. leprae infection in humans is lacking (5,22). Immunohistological analysis of lesions has shown that the CD8+ T-cell frequency and function depends on the clinical phenotype as in lesions of LL patients higher numbers of CD8+ T-cells are found than in TT lesions (23) although the ratios are again different in peripheral blood.
HLA-A*0201 is one of the most prevalent class I alleles, with a frequency of over 30% in most populations. Since the amino acid sequence of ML1419c p113-121 contains amino acids that fit the HLA-A*0201-peptide binding motif (24), we argued that this allele very likely represents the restriction element via which this peptide is in vivo presented to CD8+ T-cells. In order to address the in vivo function of ML1419c p113-121 and determine whether the M. leprae-specific CD8+ T-cells induced by this epitope have a protective or pathogenic effect, we used HLA-A*0201 transgenic (HLA-A2tg) mice. This mouse model has proved to be an appropriate tool for the identification of human HLA-A*0201-restricted T-cell epitopes (25,26). By immunizing HLA-A2tg mice with synthetic ML1419c peptides, we here demonstrate induction of cytotoxic CD8+ T-cells specific for ML1419c p113-121 that lead to killing of live mycobacteria and induction of specific IgG antibodies against ML1419c protein.
Materials And Methods
Synthetic peptides
ML1419c p113-121 (9mer; RLDGTTLEV), ML1419c p108-122 (15mer; EAVLLRLDGTTLEVE) and the synthetic long peptide ML1419c p100-129 (30mer; VGDASQPS EAVLLRLDGTTLEVEAVSVLTV), were purchased from Peptide 2.0 Inc. (Chantilly, VA, USA). Homogeneity and purity were confirmed by analytical HPLC and by mass spectrometry. Purity of all peptides was ≥ 80%. All impurities consist of shorter versions of the peptides caused by < 100% coupling efficiency in each round of synthesis.
HLA-A*0201-peptide binding
Peptide binding to HLA-A*0201 was performed as described previously (25). Briefly: recombinant HLA-A*0201 (previously determined to yield 20-40 % binding) was incubated in 96-well serocluster plates (Costar, Corning Incorporated) at 20°C for 48 h with 0.5 μl β2m (15 pmol) and 1 μl (100 fmol) fluorescent labeled peptide (HBV core 47-56 with a cysteine substitution at position 52) in 92.5 μl assay buffer (100 mM Na-phosphate, 75 mM NaCl, 1 mM CHAPS, pH 7), 2 μl protease inhibitor mixture (1 μM chymostatin, 5 μM leupeptin, 10 μM pepstatin A, 1 mM EDTA, 200 μM pefabloc; Sigma, St. Louis, MO) and 2 μl of test peptide at different concentrations to establish a dose-response curve. HLA-peptide complexes were separated from free peptide by gel filtration on a Synchropak GPC 100 column (250mm × 4.6mm; Synchrom, Inc., Lafayette, Indiana) using assay buffer containing 5% CH3CN. Fluorescent emission was measured at 528 nm on a Jasco FP-920 fluorescence detector (B&L Systems, Maarssen, The Netherlands). The percentage of labeled peptide bound was calculated as the amount of fluorescence bound to MHC divided by total fluorescence. The concentration of test peptide yielding 50% inhibition (IC50) was deduced from the dose-response curve.
Mice
HLA-A2tg mice B6.Cg-Tg (HLA-A/H2-D)Enge/J stock#: 004191; The Jackson Laboratory, Bar Harbor, ME, USA) were bred under specific pathogen free conditions at the LUMC animal facility. These mice express an interspecies hybrid class I MHC gene, AAD, which contains the α1 and α2 domains of the human HLA-A2.1 gene and the α3 transmembrane and cytoplasmic domains of the mouse H-2Dd gene, under the direction of the human HLA-A2.1 promoter (27). Immunodetection of the HLA-A2.1 recombinant transgene established that expression was at equivalent levels to endogenous mouse class I molecules. The mouse α3 domain expression enhances the immune response in this system. Surface expression of the HLA-A*0201 molecule was confirmed by FACS-analysis for each mouse.
Immunizations
Since immunization with peptide alone did not cause appreciable responses, mixtures of CpG adjuvant with antigen are routinely used. Mice (4 - 5 animals per group) were injected twice, with a two week interval, subcutaneously in the flanks with 50 μg CpG (ODN1826 5′-TCC ATG ACG TTC CTG ACG TT -3′; InvivoGen, San Diego, CA) in 200 μl PBS and either 50 μg (40 nmol) ML1419c p113-121 (nonamer) or 140 μg (40 nmol) ML1419c p100-129 (30mer). Splenocytes were harvested 7-10 days after final injections. Since ODNs containing unmethylated CpG motifs can activate immune cells to produce cytokines (28), we also routinely immunize with CpG alone as a (negative) control to assess the antigen specificity of immunization.
In vitro cultures
Splenocytes were isolated from individual animals by homogenizing spleens through a plastic cellstrainer (BD Bioscience) and splenocytes (3 × 106 cells/ml) were resuspended in IMDM (Invitrogen) supplemented with 2 mM L-glutamine (Invitrogen), 100 U/100 μg/ml penicillin/ streptomycin solution (Invitrogen), 8% heat-inactivated FCS and 5×10-5 M β-mercaptoethanol (Sigma). Cell suspensions (100 μl) were added to 96-well round-bottomed microtiter plates (Costar, Corning Incorporated). Cells were incubated in quadruplicates with 100 μl of medium, peptide (1 or 10 μg/ml), or M. leprae whole cell sonicate (1 or 10 μg/ml). The mitogen concanavalin A (conA; 2 μg/ml; Sigma) was used in all experiments as a positive control for cell viability. After 6 days supernatants were taken from each well, quadruplicates pooled and frozen at -20 °C until performing ELISA assay.
M. leprae whole cell sonicate
Irradiated armadillo-derived M. leprae whole cells were probe sonicated with a Sanyo sonicator to >95% breakage. This material was provided through the NIH/NIAID “Leprosy Research Support” Contract N01 AI-25469 from Colorado State University (these reagents are now available through the Biodefense and Emerging Infections Research Resources Repository listed at http://www.beiresources.org/TBVTRMResearchMaterials/tabid/1431/Default.aspx).
IFN-γ ELISA
Detection of IFN-γ in culture supernatants of in vitro cultured splenocytes was performed by ELISA (BD Bioscience) according to the manufacturer's instructions. OD values were converted into concentrations using Microplate Manager software, version 5.2.1 (Bio-Rad Laboratories, Veenendaal, The Netherlands). The cut-off value to define positive responses was set beforehand at 100 pg/ml. The assay sensitivity level was 20 pg/ml. Values for unstimulated whole blood cultures were typically < 30 pg/ml.
Intracellular cytokine staining
For polychromatic flow cytometry, splenocytes (3 × 106 cells/ml) were cultured in vitro with peptide (5 μg/ml). After 7 days, cells were incubated with medium or fresh peptide (5 μg/ml). After 1 hour brefeldin A (Sigma, 5 μg/ml) was added. After 5 hours, cells were permeabilized fixed using Cytofix/Cytoperm (BD Bioscience) and Perm/Wash (BD Bioscience) according to the manufacturer's instructions and stained using phycoerythrin (PE)-conjugated anti-CD8β2 (BD Pharmingen), PECy5-conjugated anti-CD4 (BD Pharmingen), ebioV405-conjugated anti-CD19 (eBioscience), Vivid (Invitrogen), APC-conjugated anti-IL-2 (BD Pharmingen), Alexafluor700-conjugated anti-IFN-γ (BD Pharmingen) and PeCy7-conjugated anti–TNF (BD Pharmingen).
