Abstract
Goal
To examine whether lipid lowering medications (LLMs) and especially statin drugs can delay cognitive decline and dementia onset in individuals with and without Mild Cognitive Impairment (MCI) at baseline.
Methods
Longitudinal, observational study of 3,069 cognitively healthy elderly, ages 75 years and older, who were enrolled in the Ginkgo Evaluation of Memory Study. Primary outcome measure was the time to adjudicated all-cause dementia and Alzheimer dementia (AD). Secondary outcome measure was the change in global cognitive function over time measured by 3MSE and ADAS-cog scores.
Findings
Among participants without MCI at baseline current use of statins was consistently associated with a reduced risk of all cause dementia (HR 0. 79, 95% confidence interval, 0.65–0.96, p=0.021) and AD (HR 0.57, 95% confidence interval, 0.39–0.85, p= 0.005). In participants who initiated statin therapy lipophilic statins tended to reduce dementia risk more than nonlipophilic agents. In contrast there was no significant association between LLM use (including statins), dementia onset or cognitive decline in individuals with baseline MCI. However, in individuals without MCI at baseline there was a trend for a neuroprotective effect of statins on cognitive decline.
Conclusions
Statins may slow the rate of cognitive decline and delay the onset of AD and all cause dementia in cognitively healthy elderly individuals whereas individuals with MCI may not have comparable cognitive protection from these agents. However, the results from this observational study need to be interpreted with caution and will require confirmation by randomized clinical trials stratifying treatment groups based on MCI status at baseline.
Keywords: Cognitive function, 3HMG-ACoA reductase inhibitors, Mild Cognitive Impairment, dementia
INTRODUCTION
If present trends continue, developed countries are likely to experience a global dementia epidemic. The most common etiologies for dementia are presently attributed to progressive neurodegenerative and vascular diseases, either alone or in combination. Because there is no curative therapy, strategies to prevent or delay dementia are emphasized.
Early treatment by modification of vascular risk factors may play an essential role as they are strongly associated with the development of AD, vascular and mixed dementia (1–4). The successful treatment of hyperlipidemia, a major risk factor for coronary artery disease and ischemic stroke, with HMG-CoA reductase inhibitors (statin drugs) as well as their pleiotropic therapeutic effects have spurred an interest in these drugs as a potential aid for dementia prevention. Many experimental studies indicate a link between cholesterol, amyloid metabolism, cellular membrane integrity and cerebral vascular function although the clinical relevance of these data remains unclear (5). Controversy continues whether increased cholesterol levels are associated with an increased risk of AD and other dementias (6–9).
Some observational studies have suggested a lower rate of dementia in individuals receiving statins whereas other studies yielded negative results (10–18). Prospective clinical trials did not show a protective effect of statins on cognition, but these studies were either not primarily designed to examine the effects of statin on cognitive function and enrolled predominantly participants with advanced vascular disease, including cerebrovascular disease (19,20) or studied patient with already established AD (21). The results of these randomized controlled studies seem to conflict with the finding that statins significantly reduce ischemic stroke risk (19,20,22–26). As randomized controlled trials of statins reduced the incidence of cerebral ischemic events by about 10–30% (19,20,27), it is surprising that the same intervention does not lead to a decreased incidence of vascular and mixed dementia.
Statins have multiple potential effects that may impact the development of dementia, for example lowering amyloid levels (28–30). However, the clinical relevance of lowering APP and Aβ remains unknown as cognitive function may not be dependent on Aβ levels (31). Observational studies have suggested a reduced risk of AD in those treated with statins during midlife (32) and analysis of data from the Cardiovascular Health Study (CHS) suggested a potential positive effect of statin drugs on cognitive function in the elderly (33). These findings may indicate that timing of statin therapy might be essential. Statins may exert a protective effect only if started early in cognitively healthy individuals.
The present study analyzes the effects of LLMs, predominantely statins, on incident dementia and cognitive function in participants with and without MCI at baseline who participated in GEMS, a clinical trial testing the ability of Ginkgo biloba to prevent or delay development of dementia (34).
METHODS
Study Population and Study Design
The GEMS study design has been described previously (34). Briefly, 3,069 cognitively healthy individuals and those with mild cognitive impairment (MCI), age 75 years and older, were enrolled at four academic medical centers (Universities of Pittsburgh, California-Davis, Johns Hopkins and Wake Forest)with a mean follow- up of six years. Similar to the primary analysis data from 3,069 of 3072 participants who were initially randomized into GEMS were included in this time adjusted analysis (34). Participants had to be able to sign informed consent and were required to have a proxy who provided an independent assessment of the participant’s functional and cognitive abilities. All participants underwent a detailed physical, neurological, and neuropsychiatric examination. At baseline and at regularly scheduled 6-month visits, medical history and current medication use were reviewed, with participants bringing to the clinic medicine bottles for currently used medications in order to record the exact name and dose of each. Fasting lipid profiles were not measured.
Cognitive Assessments
The baseline neuropsychological battery measured language, mood, executive and visuo-spatial function, memory, psychomotor speed and global cognitive function, using previously validated cut off scores in subject of similar age (34). At each follow-up visit participants were re-evaluated with an abbreviated cognitive test battery including the Modified Mini-Mental State Exam (3MSE), the Clinical Dementia Rating Scale and the cognitive subscale of the AD Assessment Scale (ADAS-Cog) (340. If scores on 2 out of the three assessments were below preset cutpoints, or when dementia was suspected by the proxy, family or a treating physician, the complete baseline neuropsychological test battery was repeated, followed by an additional neurological and medical examination and brain MRI. Final diagnosis classification was made by an expert consensus panel (34).
