Abstract
The pathogenic fungus Cryptococcus neoformans is a major cause of morbidity and mortality in immunocompromised individuals. Infection of the human host occurs through inhalation of infectious propagules following environmental exposure. In the lung, C. neoformans can reside in the extracellular environment of the alveolar spaces or, upon phagocytosis, it can survive and grow intracellularly within alveolar macrophages (AMs). In previous studies, we found that sphingosine kinase 1 (SK1) influenced the intracellular residency of C. neoformans within AMs. Therefore, with this study we aimed to examine the role of the SK1 lipid product, sphingosine-1-phosphate (S1P), in the AMs–C. neoformans interaction. It was found that extracellular S1P enhances the phagocytosis of C. neoformans by AMs. Using both genetic and pharmacological approaches we further show that extracellular S1P exerts its effect on the phagocytosis of C. neoformans by AMs through S1P receptor 2 (S1P2). Interestingly, loss of S1P2 caused a dramatic decrease in the mRNA levels of Fcγ receptors I (FcγRI), -II and -III. In conclusion, our data suggest that extracellular S1P increases antibody-mediated phagocytosis through S1P2 by regulating the expression of the phagocytic Fcγ receptors.
Introduction
Cryptococcus neoformans is a major cause of morbidity and mortality in individuals with an immunocompromised state, particularly among HIV-infected patients, as it is diagnosed in approximately 1 000 000 individuals per year and is responsible for an average of at least 600 000 deaths per year (Park et al., 2009). Upon environmental exposure, infectious C. neoformans propagules are inhaled and enter the host lungs where resident alveolar macrophages (AMs) can internalize the fungal cells to initiate the host immune response. As the central effector function of AMs, phagocytosis of C. neoformans by these phagocytes can lead to the killing of internalized fungal cells, induction of an inflammatory response and the development of a cell-mediated adapted immune response, which is required for host survival. However, C. neoformans is a facultative intracellular pathogen capable of surviving not only in the extracellular environment of the alveolar spaces but also intracellularly within AMs (Feldmesser et al., 2000). Thus, when AMs are unable to kill intracellular C. neoformans, internalization of C. neoformans is detrimental to the host as it provides a protective environment that promotes survival and can exacerbate the dissemination from the lung to other organs (Chrétien et al., 2002; Goldman et al., 2000; Kechichian et al., 2007; Levitz et al., 1999; Luberto et al., 2003; Rittershaus et al., 2006). Therefore, it is important to our understanding of the pathogenesis of C. neoformans to define the host factors modulating the effector functions of AMs, specifically phagocytosis, in order to develop new anticryptococcal therapeutic regiments.
As components of the sphingolipid biosythesis pathway in mammalian cells, sphingosine kinases 1 (SK1) and 2 (SK2) catalyse the phosphorylation of sphingosine to produce the bioactive lysophospholipid sphingosine-1-phosphate (S1P), which is well documented to regulate numerous facets of the immune system (Rivera et al., 2008). The SK1/S1P pathway is particularly important in macrophage function (Weigert et al., 2009) and greatly affects the immune response in the lungs (Garg et al., 2006; Jolly et al., 2002). Previously, we showed that SK1 has a key role in the formation of a granulomatous inflammation in response to pulmonary cryptococcosis (McQuiston et al., 2010). In particular, we found that SK1 is essential for preventing the dissemination of C. neoformans when the host and/or C. neoformans factors promote intracellular parasitism. These previous results indirectly indicate that the product of SK1 activity, S1P, affects the phagocytosis of C. neoformans by AMs.
S1P is an extracellularly secreted sphingolipid that evokes its effects on cells through its binding to a family of five G-protein-coupled receptors (S1P1–S1P5) located on the cell surface. S1P1 is universally expressed on immune cells and is well established to govern the chemotaxic effects of extracellular S1P (Rivera et al., 2008). In contrast, the expression profile of the other S1P receptors (S1PRs) is cell-type-dependent, and the effects on the functions of immune cells have not been elucidated. Extracellular S1P has been shown to have numerous effects on macrophages, such as modulating receptor expression (Duong et al., 2004), inducing a proinflammatory state (Hammad et al., 2008) and increasing the killing of internalized Mycobacterium species (Garg et al., 2004; Sali et al., 2009). In addition to these antibacterial actions, exogenous S1P increases antigen processing and presentation in Mycobacterium tuberculosis-infected AMs (Santucci et al., 2007). Currently, the identity of the S1PR(s) mediating the extrinsic actions of S1P on AMs is not known. Also unknown is whether extracellular S1P affects antimicrobial actions of AMs against C. neoformans.
In this study we examined the effects of extracellular S1P on the AMs–C. neoformans interaction and investigated which S1P receptor is involved in this interaction. We show that extracellular S1P increases the phagocytosis of C. neoformans by AMs through S1P2. Using S1P2−/− mice we found that AMs from these mice have decreased expression of Fcγ receptors and, thus, they ingest fewer C. neoformans compared with wild-type AMs. Taken together, these results suggest that the S1P–S1P2 interaction and the consequent Fcγ regulation are important for favouring phagocytosis of C. neoformans.
Methods
Mouse strains.
Five- to seven-week-old wild-type C57BL/6J mice (The Jackson Laboratory), SK1-deficient mice (SK1−/−) and S1P2-deficient mice (S1P2−/−) were used for this research. SK1−/− and S1P2−/− mice were generated previously and colonies were maintained as described previously (Allende et al., 2004; Kono et al., 2004). All mice were available to us through the Medical University of South Carolina Center of Biomedical Research Excellence (MUSC COBRE) Animal Core Facility, directed by Dr Toshihiko Kawamori, who provided breeding pairs for this study. Travis McQuiston performed all breeding, weaning and genotyping (data not shown). For all experiments, SK1−/− and S1P2−/− mice were age- and sex-matched with SK1/2+/+ wild-type mice (C57BL/6J). Both SK1−/− and S1P2−/− mice are isogenic to C57BL/6J mice. Wild-type C57BL/6J mice are also interchangeably called SK1/2+/+ or S1P2+/+ mice.
Isolation and cell culturing of AMs.
AMs were isolated from the lungs of mice using a 1× sterile PBS bronchoalveolar lavage (BAL). BAL fluid was subjected to centrifugation at 500 g for 10 min. Cell pellets were resuspended in serum-free RPMI supplemented with 0.1 % penicillin–streptomycin and cell number was determined by using a haematocytometer. For all co-incubation assays, 1×105 cells were plated on the glass portion of a poly-d-lysine-coated glass-bottomed confocal cell dish (MatTek Corporation). AMs were allowed to adhere for 30 min before the cell dishes were washed three times and fresh media was added. Afterwards, cells were incubated for an additional 90 min prior to experimentation.
C. neoformans strains and growth media.
C. neoformans var. grubii serotype A strain H99 is a facultative intracellular pathogen and is universally considered to be a wild-type strain of C. neoformans (WT). C. neoformans cells were grown in yeast extract–peptone–glucose (YPD) medium for 16–18 h at 30 °C in a shaking cell culture incubator.
Real-time reverse transcriptase (RT)-PCR.
mRNA was isolated from AMs using the RNeasy mini kit from Qiagen. cDNA was generated from 0.5 µg RNA using random hexamer primers using the SuperScript III first strand cDNA synthesis system from Invitrogen. Real-time RT-PCR was conducted using a Bio-Rad iCycler to quantify mRNA levels. The standard real-time RT-PCR volume was 25 µl, which comprised 12.5 µl SYBR Green PCR reagents (Bio-Rad), 5 µl cDNA template, 1 µl forward primer (4 µM), 1 µl reverse primer (4 µM) and 5.5 µl water. The sequences of primer pairs for SK isoforms and S1PRs, along with the RT-PCR steps for amplification, were described previously (Argraves et al., 2008; Snider et al., 2009; Xing et al., 2008). All reactions were performed in triplicate. Q-Gene software was used to analyse data, which were then expressed as fold-change mean of normalized expression from control value. As shown, the mean normalized expression is directly proportional to the amount of mRNA of the target gene relative to the amount of mRNA of the reference gene, β-actin. Melt curves were also examined to ensure that the data corresponded to production of the single desired RT-PCR fragment for each target gene. Data represent the average of three separate trials.
In vitro phagocytosis assay.
