Abstract
This study employed a multi-step, rational-empirical approach to identify dimensions of personality disturbance in brain-damaged individuals: (1) Five dimensions were hypothesized based on empirical literature and conceptual grounds. (2) Principal components analysis was performed on the Iowa Scales of Personality Change to determine the pattern of covariance among 30 personality characteristics. (3) When discrepancies existed between principal components analysis results and conceptually-based dimensions, empirical findings and clinical considerations were weighed to determine assignment of ISPC scales to dimensions. (4) The fit of data to the refined dimensions was assessed by examination of intercorrelations. (5) Differential predictions concerning the relationship of dimensions to ventromedial prefrontal (vmPFC) damage were tested. This process resulted in specification of five dimensions: Disturbed Social Behavior, Executive/Decision-Making Deficits, Diminished Motivation/Hypo-emotionality, Irascibility, and Distress. In accord with predictions, the 28 participants with vmPFC lesions, compared to 96 participants with focal lesions elsewhere in the brain, had significantly more Disturbed Social Behavior and Executive/Decision-Making Deficits, and tended to have more Diminished Motivation/Hypo-emotionality. Irascibility was not significantly higher among the vmPFC group, and the groups had very similar levels of Distress. The findings indicate that conceptually distinctive dimensions with differential relationships to vmPFC can be derived from the Iowa Scales of Personality Change.
Keywords: apathy, disinhibition, executive function, factor analysis, prefrontal cortex
Personality disturbances are common in individuals with brain dysfunction from a variety of etiologies, including traumatic brain injury (TBI) (Dywan & Segalowitz, 1996; Eslinger, Grattan, & Geder, 1996; Levin et al., 1987; Lezak, 1987), frontotemporal dementia (Kertesz, Nadkarni, Davidson, & Thomas, 2000; Malloy, Tremont, Grace, & Frakey, 2007; Miller, Darby, Benson, Cummings, & Miller, 1997), Huntington's disease (Cummings & Cunningham, 1992; Folstein, 1989), Alzheimer's disease (Malloy et al., 2007; Reisberg et al., 1987), Parkinson's disease (Kaasinen et al., 2001; Menza, 2000), epilepsy (Devinsky, 1991; Hermann, Wyler & Richey, 1987), brain tumors (Gleason, 2004; Hunter, Blackwood, & Bull, 1968), and stroke (DeLuca & Diamond, 1995). Indeed, personality disturbances following brain damage can be devastating, and in regard to psychosocial functioning in particular, can have more profound impact than acquired cognitive impairments (Lezak, 1987; Nightingale, Soo, & Tate, 2007). In this paper, the term “personality” refers broadly to enduring behavioral tendencies, with “behavior” explicitly including characteristic tendencies of drive, affect and mood; pervasive attitudes and self-awareness; and cognitive tendencies which, if disturbed, adversely impact the individual's interpersonal or psychosocial functioning (Stuss, Gow, & Hetherington, 1992). In regards to activities that are often thought of as cognitive phenomena, in this paper the focus is explicitly on behavioral tendencies that are manifest in an individual's real-life behavior over time and across situations. For example, in the present study, “lack of planning” is not concerned with deficient performance on a standardized cognitive task, but with compromised execution of real-life activities due to inadequate forethought, and thus is included as a “personality characteristic.”
Over the years, a rich literature has developed describing particularly profound personality changes in premorbidly normal individuals who sustained damage to prefrontal cortices, beginning with the striking case of Phineas Gage (Harlow, 1868), and further illuminated by Luria (1969), Blumer and Benson (1975), and Stuss and Benson (1984; 1986). Careful case descriptions of patients with damage to the ventromedial prefrontal region (vmPFC) from meningioma resection (Eslinger & Damasio, 1985; Hunter et al., 1968) or ruptures of anterior communicating artery aneurysms (Eslinger & Damasio, 1984; DeLuca & Diamond, 1995) have highlighted problems with behavioral control (especially social behavior), goal-directed behavior, decision-making and modulation of emotions. Damage to the vmPFC has been found to be associated with several specific personality changes, including problems with disinhibited social behavior (Tranel, Bechara, & Denburg, 2002; Anderson, Barrash, Bechara, & Tranel, 2006), impulsivity (Boes et al., 2009), empathy (Eslinger, 1998; Shamay-Tsoory, Aharon-Peretz, & Perry, 2009), situationally-inappropriate affect (Barrash, Tranel, & Anderson, 2000; Tang et al., 2009), judgment and decision-making (Bechara, Damasio, & Damasio, 2000; Bechara, Damasio, Damasio, & Anderson, 1994; Bechara, Damasio, Tranel, & Damasio, 1997; Godefroy & Rousseaux, 1997; Koenigs et al., 2007; Satish, Streufert, & Eslinger, 1999; Van den Bos & Guroglu, 2009; Young et al., 2010), cognitive/behavioral flexibility (Blair & Cipolotti, 2000; Satish et al., 1999), perseveration (Anderson et al., 2006; Hauser, 1999; Luria, Pribram, & Homskaya, 1964), hoarding behavior (Anderson, Damasio, & Damasio, 2005), initiative (Satish et al., 1999), planning (Owen, 1997), emotional reactivity (Anderson et al., 2006; Koenigs & Tranel, 2007; Tranel et al., 2002), impatience and irritability (Barrash et al., 2000), aggression (Giancola, 2006; Grafman et al., 1996; Scarpa & Raine, 1997), diminished experience of emotions (Bechara et al., 1997; Damasio, Tranel, & Damasio, 1990; Tranel, 1994), apathy (Barrash et al., 2000; Blumer & Benson, 1975), blunted affect or diminished emotional expressiveness (Borod, Koff, Lorch, & Nicholas, 1985; House, Rowe, & Standen, 1987; Kolb & Milner, 1981; Weddell, Trevarthen, & Miller, 1988), and insight (Anderson & Tranel, 1989). Changes such as these have been referred to as “pseudopsychopathy” (Blumer & Benson, 1975), “acquired sociopathy” (Eslinger & Damasio, 1985), and the “orbitomedial frontal syndrome” (Malloy, Bihrle, Duffy, & Cimino, 1993), and have been explicitly associated with damage to vmPFC (defined below) (Damasio, 1994; Tranel, 1994).
Historically, the literature contains rich clinical descriptions of individuals with profound personality changes after damage to the vmPFC region. However, reliable and valid assessment of possible personality changes in individuals with neurological disease presents a number of challenging methodological issues (Barrash et al., 2000). Within the past two decades, a number of standardized instruments have been developed to enhance assessment of neurobehavioral sequelae of acquired brain dysfunction, including the Dysexecutive Questionnaire (Wilson, Alderman, Burgess, Emslie, & Evans, 1996), the Frontal Behavioral Inventory (Kertesz, Davidson, & Fox, 1997), the Frontal Systems Behavior Scale (Grace & Malloy, 2001), the Iowa Rating Scales of Personality Change (Barrash & Anderson, 1993), and the Neuropsychiatric Inventory (Cummings et al., 1994). The characteristics, psychometric properties and validity data for these instruments were carefully reviewed by Malloy and Grace (2005). The review illustrates two points of particular relevance to the present discussion: First, two instruments, the Frontal Systems Behavior Scale and the Iowa Rating Scales of Personality Change, have demonstrated sensitivity to the personality changes characteristic of patients with frontal lesions. Second, most of the scales were developed for a specific purpose. The Dysexecutive Questionnaire has 20 items that were specifically selected to assess changes in personality, emotion, motivation behavior or cognition specifically related to executive dysfunction. The Frontal Behavioral Inventory was designed specifically to improve diagnosis of frontal lobe dementia. The Frontal Systems Behavior Scale was designed specifically to assess behavior associated with damage to frontal systems. The Neuropsychiatric Inventory was designed to assess behavioral disturbances in dementia patients. A sixth scale reviewed was the Behavior Rating Inventory of Executive Functions (Gioia, Isquith, Guy, & Kenworthy, 2000), which was designed to measure executive dysfunction in children. Another scale, the Current Behavior Scale (Elsass & Kinsella, 1989) assesses behavioral disturbances following TBI. Among the instruments for assessment of personality change in neurological patients, the Iowa Rating Scales of Personality Change are unique in that they were not intended for use with a specific type of patient (e.g., dementia) or specific types of personality changes (e.g., “frontal” problems); rather, they were designed to characterize patterns of personality changes that may follow from a wide range of neurological conditions.
Systematic assessment of neurological patients with these scales indicates that acquired personality disturbance is not unitary, but multidimensional. Cummings (1993) proposed that higher-order neurobehavioral consequences of frontal lobe dysfunction comprise three syndromes that are each associated with one of three frontal-subcortical circuits: executive cognitive deficits (associated with damage to the dorsolateral circuit); disinhibition and impaired self-regulation (orbitofrontal circuit); and disorders of behavioral activation and motivation, or apathy (anterior cingulate circuit). The Frontal Systems Behavior Scale was designed to assess those three syndromes. In addition to total score, ratings generate subscale scores for executive dysfunction, disinhibition and apathy. Factor analysis of the Frontal Systems Behavior Scale on a sample of 324 neurologic patients yielded three factors that reflected the three dimensions (Stout, Ready, Grace, Malloy, & Paulsen, 2003). Factor analysis with the Neuropsychiatric Inventory with a sample of 37 patients with Alzheimer's and 33 with frontotemporal dementia yielded four factors labeled as: executive dysfunction and self-care, loss of social awareness, mood changes, and stereotypic and eating behaviors (Bozeat, Gregory, Ralph, & Hodges, 2000). A factor analysis of the Current Behavior Scale in a study sample of TBI patients found two factors reflecting loss of emotional control and loss of motivation (Kinsella, Packer, & Olver, 1991).
Factor analysis of the Iowa Rating Scales of Personality Change with a sample of 115 patients with brain damage from a wide array of neuropathological conditions was performed separately on change ratings and on current level of disturbance (Barrash, Anderson, Jones, & Tranel, 1997b). The analysis of change ratings yielded five factors reflecting: executive dysfunction, impaired goal-directed behavior, interpersonal/social disturbance, distress/emotional reactivity, hypo-emotionality/apathy, and disinhibition. The analysis of current-level ratings yielded six factors that were essentially identical with the exception that the executive dysfunction factor split into two partially overlapping factors, one reflecting primarily cognitive deficits (e.g., lack of planning, indecisiveness) and one reflecting primarily behavioral manifestations of executive dysfunction (e.g., lack of initiative, perseverative behavior). Considering this set of factor analyses, it may be noted that although the different instruments vary in the range of personality changes they were designed to assess, and despite differences in the clinical characteristics of the patient samples, the factor analyses each show multiple dimensions of personality disturbances among neurological patients, disturbances concerning emotional modulation, goal-directed behavior/behavioral control (especially concerning social behavior), and – depending on the particular set of disturbances assessed – aspects of executive functioning.
Based on extensive psychometric analyses (Barrash et al., 1997b), the Iowa Rating Scales of Personality Change was revised and renamed the Iowa Scales of Personality Change (ISPC, Barrash, Anderson, Hathaway-Nepple, Jones, & Tranel, 1997a). The ISPC has shown sensitivity to personality changes in patients with Parkinson's disease (Gronchi-Perrin et al., 2007; Houeto et al., 2002, 2006; Witjas et al., 2005), frontotemporal dementia (Hallam, MacKenzie, & Feldman, 2006), multiple sclerosis (Simioni et al., 2008, 2009; Souza Lima et al., 2007), brain cancer (Gleason, 2004), traumatic brain injury (Cantagallo, Contini, & Bianchi, 2010; Rochat, Ammann, Mayer, Annoni, & Van der Linden, 2009), focal vmPFC lesions (Driscoll, 2009; Koenigs et al., 2007; Tranel, Damasio, Denburg, & Bechara, 2005; Young et al., 2010), focal posterior lesions from stroke (Annoni, Devuyst, Carota, Bruggimann, & Bogousslavsky, 2005; Hommel et al., 2000), and temporal lobectomy (Sellal et al., 2003; Tranel & Bechara, 2009). However, the factor structure of the ISPC has not been investigated. Specification of the dimensions of acquired personality changes assessed by the ISPC would allow for data reduction and interpretation heuristics that would be helpful in both clinical and research applications.