If a participant was unable to come to the clinic, the Telephone Instrument of Cognitive Status (TICS) was administered, and these scores were used to estimate the 3MSE score (35). Individuals who reached dementia endpoint were excluded from further assessments.
Exposures and Adjustment Variables
The focus of our analysis was the use of LLM as assessed every 6 months. Exposure was defined and updated at each visit as never use of LLM’s, ever use of statins, or ever use of an alternative (non-statin) LLM. Additional analyses assessed associations with former/current use of statins, and ever use of lipophilic vs. non-lipophilic statins. Adjustment variables included age, sex, race, education, clinic, treatment group, MCI at baseline, APOe4 genotype, and history of coronary heart disease (CHD) defined as definite/probable myocardial infarction, definite/probable angina pectoris, probable/definite resuscitated cardiac arrest, status post coronary artery bypass graft or coronary angioplasty/stent placement and stroke (both groups together are summarized as CVD). The latter were determined by self-report at baseline and by review of medical records if any vascular events occurred during follow-up, as previously described (36). In longitudinal analyses, CHD, stroke and LLM use were updated at each visit.
Statistical Analysis
Participant characteristics by LLM use at baseline were compared using Chi-Square tests for categorical measures and Analysis of Variance (ANOVA) for continuous measures. Cox regression models were used to compute hazard ratios as estimates of relative risk of all cause dementia, AD and mixed vascular dementia associated with ever use of statins or other LLM’s compared to no use, updating LLM use over time. There were too few participants on other LLMs to allow that group to serve as the comparison group for the statin users. We also developed models excluding participants who were taking LLMs at baseline in order to consider an inception cohort, since duration of use at baseline was unknown. In the absence of lipid levels, CVD status at baseline represents a possible indication for use of LLM’s. In order to address indication bias, analyses were repeated restricting to participants without CVD at baseline. Significant results were further explored by examining former vs. current use of statins, and lipophilic class of statins. The assumption of proportional hazards was not met for the variable MCI at baseline, leading us to stratify analyses by MCI at baseline.
Mixed effects regression models were used to estimate the association of LLM use with change in cognitive function scores over time for participants without MCI at baseline. Change was modeled with a linear and quadratic term for year, but only interactions with the linear term were assessed. Year was centered at year 3 to reduce the collinearity between year and year squared, with the result that the coefficient for LLM use by year assesses the difference in the instantaneous rate of change at year 3 attributable to LLM use compared to no use.
ApoE status was available for 80% of participants. We performed sensitivity analyses classifying all those missing ApoE genotype in turn as absent or present, then using a separate code for missing; results were consistent across all 3 approaches. The results presented used an indicator for missing ApoE. Statistical analysis was performed using STATA statistical software version 10 (StataCorp LP, Texas, USA).
RESULTS
The cohort was predominantly white and highly educated (average of 14.4 years of education). Participant characteristics by type of LLM used at baseline are summarized in Table 1. About 16% of the total cohort was classified as having MCI at baseline; the prevalence of MCI did not differ by LLM use (37). LLM use was more common in men and in participants with a history of stroke or CHD.
Table 1.
Participant characteristics by Lipid Lowering Medication (LLM) use at baseline Entries in table are N(%) unless otherwise noted
| Characteristic | No LLM N=2218 |
Statins N=778 |
Other LLM N=73 |
Total N=3069 |
p- value1 |
|---|---|---|---|---|---|
| Male | 1,147 (51.7) |
457 (58.7) |
46 (63.0) |
1,650 (53.8) |
.001 |
| Black race | 71 (3.2) |
19 (2.4) |
0 (0) |
90 (2.9) |
.18 |
| MCI | 346 (15.6) |
123 (15.8) |
13 (17.8) |
482 (15.7) |
.87 |
| Cerebrovascular disease |
147 (6.6) |
105 (13.5) |
5 (6.8) |
257 (8.4) |
<.001 |
| Coronary heart disease |
219 (9.9) |
370 (47.6) |
25 (34.2) |
614 (20.0) |
<.001 |
| Age, yrs Mean (SD) |
78.7 (3.4) |
78.4 (3.0) |
77.8 (2.9) |
78.6 (3.3) |
.007 |
| Education, yrs Mean (SD) |
14.3 (3.2) |
14.5 (3.1) |
14.8 (3.2) |
14.4 (3.2) |
.26 |
| Apoe4 yes missing |
393 (17.7) 471 (21.2) |
175 (22.5) 132 (17.0) |
10 (13.7) 14 (19.2) |
578 (18.8) 617 (20.1) |
.008 |
| 3MSE | 93.4 (4.7) |
93.2 (4.6) |
93.7 (4.7) |
93.4 (4.7) |
.34 |
| ADAS-cog | 6.5 (2.7) |
6.5 (2.7) |
6.3 (2.4) |
6.5 (2.7) |
.86 |
Comparison across all 3 groups.