AMs were plated as described above and the desired C. neoformans strain was grown as noted. C. neoformans cultures were subjected to centrifugation at 500 g for 10 min. YPD media was removed and the cell pellet was resuspended in sterile H2O. This was repeated three times. After washing, C. neoformans cells were resuspended in the desired cell media, and the cell number was calculated using a haematocytometer. The volume corresponding to 1×106 C. neoformans cells was brought up to a total volume of 1 ml with RPMI containing either 10 % mouse serum or 10 µg anti-glucuronoxylomannan (GXM) monoclonal IgG1 antibody 18B7 ml−1 (kindly provided by Arturo Casadevall, Albert Einstein College of Medicine, Bronx, NY, USA) or both 10 % mouse serum and antibody. On the day the phagocytosis assay was conducted, mouse blood collected from the hearts of wild-type mice and sera was isolated. C. neoformans suspensions were vortexed vigorously and opsonized with complement, using the freshly isolated mouse serum, and/or with 18B7 antibody for 20 min at 37 °C. This length of time and serum percentage has been shown by several groups to allow for maximum inactive complement 3b (iC3b) binding and adequate for complement-mediated phagocytosis (Dromer et al., 1989; Kozel & Pfrommer, 1986; Levitz et al., 1997). After opsonization, C. neoformans cell suspensions were centrifuged, washed three times with serum-free RPMI and finally resuspended to a concentration of 1×106 C. neoformans cells ml−1. The medium from the confocal dishes containing the AMs was removed and replaced with 100 µl opsonized C. neoformans solution containing 1×105 C. neoformans cells, thereby making the m.o.i. 1 : 1. After 2 h co-incubation, the medium was removed, and the plates were washed three times with PBS, fixed in ice-cold methanol and stained with Giemsa stain for analysis by light microscopy using a 100× objective under oil immersion. For each confocal dish, a minimum of 500 macrophages was examined for C. neoformans internalization. As described in the literature, the phagocytic index is the percentage of macrophages with internalized fungal cells multiplied by the average number of internalized fungal cells (Bianco et al., 1975; Taborda & Casadevall, 2002). It is important to note that C. neoformans attachment to AMs was not calculated as part of the phagocytic indices.
In vitro C. neoformans intracellular growth assay.
The ability of internalized C. neoformans to grow in a co-culture with AMs was examined after 4 h. C. neoformans and AMs were plated and treated as described for the in vitro phagocytosis assay above. To determine the intracellular growth of C. neoformans, the medium from co-incubations was removed after 2 h, and plates were washed three times to remove any extracellular C. neoformans. Fresh medium was added for an additional 2 h. After a total of 4 h, plates were processed for light microscopy to allow for visualization of daughter cells, also known as ‘buds’. A minimum of 100 internalized C. neoformans per plate was inspected for budding, and intracellular growth was calculated as the percentage of internalized C. neoformans cells with buds in the observed population of internalized fungal cells.
Statistics.
Data from each experimental group were subjected to an analysis of normality and variance. Statistical significance between the means of two experimental datasets composed of normally distributed values was analysed using Student’s two-tailed t test. The two-way ANOVA was used when the analysis of the effects of two independent variable concurrently was required. For all statistical tests, sd with P-values less than 0.05 were considered significant.
Results
Effect of extracellular S1P on the phagocytosis and intracellular growth of C. neoformans
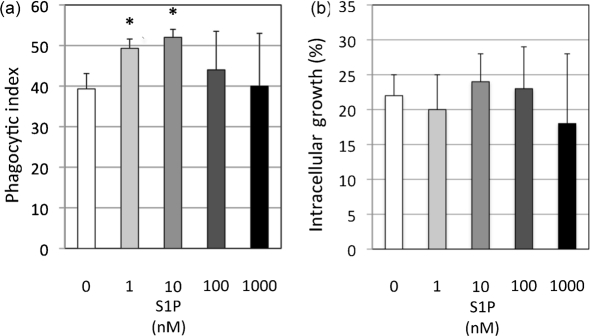
The quintessential effector function of AMs is the phagocytosis of microbes, as it is required for subsequent antimicrobial actions of these host phagocytes. Previous studies on Mycobacterium–AMs interaction led us to hypothesize that SK1 and S1P may have roles in the antimicrobial actions of AMs against C. neoformans. Therefore, to investigate if extracellular S1P affects the actions of AMs against the facultative intracellular pathogen C. neoformans, wild-type AMs and wild-type C. neoformans strain H99 were co-incubated in the presence of varying concentrations of S1P, and phagocytosis and intracellular growth of C. neoformans were analysed by light microscopy. The S1P concentrations used in this initial experiment have been demonstrated to induce optimal S1P receptor activation (Hla et al., 2001) and are physiologically relevant to the extracellular environment of the mouse lung (Ammit et al., 2001; Garg et al., 2006). C. neoformans cells were opsonized with only anti-GXM IgG1 antibody (Ab) 18B7 and the in vitro phagocytosis assay was conducted in serum-free conditions since serum possesses micromolar concentrations of S1P. Treatment with 1 nM and 10 nM S1P were observed to significantly increase the phagocytosis of C. neoformans (Fig. 1a). Greater concentrations of S1P (100 nM and 1 µM) did not significantly increase internalization, possibly due to saturation of the S1P–S1P receptor pathway. S1P increased the phagocytosis of the alveolar-macrophage-derived MH-S cell line and the peritoneal-derived J774.A cell line in nearly an identical pattern to that observed in primary AMs, suggesting the effect of S1P on the phagocytosis of C. neoformans is not exclusive to primary AMs (data not shown). Interestingly, treatment with exogenous S1P did not affect the growth of intracellular C. neoformans after 4 h (Fig. 1b), suggesting that S1P does not induce fungistatic or fungicidal actions of AMs against internalized C. neoformans as occurs in the Mycobacterium–macrophage interaction (Garg et al., 2004; Greco et al., 2010).
Fig. 1.
Extracellular S1P increases the phagocytosis of C. neoformans by AMs but does not affect C. neoformans intracellular growth. AMs from C57BL/6J wild-type mice were incubated with C. neoformans strain H99 cells opsonized with anti-GXM IgG antibody and complement at an m.o.i. of 1 : 1 in the presence of varying S1P concentrations to determine the effects of S1P on phagocytosis (a) and intracellular growth (b). (a) Co-cultures were stopped after 2 h by removing the media and fixing with ice-cold methanol. Cells were stained with Giesma stain and internalization of C. neoformans was determined using light microscopy. A minimum of 500 AMs was examined per co-culture to determine and calculate the phagocytic index. (b) To determine if S1P affects the intracellular growth of internalized C. neoformans, media was removed from co-cultures, the cultures were washed and fresh media containing the appropriate S1P concentration was added for an additional 2 h, at which time cells were stained with Giesma stain and C. neoformans budding was determined using light microscopy. A minimum of 100 internalized C. neoformans was examined per culture to determine and calculate intracellular growth (%). For both (a) and (b), data are the average from three separate experiments and error bars represent the sd. Student’s t-test was conducted to determine significance (*P<0.05) compared with untreated (0 nM S1P) AMs.
Internalization of C. neoformans by AMs occurs through a receptor-mediated phagocytosis
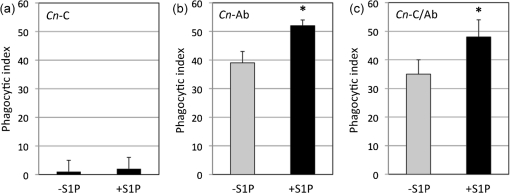
Although several different types of cell surface receptors recognize various fungal motifs (Levitz, 2002), C. neoformans internalization by host phagocytes requires opsonization. In Fig. 1(a), we found that S1P increased the phagocytosis of IgG1-Ab-opsonized C. neoformans. To determine if the S1P-induced increase in phagocytosis is dependent on the host molecule that serves as the opsonin, AMs were co-incubated with C. neoformans cells opsonized with complement, Ab or both complement and Ab with or without S1P. In our hands, AMs have a very low phagocytic index of C. neoformans when opsonized with complement only and, in this case, S1P treatment does not increase phagocytosis (Fig. 2a). On the other hand, S1P increases the internalization of C. neoformans cells when opsonized with Ab only or when opsonized with both complement and Ab (Fig. 2b and c). Notably, C. neoformans were not or very rarely internalized (less than 1 %) when they were not opsonized and the addition of S1P did not increase phagocytosis of non-opsonized fungal cells. Together, these results suggest that extracellular S1P increases C. neoformans internalization through an Ab-dependent mechanism.
Fig. 2.
