Abstract
Objective
To describe obstacles in the implementation of a controlled treatment trial of adolescent anorexia nervosa (AN).
Method
The original aim was to enter 240 participants with AN to one of 4 cells: Behavioral family therapy (BFT) plus fluoxetine; BFT plus placebo; systems family therapy (SFT) plus fluoxetine; SFT plus placebo.
Results
Recruitment was delayed pending a satisfactory resolution concerning participant safety. After 6 months of recruitment it became clear that the medication was associated with poor recruitment leading to a study redesign resulting in a comparison of two types of family therapy with a projected sample size of 160. One site was unable to recruit and was replaced.
Discussion
Problems with the delineation of safety procedures, recruitment, re-design of the study, and replacement of a site, were the main elements resulting in a 1-year delay. Suggestions are made for overcoming such problems in future AN trials.
Keywords: Anorexia nervosa, adolescents, Behavioral Family Therapy, Systems Family Therapy, RIAN trial
Introduction
There are few randomized controlled trials (RCTs) of psychosocial treatments for Anorexia Nervosa (AN), a serious psychiatric disorder typically with onset during adolescence and a clinical course leading to significant medical morbidity and mortality (1–3). Of the 15 published RCT’s 9 involved adults and 6 adolescents (4). Studies are limited by a range of problems including difficulty with recruitment limiting sample size, high attrition rates (especially in adult studies threatening the validity of randomization), use of inadequate assessments, and a lack of standardized outcome measures. As a result of these difficulties the published trials are mostly small in scale, with average cell sizes below 20 per group. Hence, they provide limited guidance to clinicians. The failure of existing studies to address these problems can be viewed as a significant factor in the overall lack of evidence-based treatments for AN.
In 2002 the National Institute of Mental Health (NIMH) convened a workshop to review the state of research in AN. (5) This revealed that less than a dozen psychotherapy trials had been published at that time and that only one had been carried out in the U.S.(6) In response to this workshop and the lack of evidence-based treatments for AN, the NIMH issued an RFA (MH-05-009 “Request for Applications for Research on Interventions for Anorexia Nervosa (RIAN)” to encourage new treatment research. This report summarizes the challenges faced by the group of investigators who were funded through this RFA to conduct a treatment study for adolescent AN. In the process of implementing the study, a number of significant obstacles arose necessitating changes in the research design and timing of the research project. In this paper we describe how we addressed these obstacles in hopes of aiding future studies. (7)
Study Background
The choice of an adolescent study was guided first by the findings that adult trials were plagued by high rates of non-compliance to assigned treatments and high study dropout.(8) Moreover, there was no evidence that any treatment in adults was effective.(4) Further, studies suggested that not only was the onset of AN largely in adolescence, but that adolescents were more likely to remain in and respond to treatment,(9) especially if it involved families.(10) Four small-scale clinical trials suggested that a specific form of family-based therapy (FBT) was likely to be effective with adolescent patients with AN.(6, 11–14) In two of these trials, FBT appeared to outperform individual therapy, though studies were modest in scale and conclusions appropriately circumspect. (6, 13, 14) Although these initial studies were encouraging, it had been hypothesized but not definitively demonstrated that the procedures unique to FBT, namely charging parents with re-feeding their child, were responsible for its effectiveness.(15) A study that targeted adolescents and compared FBT to another family approach that did not charge parents to re-feed their child, Systems Family Therapy (SFT)(16), would provided new insight into the effectiveness and mechanism of action of FBT.
Other data suggested that some characteristics of adolescents might contribute to their response to treatment. Patients with AN are frequently perfectionistic and have obsessive-compulsive personality traits in childhood. These traits are often evident before the onset of their eating disorder. (17, 18) In addition, a substantial body of literature suggests that early onset anxiety disorders are a risk factor for the development of AN in girls. (19–21) (22–24) Studies (25–29) have also found that patients who have recovered from AN continue to manifest residual anxiety, perfectionism, inflexible thinking, restraint in emotional expression, social introversion, and obsessions related to symmetry, exactness, and order. This is underscored by a finding that OC-ED symptoms may moderate the outcome of FBT in that those with higher scores on the trait did less well with short-term treatment. (30) Fluoxetine has been shown to be helpful in treating anxiety disorders and obsessive-compulsive behaviors in adolescents,(31, 32). While these medications are often prescribed for adolescents with AN, their role in treatment in this age group remains uninvestigated.(33) Therefore, an additional question to be addressed was whether the addition of fluoxetine in the context of family therapy would enhance outcome by addressing obsessive, and anxious traits that might interfere with treatment response.(30)
Original Study Design
Overview
Based on these background data, our initial study was designed to address two major specific aims:
To compare the relative effectiveness of FBT to SFT for adolescent AN in an adequately powered randomized controlled trial (RCT) to determine whether the effects of FBT are due to specific or non-specific elements of treatment.
