Abstract
Rebuilding tissues involves the creation of a vasculature to supply nutrients and this in turn means that the endothelial cells (EC) of the resulting endothelium must be a quiescent, non- thrombogenic blood contacting surface. Such EC are deployed on biomaterials that are composed of natural materials such as extracellular matrix proteins or of synthetic polymers in the form of vascular grafts or tissue-engineered constructs. Because EC function is influenced by their origin, biomaterial surface chemistry and hemodynamics, these issues must be considered to optimize implant performance. This article reviews the recent in vivo use of endothelialized biomaterials and discusses the fundamental issues that must be considered when engineering functional vasculature.
Introduction
The use of endothelialized biomaterials for tissue engineering and regenerative medicine has a history that spans many decades. To treat vascular injury and defects, bypass grafts were developed using polymers such as expanded polytetrafluoroethylene and polyethylene terephthalate (e.g. Dacron®) [1]. However, graft patency was limited for small diameter (<4 mm) situations, such as for coronary bypasses. This early work revealed the desire to incorporate a confluent, mature, quiescent and non-thrombogenic endothelial cell (EC) layer into the graft to enhance patency and prevent thrombosis (Text Box 1). Thus, endothelialization via pre-seeding was first attempted in the early 1980’s [2]. Unfortunately, early success in canines did not translate into clinical success largely because of differences between canine and human EC and their ability to migrate from the anastomosis, the region where the graft was attached to the patient’s vasculature (reviewed in [3]). Limited EC attachment to the graft further compromised the pre-seeding approach while unfavorable matrix interactions altered the phenotype of the EC. These changes included EC activation, unexpected responses to hemodynamic forces, thrombosis and ultimately graft failure (Text Box 2). Since those early days, much effort has been devoted to optimizing biomaterials to overcome these issues (reviewed in [4]).
Text Box 1. Endothelial cell function and vessel formation.
Endothelial cells (EC) have a variety of important roles which have been reviewed in [75,76]. Briefly, they create a nonthrombogenic surface through the expression of anticoagulant, and fibrinolytic agents. Thrombomodulin, heparan sulphate, nitric oxide (NO) and prostacyclin (PGI2) are antithrombotic and the endothelium specifically binds factors that prevent coagulation. Additionally, the endothelium balances interactions between the coagulation and fibrinolytic systems by forming plaminogen activator and urokinase. EC form a selectively permeable barrier through which there is exchange and active transport of macromolecular substances into the vessel wall. By acting on vascular smooth muscle cell (SMC) contraction, they regulate the overall vessel tone by the release of small molecules that regulate vasodilation (e.g. NO and PGI2) and vasoconstriction (e.g. endothelin (ET)). EC influence vascular remodeling through the formation and secretion of growth-regulatory molecules and cytokines. Additionally, EC maintain the basement membrane collagen and proteoglycans upon which they rest and have the ability to oxidize liproproteins as they are transported into the artery wall. Finally, EC mediate the inflammatory response through the expression of chemotactic and adhesion molecules on their membrane surfaces. They normally provide a nonadherent surface for leukocytes and platelets.
Some strategies for endothelialization rely on vasculogenesis where the EC from the biomaterial form vessels, which then connect with the host’s existing vasculature (a process termed anastomosis). However, many implanted biomaterials experience early vascularization through angiogenesis (new blood vessels sprouting from existing host vessels) and this is often due to the inflammatory response which is linked to both the vascular injury caused by the implantation procedure and the host’s response to the implanted biomaterial (the foreign body reaction). The local implant area remodels as the host reconciles the presence of the implant. Depending on the material of the implant, reconciliation can involve remodeling and integration into the host tissue, degradation, phagocytosis and clearance by leukocytes (e.g. macrophages) or isolation through the formation of a fibrotic capsule. These events require the formation of new blood vessels to sustain the metabolic load of the inflammatory cells. The vessels also facilitate leukocyte infiltration and material clearance (e.g. cell and implant debris). However, these vessels are not permanent and recede after reconciliation. Blood flow ceases and EC undergo apoptosis leaving empty basement membrane sheaves that can act as scaffolds for future capillary re-growth [77]. To form a stable vasculature, the provision of supporting cell types such as SMC or pericytes is required. The combination of supporting cells and the non-thrombogenic surface helps maintain vessel patency (a non-occluded lumen), which ensures continued blood flow.
Text Box 2. Hemodynamics and the effect on EC phenotype.
For strategies that favour the pre-formation of blood vessels in tissue engineered constructs, special consideration must be given to the nature of the blood flow and its impact on EC phenotype. Leukocyte adhesion receptor expression and permeability are important determinants of the fate of a tissue engineered construct in vivo. Receptors, such as Intercellular Cell Adhesion Molecule (ICAM)-1, Vascular Cell Adhesion Molecule (VCAM)-1 and E-selectin, mediate the attachment of circulating blood leukocytes to the construct’s endothelium. Once attached, the leukocytes begin their transendothelial migration and potentiate an inflammatory reaction. This reaction diminishes the therapeutic function of the implanted construct. The blood flow regime has been shown to greatly influence the expression of cell adhesion molecules and permeability (reviewed in [78]). Compared to laminar flow, disturbed flow increases the permeability of the endothelium through, for example, the disruption of Vascular endothelial (VE)-cadherin and the subsequent loss of cell-cell contacts. It also increases the expression of cell adhesion molecules which leads to the recruitment of circulating leukocytes (such as monocytes). Once attached, monocytes pass through the disrupted endothelial cell junctions and enter the vessel wall and underlying tissue. A damaged, activated endothelium or an exposed basement membrane also leads to platelet adhesion. Platelets respond quickly to injury or alterations in vessel walls, adhering to the subendothelial matrix at damage sites to initiate haemostasis. Platelets adhering to a thrombogenic biomaterial surface aggregate through the bridging function of adhesive proteins, such as fibrinogen or von Willebrand factor (VWF). The proteins adsorb to the material’s surface and the platelets in turn adhere to the proteins. Repeated cycles of platelet adhesion and aggregation result in the spatial development of mural thrombi [79], which lead to the occlusion of the vessel and a loss of patency. Additionally, areas of recirculation can add to the intensity of the response by trapping circulating monocytes and platelets, and with their increased residence time offers them ample opportunity to participate in inflammatory reactions.