In this study, we hypothesized that personality disturbances assessed by the ISPC reflect five dimensions as identified by previous factor analysis of the Iowa Rating Scales of Personality Change. Although executive dysfunction split into two factors in the previous analysis, our experience with patients with prefrontal damage is most consistent with the earlier factor analysis of personality changes and the literature generally in indicating that cognitive and behavioral aspects of executive dysfunction are mainly just different aspects of the same underlying disturbance. Following parsimony, we thus hypothesized a unitary dimension of executive/decision-making disturbance, along with dimensions of disturbed social behavior, emotional reactivity, diminished motivation/hypo-emotionality, and distress. We investigated the scales forming those dimensions employing a rational-empirical approach. It was predicted that the hypothesized dimensions would correspond to factors extracted by principal components analysis (PCA). It was further predicted that the dimensions reflecting disturbed social behavior, executive/decision-making deficits, diminished motivation/hypo-emotionality, and emotional reactivity, would be associated with damage to the vmPFC region, and that a fifth dimension, distress, would not be specifically associated with such damage. It follows from these predictions that a higher-order composite measure of vmPFC-related disturbances (i.e., the sum of the four relevant dimensional scores) should also be strongly associated with vmPFC damage. It was expected that dimensions of personality disturbances associated with vmPFC damage would not be independent of each other; that is, that they would be significantly correlated.
Method
Participants
Participants were 124 individuals with focal brain lesions selected from the Patient Registry of the Division of Behavioral Neurology and Cognitive Neuroscience at the University of Iowa. Criteria for selection were lesion onset at age 18 or older, a stable cortical lesion of at least six months duration and, in keeping with entry criteria for the Patient Registry more broadly, no history of significant alcohol or substance abuse, psychiatric disorder, or other neurologic disorder unrelated to the lesion. Eligibility required the availability of valid ratings by an individual (almost always a spouse, parent, or adult child) who knew the participant well and had regular opportunities to interact with and observe the participant in a variety of situations both before and subsequent to the lesion onset. In accordance with institutional and federal guidelines, all participants provided informed consent for participation in research and for obtaining personality ratings. All procedures were approved by the University of Iowa Institutional Review Board.
Procedure
According to protocol, neuroimaging with detailed neuroanatomical characterization was completed, and comprehensive neuropsychological characterization was completed with standard procedures of the Benton Neuropsychology Laboratory (Tranel, 2009), including standardized assessment of orientation, attention and concentration, intellectual functioning, memory, speech and language, visuoperceptual and visuospatial abilities, and executive functioning. The ISPC was completed by the informant in a separate room while the participant was engaged in neuropsychological testing.
Measures
Neuroanatomical classification
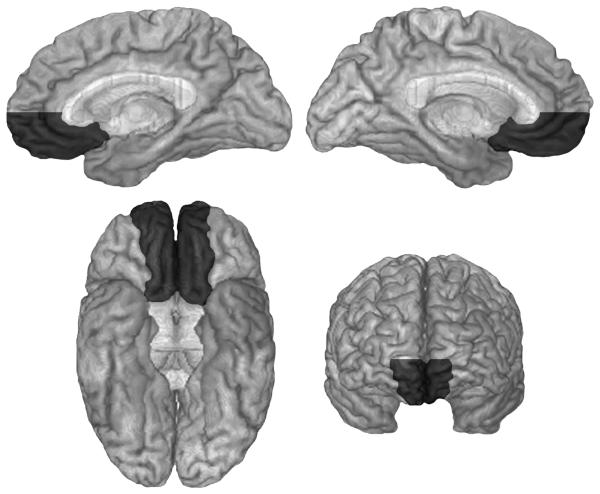
Neuroanatomical analysis was based on magnetic resonance (MR) data or, for a few participants for whom an MR was contraindicated (due to the presence of metal clips or claustrophobia), on computerized axial tomography data. All imaging data were obtained at least three months post-lesion onset, and lesion locations were mapped according to the standard procedures of the University of Iowa Human Neuroanatomy and Neuroimaging Laboratory (Damasio & Frank, 1992; Frank, Damasio, & Grabowski, 1997). Based on this neuroanatomical analysis, participants were classified into either the vmPFC group (n = 28) or the non-vmPFC group (n = 96). The vmPFC group was defined by the presence of bilateral (n = 16) or unilateral right (n = 9) or left (n = 3) lesions in ventromedial prefrontal cortex. Inclusion in the vmPFC group required lesions that involved substantial damage to one or more of the structures included in the vmPFC region, as defined below; however, it was not required that the lesions were circumscribed to the vmPFC region, so long as they were mainly in the vmPFC region. We defined the vmPFC as inclusive of: the anterior cingulate gyrus (including the subgenual part, and the lower region of the perigenual part, below the genu), and the anteromedial and ventral medial sectors anteriorly up to and including the frontal pole, including the mesial region of the orbitofrontal cortex (gyrus rectus and mesial half of orbital gyrus), in the left and right hemispheres. The region so defined is depicted in the Figure. An additional criterion was that vmPFC lesions had to include the area below a line from the anterior-most point of the genu to the anterior-most part of the frontal pole. The non-vmPFC group had lesions entirely outside the vmPFC region, or with only negligible involvement of vmPFC. Neuroanatomical classification was performed blind to study hypotheses and personality ratings. The non-vmPFC group had lesions that were neuroanatomically heterogeneous, located throughout the cerebrum, including some that were circumscribed to the right or left dorsolateral or lateral orbital region and others that were large frontotemporoparietal lesions.
Figure.
Ventromedial prefrontal region. The areas included in ventromedial prefrontal cortex are depicted in dark gray in medial (upper left, right hemisphere; upper right, left hemisphere), inferior (lower left) and anterior (lower right) views of a normalized brain.
The Iowa Scales of Personality Change (ISPC)
The ISPC (Barrash et al., 2000) provides standardized assessment of 26 characteristics that might change as a result of a neurological condition, with characteristics concerning emotional functioning, social and interpersonal behavior, decision-making and goal-directed behavior, behavioral control, and insight. Additionally, four control scales concern characteristics that are not expected to change as a consequence of brain damage. Information is elicited from an informant who has had regular, substantial contact with the patient. Each characteristic is introduced by a brief behaviorally-oriented definition. Informants make two ratings for each characteristic: a “Before” rating to describe a patient's typical functioning over their adult life, and a “Now” rating to describe their functioning in the months and years since developing their neuropathological condition. Characteristics are rated along 7-point scales, with 1 reflecting very good functioning, 3 reflecting the hypothetically “average” level of the characteristic, 5 indicating that the characteristic is present to the degree that it is considered a problem, and 7 indicating a severe problem. Points along the scale are accompanied by rating guidelines with multiple behavioral examples to enhance reliability (Schwarz, 1999).
The ISPC is a revised version of the Iowa Rating Scales of Personality Change (Barrash & Anderson, 1993). Comprehensive psychometric analyses of the earlier version found that interrater agreement weighted by the magnitude of rating discrepancy was generally very high across all scales, ranging from .80 to .96 (Barrash et al., 1997b). Validity was supported by the generally good agreement of ratings with staffing neuropsychologists' ratings. However, based on these analyses, five scales were removed for excessive overlap with other scales: Poor Frustration Tolerance (overlap with Irritability), Disorganization (with Lack of Planning), Excitability (with Emotional Lability), and Restricted Feelings (with Blunted Affect), and the scale Delusional Thinking was removed for poor validity. Additionally, several scales underwent minor revisions to enhance reliability, and one scale, Egocentricity, underwent more significant modification because it was not functioning satisfactorily in its intended role as a control scale. In the revised version (ISPC), Poor Frustration Tolerance was modified to become Impatience, with increased conceptual distinction from Irritability; Delusional Thinking was modified to be less inferential and defined more behaviorally as Suspiciousness; Egocentricity was modified substantially and renamed Vanity; and other removed scales were replaced with Aggression, Vulnerability to Pressure, and Lack of Stamina.
Dimensions of personality disturbance
By a priori conceptualization informed by the literature and the results of the 1997 factor analysis of the Iowa Rating Scales of Personality Change, it was expected that ISPC scales reflect personality disturbances along five dimensions, comprising the following scales: Executive Deficits (Lack of Planning, Perseverativeness, Lack of Initiative, Lack of Persistence, Impulsivity, Poor Judgment and Indecisiveness); Disturbed Social Behavior (Social Inappropriateness, Insensitivity, Inappropriate Affect, Lack of Insight, Inflexibility, and Aggression); Diminished Motivation/Hypo-emotionality (Apathy, Blunted Affect and Social Withdrawal); Emotional Reactivity (Irritability, Emotional Lability and Impatience); and Distress (Depression, Anxiety, Vulnerability to Pressure and Dependency). Regarding the latter, Distress was conceptualized as negative emotionality and dependency that tend to be relatively enduring aspects of the individual's way of being (Stuss et al., 1992). This contrasts with Emotional Reactivity, which is more about emotionality or aggravation, often manifest as a poorly-modulated response to a situation at hand, and typically short-lived. Lack of Stamina was considered a nonspecific change (i.e., not an aspect of hypothesized dimensions, and not hypothesized to be associated with damage to any particular region), and two scales, Obsessiveness and Suspiciousness, were conceptualized as freestanding characteristics that are neurobehavioral symptoms in their own right and are not part of any broader syndromes of personality change. Two sets of control scales reflect “negative” or socially undesirable characteristics (Manipulativeness, Vanity), and “positive” characteristics (Frugality, Type A Behavior) that are not expected to change systematically with acquired brain damage.
“Acquired ventromedial prefrontal personality disturbances”
For the purpose of hypothesis-testing, “Now” ratings on scales comprising Disturbed Social Behavior, Executive Deficits, Diminished Motivation/Hypo-emotionality and Emotional Reactivity were summed to form a higher-order composite measure referred to as “Acquired Ventromedial Prefrontal Personality Disturbances.” “Now” ratings for the scales comprising Distress and the individual scales not belonging to any of the dimensions were summed to form a composite measure referred to as “Other Personality Disturbances.”
Analysis
All analyses were with SPSS for Windows (Release 17.0). The general analytic strategy includes principal components analysis of the ISPC to examine the correspondence of scales' co-variation to the hypothesized dimensions. Next, the scales comprising dimensions were re-considered in light of both a priori conceptualizations and the empirical findings, and adjusted if indicated. Intercorrelations of scales to dimensions were then calculated to examine the degree to which individual personality characteristics related to their assigned dimension and to other dimensions in expected fashion. Finally, we tested predictions concerning the expected neuroanatomical correlates of the dimensions. Exploratory rather than confirmatory factor analysis was employed in order to examine covariance among scales to provide empirical grounding to the composition of ISPC dimensions, making minimal assumptions, rather than to test highly developed hypotheses regarding ISPC factors.
PCA was conducted on the “Now” ratings of the 30 ISPC scales. “Now” ratings were analyzed for two reasons: First, “acquired disturbances,” a variable we have employed in the past, is a compound variable requiring (a) a significant increase in the characteristic from premorbid status, and (b) the postmorbid level of the characteristic reflects disturbance; and compound variables do not lend themselves to factor analysis. Second, change scores (i.e., “Now” rating minus “Before” rating) could have been used; however, higher ratings of change do not necessarily mean that disturbance is present. That is, an individual may have had a low level of characteristic premorbidly, and the rating can increase two points but still not reflect a disturbance). On the other hand, elevated “Now” ratings are a direct index of disturbance, and the strong likelihood that the disturbance is acquired as a result of the neurological condition can be inferred: Enrollees in the Patient Registry have no premorbid history of psychiatric disorder and, as a group, participants were largely free of premorbid disturbances. Results to be presented indicated that mean “Before” ratings of vmPFC were at or most often below the “average” level (i.e., a rating of 3) for clinical scales other than Blunted Affect and Obsessiveness (at 3.4 and 3.3, respectively), and similar findings apply to non-vmPFC.
PCA was employed, and varimax rotation was performed. The number of components to be extracted was determined by parallel analysis (O'Connor, 2002). The results of the PCA were then reviewed and, based on this review, the a priori assignments of scales to the conceptually-derived dimensions were reconsidered. It is important to note that component scores are mathematically distinct from dimension scores, as the former are determined by the scores on all 30 scales weighted by the loadings of those scales to the component, while the dimension scores are simply the summed ratings of each scale assigned to the dimension. Following the review of the PCA, a scale might be re-assigned to a different dimension if the principal components analysis indicated that the scale was more highly associated with a dimension other than the one to which it was initially assigned, and if re-assignment was judged to make sense conceptually.
Following re-formulation of the dimensions, correlations of each scale to each of the dimensions were calculated. As the ratings are ordinal scale variables, Spearman's rho was employed to quantify correlations. Part-whole correlations were calculated to characterize the correlations between individual scales and the dimension to which it was assigned to avoid spuriously inflated correlations. Additionally, Spearman correlations were calculated to examine the relationships among the dimensions, and between the dimensions and the higher-order composite indices, “acquired ventromedial prefrontal personality disturbances” and other personality disturbances (including part-whole correlations where appropriate).