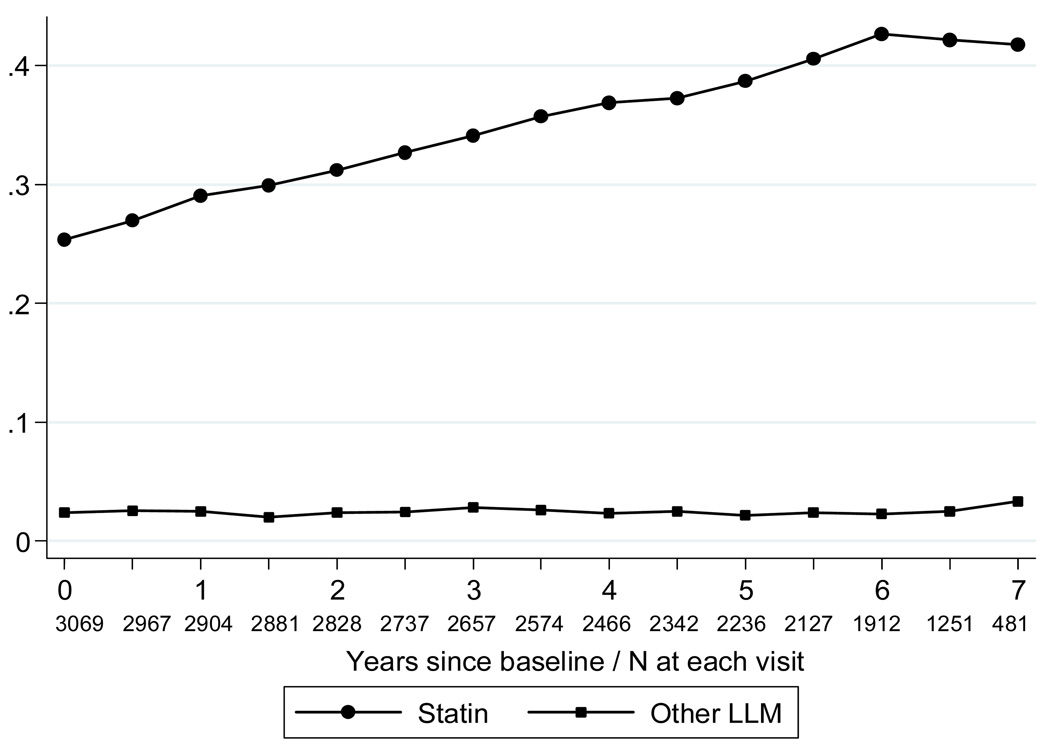
At baseline, 25.3% of participants were taking statins, and 2.4% were taking other LLMs including bile-acid sequestrants, fibrates, niacin, or nicotinic acid. The percentage of statin users increased to more than 40% over time, but the percent taking other LLM’s did not change (Figure 1).
Figure 1.
Proportion of participants taking statins and other lipid lowering medications by year. Circles=Statins; squares=Other LLM
Cross-sectional analysis of LLM use with cognitive function scores at baseline
Cross sectional analysis showed no association of LLM use with either 3MSE (p=0.71) or ADAS cog scores (p=0.81). This was consistent with a lack of association with MCI at baseline, indicating that at entry participants with MCI were not less likely to receive a LLM than those without MCI.
Survival analysis of time to dementia
A total of 523 GEMS participants reached the dementia endpoint during the trial, including 353 classified as having AD without vascular disease, and 24 with pure vascular dementia (34). For analysis, those with vascular dementia were combined with the mixed dementia cases to form a group with a vascular component. Among participants without MCI at baseline, there was some evidence for a reduced risk of all cause dementia among ever users of statins, and although hazard ratios were often similar for users of other LLM, our data were insufficient to confirm a statistically significant effect in this group (Table 2). The strongest results were a reduction in risk of AD and all cause dementia among those who initiated statin use during the study (HR 0.46, 95% confidence interval, 0.29–0.74, p< 0.001), and (HR 0.53, 95% confidence interval, 0.37–0.75, p<.001), respectively. Hazard ratios for ever use of statins and dementia with a vascular component were consistently less than 1.0, but did not reach statistical significance. There were no significant associations of LLM use and dementia in the group with MCI at baseline.
Table 2.
Results by Dementia type
| All cause dementia N=523 |
AD only N=353 |
Vascular component N=148 |
|||||
|---|---|---|---|---|---|---|---|
| Risk Group | Exposure | HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
HR (95% CI) |
p- value |
|
No MCI at baseline |
|||||||
|
N=2587 |
Statin ever Other LLM ever |
0.77 (0.60, 0.98) 0.65 (0.37, 1.14) N=324 |
.034 .135 |
0.83 (0.61, 1.12) 0.48 (0.21, 1.08) N=212 |
.21 .076 |
0.72 (0.46, 1.13) 0.98 (0.42, 2.28) N=97 |
.15 .96 |
| No CVD at baseline N=1941 |
Statin ever Other LLM ever |
0.75 (0.56, 1.01) 0.89 (0.46, 1.75) N=223 |
.060 .74 |
0.78 (0.55, 1.12) 0.55 (0.20, 1.49) N=155 |
.18 .24 |
0.79 (0.44, 1.43) 2.26 (0.88, 5.80) N=58 |
.44 .09 |
| Initiators only N=1872 |
Statin ever Other LLM ever |
0.53 (0.37, 0.75) 0.43 (0.13, 1.33) N=220 |
<.001 .14 |
0.46 (0.29, 0.74) 0.21 (.03, 1.49) N=142 |
<.001 .12 |
0.67 (0.36, 1.22) 0.97 (0.23, 4.03) N=68 |
.19 .96 |
|
MCI at baseline |
|||||||
|
N=482 |
Statin ever Other LLM ever |
0.88 (0.64, 1.21) 0.78 (0.36, 1.68) N=199 |
.43 .52 |
0.70 (0.47, 1.04) 0.83 (0.33, 2.07) N=141 |
.075 .69 |
1.34 (0.72, 2.50) 0.73 (0.17, 3.17) N=51 |
.36 .68 |
| Initiators only N=346 |
Statin ever Other LLM ever |
0.73 (0.45, 1.20) 0.47 (0.11, 1.97) N=143 |
.22 .30 |
0.64 (0.35, 1.15) 0.63 (0.15, 2.70) N=109 |
.13 .54 |
1.15 (0.45, 2.92) n/a N=32 |
.77 |
All models were adjusted for age, sex, race, field center, years of education, ginkgo biloba randomization group, Apoe(4), and time-varying stroke and CHD.