Extracellular S1P increases phagocytosis in an antibody-dependent manner. AMs from C57BL/6J wild-type mice were incubated with C. neoformans strain H99 cells opsonized either with complement from mouse serum [C. neoformans-C (Cn-C)] (a), anti-GXM IgG1 antibody 18B7 [C. neoformans-Ab (Cn-Ab)] (b), or both C and Ab [C. neoformans-C/Ab (Cn-C/Ab)] (c) at an m.o.i. of 1 : 1 with or without S1P (10 nM). After 2 h co-incubation, co-cultures were processed and analysed for C. neoformans phagocytosis by AMs. A minimum of 500 AMs was examined per co-culture to determine and calculate phagocytic index. Data represent the average from three separate experiments and error bars represent the sd. Student’s t-test was conducted to determine significance (*P<0.05).
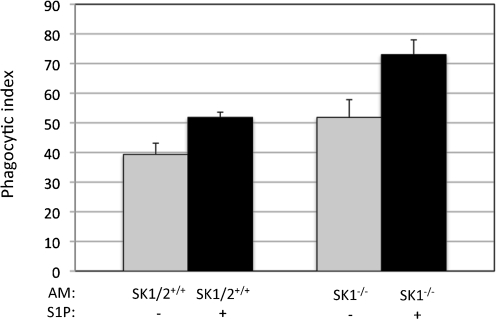
In a previous study, we showed that AMs isolated from mice lacking SK1 (SK1−/−) internalize a significantly greater number of C. neoformans cells compared with AMs from wild-type mice SK1/2+/+ (McQuiston et al., 2010), suggesting that a decrease of S1P due to lack of SK1 (please note that S1P is not totally absent in SK1−/− AMs because of the presence of SK2) enhances phagocytosis of C. neoformans by AMs. In the present study, we found that addition of extracellular S1P increases phagocytosis of C. neoformans by AMs. This apparent contradiction could be explained by considering that extracellular S1P regulates phagocytosis differently from intracellular S1P. To address this hypothesis, we sought to determine the effect of extracellular S1P on phagocytosis of C. neoformans in conditions in which the intracellular pool of S1P is significantly decreased using AMs from SK1−/− mice. Thus, AMs from SK1−/− or from SK1/2+/+ wild-type mice were co-incubated with C. neoformans in the presence of 10 nM S1P, which is the concentration of S1P we found to induce the greatest enhancement in C. neoformans phagocytosis. We observed extracellular S1P to increase the internalization of C. neoformans in both wild-type (SK1+/+) AMs and SK1-deficient AMs and, as previously observed (McQuiston et al., 2010), deficiency in SK1 increases phagocytosis (Fig. 3). Using the two-way ANOVA, we found that both S1P treatment and SK1 deficiency significantly increase phagocytosis (Fig. 3). Intriguingly, the interaction between extracellular S1P and SK1 deficiency is also significant. These data suggest that extracellular S1P modulates the phagocytosis of C. neoformans by AMs through an SK1-indepedent mechanism but extracellular S1P and SK1 affect the actions of each other on C. neoformans internalization.
Fig. 3.
Extracellular S1P increases the phagocytosis of C. neoformans by AMs independent of SK1. AMs from C57BL/6J wild-type (SK1/2+/+) and SK1-deficient (SK1−/−) mice were incubated with C. neoformans strain H99 cells at an m.o.i. of 1 : 1 in the presence of 10 nM S1P to determine if the S1P-induced increase on phagocytosis requires SK1. Co-cultures were stopped after 2 h by removing the media and fixing with ice-cold methanol. Cells were stained with Giesma stain, and internalization of C. neoformans was determined using light microscopy. A minimum of 500 AMs was examined per co-culture to determine and calculate phagocytic index. Data are the average from three separate experiments. Two-way ANOVA was conducted to determine significance of SK1 and S1P treatment. The effects of S1P and SK1 on phagocytosis are significant (P<0.0002 SK1/2+/+ compared with SK1−/− and P<0.00001 no S1P compared with S1P treatment). The interactions between S1P treatment compared with SK1 deficiency are also significant (P<0.02).
Identifying the S1PR(s) mediating the effects of extracellular S1P on AMs
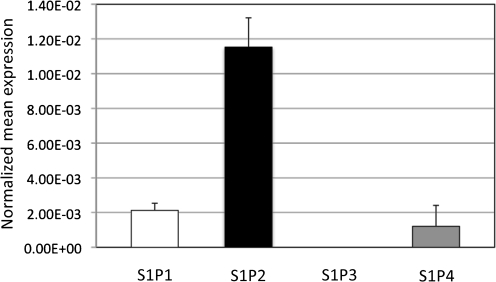
Human and mouse monocytes and macrophages have been shown to express only S1P1 and S1P2 under in vitro conditions (Rivera et al., 2008). However, since S1PR expression profiles on monocytes and macrophages are greatly influenced by differentiation, activation and microenvironmental factors, such as extracellular S1P levels (Duong et al., 2004), it should not be assumed that all macrophages possess the same S1PR profile. Therefore, it was imperative to determine the S1P expression repertoire of each macrophage cell type under specific experimental conditions in order to resolve the S1P–S1PR pathway mediating the effects of extracellular S1P. For this, mRNA from WT AMs was isolated and S1P receptor mRNA levels were analysed using real-time RT-PCR. It is important to note that, unlike other reports in which the expression of S1PR in AMs was examined upon incubation in serum-containing media (which contains S1P) (Hornuss et al., 2001), in this study the S1P expression profiles were assessed in freshly isolated AMs incubated in serum-free media in order to preserve the in vivo conditions of the alveolar environment, in which serum is not present. Fig. 4 shows that AMs from WT mice express S1P1, S1P2 and S1P4. S1P2 was expressed approximately sixfold more than S1P1, making it the prevalent S1PR expressed by AMs under these conditions. S1P4 was inconsistently expressed between trials, as it was poorly detected.
Fig. 4.
Expression of S1P receptors on mouse AMs. AMs were isolated and pooled together from four C57BL/6J (wild-type) mice. RNA was extracted from these AMs, and real-time RT-PCR was conducted to determine the S1P receptor expression profile. All values were normalized against the expression of a reference gene, β-actin. Data are the average from three separate experiments.
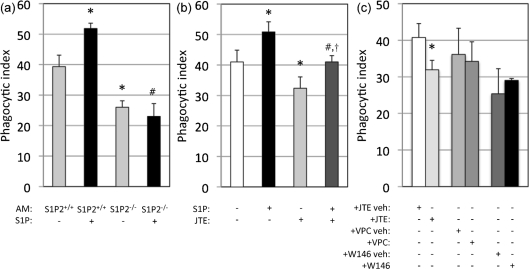
Since S1P2 was found to be the predominant S1P receptor expressed by AMs, and previous research has implicated S1P2 in regulating effector functions of innate immune cells (Jolly et al., 2004), we hypothesized that extracellular S1P activates S1P2 on the cell surface of AMs to initiate signalling cascades that enhance phagocytosis. To investigate this hypothesis, AMs from WT (S1P2+/+) and S1P2-deficient (S1P2−/−) mice were co-incubated with wild-type C. neoformans strain H99 opsonized with anti-GXM 18B7 Ab in the presence of 10 nM S1P for 2 h and analysed for phagocytosis. Untreated S1P2-deficient AMs had significantly decreased phagocytosis of C. neoformans compared with untreated S1P2+/+ AMs (39.3± 3.8 versus 24.5± 2.12, P<0.01) (Fig. 5a). Additionally, in contrast with S1P2+/+, S1P treatment did not increase the internalization of C. neoformans by S1P2−/− AMs (51.8 ± 1.8 versus 24.5 ± 7.7, P<0.01) (Fig. 5a). To further corroborate the S1P2 involvement, S1P2+/+ AM–C. neoformans co-cultures were treated with the S1P2 antagonist N-(2,6-dichloro-4-pyridinyl)-2-[1,3-dimethyl-4-(1-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-hydrazinecarboxamide (JTE-013) and analysed for phagocytosis. JTE-13 is a pyrazopyridine derivative that specifically binds to S1P2 to prevent the extrinsic effects of S1P (Ikeda et al., 2003; Osada et al., 2002). Several concentrations of JTE-013 were used to determine if this S1P2-specific antagonist affects phagocytosis in our model and to determine the concentration with the greatest inhibitory effect (data not shown). We found that 1 µM JTE-013 treatment significantly decreased phagocytosis of C. neoformans by S1P2+/+ AMs (Fig. 5b). Importantly, the addition of S1P to AMs at the same time as JTE-013 treatment induced a slight but statistically insignificant increase in phagocytosis compared with AMs treated with JTE-013 alone, thereby demonstrating that antagonist partially inhibits the effects of S1P specifically through S1P2. Furthermore, AMs treated with 1 µM JTE-013 and 10 nM S1P at the same time had a phagocytic index nearly identical to untreated AMs (40.7 ± 3.8 versus 40.8 ± 2.5), (Fig. 5b). Therefore, by using both S1P2-deficient AMs and an S1P2-specific antagonist, these data show that extracellular S1P mediates its effect on the phagocytosis of C. neoformans by AMs through S1P2.