The prediction that four of the dimensions would be associated with damage to the vmPFC region, and that the fifth dimension, Distress, would not be specifically associated with damage to this region were evaluated by group comparisons between vmPFC and non-vmPFC. Mann-Whitney U tests were employed since the dimensions are based on ordinal-scale ISPC ratings. Similarly, Mann-Whitney U tests were used to test the hypothesis that the vmPFC group would be characterized by a higher score on the composite measure, “acquired ventromedial prefrontal personality disturbances,” but that these groups would not differ in other personality disturbances. A .05 alpha level was employed. Significance tests were one-tailed when directional predictions were being evaluated, two-tailed tests were used for all other statistical tests.
Results
Demographic characteristics and selected clinical data of the full sample and the two study groups, vmPFC and non-vmPFC (as described below), are presented in Table 1. The groups were similar in terms of age, education, sex ratio, age at lesion onset, years known by the rater, and WAIS-III/IV Verbal and Full Scale IQs. The vmPFC group had a significantly lower Performance IQ than the non-vmPFC group (t = −2.08, p < .04) (all mean IQ indexes fell in the average range, however, and there are no meaningful differences between the groups in terms of overall intellectual abilities).
Table 1.
Demographic and Clinical Characteristics of Study Groups
| vmPFC (n = 28) |
Non-vmPFC (n = 96) |
||
|---|---|---|---|
| Variable | M (SD) | M (SD) | Group comparison |
| Sex (male:female)a | 17:11 | 46:50 | χ2(1, N = 124) = 1.42, p = .29 |
| Age (years) | 52.5 (15.6) | 52.1 (13.0) | t(122) = 0.14, p = .89 |
| Education (years) | 14.1 (2.5) | 13.8 (2.3) | t(122) = 0.64, p = .52 |
| Age at Onset (years) | 47.6 (16.4) | 47.1 (13.2) | t(122) = 0.19, p = .85 |
| Interval (years) | 4.8 (6.2) | 5.0 (6.3) | t(122) = −0.13, p = .90 |
| Years known | 32.2 (15.8) | 33.1 (13.6) | t(122) = −0.30, p = .77 |
| WAIS VIQ | 97.8 (15.4) | 101.1 (15.3) | t(122) = −0.84, p = .41 |
| WAIS PIQ | 95.2 (14.6) | 102.7 (13.6) | t(122) = −2.08, p = .04 |
| WAIS FSIQ | 96.3 (11.2) | 102.1 (14.0) | t(122) = −1.64, p = .11 |
Note. Interval = Interval between lesion onset and personality ratings; WAIS = Wechsler Adult Intelligence Scale-III; VIQ = Verbal Intelligence Quotient; PIQ = Performance Intelligence Quotient; FSIQ = Full Scale Intelligence Quotient.
Mean “Before” and “Now” ratings are presented in Table 2. The “Before” ratings indicate that both groups were highly comparable in terms of premorbid personality characteristics, and neither group had mean ratings indicative of premorbid problems on any assessed personality characteristic. In contrast, “Now” ratings indicated that the vmPFC group had mean ratings of 4.0 or higher (suggestive of disturbances) on several characteristics, and damage to the vmPFC was associated with significantly higher ratings of disturbance on 11 of the 19 scales conceptualized as belonging to dimensions predicted to be associated with vmPFC lesions. With stringent Bonferroni corrections for multiple (19) tests, only the difference for Lack of Planning would remain statistically significant. Considering scales not belonging to those predicted to be associated with vmPFC lesions, only one – Lack of Stamina – show a significant difference (higher among vmPFC). The non-vmPFC group did not show a higher level of disturbance on any characteristic.
Table 2.
“Before” and “Now” Ratings by Study Group, with Comparison of Group Means
| Ratings | ||||
|---|---|---|---|---|
| Beforec | Nowd | |||
| vmPFC | Non-vmPFC | vmPFC | Non-vmPFC | |
| ISPC Scale | M (SD) | M (SD) | M (SD) | M (SD) |
| Associated with vmPFCa | ||||
| Lack of Planning | 2.6 (1.4) | 2.4 (1.2) | 4.7 (2.0) | 3.3 (1.7)*** |
| Poor Judgment | 2.2 (1.1) | 2.6 (1.2) | 4.2 (1.7) | 3.5 (1.4)* |
| Lack of Persistence | 2.5 (1.1) | 2.4 (1.2) | 4.1 (1.7) | 3.3 (1.4)** |
| Perseveration | 2.6 (1.2) | 2.6 (1.1) | 4.2 (1.5) | 3.6 (1.4)* |
| Lack of Initiative | 2.0 (1.1) | 2.4 (1.3) | 4.5 (2.0) | 3.5 (1.5)** |
| Impulsivity | 3.0 (1.6) | 2.6 (1.3) | 4.0 (1.8) | 3.2 (1.5)* |
| Indecisiveness | 2.5 (1.3) | 2.7 (1.3) | 4.0 (2.0) | 3.9 (1.5) |
| Insensitivity | 2.8 (1.1) | 2.8 (1.3) | 4.1 (1.7) | 3.3 (1.7)* |
| Social Inappropriateness | 2.0 (1.0) | 2.4 (1.2) | 3.9 (1.5) | 3.2 (1.8)* |
| Inappropriate Affect | 2.3 (1.0) | 2.5 (1.1) | 3.4 (1.5) | 3.0 (1.3) |
| Aggression | 2.5 (1.3) | 2.6 (1.4) | 3.0 (1.2) | 2.6 (1.4) |
| Lack of Insight | N/A | N/A | 3.4 (1.4) | 2.7 (1.4)* |
| Impatience | 2.9 (1.2) | 3.0 (1.3) | 3.8 (2.0) | 3.6 (1.8) |
| Irritability | 2.6 (1.3) | 2.9 (1.2) | 4.1 (1.6) | 3.5 (1.6)* |
| Emotional Lability | 2.8 (1.1) | 3.0 (1.3) | 3.9 (1.7) | 3.8 (1.5) |
| Inflexibility | 3.0 (1.5) | 3.3 (1.4) | 4.2 (1.5) | 3.9 (1.5) |
| Apathy | 2.6 (1.1) | 2.7 (0.9) | 3.7 (1.4) | 3.4 (1.4) |
| Social Withdrawal | 2.6 (1.2) | 2.7 (1.3) | 3.2 (1.8) | 3.4 (1.6) |
| Blunted Affect | 3.4 (1.3) | 3.3 (1.3) | 3.9 (1.8) | 2.9 (1.6)** |
| Not Associated with vmPFCb | ||||
| Vulnerability to Pressure | 2.4 (1.1) | 2.8 (1.4) | 4.3 (1.8) | 4.0 (1.8) |
| Anxiety | 2.5 (1.0) | 3.0 (1.4) | 3.4 (1.5) | 3.7 (1.6) |
| Depression | 2.6 (1.3) | 2.6 (1.3) | 3.5 (1.5) | 3.5 (1.4) |
| Dependency | 2.2 (0.9) | 2.3 (1.3) | 3.4 (1.8) | 3.3 (1.6) |
| Lack of Stamina | 2.5 (1.3) | 2.6 (1.5) | 5.0 (1.7) | 4.3 (1.7)* |
| Suspiciousness | 2.6 (1.0) | 2.9 (1.0) | 3.0 (1.7) | 3.1 (1.2) |
| Obsessiveness | 3.3 (1.2) | 3.5 (1.3) | 3.5 (1.6) | 4.0 (1.5) |
| Vanity | 2.4 (1.2) | 2.4 (1.3) | 2.4 (1.5) | 2.4 (1.2) |
| Manipulativeness | 2.3 (1.1) | 2.4 (1.2) | 3.0 (1.7) | 2.7 (1.4) |
| Type A Behavior | 3.0 (1.5) | 3.3 (1.6) | 2.7 (1.7) | 2.9 (1.6) |
| Frugality | 3.4 (1.1) | 3.3 (1.1) | 3.3 (1.4) | 3.5 (1.3) |
Note. Control scales are in italics.
These scales were expected to belong to dimensions hypothesized to be associated with vmPFC damage.
These scales were not expected to belong to dimensions hypothesized to be associated with vmPFC damage.
There were no significant differences between study groups in “Before” ratings (all p's > 0.1).
Significance levels refer to differences between study group means in “Now” ratings:
= p < .05.
= p < .01.
= p < .001.
The principal components analysis, presented in Table 3, extracted seven components, all exceeding an eigenvalue of 1.0 and significant at the .05 level by parallel analysis, and accounting for a total 63.9% of variance. After rotation, the first component was quite robust, accounting for 17.3% of variance. Four of the five scales loading most highly on this component reflected disturbances in interpersonal relations or social behavior more generally, and poor modulation of emotional responses. This component was labeled “Disturbed Social Behavior/Emotional Reactivity.” The second component, accounting for 17.2% of variance, was loaded on highly by several scales that are characteristic of executive dyscontrol and impaired decision-making, and was labeled “Executive Deficits.” The third component, accounting for 10.8% of variance, was characterized most strongly by anxiety and vulnerability to pressure, and was labeled “Distress.” The fourth component, accounting for 6.4% of variance, was not conceptually coherent in an obvious way, but high loadings by vanity and suspiciousness, and lesser loadings including manipulativeness and impatience, led us to label this component “Self-Centered.” The fifth component, accounting for 4.3% of variance, was primarily defined by a high negative loading by frugality, and a moderate loading by manipulativeness, and was labeled “Self-Serving.” The sixth component, accounting for 4.1% of variance, had very high loadings from blunted affect and social withdrawal, and more moderately from apathy, and was labeled “Hypo-Emotionality.” The seventh component, accounting for 3.8% of variance, had significant loadings only from obsessiveness and type A behavior, and was labeled “Obsessive/Type A.”
Table 3.
Component Loadings for Principal Components Analysis with Varimax Rotation of ISPC Scales
| ISPC Scale | Disturbed Social Behavior/Emotional Reactivity |
Executive Deficits |
Distress | Self-Centered | Self-Serving | Hypo-Emotionality | Obsessive/ Type A |
|---|---|---|---|---|---|---|---|
| Insensitivity | .80 | .21 | .19 | .08 | .14 | .16 | −.01 |
| Aggression | .79 | .17 | −.10 | .12 | −.04 | .06 | .10 |
| Irritability | .75 | .06 | .20 | .26 | −.03 | −.05 | −.09 |
| Impatience | .65 | .10 | .31 | .31 | .16 | −.17 | −.07 |
| Social Inappr'ness | .61 | .37 | .22 | −.12 | .40 | .07 | .18 |
| Emotional Lability | .60 | .20 | .40 | .31 | −.03 | −.23 | .07 |
| Lack of Insight | .58 | .28 | .01 | −29 | .09 | .26 | .07 |
| Inflexibility | .56 | .14 | .25 | .24 | .33 | −.02 | .21 |
| Inappropriate Affect | .55 | .37 | .13 | .01 | .30 | .07 | .12 |
| Lack of Planning | .21 | .81 | .02 | .10 | .03 | .22 | .14 |
| Lack of Initiative | .21 | .78 | .19 | .01 | −.08 | −.01 | −.17 |
| Lack of Persistence | .24 | .78 | .10 | .15 | −.02 | .20 | −.17 |
| Poor Judgment | .32 | .73 | .12 | .06 | .28 | .21 | .08 |
| Lack of Stamina | −.11 | .70 | .36 | .01 | −.01 | .02 | −.15 |
| Perseveration | .31 | .64 | .27 | −.19 | −.04 | .17 | .14 |
| Impulsivity | .48 | .56 | .11 | .18 | .26 | −.18 | .17 |
| Indecisiveness | .04 | .51 | .45 | .01 | .23 | .25 | .23 |
| Anxiety | .34 | .13 | .80 | .07 | −.10 | −.11 | .20 |
| Vulnerability to Pressure | .31 | .46 | .65 | .07 | .13 | .02 | .02 |
| Depression | .41 | .32 | .57 | .12 | −.15 | .14 | −.07 |
| Dependency | .09 | .23 | .54 | .13 | .48 | .14 | −.10 |
| Vanity | .13 | .10 | .03 | .78 | .24 | .03 | .11 |
| Suspiciousness | .32 | .05 | .14 | .73 | −.24 | .01 | .11 |
| Frugality | −.09 | .00 | .15 | .06 | −.68 | .02 | .07 |
| Manipulativeness | .35 | −.07 | .26 | .30 | .50 | .07 | −.13 |
| Blunted Affect | −.04 | .24 | −.19 | .05 | .13 | .77 | −.17 |
| Social Withdrawal | .08 | .25 | .43 | −.01 | −.17 | .64 | .12 |
| Apathy | .23 | .44 | .47 | −.06 | −.05 | .47 | −.04 |
| Obsessiveness | −.01 | .04 | .05 | .05 | −.07 | −.16 | .84 |
| Type A Behavior | .32 | −.25 | .32 | .32 | −.11 | .21 | .62 |
Note. Component loadings > .50 are in bold. Scales in italics are control scales that are not expected to change as a result of a brain lesion.