Multiplex determination of cytokines and chemokines
According to the manufacturer's guidelines, 16 inflammatory and immunomodulatory cytokines or chemokines (MIG, VEGF, IP-10, IFN-γ, GM-CSF, IL-4, IL-6, MIP-1β, IL-10, IL12p70, IL-17, IL-1α, IL-1β, IL-2, TNF, MCP-1) were measured in unstimulated, antigen-stimulated or mitogen-stimulated samples by Milliplex® Multi-Analyte Profiling (MAP) (Millipore, Billerica, MA, USA): 96-well microtiter filter plates were pre-wetted with washing buffer (Millipore; 200 μl/well), sealed and shaken at room temperature (RT). After 10 min. washing buffer was removed by vacuum and subsequently assay buffer (12.5 μl), test sample (12.5 μl) and mixed cytokine beads (12.5 μl) were added to each well. Four-fold dilutions of standards were used for each analyte starting from 10,000 pg/ml. Plates were sealed and incubated at RT on a microtitre plate shaker. After 2 hours fluids were removed and plates were washed twice with washing buffer (Millipore; 200 μl/well). To each well detection antibody was added (Millipore; 12.5μl) and plates were incubated at RT on a plate shaker at 300 rpm. After 1 hour phycoerythrin (PE)-labelled streptavidin (Millipore; 12.5 μl) was added to each well and incubated at RT. After 30 min. fluids were removed and plates washed twice with washing buffer (Millipore; 200 μl/well). To each well sheath fluid was added (Millipore; 80 μl) and mixed well for 5 min. on a plate shaker at 300 rpm after which plates were placed in the Bio-Plex System (Bio-Rad Laboratories, Veenendaal, The Netherlands). From each well, a minimum of 50 analyte-specific beads were analyzed for fluorescence with the Bio-Plex Manager™ Software 4.0 (Bio-Rad Laboratories, Veenendaal, The Netherlands). A curve fit was applied to each standard curve according to the manufacturer's manual. Sample concentrations were interpolated from these standard curves. Analyte concentrations outside the upper- or lower limits of quantification were assigned the values of the limits of quantification of the cytokine or chemokine.
HLA-A*0201/ML1419C p113-121 tetramer production and staining
Tetrameric complexes were prepared essentially as described (1). Briefly, recombinant HLA-A*0201/Kd and human β2 microglobulin were produced in Escherichia coli as inclusion bodies. Pre-folded human β2 microglobulin and HLA-A-*0201/Kd solubilized in urea were added with synthetic peptide (ML1419c p113-121) into a refolding buffer consisting of 100 mM Tris (pH 8.0), 400 mM arginine, 2 mM EDTA, 5 mM reduced glutathione, and 0.5 mM oxidized glutathione. Refolded complexes were biotinylated by incubation for 90 min at 30°C with BirA enzyme (Avidity, Denver), and the biotinylated complex was purified by gel filtration on a Superdex 75 column (Amersham Pharmacia Biotech). Tetrameric HLA-peptide complexes were produced by the stepwise addition of streptavidin-conjugated allophycocyanin (APC) (Sigma) to achieve a 1:6 molar ratio (streptavidin-APC:biotinylated HLA class I).
Splenocytes were stained in PBS with 0.1% BSA using APC-conjugated HLA-A*0201/ML1419c p113-121 tetramer (HLA-A2/p113 TM; 50 μl; 1:50), phycoerythrin (PE)-conjugated anti-CD8β2 (Ly-3.2 clone RM4-5; BD Pharmingen, San Jose, CA, USA; 50 μl; 1:100) and propidium iodide (Sigma, 50 μl; 1:2000)
Determination of anti-ML1419c antibodies
Levels of antibody directed against ML1419c in serum from immunized mice were determined by ELISA. Briefly, plates were coated overnight at 4 °C with recombinant ML1419c antigen (5 ug/ml) or PBS (0.4% BSA) as a negative control. Plates were blocked for 2 hours using PBS containing 1% BSA and 1% Tween-20. Different sample dilutions (100 μl/well) were added to wells and incubated at 37°C for 2 hours. Plates were washed three times using PBS containing 0.05% Tween-20 and 100 μl/well horse radish peroxide (HRP)-labeled, rabbit-anti mouse total IgG (Dako, Denmark). After 2 hours at 37°C, plates were washed three times using PBS containing 0.05% Tween-20 and 100 μl/well tetramethylbenzidine substrate (TMB; Sigma) was added for 15 min at RT. The reaction was stopped by addition of H2SO4 (1M; 100 μl/well). OD values at 450 nm were determined using BioRad Microplate reader 680 (BioRad Laboratories, Veenendaal, The Netherlands). Mean Ab concentration was calculated from the linear part of the titration curve.
In vivo cytoxicity assay
Erythrocytes in splenocytes-suspension were lysed with ammonium chloride treatment and the single cell suspension was split into two equal fractions. Cells were differentially labelled at 37°C for 10 min. with CFSE (Invitrogen, Carlsbad, CA) to 5 μM (target = CFSEhigh) or 0.02 μM CFSE (control = CFSElow) concentration in PBS with 0.1% BSA. The reaction was stopped by addition of FCS (Invitrogen) to a final 10% v/v. The target population was pulsed for 2 hours with 5 μg/ml ML1419c nonamer and the control population remained unpulsed. Cells were washed four times with PBS before the two populations were mixed in 1:1 ratio and a total of 15 × 106 cells was injected intravenously in the tail. After two days spleens were removed and splenocytes analyzed for specific killing by FACs cytometry. The ratio of CFSElow/CFSEhigh cells was determined by flowcytometry. Specific killing of ML1419c p113-121-pulsed CFSEhigh target cells was calculated as follows: [1-(CFSEhigh/CFSElow)] × 100%.
Mycobacterium smegmatis strains
M. smegmatis strains were produced including empty vector control (pVV16) or M. smegmatis expressing ML1419c (pVV:ML1419). Western blot analysis using mouse antibodies directed against ML1419c was used to check the expression level of ML1419c in the latter two M. smegmatis strains. In order to compare viability of M. smegmatis strains three clones per strain were grown in Luria-Bertani (LB) broth containing 0.05% Tween 80 (Sigma), kanamycin (25 ug/ml) (Sigma) and hygromycin (25 ug/ml) (Invitrogen). The OD (at 600 nm) was checked every two hours and indicated similar growth rates for all three strains. The pVV16, E.coli-Mycobacterium shuttle vector was generously provided by Dr. V. Vissa (Dept. of Microbiology, Immunology and Pathology, Colorado State University, USA).
Killing of recombinant M. smegmatis by splenocytes of immunized mice
M. smegmatis strains were grown in Middlebrook 7H9 medium supplemented with 10% ADC (BD Biosciences) until log phase. Splenocytes (107/well) derived from immunized HLA-A2tg mice were plated in 48-well cell cluster plates (Costar, Corning Incorporated) at 37°C together in IMDM supplemented with 8% FCS and 2 mM glutamine (Gibco, Paisley, UK) together with 1.5 × 107 colony forming units (CFU) M. smegmatis (pVV:ML1419) or M. smegmatis (pVV16) and plates were centrifuged for 3 min. at 1,000 rpm. Based on our findings that approximately 30% of the splenocytes (= 3×106/ well) are macrophages and since macrophages are specifically infected by the added mycobacteria (1.5 × 107/ well) the multiplicities of infection (MOI) used in these experiments was 5. The number of CFU used for infection was calculated using OD (600 nm) and standard growth curves, and the inoculums were confirmed by growth on Middlebrook 7H10 agar medium supplemented with 10% OADC (BD Biosciences). After 1h incubation splenocytes were washed three times with PBS. To prevent extracellular growth of M. smegmatis cells were incubated at 37°C with 50 μg/ml gentamycin (Sigma). After 1h splenocytes were washed three times with and incubated for 24 h at 37°C in the presence of 5 μg/ml gentamycin in IMDM supplemented with FCS and glutamine. Splenocytes were lysed with 0.1% Triton X-100 (Sigma) for 5 min. and plated on Middlebrook 7H10 agar plates. CFU were determined by counting after 3 days.
Results
Immunization of HLA-A2tg mice with ML1419c peptides induces high levels of IFN-γ-producing, ML1419c-specific CD8+ T-cells
In view of its unique T-cell recognition pattern in PBMC of leprosy patients and their contacts (21), we decided to study the role of ML1419c p113-121-reactive T-cells in more detail and analyse whether this nonamer can induce protective T-cell responses in vivo. For this purpose we used the HLA-A2tg mouse model that has been shown to be suitable for identification of human HLA-A*0201-restricted T-cell epitopes (25,26). First, after immunisation of mice with ML1419c p113-121 (9mer) or ML1419c p100-129 (30mer), IFN-γ secretion induced by in vitro stimulation of splenocytes with ML1419c peptide or M. leprae whole cell sonicate was analyzed by ELISA (Figure 1A). Naive mice and mice immunized with CpG alone showed no IFN-γ secretion in response to M. leprae antigens. In contrast, mice immunized with either ML1419c p113-121 or p100-129 both induced high levels of IFN-γ to the ML1419c 9mer. Since the HLA-A*0201-restricted epitope Rv1886 (M. tuberculosis Ag85B) p143-152 (25) has a high binding affinity for HLA-A*0201 (Table I), it was used as a control for ML1419c-specificity. Even though Rv1886 p143-152 binds with higher affinity to HLA-A*0201 than ML1419c p113-121 (IC50: 0.01 μM vs. 0.035 μM, respectively), no IFN-γ was produced in response to this M. tuberculosis epitope, demonstrating the specificity for ML1419c in HLA-A2tg mice immunized with ML1419c peptides (Figure 1A).