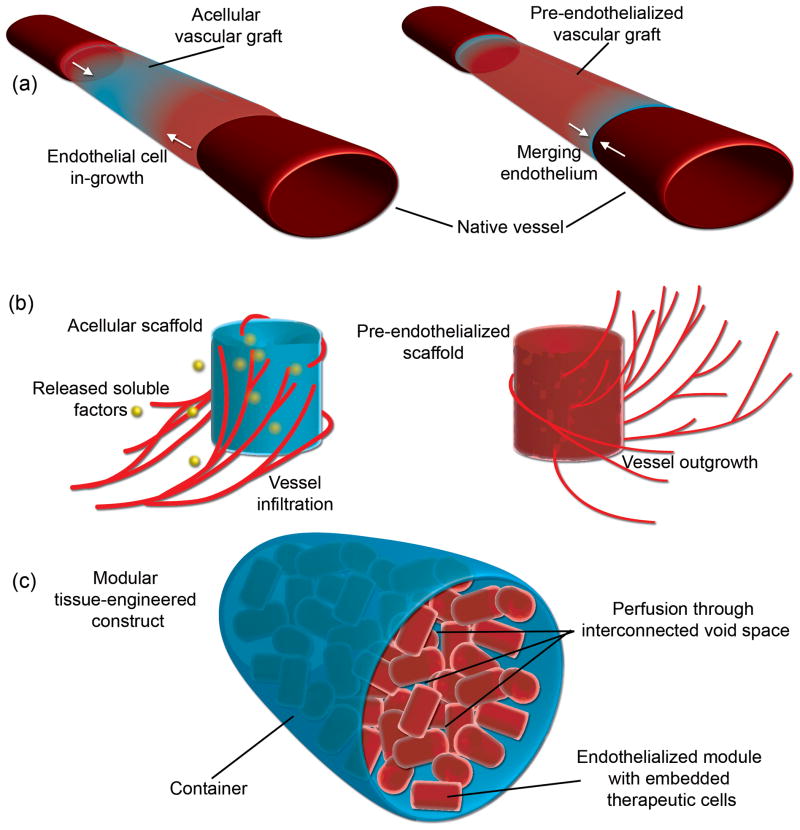
Beyond the creation of vascular grafts, much effort has been placed on the creation of three-dimensional vascularized constructs for tissue engineering applications. Due to the ~150 μm diffusion limit imposed by Fick’s law [5], thick tissues require an internal vasculature to supply adequate amounts of nutrients and oxygen to avoid core necrosis. Hence, our interest in modular tissue engineering [6] while others are interested in controlled release strategies (e.g., [7]). These approaches reflect different ways of deploying biomaterials (Figure 1). One way is to use them as scaffolds where cells are seeded on and within the material. The scaffold organizes the cells for delivery into the host. Alternatively, the material is used as a platform for bioactive agonists that modulate the host response, such as a scaffold material that slowly releases soluble factors to encourage host blood vessel infiltration.
Figure 1. Strategies to endothelialize biomaterials.
(a) Endothelialized vascular grafts for bypass surgery. Grafts are made of natural materials, such as collagen, or synthetic materials, such as Dacron®. They can be further modified to release soluble factors that encourage endothelium in-growth (shown on left) or be pre-endothelialized prior to implantation (shown on right) such that the endothelial cells in the graft merge with the in-growing endothelial cells from the host. (b) Scaffolds for vascularized constructs for tissue engineering. Some scaffolds rely on the controlled release or immobilization of pro-angiogenic factors to initiate host endothelial cell in-growth [8–13] (shown on left) while others make use of specific internal geometric structures that act as spatial and organizational cues for infiltrating cells [73]. Alternatively, pre-seeded scaffolds are cultured in vitro prior to implantation in order to create a rudimentary internal vasculature (shown on the right). Upon implantation, the embedded endothelial cells are expected to connect (anastomose) with the host tissue in order to generate a perfusable construct. (c) Modular tissue engineering. Pre-seeded scaffolds can be used to produce larger tissue engineered constructs using a modular approach. Sub-millimeter-sized cylindrical scaffolds containing embedded therapeutic cells have their surfaces pre-seeded with endothelial cells. The modular scaffolds are then implanted directly or randomly packed into a larger container. Due to the random packing, an interconnecting, endothelial cell-lined void space capable of blood perfusion is created. Thus, a pre-vascularized construct capable of sustaining the embedded therapeutic cells is generated in vitro prior to implantation [6,19].
Endothelialized biomaterials may be comprised of a variety of materials including extracellular matrix (ECM)-based proteins, surface or bulk modified synthetic polymers and synthetic peptides. These materials are substrates for EC and it is the cells that are intended to circumvent the difficulties associated with thrombosis and diffusion. Hence, this review highlights the challenges associated with the creation of these biomaterials and surveys selected recent examples of soft-tissue biomaterials with an emphasis placed on in vivo results to illustrate the range of substrates (natural and synthetic) that are being exploited for this purpose.
General considerations
Endothelialized biomaterials are formed by seeding EC prior to implantation or by relying on the infiltration of the host’s vasculature into the material (refer to Text Box 1 and Figure 1). Often, to promote angiogenesis (the in-growth of blood vessels from the host), scaffolds are modified to incorporate or immobilize pro-angiogenic cytokines and growth factors [8–13]. In the pre-vascularization approach, scaffolds are seeded with EC under static or dynamic (e.g. perfusion) conditions to ensure the penetration of EC deep within the scaffold [14,15]. Elements such as scaffold pore size and density influence the speed of pre-vascularization. These elements are tuned by controlling the ratios of the scaffold’s components, curing conditions, or by using special fabrication techniques such as self-assembly or solid free-form fabrication [16–18]. The EC organize to form new blood vessels (vasculogenesis) and grow outwards to anastomose with the host’s vasculature. A scalable, pre-vascularized tissue can also be created by using a modular approach. EC-covered cylindrical modules are randomly packed within a larger container, thus creating an EC-lined interconnected void space amenable to perfusion [6,19]. Thus, perfusion-capable blood vessels are pre-formed within the tissue engineered construct prior to implantation. Another approach involves the pre-determined design of the internal blood vessels. For example, a sacrificial hydrogel material is cast in the desired vessel pattern around which a second hydrogel is cast [20]. In this way, a regular geometric structure is formed that is potentially more predictable, although cell-based remodeling can still change the structure over time in vivo. Pre-cellularized biomaterials can also enhance angiogenesis through hypoxia. Scaffolds containing embedded cells (such as liver, islet or EC) experience decreased oxygen tension and the resulting hypoxia is a powerful angiogenesis stimulant and can improve the rate of biomaterial endothelialization in vivo [21,22].
Matrix effects
Robust EC attachment to its underlying matrix (detailed in [23]) is necessary to prevent EC ablation due to blood flow shear stress and to prevent the exposure of the underlying matrix to blood. Matrix cues also influence EC phenotype. Poor attachment leads to anoikis, where cells detach and undergo apoptosis [24]. Cues result from the binding of the integrins on the cell surface and the corresponding ligands on the ECM. In vivo, the ECM is complex and is composed of a variety of proteins including collagen I, collagen IV, laminin, elastin, proteoglycans and glycosaminoglycans [25]. To create biomaterials and scaffolds that preserve the ECM’s complexity, decellularized matrices are often used [26–28]. Alternatively, a small subset of the naturally occurring ECM proteins is used. For example, systems composed exclusively of collagen I have been used, partly due to its commercial availability [6]. The major caveat is that EC behaviour and angiogenic potential is altered by differences in material composition, which is further exacerbated by the fact that EC from different sources also exhibit different behaviours on each type of matrix [29]. Rather than using an entire protein, peptide sequences may be incorporated into the scaffold to promote adhesion and generate beneficial matrix cues. Many synthetic polymer materials are modified to contain immobilized peptide adhesion sequences to provide robust cell attachment in place of the ligands found in real ECM [11,29–31]. Alternatively, synthetic materials can be coated with a film of protein such as laminin or gelatin [32,33] in order to provide additional EC binding sites. However, synthetic biomaterial-cell interactions can cause EC activation. For example, EC grown on polystyrene, polypropylene, polyethylene terephthalate and poloxamine become activated (i.e. non-quiescent) [34–37] in contrast to EC grown on polymer surfaces that were pre-coated with ECM proteins (such as polyethersulfone pre-coated with gelatin) or on collagen-only gels [33,36]. Moreover, the initial seeding density of EC and the time taken to proliferate to confluence can increase Intercellular Cell Adhesion Molecule (ICAM)-1 expression [38], an indication of EC activation and the loss of quiescence.