When use of statins was separated into former and current use, there was a consistent association of current use with reduced risk of all cause dementia and AD among participants without MCI at baseline (Table 3). Duration of use could only be determined in participants who initiated statins during the study, and there was a linear trend of reduced risk with longer duration of use. This result needs to be interpreted with caution because duration of use is correlated with length of dementia-free follow-up time. Lipophilic statins tended to reduce risk of dementia more than nonlipophilic statins, especially among initiators of statin therapy.
Table 3.
Further classification of statin use for significant results above.
| All cause dementia | AD only | ||||
|---|---|---|---|---|---|
| Risk Group | Exposure | HR (95% CI) | p-value | HR (95% CI) | p-value |
| No MCI at baseline |
Statin use Former Current |
0.98 (0.66, 1.46) 0.71 (0.55, 0.93) |
.92 .012 |
1.39 (0.89, 2.15) 0.69 (0.49, 0.97) |
.14 .03 |
| Type of statin (ever use) Lipophilic Nonlipophilic |
0.76 (0.60, 0.98) 0.89 (0.61, 1.29) |
.033 .54 |
0.79 (0.58, 1.08) 1.01 (0.65, 1.58) |
.14 .96 |
|
| No CVD at baseline |
Statin use Former Current |
1.01 (0.62, 1.64) 0.69 (0.49, 0.96) |
.97 .026 |
1.35 (0.80, 2.27) 0.64 (0.42, 0.96) |
.26 .032 |
| Type of statin (ever use) Lipophilic Nonlipophilic |
0.82 (0.60, 1.11) 0.70 (0.41, 1.20) |
.19 .19 |
0.85 (0.59, 1.22) 0.70 (0.36, 1.34) |
.37 .28 |
|
| Initiators only | Statin use Former Current |
0.47 (0.23, 0.95) 0.53 (0.36, 0.79) |
.035 .002 |
0.48 (0.20, 1.13) 0.45 (0.27, 0.76) |
.091 .003 |
| Duration < 1 year 1 – 3 years > 3 years |
0.69 (0.41, 1.14) 0.56 (0.34, 0.91) 0.26 (0.11, 0.60) |
<.001* |
0.31 (0.12, 0.76) 0.71 (0.41, 1.22) 0.22 (0.07, 0.70) |
.003* | |
| Type of statin (ever use) Lipophilic Nonlipophilic |
0.53 (0.36, 0.77) 0.89 (0.46, 1.69) |
.001 .71 |
0.47 (0.29, 0.77) 0.78 (0.34, 1.80) |
.003 .56 |
|
All models were adjusted for age, sex, race, field center, years of education, ginkgo biloba randomization group, Apoe(4), and time-varying stroke, CHD and ever use of other lipid lowering medications.
Test for linear trend.
Longitudinal analysis of cognitive decline: 3MSE and ADAS-cog
Trajectories of cognitive function over time showed a significant quadratic trend, with scores initially improving after baseline, probably due to a practice effect, and then gradually declining. The amount of cognitive decline as assessed by these measures was minimal in this cohort. Among participants without MCI at baseline, the mean (95% CI) linear rate of change at the third year of follow-up was −.09 (−.13, −.06) points per year for the 3MSE and .03 (.01, .05) for the ADAS-cog. The subscore of the 3MSE associated with memory showed no linear rate of change at year 3, with an estimated slope of 0.00 (−.02, .02). Participants with MCI at baseline had a greater change of −.45 (−.58 −.32) points per year for the 3MSE, and .18 (.12, .24) for the ADAS-cog. The subscore of the 3MSE associated with memory showed a modest linear rate of change at year 3, with an estimated slope of −.08 (−.14, −.02) points per year.
Similar to the results for dementia, there was no association of statin use with rate of cognitive decline among participants with MCI at baseline. Table 4 summarizes the results for participants free of MCI at baseline. For the 3MSE, statin users tended to have a slower rate of decline compared to non-users of the medication, with current users having half the rate of change of non-users at the midpoint of follow-up. The scale of the ADAS-cog is reversed, such that a higher score indicates worse cognitive function, so that a larger slope coefficient indicates a faster rate of cognitive decline. As with the 3MSE, there was a trend toward slower decline among statin users. These results are not striking by themselves, but they are consistent with the results of our time to dementia analysis showing a trend towards neuroprotection with statin use. Results were similar when restricted to participants without CVD at baseline.
Table 4.
Results of longitudinal models of cognitive function among participants free of MCI at baseline.
| 3MSE | ADAS-cog | |||
|---|---|---|---|---|
| Exposure | Slope1 (95% CI) | p- value2 |
Slope 1 (95% CI) | p- value2 |
| No LLM Statin ever Other LLM ever |
−0.11 (−0.15, − 0.07) −0.06 (−.10, −.01) −0.16 (−0.29, − 0.04) |
.066 .43 |
0.05 (0.02, 0.07) 0.015 (−0.01, 0.04) −0.03 (−0.11, − 0.04) |
.058 .041 |
| Statin use None Former Current |
−0.14 (−0.18, − 0.10) − 0.06 (−0.18, 0.07) −0.07 (−0.12, − 0.02) |
.19 .014 |
0.04 (0.02, 0.07) 0.03(−0.05, 0.11) 0.01 (−0.02, 0.04) |
.73 .045 |
| Type of statin (ever use) None Lipophilic Nonlipophilic |
−0.10 (−0.14, − 0.06) −0.06 (−0.11, − 0.01) −0.16 (−0.26, − 0.06) |
.13 .26 |
0.04 (0.02, 0.06) 0.01 (−0.02, 0.03) 0.05 (−0.00, 0.11) |
.044 .66 |
Estimate of rate of change at midpoint of follow-up time.