Figure 1.
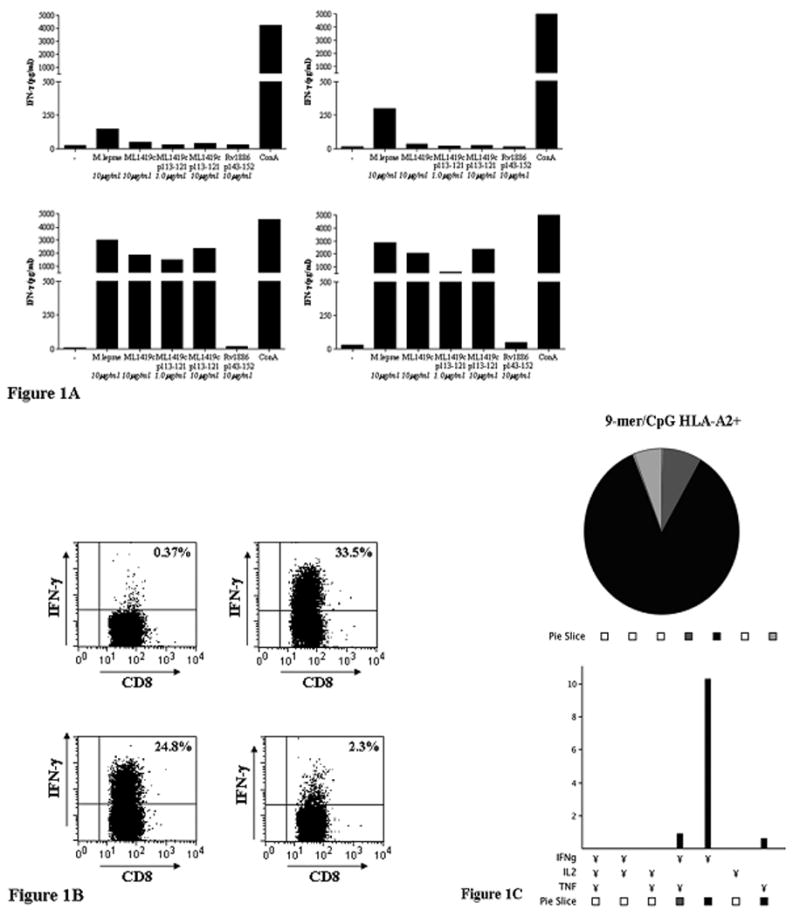
Figure 1A: IFN-γ secretion. IFN-γ secretion was analyzed after 5 days in vitro stimulation of splenocytes with ML1419c p113-121, M. leprae sonicate or mitogen (conA). HLA-A2tg mice were unimmunized (upper left panel) or immunized with CpG alone (upper right panel), ML1419c p113-121 (9mer/ CpG; lower left panel) or ML1419c p100-129 (30mer/ CpG; lower right panel). The HLA-A*0201-restricted epitope Rv1886 (M. tuberculosis Ag85B) p143-152 (25) was used as a control for ML1419c-specificity. All test groups included five mice. All mice were separately analyzed. Results are shown for one animal and are representative for each test group.
Figure 1B: Intracellular IFN-γ production by CD8+ T-cells after ML1419c p113-121 immunization of HLA-A2tg mice. Mice were immunized twice with CpG alone (upper left), ML1419c p113-121 (9mer/ CpG; upper right), p108-122 (15mer/ CpG; lower left) or p100-129 (30mer/ CpG; lower right). Splenocytes were stimulated in vitro with the same peptides used for immunization. After 7 days, cells were incubated with medium or fresh peptide for 1h before addition of brefeldin A and analysis for intracellular IFN-γ production.
Figure 1C: Frequency of polyfunctional CD8+ T-cells. Percentage of CD8+ T-cells in HLA-A2tg mice, producing combinations of IFN-γ, TNF-α or IL-2 after in vitro stimulation with peptide (see 1B). Mice were immunized with ML1419c p113-121 (9mer/ CpG). The total number of CD8+ T-cells analysed in immunized mice was 56,000. Slices in pie chart represent the proportion of single, double or triple positive CD8+ T-cells for each antigen. Only CD8+ populations of > 5 × 104 events were analyzed. In naïve mice the number of CD8+ T-cells producing cytokines was less than 5 × 104c.
TABLE I. ML1419c 9mer, 15mer and 30mer peptides.
| Peptide | amino acid sequence | HLA-A*0201 binding affinity (IC50)* | in vivo CTL induction |
|---|---|---|---|
| ML1419c p113–121 | RLDGTTLEV | 0.035 μM | ++ |
| ML1419c p108–122 | EAVLLRLDGTTLEVE | > 50 μM | + |
| ML1419c p100-129 | VGDASQPS EAVLLRLDGTTLEVEAVSVLTV | > 50 μM | + |
| Rv1886 p143-152 | FIYAGSLSAL | 0.01 μM | - |
Peptide binding affinity (IC50) was defined as high-affinity (< 1 μM), intermediate-affinity (1 μM to 10 μM), weak-affinity (10 μM to 100 μM), or nonbinding (>100 μM), according to reference (46).
In order to define the phenotype of ML1419c-responsive T-cells more precisely, splenocytes of HLA-A2tg mice immunized with ML1419c p113-121 (9mer), p108-122 (15mer) or p100-129 (30mer) were analyzed directly ex vivo or stimulated in vitro for one week with the same ML1419c peptides they had been immunized with, after which intracellular IFN-γ production was assessed by FACs analysis. As shown in Figure 1B, CD8+ T-cells were responsible for the ML1419c-specific IFN-γ production after in vitro stimulation with both 9mer and 15mer peptides. Directly ex vivo ICS of splenocytes of ML1419c peptide immunized HLA-A2tg mice resulted in specific CD8+ T cells as well, but percentages of CD8+ IFN-γ+ T cells were slightly lower, ranging from 4.3 to 5.6 % (data not shown). Thus, directly ex vivo analysis of splenocytes of HLA-A2tg mice immunized with ML1419c peptide showed the presence of CD8+ T cells specific for ML1419c 9mer (19.5 %, Figure 2), part of which (4.3 to 5.6 %) also produced IFN-γ+ex vivo (data not shown), and which was further expanded by in vitro ML1419c peptide stimulation (Figure 1B). This in vitro expansion was specific as in vitro stimulation with ML1419c peptides of splenocytes of unimmunized mice did not induce CD8+ IFN-γ+ T cells (Figure 1B).
Figure 2. Tetramer staining.
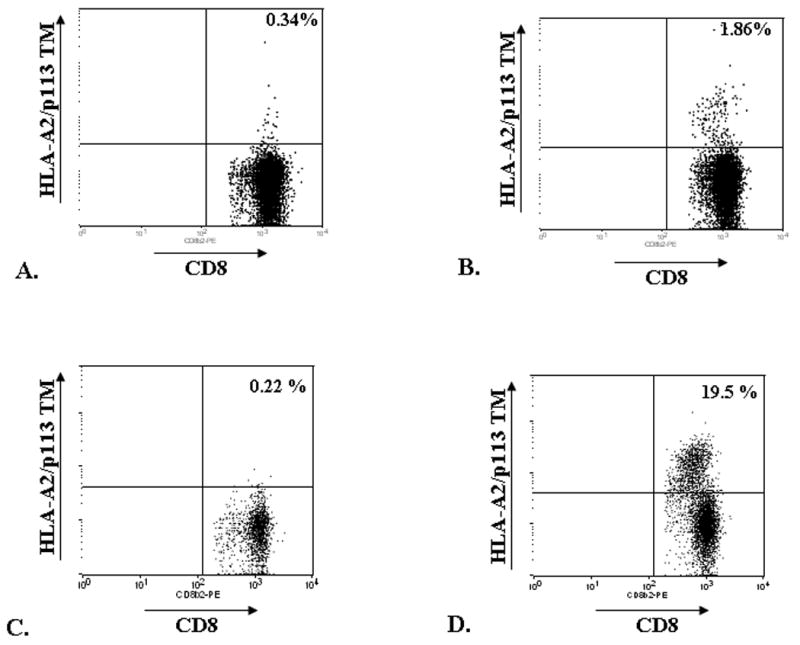
Splenocytes of unimmunized (A, C) or ML1419c p113-121-immunized (B, D) HLA- A2 tg mice were stained with PE-conjugated anti-CD8 and APC-conjugated HLA-A*0201/ml1419c p113-121 tetramer (HLA-A2/p113 TM) directly ex vivo (A, B) or after 1 week in vitro restimulation with ML1419c p113-121 (C, D).