To compare the relative efficacy of fluoxetine versus placebo in augmenting recovery and preventing relapse after treatment with either FBT or SFT in adolescents with AN.
To accomplish these aims, the initial proposal called for 240 adolescents with a diagnosis of AN to be randomly assigned to one of the two family therapy treatments at six sites (40 per site) with the addition of either fluoxetine or placebo. Medication was to be continued for 6-months following the end of family therapy. Participants would then be followed for an additional six months. This design would allow evaluation of the added effects of fluoxetine to family therapy during both treatment and maintenance of weight restoration as suggested by two small-scale studies in adults at the time. (34, 35) Comprehensive assessments of weight, eating psychopathology, psychological, and family factors would occur at baseline, end of treatment, and six-month and one-year follow-up. The primary outcome was change in weight as measured by age adjusted body mass index (BMI).(36, 37) In addition, both parents were assessed. The assessments involved standardized research interviews, patient reports, and questionnaires. An independent assessor not involved in treatment delivery and therefore blind to randomization would conduct all assessment interviews. Assessments were selected to evaluate outcome, as well as moderators, and mediators of treatment.
Defining AN
As the literature attests there are several problems with the DSM-IV criteria for the diagnosis of AN.(38) In line with these criticisms we made two modifications to the criteria. We dropped the amenorrhea criteria (39). Second, we modified the weight criterion for entry to this study to an Ideal Body Weight (IBW) of 87% or below based on exclusions in previous studies (30, 40). By making these changes, we believe we identified a research population that is typical of most adolescents presenting with AN in clinical settings. In addition, based on inconsistencies between different observers when the CDC charts were used by hand to calculate IBW, a computerized system was developed to calculate IBW using an Excel program. When using this program the assessment date is first entered, then the patient’s gender, date of birth, height in inches and weight in pounds. The program then calculates and displays the % IBW based on the CDC tables for height and weight adjusted for age.
Collaborating Sites
Highly specialized clinical and research sites were identified in North America to carry out this research (see Table 1). The treatment sites chosen were directed by established clinicians and researchers in the field of eating disorders. As such, these sites were considered likely to be successful in the recruitment, evaluation and treatment of participants. In addition an independent Data and Coordinating center (DCC) with experience in the management and coordination of multisite clinical trials with eating disorders was included.(8, 30, 41) Based on recruitment rates of 20 adolescent participants/year from previous studies (30, 40) it was thought that 6 treatment sites would allow for recruitment of 240 participants over a 2 year period. In addition, this number of sites permitted geographical and sociodemographic diversity that could lead to better generalization of the findings. The study was funded using an NIH U-Mechanism grant structure. NIMH appointed a Data and Safety Monitoring Board (DSMB) to provide oversight of the study in addition to each site obtaining IRB approval. See Table 1 for Intervention sites and DCC involved.
Table 1.
Intervention Sites and Data Center
|
Family Treatments
Both FBT(42) and SFT(43) consist of 14 treatment sessions conducted over a 9 month period, each lasting one hour. Sessions are spaced weekly for the first 8 weeks, bi-weekly for the next 4 weeks, and monthly for the remaining 2 sessions although some flexibility is allowed.
Family Based Treatment (FBT ) is a 3 phase manualized treatment that has been used in previous treatment studies of adolescent AN.(30, 42) In the first phase (sessions 1–8), therapy is focused on the eating disorder, and includes a family meal at session 2. The latter provides the therapist with an opportunity for direct observation of the familial interaction patterns around eating. The therapist makes careful and persistent requests for united parental action directed toward weight restoration, which is the primary concern at this early point of the treatment. In addition, the therapist directs the discussion in such a way as to create and reinforce a strong parental alliance around their efforts at weight restoration of their offspring on the one hand, and aligning the patient with the sibling sub-system on the other. Phase 2 (sessions 9–12) begins after patient demonstrates steady weight gain under parental supervision and the parents feel their child is able to begin age-appropriate eating more independently again. Symptoms remain central in the discussions and weight gain with minimum tension is encouraged. Only when the adolescent is able to eat independently and demonstrate freedom from the preoccupations of AN, do other issues about adolescent development and termination come to the fore (Phase 3, sessions 13–14).