Fig. 5.
S1P2 mediates the phagocytosis of C. neoformans by AMs. S1P1/2+/+ and S1P2−/− AMs were incubated with C. neoformans strain H99 opsonized with the anti-GXM antibody 18B7 at an m.o.i. of 1 : 1 with or without 10 nm S1P to determine if S1P increases phagocytosis through S1P2 (a). Student’s t-test was conducted to determine significance (*P<0.01 compared with S1P1/2+/+ AMs without S1P; †P<0.005 compared with S1P1/2+/+ AMs with S1P). (b) Wild-type (S1P1/2+/+) AMs were co-incubated with 18B7-opsonized C. neoformans strain H99 cells in the presence of the S1P2 antagonist JTE-013 (1 µM) with and without S1P (10 nM). Student’s t-test was conducted to determine significance (*P<0.01 compared with S1P1/2+/+ AMs without S1P or JTE-013; †P<0.01 compared with S1P1/2+/+ AMs with S1P; ‡P>0.05, compared with SIP1/2+/+ AMs with JTE-013). (c) Wild-type (S1P1/2+/+) AMs were co-incubated with C. neoformans strain H99 cells opsonized with the anti-GXM antibody 18B7 in the presence of either vehicle only (+veh) the S1P2 antagonist JTE-013 (1 µM), the S1P1/3 antagonist VPC 23019 (10 µM) or the S1P1 antagonist W146 (10 µM). Note that the vehicle for each antagonist was different. Student’s t-test was conducted to determine significance (*P<0.05 compared with the applicable vehicle only). For all experiments, co-incubations were stopped after 2 h by removing the media and fixing with ice-cold methanol. Phagocytic indices were determined using light microscopy to determine C. neoformans internalization. Data are the average from three separate experiments.
On the other hand, AMs also express S1P1 (Fig. 4) and, therefore, it is possible that S1P1 may participate in the modulation of phagocytosis. Studies have shown that S1P1 and S1P2 have opposing functions and even negatively regulate the activation of each other (Jolly et al., 2004; Okamoto et al., 2000). To determine if S1P1 affects the observed increase in phagocytosis following S1P treatment, S1P2+/+ AMs were treated with either the S1P1 antagonist W146 or VPC 23901. A set of control AMs was treated with the specific vehicle solution used to resuspend each antagonist. In contrast with JTE-013 treatment, neither W146 nor VPC 23901 affected the phagocytosis of C. neoformans by wild-type AMs (Fig. 5c). Additional concentrations of these antagonists were also examined, and similar results (e.g. no effect on phagocytosis) were obtained (data not shown). The variation in the phagocytic index of control AMs (e.g. AMs treated with vehicle solution alone) is most likely to be due to the differences in the vehicle solutions, as the manufacturer’s directions of each compound required a different concentration of DMSO for reconstitution.
Effect of extracellular S1P on the Fcγ receptor (FcγR) cell surface expression of AMs
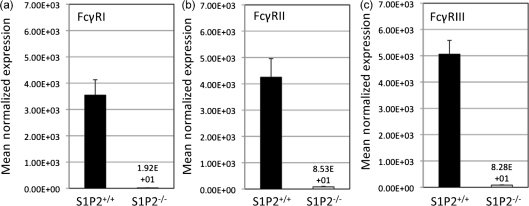
The data presented here show that S1P2 regulates internalization of Ab-opsonized C. neoformans by AMs. The mechanism by which S1P2 affects phagocytosis of C. neoformans under these conditions is unknown. Duong et al. (2004) examined the effects of lysophospholipids on human monocyte-derived macrophages and reported that S1P induces mRNA levels and cell-surface expression of FcγRII, thereby implicating S1P in the regulation of phagocytic receptors recognizing Abs (Duong et al., 2004). In light of the data presented here, it was hypothesized that S1P binds to S1P2 to initiate signalling cascades governing FcγR expression on the cell surface of AMs, thereby modulating the phagocytosis of Ab-opsonized C. neoformans. Since antibodies specific to each FcγR isoform (e.g. due to high level of homology between FcγII and FcγIII) are not commercially available, we examined the level of FcγR mRNA in AMs from S1P2+/+ and S1P2−/− mice to address this hypothesis. The deficiency of S1P2 resulted in a dramatically decreased mRNA levels of FcγRI, -II and -III (Fig. 6). Thus, the data presented here suggest that S1P2 affects internalization of Ab-opsonized C. neoformans by modulating the expression of the phagocytic FcγRs.
Fig. 6.
S1P2-deficient AMs have decreased FcγR expression. AMs were isolated and pooled together from four S1P2+/+ (wild-type) and S1P2−/− mice. RNA was extracted from these AMs and real-time RT-PCR was conducted to determine whether the presence of S1P2 affects FcγR expression profile. All values were normalized against the expression of a reference gene, β-actin. Data are the average from three separate experiments.
Effect of S1P2 on host susceptibility to cryptococcosis
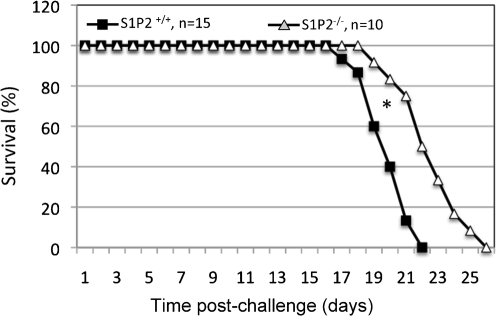
To determine if S1P2 affects host susceptibility to C. neoformans infection, S1P2+/+ and S1P2−/− mice were intranasally challenged with highly virulent wild-type C. neoformans strain H99 and monitored for their susceptibility to cryptococcosis. Interestingly, S1P2−/− mice survived significantly longer than S1P2+/+ mice (22.5±2.1 days versus 19.8±1.5 days, P<0.001) (Fig. 7). Along with our data showing that S1P2 modulates the internalization of C. neoformans by AMs in vivo, these data demonstrate that the deficiency in S1P2 provides protection against cryptococcosis.
Fig. 7.
S1P2 increases host susceptibility to crypotococcosis. Wild-type C57BL/6J (S1P1/2+/+) (n = 15) and S1P2-deficient (S1P/2−/−) (n = 10) mice were challenged intranasally with 5×105 C. neoformans strain H99 cells. Mice were monitored and closely inspected twice a day. Using the Wilcoxon rank-sum test, S1P1/2−/− mice were significantly less susceptible than S1P1/2+/+ mice to C. neoformans infections (22.4±2.0 days compared with 19.5±1.4 days; *P<0.001).
Discussion
In this study, we show that extracellular S1P mediates the phagocytosis of the pathogenic fungus C. neoformans by AMs through S1P2. Furthermore, we present data suggesting that S1P2 affects Ab-dependent phagocytosis by modulating FcγR expression.
Unlike previously published studies examining the effects of extracellular S1P on the antimicrobial actions of AMs against internalized Mycobacterium species (Garg et al., 2004; Sali et al., 2009), treatment with S1P did not affect the intracellular growth of C. neoformans within AMs. This is not necessarily surprising since C. neoformans, in contrast with Mycobacterium, does not actively prevent the acidification or maturation of the phagosome (Feldmesser et al., 2000; Levitz et al., 1999), a process that in Mycobacterium-infected AMs is indeed controlled by S1P (Garg et al., 2004; Kusner, 2005; Malik et al., 2000; Thompson et al., 2005). In addition, C. neoformans possesses virulence factors enabling internalized C. neoformans cells to survive within this microbicidal intracellular environment (Cox et al., 2001, 2003; Shea et al., 2006; Wright et al., 2007; Zaragoza et al., 2008).