Intracellular IFN-γ production in response to the 30mer was much less probably because presentation in the context of HLA class I requires processing of the 30mer whereas the 15mer can induce IFN-γ by CD8+ T-cells as well (21). The one hour incubation time with freshly added peptide followed by five hours in combination with brefeldin A (Figure 1B) may have been too short for the 30mer to be processed. This was in agreement with our finding that immunization with 30mer followed by in vitro stimulation with the 9mer induced 29% IFN-γ producing CD8+ T-cells showing that 30mer induced ML1419c-specific cellular responses (data not shown).
In order to estimate the frequency of polyfunctional CD8+ T-cells, intracellular TNF-α and IL-2 production was assessed simultaneously with IFN-γ (Figure 1C) by polychromatic flow cytometry. After 9mer immunization, the majority (85%) of these CD8+ T-cells produced IFN-γ but distinct IFN-γ+/TNF-α+/CD8+ and TNF+/CD8+ populations were observed as well. Similar results were observed using 15mer immunization, in which case we found 78% of the CD8+ T-cells to be single positive for IFN-γ (data not shown).
Finally, in the ICS analyses no IFN-γ production by CD4+ T-cells was observed after in vitro ML1419c peptide restimulation (data not shown). Mice with a genetic background identical to the HLA-A2tg mice but lacking the human HLA-A2 molecule did, however, not show ML1419c-specific IFN-γ producing CD8+ (nor CD4+) T-cells. Both these findings strongly support the HLA class I-restriction of ML1419c p113-121-specific T cells.
Multiplex determination of cytokines and chemokines in response to ML1419c stimulation
Immunological correlates of protection in leprosy are still lacking: although antigen-specific IFN-γ production is often used as a biomarker for M. leprae infection (18), it is possible that additional cytokines might allow more specific or qualitatively different detection of immune responses against M. leprae peptides. In order to further characterize the cellular immune response directed against ML1419c, 15 additional cytokines and chemokines were tested in multiplex assays on supernatants of splenocytes of ML1419c p113-121 immunized HLA-A2tg mice after in vitro 6 days stimulation with ML1419c p113-121 (Table II). As expected based on the ELISA data (Figure 1A), immunization with ML1419c p113-121, but not with CpG only (data not shown), induced IFN-γ production in response to this peptide but not to Rv1886 p143-152. Similar responses were observed for two proteins that can be induced by IFN-γ: the 10 kDa protein IP-10/CXCL10 and the T-cell chemo-attractant MIG/CXCL9. Production of MIP-1β (macrophage inflammatory protein1β) which is produced by macrophages after stimulation with bacterial endotoxins as well as by regulatory CD8+ T-cells in humans (29), was observed to a higher extent in ML1419c p113-121-immunized mice but production in supernatants from medium-stimulated cultures of peptide immunized mice was already substantial indicating only partial M. leprae-specificity in the assay. Immunization with ML1419c p113-121 specifically induced VEGF (vascular endothelial growth factor) secretion since significant production was only observed in splenocytes in response to ML1419c p113-121 and not to Rv1886 p143-152, and additionally, since immunization with ML1419c p113-121, and not CpG alone (data not shown), induced VEGF. Similarly, for TNF-α and IL-6 secretion was only observed in response to the M. leprae peptide but the amounts of the cytokines measured in the supernatants were only marginal.
TABLE II.
Multiplex analysis of ML1419c peptide-immunized HLA-A2tg
| in vitro stimuli | |||||
|---|---|---|---|---|---|
| Analyte | medium | ML1419c p113-121(1 μg/ml) | ML1419c p113-121(10 μg/ml) | Rv1886 p143-152(10 μg/ml) | conA |
| IFN-γ (pg/ml) | 0 | 390 | 287 | 0 | 5112 |
| IP-10 (pg/ml) | 20 | 289 | 147 | 23 | 251 |
| MIG (pg/ml) | 29 | 370 | 236 | 36 | 533 |
| MIP-β (pg/ml) | 272 | 917 | 731 | 248 | 787 |
| TNF-α (pg/ml) | 1.0 | 21 | 9.3 | 2.9 | 33 |
| VEGF (pg/ml) | 2.8 | 48 | 24 | 1.2 | 83 |
| IL-6 (pg/ml) | 3.8 | 41 | 26 | 4.4 | 299 |
* In response to in vitro stimulation of splenocytes with ML1419c p113-121 for 5 days, production was assessed for 16 analytes. Shown here are IFN-γ, IP-10, MIG, MIP-1β, TNF-α, VEGF and IL-6. HLA-A2tg mice were tested after immunization with ML1419c p113-121 or CpG alone (data not shown). Levels of IL-2, IL-4, IL-5, IL-10, IL-12p70 and IL-17 in samples stimulated with ML1419c p113-121 were below detection threshold; for IL-1α, IL-1β and GM-CSF all stimuli induced secretion levels equal to unstimulated samples. Both groups included five mice. All mice were separately analyzed. Results are shown for one animal and are representative for each test group.
Finally, levels of IL-2, IL-4, IL-5, IL-10, IL-12p70 and IL-17 in samples stimulated with ML1419c p113-121 were below detection threshold, whereas for IL-1α, IL-1β and GM-CSF all stimuli induced secretion levels in splenocytes that were equal to unstimulated samples.
Frequency of ML1419c p113-121-specific, HLA-A*0201-restricted CD8+ T-cells
Using APC-conjugated tetramers composed of HLA-A*0201 and ML1419c p113-121 (HLA-A2/p113 TM) the frequency of CD8+ T-cells after immunization of HLA-A2tg mice with ML1419c p113-121 was addressed. Tetramer staining of splenocytes was determined directly ex vivo (Figure 2A, B) or after 7 days in vitro incubation with 5 μg/ml peptide (Figure 2C, D).
The percentage of TM+/CD8+ splenocytes induced by peptide immunization was 1.86% (CpG only: 0.34%) which could be increased further to 19.5% by in vitro stimulation with ML1419c peptide. This increase was specific for ML1419c as no increase in frequency of TM+CD8+ T-cells was observed after in vitro peptide restimulation of splenocytes derived from HLA-A2tg mice immunized with CpG only (0.22%).
Immunization with ML1419c nonamer induces Ab specific for ML1419c protein
Contrasting to the high cell mediated immune response against M. leprae in tuberculoid leprosy is the strong M.leprae specific humoral response in lepromatous leprosy. This response is primarily directed to PGL-I but also M. leprae protein antigens can be recognized by sera from lepromatous patients (30,31). In view of this, antibody levels against the ML1419c protein were analyzed after immunisation with its HLA-A*0201-restricted nonamer (Figure 3). Immunisation with ML1419c p113-121 induced high antibody titers not only to the nonamer itself but also to the 30mer and even to the whole recombinant ML1419c protein. Mock-immunized mice, on the other hand, did not show any Ab reactivity, indicating that the ML1419c p113-121 is capable of not only inducing a cellular but a humoral immune response as well. Furthermore, the Ab levels present after this peptide immunisation indicate that CD8+ T-cells are capable of providing T-cell help to induce the production of Ab by plasma cells, as no CD4 T-cells could be detected after peptide immunisation.
Figure 3. Quantification of serum antibodies to ML1419c.
