Abstract
Background
Saponosides (horse chestnut seed extract, escin) and flavonoids (diosmin, Daflon 500) exhibit venotonic properties that have been utilized in treatment of varicose veins. However, the cellular mechanisms underlying the venotonic properties of escin and diosmin are unclear. Because Ca2+ is a major regulator of venous smooth muscle (VSM) function, we tested the hypothesis that escin and diosmin promote Ca2+-dependent venous contraction.
Methods
Rings of inferior vena cava (IVC) from male rats were suspended in a tissue bath for measurement of isometric contraction. Following control contraction to 96 mM KCl, the effects of escin and diosmin (10−10 to 10−4 M) on vein contraction were measured. To test the role of intracellular Ca2+ release, the vein response to escin and diosmin was measured in Ca2+-free (2mM EGTA) Krebs. To test for Ca2+-dependent effects, IVC segments were pretreated with escin or diosmin (10−4 M) in 0 Ca2+ Krebs, then extracellular CaCl2 (0.1, 0.3, 0.6, 1, 2.5 mM) was added and the [Ca2+]e-contraction relationship was constructed. To test for synergistic effects of diosmin, IVC segments were pretreated with diosmin (10−4 M) then stimulated with KCl (16 to 96 mM) or escin (10−10 to 10−4 M) and vein contraction was measured. Contraction data were presented as mg/mg tissue (means±SEM).
Results
In IVC segments incubated in normal Krebs (2.5 mM Ca2+), escin caused concentration-dependent contraction (max 104.3±19.6 at 10−4 M). Escin-induced contraction was not a rigor state, because after washing with Krebs the veins returned to a relaxed state. In Ca2+-free Krebs, there was essentially no contraction to escin. In escin-treated veins incubated in 0 Ca2+ Krebs, stepwise addition of extracellular CaCl2 caused corresponding increases in contraction (max 80.0±11.1 at 2.5 mM). In the absence of escin, the α-adrenergic agonist phenylephrine (PHE, 10−5 M), angiotensin II (AngII, 10−6 M), and membrane depolarization by KCl (96 mM) caused significant contraction (122.5±45.1, 114.2±12.2 and 221.7±35.4, respectively). In IVC segments pretreated with escin (10−4 M), the contractile response to PHE (9.7±2.6), AngII (36.0±9.1) and KCl (82.3±10.2) was significantly reduced. Diosmin (10−4 M) caused small contraction in normal Krebs (11.7±1.9) and Ca2+-free Krebs (4.2±2.2). In diosmin-treated veins incubated in 0 Ca2+ Krebs, addition of extracellular CaCl2 caused minimal contraction. Diosmin did not enhance the IVC contraction to PHE, AngII, or escin, but enhanced the contractile response to KCl (24 to 51 mM).
Conclusion
In rat IVC, escin induces extracellular Ca2+-dependent contraction, but disrupts α-adrenergic and AT1R receptor-mediated pathways, and depolarization-induced contraction. The initial venotonic benefits of escin may be offset by disruption of vein response to endogenous venoconstrictors, limiting its long-term therapeutic benefits in varicose veins. Diosmin does not cause venous contraction or potentiate the venotonic effects of endogenous venoconstrictors or escin ex vivo, and its use as venotonic may need to be further evaluated.
Keywords: escin, diosmin, varicose veins, vascular smooth muscle, calcium
INTRODUCTION
Varicose veins is a common disease of the lower extremity characterized by vein valve dysfunction, vein dilation and tortuosity.1–3 If untreated, varicose veins can progress causing severe venous insufficiency, and may lead to significant pain, venous thrombophlebitis and lower extremity skin unlceration.1, 4–6 Surgical treatment approaches of varicose veins include vein ablation and stripping. Other more conservative treatment strategies to reduce the pain and edema associated with varicose veins include compression stockings and oral venotonic medication.7, 8
Saponosides such as horse chestnut seed extract (Aesculus hippocastanum, Aescin, Escin, β-Escin) and flavonoids such as diosmin (Daflon 500, Servier, France) exhibit venotonic properties that have been utilized in treatment of chronic venous disorders including varicose veins and lower extremity venous ulcers.9–11 Escin is also used clinically to treat edema, pain, leg fatigue/heaviness, calf cramps, and itching.12, 13 Some of the suggested mechanisms of venotonic action of escin include increasing endothelial cell permeability to Ca2+ and the release of vasoconstrictor prostanoids such as prostaglandin F2α, and sensitization of the vein wall to the contractile effects of serotonin and histamine.10, 14, 15 Escin also forms small pores in the cell membrane and has been used as a pharmacological tool to assess the sensitivity of the contractile mechanisms of blood vessels to different agonists,10, 16 and for permeabilization of VSM membranes and manipulation of intracellular ionic environment and regulatory proteins.15, 17 The escin-induced pores in the plasma membrane permit Ca2+ to diffuse freely across, and make the cell membrane permeable to higher molecular weight solutes (>3000) of biological interest, such as heparin and calmodulin, yet retain coupled receptors.18 On the other hand, diosmin has been suggested to decrease the vein tissue inflammatory response, increase lymph drainage, and to inhibit venous catechol-O-methyltransferase (COMT) and thereby decreases the metabolism of norepinephrine and prolongs its venoconstrictor effects.19, 20 Another study has shown that diosmin enhances the effects of other venotonics such as escin.16 However, the cellular mechanisms underlying the venotonic properties of escin and diosmin have not been clearly established. Also, the direct effects of escin and diosmin on vein tissue function and their synergistic effects with each other and with other endogenous venoconstrictors have not been fully examined. Because Ca2+ is a major regulator of VSM function,21 we tested the hypothesis that escin promotes Ca2+-dependent mechanisms of venous contraction, and that diosmin potentiates these mechanisms.