Implant remodeling
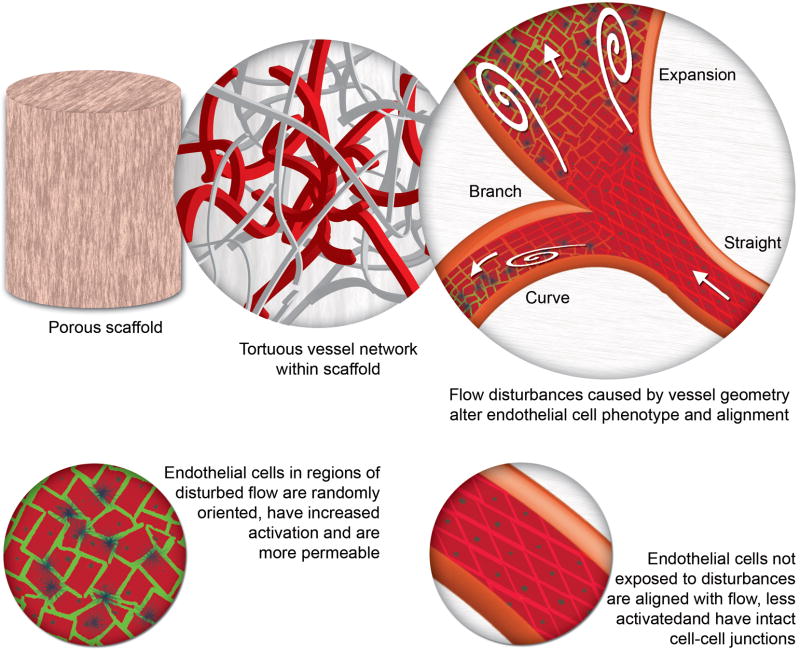
Both biodegradable implants and the ECM found within tissues are remodeled by the host through a process of cell invasion, enzymatic breakdown, and new protein synthesis and deposition (reviewed in [39] and highlighted in Text Box 3). These processes alter the structure of the implants over time and further influence the hemodynamics within the implants (Figure 3). For scaffolds that contain randomly oriented fibers, the tortuous interconnected pore space will, in part, guide EC migration and vessel formation to create tortuous vessels. If excessively tortuous, the hemodynamics may have negative effects on the EC (Text Box 2 and Figure 3). Channel geometry, flow rate and Reynolds number are also important factors. Slowly percolating laminar flow will have a different effect from higher speed flow through larger, tortuous, and flow separation-prone channels. Due to ongoing remodeling, the structure and organization of the scaffold (particularly biodegradable ones) is in flux and the internal vessels and flow regimes will change over time. Hence, remodeling might improve the patency of some vessels while diminishing others [40].
Text Box 3. Animal implant models.
The innate immune and the foreign body reactions are initiated by the vascular injury caused by the biomaterial’s implantation procedure where neutrophils, macrophages and foreign body giant cells aid in wound repair and also attempt to remove, destroy or isolate (i.e., form a fibrotic capsule) the foreign implant. Additionally, if the biomaterial is blood incompatible and exposed to blood, the complement system is activated. As integral parts of the adaptive immune system, T-cells, natural killer (NK) cells and B-cells make use of antibodies to identify foreign cell-containing implants, which facilitates their destruction and clearance from the host. Xenogeneic implants have cells from one species implanted into another. Allogeneic implants are species, but not genotype, matched while syngeneic implants match both the species and genotype. To correctly gauge the biomaterial’s in vivo ability to integrate with the host’s vasculature when species or genotype differences exist, immunosuppressants such as cyclosporin A, cyclophosphhamide, atorvastatin or tacrolimus, are used. Several animal models that limit the adaptive immune response are also available. Nude animal models lack T-cells but contain macrophages, B-cells, NK cells and complement. Some nude strains, such as the NIH III, also lack B-cells and have impaired NK cells. Severe Combined Immunodeficiency (SCID) models lack T-cells and B-cells but contain NK cells, macrophages and complement. SCID Beige models only contain macrophages, complement and impaired NK cells. Non Obese Diabetic (NOD) SCID models lack T-cells, B-cells and complement, but have macrophages and impaired NK cells. Moreover, it is possible to reduce the function of the innate immune system by depleting macrophages through the use of, for example, clodronate liposomes [80]. It is also imperative to recognize that differences in human and animal immune systems exist, which can confound experimental data. For example, unlike humans, rodent microvascular EC lack LFA-3 and class II MHC which facilitate T-helper cell contact and antigen recognition [81]. To track implanted cells, Green Fluorescent Protein (GFP)-expressing cells harvested from GFP animals can be visualized or labeled via antibodies. Similarly, cultured cells are virally transfected to incorporate genes, such as luciferase, for bioluminescence applications or chemically labeled using reagents such as carboxyfluorescein diacetate succinimidyl ester (CFDA-SE). Fluorescence in-situ hybridization (FISH) for X and Y chromosomes is also useful for cross-sex transplants. For cross-species implants, the antibody labeling of species-specific proteins, such as a UEA-1 lectin that targets human but not rat EC, is also useful.
Figure 3. Hemodynamics and its effect on endothelial cell phenotype.
In a porous scaffold that contains a very tortuous network of pores, flow disturbances may arise which cause endothelial cell activation, loss of cell alignment and an increase in endothelium permeability (see also Text Box 2). As seen in the top right and bottom right images, endothelial cells in straight sections are aligned with the direction of flow. Flow disturbances (indicated by white swirls) are created by the separation of flow and thus occur when the vessel geometry undergoes rapid changes along its length (e.g. branches, obstructions, bends contractions and sudden expansions). These types of tortuous vessels are often seen in newly vascularized biomaterial implants in vivo. As illustrated in the expansion region (top right image), cells are no longer aligned and are randomly oriented. Cells located within these disturbances also become more permeable (illustrated by green colour in the bottom left image) as their cell-cell junctions are disrupted through, for example, the dismantling of VE-cadherin junctions. Moreover, they become activated, which increases their cell adhesion molecule expression (dark blue dots). As the implant is remodeled by the endothelial cells and the surrounding host tissue, the vessel shape and structure can change over time, which creates further opportunity for changes in vessel geometry.