The results of PCA were reviewed with regard to the a priori conceptualization of dimensions. As the first component extracted revealed interdigitation of scales hypothesized to form two distinct dimensions, Disturbed Social Behavior and Emotional Reactivity, the question arose as to whether those eight scales in fact comprise a unitary dimension, or whether they reflect two dimensions brought together by the statistics of component analysis in which the pattern of covariance among all 30 ISPC scales determines the composition of each component. Thus, a post hoc PCA with varimax rotation was performed on the eight scales loading most highly on component 1. The results, presented in Table 4, yielded two distinct components. The first component, labeled “Irascibility,” accounting for 38.5% of the variance in this analysis, was loaded on most highly by the three scales conceptualized a prior as comprising emotional reactivity, plus the scale Inflexibility. None of these scales loaded significantly on the other component, which accounted for 12.3% of the variance. With the exception of Inflexibility, the other five scales conceptualized a priori as comprising disturbed social behavior loaded highly on the second component, although Insensitivity and Aggression also loaded highly on component 1 at levels broadly comparable to the loading on component 2. These results were considered to support retaining two conceptually distinct dimensions. Based on the results of the post hoc component analysis, Inflexibility was included with the dimension originally referred to as “Emotional Reactivity” but, in light of the altered composition, is now referred to as “Irascibility.” It was noted that Insensitivity loaded somewhat more highly on the first component than on the second, but it was decided that, conceptually, Insensitivity is an integral aspect of the dimension, Disturbed Social Behavior.
Table 4.
Principal Components Analysis with Varimax Rotation of Scales Loading on Component 1
| ISPC Scale | Irascibility | Disturbed Social Behavior |
|---|---|---|
| Impatience | .85 | .20 |
| Irritability | .83 | .18 |
| Emotional Lability | .76 | .23 |
| Inflexibility | .74 | .28 |
| Insensitivity | .66 | .57 |
| Lack of Insight | .07 | .82 |
| Inappropriate Affect | .23 | .80 |
| Social Inappropriateness | .46 | .70 |
| Aggression | .48 | .57 |
Note. Component loadings > .50 are in bold.
Scales conceptualized as being associated with Executive Deficits loaded highly on the second component. Additionally, Lack of Stamina, initially conceptualized as a nonspecific disturbance (expected to be) associated with brain damage in general, loaded highly on the second component. A post hoc analysis parallel to that performed on scales loading on component 1 was performed to address two questions: Do cognitive and behavioral aspects of executive dysfunction warrant assessment as distinctive dimensions of personality disturbance in the brain-damaged population (as suggested by earlier factor analysis of the IRSPC)? Might the loading of Lack of Stamina on the Executive Deficits component reflect a subcomponent of deficient drive or energization? Post hoc PCA was performed on the eight scales loading most highly on component 1. Only one factor could be extracted, with the eight scales loading from .85 (Lack of Planning) to .68 (Indecisiveness), indicating a unitary nature to this component. However, as Lack of Stamina was not conceptualized as an aspect of deficits in decision-making and executive functions, this scale was not incorporated into the Executive/Decision-Making Deficits dimension. Scales loading on the third and sixth components supported the a priori conceptualizations of the dimensions Diminished Motivation/Hypo-emotionality and Distress.
The remaining components were not related to a priori dimensions. Each of these components had only two scales with high or moderately high loadings, and these included one freestanding scale (Obsessiveness or Suspiciousness) and one control scale for two of the components (Self-Centered and Obsessive/Type A), and two control scales for the third (Self-Serving). The presence and composition of these components supports treatment of Obsessiveness and Suspiciousness as free-standing characteristics rather than components of broader dimensions of acquired personality disturbance.
The correlations of each scale to the re-formulated dimensional scores are presented in Table 5. In general, the pattern of part-whole correlations showed that scales correlated most highly with the dimensions to which they were assigned, supporting the assignment of scales to dimensions. It is noted, however, that the magnitude of many of the differences in correlations was small, and several scales correlated about as highly with one or more other dimensions as with the assigned dimension. Additionally, there were two cases in which the correlation coefficient of a scale with another dimension was (non-significantly) higher than the coefficient with the dimension to which it was assigned (Insensitivity and Apathy), and these scales are considered further in the discussion.
Table 5.
Spearman correlations of scales to dimensional scores
| Scale | Executive/ Decision- Making Deficits |
Disturbed Social Behavior |
Irascibility | Diminished Motivation/ Hypo- Emotionality |
Distress | Lack of Stamina |
Suspiciousness | Obsessiveness | Control Scales- Negative |
Control Scales- Positive |
|---|---|---|---|---|---|---|---|---|---|---|
|
Executive/Decision-
Making Deficits |
||||||||||
| Lack of Planning | .82 | .46 | .30 | .49 | .41 | .50 | .10 | .08 | .03 | −.02 |
| Poor Judgment | .79 | .60 | .40 | .47 | .53 | .50 | .16 | .02 | .18 | −.17 |
| Lack of Persistence | .76 | .48 | .33 | .48 | .51 | .55 | .15 | −.13 | .12 | −.14 |
| Perseveration | .73 | .54 | .39 | .44 | .55 | .49 | .02 | .07 | −.01 | .05 |
| Lack of Initiative | .69 | .45 | .29 | .41 | .50 | .53 | .13 | −.03 | .05 | −.20 |
| Impulsivity | .61 | .56 | .59 | .22 | .53 | .29 | .22 | .14 | .31 | −.06 |
| Indecisiveness | .60 | .40 | .28 | .46 | .56 | .39 | .09 | .13 | .09 | −.06 |
| Disturbed Social Behavior | ||||||||||
| Insensitivity | .49 | .71 | .70 | .34 | .47 | .15 | .32 | −.02 | .22 | .03 |
| Social Inappropriateness | .60 | .70 | .59 | .33 | .52 | .27 | .05 | .10 | .19 | −.12 |
| Inappropriate Affect | .52 | .59 | .39 | .26 | .47 | .12 | .20 | .03 | .29 | −.09 |
| Aggression | .37 | .58 | .53 | .22 | .25 | −.01 | .33 | .04 | .19 | .17 |
| Lack of Insight | .44 | .52 | .32 | .29 | .32 | .17 | .01 | −.03 | .08 | .02 |
| Irascibility | ||||||||||
| Impatience | .34 | .53 | .74 | .09 | .49 | .14 | .39 | −.01 | .35 | −.01 |
| Irritability | .35 | .56 | .70 | .19 | .47 | .08 | .34 | .01 | .30 | .13 |
| Emotional Lability | .43 | .50 | .67 | .11 | .57 | .23 | .49 | .12 | .33 | .06 |
| Inflexibility | .40 | .53 | .64 | .18 | .42 | .12 | .28 | .16 | .36 | .05 |
| Diminished Motivation/Hypo-Emotionality | ||||||||||
| Apathy | .62 | .47 | .37 | .54 | .57 | .45 | .17 | .01 | .12 | −.10 |
| Social Withdrawal | .43 | .30 | .21 | .54 | .42 | .34 | .15 | .05 | −.01 | .07 |
| Blunted Affect | .23 | .11 | −.10 | .27 | −.01 | .12 | −.06 | −.19 | .01 | −.08 |
| Distress | ||||||||||
| Vulnerability to Pressure | .61 | .46 | .48 | .38 | .70 | .50 | .22 | .11 | .20 | −.11 |
| Anxiety | .36 | .37 | .50 | .21 | .64 | .28 | .32 | .27 | .19 | .14 |
| Depression | .55 | .48 | .55 | .41 | .57 | .40 | .33 | .06 | .26 | .03 |
| Dependency | .40 | .35 | .34 | .29 | .44 | .34 | .13 | .00 | .34 | −.24 |
| Freestanding Characteristics | ||||||||||
| Lack of Stamina | .57 | .22 | .17 | .38 | .49 | ---- | −.01 | −.07 | −.04 | −.17 |
| Suspiciousness | .14 | .20 | .44 | .10 | .33 | −.01 | ---- | .12 | .31 | .29 |
| Obsessiveness | .06 | .04 | .07 | −.03 | .12 | −.07 | .12 | ---- | −.03 | .41 |
| Control Scales- Negative | ||||||||||
| Vanity | .11 | .07 | .28 | .04 | .20 | .04 | .41 | .04 | .24 | .09 |
| Manipulativeness | .11 | .23 | .34 | .02 | .28 | −.10 | .13 | −.07 | .24 | −.04 |
| Control Scales- Positive | ||||||||||
| Type A Behavior | −.11 | .06 | .17 | −.04 | −.07 | −.27 | .33 | .37 | .09 | .08 |
| Frugality | −.06 | −.13 | −.11 | .02 | −.01 | .03 | .08 | .20 | −.12 | .08 |
Note. Part-whole correlations of scales to the dimension to which they were assigned are in bold. Correlations greater than .30 were significant at the .001 level for two-tailed tests, and correlations greater than .27 were significant at the .001 level for one-tailed tests (i.e., part-whole correlations of scales to the assigned dimension).
The correlations of dimensions with each other are presented in Table 6. Executive/Decision-Making Deficits, Disturbed Social Behavior, and Irascibility were each significantly correlated with each other. In contrast, Diminished Motivation/Hypo-Emotionality showed a robust correlation only with Executive/Decision-Making Deficits, and a marginal correlation with Disturbed Social Behavior, while the correlation with Irascibility was non-significant. Distress was significantly correlated with each of the other dimensions.
Table 6.
Intercorrelations of dimensional scores and freestanding scales
| Dimensions/Scales | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1.Executive/Decision-Making Deficits | ---- | ||||||||
| 2. Disturbed Social Behavior | .62 | ---- | |||||||
| 3. Irascibility | .45 | .64 | ---- | ||||||
| 4. Diminished Motivation/Hypo-Emotionality | .54 | .37 | .18 | ---- | |||||
| 5. Distress | .63 | .53 | .59 | .43 | ---- | ||||
| 6. Lack of Stamina | .57 | .22 | .17 | .38 | .49 | ---- | |||
| 7. Suspiciousness | .14 | .20 | .44 | .10 | .33 | −.01 | ---- | ||
| 8. Obsessiveness | .06 | .04 | .07 | −.03 | .12 | −.07 | .12 | ---- | |
| 9. Control Scales- Negative | .14 | .19 | .38 | .04 | .31 | −.04 | .31 | −.03 | ---- |
| 10. Control Scales- Positive | −.10 | −.01 | .05 | −.04 | −.07 | −.17 | .29 | .41 | .01 |
Note. Correlations greater than .24 were significant at the .01 level. Correlations greater than .30 were significant at the .001 level.
The associations of dimensions and higher-order composite scores with lesion location are presented in Table 7. The vmPFC groups was rated as having significantly more disturbance in Executive/Decision-Making Deficits and Disturbed Social Behavior than the non-vmPFC group, and more disturbance in Diminished Motivation/Hypo-Emotionality at a level approaching significance. There was no significant difference between the groups in Irascibility or in Distress. The groups also did not differ in ratings of Suspiciousness or Obsessiveness, but the vmPFC group had higher ratings for Lack of Stamina. Mean group ratings were comparable for negative and positive control scales, as should be the case. Regarding higher-order composite measures, the vmPFC group showed significantly greater disturbance on “acquired ventromedial prefrontal personality disturbances” than did the non-vmPFC group. The groups had nearly identical summary scores for other personality disturbances, and they did not differ significantly in total ISPC score (exclusive of control scales), although the vmPFC had a mildly higher summary score.
Table 7.
Comparisons between vmPFC and non-vmPFC on dimensional scores, higher-order indices, and total score
| vmPFC (n=28) |
Non-vmPFC (n=96) |
Mann- Whitney U |
p | |
|---|---|---|---|---|
| Dimensions/Scales | ||||
| Executive/Decision-Making Deficits | 29.8 (10.6) | 24.2 (7.7) | 874.5 | .003 |
| Disturbed Social Behavior | 17.6 (5.6) | 14.9 (6.1) | 940.0 | .020 |
| Irascibility | 16.0 (5.7) | 14.7 (5.3) | 1171.0 | .186 |
| Diminished Motivation/Hypo-Emotionality | 10.8 (4.0) | 9.7 (3.5) | 1102.0 | .073 |
| Distress | 14.6 (4.9) | 14.6 (5.1) | 1334.5 | .960 |
| Lack of Stamina | 5.1 (1.7) | 4.3 (1.7) | 1007.5 | .041 |
| Suspiciousness | 3.0 (1.7) | 3.1 (1.2) | 1172.5 | .272 |
| Obsessiveness | 3.5 (1.6) | 4.0 (1.5) | 1116.5 | .160 |
| Control Scales- Negative | 5.4 (2.7) | 5.0 (2.1) | 1288.5 | .736 |
| Control Scales- Positive | 6.0 (2.2) | 6.4 (2.0) | 1195.5 | .366 |
| Higher-Order Indices | ||||
| Acquired Ventromedial Prefrontal Personality Disturbances | 73.2 (20.1) | 63.6 (18.2) | 906.5 | .014 |
| Other Personality Disturbances | 26.2 (6.8) | 26.0 (7.0) | 1326.0 | .914 |
| ISPC Totala | 99.1 (25.8) | 89.6 (23.5) | 990.5 | .096 |
Note.