MATERIALS AND METHODS
Solutions, drugs and chemicals
Normal Krebs solution contained in mM: NaCl 120, KCl 5.9, NaHCO3 25, NaH2PO4 1.2, dextrose 11.5 (Fisher Scientific, Fair Lawn, NJ), CaCl2 2.5 (BDH Laboratory Supplies Poole, England), MgCl2 1.2 (Sigma, St. Louis, MO). Krebs solution was bubbled with 95% O2 and 5% CO2 for 30–45 min, at an adjusted pH 7.4. For nominally 0 Ca2+ Krebs, CaCl2 was omitted. For Ca2+-free Krebs, CaCl2 was omitted and 2 mM ethylene glycolbis[β-aminoethyl ether]-N,N,N',N'-tetraacetic acid (EGTA, Sigma) was added. KCl depolarizing solution (16 to 96 mM) was prepared as normal Krebs but with equimolar substitution of NaCl with KCl. Stock solutions (10−1 M) of β-escin, diosmin, phenylephrine (PHE), angiotensin II (AngII, Sigma), and diltiazem (Calbiochem, La Jolla, CA) were prepared in distilled water. Other chemicals were of reagent grade or better.
Animals and tissues
Male Sprague-Dawley rats (12 wk, 250–300g, Charles River lab, Wilmington, MA) were housed in the animal facility and maintained on ad libitum standard rat chow and tap water in 12 hr/12 hr light/dark cycle. Rats were euthanized by inhalation of CO2. The inferior vena cave (IVC) was rapidly excised, placed in oxygenated Krebs solution, and carefully dissected and cleaned of connective tissue under microscopic visualization. The IVC was portioned into 3 mm rings in preparation for isometric contraction experiments. Extreme care was taken throughout the vein isolation, dissection procedure, and mounting in the tissue bath in order to minimize injury to the vein wall and endothelium. Using this careful approach we have previously shown that acetylcholine causes significant relaxation of IVC segments precontracted with PHE, indicating that the endothelium is intact and functional.22 All procedures followed the NIH guide for the Care of Laboratory Animal Welfare Act, and the guidelines of the Animal Care and Use Committee at Harvard Medical School.
Isometric contraction
Circular segments of IVC were suspended between two stainless-steel hooks, one hook was fixed at the bottom of a tissue bath and the other hook was connected to a Grass force displacement transducer (FT03, Astro-Med Inc., West Warwick, RI). Vein segments were stretched under 0.5 gm of resting tension and allowed to equilibrate for 40 min in a tissue bath filled with 50 ml Krebs solution continuously bubbled with 95% O2 5% CO2 at 37°C. The changes in isometric contraction/relaxation were recorded on a Grass polygraph (Model 7D, Astro-Med Inc.). Control IVC contraction in response to 96 mM KCl was first elicited. Once the KCl contraction reached a maximum and a plateau was achieved (within 10 to 15 min) the vein was washed 3 times in Krebs, 10 min each. The control contraction to 96 mM KCl was repeated twice prior to further experimentation.
Escin and diosmin concentration-response curve and Ca2+-dependent contraction
Following KCl-induced control contractions, increasing concentrations of escin or diosmin (10−10 to 10−4 M) were added and their effect on IVC contraction was measured. In vivo studies in 18 healthy human volunteers have shown that after administration of escin 50 mg tablet daily the average plasma levels reach 10 ng/ml (~ 10−5 M) at 12 hr and 7 ng/ml (~ 7×10−6 M) at 24 hr. Over the course of a week, escin plasma levels were maintained at 8 ng/ml (~ 8×10−6 M).10 The present experiments demonstrated measurable effects of escin on vein contraction at 1, 10 and 100 micromolar concentrations. To test for the role of Ca2+ entry from the extracellular space through Ca2+ channels, the veins were pretreated with the Ca2+ channel blocker diltiazem (10−5 M) followed by increasing concentrations of escin (10−10 to 10−4 M) and the venous contraction was measured. To test for the extracellular Ca2+-independent effects and possible intracellular Ca2+ release mechanisms, the vein response to increasing concentrations of escin or diosmin was measured in Ca2+-free (2 mM EGTA) Krebs. To test for extracellular Ca2+-dependent effects, IVC segments were pretreated with escin or diosmin (10−4 M) in 0 Ca2+ Krebs for 5 min, then increasing extracellular CaCl2 concentrations (0.1, 0.3, 0.6, 1, 2.5 mM) were added and the [Ca2+]e-contraction relationship was constructed.
Effects of escin and diosmin on PHE, AngII, and KCl contraction
IVC segments were incubated in normal Krebs solution containing 2.5 mM Ca2+, nontreated or pretreated with escin or diosmin (10−4 M) for 2 hr. The veins were washed with Krebs solution 3 times 10 minutes each, then stimulated with PHE (10−5 M), AngII (10−6 M), or KCl (96 mM), and the vein contraction was measured. These doses of PHE, AngII and KCl have previously been shown to produce maximal IVC contraction.22, 23
Synergistic effects of diosmin
To test if diosmin pontentiates the Ca2+-dependent mechanisms of VSM contraction, IVC segments were pretreated with diosmin (10−4 M) then subjected to increasing concentrations of KCl (16 to 96 mM). To test if diosmin pontentiates the effects of other venotonics, IVC segments were pretreated with diosmin (10−4 M) then subjected to increasing concentrations of escin (10−10 to 10−4 M), and the venous contraction was measured.
Statistical analysis
Each rat IVC produced 4 circular segments. One IVC segment was used for each experimental protocol, and the average data from different IVC segments for each experimental protocol were measured. The “n” value represented the number of experiments on different IVC segments from different rats. Vein contractions in mg/mg tissue weight were presented as means±SEM (n) and compared using Student’s t-test for paired and unpaired data. Differences were considered statistically significant if P < 0.05.