If the biomaterial is degradable and able to be remodeled by cells, one must consider that as remodeling occurs, the EC monolayer enters a state of flux and its integrity diminishes. Vascular endothelial (VE)-cadherin bonds are broken as EC reorient, migrate and even proliferate [19,29,41,42]. This results, for example, in an increase in EC monolayer permeability to macromolecules, the exposure of underlying matrix and it can facilitate transendothelial migration for leukocytes during foreign body reactions [43,44], the consequences of which are outlined in Text Boxes 1 and 3. Similarly, changes in EC activation and barrier function occur during angiogenesis (reviewed in [45]).
EC source
In terms of in vivo experiments, EC source, species and implant models are important considerations and may, at times, alter the outcome and context of the experimental data (reviewed in Text Box 3). EC from varying sources (e.g., small versus large diameter vessels; see Figure 2) are known to exhibit different behaviours in terms of angiogenic potential, molecular permeability, leukocyte transmigration, hemostasis, vascular tone, humidification, thermoregulation and even immune tolerance [46]. On the other hand, EC are considered to be fairly plastic [47] and the vessel source may not be that crucial when other considerations such as availability are important. In addition to vessel source concerns, there are also species differences, as illustrated when the success with canine vascular grafts did not translate into clinical efficacy [3,4].
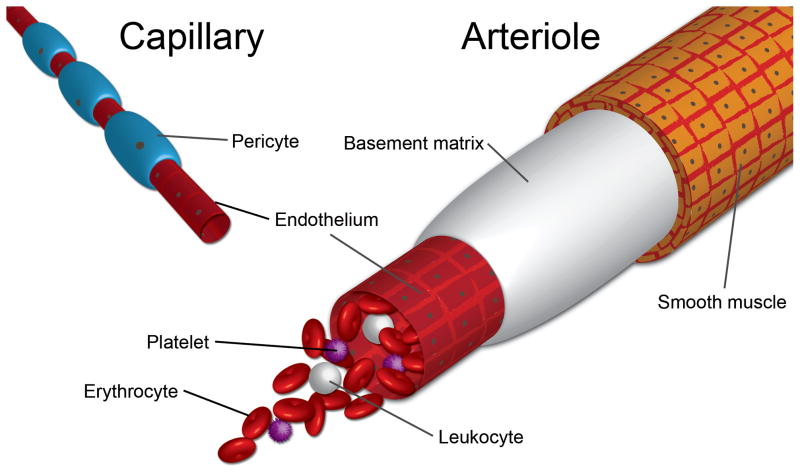
Figure 2. Endothelial cell sources and blood vessel structures.
The lumen of the blood vessel contains an intact endothelium which carries blood. The blood contains plasma and cells such as erythrocytes, platelets and leukocytes. The structure of the vessel depends on its size and location within the vasculature. Larger vessels, such as arteries and arterioles, are surrounded by concentric layers of smooth muscle cells which aid in the maintenance of vascular tone and endothelial cell quiescence through, for example, a nitric oxide pathway [82]. Smaller vessels, such as capillaries, lack smooth muscle and instead are surrounded by pericytes, which actively maintain vessel maturity and are thought now to be the source of mesenchymal stromal cells (refer to review by Corselli et al [83]). The source of the EC is an important consideration in this field.
For biomaterials and constructs that contain multiple cell types, special considerations must be given to co-culture conditions. For instance, large-scale vascular grafts require both EC and a supportive smooth muscle cell (SMC) layer. Tissue engineered constructs often contain therapeutic cells in addition to EC. Typically, the culture medium for each cell type is distinct and only optimized for that particular cell type. Other cells exposed to this medium often undergo undesired phenotypic changes. For example, in a cardiomyocyte and EC co-culture system, the medium formulation (e.g. serum type and glucose concentration) was found to impact the electrical performance of the cardiomyocytes and EC VE-cadherin organization [48]. Microscale technologies may permit high throughput screening of medium and the assessment of cell phenotype. Such high-throughput systems may also lead to the elucidation of an ideal, fully defined medium without serum. Cells cultured in 3-dimensional matrices within microfluidic devices can further improve screening quality by recapitulating the spatial orientation found in vivo [19,49].
Natural materials used for endothelialization
Natural materials generally have favorable properties from the perspective of cell attachment and behavior, and are typically amenable to cell-based remodeling.
Collagen
Collagen is an abundant ECM protein that has excellent cell-binding properties and is amenable to host integration. A number of methods exist to fabricate collagen into mechanically robust, endothelializable materials. For example, to create a vascular graft, a collagen membrane was lyophilized, seeded with EC and SMC, fashioned into a tubular structure and then implanted to regenerate a rat inferior vena cava [50]. After 12 weeks, the graft was of sufficient tensile strength and showed no signs of significant thrombogeneity and intima hyperplasia.
Another method for deploying collagen combines scaffold creation and modular assembly [6,19,51]. Sub-millimeter-sized collagen cylinders were coated with a confluent layer of EC and randomly assembled within a larger container. The random packing created an interconnected void space, lined with non-thrombogenic EC, through which blood perfusion occured. The technique was also amenable to the creation of more complex tissues by embedding cells within the cylindrical modules, such as SMC, mesenchymal stromal cells (MSC) and cardiomyocytes [48,52,53].
Growth factors such as Vascular Endothelial Growth Factor (VEGF), Fibroblast Growth Factor 2 (FGF2) and Angiopoietin-1 (Ang1) have also been incorporated into collagen to increase its angiogenic potential. When collagen scaffolds containing both VEGF and FGF2 were subcutaneously implanted in rats, a greater amount of mature blood vessels containing smooth muscle actin-positive cells were observed, as compared to scaffolds without growth factors and scaffolds made up of heparinized collagen [10]. Moreover, no hypoxic cells were found at days 7 and 21, as indicated by low levels of hypoxia inducible factor 1-α (HIF1-α).
Covalently tethering growth factors to collagen can both prolong signaling and generate a localized effect. For example, angiogenesis in VEGF-tethered scaffolds contributed to improved cell survival and tissue formation [9]. VEGF-tethered porous collagen scaffolds showed enhanced growth of endothelial and bone marrow cells in vitro, and higher blood vessel density and scaffold thickness in vivo after 28 days. Similarly, enhanced proliferation, EC tube formation and angiogenesis were observed after 7 days (both in vivo and in an assay that quantifies the formation of new blood vessels using fertilized eggs) when both VEGF and Ang1 had been immobilized on collagen scaffolds, as compared to unmodified scaffolds [8].