The results presented here show that exogenous (i.e. extracellular) S1P increases the phagocytosis of C. neoformans by AMs whereas our previous studies showed that SK1 decreases it (McQuiston et al., 2010). One plausible explanation is that intracellular S1P produced by SK1 mediates phagocytosis through a different signalling pathway than that initiated upon extracellular S1P ligation to S1PRs. This is supported by the observation that SK1 seems not to be required for the action of extracellular S1P since addition of extracellular S1P increases phagocytosis of C. neoformans in AMs in which SK1 is deleted (SK1−/− AMs) (Fig. 3).
The use of appropriate and physiological concentrations of S1P is also very important for studying its effect on biological systems. For instance, in studies examining the extrinsic effects of S1P on AMs (Garg et al., 2004, 2006; Hammad et al., 2008; Hughes et al., 2008; Jiang et al., 2007; Santucci et al., 2007), the concentrations of S1P ranged from 0.5 to 50 µM S1P and/or were conducted in the presence of serum, which has an S1P concentration of 0.4–1.1 µM (Yatomi et al., 1997). With the exception of the blood, in which S1P concentration ranges from 1 to 4 µM, the S1P content in all other tissue ranges from 0.5 to 75 pmol (mg tissue)−1 (Allende et al., 2004; Edsall & Spiegel, 1999; Schwab et al., 2005). S1P concentration in the mucosal airway surface, as measured in BAL fluid, from healthy patients has been shown to range from 2 to 4 nM (Ammit et al., 2001). Antigen challenge significantly increases S1P levels in the BAL fluid from asthmatic patients to concentrations ranging between 8 and 14 nM (Ammit et al., 2001). In addition, the S1P concentration in the BAL fluid from Mycobactrium tuberculosis-infected patients has been shown to have a range of 47.5±36.2 nM (Garg et al., 2006). Thus, previous studies conducted in the presence of micromolar concentrations of S1P may not represent how S1P modulates the actions of resident AMs, since, in non-blood tissues, S1P concentrations are in the nanomolar range. The high affinities of the S1PRs, which range from 2 to 63 nM (Kd values) (Hla et al., 2001), suggest that S1P can evoke its effects on cells and in tissue with low S1P content. The research presented here shows that similar S1P concentrations to the ones present in the extracellular spaces of the lung can modulate the phagocytosis of C. neoformans by AMs through S1P2. To our knowledge, this represents the first report showing the ability of extracellular S1P to regulate microbial phagocytosis through an S1PR.
In this research, we show that S1P treatment increases AM phagocytosis of C. neoformans by approximately 30–40 % (Fig. 3). Although this effect on phagocytosis may seem modest, this increase in C. neoformans internalization may have a larger role in the antimicrobial actions of macrophages required to contain cryptococcal infection. C. neoformans cells evoke AMs to secrete chemokines and cytokines to recruit other inflammatory cells, which further enhance the antimicrobial actions of AMs through cytokines and, ultimately, result in the development of an adaptive immune response that controls the cryptococcal infection (Abe et al., 1998; Buchanan & Murphy, 1997; Vecchiarelli et al., 1994; Voelz et al., 2009). Intravenous administration of S1P decreases disease severity and bacterial burden of macrophages during Mycobacterium infections, suggesting that S1P could also modulate the host inflammatory response to C. neoformans (Garg et al., 2004, 2006; Sali et al., 2009). The effects of exogenous S1P on cryptococcosis were not examined in this research but the role of S1P and its respective receptors, particularly S1P1 and S1P2, in cryptococcosis should be examined in future studies.
Research studies addressing S1P and S1P2 suggest that S1P1 and S1P2 may have opposing functions in controlling host immune cells. S1P1 expression is required for immune cell egress from lymphoid tissues (low nanomolar S1P concentrations) into the blood stream (micromolar concentrations) (Chiba, 2005; Singer et al., 2005; Vora et al., 2005). In contrast, binding of extracellular S1P to S1P2 in mast cells results in the inhibition of cell migration through the activation of the small GTPase Rho, which negatively regulates S1P1-induced Rac activity (Okamoto et al., 2000; Yokoo et al., 2004). Furthermore, upregulation of S1P2 expression in mast cells following FcγRI cross-linking via immunoglobulin E inhibits cell migration (Jolly et al., 2004). These conflicting actions are also observed in terms of the inflammatory state of macrophages. Hughes et al. (2008) have shown that S1P binds to S1P1 and prevents the proinflammatory stimulus of lipopolysaccharide on mouse peritoneal macrophages. Extracellular S1P increases the levels of pro-inflammatory molecules COX-2 and PGE2 in the mouse monocyte/macrophage RAW264.7 cell line (Hammad et al., 2008). Similarly, S1P binding to S1P2 increased cAMP levels in PGE2- and isopreteronol-stimulated RAW264.7 cells (Jiang et al., 2007). But, since the expression pattern of the S1P receptors ultimately determines the net effect of S1P–S1P2 interaction (Okamoto et al., 2000; Yokoo et al., 2004), the ability of S1P to evoke either an anti-inflammatory or pro-inflammatory state in macrophages may depend on the S1P1 : S1P2 expression ratio. In this research, AMs were found to express S1P2 at a sixfold greater level than S1P1, and S1P increases C. neoformans phagocytosis. Thus, the research presented here further suggests that the relative ratio of the S1PRs may be the major factor deciding the effect of extracellular S1P.
S1P increased internalization of C. neoformans cells by AMs only when the fungal cells were opsonized with Ab, specifically an IgG1 molecule targeting capsular GXM. We hypothesized that the binding of extracellular S1P to S1P2 of AMs may augment C. neoformans internalization by modulating the cell-surface expression of FcγRs on AMs. Macrophages express three FcγRs (FcγRI, -II and -III) capable of binding IgG1 molecules to illicit an effector function. The activating FcγRI is a high-affinity receptor for IgG1 (Kd 1×10−9) that can bind to monomeric IgG1 and IgG1 immune complexes while FcγRII and -III are considered low-affinity receptors for IgG1 and, therefore, can only bind to IgG1 immune complexes (Daëron, 1997; Nimmerjahn et al., 2005). This hypothesis of S1P augmenting phagocytosis through modulation of FcγR expression was also suggested in the previous work by Duong et al. (2004), in which they showed, using a GeneChip assay, that S1P treatment affects expression levels of FcγRII isoforms in human differentiated macrophages. The mechanism by which extracellular S1P regulates the Fcγ receptor expression is not known and our results suggest that this regulation occurs through S1P2.
Human FcγRIIa is an activatory isoform homologous to mouse FcγRIII while human FcγRIIb is an inhibitory isoform homologous to mouse FcγRII. Since the relative expression levels of the activatory FcγRI and -III compared with the inhibitory FcγRII determine if phagocytosis occurs (Ravetch & Bolland, 2001), S1P2 could promote phagocytosis by triggering signalling cascades that either increase the expression of FcγRI and/or FcγRIII or decrease the expression of FcγRII. It is important to note that, although FcγRII and FcγRIII are both considered low-affinity IgG1 receptors compared with the high affinity of FcγRI for IgG1 (Kd 1×10−9), the inhibitory FcγRII has a tenfold greater affinity for IgG1 immune complexes than activatory FcγRIII (Kd 3.01×10−7 versus Kd 3.2×10−6) (Nimmerjahn et al., 2005). Therefore, modest alteration in expression of the inhibitory FcγRII compared with the activatory FcγRIII could result in a much more pronounced change in the activation threshold and, subsequently, effector cell responses of AMs. Additional investigations are needed to elucidate the molecular mechanism by which S1P2 affects FcγR expression.
It is important to note that even if the effect of S1P on phagocytosis is dependent on the ligation of IgG-opsonized C. neoformans with FcγRs, it is possible that other receptors are also affected by S1P treatment and contribute to the S1P-induced increase in C. neoformans internalization. Complement receptors 3 (CR3) and 4 (CR4) can internalize Ab-opsonized C. neoformans in a complement-independent mechanism (Taborda & Casadevall, 2002). Both antibodies against CR3 and CR4 were shown to significantly decrease phagocytosis of IgG1-opsonized C. neoformans by macrophages (Taborda & Casadevall, 2002). The effect of S1P on the phagocytosis of C. neoformans opsonized only with IgG1 was examined in this research since IgG1 serves as an excellent opsonin for macrophage phagocytosis (Mukherjee et al., 1995, 1996; Netski & Kozel, 2002), shows therapeutic potential in mouse models of C. neoformans (Feldmesser & Casadevall, 1997; Mukherjee et al., 1994; Shapiro et al., 2002) and is produced during cryptococcosis in humans (Abadi & Pirofski, 1999; Deshaw & Pirofski, 1995). However, similar to IgG, IgM that is innately produced by naïve mature B cells (prior to activation and immunoglobulin class switching) is also recognized by CR3 and CR4 in a complement-independent mechanism (Taborda & Casadevall, 2002). Interestingly, though, hyper-IgM syndrome increases susceptibility to crypotococcosis (Escárcega-Barbosa et al., 2002; Jo et al., 2002). Thus, other Ig classes, particularly IgM, may be an important link between the innate and adaptive immune system during cryptococcosis, affecting disease outcome. Several cell-surface receptors expressed by macrophages, including CD14, TLR2, TLR4 and CD18, that recognize C. neoformans capsular GXM do not work independently to internalize C. neoformans but rather modulate the phagocytosis and other effector functions as co-receptors and/or by initiating various signalling cascades (Levitz, 2002; Monari et al., 2005; Yauch et al., 2005). Thus, these receptors could act in concert with FcγRs or independently to affect C. neoformans internalization by AMs.