RESULTS
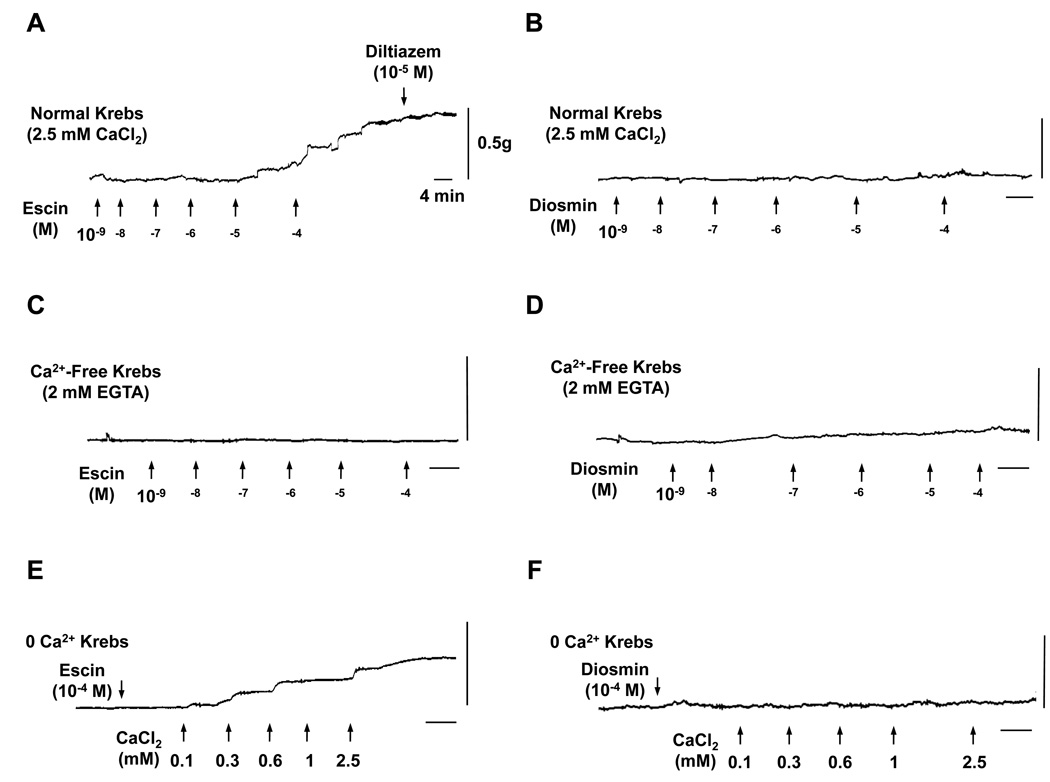
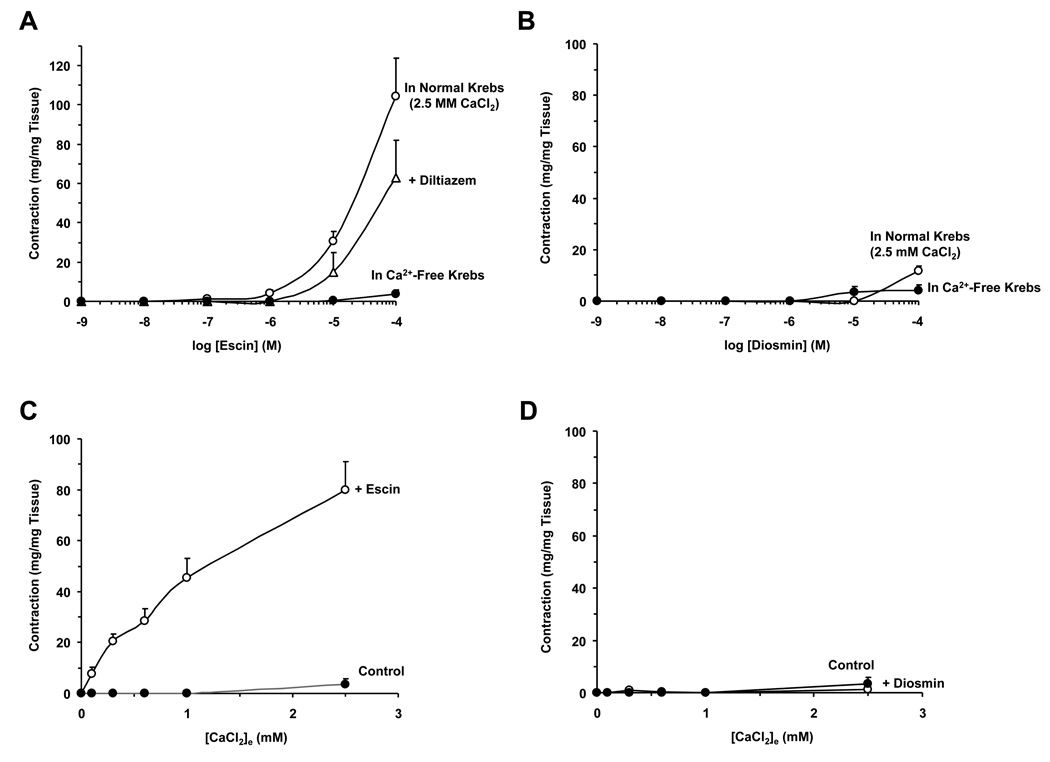
In IVC segments incubated in normal Krebs (2.5 mM Ca2+), escin caused concentration-dependent contraction reaching a maximum of 104.3±19.6 mg/mg tissue or 48.3±4.4% of control KCl contraction (Fig. 1A, 2A). The escin-induced contraction was reversible, and after washing 3 times with Krebs, the veins returned to a relaxed state, suggesting that the escin-induced contraction does not constitute a rigor state. Topical application or pretreatment of IVC segments with the Ca2+ channel blocker diltiazem (10−5 M) did not cause significant reduction in escin-induced contraction (Fig. 1A, 2A). In Ca2+-free (2 mM EGTA) Krebs, escin caused minimal IVC contraction (3.8±2.0 at 10−4 M) (Fig. 1C, 2A), supporting that escin-induced contraction is not solely due to its pore forming properties and potential loss of intracellular ATP and consequently contractile rigor. In comparison with escin, increasing concentrations of diosmin caused a very small vein contraction in normal Krebs solution (maximum 11.7±1.9 mg/mg tissue or 13.3±3.3% of control KCl contraction) (Fig. 1B, 2B) and Ca2+-free (2 mM EGTA) Krebs (4.2±2.2 at 10−4 M) (Fig. 1D, 2B).
Fig. 1.