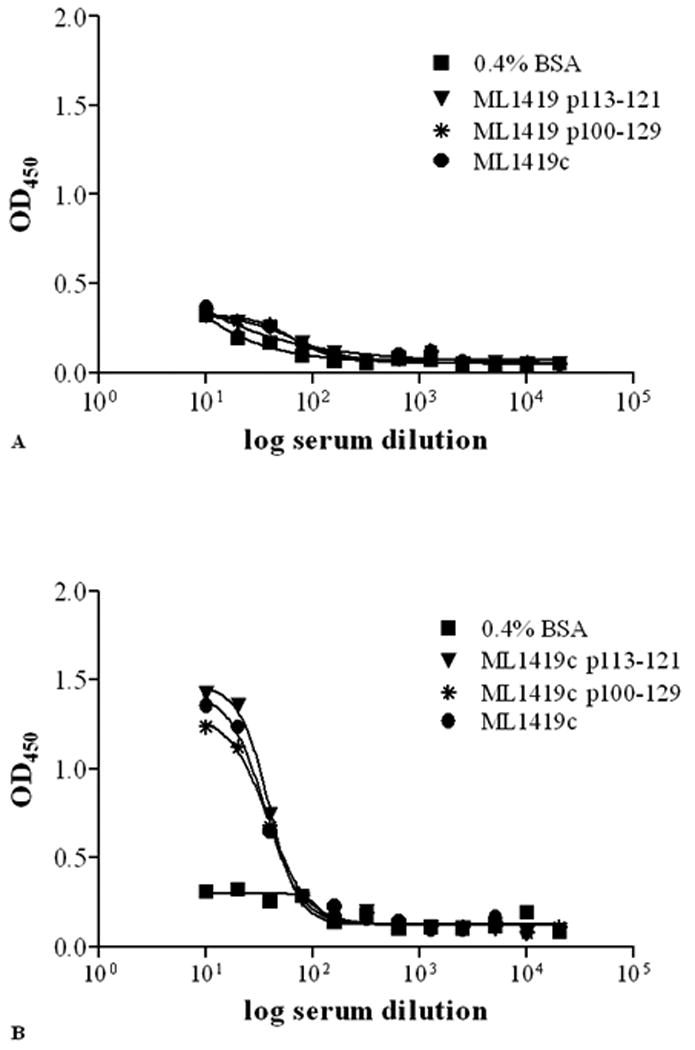
Following immunization of HLA-A2tg mice with CpG alone (A) or with ML1419c p113-121/ CpG (B) antibody titers (OD450) against ML1419c p113-121 (▼), ML1419c p100-129 (*) or ML1419c protein (●) were determined by ELISA. As a control affinity for BSA (0.4% in PBS) alone (■) is shown. Serum dilutions are shown on the x-axis. All test groups included five mice. All mice were separately analyzed. Results are shown for one animal and are representative for each test group.
Immunization with ML1419c peptides in HLA-A2tg mice induces in vivo CTL activity
Besides producing IFN-γ, CD8+ T-cells also contribute to protection by exerting cytolytic functions. Therefore, we determined whether ML1419c p113-121 could induce HLA-A*0201-restricted CTL using in vivo cytotoxicity assays (32). For this purpose mice were immunized with CpG alone or with ML1419c 9mer and 30mer peptides combined with CpG. As shown in Figure 4B, ML1419c p113-121 immunization in HLA-A2tg mice induced high levels of in vivo cytotoxic activity specific for the ML1419c nonamer ranging from 88% - 94%. Likewise immunization with the 30mer ML1419c p100-129 induced efficient specific lysis in a similar range (83% - 95%; Figure 4C). As expected, no lysis was observed in CpG immunized mice.
Figure 4. In vivo cytotoxicity.
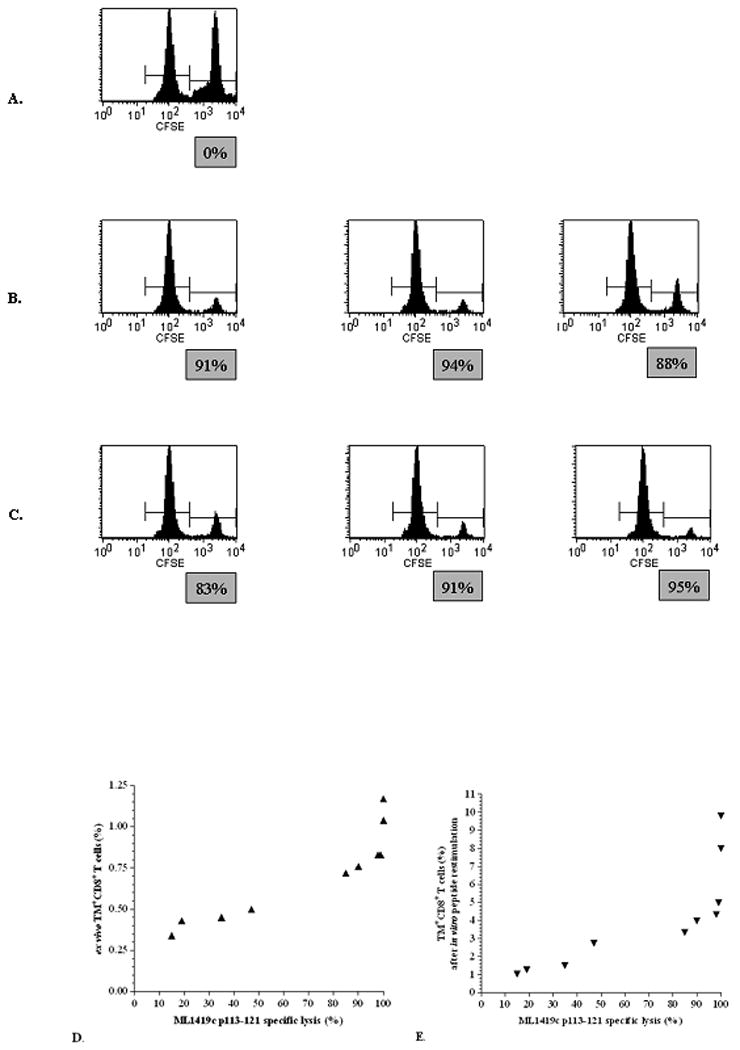
CTL response in HLA-A2tg mice against ML1419c p113-121 as detected by lysis of ML1419c p113-121-pulsed, CFSEhigh labeled syngeneic target cells using flow cytometry. The y-axis indicates the number of cells and the x-axis the CFSE intensity. The figure shows one representative CpG-immunized animal (A; n = 5), three representative animals from the groups immunized with ML1419c p113-121/CpG (B; n = 5) and ML1419c p100-129/CpG (C; n =5). Results shown are representative for at least four separate experiments. Correlation of ML1419c p113-121-specific in vivo lysis in various experiments with the percentage of HLA-A2/p113 TM+ CD8+ T-cells directly ex vivo (D) or after in vitro peptide restimulation (E).
Killing of M. smegmatis expressing ML1419c by splenocytes of ML1419c p113-121-immunized mice
Finally to assess the ability of ML1419c-specific, HLA-A*0201-restricted T-cells to kill live mycobacteria, M. smegmatis was transfected with ML1419c (pVV:ML1419). The two strains, M. smegmatis (pVV:ML1419) and M. smegmatis transduced with the with empty vector (pVV16), were used to infect splenocytes derived from CpG-, ML1419c p113-121- or ML1419c p100-129-immunized HLA-A2tg mice and after 3 days colony forming units (CFU) of both strains were determined (Figure 5).
Figure 5. Determination of colony forming units (CFU) of recombinant M. smegmatis expressing ML1419c antigen.
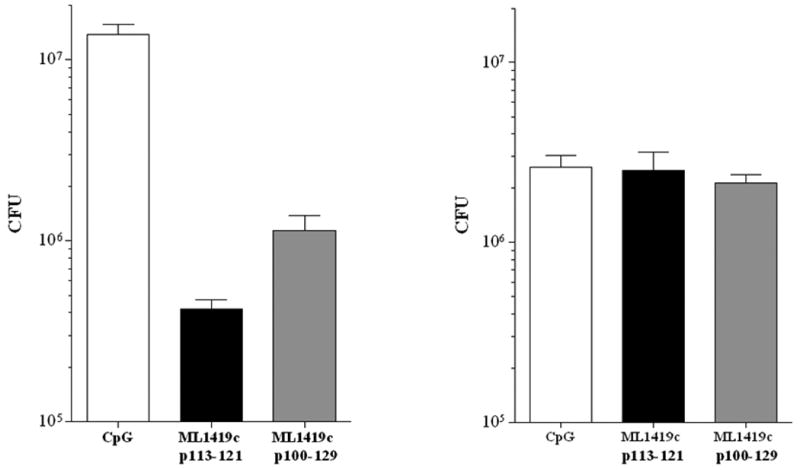
CFU were determined for M. smegmatis expressing ML1419C (pVV:ML1419) (A) or M. smegmatis with empty vector (pVV16) (B) after incubation with splenocytes derived from HLA-A2tg mice immunized with CpG (white bar), ML1419c p113-121/CpG (black bar) or ML1419c p100-129/CpG (grey bar).
Splenocytes of mice that had been immunized with either 9mer or 30mer ML1419c peptide in CpG respectively caused 95% (1,850,000 to 420,000 CFU) and 62% (1,850,000 to 1,140,000 CFU) reduction in CFU of M. smegmatis (pVV:ML1419) which was not observed for mice immunized with CpG only (Figure 5A). In contrast, no difference in CFU of wild type M. smegmatis was observed when this strain was cultured in the presence of splenocytes from ML1419c peptide-immunized mice compared to CpG-immunized mice (Figure 5B).