Hyaluronan
Hyaluronan (HA, reviewed in [54]) is an acidic glycosaminoglycan made entirely of a repeating disaccharide (d-glucuronic acid β-1,3-N-acetylglucosamine-β1,4). It is important in cell proliferation, migration and angiogenesis (reviewed in [55]), which makes it attractive for use in grafts. HA-based biodegradable grafts (HYAFF-11™) were used to prepare large blood vessels (4 mm diameter and 5 cm length) and in a 5 month-long porcine implant model, the material guided the development of a well-functioning neoartery that contained luminal EC, mature SMC and organized layers of elastin fiber [56]. However, while 7 of 10 animals showed no signs of stenoses or aneurysms, the remaining 3 did experience intimal hyperplasia (initiating at the anastomotic site) and graft thrombosis. Additionally, injecting HA along with Human Umbilical Vein EC (HUVEC) into mouse ischemic hindlimbs improved angiogenesis, arteriogenesis, HUVEC survival and blood perfusion, as compared to injection of HA or HUVEC alone [57].
Alginate
Alginate undergoes solid/gel transition in the presence of multivalent cations such as Ca2+ and is thus amenable to cell encapsulation applications (reviewed in [58]). These properties make it useful for embedding and transplanting therapeutic cells. Recently, a modified macroporous alginate that incorporated the Arg-Gly-Asp (RGD) cell adhesion sequence was used as an in vivo depot for what was termed endothelial progenitor cells and showed success with respect to angiogenesis and cell engraftment when implanted into ischemic murine hindlimb musculature [59]. Here, blood vessel densities increased from 260 to 670 vessels/mm2, as compared to bolus cell injection, and perfusion returned to normal in 40 days whereas bolus cell injections were minimally effective in improving limb perfusion. Similarly, in a chronic infarct model, RGD peptides conjugated to alginate improved HUVEC proliferation and adhesion when compared to a non-modified alginate group [60].
Matrigel
Matrigel, a mouse sarcoma-derived ECM, contains a variety of ECM proteins and growth factors. Because it is available and it influences cell behaviour, investigators have used it to promote blood vessel in-growth into poly-D,L-lactic-co-glycolic acid (PLGA) scaffolds [61]. The porous scaffolds were impregnated with Matrigel and implanted into the dorsal skinfold chamber of balb/c mice. The Matrigel accelerated the in-growth of blood vessels, promoted their maturation (including pericyte coverage) and did not increase inflammatory leukocyte-EC interactions, as compared to growth factor reduced Matrigel-containing scaffolds and PLGA-only controls. A synthetic mimic of Matrigel would need to be produced to exploit these results in the clinic.
Fibrin
Fibrin is harvestable from human blood and is a completely autologous scaffold material (reviewed in [62]). Chen and colleagues capitalized on these properties in order to prevascularize fibrin-based constructs [63]. The constructs were prevascularized with capillary networks by co-culturing HUVEC and fibroblasts in fibrin gels for 1 week. When subcutaneously implanted within immune-deficient mice, functional anastomosis (red blood cell perfusion) was observed. The number and area of perfused lumens in the prevascularized tissue were significantly larger than with the non-prevascularized controls. Moreover, new collagen deposition and increased cell proliferation were also observed.
Silk fibroin
Silk fibroin is one of two proteins that make up the silk produced by silkworms. Scaffolds made from silk fibroin were seeded with human osteoblast cells and human microcapillary EC (HDMEC), the latter of which self-assembled in vitro into lumen-containing microcapillary-like structures that were intertwined between the osteoblasts and biomaterial [64]. When implanted into immunodeficient mice, chimeric vessels were formed, i.e. the HDMEC integrated with the host’s in-growing capillaries. A similar material was used as a stent coating [65].
Peptides
Some self-assembling peptides spontaneously assemble into stable gels with an ECM-like microarchitecture without the use of potentially cytotoxic chemical crosslinkers (which are often needed for many synthetic polymers). Sieminski et al [31] observed that HUVEC cultured within RAD16-I ((RADA)4) or RAD16-II ((RARADADA)2) gels elongated and formed interconnected capillary-like networks resembling in vivo capillaries, while they remained round and formed clusters within KFE-8 ((FKFE)2), or KLD-12 ((KLDL)3) gels. The differences in attachment were attributed to differences in the peptides’ primary sequences. Similarly, when multi-peptide co-assembling hydrogels based on the β-sheet fibrillizing peptide Q11 (QQKFQFQFEQQ) presenting either RGDS or IKVAV ligands on their fibril surfaces were compared, the RGDS-Q11 increased HUVEC attachment, spreading and growth when co-assembled with Q11 gels [30].
A slightly different use of peptides is the isolation of specific sequences from biologically active proteins and their incorporation into hybrid scaffolds. For example, the osteopontin-derived Ser-Val-Val-Tyr-Gly-Leu-Arg (SVVYGLR) peptide exhibited potent angiogenic activity and as such, was incorporated into a CO3-apatite-collagen composite [66]. When implanted as a graft into a tissue defect created in a rat tibia, increased angiogenesis and vascular EC infiltration was observed.
Decellularized matrix
Decellularized matrices are generated by flushing and perfusing tissues with detergents and buffers. Though the cells are removed in the process, the matrix and geometry of the tissue is preserved. Ott et al [27] and Petersen et al [28] demonstrated the efficacy of decellularized matrices during the creation and transplantation of tissue-engineered lungs capable of blood perfusion and gas exchange in vivo. Decellularized rat lungs were seeded with epithelial cells and EC. To establish function, lungs were perfused and ventilated in a bioreactor. After 4 to 8 days of in vitro culture, regenerated lungs were implanted into rats where they functioned for up to 6 hours. Vascularized heart tissue has also been produced in a similar fashion [26]
To create an artery, autologous decellularized ovine carotid arteries were seeded with SMC- and EC-like cells (differentiated from MSC) in vitro and interposed into the carotid arteries of an ovine host [67]. Grafts were patent, non-thrombogenic and mechanically stable for 5 months in vivo, whereas non-seeded grafts lost patency within 2 weeks. After 2 and 5 months in vivo, grafts contained endothelium, smooth muscle, collagen and elastin. MSC labeled with a fluorescent dye prior to implantation were detected in 2 month graft explants, indicating that the MSC survived and contributed to the vascular tissue regeneration. In another study, vascular grafts were produced by seeding SMC and EC on decellularized porcine ureters [68]. This highlighted the notion that a blood vessel can be generated from a non-blood vessel decellularized matrix, so long as the geometry is similar.
Synthetic materials used for endothelialization
Natural materials often have favorable properties from the perspective of cell attachment and behavior, but are more limited in their mechanical characteristics and their versatility in forming different structures. Synthetic materials are the opposite: unmatchable versatility in processing and mechanical characteristics but poor ability to enable cell attachment (at least without chemical modification).
Poly(ε-caprolactone)
Poly(ε-caprolactone) (PCL) is a degradable polymer that is formed by the ring opening polymerization of ε-caprolactone. Small, 2 mm internal diameter electrospun PCL vascular grafts were implanted into the abdominal aorta of rats [69]. After 24 weeks, both the PCL grafts and expanded polytetrafluoroethylene (ePTFE) control grafts showed patency after 24 weeks; however, only the PCL grafts showed fiber degradation, faster endothelialization and rapid ECM formation. Moreover, stenosis was observed in ePTFE groups but not with PCL. None of the grafts showed aneurysmal dilatation. In terms of neointimal formation, macrophage and fibroblast in-growth with ECM formation and neoangiogenesis were better in the PCL group. When PCL was pre-treated with NaOH, EC graft coverage and nitric oxide (NO) production was improved [70]. PCL blended with starch (SPCL) also showed promise in terms of enhanced EC graft coverage [71]. Both macro- and microvascular EC adhered to SPCL fiber-mesh scaffolds and grew to cover much of the available surface.