Representative traces of the effect of escin and diosmin on rat IVC. Rat IVC segments were treated with escin or diosmin (10−10 to 10−4 M) in normal Krebs (2.5 mM Ca2+) (A and B) or in Ca2+-free (2 mM EGTA) Krebs (C and D). In other experiments, IVC segments incubated in 0 Ca2+ Krebs were treated with 10−4 M escin (E) or diosmin (F) then increasing concentrations of extracellular CaCl2 (0.1 to 2.5 mM) were added and the contractile response to Ca2+ was observed. Traces are representative of 4 to 9 experiments. Horizontal bar = 4 min, Vertical bar = 0.5 g.
Fig. 2.
Effect of escin and diosmin on rat IVC. Rat IVC segments were treated with increasing concentrations (10−10 to 10−4 M) of escin (A) or diosmin (B) in normal Krebs (2.5 mM Ca2+), in the presence of diltiazem (10−5 M), and in Ca2+-free (2 mM EGTA) Krebs. In other experiments, IVC segments incubated in 0 Ca2+ Krebs were either nontreated (control) or treated with 10−4 M escin (C) or diosmin (D) then increasing concentrations of extracellular CaCl2 (0.1 to 2.5 mM) were added and the Ca2+ concentration-contraction curve was constructed. Data represent the means±SEM (n=4 to 9).
We tested the Ca2+-dependent component of escin contraction. In IVC pretreated with escin (10−4 M) in 0 Ca2+ Krebs, stepwise addition of extracellular CaCl2 (0.1 to 2.5 mM) caused corresponding increases in contraction that reached a maximum of 80.0±11.1 at 2.5 mM CaCl2 (Fig. 1E, 2C). Control experiments in IVC segments nontreated with escin demonstrated minimal contraction in response to increasing concentrations of CaCl2 (3.8±2.0 at 2.5mM CaCl2) (Fig. 2C). In comparison with escin, IVC segments pretreated with diosmin (10−4 M) in 0 Ca2+ Krebs, stepwise addition of extracellular CaCl2 elicited only a very small contraction (1.2±1.2 at 2.5 mM CaCl2) (Fig. 1F, 2D).
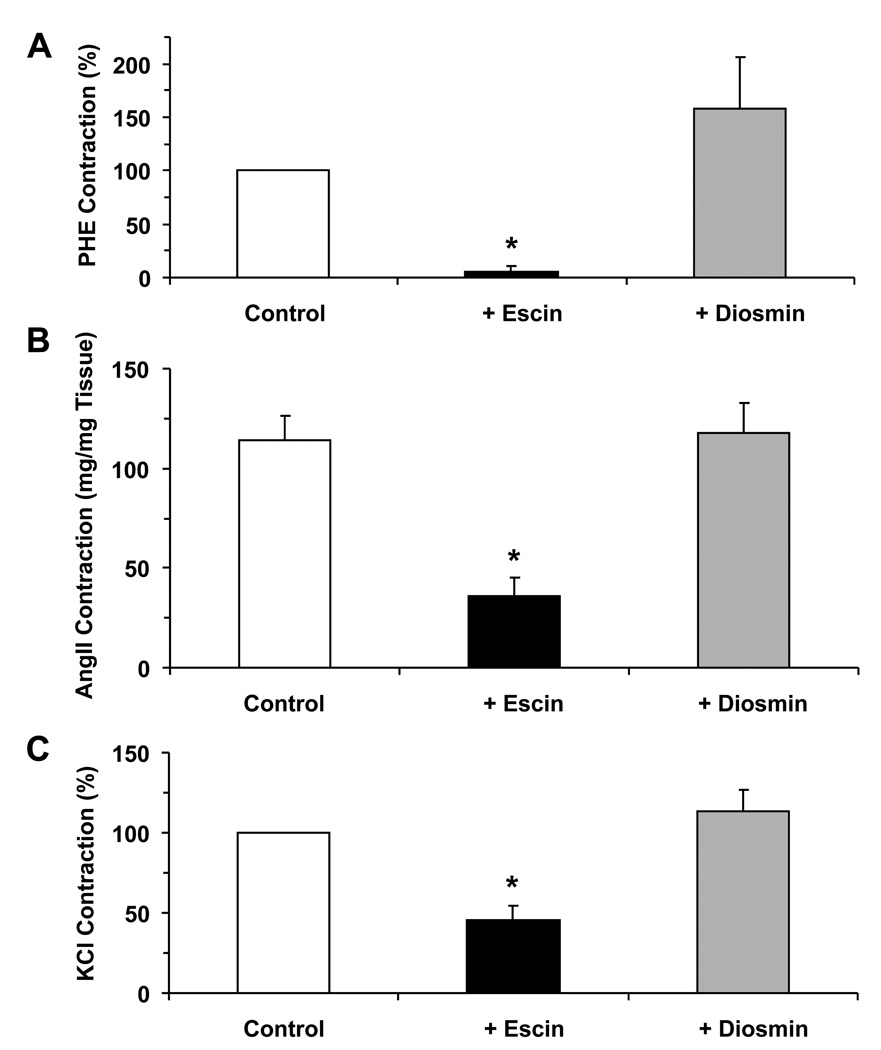
Because escin and diosmin demonstrated different vein contraction profile and Ca2+-dependent contractile responses, we tested whether they also differentially affect the venous contractile response to other vasoconstrictor agonists. In the absence of escin, the IVC demonstrated significant contraction in response to the α-adrenergic agonist PHE (122.5±45.1 at 10−5 M), AngII (114.2±12.2 at 10−6 M), and membrane depolarization by 96 mM KCl (221.7±35.4) (Fig. 3). In IVC segments pretreated with escin (10−4 M) for 2 hr, the PHE, AngII and KCl-induced contraction were significantly reduced (Fig. 3). In contrast with the experiments with escin, the PHE, AngII, and 96 mM KCl-induced contractions were not significantly different in IVC segments pretreated with diosmin (10−4 M) as compared to control contractions in the absence of diosmin (Fig. 3).
Fig. 3.
Effect of escin and diosmin on PHE, AngII and depolarization-induced contraction in rat IVC. Rat IVC segments were treated with PHE (10−5 M) (A), AngII (10−6 M) (B) or 96 mM KCl (C) in the absence (open bars) or presence of escin (10−4 M) (black bars) or diosmin (10−4 M) (shaded bars). The contractile response was recorded and presented as % of control contraction (A, C) or as mg/mg tissue weight (B). Data represent the means±SEM (n=4 to 19). * Significant p<0.05.