ISPC total does not include control scales.
Discussion
The findings from this study support the hypothesis that there are multiple distinctive dimensions of personality disturbance following brain damage, including the five hypothesized dimensions: Disturbed Social Behavior, Executive/Decision-Making Deficits, Diminished Motivation/Hypo-emotionality, Irascibility, and Distress. Additionally, principal components analysis extracted three additional components appearing to reflect premorbid characterological issues (Self-Centered, Self-Serving, and Obsessive/Type A). The four dimensions hypothesized to be associated with vmPFC damage showed more disturbance among participants with vmPFC lesions, compared to participants with lesions outside the vmPFC, although this was statistically significant for only two of the four dimensions. A goal of the study was to develop ISPC subscales for assessment of these dimensions. In the discussion that follows, we consider the results bearing on composition of the dimensions in more detail.
Executive/Decision-Making Deficits
The dimension Executive/Decision-Making Deficits reflects classic aspects of executive dysfunction widely associated with prefrontal damage (e.g., lack of planning and initiative, poor judgment, perseveration, and impaired decision-making). As the ISPC is a measure of personality (i.e., behavioral tendencies rather than performance on cognitive tasks), the rater is presented with behavioral guidelines to inform their ratings. Consistent with the emphasis on behavioral tendencies, post hoc analysis showed no dissociation between the more “cognitive” and more “behavioral” aspects of executive dysfunction assessed by the ISPC. Lack of Stamina, which had been viewed a priori as a freestanding characteristic, loaded highly on the Executive deficits component, so assignment of Lack of Stamina to the corresponding dimension was considered. However, as this characteristic is not conceptually related to deficits in decision-making and executive functions, it was decided not to incorporate it into this dimension (a) to keep the Executive/Decision-Making Deficits dimension a more conceptually “pure” dimension for clinical and research purposes, and (b) to facilitate future research into this potentially important consequence of brain damage—viz., lack of stamina or what has been often referred to as “fatiguability” (see below). As predicted, greater disturbance in Executive/Decision-Making Deficits was significantly associated with vmPFC damage. Lack of Stamina was also significantly associated with vmPFC damage.
Disturbed Social Behavior
Principle component analysis demonstrated a strong relationship between disturbed social behavior and emotionality/interpersonal prickliness. Post hoc PCA demonstrated they are dissociable dimensions, a finding that is highly consistent with the results of an independent factor analysis of the Iowa Rating Scales of Personality Change, forerunner of the ISPC, with a clinically heterogeneous sample of neurological patients (Barrash et al., 1997b). The first two factors extracted in that earlier analysis found Emotional Reactivity to be the most powerful factor, while a factor labeled “Interpersonal Disturbance” – highly overlapping with the dimension labeled “Disturbed Social Behavior” in the present study – was the second factor to emerge. It is logical that disturbance in emotional modulation would play an important role in disturbance in social/interpersonal behavior (Damasio, 1994); however, empirical investigation of such relationships requires distinctive measures of the two, and it was decided to maintain two distinct dimensions.
The post hoc PCA revealed that Inflexibility covaried strongly with the latter rather than the former, and so it was re-assigned. It also indicated that the insensitivity covaried at least as strongly with ratings of the latter as with ratings of disturbed social behavior. However, we view insensitivity as a cardinal feature of disturbed social behavior, and so this scale was kept with this dimension. The appropriateness of this decision was supported by subsequent part-whole correlations that found that it had the highest part-whole correlation coefficient with the dimensional score of all the scales comprising this dimension (although at essentially the same level as the other cardinal feature, social inappropriateness). The scale Aggression could reasonably be considered for inclusion in the Irascibility dimension. However, Aggression tended to be described by informants as an alteration of characteristic interpersonal behavior rather than as uncharacteristic behaviors due to emotional dyscontrol provoked by circumstances. Its correlations with the dimensions of Disturbed Social Behavior and Irascibility suggest slightly more covariance with the former. The dimension, Disturbed Social Behavior, was significantly associated with damage to the vmPFC region.
Irascibility
The hypothesized dimension, Emotional Reactivity, was expected to comprise three scales: Irritability, Impatience and Emotional Lability. However, with the inclusion of the Inflexibility, the resultant set of four scales may be seen as forming a coherent set of cross-situational, affective-interpersonal tendencies (rather than emotional reactivity to external circumstances). Accordingly, this re-formulated dimension was renamed “Irascibility.” Part-whole correlations showed that each of the four component scales was highly related to the overall dimension, Irascibility. Comparison of the study groups found that the higher level of Irascibility found among vmPFC compared to non-vmPFC approached but did not reach statistical significance. Of note, this contrasts with the significant association of Disturbed Social Behavior to vmPFC damage.
There is a conceptual distinction between the dimensions that would be expected to have important clinical implications, such as differential treatment of choice for disturbances of emotional control versus social behavior. The clinical utility of maintaining Irascibility as a distinct dimension of acquired personality disturbance is also highlighted by findings that disruption of emotional modulation following brain damage resulting in Irascibility was highly predictive of real-world competencies such as everyday functioning, financial management, health and safety-related behavior, and employment/academic status (Anderson et al., 2006).
Diminished Motivation/Hypo-emotionality
In accordance with a priori expectation, PCA extracted a component loaded on by blunted affect, apathy, and social withdrawal, and reflective of diminished motivation and hypo-emotionality. Of note, this component was the sixth component to emerge and it accounted for only four percent of scale co-variation. This is consistent with the finding that a Hypo-emotionality factor comprised of blunted affect, social withdrawal and apathy was the weakest of all factors extracted in the earlier factor analyses of the Iowa Rating Scales of Personality Change (Barrash et al., 1997b), and the factor reflecting apathy was the weakest of all factors extracted in factor analyses of other instruments (Kinsella et al., 1991; Stout et al., 2003). One component scale, Apathy, loaded about as highly with factors labeled Distress and Executive Deficits as with the factor labeled Hypo-emotionality. These findings are consistent with evidence that apathy is a complex phenomenon, with aspects reflecting loss of interest as is frequently seen in major depression (American Psychiatric Association, 1994). However, in brain-damaged populations this symptom is often not associated with low mood (Cahn-Weiner, Grace, Ott, Fernandez, & Friedman, 2002), but rather with cognitive disengagement associated with indecisiveness and impaired attention (Siegert, Walkey, & Turner-Stokes, 2009) or, in patients with damage to frontal systems, a disorder of drive and self-activation (Ready, Ott, Grace, & Cahn-Weiner, 2003; Stuss et al., 1992). Even though apathy may be associated with severe depression, in a population with neuropathological conditions it may be more accurate and clinically useful to see apathy as a neuropathological symptom with differential treatment implications than apathy-as-depression (Boyle & Malloy, 2004). Thus, in accordance with a priori conceptualization based on our experience with patients with vmPFC lesions, it was decided to retain apathy in the Diminished Motivation/Hypo-emotionality dimension, although it is understood that this complex symptom is relevant to other dimensions as well.
Although mean ratings suggest that Diminished Motivation/Hypo-emotionality may not be an especially prominent aspect of personality change after brain damage, it is of significant theoretical and clinical import. This dimension corresponds to the syndrome of apathy and loss of motivation theorized by Cummings (1993) to result from damage to the anterior cingulate circuit. However, there is extensive evidence suggesting that the syndrome involves deficiencies in emotional experience as well as motivation (apathy) in patients with vmPFC damage (Damasio, 1994; Tranel & Damasio, 1994). The clinical importance of the deficit in emotional experience is suggested by a body of research indicating that this disturbance contributes prominently to impairments in the decision-making (Bechara et al., 2000; Bechara, Tranel, Damasio, & Damasio, 1996; Tranel, Bechara, & Damasio, 1999) and psychosocial functioning (Anderson et al., 2006; Damasio, 2000). Stuss and Benson (1986) have emphasized that diminished motivation is a core deficit following prefrontal damage in that it contributes to disturbances in other abilities. Accordingly, in the present study, intercorrelations with other dimensions show that Diminished Motivation/Hypo-emotionality is highly correlated with Executive Decision-Making Deficits consistent with their crucial theoretical connection (Damasio, 1994; Tranel et al., 1999). In contrast, Diminished Motivation/Hypo-emotionality is only modestly correlated with Distress, with which it shares only 18% of variance. Although participants with vmPFC lesions were rated as having a higher level of Diminished Motivation/Hypo-emotionality, the difference from non-vmPFC failed to reach statistical significance, possibly reflecting difficulty that laypersons may have appreciating the degree of disturbance in characteristics characterized by the absence of activity or emotional experience (Anderson et al., 2006).
Distress
Depression following brain injury is common (House, 1987; Jorge et al., 1993b, 2004; Starkstein & Tranel, in press), frequently with co-morbid anxiety (Jorge et al., 2004). Consistent with the prediction that Distress is a non-specific consequence of brain damage rather than a consequence of damage to specific structures, the vmPFC group and non-vmPFC group had identical summary scores on this dimension. Furthermore, Distress was generally highly correlated with disturbances on all other dimensions. This is consistent with an earlier study with the Iowa Rating Scales of Personality Change finding that depression and anxiety were not specifically associated with damage to vmPFC (Barrash et al., 2000), and findings that depression developing after the acute phase was not associated with lesion location (Jorge et al., 1993a, 1993b). Although depression has been associated with prefrontal lesions in the acute stage (Jorge et al., 1993a, 1993b, 1993c, 2004), meta-analysis revealed that mood disorder was related to lesion location only in the first half-year following stroke (Narushima, Kosier, & Robinson, 2003)—leading to the suggestion that depression developing after the acute phase may be more related to psychosocial and psychological mechanisms (Jorge et al., 1993a). The lack of association between distress and vmPFC damage in the present study is consistent with such speculation, as we were explicitly concerned with chronic, stable personality and mood disturbances and so restricted our sample to participants rated a minimum of six months after lesion onset.
Freestanding Disturbances
The composition of the remaining components—Self-Centered, Self-Serving and Obsessive/Type A—including control scales with the highest or second highest loading, suggests primarily characterological problems rather than acquired disturbances secondary to brain damage. These components will not be discussed further.
Three scales—Lack of Stamina, Suspiciousness and Obsessiveness—were hypothesized to be freestanding disturbances; that is, scales that are not components of multivariate dimensions. Results supported those expectations for the latter two scales, which were conceptualized as being idiosyncratic characteristics which, if present premorbidly, might be exacerbated in an individual sustaining brain damage due to diminished control over these tendencies. This conceptualization was supported by significant correlations between the “Now” and “Before” ratings for Suspiciousness (.49) and Obsessiveness (.39)—higher than such correlations for other scales.
In contrast, Lack of Stamina was conceptualized as a nonspecific consequence of brain damage, and thus a characteristic that would not especially related to any one dimension. However, Lack of Stamina loaded highly on the Executive Deficits scale, prompting re-consideration of its hypothesized status as a freestanding characteristic. Lack of Stamina is typically referred to as “fatigue” in the literature, and the term “central fatigue” has been defined as a feeling of constant exhaustion and difficulty in initiation or sustaining a voluntary activity (Chaudhuri & Behan, 2004), to distinguish this symptom from muscle fatigability (or “peripheral fatigue”). Central fatigue is a common symptom, and may among the most disabling, in many neurological disorders, including cerebrovascular disease, TBI, Parkinson's disease, and multiple sclerosis (Van der Werf et al., 1998). In a study of the late consequences of mild stroke at one year, fatigue was the most common symptom (72%); in comparison, the next most frequent complaints were memory dysfunction and stressfulness (55% for both) (Carlsson, Möller, & Blomstrand, 2003). Staub and Bogousslavsky (2001) reported that a major sequela of stroke is “primary poststroke fatigue” (i.e., in the absence of depression or significant cognitive sequelae), defined as “a feeling of early exhaustion developing during mental activity, with weariness, lack of energy and aversion to effort.” Possible causal mechanisms are many, including lesions affecting circuits that connect thalamus, basal ganglia, amygdala and frontal cortex (Bruno, Crenage, & Fick, 1998; Chaudhuri & Behan, 2000; Glader, Stegmayr, & Asplund, 2002); conditions compromising the availability of neurotransmitters (Bradley & Alarcon, 1999; Bruno et al., 1998); endocrinological disturbances (Chaudhuri & Behan, 2004); or the psychobiological effects of depression and anxiety (Chaudhuri & Behan, 2004). It is important to note that the presence of fatigue is commonly overlooked, but it is sometimes the only persisting sequela of stroke, and it may severely limit a patient's return to their previous level of functioning (Staub & Bogousslavsky, 2001).