These data show that immunization with ML1419c 9mer or 30mer induced a strong CD8+ T-cell response with Th1-, cytolytic- as well as B-cell help-functional activity, specific for the M. leprae peptide antigen ML1419c p113-121 as well as whole protein, and which -importantly- are able to inhibit mycobacterial growth. Thus, ML1419c-specific CD8+ T-cells (as present in BT/TT patients and contacts) have all characteristics of a protective host response against M. leprae.
Discussion
Identification of M. leprae antigens that induce protective CD4+ and CD8+ Th1 immune responses is important to the development of both diagnostic tools and new leprosy vaccines. Previously we demonstrated that the M. leprae unique nonamer ML1419c p113-121 induced ex vivo IFN-γ production by CD8+ T-cells of BT leprosy patients and healthy household contacts (HHC) of leprosy patients allowing more sensitive detection of M. leprae-specific immunity in these individuals than by PGL-I-based tests (21). However, since the in vivo function and genetic restriction of these responding CD8+ T-cells and their possible link to protection (e.g. their ability to inhibit growth of M. leprae) was unknown, the characteristics of these cells in vivo were analysed in detail in the current study.
In the absence of a relevant experimental infection model for leprosy which allows analysis of HLA-restricted T-cell responses directed against M. leprae peptides, and because of the impossibility to grow the causative agent in vitro, we used an HLA-A2tg mouse strain which enables the modeling and identification of human T-cell immune responses presented in the context of HLA-A*0201. These mice express chimeric HLA-A*0201/ H2-Dd MHC class I molecules which, compared to unmodified HLA-A*0201, mediate efficient positive selection of mouse T-cells to provide a more complete T-cell repertoire capable of recognizing peptides presented by HLA-A*0201 class I molecules.
Immunization of peptides with adjuvant has been shown to induce stronger responses and better protection than immunization of the whole protein alone (33). Thus, we administered both the HLA-A*0201-restricted, ML1419c minimal peptide epitope and a synthetic long peptide (SLP; 30mer) containing the 9mer in combination with CpG. The advantage of using SLP for immunization is that these peptides are targeted to and processed by professional antigen presenting cells (APC), namely DC, resulting in efficient CD4+ and CD8+ T-cell responses (34) Minimal HLA class I binding epitopes, on the other hand, can in addition bind to non-professional APC bearing the risk of tolerance induction.
Immunization of HLA-A2tg mice with ML1419c peptides led to production of IFN-γ by HLA-A2-restricted CD8+ T-cells that were specific for M. leprae, ML1419c and ML1419c peptides, whereas other HLA-A2-restricted mycobacterial peptides such as the M. tuberculosis Ag85B epitope Rv1886 p143-152 (25) did not induce any IFN-γ production against ML1419c (Figure 1). As expected, splenocytes derived from C57Bl/6 mice immunized with ML1419c p113-121 that did not express the chimeric HLA-A*0201/H2-Dd MHC class I molecule, did not produce IFN-γ in response to similar in vitro stimulation with this peptide (data not shown), further indicating that the response to ML1419c peptides is restricted by HLA-A*0201 and not by murine MHC.
After ML1419c p113-121 immunization, the majority (85%) of the ML1419c-specific CD8+ T-cells produced only IFN-γ although distinct, yet less significant, IFN-γ+TNF-α+CD8+ and TNF+CD8+ T-cell populations were observed as well. Research on anti-viral immunity has shown that the presence of polyfunctional (IFN-γ+IL-2+TNF-α+) profiles of virus-specific T-cell responses correlated with disease activity (35) but recent studies on M. tuberculosis-infected cohorts demonstrated a substantial increase of TNF-α single positive M. tuberculosis-specific CD4+ T-cells in active disease (36). On the other hand, vaccine-induced protection against Leishmania major infection in mice has been associated with polyfunctional (IFN-γ+IL-2+TNF-α+) CD4+ T-cells (37). In contrast, however, polyfunctional CD4+ T-cells were specifically detected in patients with active M. tuberculosis infection (38). Although the exact contribution of the IFN-γ produced by ML1419c-specific CD8+ T-cells to protection against mycobacterial infection is not exactly clear, because specific antibodies and CTL activity were induced simultaneously, our finding that ML1419c p113-121/CpG adjuvant immunization induces a protective immune response dominated by IFN-γ-single positive CD8+ T-cells with CTL activity against mycobacterium infected cells, suggests that these cells may well have a protective role. Moreover, it has been shown recently that the frequency and cytokine profile of M. tuberculosis-specific T cells did not correlate with protection against TB (39). Therefore, critical components of immunity against mycobacteria, such as IFN-γ production by CD4+ T cells, may not necessarily translate into immune correlates of protection against mycobacterial disease by itself, and other functions (cytotoxicity or help for Ab production) may be required as well.
Besides IFN-γ, specific production was detected for IP-10, MIG, VEGF and to a lesser extent for TNF-α that were also specific for ML1419c p113-121 (Table II), indicating the pro-inflammatory nature of the response against ML1419c. VEGF has recently been found to have potential to differentiate between M. tuberculosis infection states as levels of VEGF in combination with EGF, TGF-α and sCD40L levels were higher in TB patients (40). MIP (CXCL9) and IP-10 (CXCL10) are potent chemo-attractants for monocytes, both induced by IFN-γ and have potential as biomarkers for TB as well (41,42). Multiplex biomarker signatures will probably be more informative as candidate signatures of vaccine-induced immunological protection.
The striking observation that the 9mer ML1419c p113-121 unexpectedly appeared to induce efficient IgG antibodies at high titers in sera of immunized mice indicated the multi-functionality of this M.leprae epitope: the IgG antibodies specifically recognized both the nonamer peptide, the 30mer peptide and the whole ML1419c protein. Classically B-cells and antibodies are thought to offer no significant contribution towards protection against M. tuberculosis or other mycobacterial pathogens. However, emerging experimental evidence suggest that B-cells play a role in many intracellular infections, probably by interacting with T cells, and thereby contributing to long-lived protection in vaccination settings (43). Our findings also suggest that B-cells may play a more important role in anti-mycobacterial immunity than hitherto appreciated (44).
Thus, ML1419c p113-121 immunisation induces specific CD8+ T-cells capable of providing B-cell help for production of IgG. It is generally thought that only CD4+ T-cells provide help for B-cells and this unusual phenomenon has only been observed rarely (45). In contrast to the previously reported CD8+ helper T-cell clones that provided B-cell help by secreting IL-4 (10), no IL-4 production by the ML1419c-specific CD8+ T-cells was detected in our study. Expression of CD40L in ML1419c-specific CD8+ T-cells was slightly increased compared to those in CpG-immunized mice (data not shown) indicating that CD40-CD40L interaction may activate B-cells to produce antibodies. Thus, these data provide a novel function of CD8+ T-cells by which they participate in anti-mycobacterial immunity.
P113-121- or 30mer immunized HLA-A2tg mice showed specific in vivo killing of p113-121-labeled, HLA-A*0201+ splenocytes, whereas no such lysis was observed in unimmunized mice or after immunization with an irrelevant HLA-*0201-binding peptide. Lysis was even detected in immunized mice after 9 months (data not shown). Importantly, p113-121-specific HLA-A*0201+ CD8+ T-cells directly and strongly inhibited mycobacterial growth using recombinant M. smegmatis expressing ML1419c. Immunisation with both 9mer and 30mer peptides inhibited growth of M. smegmatis strains expressing ML1419c while no such inhibition was observed using wild-type mycobacteria without the M. leprae protein. Analysis of leprosy lesions has revealed that CD8+CD28+ (T cytotoxic) cells are more prevalent in tuberculoid than in lepromatous lesions (23) which is in line with our findings in Brazilian BT patients (21). Thus, it is possible that the CD8+ T cells induced by ML1419c-vaccination, are able to kill M. leprae-infected cells in vivo, and contribute to reducing the mycobacterial load in infected individuals. Analysing the killing of M. smegmatis expressing M. leprae antigens like ML1419c may be a useful correlate of the efficacy of vaccines against M. leprae.
The data described here show that immunization with 9- or 30mer peptides from M. leprae specific ML1419c induced HLA-A*0201-restricted, multifunctional CD8+ T-cells that produce various Th1 and pro-inflammatory cytokines, have a strong cytolytic capability that is specific for the M. leprae antigen ML1419c, mediate B-cell help for specific antibody production and induce mycobacterial killing. The novel characteristics of these peptide-specific CD8+ T-cells may be exploited for the development of diagnostic tools as well as subunit vaccines to augment protection against leprosy.