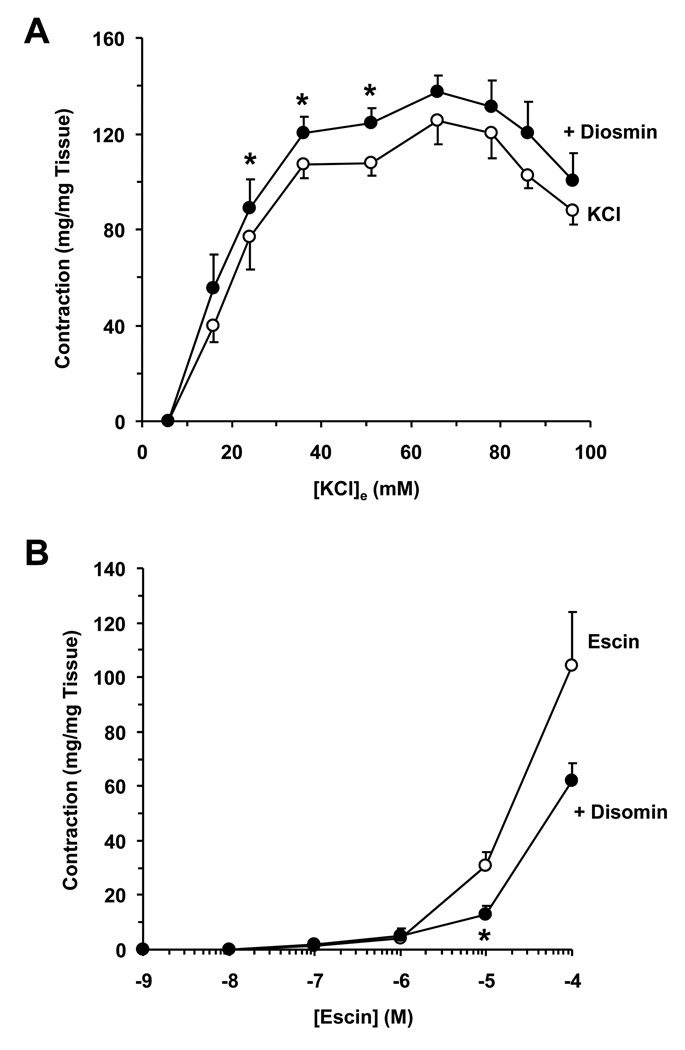
Membrane depolarization by KCl is known to stimulate Ca2+ entry via voltage-gated Ca2+ channels.21 Some studies also suggest that KCl may activate Ca2+ sensitization pathways.24, 25 To evaluate possible potentiation and Ca2+ sensitization effects of diosmin, we tested whether pretreatment with diosmin (10−4 M) enhances the vein response to increasing concentrations of KCl (16 to 96 mM). At KCl concentrations 24 to 51 mM, diosmin caused a small but significant enhancement of vein contraction (Fig. 4A). On the other hand, vein pretreatment with diosmin (10−4 M) did not enhance the contractile response to increasing concentrations of escin (Fig. 4B). In effect, a significant inhibition of contraction to escin (10−5 M) was observed in veins pretreated with diosmin (Fig. 4B).
Fig. 4.
Effect of diosmin on KCl and escin-induced IVC contraction. IVC segments were either non-treated (open circles) or pretreated with diosmin (10−4 M) (closed circles) then stimulated with increasing concentrations of KCl (16 to 96 mM) (A) or escin (10−10 to 10−4 M) (B) and the contractile response was recorded. Data represent the means±SEM (n=7 to 8). * Significant p<0.05.
DISCUSSION
The main findings of the present study are: 1) escin causes Ca2+ dependent venous contraction, 2) escin decreases contraction to venoconstrictors such as α-adrenergic and AngII (AT1R) receptor stimulation, and membrane depolarization, 3) diosmin does not cause contraction or enhance contraction to endogenous venoconstrictors such as PHE and AngII or other venotonics such as escin, but enhances vein contraction to KCl-induced membrane depolarization at low concentrations.
An important property of escin is that it forms small plasma membrane pores, allowing investigators to examine various Ca2+-dependent and Ca2+-sensitization pathways of vascular contraction.10, 15–17, 26, 27 The present study demonstrated that escin induced Ca2+-dependent venous contraction because: 1) escin contraction was observed in normal Krebs (2.5 mM Ca2+) and was significantly attenuated in Ca2+-free Krebs, and 2) increasing concentrations of extracellular Ca2+ caused significant contraction in escin-treated IVC, but not in control veins in the absence of escin. Previous studies have shown that escin-induced contraction of the rat aorta is reduced by 25% in endothelium-denuded vessels or in the presence of the cyclooxygenase inhibitor indomethacin, and suggested a partial role of a vasoconstrictor prostaglandin-dependent pathway.15 We should note that, when compared to the rat aorta, the rat IVC is very delicate and mechanical removal of the endothelium often causes injury to the adjacent VSM and considerable reduction in vein contraction. Although we did not test the effects of escin in endothelium-denuded IVC, the potential role of escin-induced prostaglandin release needs to be further examined. It could be argued that the observed escin-induced vein contraction is solely due to formation of pores in the plasma membrane which would allow loss and depletion of intracellular ATP and consequently VSM contractile rigor.28, 29 If this is the case then the escin-induced contractile rigor would be difficult to reverse, which is opposite from the observed return of the veins to the relaxed state upon washing with normal Krebs. Also, if escin contraction is a rigor state due to loss of intracellular ATP, then escin should also produce contraction in Ca2+-free solution. The observation that escin contraction was almost abolished in IVC incubated in Ca2+-free Krebs is consistent with reports that the contractile response of escin is abolished in a Ca2+-free perfusion fluid.15 These data indicate that escin-induced contraction is not solely due to its pore forming properties, loss of intracellular ATP and VSM contractile rigor. However, upon the addition of extracellular Ca2+ up to 2.5 mM, escin caused contraction of the IVC, suggesting that Ca2+ entry from the extracellular environment is the major cause of the contractile response. One possibility is that escin may enhance Ca2+ entry through specific Ca2+ channels. Ca2+ antagonists such as diltiazem block Ca2+ entry through voltage-gated L-type Ca2+ channels.30 The observation that diltiazem did not significantly affect escin-induced contraction suggests that escin may not be acting by enhancing Ca2+ entry through L-type Ca2+ channels. The data suggest that the mechanism of escin-induced contraction more likely involves formation of non-specific membrane pores small enough to allow Ca2+ movement across the plasma membrane, rather than activation of specific Ca2+ channels. However, VSM also express N- and T-type Ca2+ channels,31, 32 and the effects of escin on these channels need to be examined in future studies.