Despite its pervasiveness in many patients' lives and highly negative impact on functioning, fatigue is often not assessed systematically—when it is assessed, it is almost always by subjective self-report. Objective measures of fatigue during performance on cognitive tasks are being developed (e.g., Peter, Zoltan, Ivan et al., 2009; Lal, Craig, Borod, Kirkup, & Nguyen, 2003), but it is an open question whether “central fatigue” is most meaningfully assessed as a physiological variable, or whether it may be informatively conceptualized as a multifaceted final common pathway related to personality (i.e., enduring behavioral tendencies—potentially related to drive, mood, and pervasive attitudes—that, when disturbed, adversely impact psychosocial functioning in ongoing fashion). This is not intended to diminish the important role of biological factors. Rather, if fatigue or lack of stamina is related to multiple interacting factors including “psychological” factors such motivation and attitude, then investigation of the degree of “lack of stamina” as an enduring real-life characteristic will be critical to greater understanding of this important disturbance. Accordingly, it was decided that, while lack of stamina clearly co-varies with executive dysfunction, maintaining this characteristic as a freestanding disturbance would facilitate research and clinical utility.
Control Scales
As the goal of this study is investigation of distinctive dimensions of acquired personality disturbances, control scales are not a primary concern, and we offer only a few brief comments. The mean “Now” rating was 2.9, essentially at the hypothetically “average” or typical level of 3. Collectively, the four scales showed a negligible mean increase in ratings of 0.13 from “Before” to “Now” ratings. These findings are consistent with the expectation that the control scales assess aspects of behavior that do not develop disturbances following brain damage. Ratings of increased disturbance from premorbidly normal levels on these scales may suggest response bias, and further investigation of this idea is warranted.
Returning to the broader landscape of personality changes following brain damage, our results are comparable to factor analyses of other instruments assessing personality disturbances after brain damage involving prefrontal region. Factors reflecting loss of motivation were found for the Frontal Systems Behavior Scale (Stout et al., 2003), the Neuropsychiatric Inventory (Bozeat et al., 2000) and the CBC (Kinsella et al., 1991), and others have found factors paralleling executive dysfunction (Stout et al., 2003; Bozeat et al., 2000), disinhibition or disturbed social behavior (Stout et al., 2003; Bozeat et al., 2000), emotional dyscontrol (Bozeat et al., 2000; Kinsella et al., 1991). As in the present principal components analysis, Stout and colleagues found that some items loaded significantly on more than one dimension. Even though the Frontal Systems Behavior Scale was designed to assess the specific syndromes of executive dysfunction, disinhibition and apathy, some items did not load most highly on the expected factor (Stout et al., 2003), as was found in the present analysis. They also found significant correlations between the three factor scores, as found in the present analysis, indicating that personality changes after brain damage are not completely independent phenomena, and individuals with significant personality disturbances in one area tend to have some degree of disturbance in other dimensions.
There are also differences between the present PCA and the factor analyses of the Frontal Systems Behavior Scale, Neuropsychiatric Inventory and CBC. Factor analyses of those instruments extracted from two to four factors, factors which left approximately 55%-60% of the variance unaccounted for, raising the question whether there might be other aspects of acquired personality disturbances not sufficiently assessed with sufficient coverage for the formation of additional factors (Stout et al., 2003). PCA of the ISPC yielded seven components accounting for all but 36% of the variance in this instrument. It is possible that the identification of additional components with the ISPC, and the corresponding increase in variance accounted for, reflects a difference in the goals for the design of the different instruments. The Frontal Systems Behavior Scale, Neuropsychiatric Inventory and CBC were expressly developed for more specific assessment goals, respectively: personality changes related to prefrontal dysfunction (Grace & Malloy, 2001), neurobehavioral symptoms associated with dementia (Cummings et al., 1994), and behavioral disturbances following TBI (Elsass & Kinsella, 1989). In contrast, the Iowa Rating Scales of Personality Change/ISPC was expressly designed to assess a wide range of possible personality changes after development of any adult-onset neuropathological changes in the brain. The lack of constraints on item selection in the design of the ISPC may allow it to be sensitive to a broader range of personality changes following brain damage. In particular, the ISPC offers greater assessment of mood-related disturbances: irascibility, diminished emotionality and distress. We believe these dimensions of personality changes after brain damage are each important aspects. A recent cluster analysis of personality disturbances after TBI (Velikonja, Warriner, & Brum, 2010) revealed 13 different personality profiles, with various mood disturbances playing a prominent role in those profiles. This prompted the investigators to emphasize the need for multidimensional measures that assess both behavioral changes and mood disturbance to adequately characterize the diverse nature of symptoms in the brain injury population, noting that comprehensive measures yield superior information to use of multiple measures assessing symptoms of a single disorder.
Since the ISPC replaced the Iowa Rating Scales of Personality Change in 1997, findings regarding personality disturbances have been reported for a wide range of neurological populations in North America and Europe. Several investigators have summed all of the ISPC scales into one summary measure to reflect total personality disturbance. This approach has yielded marginal or non-significant results. For example, comparing multiple sclerosis patients with normal or weak decision-making ability, the difference in total score on a French version of the ISPC (Juillerat, Peter-Favre, & Van der Linden, 1998) was not significant (Simioni et al., 2008). In a second study, patients with multiple sclerosis did not differ from controls in total ISPC score, although there were differences in particular characteristics such as lack of stamina, inflexibility, social withdrawal and lack of insight (Souza Lima et al., 2007). Only relatively mild differences in ISPC total score (French version) were found between first-ever stroke patients with normal, mildly impaired, or severely impaired psychosocial functioning (Hommel et al., 2009). Parkinson's patients successfully treated with bilateral deep brain stimulation did not show significant pre-post personality change when the total ISPC score (French version) was examined, but significant improvement was found when specific scales were examined—scales closely mapping onto the dimensions of Executive Dysfunction and Diminished Emotion (Houeto et al., 2002). Gleason (2004) found only weak associations between frontal vs. nonfrontal tumors and overall score on the ISPC or on the Frontal Systems Behavior Scale. However, greater disturbance was associated with frontal tumors when specific scales were examined on the Frontal Systems Behavior Scale, but specific scales of the ISPC were not examined.
In contrast to the generally weak findings when analyses are conducted with total ISPC score, investigators have found it fruitful to examine subsets of scales selected to address hypotheses regarding specific relationships of personality disturbances to varying clinical issues. As noted above, Anderson and colleagues (2006) hypothesized that the presence of acquired disturbances in Irascibility and hypo-emotionality would be associated with the development of impairments in real-world psychosocial functioning, and these hypotheses were well supported. In another study, Nguyen and colleagues (2010) found that a subset of ISPC scales relevant to executive and decision-making deficits were predictive of impaired performance on a decision-making task in healthy, community dwelling elderly adults. To investigate the personality and cognitive changes underlying psychosocial dysfunction after TBI, Rochat and colleagues (2009) selected relevant ISPC scales to form measures of internalizing and externalizing personality changes. They found that externalizing changes (e.g., irritability, impatience, aggression, insensitivity, social inappropriateness, inappropriate affect and impulsivity) and, to a lesser extent, internalizing changes (depression, anxiety and social withdrawal) were associated with more severe executive defects. Taken together, studies with the ISPC indicate that investigation with particular scales, or sets of scales, is more informative than comparison of total ISPC score. The results of the present study provide a basis for employing subscale scores to test specific hypotheses in future studies.
“Acquired Ventromedial Prefrontal Personality Disturbances”
Only two of the four dimensions predicted to be associated with vmPFC damage were significantly higher in the vmPFC group, with a third being marginally significant. However, “acquired ventromedial prefrontal personality disturbances”—the composite measure comprised of those four dimensions—was strongly associated with vmPFC damage. Heretofore, we have used the term “acquired sociopathy” as a shorthand to highlight the self-defeating behaviors primarily in the social/interpersonal domain that are so salient in individuals with vmPFC damage (Eslinger & Damasio, 1985; Tranel, 1994). However, over time we have developed concern that this term connotes more than intended—in particular, it may convey the implication that the socially problematic behavior of these patients is motivated and willful, and therefore warranting punishment or scorn. This connotation is not accurate, as most vmPFC patients do things that are harmful to their own best interests, but do not engage in serious or violent behavior towards others or others' property. Hence, we recommend a different rubric, viz., “acquired ventromedial prefrontal personality disturbances.” Also, we note that this term does not imply that this syndrome occurs only with lesions to vmPFC, as damage elsewhere may result in dysfunction in the systems subserved by vmPFC cortex (Cummings, 1993; Damasio & Anderson, 2003). While acquired ventromedial prefrontal personality disturbances summary scores were strongly associated with ventromedial prefrontal damage, the sum of other personality disturbances was virtually identical in the vmPFC and non-vmPFC groups. Accordingly, if a total ISPC score is calculated by including other personality disturbances along with acquired ventromedial prefrontal personality disturbances, the difference between vmPFC and non-vmPFC is diminished to the point that there is not a significant difference between the two groups. This highlights the recommendation that total ISPC score not be used for analyses because the heterogeneity of behavioral disturbances assessed by the ISPC is such that combining them all together risks obscuring meaningful relationships that may be present between specific dimensions and other variables of interest.
This study is not without limitations. A larger sample size would have been advantageous, although for research with focal lesions, the sample size is larger than in most of the literature. However, the present sample size did not appear to undermine the validity of the study as the results of the present PCA were highly consistent with factor analysis of the earlier version of the Iowa Scales, and with factor analysis of other instruments assessing personality disturbances in neurological patients. Nevertheless, it is possible that more subjects may have provided stronger statistical support for the relationships of Irascibility and Diminished Motivation/Hypo-Emotionality to vmPFC damage. Perhaps a more significant limitation was the limited number of patients with unilateral vmPFC lesions. A greater number of such patients would have allowed for examination of the relationships of ISPC dimensions to unilateral lesions and to possible hemispheric differences. An earlier study (with Iowa Rating Scales of Personality Change ratings) investigating the nature of acquired personality disturbances in patients with focal lesions (Barrash et al., 2000) yielded quite robust differences for between patients with bilateral vmPFC lesions versus lesions elsewhere. However, accrual is slow for individuals with discrete unilateral focal lesions in the vmPFC region, and meeting the restrictive inclusion/exclusion criteria for investigation of acquired personality changes. Enrollment of additional subjects will permit further investigation of distinctive personality changes specifically associated with unilateral left or right VMC lesions (Tranel et al., 2002), and the possibility of gender effects on the hemispheric differences in the contribution of vmPFC to social-emotional functioning (Tranel et al., 2005).
Limitations notwithstanding, study findings identifying ISPC subscales reflecting disturbances in aspects of emotional functioning, executive functions/decision-making, and behavioral control may be useful clinically for the characterizing social and emotional changes with significant impact on real-world functioning and important implications for neuropsychological rehabilitation strategies (Anderson et al., 2006). Research regarding personality changes in neurological populations will also benefit from attention to specific dimensions of personality disturbance assessed with the ISPC as analyses with the total scores often obscures more specific relationships.
Future directions for research with the ISPC include independent replication of dimensions and development of normative information regarding dimension scores. Investigations of the clinical correlates of individuals with relevant disturbances may contribute to development of sensitive and specific criteria for “Acquired Ventromedial Prefrontal Personality Disorder.” Additionally, it will be informative to investigate relationships between specific acquired personality disturbances and subregions within prefrontal cortices and elsewhere in the brain. We anticipate performing such analyses in the future, including examination of potential effects of laterality and gender.
Acknowledgments
This research was supported by NIH grants NIDA DA022549 and NINDS NS19632, and the Kiwanis Foundation. The authors thank Joel Bruss for his assistance creating the figure of the brain.
Contributor Information
Joseph Barrash, Departments of Neurology and Psychology, University of Iowa.
Erik Asp, Department of Neurology and Neuroscience Program, University of Iowa.
Kristian Markon, Department of Psychology, University of Iowa.
Kenneth Manzel, Department of Neurology, University of Iowa.
Steven W. Anderson, Department of Neurology, University of Iowa
Daniel Tranel, Departments of Neurology and Psychology, University of Iowa..