In summary, these data show that immunization with 9- or 30mer peptides from the M. leprae specific protein ML1419c induces a strong CD8+ T-cell response with Th1-, cytolytic-, as well as B-cell help-functional activity. These responses are directed to the M. leprae ML1419c peptide, ML1419c protein and whole M. leprae, and, importantly, are able to inhibit mycobacterial growth. Thus, ML1419c-specific CD8+ T-cells possess all key functions characteristic of a protective host response against M. leprae.
Acknowledgments
LUMC, Fiocruz and CSU are part of the IDEAL (Initiative for Diagnostic and Epidemiological Assays for Leprosy) Consortium that provided a forum for interaction in the context of this study.
Footnotes
This study was supported by the Netherlands Leprosy Relief Foundation (NLR) ILEP#: 702.02.65 and ILEP#: 701.02.49 and the Q.M. Gastmann-Wichers Foundation. Additional support for this study was received from NLR (ILEP#: 7.01.02.48) and the Turing Foundation as part of the IDEAL Consortium, and the NIH/NIAID Leprosy Contract N01-AI-25469.
References
- 1.Mogues T, Goodrich ME, Ryan L, LaCourse R, North RJ. The relative importance of T cell subsets in immunity and immunopathology of airborne Mycobacterium tuberculosis infection in mice. J Exp Med. 2001;193:271–280. doi: 10.1084/jem.193.3.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaufmann SH. CD8+ T lymphocytes in intracellular microbial infections. Immunol Today. 1988;9:168–174. doi: 10.1016/0167-5699(88)91292-3. [DOI] [PubMed] [Google Scholar]
- 3.van Pinxteren LA, Cassidy JP, Smedegaard BH, Agger EM, Andersen P. Control of latent Mycobacterium tuberculosis infection is dependent on CD8 T cells. Eur J Immunol. 2000;30:3689–3698. doi: 10.1002/1521-4141(200012)30:12<3689::AID-IMMU3689>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 4.Bruns H, Meinken C, Schauenberg P, Harter G, Kern P, Modlin RL, Antoni C, Stenger S. Anti-TNF immunotherapy reduces CD8+ T cell-mediated antimicrobial activity against Mycobacterium tuberculosis in humans. J Clin Invest. 2009;119:1167–1177. doi: 10.1172/JCI38482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ottenhoff TH, T Mutis. Role of cytotoxic cells in the protective immunity against and immunopathology of intracellular infections. Eur J Clin Invest. 1995;25:371–377. doi: 10.1111/j.1365-2362.1995.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 6.Britton WJ, DN Lockwood. Leprosy. Lancet. 2004;363:1209–1219. doi: 10.1016/S0140-6736(04)15952-7. [DOI] [PubMed] [Google Scholar]
- 7.Ridley DS, WH Jopling. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34:255–273. [PubMed] [Google Scholar]
- 8.Adams LB, Scollard DM, Ray NA, Cooper AM, Frank AA, Orme IM, Krahenbuhl JL. The study of Mycobacterium leprae infection in interferon-gamma gene--disrupted mice as a model to explore the immunopathologic spectrum of leprosy. J Infect Dis. 2002;185 1:S1–S8. doi: 10.1086/338002. [DOI] [PubMed] [Google Scholar]
- 9.Ottenhoff TH, de Vries RR. HLA class II immune response and suppression genes in leprosy. Int J Lepr Other Mycobact Dis. 1987;55:521–534. [PubMed] [Google Scholar]
- 10.Ottenhoff TH, Haanen JB, Geluk A, Mutis T, Ab BK, Thole JE, van Schooten WC, van den Elsen PJ, de Vries RR. Regulation of mycobacterial heat-shock protein-reactive T cells by HLA class II molecules: lessons from leprosy. Immunol Rev. 1991;121:171–191. doi: 10.1111/j.1600-065x.1991.tb00828.x. [DOI] [PubMed] [Google Scholar]
- 11.Misch EA, Berrington WR, Vary JC, Jr, Hawn TR. Leprosy and the human genome. Microbiol Mol Biol Rev. 2010;74:589–620. doi: 10.1128/MMBR.00025-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang FR, Huang W, Chen SM, Sun LD, Liu H, Li Y, Cui Y, Yan XX, Yang HT, Yang RD, Chu TS, Zhang C, Zhang L, Han JW, Yu GQ, Quan C, Yu YX, Zhang Z, Shi BQ, Zhang LH, Cheng H, Wang CY, Lin Y, Zheng HF, Fu XA, Zuo XB, Wang Q, Long H, Sun YP, Cheng YL, Tian HQ, Zhou FS, Liu HX, Lu WS, He SM, Du WL, Shen M, Jin QY, Wang Y, Low HQ, Erwin T, Yang NH, Li JY, Zhao X, Jiao YL, Mao LG, Yin G, Jiang ZX, Wang XD, Yu JP, Hu ZH, Gong CH, Liu YQ, Liu RY, Wang DM, Wei D, Liu JX, Cao WK, Cao HZ, Li YP, Yan WG, Wei SY, Wang KJ, Hibberd ML, Yang S, Zhang XJ, Liu JJ. Genomewide association study of leprosy. N Engl J Med. 2009;361:2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 13.Global leprosy situation, 2010. Wkly Epidemiol Rec. 2010;85:337–348. [PubMed] [Google Scholar]
- 14.Lockwood DN, Suneetha S. Leprosy: too complex a disease for a simple elimination paradigm. Bull World Health Organ. 2005;83:230–235. [PMC free article] [PubMed] [Google Scholar]
- 15.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. The continuing challenges of leprosy. Clin Microbiol Rev. 2006;19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moet FJ, Meima A, Oskam L, Richardus JH. Risk factors for the development of clinical leprosy among contacts, and their relevance for targeted interventions. Lepr Rev. 2004;75:310–326. [PubMed] [Google Scholar]
- 17.Monot M, Honore N, Garnier T, Zidane N, Sherafi D, Paniz-Mondolfi A, Matsuoka M, Taylor GM, Donoghue HD, Bouwman A, Mays S, Watson C, Lockwood D, Khamispour A, Dowlati Y, Jianping S, Rea TH, Vera-Cabrera L, Stefani MM, Banu S, Macdonald M, Sapkota BR, Spencer JS, Thomas J, Harshman K, Singh P, Busso P, Gattiker A, Rougemont J, Brennan PJ, Cole ST. Comparative genomic and phylogeographic analysis of Mycobacterium leprae. Nat Genet. 2009;41:1282–1289. doi: 10.1038/ng.477. [DOI] [PubMed] [Google Scholar]
- 18.Geluk A, Klein MR, Franken KL, van Meijgaarden KE, Wieles B, Pereira KC, Buhrer-Sekula S, Klatser PR, Brennan PJ, Spencer JS, Williams DL, Pessolani MC, Sampaio EP, Ottenhoff TH. Postgenomic approach to identify novel Mycobacterium leprae antigens with potential to improve immunodiagnosis of infection. Infect Immun. 2005;73:5636–5644. doi: 10.1128/IAI.73.9.5636-5644.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geluk A, Spencer JS, Bobosha K, Pessolani MC, Pereira GM, Banu S, Honore N, Reece ST, Macdonald M, Sapkota BR, Ranjit C, Franken KL, Zewdie M, Aseffa A, Hussain R, Stefani MM, Cho SN, Oskam L, Brennan PJ, Dockrell HM. From genome-based in silico predictions to ex vivo verification of leprosy diagnosis. Clin Vaccine Immunol. 2009;16:352–359. doi: 10.1128/CVI.00414-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geluk A, Ploeg J, Teles RO, Franken KL, Prins C, Drijfhout JW, Sarno EN, Sampaio EP, Ottenhoff TH. Rational combination of peptides derived from different Mycobacterium leprae proteins improves sensitivity for immunodiagnosis of M. leprae infection. Clin Vaccine Immunol. 2008;15:522–533. doi: 10.1128/CVI.00432-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spencer JS, Dockrell HM, Kim HJ, Marques MA, Williams DL, Martins MV, Martins ML, Lima MC, Sarno EN, Pereira GM, Matos H, Fonseca LS, Sampaio EP, Ottenhoff TH, Geluk A, Cho SN, Stoker NG, Cole ST, Brennan PJ, Pessolani MC. Identification of specific proteins and peptides in mycobacterium leprae suitable for the selective diagnosis of leprosy. J Immunol. 2005;175:7930–7938. doi: 10.4049/jimmunol.175.12.7930. [DOI] [PubMed] [Google Scholar]