An important finding of this study is that pretreatment of IVC with escin decreased the contractile response to the α-adrenergic receptor agonist PHE. The decreased PHE contraction in escin-treated veins may not be due to damage of α-adrenergic receptors because: 1) previous studies demonstrated robust response to PHE in rabbit portal vein and mesenteric artery permeabilized with escin (75 µM) for 35 min,18, 33, 34 2) escin decreased another receptor-mediated contractile response induced by AngII which is known to activate AT1R receptor-mediated signaling pathways, and 3) escin decreased the contractile response to membrane depolarization by KCl, a receptor-independent response that involves Ca2+ entry from the extracellular space. We have previously shown that matrix metalloproteinases (MMPs) are expressed in rat IVC, and cause membrane hyperpolarization and inhibition of vein contraction.23 Because MMPs are Ca2+-dependent, it is likely that escin-induced increase in venous Ca2+ could activate MMPs which may in turn cause membrane hyperpolarization and inhibition of vein contraction. Another possibility is that prolonged treatment of the rat IVC with escin may cause structural or chemical changes in the vein that reduce the vein responsiveness to various contractile stimuli. Future histology studies would further examine any potential phenotypic damage following vein treatment with escin.
Escin is a major constituent of horse chestnut seed extract that is used clinically to treat chronic venous disorders including varicose veins, edema, pain, leg fatigue/heaviness, calf cramps, and itching.12, 13 The escin-induced IVC contraction suggests a measurable venotonic effect that, in the short-term, could be useful in improving the venous tone of varicose veins. However, the observed reduction in the contractile response to endogenous venoconstrictors in escin-treated veins suggests that the initial venotonic benefits of escin may be offset in the long-term due to decreased VSM responsiveness to venoconstrictor stimuli, and possible disruption of the normal physiologic vein response to increased sympathetic activity and changes in the hormone environment during exercise and stress. Further studies in human veins are needed to determine if prolonged treatment with escin inhibits the physiological receptor-mediated signaling pathways and disrupts the VSM contractile mechanisms.
Diosmin is a flavonoid processed as a micronized purified flavonoid fraction (Daflon 500). Diosmin has been promoted for its inhibitory effects on inflammation, improved lymphatic drainage and venotonic properties, and is used in the treatment of chronic venous insufficiency and venous leg ulcers.35, 36 Previous studies have suggested that the vasoconstrictor effects of diosmin involve inhibition of COMT, decreased metabolism of norepinephrine and enhanced sympathetic activity.19, 20 Other studies have demonstrated potentiation effects of diosmin on norepinephrine induced contraction of both human normal saphenous vein and varicose veins in vitro.37 The present study demonstrates that diosmin alone does not cause significant Ca2+-dependent or Ca2+-independent contraction in rat IVC. Also, pretreatment with diosmin does not enhance the rat IVC contraction to PHE. The differences in the results could be related to the species or vein tissue studied, or the activator of the adrenergic receptor, where norepinephrine activates α-receptors and some β-receptors, while PHE is a specific α1-receptor agonist. Future studies should compare the effects of diosmin on vein contraction induced by different α-adrenergic receptor activators including epinephrine, norepinephrine and PHE.
Our experiments with AngII and high concentrations of KCl supported that diosmin did not potentiate IVC contraction. Since various concentrations of KCl cause various degrees of membrane depolarization and consequently extracellular Ca2+ entry, we explored whether disomin potentiates the contractile effects of low KCl concentrations. Diosmin potentiated KCl-induced contraction at concentrations 24 to 51 mM. Other studies have shown that diosmin at 10−6 M concentration enhances Ca2+ sensitivity in escin skinned rat femoral vein.16 However, we were not able to demonstrate any significant contraction elicited by diosmin at a concentration range of 10−10 to 10−4 M. Also, diosmin at 10−4 M did not potentiate escin-induced venous contraction. The difference in the results could be related to the tissue studied (IVC versus femoral vein), and the temperature of the bathing medium (37°C versus 25°C). Other explanations are that diosmin requires specific cofactor(s) to increase venous Ca2+ sensitivity and produce an effective contraction, or that diosmin itself may need to penetrate the veins in order to sensitize the VSM cells to Ca2+.
Thus in rat IVC, escin induces extracellular Ca2+-dependent contraction that could translate into measurable venotonic effects, but may also disrupt α-adrenergic and AT1R receptor-mediated pathways, and depolarization-induced vein contraction. The initial venotonic benefits of escin may be offset by disruption of VSM response to endogenous venoconstrictors and thereby limit its long-term therapeutic benefit in varicose veins. The present ex vivo experiments also demonstrate that diosmin does not cause venous contraction or potentiate the venotonic effects of endogenous venoconstrictors or escin, and its use as venotonic may need to be further evaluated in carefully-designed clinical trials.
Limitations of the present study include the use of rat veins, the absence of circulating blood, and the concentrations of α-adrenergic receptor agonist and AngII that may exceed the physiologic levels. It should be noted that diosmin has been shown to decrease inflammation in the microcirculation, and to reduce leukocyte activation and the surface expression of CD62L by neutrophils and monocytes.38–42 Given the ex vivo conditions of the present study and the absence of circulating cellular elements, the lack of venotonic effects of diosmin should not minimize other potential beneficial anti-inflammatory effects of diosmin on varicose veins in vivo.