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association Press; Washington, DC: 2000. Text Revision. [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real world complex behavior following childhood-or adult-onset lesions in ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Damasio H, Damasio AR. A neural basis for collecting behaviour in humans. Brain. 2005;128:201–212. doi: 10.1093/brain/awh329. [DOI] [PubMed] [Google Scholar]
- Anderson SW, Tranel D. Awareness of disease states following cerebral infarction, dementia, and head trauma. The Clinical Neuropsychologist. 1989;3:327–339. [Google Scholar]
- Annoni J-M, Devuyst G, Carota A, Bruggimann L, Bogousslavsky J. Changes in artistic style after minor posterior stroke. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:797–803. doi: 10.1136/jnnp.2004.045492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrash J, Anderson SW. The Iowa Rating Scales of Personality Change. University of Iowa, Department of Neurology; Iowa City: 1993. [Google Scholar]
- Barrash J, Anderson SW, Jones RD, Tranel D. The Iowa Rating Scales of Personality Change: Reliability and Validity; Paper presented at the 25th annual meeting of the International Neuropsychological Society; Orlando, FL. Feb, 1997b. [Google Scholar]
- Barrash J, Anderson SW, Hathaway-Nepple J, Jones RD, Tranel D. The Iowa Scales of Personality Change. University of Iowa College of Medicine, Dept. of Neurology; Iowa City, Iowa: 1997a. [Google Scholar]
- Barrash J, Tranel D, Anderson SW. Acquired personality disturbances associated with bilateral damage to the ventromedial prefrontal region. Developmental Neuropsychology. 2000;18:355–381. doi: 10.1207/S1532694205Barrash. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cerebral Cortex. 2000;10:295–225. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–12. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H, Damasio AR. Failure to respond autonomically to anticipated future outcomes following damage to prefrontal cortex. Cerebral Cortex. 1996;6:215–225. doi: 10.1093/cercor/6.2.215. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Cipolotti L. Impaired social response reversal and “acquired sociopathy”. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blumer D, Benson DF. Personality changes with frontal and temporal lobe lesions. In: Benson DF, Blumer D, editors. Psychiatric aspects of neurological disease. Grune & Stratton; New York: 1975. pp. 151–169. [Google Scholar]
- Boes AD, Bechara A, Tranel D, Anderson SW, Richman L, Nopoulos P. Right ventromedial prefrontal cortex: A neuroanatomical correlate of impulse control in boys. Scan. 2009;4:1–9. doi: 10.1093/scan/nsn035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borod JC, Koff E, Lorch MP, Nicholas M. Channels of emotional expression in patients with unilateral brain damage. Archives of Neurology. 1985;42:345–348. doi: 10.1001/archneur.1985.04060040055011. [DOI] [PubMed] [Google Scholar]
- Boyle PA, Malloy P. Treating apathy in Alzheimer's disease. Dementia and Geriatric Cognitive Disorders. 2004;17:1–9. doi: 10.1159/000074280. [DOI] [PubMed] [Google Scholar]
- Bozeat S, Gregory CA, Ralph MAL, Hodges JR. Which neuropsychiatric and behavioral features distinguish frontal and temporal variants of frontotemporal dementia from Alzheimer's disease? Journal of Neurology, Neurosurgery, and Psychiatry. 2000;69:178–186. doi: 10.1136/jnnp.69.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LA, Alarcon GS. Is Chiari malformation associated with increased levels of substance P and clinical symptoms in persons with fibromyalgia? Arthritis & Rheumatism. 1999;60:471–473. doi: 10.1002/1529-0131(199912)42:12<2731::AID-ANR36>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Bruno RL, Crenage SJ, Fick NM. Parallels between post-polio fatigue and chronic fatigue syndrome: A common pathophysiology? American Journal of Medicine. 1998;105:66S–73S. doi: 10.1016/s0002-9343(98)00161-2. [DOI] [PubMed] [Google Scholar]
- Cahn-Weiner DA, Grace J, Ott BR, Fernandez HH, Friedman JH. Cognitive and behavioral features discriminate between Alzheimer's and Parkinson's disease. Neuropsychiatry, Neuropsychology, & Behavioral Neurology. 2002;15:79–87. [PubMed] [Google Scholar]
- Cantagallo A, Contini E, Bianchi A. Personality changes after traumatic brain injury assessed with the Iowa Scales of Personality Change; Presented at the 17th Congress of the European Society of Physical and Rehabilitation Medicine; Venice, Italy. May, 2010. [Google Scholar]
- Carlsson GE, Möller A, Blomstrand C. Consequences of mild stroke in persons <75 years – a 1 year follow-up. Cerebrovascular Diseases. 2003;16:383–388. doi: 10.1159/000072561. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue and basal ganglia. Journal of Neurological Science. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue in neurological disorders. Lancet. 2004;363:978–988. doi: 10.1016/S0140-6736(04)15794-2. [DOI] [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Cunningham K. Obsessive-compulsive disorder in Huntington's disease. Biological Psychiatry. 1992;31:263–270. doi: 10.1016/0006-3223(92)90049-6. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–2314. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes' error: Emotion, reason, and the human brain. Grosset & Dunlap; New York: 1994. [Google Scholar]
- Damasio AR. Commentary: A neural basis for sociopathy. Archives of General Psychiatry. 2000;57:128–129. [Google Scholar]
- Damasio AR, Anderson SW. The frontal lobes. In: Heilman K, Valenstein E, editors. Clinical neuropsychology. 3rd ed. Chap. 15. Oxford University Press; New York: 2003. pp. 404–446. [Google Scholar]
- Damasio H, Frank R. Three-dimensional in vivo mapping of brain lesions in humans. Archives of Neurology. 1992;49:137–143. doi: 10.1001/archneur.1992.00530260037016. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- DeLuca J, Diamond BJ. Aneurysm of the anterior communicating artery: A review of neuroanatomical and neuropsychological sequelae. Journal of Clinical and Experimental Neuropsychology. 1995;17:100–121. doi: 10.1080/13803399508406586. [DOI] [PubMed] [Google Scholar]
- Devinsky O. Interictal behavioral changes in epliepsy. In: Devinsky O, Theodore W, editors. Epilepsy and behavior. Chap. 1. Wiley-Liss, Inc; New York: 1991. pp. 1–21. [Google Scholar]
- Driscoll D. The effects of prefrontal cortex damage on the regulation of emotion. University of Iowa; Iowa City, Iowa: 2009. Theses and Dissertations. [Google Scholar]
- Dywan J, Segalowitz SJ. Self- and family ratings of adaptive behavior after traumatic brain injury: Psychometric scores and frontally generated ERPs. Journal of Head Trauma Rehabilitation. 1996;11:79–95. [Google Scholar]
- Elsass L, Kinsella G. Development of a scale for measuring behavior change following closed head injury. In: Anderson V, Bailey M, editors. Proceedings of the Fourteenth Annual Brain Impairment Conference. Australian Society for the Study of Brain Impairment; Melbourne, Australia: 1989. pp. 124–131. [Google Scholar]
- Eslinger P. Neurological and neuropsychological bases of empathy. European Neurology. 1998;39:193–199. doi: 10.1159/000007933. [DOI] [PubMed] [Google Scholar]
- Eslinger P, Damasio AR. Behavioral disturbances associated with rupture of anterior communicating artery aneurysms. Seminars in Neurology. 1984;4:385–389. [Google Scholar]
- Eslinger P, Damasio AR. Severe disturbances of higher cognition after bilateral frontal lobe ablation. Neurology. 1985;35:1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Eslinger P, Grattan LM, Geder L. Neurologic and neuropsychologic aspects of frontal lobe impairments in postconcussive syndrome. In: Rizzo M, Tranel D, editors. Head injury and postconcussive syndrome. Churchill; New York: 1996. pp. 415–440. [Google Scholar]
- Folstein SE. Huntington's disease: A disorder of families. Johns Hopkins University Press; Baltimore, MD: 1989. [Google Scholar]
- Frank R, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal, visualization and analysis system for neuroanatomical imaging. NeuroImage. 1997;5:13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Evidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behavior. Aggressive Behavior. 2006;21:431–450. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function. Child Neuropsychology. 2000;6:235–238. doi: 10.1076/chin.6.3.235.3152. [DOI] [PubMed] [Google Scholar]
- Glader EL, Stegmayr B, Asplund K. Poststroke fatigue: A 2-year follow-up study of stroke patients in Sweden. Stroke. 2002;33:1327–1333. doi: 10.1161/01.str.0000014248.28711.d6. [DOI] [PubMed] [Google Scholar]
- Gleason AC. Personality and behavior changes in patients with primary brain tumors. Department of Psychology, University of Houston; Houston, TX: 2004. Doctoral Dissertation. [Google Scholar]
- Godefroy O, Rousseaux M. Novel decision making in patients with prefrontal or posterior brain damage. Neurology. 1997;49:695–701. doi: 10.1212/wnl.49.3.695. [DOI] [PubMed] [Google Scholar]
- Grace J, Malloy P. Frontal Systems Behavior Scale (FrSBe): Professional Manual. Psychological Assessment Resources; Lutz: FL: 2001. [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: A report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Gronchi-Perrin A, Aybek S, Jaques A, Pollo C, Burkhard P, Vingerhoets F. How the study design influences perceived personality trait changes in Parkinson's Disease after subthalamic deep brain stimulation. Parkinsonism and Related Disorders. 2007;13(Suppl. 2):S151. [Google Scholar]
- Hallam BJ, Mackenzie IRA, Feldman HH. Pilot data on Personality Changes in Prodromal Frontotemporal Dementia presented to the Canadian Institute for Health Research. Department of Neurology, University of British Columbia; Vancouver, BD: 2006. [Google Scholar]
- Harlow JM. Recovery from the passage of an iron bar through the head. Publications of the Massachusetts Medical Society. 1868;2:327–347. [Google Scholar]
- Hauser MD. Perseveration, inhibition and the prefrontal cortex: A new look. Current Opinion in Neurobiology. 1999;9:214–222. doi: 10.1016/s0959-4388(99)80030-0. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Wyler AR, Richey ET. Epilepsy, frontal lobe and personality. Biological Psychiatry. 1987;22:1055–1057. doi: 10.1016/0006-3223(87)90047-3. [DOI] [PubMed] [Google Scholar]
- Hommel M, Trabucco-Miguel S, Joray S, Naegele B, Gonnet N, Jaillard A. Social dysfunction after mild to moderate first-ever stroke at vocational age. Journal of Neurology, Neurosurgery and Psychiatry. 2009;80:371–375. doi: 10.1136/jnnp.2008.157875. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Mallet L, Mesnage V, Tezenas du Moncet S, Behar C, Gargiulo M, Torny F, Pelissolo A, Welter M-L, Agid Y. Subthalamic stimulation in Parkinson's Disease. Archives of Neurology. 2006;63:1090–1095. doi: 10.1001/archneur.63.8.1090. [DOI] [PubMed] [Google Scholar]
- Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, Tezenas du Moncet S, Bonnet AM, Pidoux B, Dormont D, Cornu P, Agid Y. Behavioural disorders, Parkinson's Disease and subthalamic stimulation. Journal of Neurology, Neurosurgery and Psychiatry. 2002;72:701–707. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House A. Depression after stroke. British Medical Journal. 1987;294:76–78. doi: 10.1136/bmj.294.6564.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House A, Rowe D, Standen PJ. Affective prosody in the reading voice of stroke patients. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:910–912. doi: 10.1136/jnnp.50.7.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter R, Blackwood W, Bull J. Three cases of frontal meningiomas presenting psychiatrically. British Medical Journal. 1968;3:9–16. doi: 10.1136/bmj.3.5609.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Arndt SV, Forrester AW, Geisler F, Starkstein SE. Comparison between acute- and delayed-onset depression following traumatic brain injury. Journal of Neuropsychiatry. 1993a;5:43–49. doi: 10.1176/jnp.5.1.43. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Arndt SV, Starkstein SE, Forrester AW, Geisler F. Depression following traumatic brain injury: A 1 year longitudinal study. Journal of Affective Disorders. 1993b;27:233–243. doi: 10.1016/0165-0327(93)90047-n. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Moser D, Tateno A, Crespo-Facorro B, Arndt SV. Major depression following traumatic brain injury. Archives of General Psychiatry. 2004;61:42–50. doi: 10.1001/archpsyc.61.1.42. [DOI] [PubMed] [Google Scholar]