The intravenous administration of S1P to Mycobacterium-challenged mice during the acute phase of infection decreases Mycobacterium organ burden, improves lung histopathology and reduces dissemination and disease progression (Garg et al., 2004; Sali et al., 2009). However, how a systemic treatment with this bioactive molecule enhances the localized host immune response in the lungs is unknown. The studies on Mycobacterium presuppose S1PR involvement in virulence but that assumption was not investigated. When we tested the susceptibility of S1P2−/− mice to C. neoformans we found, surprisingly, that they survived longer compared with S1P2+/+ wild-type mice (Fig. 7). Since S1P2 is expressed in many different immune cells, it is very difficult to conclude that the specific role of S1P2 in virulence is due to its regulation of phagocytosis of C. neoformans by AMs. For instance, activation of S1P2 increases the vascular permeability of lung endothelial cells while antagonist inhibition using JTE-013 improved barrier integrity (Sanchez et al., 2007). Therefore, the deficiency in S1P2 may decrease the ability of C. neoformans cells to pass through the endothelial lining of the lungs to enter the bloodstream and cause disseminated disease. Thus, even though less phagocytosis by AMs occurs in the lungs of S1P2−/− mice, this host may be less permissive to fungal dissemination in the lung tissue than S1P2+/+ mice.
In conclusion, our studies suggest that extracellular S1P mediates phagocytosis of Ab-opsonized C. neoformans by AMs through an S1P2-dependent mechanism.
Acknowledgements
We thank all members of Dr Del Poeta’s and Dr Luberto’s laboratories for helpful and constructive discussion. This work was supported by National Institutes of Health ROI grants AI56168 and AI71142 (to M. D. P.) and was conducted in a facility constructed with support from the National Institutes of Health, grant no. C06 RR015455 from the Extramural Research Facilities Program of the National Center for Research Resources. T. M. was supported by the Graduate Assistance in Areas of National Need (GAANN) training grant in ‘Lipidology and New Technologies’ (to M. D. P.) from the United States Department of Education. The data in this paper are from a dissertation submitted by T. M. in partial fulfilment of the requirements for the degree of doctor of philosophy in the Department of Biochemistry, Medical University of South Carolina, Charleston, SC. M. D. P. is a Burroughs Wellcome New Investigator in Pathogenesis of Infectious Diseases.
Abbreviations:
- Ab
antibody
- AM
alveolar macrophage
- BAL
bronchoalveolar lavage
- GXM
glucuronoxylomannan
- JTE-013
N-(2,6-dichloro-4-pyridinyl)-2-[1,3-dimethyl-4-(1-methylethyl)-1H-pyrazolo[3,4-b]pyridin-6-yl]-hydrazinecarboxamide
- RT
reverse transcriptase
- S1P
sphingosine-1-phosphate
- S1PR
S1P receptor
- SK
sphingosine kinase
Footnotes
Edited by: J. Pla
References
- Abadi J., Pirofski L. (1999). Antibodies reactive with the cryptococcal capsular polysaccharide glucuronoxylomannan are present in sera from children with and without human immunodeficiency virus infection. J Infect Dis 180, 915–919. 10.1086/314953. [DOI] [PubMed] [Google Scholar]
- Abe K., Yoshinaga M., Ishimatsu Y., Iwashita T., Matsubara Y., Maesaki S., Tomono K., Kadota J., Kohno S. (1998). [Cytokines produced by cells in bronchoalveolar lavage fluid from a patient with primary pulmonary cryptococcosis]. Nihon Kokyuki Gakkai Zasshi 36, 299–305 (in Japanese). [PubMed] [Google Scholar]
- Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., et al. (2004). Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J Biol Chem 279, 52487–52492. 10.1074/jbc.M406512200. [DOI] [PubMed] [Google Scholar]
- Ammit A. J., Hastie A. T., Edsall L. C., Hoffman R. K., Amrani Y., Krymskaya V. P., Kane S. A., Peters S. P., Penn R. B., et al. (2001). Sphingosine 1-phosphate modulates human airway smooth muscle cell functions that promote inflammation and airway remodeling in asthma. FASEB J 15, 1212–1214. [DOI] [PubMed] [Google Scholar]
- Argraves K. M., Gazzolo P. J., Groh E. M., Wilkerson B. A., Matsuura B. S., Twal W. O., Hammad S. M., Argraves W. S. (2008). High density lipoprotein-associated sphingosine 1-phosphate promotes endothelial barrier function. J Biol Chem 283, 25074–25081. 10.1074/jbc.M801214200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco C., Griffin F. M., Jr, Silverstein S. C. (1975). Studies of the macrophage complement receptor. Alteration of receptor function upon macrophage activation. J Exp Med 141, 1278–1290. 10.1084/jem.141.6.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan K. L., Murphy J. W. (1997). Kinetics of cellular infiltration and cytokine production during the efferent phase of a delayed-type hypersensitivity reaction. Immunology 90, 189–197. 10.1046/j.1365-2567.1997.00144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K. (2005). FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther 108, 308–319. 10.1016/j.pharmthera.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Chrétien F., Lortholary O., Kansau I., Neuville S., Gray F., Dromer F. (2002). Pathogenesis of cerebral Cryptococcus neoformans infection after fungemia. J Infect Dis 186, 522–530. 10.1086/341564. [DOI] [PubMed] [Google Scholar]
- Cox G. M., McDade H. C., Chen S. C., Tucker S. C., Gottfredsson M., Wright L. C., Sorrell T. C., Leidich S. D., Casadevall A., et al. (2001). Extracellular phospholipase activity is a virulence factor for Cryptococcus neoformans. Mol Microbiol 39, 166–175. 10.1046/j.1365-2958.2001.02236.x. [DOI] [PubMed] [Google Scholar]
- Cox G. M., Harrison T. S., McDade H. C., Taborda C. P., Heinrich G., Casadevall A., Perfect J. R. (2003). Superoxide dismutase influences the virulence of Cryptococcus neoformans by affecting growth within macrophages. Infect Immun 71, 173–180. 10.1128/IAI.71.1.173-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daëron M. (1997). Structural bases of FcγR functions. Int Rev Immunol 16, 1–27. 10.3109/08830189709045701. [DOI] [PubMed] [Google Scholar]
- Deshaw M., Pirofski L. A. (1995). Antibodies to the Cryptococcus neoformans capsular glucuronoxylomannan are ubiquitous in serum from HIV+ and HIV− individuals. Clin Exp Immunol 99, 425–432. 10.1111/j.1365-2249.1995.tb05568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromer F., Perronne C., Barge J., Vilde J. L., Yeni P. (1989). Role of IgG and complement component C5 in the initial course of experimental cryptococcosis. Clin Exp Immunol 78, 412–417. [PMC free article] [PubMed] [Google Scholar]
- Duong C. Q., Bared S. M., Abu-Khader A., Buechler C., Schmitz A., Schmitz G. (2004). Expression of the lysophospholipid receptor family and investigation of lysophospholipid-mediated responses in human macrophages. Biochim Biophys Acta 1682, 112–119. [DOI] [PubMed] [Google Scholar]
- Edsall L. C., Spiegel S. (1999). Enzymatic measurement of sphingosine 1-phosphate. Anal Biochem 272, 80–86. 10.1006/abio.1999.4157. [DOI] [PubMed] [Google Scholar]
- Escárcega-Barbosa D., Ortiz-Jiménez M. P., Juárez-García J., Miranda-Feria A. J. (2002). [Hyper-IgM syndrome: mucocutaneous lesions and neutropenia]. Rev Alerg Mex 49, 57–59 (in Spanish). [PubMed] [Google Scholar]
- Feldmesser M., Casadevall A. (1997). Effect of serum IgG1 to Cryptococcus neoformans glucuronoxylomannan on murine pulmonary infection. J Immunol 158, 790–799. [PubMed] [Google Scholar]