CLINICAL RELEVANCE.
Venotonic agents are used in clinical practice for treatment of varicose veins. Saponosides such as escin (horse chestnut seed extract) and flavonoids such as disomin (active ingredient in Daflon 500) are commonly prescribed venotonic agents. Although escin is known to form pores in the plasma membrane, the mechanisms of its venotonic action are less clear. Also, while diosmin may affect the venous tissue inflammatory response and lymphatic drainage, its direct effects on vein function have not been fully examined. The present results suggest that escin has significant Ca2+-dependent venotonic effects, but also reduces the effects of endogenous venoconstrictors, which could limit its long-term therapeutic benefits in varicose veins. Disomin has negligible direct venotonic effects, and does not potentiate the effects of endogenous venoconstrictors or escin, and its use as a venotonic agent may need further evaluation.
ACKNOWLEDGEMENTS
This work was supported by grants from National Heart, Lung, and Blood Institute (HL-65998, HL-70659, and HL-98724) and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (HD-60702).
List of Abbreviations
- AngII
angiotensin II
- Ca2+
calcium
- PHE
phenylephrine
- VSM
vascular smooth muscle
REFERENCES
- 1.Bergan JJ, Schmid-Schonbein GW, Smith PD, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;335(5):488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 2.Raffetto JD, Khalil RA. Mechanisms of varicose vein formation: valve dysfunction and wall dilation. Phlebology. 2008;23(2):85–98. doi: 10.1258/phleb.2007.007027. [DOI] [PubMed] [Google Scholar]
- 3.Lim CS, Davies AH. Pathogenesis of primary varicose veins. Br J Surg. 2009;96(11):1231–1242. doi: 10.1002/bjs.6798. [DOI] [PubMed] [Google Scholar]
- 4.Labropoulos N, Leon M, Nicolaides AN, Giannoukas AD, Volteas N, Chan P. Superficial venous insufficiency: correlation of anatomic extent of reflux with clinical symptoms and signs. J Vasc Surg. 1994;20(6):953–958. doi: 10.1016/0741-5214(94)90233-x. [DOI] [PubMed] [Google Scholar]
- 5.Kakkos SK, Lampropoulos G, Papadoulas S, Ntouvas I, Tsolakis I. Seasonal variation in the incidence of superficial venous thrombophlebitis. Thromb Res. 126(2):98–102. doi: 10.1016/j.thromres.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Kostas TI, Ioannou CV, Drygiannakis I, Georgakarakos E, Kounos C, Tsetis D, Katsamouris AN. Chronic venous disease progression and modification of predisposing factors. J Vasc Surg. 51(4):900–907. doi: 10.1016/j.jvs.2009.10.119. [DOI] [PubMed] [Google Scholar]
- 7.Partsch H. Varicose veins and chronic venous insufficiency. Vasa. 2009;38(4):293–301. doi: 10.1024/0301-1526.38.4.293. [DOI] [PubMed] [Google Scholar]
- 8.Gohel MS, Davies AH. Pharmacological agents in the treatment of venous disease: an update of the available evidence. Curr Vasc Pharmacol. 2009;7(3):303–308. doi: 10.2174/157016109788340758. [DOI] [PubMed] [Google Scholar]
- 9.Frick RW. Three treatments for chronic venous insufficiency: escin, hydroxyethylrutoside, and Daflon. Angiology. 2000;51(3):197–205. doi: 10.1177/000331970005100303. [DOI] [PubMed] [Google Scholar]
- 10.Sirtori CR. Aescin: pharmacology, pharmacokinetics and therapeutic profile. Pharmacol Res. 2001;44(3):183–193. doi: 10.1006/phrs.2001.0847. [DOI] [PubMed] [Google Scholar]
- 11.Coleridge Smith PD. From skin disorders to venous leg ulcers: pathophysiology and efficacy of Daflon 500 mg in ulcer healing. Angiology. 2003;54 Suppl 1:S45–S50. doi: 10.1177/0003319703054001s06. [DOI] [PubMed] [Google Scholar]
- 12.Siebert U, Brach M, Sroczynski G, Berla K. Efficacy, routine effectiveness, and safety of horsechestnut seed extract in the treatment of chronic venous insufficiency A meta-analysis of randomized controlled trials and large observational studies. Int Angiol. 2002;21(4):305–315. [PubMed] [Google Scholar]
- 13.Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochrane Database Syst Rev. 2006;(1) doi: 10.1002/14651858.CD003230.pub3. CD003230. [DOI] [PubMed] [Google Scholar]
- 14.Berti F, Omini C, Longiave D. The mode of action of aescin and the release of prostaglandins. Prostaglandins. 1997;14(2):241–249. doi: 10.1016/0090-6980(77)90169-1. [DOI] [PubMed] [Google Scholar]
- 15.Carrasco OF, Vidrio H. Endothelium protectant and contractile effects of the antivaricose principle escin in rat aorta. Vascul Pharmacol. 2007;47(1):68–73. doi: 10.1016/j.vph.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 16.Savineau JP, Marthan R. Diosmin-induced increase in sensitivity to Ca2+ of the smooth muscle contractile apparatus in the rat isolated femoral vein. Br J Pharmacol. 1994;111(4):978–980. doi: 10.1111/j.1476-5381.1994.tb14838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Heijs BG, Blange T, Jongsma HJ, De Beer EL. The length dependency of calcium activated contractions in the femoral artery smooth muscle studied with different methods of skinning. J Muscle Res Cell Motil. 2000;21(1):59–66. doi: 10.1023/a:1005609319445. [DOI] [PubMed] [Google Scholar]
- 18.Kobayashi S, Kitazawa T, Somlyo AV, Somlyo AP. Cytosolic heparin inhibits muscarinic and alpha-adrenergic Ca2+ release in smooth muscle. Physiological role of inositol 1,4,5-trisphosphate in pharmacomechanical coupling. J Biol Chem. 1989;264(30):17997–18004. [PubMed] [Google Scholar]
- 19.Boudet C, Peyrin L. Comparative effect of tropolone and diosmin on venous COMT and sympathetic activity in rat. Arch Int Pharmacodyn Ther. 1986;283(2):312–320. [PubMed] [Google Scholar]