PEG
Polyethylene glycol (PEG) is a synthetic, hydrophilic polymer that can form hydrogels with low protein and cell adsoption/adhesion. PEG-based hydrogel matrices incorporating protease degradable sites, cell-adhesion motifs and controlled VEGF release were produced to induce the growth of vasculature in vivo [11]. When implanted subcutaneously in rats, these degradable constructs induced a significant number of vessels to grow into the implant at 2 weeks with increasing vessel density at 4 weeks. Moreover, in a mouse model of hindlimb ischemia, the matrices significantly increased the rate of reperfusion.
Similarly, a synthetic matrix metalloproteinase (MMP)-responsive PEG-based hydrogel was used as an in situ forming scaffold to deliver thymosin β4 (Tβ4), a pro-angiogenic and pro-survival factor, and vascular cells derived from human embryonic stem cells (hESC), to ischemic rat hearts [72]. The gel supported the infarct’s degrading ECM and promoted structural organization of native EC. Moreover, some of the hESC-derived vascular cells formed de novo capillaries in the infarct zone.
pHEMA
Recently, Madden et al created a vascularized tissue in vivo through the use of spatial control and directional cues [73]. Microtemplating was used to shape poly(2-hydroxyethyl methacrylate-co-methacrylic acid) (pHEMA) hydrogels into acellular scaffolds that contained parallel channels to organize cardiomyocyte bundles, which were supported by micrometer-sized, spherical, interconnected pores. Cardiac implantation of acellular scaffolds with pore diameters of 40 μm showed some evidence of angiogenesis, as compared to 20 and 80 μm diameter pore scaffolds. Moreover, 30–40 μm pore scaffolds showed reduced fibrotic response.
Other materials
Poly(lactic-co-glycolic acid) (PLGA) is a common, FDA-approved material used in tissue engineering. A PLGA scaffold incorporating Phthalimide Neovascular Factor 1 (PNF1), a potent stimulator of proangiogenic signaling pathways in EC, was used to significantly expand microvascular networks within a 2 mm radius from implants after 3 and 7 days by increasing the microvessel length, density and lumen diameter of local arterioles and venules [12]. An enhanced recruitment of circulating leukocytes, including monocytes, which are critical for the process of vessel enlargement through arteriogenesis, was also observed as a consequence of the controlled release of PNF1.
PLGA is not a soft, elastic material and elasticity is important for vascular grafts, particularly in the context of regulating vascular tone. The biodegradable elastomeric copolymer poly(1,8-octanediol citrate) (POC), a PLGA alternative, supported HAEC attachment without any premodification of the surface and relative to ePTFE, exhibited decreased platelet adhesion and clotting, negligible hemolysis, and comparable protein adsorption [74]. Poly(glycerol sebacate) (PGS) is also mechanically suitable, though to achieve improved EC coverage, survival and proliferation, it required a laminin pre-coat [32] to provide cells with the additional integrin binding sites that were otherwise lacking.
Conclusions
Many techniques exist to create endothelialized biomaterials for applications in vascular grafts and tissue engineered organs. Nonetheless, validation through in vivo experimentation is vital in order to hone in on the subset that promotes long term functionality, patency and integration. Focus must be placed on material and cell sourcing in order to ensure experimental results are not confounded by inherent differences in phenotype. In order to ensure a non-thrombogenic blood contacting surface, characterization must go beyond basic EC attachment studies and focus on cell function, including activation status and monolayer integrity, as modified by the hemodynamic forces to which the cells are subjected. Endothelialized biomaterials are essential components of the next generation of medical devices. Success will require careful attention to the dynamic behavior of these cells in the variety of configurations that are being proposed.
Acknowledgments
The authors acknowledge the financial support of the Natural Sciences and Engineering Research Council, the Canadian Institutes of Health Research and the US National Institutes of Health (EB006903). O.F. Khan acknowledges scholarship support from the Ontario Graduate Scholarship Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.L’Heureux N, et al. Technology Insight: The evolution of tissue-engineered vascular grafts - From research to clinical practice. Nat Clin Pract Cardiovasc Med. 2007;4:389–395. doi: 10.1038/ncpcardio0930. [DOI] [PubMed] [Google Scholar]
- 2.Graham LM, et al. Endothelial cell seeding of prosthetic vascular grafts. Early experimental studies with cultured autologous canine endothelium. Arch Surg. 1980;115:929–933. doi: 10.1001/archsurg.1980.01380080025005. [DOI] [PubMed] [Google Scholar]
- 3.Bordenave L, et al. Clinical performance of vascular grafts lined with endothelial cells. Endothelium. 1999;6:267–275. doi: 10.3109/10623329909078494. [DOI] [PubMed] [Google Scholar]
- 4.Zilla P, et al. The endothelium: A key to the future. J Card Surg. 1993;8:32–60. doi: 10.1111/j.1540-8191.1993.tb00574.x. [DOI] [PubMed] [Google Scholar]
- 5.Folkman JH, Hochberg M. Self regulation of growth in three dimensions. J Exp Med. 1973;138:745–753. doi: 10.1084/jem.138.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci USA. 2006;103:11461–11466. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuen WW, et al. Mimicking nature by codelivery of stimulant and inhibitor to create temporally stable and spatially restricted angiogenic zones. Proc Natl Acad Sci USA. 2010;107:17933–17938. doi: 10.1073/pnas.1001192107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiu LLY, Radisic M. Scaffolds with covalently immobilized VEGF and Angiopoietin-1 for vascularization of engineered tissues. Biomaterials. 2010;31:226–241. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 9.Miyagi Y, et al. Biodegradable collagen patch with covalently immobilized VEGF for myocardial repair. Biomaterials. 2011;32:1280–1290. doi: 10.1016/j.biomaterials.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Nillesen STM, et al. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–1131. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 11.Phelps EA, et al. Bioartificial matrices for therapeutic vascularization. Proc Natl Acad Sci USA. 2010;107:3323–3328. doi: 10.1073/pnas.0905447107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wieghaus KA, et al. Expansion of microvascular networks in vivo by phthalimide neovascular factor 1 (PNF1) Biomaterials. 2008;29:4698–4708. doi: 10.1016/j.biomaterials.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao C, et al. The effect of cross-linking of collagen matrices on their angiogenic capability. Biomaterials. 2008;29:66–74. doi: 10.1016/j.biomaterials.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Kyriakidou K, et al. Dynamic co-seeding of osteoblast and endothelial cells on 3D polycaprolactone scaffolds for enhanced bone tissue engineering. J Bioact Compat Polym. 2008;23:227–243. [Google Scholar]