- 22.Kaleab B, Ottenoff B, Converse P, Halapi E, Tadesse G, Rottenberg M, Kiessling R. Mycobacterial-induced cytotoxic T cells as well as nonspecific killer cells derived from healthy individuals and leprosy patients. Eur J Immunol. 1990;20:2651–2659. doi: 10.1002/eji.1830201219. [DOI] [PubMed] [Google Scholar]
- 23.Modlin RL, Melancon-Kaplan J, Young SM, Pirmez C, Kino H, Convit J, Rea TH, Bloom BR. Learning from lesions: patterns of tissue inflammation in leprosy. Proc Natl Acad Sci U S A. 1988;85:1213–1217. doi: 10.1073/pnas.85.4.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruppert J, Sidney J, Celis E, Kubo RT, Grey HM, Sette A. Prominent role of secondary anchor residues in peptide binding to HLA-A2.1 molecules. Cell. 1993;74:929–937. doi: 10.1016/0092-8674(93)90472-3. [DOI] [PubMed] [Google Scholar]
- 25.Geluk A, van Meijgaarden KE, Franken KL, Drijfhout JW, D'Souza S, Necker A, Huygen K, Ottenhoff TH. Identification of major epitopes of Mycobacterium tuberculosis AG85B that are recognized by HLA-A*0201-restricted CD8+ T cells in HLA-transgenic mice and humans. J Immunol. 2000;165:6463–6471. doi: 10.4049/jimmunol.165.11.6463. [DOI] [PubMed] [Google Scholar]
- 26.Gram GJ, Karlsson I, Agger EM, Andersen P, Fomsgaard A. A novel liposome-based adjuvant CAF01 for induction of CD8(+) cytotoxic T-lymphocytes (CTL) to HIV-1 minimal CTL peptides in HLA-A*0201 transgenic mice. PLoS ONE. 2009;4:e6950. doi: 10.1371/journal.pone.0006950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newberg MH, Smith DH, Haertel SB, Vining DR, Lacy E, Engelhard VH. Importance of MHC class 1 alpha2 and alpha3 domains in the recognition of self and non-self MHC molecules. J Immunol. 1996;156:2473–2480. [PubMed] [Google Scholar]
- 28.Juffermans NP, Leemans JC, Florquin S, Verbon A, Kolk AH, Speelman P, van Deventer SJ, van der PT. CpG oligodeoxynucleotides enhance host defense during murine tuberculosis. Infect Immun. 2002;70:147–152. doi: 10.1128/IAI.70.1.147-152.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joosten SA, van Meijgaarden KE, Savage ND, de BT, Triebel F, van der WA, de HE, Klein MR, Geluk A, Ottenhoff TH. Identification of a human CD8+ regulatory T cell subset that mediates suppression through the chemokine CC chemokine ligand 4. Proc Natl Acad Sci U S A. 2007;104:8029–8034. doi: 10.1073/pnas.0702257104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Duthie MS, Goto W, Ireton GC, Reece ST, Cardoso LP, Martelli CM, Stefani MM, Nakatani M, de Jesus RC, Netto EM, Balagon MV, Tan E, Gelber RH, Maeda Y, Makino M, Hoft D, Reed SG. Use of protein antigens for early serological diagnosis of leprosy. Clin Vaccine Immunol. 2007;14:1400–1408. doi: 10.1128/CVI.00299-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Groathouse NA, Amin A, Marques MA, Spencer JS, Gelber R, Knudson DL, Belisle JT, Brennan PJ, Slayden RA. Use of protein microarrays to define the humoral immune response in leprosy patients and identification of disease-state-specific antigenic profiles. Infect Immun. 2006;74:6458–6466. doi: 10.1128/IAI.00041-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Billeskov R, Vingsbo-Lundberg C, Anderson P, Dietrich J. Induction of CD8 T cells against a novel epitope in TB10.4: correlation with mycobacterial virulence and the presence of a functional region of difference-1. J Immunol. 2007;179:3973–3981. doi: 10.4049/jimmunol.179.6.3973. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Hong H, Li D, Ma S, Di Y, Stoten A, Haig N, Di GK, Yu Z, Xu XN, McMichael A, Jiang S. Comparing pooled peptides with intact protein for accessing cross-presentation pathways for protective CD8+ and CD4+ T cells. J Biol Chem. 2009;284:9184–9191. doi: 10.1074/jbc.M809456200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bijker MS, van den Edden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund's adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179:5033–5040. doi: 10.4049/jimmunol.179.8.5033. [DOI] [PubMed] [Google Scholar]
- 35.Pantaleo G, Harari A. Functional signatures in antiviral T-cell immunity for monitoring virus-associated diseases. Nat Rev Immunol. 2006;6:417–423. doi: 10.1038/nri1840. [DOI] [PubMed] [Google Scholar]
- 36.Harari A, Rozot V, Enders FB, Perreau M, Stalder JM, Nicod LP, Cavassini M, Calandra T, Blanchet CL, Jaton K, Faouzi M, Day CL, Hanekom WA, Bart PA, Pantaleo G. Dominant TNF-alpha(+) Mycobacterium tuberculosis-specific CD4(+) T cell responses discriminate between latent infection and active disease. Nat Med. 2011;17:372–376. doi: 10.1038/nm.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat Med. 2007;13:843–850. doi: 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 38.Caccamo N, Guggino G, Joosten SA, Gelsomino G, Di CP, Titone L, Galati D, Bocchino M, Matarese A, Salerno A, Sanduzzi A, Franken WP, Ottenhoff TH, Dieli F. Multifunctional CD4(+) T cells correlate with active Mycobacterium tuberculosis infection. Eur J Immunol. 2010;40:2211–2220. doi: 10.1002/eji.201040455. [DOI] [PubMed] [Google Scholar]
- 39.Kagina BM, Abel B, Scriba TJ, Hughes EJ, Keyser A, Soares A, Gamieldien H, Sidibana M, Hatherill M, Gelderbloem S, Mahomed H, Hawkridge A, Hussey G, Kaplan G, Hanekom WA. Specific T cell frequency and cytokine expression profile do not correlate with protection against tuberculosis after bacillus Calmette-Guerin vaccination of newborns. Am J Respir Crit Care Med. 2010;182:1073–1079. doi: 10.1164/rccm.201003-0334OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chegou NN, Black GF, Kidd M, van Helden PD, Walzl G. Host markers in QuantiFERON supernatants differentiate active TB from latent TB infection: preliminary report. BMC Pulm Med. 2009;9:21. doi: 10.1186/1471-2466-9-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruhwald M, Petersen J, Kofoed K, Nakaoka H, Cuevas LE, Lawson L, Squire SB, Eugen-Olsen J, Ravn P. Improving T-cell assays for the diagnosis of latent TB infection: potential of a diagnostic test based on IP-10. PLoS ONE. 2008;3:e2858. doi: 10.1371/journal.pone.0002858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Abramo C, Meijgaarden KE, Garcia D, Franken KL, Klein MR, Kolk AJ, Oliveira SC, Ottenhoff TH, Teixeira HC. Monokine induced by interferon gamma and IFN-gamma response to a fusion protein of Mycobacterium tuberculosis ESAT-6 and CFP-10 in Brazilian tuberculosis patients. Microbes Infect. 2006;8:45–51. doi: 10.1016/j.micinf.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 43.Maglione PJ, Chan J. How B cells shape the immune response against Mycobacterium tuberculosis. Eur J Immunol. 2009;39:676–686. doi: 10.1002/eji.200839148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abebe F, Bjune G. The protective role of antibody responses during Mycobacterium tuberculosis infection. Clin Exp Immunol. 2009;157:235–243. doi: 10.1111/j.1365-2249.2009.03967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cronin DC, Stack R, Fitch FW. IL-4-producing CD8+ T cell clones can provide B cell help. J Immunol. 1995;154:3118–3127. [PubMed] [Google Scholar]
- 46.Geluk A, Taneja V, van Meijgaarden KE, Zanelli E, bou-Zeid C, Thole JE, de Vries RR, David CS, Ottenhoff TH. Identification of HLA class II-restricted determinants of Mycobacterium tuberculosis-derived proteins by using HLA-transgenic, class II-deficient mice. Proc Natl Acad Sci U S A. 1998;95:10797–10802. doi: 10.1073/pnas.95.18.10797. [DOI] [PMC free article] [PubMed] [Google Scholar]