Test for difference in slope compared to non-users.
DISCUSSION
The present analysis focuses mainly on the effects of statins on cognitive function as the number of participants on alternative lipid lowering agents was relatively low. Among participants without MCI at baseline, current use of statins was associated with a reduced risk of all cause dementia (HR 0. 79, 95% confidence interval, 0.65–0.96, p=0.021) and AD (HR 0.57, 95% confidence interval, 0.39–0.85, p= 0.005). Results were strongest when restricted to initiators of statins during the study, and in this group, there was a significant association of lipophilic statins with reduced risk of dementia which was not seen for non-lipophilic statins. In contrast there was no significant association of LLM use (including statins) and dementia onset in individuals with baseline MCI. Consistent with these results there was no association of statin use with rate of cognitive decline among participants with MCI at baseline as measured by 3MSE and ADAS-cog. However, in individuals without MCI at baseline there was a trend for a neuroprotective effect of statins on cognitive decline.
Previous observational studies have shown mixed results. While some studies have been negative, others have shown a lower rate of dementia in individuals receiving statins (10–18). Similar to our results, a Canadian study found that the use of LLMs was associated with a lower risk of dementia, specifically AD (11) and in the recently published Rotterdam study, statin use was associated with lower risk of AD in the general population (16).
However, data from two prospective randomized trials comparing statin users with non-users showed no significant effects of statins on cognition (19,20). Both studies used cognitive function only as a tertiary outcome measure, and in contrast to GEMS, enrolled individuals with advanced vascular disease who were at increased risk for stroke and other vascular events potentially compromising cerebral blood supply. In the Heart Protection Study 33% of all participants had cerebrovascular disease and 51% had vascular disease (38) and PROSPER enrolled 11% of individuals with stroke and 50% with vascular disease (39). The Heart Protection Study utilized one phone interview during final follow-up to assess risk of cognitive decline but did not include routine detailed neuropsychological evaluations. The PROSPER study used a comprehensive neuropsychological testing battery consisting of Mini-Mental State Examination, the Picture-Word Learning Test, the Stroop Color Word Test and the Letter Digit Coding Test at baseline and during follow-ups. Detailed neuropsychological testing with screening for MCI at baseline or brain MRIs were not performed in these high risk populations so that a relatively high proportion of individuals with cognitive impairment due to significant vascular disease at baseline may have been enrolled in both studies. Conflicting results from our analysis and those studies may thus be due to that fact that study populations are not comparable and may be consistent with our finding that statins may not exert a protective cognitive effect when treatment is initiated after MCI and cerebrovascular disease have developed.
Similar to results from those randomized trials there was no benefit of statins on cognitive function in individuals with mild to moderate AD in the LEADe trial (21). Interestingly, the investigators raised the question whether different timing of statin therapy could have yielded different results. Findings from the Rotterdam study suggest that statins are associated with a reduced AD risk in the general population, a sample that consists of a greater group of cognitively healthy individuals at baseline compared to the discussed studies, which again could indicate that timing of statin therapy is crucial.
Our analysis has several limitations. The data were derived from the observational component of a clinical trial in which LLM use was not the primary exposure of interest and cholesterol levels were not measured, so we were unable to restrict our analysis to those with an indication for LLMs. To address this limitation we repeated our complete statistical analysis in participants without CVD, which produced similar estimates of reduced risk. We recognize, however, that there remains substantial potential for confounding by indication.
Whereas our results do not point to any specific advantage for statin over alternative LLM use, the group comparison is limited by statistical power. Given that participants on LLMs might differ from those not on LLMs in ways for which we are unable to adjust, the ideal analysis to identify a particular benefit of statin use would have been a comparison of statin users with users of other LLMs. Unfortunately, the number of alternative LLM users was too small to allow for this comparison.
Our results were stronger for current statin users, which could suggest treatment bias. As participants loose cognitive function, treating physicians may reduce the number of medications, with the result that current statin use appears protective. Although cross sectional analysis showed no association of LLM use with cognitive scores or MCI at baseline, we cannot rule out that physicians are less likely to prescribe new medications to patients experiencing cognitive difficulty during study follow up. Our results for duration of treatment show an increasing protective effect with longer treatment, but again, we cannot rule out treatment bias. Additionally there is theoretical concern of confounding by selection bias. Participants with low socioeconomic status may be less likely to receive a statin drug. However, we found no association between race, education or income status and LLM use, and adding those covariates to the model did not change the results.
We have examined the effects of different statin types in a secondary analysis dividing lipophilic and hydrophilic statins (see Table 3 and 4). Initiators of lipophilic statins seemed to experience the greatest risk reduction of dementia and AD onset. Lipophilic statins may be more beneficial as they cross the blood-brain barrier much more easily than their hydrophilic counterparts. The mechanism by which LLMs may exert their effect on cognition remains unclear but could be related to direct effects within the nervous system. It has been hypothesized that statins not only lower lipid levels and reduce atherosclerosis related vascular disease but that they also have multiple pleiotropic effects (28–30,40). However, due to study limitations these results need to be interpreted with caution.