- 15.Maidhof R, et al. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnol Prog. 2010;26:565–572. doi: 10.1002/btpr.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drury JL, Mooney DJ. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/s0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang S, et al. The design of scaffolds for use in tissue engineering. Part I. Traditional factors. Tissue Eng. 2001;7:679–689. doi: 10.1089/107632701753337645. [DOI] [PubMed] [Google Scholar]
- 18.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat Biotechnol. 2003;21:1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 19.Khan OF, Sefton MV. Perfusion and characterization of an endothelial cell-seeded modular tissue engineered construct formed in a microfluidic remodeling chamber. Biomaterials. 2010;31:8254–8261. doi: 10.1016/j.biomaterials.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7:720–725. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 21.Landman KA, Cai AQ. Cell proliferation and oxygen diffusion in a vascularising scaffold. Bull Math Biol. 2007;69:2405–2428. doi: 10.1007/s11538-007-9225-x. [DOI] [PubMed] [Google Scholar]
- 22.Malda J, et al. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007;13:2153–2162. doi: 10.1089/ten.2006.0417. [DOI] [PubMed] [Google Scholar]
- 23.Albelda SM, Buck CA. Integrins and other cell adhesion molecules. FASEB J. 1990;4:2868–2880. [PubMed] [Google Scholar]
- 24.Frisch SM, Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994;124:619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bosman FT, Stamenkovic I. Functional structure and composition of the extracellular matrix. J Pathol. 2003;200:423–428. doi: 10.1002/path.1437. [DOI] [PubMed] [Google Scholar]
- 26.Ott HC, et al. Perfusion-decellularized matrix: Using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 27.Ott HC, et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–933. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 28.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–541. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dye JF, et al. Distinct patterns of microvascular endothelial cell morphology are determined by extracellular matrix composition. Endothelium. 2004;11:151–167. doi: 10.1080/10623320490512093. [DOI] [PubMed] [Google Scholar]
- 30.Jung JP, et al. Co-assembling peptides as defined matrices for endothelial cells. Biomaterials. 2009;30:2400–2410. doi: 10.1016/j.biomaterials.2009.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sieminski AL, et al. Primary sequence of ionic self-assembling peptide gels affects endothelial cell adhesion and capillary morphogenesis. J Biomed Mater Res A. 2008;87:494–504. doi: 10.1002/jbm.a.31785. [DOI] [PubMed] [Google Scholar]
- 32.Lee EJ, et al. A biocompatible endothelial cell delivery system for in vitro tissue engineering. Cell Transplant. 2009;18:731–743. doi: 10.3727/096368909X470919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unger RE, et al. Vascularization and gene regulation of human endothelial cells growing on porous polyethersulfone (PES) hollow fiber membranes. Biomaterials. 2005;26:3461–3469. doi: 10.1016/j.biomaterials.2004.09.047. [DOI] [PubMed] [Google Scholar]
- 34.Cenni E, et al. Expression of adhesion molecules on endothelial cells after contact with knitted Dacron. Biomaterials. 1997;18:489–494. doi: 10.1016/s0142-9612(96)00160-3. [DOI] [PubMed] [Google Scholar]
- 35.Granchi D, et al. Adhesive protein expression on human endothelial cells after in vitro contact with woven Dacron. Biomaterials. 1998;19:93–98. doi: 10.1016/s0142-9612(97)00161-0. [DOI] [PubMed] [Google Scholar]
- 36.Khan OF, Sefton MV. Patterning Collagen/Poloxamine-Methacrylate Hydrogels for Tissue-Engineering-Inspired Microfluidic and Laser Lithography Applications. J Biomater Sci Polym Ed. 2010 doi: 10.1163/092050610X540693. In press. [DOI] [PubMed] [Google Scholar]
- 37.Margiotta MS, et al. The adherence of endothelial cells to Dacron induces the expression of the intercellular adhesion molecule (ICAM-1) Ann Surg. 1992;216:600–604. doi: 10.1097/00000658-199211000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Der Zijpp YJT, et al. ICAM-1 and VCAM-1 expression by endothelial cells grown on fibronectin-coated TCPS and PS. J Biomed Mater Res A. 2003;65:51–59. doi: 10.1002/jbm.a.10327. [DOI] [PubMed] [Google Scholar]
- 39.Jacob MP, et al. Extracellular matrix remodeling in the vascular wall. Pathologie Biologie. 2001;49:326–332. doi: 10.1016/s0369-8114(01)00151-1. [DOI] [PubMed] [Google Scholar]
- 40.Sundström J, Vasan RS. Circulating biomarkers of extracellular matrix remodeling and risk of atherosclerotic events. Curr Opin Lipidol. 2006;17:45–53. doi: 10.1097/01.mol.0000203891.34890.b5. [DOI] [PubMed] [Google Scholar]
- 41.Lampugnani MG, et al. The molecular organization of endothelial cell to cell junctions: Differential association of plakoglobin, β-catenin, and alpha-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noria S, et al. Transient and steady-state effects of shear stress on endothelial cell adherens junctions. Circ Res. 1999;85:504–514. doi: 10.1161/01.res.85.6.504. [DOI] [PubMed] [Google Scholar]
- 43.Corada M, et al. Vascular endothelial-cadherin is an important determinant of microvascular integrity in vivo. Proc Natl Acad Sci USA. 1999;96:9815–9820. doi: 10.1073/pnas.96.17.9815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hordijk PL, et al. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112:1915–1923. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 45.Stupack DG, Cheresh DA. Integrins and Angiogenesis. Curr Top Dev Biol. 2004;64:207–238. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- 46.Aird WC. Phenotypic heterogeneity of the endothelium: I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- 47.Kang J, et al. An exquisite cross-control mechanism among endothelial cell fate regulators directs the plasticity and heterogeneity of lymphatic endothelial cells. Blood. 2010;116:140–150. doi: 10.1182/blood-2009-11-252270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leung BM, Sefton MV. A modular approach to cardiac tissue engineering. Tissue Eng - Part A. 2010;16:3207–3218. doi: 10.1089/ten.tea.2009.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung S, et al. Cell migration into scaffolds under co-culture conditions in a microfluidic platform. Lab Chip. 2009;9:269–275. doi: 10.1039/b807585a. [DOI] [PubMed] [Google Scholar]