- Feldmesser M., Kress Y., Novikoff P., Casadevall A. (2000). Cryptococcus neoformans is a facultative intracellular pathogen in murine pulmonary infection. Infect Immun 68, 4225–4237. 10.1128/IAI.68.7.4225-4237.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S. K., Volpe E., Palmieri G., Mattei M., Galati D., Martino A., Piccioni M. S., Valente E., Bonanno E., et al. (2004). Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J Infect Dis 189, 2129–2138. 10.1086/386286. [DOI] [PubMed] [Google Scholar]
- Garg S. K., Santucci M. B., Panitti M., Pucillo L., Bocchino M., Okajima F., Bisen P. S., Saltini C., Fraziano M. (2006). Does sphingosine 1-phosphate play a protective role in the course of pulmonary tuberculosis? Clin Immunol 121, 260–264. 10.1016/j.clim.2006.09.002. [DOI] [PubMed] [Google Scholar]
- Goldman D. L., Lee S. C., Mednick A. J., Montella L., Casadevall A. (2000). Persistent Cryptococcus neoformans pulmonary infection in the rat is associated with intracellular parasitism, decreased inducible nitric oxide synthase expression, and altered antibody responsiveness to cryptococcal polysaccharide. Infect Immun 68, 832–838. 10.1128/IAI.68.2.832-838.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco E., Santucci M. B., Sali M., De Angelis F. R., Papi M., De Spirito M., Delogu G., Colizzi V., Fraziano M. (2010). Natural lysophospholipids reduce Mycobacterium tuberculosis-induced cytotoxicity and induce anti-mycobacterial activity by a phagolysosome maturation-dependent mechanism in A549 type II alveolar epithelial cells. Immunology 129, 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad S. M., Crellin H. G., Wu B. X., Melton J., Anelli V., Obeid L. M. (2008). Dual and distinct roles for sphingosine kinase 1 and sphingosine 1 phosphate in the response to inflammatory stimuli in RAW macrophages. Prostaglandins Other Lipid Mediat 85, 107–114. 10.1016/j.prostaglandins.2007.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hla T., Lee M. J., Ancellin N., Paik J. H., Kluk M. J. (2001). Lysophospholipids–receptor revelations. Science 294, 1875–1878. 10.1126/science.1065323. [DOI] [PubMed] [Google Scholar]
- Hornuss C., Hammermann R., Fuhrmann M., Juergens U. R., Racké K. (2001). Human and rat alveolar macrophages express multiple EDG receptors. Eur J Pharmacol 429, 303–308. 10.1016/S0014-2999(01)01329-2. [DOI] [PubMed] [Google Scholar]
- Hughes J. E., Srinivasan S., Lynch K. R., Proia R. L., Ferdek P., Hedrick C. C. (2008). Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res 102, 950–958. 10.1161/CIRCRESAHA.107.170779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H., Satoh H., Yanase M., Inoue Y., Tomiya T., Arai M., Tejima K., Nagashima K., Maekawa H., et al. (2003). Antiproliferative property of sphingosine 1-phosphate in rat hepatocytes involves activation of Rho via Edg-5. Gastroenterology 124, 459–469. 10.1053/gast.2003.50049. [DOI] [PubMed] [Google Scholar]
- Jiang L. I., Collins J., Davis R., Lin K. M., DeCamp D., Roach T., Hsueh R., Rebres R. A., Ross E. M., et al. (2007). Use of a cAMP BRET sensor to characterize a novel regulation of cAMP by the sphingosine 1-phosphate/G13 pathway. J Biol Chem 282, 10576–10584. 10.1074/jbc.M609695200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo E. K., Kim H. S., Lee M. Y., Iseki M., Lee J. H., Song C. H., Park J. K., Hwang T. J., Kook H. (2002). X-linked hyper-IgM syndrome associated with Cryptosporidium parvum and Cryptococcus neoformans infections: the first case with molecular diagnosis in Korea. J Korean Med Sci 17, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly P. S., Rosenfeldt H. M., Milstien S., Spiegel S. (2002). The roles of sphingosine-1-phosphate in asthma. Mol Immunol 38, 1239–1245. 10.1016/S0161-5890(02)00070-6. [DOI] [PubMed] [Google Scholar]
- Jolly P. S., Bektas M., Olivera A., Gonzalez-Espinosa C., Proia R. L., Rivera J., Milstien S., Spiegel S. (2004). Transactivation of sphingosine-1-phosphate receptors by FcϵRI triggering is required for normal mast cell degranulation and chemotaxis. J Exp Med 199, 959–970. 10.1084/jem.20030680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kechichian T. B., Shea J., Del Poeta M. (2007). Depletion of alveolar macrophages decreases the dissemination of a glucosylceramide-deficient mutant of Cryptococcus neoformans in immunodeficient mice. Infect Immun 75, 4792–4798. 10.1128/IAI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kono M., Mi Y., Liu Y., Sasaki T., Allende M. L., Wu Y. P., Yamashita T., Proia R. L. (2004). The sphingosine-1-phosphate receptors S1P1, S1P2, and S1P3 function coordinately during embryonic angiogenesis. J Biol Chem 279, 29367–29373. 10.1074/jbc.M403937200. [DOI] [PubMed] [Google Scholar]
- Kozel T. R., Pfrommer G. S. (1986). Activation of the complement system by Cryptococcus neoformans leads to binding of iC3b to the yeast. Infect Immun 52, 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusner D. J. (2005). Mechanisms of mycobacterial persistence in tuberculosis. Clin Immunol 114, 239–247. 10.1016/j.clim.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Levitz S. M. (2002). Receptor-mediated recognition of Cryptococcus neoformans. Nippon Ishinkin Gakkai Zasshi 43, 133–136. 10.3314/jjmm.43.133. [DOI] [PubMed] [Google Scholar]
- Levitz S. M., Tabuni A., Kozel T. R., MacGill R. S., Ingalls R. R., Golenbock D. T. (1997). Binding of Cryptococcus neoformans to heterologously expressed human complement receptors. Infect Immun 65, 931–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitz S. M., Nong S. H., Seetoo K. F., Harrison T. S., Speizer R. A., Simons E. R. (1999). Cryptococcus neoformans resides in an acidic phagolysosome of human macrophages. Infect Immun 67, 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luberto C., Martinez-Mariño B., Taraskiewicz D., Bolaños B., Chitano P., Toffaletti D. L., Cox G. M., Perfect J. R., Hannun Y. A., et al. (2003). Identification of App1 as a regulator of phagocytosis and virulence of Cryptococcus neoformans. J Clin Invest 112, 1080–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik Z. A., Denning G. M., Kusner D. J. (2000). Inhibition of Ca2+ signaling by Mycobacterium tuberculosis is associated with reduced phagosome–lysosome fusion and increased survival within human macrophages. J Exp Med 191, 287–302. 10.1084/jem.191.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuiston T., Luberto C., Del Poeta M. (2010). Role of host sphingosine kinase 1 in the lung response against cryptococcosis. Infect Immun 78, 2342–2352. 10.1128/IAI.01140-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monari C., Bistoni F., Casadevall A., Pericolini E., Pietrella D., Kozel T. R., Vecchiarelli A. (2005). Glucuronoxylomannan, a microbial compound, regulates expression of costimulatory molecules and production of cytokines in macrophages. J Infect Dis 191, 127–137. 10.1086/426511. [DOI] [PubMed] [Google Scholar]
- Mukherjee S., Lee S., Mukherjee J., Scharff M. D., Casadevall A. (1994). Monoclonal antibodies to Cryptococcus neoformans capsular polysaccharide modify the course of intravenous infection in mice. Infect Immun 62, 1079–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Lee S. C., Casadevall A. (1995). Antibodies to Cryptococcus neoformans glucuronoxylomannan enhance antifungal activity of murine macrophages. Infect Immun 63, 573–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S., Feldmesser M., Casadevall A. (1996). J774 murine macrophage-like cell interactions with Cryptococcus neoformans in the presence and absence of opsonins. J Infect Dis 173, 1222–1231. [DOI] [PubMed] [Google Scholar]