- Jorge RE, Robinson RG, Starkstein SE, Arndt SV. Depression and anxiety following traumatic brain injury. Journal of Neuropsychiatry. 1993c;5:369–374. doi: 10.1176/jnp.5.4.369. [DOI] [PubMed] [Google Scholar]
- Juillerat A-C, Peter-Favre C, Van der Linden M. Adaptation française de l'échelle d'Iowa des Changements de Personnalité. Hôpitaux Universitaires de Genève; Geneva, Switzerland: 1998. [Google Scholar]
- Kaasinen V, Nurmi E, Bergman J, Eskola O, Solin O, Sonninen P, Rinne JO. Personality traits and brain dopaminergic function in Parkinson's disease. Proceedings of the National Academy of Science (USA) 2001;98:13272–13277. doi: 10.1073/pnas.231313198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz A, Davidson W, Fox H. Frontal Behavioral Inventory: Diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Science. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- Kertesz A, Nadkarni N, Davidson W, Thomas AW. The Frontal Behavioral Inventory in the differential diagnosis of frontotemporal dementia. Journal of the International Neuropsychological Society. 2000;6:460–468. doi: 10.1017/s1355617700644041. [DOI] [PubMed] [Google Scholar]
- Kinsella G, Packer S, Olver J. Maternal reporting of behaviour following very severe blunt head injury. Journal of Neurology, Neurosurgery and Psychiatry. 1991;54:422–426. doi: 10.1136/jnnp.54.5.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal: Evidence from the Ultimatum Game. Journal of Neuroscience. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenigs M, Young L, Adolphs R, Tranel D, Cushman F, Hauser M, Damasio AR. Damage to prefrontal cortex increases utilitarian moral judgments. Nature. 2007;446:908–911. doi: 10.1038/nature05631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolb B, Milner B. Observations on spontaneous facial expression after focal cerebral excisions and after intracarotid injection of amytal sodium. Neuropsychologia. 1981;19:505–514. doi: 10.1016/0028-3932(81)90017-8. [DOI] [PubMed] [Google Scholar]
- Lal SKL, Craig A, Borod P, Kirkup L, Nguyen H. Development of an algorithm for an EEG-based driver fatigue countermeasure. Journal of Safety Research. 2003;34:321–328. doi: 10.1016/s0022-4375(03)00027-6. [DOI] [PubMed] [Google Scholar]
- Levin HS, High WM, Goethe KE, Sisson RA, Overall JE, Rhoades HM, Eisenberg HM, Kalisky Z, Gary HE. The neurobehavioral rating scale: Assessment of the behavioral sequelae of head injury by the clinician. Journal of Neurology, Neurosurgery, and Psychiatry. 1987;50:183–193. doi: 10.1136/jnnp.50.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD. Relationships between personality disorders, social disturbances, and physical disability following traumatic brain injury. Journal of Head Trauma Rehabilitation. 1987;2:57–69. [Google Scholar]
- Luria AR. Frontal lobe syndromes. In: Vinken PJ, Bruyn GW, editors. Handbook of clinical neurology. Vol. 2: Localization in clinical neurology. Elsevier; New York: 1969. pp. 725–757. [Google Scholar]
- Luria AR, Pribram KH, Homskaya ED. An experimental analysis of the behavioral disturbance produced by a left frontal arachnoidal endothelioma. Neuropsychologia. 1964;2:257–280. [Google Scholar]
- Malloy P, Bihrle A, Duffy J, Cimino C. The orbitomedial frontal syndrome. Archives of Clinical Neuropsychology. 1993;8:185–201. [PubMed] [Google Scholar]
- Malloy P, Grace J. A review of rating scales for measuring behavior change due to frontal systems damage. Cognitive and Behavioral Neurology. 2005;18:18–27. doi: 10.1097/01.wnn.0000152232.47901.88. [DOI] [PubMed] [Google Scholar]
- Malloy P, Tremont G, Grace J, Frakey L. The Frontal Systems Behavior Scale discriminates frontotemporal dementia from Alzheimer's disease. Alzheimer's and Dementia. 2007;3:203–203. doi: 10.1016/j.jalz.2007.04.374. [DOI] [PubMed] [Google Scholar]
- Menza M. The personality associated with Parkinson's disease. Current Psychiatry Reports. 2000;2:421–426. doi: 10.1007/s11920-000-0027-1. [DOI] [PubMed] [Google Scholar]
- Miller BL, Darby A, Benson DF, Cummings JL, Miller MH. Aggressive, socially disruptive and antisocial behavior associated with fronto-temporal dementia. British Journal of Psychiatry. 1997;170:150–154. doi: 10.1192/bjp.170.2.150. [DOI] [PubMed] [Google Scholar]
- Narushima K, Kosier JT, Robinson RG. A reappraisal of poststroke depression, intra- and inter-hemispheric lesion location using meta-analysis. Journal of Neuropsychiatry and Clinical Neuroscience. 2003;15:422–430. doi: 10.1176/jnp.15.4.422. [DOI] [PubMed] [Google Scholar]
- Nightingale E, Soo C, Tate R. A systematic review of early prognostic factors for return to work after traumatic brain injury. Brain Impairment. 2007;8:101–142. [Google Scholar]
- Nguyen C, Denburg N, Laloggia A, Tranel D, Bechara A, Barrash J. Personality characteristics associated with poor decision-making in normal elderly. Manuscript submitted for publication. [Google Scholar]
- O'Connor BP. SPSS and SAS programs for determining the number of components using parallel analysis and Velicer's MAP test. Behavior Research Methods, Instrumentation, and Computers. 2000;32:396–402. doi: 10.3758/bf03200807. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive planning in humans: Neuropsychological, neuroanatomical and neuropharmacological perspectives. Progress in Neurobiology. 1997;53:431–450. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Peter R, Zoltan H, Ivan P, Judit V, Balint S. Measurement of mental fatigability by task related spectral EEG: A pilot study. Ideggyogy Sz. 2009;62:36–40. Abstract retrieved from PubMed (PMID: 19248725) [PubMed] [Google Scholar]
- Ready RE, Ott BR, Grace J, Cahn-Weiner DA. Apathy and executive dysfunction in mild cognitive impairment and Alzheimer disease. American Journal of Geriatric Psychiatry. 2003;11:222–228. [PubMed] [Google Scholar]
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas A. Description and validation of the characteristic potentially remediable behavioral symptoms in Alzheimer's disease. Journal of Clinical Psychiatry. 1987;48:9–15. [PubMed] [Google Scholar]
- Rochat L, Ammann J, Mayer E, Annoni J-M, Van der Linden M. Executive disorders and perceived socio-emotional changes after traumatic brain injury. Journal of Neuropsychology. 2009;3:213–227. doi: 10.1348/174866408X397656. [DOI] [PubMed] [Google Scholar]
- Satish U, Streufert S, Eslinger P. Complex decision-making after orbitofrontal damage: Neuropsychological and strategic management simulation assessment. Neurocase. 1999;5:355–364. [Google Scholar]
- Schwarz N. Self-reports: How the questions shape the answers. American Psychologist. 1999;54:93–105. [Google Scholar]
- Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatric Clinics of North America. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Sellal F, Adriantseheno M, Vercueil L, Hirsch E, Kahane P, Pellat J. Dramatic changes in artistic preference after left temporal lobectomy. Epilepsy and behavior. 2003;4:449–451. doi: 10.1016/s1525-5050(03)00146-x. [DOI] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Aharon-Peretz J, Perry D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain. 2009;132:617–627. doi: 10.1093/brain/awn279. [DOI] [PubMed] [Google Scholar]
- Siegert RJ, Walkey FH, Turner-Stokes L. An examination of the factor structure of the Beck Depression Inventory-II in a neurorehabilitation inpatient sample. Journal of the International Neuropsychological Society. 2009;15:142–147. doi: 10.1017/S1355617708090048. [DOI] [PubMed] [Google Scholar]
- Simioni S, Ruffieux C, Kleeberg J, Bruggimann L, Annoni J-M, Schluep M. Preserved decision-making ability in early multiple sclerosis. Journal of Neurology. 2008;255:1762–1769. doi: 10.1007/s00415-008-0025-5. [DOI] [PubMed] [Google Scholar]
- Simioni S, Ruffieux C, Kleeberg J, Bruggimann L, Du Pasquier RA, Annoni J-M, Schluep M. Progressive decline of decision-making performances during multiple sclerosis. Journal of the International Neuropsychological Society. 2009;15:291–295. doi: 10.1017/S1355617709090262. [DOI] [PubMed] [Google Scholar]
- Souza Lima F, Simioni S, Bruggimann L, Ruffieux C, Dudler J, Felley C, Michetti P, Annoni J-M, Schluep M. Perceived behavioral changes in early multiple sclerosis. Behavioural Neurology. 2007;18:81–90. doi: 10.1155/2007/674075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Tranel D. Neurological and psychiatric aspects of emotion. In: Boller F, editor. Handbook of clinical neurology. Elsevier Press; Amsterdam: In press. [DOI] [PubMed] [Google Scholar]
- Staub F, Bogousslavsky J. Fatigue after stroke: A major but neglected issue. Cerebrovascular Diseases. 2001;12:75–81. doi: 10.1159/000047685. [DOI] [PubMed] [Google Scholar]
- Stout JC, Ready RE, Grace J, Malloy PF, Paulsen JS. Factor analysis of the Frontal Systems Behavior Scale (FrSBe) Assessment. 2003;10:79–85. doi: 10.1177/1073191102250339. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. Neuropsychological studies of the frontal lobes. Psychological Bulletin. 1984;95:3–28. [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. Raven Press; New York: 1986. [Google Scholar]
- Stuss DT, Gow CA, Hetherington CR. “No longer Gage”: Frontal lobe dysfunction and emotional changes. Journal of Consulting and Clinical Psychology. 1992;60:349–359. doi: 10.1037//0022-006x.60.3.349. [DOI] [PubMed] [Google Scholar]
- Tang WK, Chen Y, Lam WWM, Mok V, Wong A, Yngvari GS, Xiang YT, Wong KS. Emotion incontinence and executive function in ischemic stroke: A case-controlled study. Journal of the International Neuropsychological Society. 2009;15:62–68. doi: 10.1017/S1355617708090061. [DOI] [PubMed] [Google Scholar]
- Tranel D. “Acquired sociopathy”: The development of sociopathic behavior following focal brain damage. In: Fowles DC, Sutker P, Goodman SH, editors. Progress in experimental personality and psychopathology research. Vol. 17. Springer; New York: 1994. pp. 285–311. [PubMed] [Google Scholar]
- Tranel D. The Iowa-Benton school of neuropsychological assessment. In: Grant I, Adams KM, editors. Neuropsychological Assessment of Neuropsychiatric and Neuromedical Disorders. 3rd ed. Chapter 4. Oxford University Press; New York: 2009. pp. 66–83. [Google Scholar]
- Tranel D, Bechara A. Sex-related functional asymmetry of the amygdala: Preliminary evidence using a case-matched lesion approach. Neurocase. 2009;15:217–234. doi: 10.1080/13554790902775492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Damasio AR. Decision making and the somatic marker hypothesis and the possible functions of the prefrontal cortex. In: Gazzaniga MS, editor. The new cognitive neuroscience. 2nd ed. MIT Press; Cambridge, MA: 1999. pp. 1047–1061. [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:1227–1241. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Van den Bos W, Guroglu B. The role of the ventral medial prefrontal cortex in social decision-making. Journal of Neuroscience. 2009;29:7631–7632. doi: 10.1523/JNEUROSCI.1821-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Werf SP, Jongen PJ, Lyclama A, Nijeholt GJ, Barkhof F, Hommes OR, Bleijenberg G. Fatigue in multiple sclerosis: Interrelations between fatigue complaints, cerebral MRI abnormalities and neurological disability. Journal of Neurological Science. 1998;160:164–170. doi: 10.1016/s0022-510x(98)00251-2. [DOI] [PubMed] [Google Scholar]
- Velikonja D, Warriner E, Brum C. Profiles of emotional and behavioral sequelae following acquired brain injury: Cluster analysis of the Personality Assessment Inventory. Journal of Experimental and Clinical Neuropsychology. 2010;32:610–621. doi: 10.1080/13803390903401302. [DOI] [PubMed] [Google Scholar]
- Weddell RA, Trevarthen C, Miller JD. Reactions of patients with focal cerebral lesions to success or failure. Neuropsychologia. 1988;26:373–385. doi: 10.1016/0028-3932(88)90092-9. [DOI] [PubMed] [Google Scholar]
- Wilson BA, Alderman N, Burgess PW, Emslie H, Evans JJ. The behavioural assessment of the dysexecutive syndrome. Thames Valley Test Company; Bury St. Edmunds, England: 1996. [Google Scholar]
- Witjas T, Baunez C, Henry JM, Delfini M, Regis J, Cherif AA, Peragut JC, Azulay JP. Addiction in Parkinson's Disease: Impact of subthalamic nucleus deep brain stimulation. Movement Disorders. 2005;20:1052–1055. doi: 10.1002/mds.20501. [DOI] [PubMed] [Google Scholar]
- Young L, Bechara A, Tranel D, Damasio H, Hauser M, Damasio AR. Damage to ventromedial prefrontal cortex impairs judgment of harmful intent. Neuron. 2010;65:845–851. doi: 10.1016/j.neuron.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]