The observed rate of cognitive decline was relatively low, in part due to the relative insensitivity of the 3MSE and ADAS among highly educated people who exhibit more cognitive reserve, and in part because subjects who showed decline were excluded when they reached the dementia endpoint. Follow-up data from these individuals are thus not available to comment on the effect of LLMs on cognitive function in demented participants over time which may differ from the effects observed in cognitively healthy individuals.
The strengths of this study include that it followed a population of highly functional and cognitively healthy individuals with regular cognitive assessments over a relatively long observation period. Medication intake was verified by inspection of pill bottles and medication lists at each study visit. The primary outcome measure of adjudicated dementia was based on a detailed testing battery, neurological examination and review by a neuropsychologist and was well defined, yielding robust data of dementia onset and dementia subtype. The effects of different statin types and alternative LLMs were analyzed and data analysis was stratified by cognitive status at baseline and included participants with and without MCI, thus allowing to monitor the effects on statins on early disease stages. The current analysis adds novel information to the existing literature as it raises the intriguing question whether statins may be protective for cognitive function in the elderly if treatment is initiated before cognitive impairment due to vascular or neurodegenerative disease has developed.
Overall the effects of LLMs on cognitive scores over time were modest and thus these findings may not translate into a clinically meaningful functional improvement. However, the effect size may become more significant when treatment is initiated in midlife or if the observational period is extended. It is unclear when to expect an effect of statins on cognition. If there is any benefit, it may depend on exposure time, but also on LLM type and dose.
In summary we found that statins reduce the risk of incident AD and all cause dementia in elderly individuals without MCI at baseline. However, confirmation of these results by randomized trials stratifying participants by MCI status would be required.
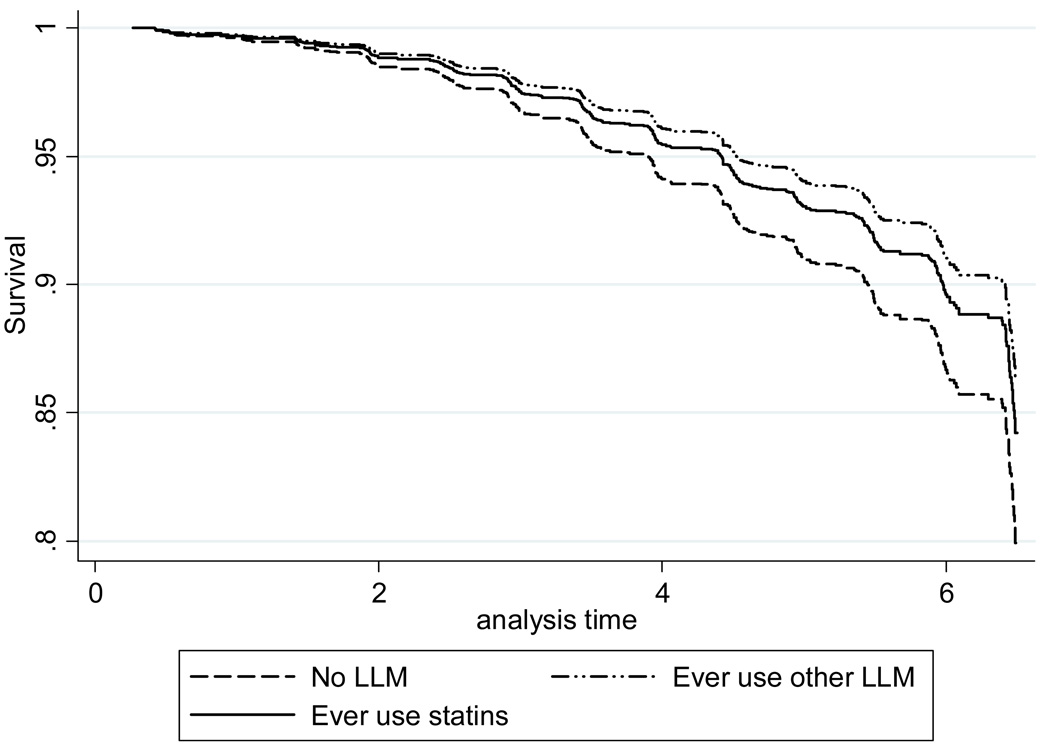
Figure 2.
Estimated survival curves for time to dementia in all participants Dashed line= No LLM; solid line=Statins; Dash-dot line= Other LLM.
Acknowledgment
We are indebted to Stephen Straus, MD, the late former director of NCCAM, who championed efforts to evaluate complementary and alternative therapies in a rigorous scientific fashion. We gratefully acknowledge the contribution of Dr. Willmar Schwabe GmbH & Co. KG, Karlsruhe, Germany, for their donation of the G. biloba tablets and identical placebos, in blister packs, for the study. We are also grateful to our volunteers, whose faithful participation in this longitudinal study made it possible. We also thank Susan Margitic for excellent administrative support.
Funding/Support: Supported by U01 AT000162 from the National Center for Complementary and Alternative Medicine (NCCAM) and the Office of Dietary Supplements, and support from the National Institute on Aging, National Heart, Lung, and Blood Institute, the University of Pittsburgh Alzheimer’s Disease Research Center (P50AG05133), the Roena Kulynych Center for Memory and Cognition Research, and National Institute of Neurological Disorders and Stroke. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCCAM, or the National Institutes of Health.