- Netski D., Kozel T. R. (2002). Fc-dependent and Fc-independent opsonization of Cryptococcus neoformans by anticapsular monoclonal antibodies: importance of epitope specificity. Infect Immun 70, 2812–2819. 10.1128/IAI.70.6.2812-2819.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J. V. (2005). FcγRIV: a novel FcR with distinct IgG subclass specificity. Immunity 23, 41–51. 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Okamoto H., Takuwa N., Yokomizo T., Sugimoto N., Sakurada S., Shigematsu H., Takuwa Y. (2000). Inhibitory regulation of Rac activation, membrane ruffling, and cell migration by the G protein-coupled sphingosine-1-phosphate receptor EDG5 but not EDG1 or EDG3. Mol Cell Biol 20, 9247–9261. 10.1128/MCB.20.24.9247-9261.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osada M., Yatomi Y., Ohmori T., Ikeda H., Ozaki Y. (2002). Enhancement of sphingosine 1-phosphate-induced migration of vascular endothelial cells and smooth muscle cells by an EDG-5 antagonist. Biochem Biophys Res Commun 299, 483–487. 10.1016/S0006-291X(02)02671-2. [DOI] [PubMed] [Google Scholar]
- Park B. J., Wannemuehler K. A., Marston B. J., Govender N., Pappas P. G., Chiller T. M. (2009). Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530. 10.1097/QAD.0b013e328322ffac. [DOI] [PubMed] [Google Scholar]
- Ravetch J. V., Bolland S. (2001). IgG Fc receptors. Annu Rev Immunol 19, 275–290. 10.1146/annurev.immunol.19.1.275. [DOI] [PubMed] [Google Scholar]
- Rittershaus P. C., Kechichian T. B., Allegood J. C., Merrill A. H., Jr, Hennig M., Luberto C., Del Poeta M. (2006). Glucosylceramide synthase is an essential regulator of pathogenicity of Cryptococcus neoformans. J Clin Invest 116, 1651–1659. 10.1172/JCI27890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera J., Proia R. L., Olivera A. (2008). The alliance of sphingosine-1-phosphate and its receptors in immunity. Nat Rev Immunol 8, 753–763. 10.1038/nri2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sali M., Delogu G., Greco E., Rocca S., Colizzi V., Fadda G., Fraziano M. (2009). Exploiting immunotherapy in Mycobacterium tuberculosis-infected mice: sphingosine 1-phosphate treatment results in a protective or detrimental effect depending on the stage of infection. Int J Immunopathol Pharmacol 22, 175–181. [DOI] [PubMed] [Google Scholar]
- Sanchez T., Skoura A., Wu M. T., Casserly B., Harrington E. O., Hla T. (2007). Induction of vascular permeability by the sphingosine-1-phosphate receptor-2 (S1P2R) and its downstream effectors ROCK and PTEN. Arterioscler Thromb Vasc Biol 27, 1312–1318. 10.1161/ATVBAHA.107.143735. [DOI] [PubMed] [Google Scholar]
- Santucci M. B., Greco E., De Spirito M., Arcovito G., De Angelis G., Cauda R., Fraziano M. (2007). Sphingosine 1-phosphate promotes antigen processing and presentation to CD4+ T cells in Mycobacterium tuberculosis-infected monocytes. Biochem Biophys Res Commun 361, 687–693. 10.1016/j.bbrc.2007.07.087. [DOI] [PubMed] [Google Scholar]
- Schwab S. R., Pereira J. P., Matloubian M., Xu Y., Huang Y., Cyster J. G. (2005). Lymphocyte sequestration through S1P lyase inhibition and disruption of S1P gradients. Science 309, 1735–1739. 10.1126/science.1113640. [DOI] [PubMed] [Google Scholar]
- Shapiro S., Beenhouwer D. O., Feldmesser M., Taborda C., Carroll M. C., Casadevall A., Scharff M. D. (2002). Immunoglobulin G monoclonal antibodies to Cryptococcus neoformans protect mice deficient in complement component C3. Infect Immun 70, 2598–2604. 10.1128/IAI.70.5.2598-2604.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea J. M., Kechichian T. B., Luberto C., Del Poeta M. (2006). The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun 74, 5977–5988. 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Tian M., Wickham L. A., Lin J., Matheravidathu S. S., Forrest M. J., Mandala S., Quackenbush E. J. (2005). Sphingosine-1-phosphate agonists increase macrophage homing, lymphocyte contacts, and endothelial junctional complex formation in murine lymph nodes. J Immunol 175, 7151–7161. [DOI] [PubMed] [Google Scholar]
- Snider A. J., Kawamori T., Bradshaw S. G., Orr K. A., Gilkeson G. S., Hannun Y. A., Obeid L. M. (2009). A role for sphingosine kinase 1 in dextran sulfate sodium-induced colitis. FASEB J 23, 143–152. 10.1096/fj.08-118109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taborda C. P., Casadevall A. (2002). CR3 (CD11b/CD18) and CR4 (CD11c/CD18) are involved in complement-independent antibody-mediated phagocytosis of Cryptococcus neoformans. Immunity 16, 791–802. 10.1016/S1074-7613(02)00328-X. [DOI] [PubMed] [Google Scholar]
- Thompson C. R., Iyer S. S., Melrose N., VanOosten R., Johnson K., Pitson S. M., Obeid L. M., Kusner D. J. (2005). Sphingosine kinase 1 (SK1) is recruited to nascent phagosomes in human macrophages: inhibition of SK1 translocation by Mycobacterium tuberculosis. J Immunol 174, 3551–3561. [DOI] [PubMed] [Google Scholar]
- Vecchiarelli A., Pietrella D., Dottorini M., Monari C., Retini C., Todisco T., Bistoni F. (1994). Encapsulation of Cryptococcus neoformans regulates fungicidal activity and the antigen presentation process in human alveolar macrophages. Clin Exp Immunol 98, 217–223. 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelz K., Lammas D. A., May R. C. (2009). Cytokine signaling regulates the outcome of intracellular macrophage parasitism by Cryptococcus neoformans. Infect Immun 77, 3450–3457. 10.1128/IAI.00297-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vora K. A., Nichols E., Porter G., Cui Y., Keohane C. A., Hajdu R., Hale J., Neway W., Zaller D., Mandala S. (2005). Sphingosine 1-phosphate receptor agonist FTY720-phosphate causes marginal zone B cell displacement. J Leukoc Biol 78, 471–480. 10.1189/jlb.0904487. [DOI] [PubMed] [Google Scholar]
- Weigert A., Weis N., Brüne B. (2009). Regulation of macrophage function by sphingosine-1-phosphate. Immunobiology 214, 748–760. 10.1016/j.imbio.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Wright L. C., Santangelo R. M., Ganendren R., Payne J., Djordjevic J. T., Sorrell T. C. (2007). Cryptococcal lipid metabolism: phospholipase B1 is implicated in transcellular metabolism of macrophage-derived lipids. Eukaryot Cell 6, 37–47. 10.1128/EC.00262-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing D., Hage F. G., Chen Y. F., McCrory M. A., Feng W., Skibinski G. A., Majid-Hassan E., Oparil S., Szalai A. J. (2008). Exaggerated neointima formation in human C-reactive protein transgenic mice is IgG Fc receptor type I (FcγRI)-dependent. Am J Pathol 172, 22–30. 10.2353/ajpath.2008.070154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatomi Y., Igarashi Y., Yang L., Hisano N., Qi R., Asazuma N., Satoh K., Ozaki Y., Kume S. (1997). Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem 121, 969–973. [DOI] [PubMed] [Google Scholar]
- Yauch L. E., Mansour M. K., Levitz S. M. (2005). Receptor-mediated clearance of Cryptococcus neoformans capsular polysaccharide in vivo. Infect Immun 73, 8429–8432. 10.1128/IAI.73.12.8429-8432.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoo E., Yatomi Y., Takafuta T., Osada M., Okamoto Y., Ozaki Y. (2004). Sphingosine 1-phosphate inhibits migration of RBL-2H3 cells via S1P2: cross-talk between platelets and mast cells. J Biochem 135, 673–681. 10.1093/jb/mvh081. [DOI] [PubMed] [Google Scholar]
- Zaragoza O., Chrisman C. J., Castelli M. V., Frases S., Cuenca-Estrella M., Rodríguez-Tudela J. L., Casadevall A. (2008). Capsule enlargement in Cryptococcus neoformans confers resistance to oxidative stress suggesting a mechanism for intracellular survival. Cell Microbiol 10, 2043–2057. 10.1111/j.1462-5822.2008.01186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]