- 20.Araujo D, Viana F, Osswald W. Diosmin therapy alters the in vitro metabolism of noradrenaline by the varicose human saphenous vein. Pharmacol Res. 1991;24(3):253–256. doi: 10.1016/1043-6618(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 21.Khalil RA, van Breemen C. Sustained contraction of vascular smooth muscle: calcium influx or C-kinase activation? J Pharmacol Exp Ther. 1988;244(2):537–542. [PubMed] [Google Scholar]
- 22.Raffetto JD, Qiao X, Beauregard KG, Khalil RA. Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins. J Vasc Surg. 51(4):972–981. doi: 10.1016/j.jvs.2009.11.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raffetto JD, Ross RL, Khalil RA. Matrix metalloproteinase 2-induced venous dilation via hyperpolarization and activation of K+ channels: relevance to varicose vein formation. J Vasc Surg. 2007;45(2):373–380. doi: 10.1016/j.jvs.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wray S, Burdyga T, Noble K. Calcium signalling in smooth muscle. Cell Calcium. 2005;38(3–4):397–407. doi: 10.1016/j.ceca.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Bolton TB. Calcium events in smooth muscles and their interstitial cells; physiological roles of sparks. J Physiol. 2006;570(Pt 1):5–11. doi: 10.1113/jphysiol.2005.095604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bruce L, Nixon GF. Increased sensitization of the myofilaments in rat neonatal portal vein: a potential mechanism. Exp Physiol. 1997;82(6):985–993. doi: 10.1113/expphysiol.1997.sp004084. [DOI] [PubMed] [Google Scholar]
- 27.Nilsson BO, Gomez M, Santiago Carrilho R, Nordstrom I, Hellstrand P. Differential actions of exogenous and intracellular spermine on contractile activity in smooth muscle of rat portal vein. Acta Physiol Scand. 1995;154(3):355–365. doi: 10.1111/j.1748-1716.1995.tb09919.x. [DOI] [PubMed] [Google Scholar]
- 28.Veigel C, von Maydell RD, Kress KR, Molloy JE, Fink RH. The effect of ionic strength on the kinetics of rigor development in skinned fast-twitch skeletal muscle fibres. Pflugers Arch. 1998;435(6):753–761. doi: 10.1007/s004240050580. [DOI] [PubMed] [Google Scholar]
- 29.Stowe DF. Understanding the temporal relationship of ATP loss, calcium loading, and rigor contracture during anoxia, and hypercontracture after anoxia in cardiac myocytes. Cardiovasc Res. 1999;43(2):285–287. doi: 10.1016/s0008-6363(99)00164-9. [DOI] [PubMed] [Google Scholar]
- 30.Triggle DJ. L-type calcium channels. Curr Pharm Des. 2006;12(4):443–457. doi: 10.2174/138161206775474503. [DOI] [PubMed] [Google Scholar]
- 31.Triggle DJ. Calcium channel antagonists: clinical uses--past, present and future. Biochem Pharmacol. 2007;74(1):1–9. doi: 10.1016/j.bcp.2007.01.016. [DOI] [PubMed] [Google Scholar]
- 32.Romero M, Sanchez I, Pujol MD. New advances in the field of calcium channel antagonists: cardiovascular effects and structure-activity relationships. Curr Med Chem Cardiovasc Hematol Agents. 2003;1(2):113–141. doi: 10.2174/1568016033477487. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki M, Hattori Y, Tomita F, Moriishi K, Kanno M, Kohya T, Oguma K, Kitabatake A. Tyrosine phosphorylation as a convergent pathway of heterotrimeric G protein- and rho protein-mediated Ca2+ sensitization of smooth muscle of rabbit mesenteric artery. Br J Pharmacol. 1998;125(8):1651–1660. doi: 10.1038/sj.bjp.0702242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gong MC, Gorenne I, Read P, Jia T, Nakamoto RK, Somlyo AV, Somlyo AP. Regulation by GDI of RhoA/Rho-kinase-induced Ca2+ sensitization of smooth muscle myosin II. Am J Physiol Cell Physiol. 2001;281(1):C257–C269. doi: 10.1152/ajpcell.2001.281.1.C257. [DOI] [PubMed] [Google Scholar]
- 35.Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. Eur J Vasc Endovasc Surg. 2005;30(2):198–208. doi: 10.1016/j.ejvs.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 36.Bergan JJ. Chronic venous insufficiency and the therapeutic effects of Daflon 500 mg. Angiology. 2005;56 Suppl 1:S21–S24. doi: 10.1177/00033197050560i104. [DOI] [PubMed] [Google Scholar]
- 37.Juteau N, Bakri F, Pomies JP, Foulon C, Rigaudy P, Pillion G, Lange G, Genre O, Cron JP. The human saphenous vein in pharmacology: effect of a new micronized flavonoidic fraction (Daflon 500 mg) on norepinephrine induced contraction. Int Angiol. 1995;14 3 Suppl 1:8–13. [PubMed] [Google Scholar]
- 38.Bergan JJ, Schmid-Schonbein GW, Takase S. Therapeutic approach to chronic venous insufficiency and its complications: place of Daflon 500 mg. Angiology. 2001;52 Suppl 1:S43–S47. doi: 10.1177/0003319701052001S06. [DOI] [PubMed] [Google Scholar]
- 39.Takase S, Lerond L, Bergan JJ, Schmid-Schonbein GW. The inflammatory reaction during venous hypertension in the rat. Microcirculation. 2000;7(1):41–52. [PubMed] [Google Scholar]
- 40.Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schonbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484–493. doi: 10.1016/j.ejvs.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 41.Shoab SS, Porter JB, Scurr JH, Coleridge-Smith PD. Effect of oral micronized purified flavonoid fraction treatment on leukocyte adhesion molecule expression in patients with chronic venous disease: a pilot study. J Vasc Surg. 2000;31(3):456–461. [PubMed] [Google Scholar]
- 42.Labrid C. Pharmacologic properties of Daflon 500 mg. Angiology. 1994;45(6 Pt 2):524–530. [PubMed] [Google Scholar]