Systemic Family Therapy (SFT) is a slightly modified family psychotherapy that was developed and manualized by researchers at Leeds University as a model of treatment that is used in practice. It is based on family-systems therapy. (43, 44) SFT is focused on patterns of behavior and beliefs that have developed in the family over the course of time. Understanding these patterns provides the therapist with the opportunity to give new information through which different solutions can be generated. This treatment deals with the family as a system and how they ‘organize’ themselves as a family in terms of their different roles and relationships. The goals of Stage 1 (sessions 1 and 2) are to 1) outline therapy boundaries and structure; 2) engage and involve all family members; 3) gather and clarify information; and 4) establish goals and objectives of the therapy. In undertaking these tasks the therapist provides a supportive environment, remains neutral, and enlists everyone’s involvement. During Stage 2 (sessions 3–8) the goals are to 1) explore beliefs and assumptions, challenging existing patterns and assumptions; reframe constraining ideas; and open new possibilities for examination. During stage 3 (sessions 9–14) the therapist focuses on information gathering and reviewing and refining the material brought by the family to sessions as in Stage 2, though the focus of the information is likely to be considerably different. It is more common in the end sessions for the focus to be on amplifying change, enhancing mastery, re-framing, and developing new explanations. Termination occurs with the therapist inviting the family to review the process of therapy with the aim of preventing future difficulties and planning for any future needs for therapeutic services.
To ensure that the two family treatments were delivered in a comparable fashion across sites, special attention was given to the identification, training, supervision, and monitoring of therapists over the study period. The criteria for selection of potential therapists included requirements that they hold a Master’s or PhD level qualification in psychology, social work or family therapy, and that they have had at least one years’ experience in treating individuals with eating disorders. With six sites, an average of 2 therapists per site per condition allowed for the decision to be made that therapists would be trained in only one of the two treatments. It was felt that with this number of therapists, therapist non-specific factors would be spread across a relatively large group, thereby mitigating potential confounding effects of these factors on outcome. Training events were therefore held separately for each treatment type. Therapists from all sites using each type of therapy were trained together, beginning with a two-day intensive workshop. Both training sessions employed manuals that formed the basis of treatment in both conditions. Training was conducted by highly experienced and trained therapists in each condition (JL in FBT and LD in SFT). In addition to training therapists, each site had a supervisor in both conditions.
After the initial workshops, therapists were assigned two pilot cases to complete under the direct supervision of local site supervisors. Sessions were taped and reviewed by the site supervisor, and supplemented by weekly face-to-face supervision. Once a therapist had successfully completed a phase of the treatment, tapes were forwarded to the relevant training supervisor (JL for FBT, LD for SFT) at the DCC. Supervisors would provide feedback and eventually certify the therapist as competent in the treatment. After competence was achieved, the therapist could accept randomized subjects.
Once cases began to be randomized, therapists continued to be involved in weekly face-to-face supervision at their local site. All sessions were recorded and viewed by the site supervisor to assess adherence to the manuals and to assist with the clinical supervision of the cases. Two additional group training sessions were held for each treatment type under the direction of the training supervisors at one-year intervals to allow for therapists from all sites to meet and to review problems that had arisen related to adherence to the models, and clinical difficulties that had been encountered in the treatment of the families. In addition to site supervision, a schema of paired-site teleconferences was developed, where two sites would be rotated in pairs for several months to minimize site drift. There was a monthly site supervisors’ supervision conducted by the training therapists in the DCC to support the site supervisors with particularly challenging cases and to increase supervisory consistency across sites.
Medication treatment
A secondary aim of the original design called for fluoxetine or placebo treatment to begin at the point of randomization. The plan was for medication treatment to be supervised by psychiatrists at each site. Changes in symptoms and side effects would be monitored at each session with particular attention to suicidal ideation. Treatment would begin with a dose of 20mg fluoxetine, and could be increased to 60mg based on clinical status. Medication could be increased at 2-week or longer intervals if indicated. Treatment was scheduled to continue for 6-months after the end of family therapy. At the end of this period the blind would be broken and the family and participant informed of whether the patient had been on active medication. For those wishing to continue on active medication their physician would be contacted and, with the family’s permission, given details regarding dosage, side effects, etc. Those wishing to stop medication would be withdrawn from medication under supervision of the site psychiatrist.