APPENDIX
GEM Study Personnel
Project Office
Richard L. Nahin, PhD, MPH, Barbara C. Sorkin, PhD, National Center for Complementary and Alternative Medicine
Clinical Centers
Michelle Carlson, PhD, Linda Fried, MD, MPH, Pat Crowley, MS, Claudia Kawas, MD, Paulo Chaves, MD, PhD, Sevil Yasar, MD, PhD, Patricia Smith, Joyce Chabot, John Hopkins University; John Robbins, MD, MHS, Katherine Gundling, MD, Sharene Theroux, CCRP, Lisa Pastore, CCRP, University of California-Davis; Lewis Kuller, MD, DrPH, Roberta Moyer, CMA, Cheryl Albig, CMA, University of Pittsburgh; Gregory Burke, MD, Steve Rapp, PhD, Dee Posey, Margie Lamb, RN, Wake Forest University School of Medicine
Schwabe Pharmaceuticals
Robert Hörr, MD, Joachim Herrmann, PhD.
Data Coordinating Center
Richard A. Kronmal, PhD, Annette L. Fitzpatrick, PhD, Fumei Lin, PhD, Cam Solomon, PhD, Alice Arnold, PhD, University of Washington
Cognitive Diagnostic Center
Steven DeKosky, MD, Judith Saxton, PhD, Oscar Lopez, MD, Beth Snitz PhD, M. Ilyas Kamboh PhD, Diane Ives, MPH, Leslie Dunn, MPH, University of Pittsburgh
Clinical Coordinating Center
Curt Furberg, MD, PhD, Jeff Williamson, MD, MHS; Nancy Woolard, Kathryn Bender, Pharm.D., Susan Margitić, MS, Wake Forest University School of Medicine
Central Laboratory
Russell Tracy, PhD, Elaine Cornell, UVM, University of Vermont
MRI Reading Center
William Rothfus MD, Charles Lee MD, Rose Jarosz, University of Pittsburgh
Data Safety Monitoring Board
Richard Grimm, MD, PhD (Chair), University of Minnesota; Jonathan Berman, MD, PhD (Executive Secretary), National Center for Complementary and Alternative Medicine; Hannah Bradford, M.Ac., L.Ac., MBA, Carlo Calabrese, ND MPH, Bastyr University Research Institute; Rick Chappell, PhD, University of Wisconsin Medical School; Kathryn Connor, MD, Duke University Medical Center; Gail Geller, ScD, Johns Hopkins Medical Institute; Boris Iglewicz, Ph.D, Temple University; Richard S. Panush, MD, Department of Medicine Saint Barnabas Medical Center; Richard Shader, PhD, Tufts University.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosure: Dr. DeKosky disclaims that he serves as consultant to various pharmaceutical companies and as editor for “Up to date” and that none of these interactions provides more than $10 000/year. None of the other authors has anything to disclaim.
REFERENCES
- 1.Nash DT, Fillit H. Cardiovascular disease risk factors and cognitive impairment. Am J Cardiol. 2006;97:1262–1265. doi: 10.1016/j.amjcard.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 2.Jellinger KA. The enigma of vascular cognitive disorder and vascular dementia. Acta Neuropathol (Berl) 2007 Apr;113(4):349–388. doi: 10.1007/s00401-006-0185-2. [DOI] [PubMed] [Google Scholar]
- 3.Ritchie K, Lovestone S. The dementias. Lancet. 2002;360:1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- 4.Kukull WA, Ganguli M. Epidemiology of dementia: concepts and overview. Neurol Clin. 2000;18:923–949. doi: 10.1016/s0733-8619(05)70233-4. [DOI] [PubMed] [Google Scholar]
- 5.Chauhan NB. Membrane dynamics, cholesterol homeostasis, and Alzheimer's disease. J Lipid Res. 2003;44:2019–2029. doi: 10.1194/jlr.R300010-JLR200. [DOI] [PubMed] [Google Scholar]
- 6.Helzner EP, Luchsinger JA, Scarmeas N, et al. Contribution of vascular risk factors to the progression in Alzheimer disease. Arch Neurol. 2009 Mar;66(3):343–348. doi: 10.1001/archneur.66.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reitz C, Tang MX, Luchsinger J, et al. Relation of plasma lipids to Alzheimer disease and vascular dementia. Arch Neurol. 2004;61(5):705–714. doi: 10.1001/archneur.61.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Evans RM, Hui S, Perkins A, et al. Cholesterol and APOE genotype interact to influence Alzheimer disease progression. Neurology. 2004;62(10):1869–1871. doi: 10.1212/01.wnl.0000125323.15458.3f. [DOI] [PubMed] [Google Scholar]
- 9.Moroney JT, Tang MX, Berglund L. Low-density lipoprotein cholesterol and the risk of dementia with stroke. JAMA. 1999;282(3):254–260. doi: 10.1001/jama.282.3.254. [DOI] [PubMed] [Google Scholar]
- 10.Wolozin B, Kellman W, Ruosseau P, et al. Decreased prevalence of Alzheimer disease associated with 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors. Arch Neurol. 2000;57:1439–1443. doi: 10.1001/archneur.57.10.1439. [DOI] [PubMed] [Google Scholar]
- 11.Rockwood K, Kirkland S, Hogan DB, et al. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe K, Barrett-Connor E, Lin F, et al. Serum lipoprotein levels, statin use, and cognitive function in older women. Arch Neurol. 2002;59:378–384. doi: 10.1001/archneur.59.3.378. [DOI] [PubMed] [Google Scholar]
- 13.Li G, Higdon R, Kukull WA, et al. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 14.Zandi PP, Sparks DL, Khachaturian AS, et al. Do statins reduce risk of incident dementia and Alzheimer disease? The Cache County Study. Arch Gen Psychiatry. 2005;62:217–224. doi: 10.1001/archpsyc.62.2.217. [DOI] [PubMed] [Google Scholar]
- 15.Rea TD, Breitner JC, Psaty BM, et al. Statin use and the risk of incident dementia: The Cardiovascular Health Study. Arch Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 16.Haag MD, Hofman A, Koudstaal PJ, et al. Statins are associated with a reduced risk of Alzheimer disease regardless of lipophilicity. The Rotterdam Study. J Neurol Neurosurg Psychiatry. 2009 Jan;80(1):13–17. doi: 10.1136/jnnp.2008.150433. [DOI] [PubMed] [Google Scholar]