- 50.Wu HC, et al. Coculture of endothelial and smooth muscle cells on a collagen membrane in the development of a small-diameter vascular graft. Biomaterials. 2007;28:1385–1392. doi: 10.1016/j.biomaterials.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 51.McGuigan AP, Sefton MV. The thrombogenicity of human umbilical vein endothelial cell seeded collagen modules. Biomaterials. 2008;29:2453–2463. doi: 10.1016/j.biomaterials.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khan OF, et al. Differentiation of mesenchymal stromal cells embedded within an endothelial cell-seeded modular tissue engineered construct formed in a microfluidic flow chamber. Tissue Eng. 2010 Submitted. [Google Scholar]
- 53.Leung BM, Sefton MV. A modular tissue engineering construct containing smooth muscle cells and endothelial cells. Ann Biomed Eng. 2007;35:2039–2049. doi: 10.1007/s10439-007-9380-0. [DOI] [PubMed] [Google Scholar]
- 54.Toole BP, et al. Hyaluronan-cell interactions in cancer and vascular disease. J Biol Chem. 2002;277:4593–4596. doi: 10.1074/jbc.R100039200. [DOI] [PubMed] [Google Scholar]
- 55.Chen WYJ, Abatangelo G. Functions of hyaluronan in wound repair. Wound Repair Regen. 1999;7:79–89. doi: 10.1046/j.1524-475x.1999.00079.x. [DOI] [PubMed] [Google Scholar]
- 56.Zavan B, et al. Neoarteries grown in vivo using a tissue-engineered hyaluronan-based scaffold. FASEB J. 2008;22:2853–2861. doi: 10.1096/fj.08-107284. [DOI] [PubMed] [Google Scholar]
- 57.Tang ZCW, et al. The enhancement of endothelial cell therapy for angiogenesis in hindlimb ischemia using hyaluronan. Biomaterials. 2011;32:75–86. doi: 10.1016/j.biomaterials.2010.08.085. [DOI] [PubMed] [Google Scholar]
- 58.Draget KI, Taylor C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocolloids. 2011;25:251–256. [Google Scholar]
- 59.Silva EA, et al. Material-based deployment enhances efficacy of endothelial progenitor cells. Proc Natl Acad Sci USA. 2008;105:14347–14352. doi: 10.1073/pnas.0803873105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yu J, et al. The effect of injected RGD modified alginate on angiogenesis and left ventricular function in a chronic rat infarct model. Biomaterials. 2009;30:751–756. doi: 10.1016/j.biomaterials.2008.09.059. [DOI] [PubMed] [Google Scholar]
- 61.Laschke MW, et al. Incorporation of growth factor containing Matrigel promotes vascularization of porous PLGA scaffolds. J Biomed Mater Res A. 2008;85:397–407. doi: 10.1002/jbm.a.31503. [DOI] [PubMed] [Google Scholar]
- 62.Shaikh FM, et al. Fibrin: A natural biodegradable scaffold in vascular tissue engineering. Cells Tissues Organs. 2008;188:333–346. doi: 10.1159/000139772. [DOI] [PubMed] [Google Scholar]
- 63.Chen X, et al. Prevascularization of a fibrin-based tissue construct accelerates the formation of functional anastomosis with host vasculature. Tissue Eng - Part A. 2009;15:1363–1371. doi: 10.1089/ten.tea.2008.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Unger RE, et al. The rapid anastomosis between prevascularized networks on silk fibroin scaffolds generated in vitro with cocultures of human microvascular endothelial and osteoblast cells and the host vasculature. Biomaterials. 2010;31:6959–6967. doi: 10.1016/j.biomaterials.2010.05.057. [DOI] [PubMed] [Google Scholar]
- 65.Wang X, et al. Controlled release from multilayer silk biomaterial coatings to modulate vascular cell responses. Biomaterials. 2008;29:894–903. doi: 10.1016/j.biomaterials.2007.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamada Y, et al. Synthetic osteopontin-derived peptide SVVYGLR can induce neovascularization in artificial bone marrow scaffold biomaterials. Dent Mater J. 2007;26:487–492. doi: 10.4012/dmj.26.487. [DOI] [PubMed] [Google Scholar]
- 67.Zhao Y, et al. The development of a tissue-engineered artery using decellularized scaffold and autologous ovine mesenchymal stem cells. Biomaterials. 2010;31:296–307. doi: 10.1016/j.biomaterials.2009.09.049. [DOI] [PubMed] [Google Scholar]
- 68.Derham C, et al. Tissue engineering small-diameter vascular grafts: Preparation of a biocompatible porcine ureteric scaffold. Tissue Eng - Part A. 2008;14:1871–1882. doi: 10.1089/ten.tea.2007.0103. [DOI] [PubMed] [Google Scholar]
- 69.Pektok E, et al. Degradation and healing characteristics of small-diameter poly(epsilon-caprolactone) vascular grafts in the rat systemic arterial circulation. Circulation. 2008;118:2563–2570. doi: 10.1161/CIRCULATIONAHA.108.795732. [DOI] [PubMed] [Google Scholar]
- 70.Serrano MC, et al. Nitric oxide production by endothelial cells derived from blood progenitors cultured on NaOH-treated polycaprolactone films: A biofunctionality study. Acta Biomaterialia. 2009;5:2045–2053. doi: 10.1016/j.actbio.2009.02.034. [DOI] [PubMed] [Google Scholar]
- 71.Santos MI, et al. Response of micro- and macrovascular endothelial cells to starch-based fiber meshes for bone tissue engineering. Biomaterials. 2007;28:240–248. doi: 10.1016/j.biomaterials.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 72.Kraehenbuehl TP, et al. Human embryonic stem cell-derived microvascular grafts for cardiac tissue preservation after myocardial infarction. Biomaterials. 2011;32:1102–1109. doi: 10.1016/j.biomaterials.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madden LR, et al. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc Natl Acad Sci USA. 2010;107:15211–15216. doi: 10.1073/pnas.1006442107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motlagh D, et al. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J Biomed Mater Res A. 2007;82:907–916. doi: 10.1002/jbm.a.31211. [DOI] [PubMed] [Google Scholar]
- 75.Cunningham KS, Gotlieb AI. The role of shear stress in the pathogenesis of atherosclerosis. Lab Invest. 2005;85:9–23. doi: 10.1038/labinvest.3700215. [DOI] [PubMed] [Google Scholar]
- 76.Li YS, et al. Molecular basis of the effects of shear stress on vascular endothelial cells. J Biomech. 2005;38:1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 77.Baffert F, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 78.Sima AV, et al. Vascular endothelium in atherosclerosis. Cell Tissue Res. 2009;335:191–203. doi: 10.1007/s00441-008-0678-5. [DOI] [PubMed] [Google Scholar]
- 79.Mizuno T, et al. Visual evaluation of blood coagulation during mural thrombogenesis under high shear blood flow. Thromb Res. 2007 doi: 10.1016/j.thromres.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 80.Gupta R, et al. Fate of endothelialized modular constructs implanted in an omental pouch in nude rats. Tissue Eng - Part A. 2009;15:2875–2887. doi: 10.1089/ten.tea.2008.0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pober JS. Immunobiology of human vascular endothelium. Immunol Res. 1999;19:225–232. doi: 10.1007/BF02786490. [DOI] [PubMed] [Google Scholar]
- 82.Aird WC. Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ Res. 2007;100:174–190. doi: 10.1161/01.RES.0000255690.03436.ae. [DOI] [PubMed] [Google Scholar]
- 83.Corselli M, et al. Perivascular ancestors of adult multipotent stem cells. Arterioscler Thromb Vasc Biol. 2010;30:1104–1109. doi: 10.1161/ATVBAHA.109.191643. [DOI] [PubMed] [Google Scholar]