- 17.Cramer C, Haan MN, Galea S, et al. Use of statins and incidence of dementia and cognitive impairment without dementia in a cohort study. Neurology. 2008 Jul 29;71(5):344–350. doi: 10.1212/01.wnl.0000319647.15752.7b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez EG, Dodge HH, Birzescu MA, et al. Use of lipid-lowering drugs in older adults with and without dementia: a community-based epidemiological study. J Am Geriatr Soc. 2002 Nov;50(11):1852–1856. doi: 10.1046/j.1532-5415.2002.50515.x. [DOI] [PubMed] [Google Scholar]
- 19.Shepherd J, Blauw GJ, Murphy MB, et al. PROSPER study group. Prospective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomized controlled trial. Lancet. 2002;360:1623–1630. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 20.Heart Protection Study Collaborative Group. MRC/BHF Heart protection study of cholesterol lowering with simvastatin in 20 536 high-risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. No authors listed. [Google Scholar]
- 21.Feldman HH, Doody RS, Kivipelto M, et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease. LEADe. Neurology. 2010;74:956–964. doi: 10.1212/WNL.0b013e3181d6476a. [DOI] [PubMed] [Google Scholar]
- 22.Scandinavian Simvastatin Survival Study Group. Randomized trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 23.Sacks FM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 24.White HD, Simes RJ, Anderson NE, et al. Pravastatin therapy and the risk of stroke. N Engl J Med. 2000;343:317–326. doi: 10.1056/NEJM200008033430502. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 26.Vaughan CJ. Prevention of stroke and dementia with statins: Effects beyond lipid lowering. Am J Cardiol. 2003;91(4A):23B–29B. doi: 10.1016/s0002-9149(02)03270-8. [DOI] [PubMed] [Google Scholar]
- 27.Miida T, Takahashi A, Ikeuchi T. Prevention of stroke and dementia by statin therapy: experimental and clinical evidence of their pleiotropic effects. Pharmacol Ther. 2007;113:378–393. doi: 10.1016/j.pharmthera.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Ostrowski SM, Wilkinson BL, Golde TE, et al. Statins reduce amyloid-beta production through inhibition of protein isoprenylation. J Biol Chem. 2007;282:26832–26844. doi: 10.1074/jbc.M702640200. [DOI] [PubMed] [Google Scholar]
- 29.Cordle A, Koenigsknecht-Talboo J, Wilkinson B, et al. Mechanisms of statin-mediated inhibition of small G-protein function. J Biol Chem. 2005;280:34202–34209. doi: 10.1074/jbc.M505268200. [DOI] [PubMed] [Google Scholar]
- 30.Refolo LM, Pappola J, LaFrancois J, et al. A cholesterol-lowering drug reduces beta-amyloid pathology in a transgenic mouse model of Alzheimer disease. Neurobiol Dis. 2001;8:890–899. doi: 10.1006/nbdi.2001.0422. [DOI] [PubMed] [Google Scholar]
- 31.Li L, Cao D, Kim H, et al. Simvastatin enhances learning and memory independent of amyloid load in mice. Ann Neurol. 2006;60:729–739. doi: 10.1002/ana.21053. [DOI] [PubMed] [Google Scholar]
- 32.DeKosky ST. Statin therapy in the treatment of Alzheimer disease: what is the rationale? Am J Med. 2005;118 Suppl 12A:48–53. doi: 10.1016/j.amjmed.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Bernick C, Katz R, Smith NL, et al. Statins and cognitive function in the elderly. The Cardiovascular Health Study. Neurology. 2005;65:1388–1394. doi: 10.1212/01.wnl.0000182897.18229.ec. [DOI] [PubMed] [Google Scholar]
- 34.DeKosky ST, Williamson JD, Fitzpatrick AL, et al. Ginkgo biloba for prevention of dementia: a randomized controlled trial. JAMA. 2008 Nov 19;300(19):2253–2262. doi: 10.1001/jama.2008.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Arnold AM, Newman AB, Dermond N, et al. Using telephone and informant assessments to estimate missing Modified Mini-Mental State Exam scores and rates of cognitive decline. The cardiovascular health study. Neuroepidemiology. 2009;33(1):55–65. doi: 10.1159/000215830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeKosy ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: Design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials. 2006;27:238–253. doi: 10.1016/j.cct.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 37.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the cardiovascular health study cognition study: part 1. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 38.MRC/BHF Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol-lowering therapy and of antioxidantvitamin supplementation in a wide range of patients at increased risk of coronary heart disease death: early safety and efficacy experience. Eur Heart J. 1999;20:725–741. doi: 10.1053/euhj.1998.1350. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd J, Blauw GJ, Murphy MB. The Design of a Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Am J Cardiol. 1999;84:1192–1197. doi: 10.1016/s0002-9149(99)00533-0. [DOI] [PubMed] [Google Scholar]
- 40.Fassbender K, Stroick M, Bertsch T, et al. Effects of statins on human cerebral cholesterol metabolism and secretion of Alzheimer amyloid peptide. Neurology. 2002;59:1257–1258. doi: 10.1212/wnl.59.8.1257. [DOI] [PubMed] [Google Scholar]