Participant safety
There can be serious medical problems associated with severe malnutrition and emaciation, including bradycardia, hypotension, hypothermia, orthostatic hypotension, as well as electrolyte and fluid abnormalities.(45) Emergency coverage of participants was to be provided at each site by the therapists, study PI and the participant’s pediatrician. Initial and ongoing medical oversight of participants was a requirement for all participants to ensure medically stability for outpatient treatment. The criteria for medical stability of adolescents with eating disorders used in the study were those identified by the Academy of Pediatrics and the Society of Adolescent Medicine.(45, 46) The decision to hospitalize patients was to be made on these standardized criteria by a pediatrician blind to the treatment group to which the patient was assigned. These specific criteria included: vital sign instability (heart rate less that 50, orthostatic blood pressure changes greater than 35 points, or clinically significant symptoms or findings e.g. evidence of gastrointestinal bleeding, dizziness, syncope), IBW < 75%, hypothermia (body temperature less than 36 degrees centigrade), electrolyte abnormalities, or prolonged QTC interval on electrocardiogram. After hospitalization, participants would return to their allocated therapeutic arm unless they were hospitalized for more than 28 consecutive days, in which case they would be withdrawn from treatment. Records of all hospitalizations (date, reason for hospitalization, length of stay and types of treatment received) were to be obtained for all patients hospitalized and entered into the database to examine any potential differences in hospital use between treatment groups.
Challenges with Implementation of the Original Design
The limited data base on treatment studies for AN is related to a variety of difficulties that can interfere with the completion of such studies.(7, 8) Gowers et al (1989) in one of the first papers describing the methodological problems encountered in a treatment study of anorexia nervosa commented “From the onset methodological problems were evident. As the study progressed more have relentlessly emerged.” We also encountered a number of problems during the early implementation of the study. We describe these difficulties and our response to them to illustrate problems that others might face, We also wanted to explain how the study design changed in response to these problems.
Potential need for Hospitalization During the Study
In the original design, pediatric monitoring, including physical examinations and laboratory tests, was to be accomplished by physicians outside the study in order to minimize study costs. This followed the practice of previous controlled trials in adult and adolescent populations.(30, 40) It was expected that the required laboratory tests and medical examinations would be paid for by the participant’s insurance. However in this study because there were insufficient funds to cover hospitalization costs, in the consenting process participants and their parents were notified that they would be responsible for the costs of hospitalization either through insurance or personal finances should it become necessary. This was particularly important as the study design mandated hospitalization for safety reasons at 75% of Ideal Body Weight (IBW) or other signs of significant medical instability (45–46). Interestingly only 2 potential participants were excluded for this reason.
Medication use with Anorexia Nervosa
During the first year of the study there was considerable discussion between the investigators and the DSMB concerning participant safety particularly because of the use of fluoxetine.(47) The basic issue was that the DSMB considered that responsibility for enhanced safety monitoring must reside within the trial rather than with an outside pediatrician. Ultimately, the DSMB required recruitment to cease until these issues were resolved. Following detailed discussions with the DSMB a schedule for surveillance of weight, vital signs, suicidality, liver function, EKG, and other tests was developed (see Tables 2 and 3). These safety assessments were to be paid for by the trial intervention sites and decisions regarding participant safety were to be made by the trial psychiatrist and the PI at each site in consultation with the outside pediatrician when necessary. Outside pediatricians continued to monitor each participant’s medical stability and the within trial test results were sent to these pediatricians. Establishment of the new safety guidelines with clearance from the DSMB and updating consent forms led to a 6-month delay in recruitment. Hence recruitment began on January 1, 2008.
Table 2.
Within site medical and psychiatric assessments for medical safety
| Assessment | Baseline | Week 1 | Weeks 2–4 | Weeks 5–20 | Months 6–9 |
|---|---|---|---|---|---|
| Vital signs | X1 | x 2 | Weekly | Bi-weekly | Monthly |
| Weight and Height | x 1 | x 2 | Weekly | Weekly | Monthly |
| Symptom functioning, suicidality | x 1 | x 2 | Weekly | Bi-weekly | Monthly |
Table 3.
Medical monitoring and laboratory testing
| Baseline | Week-8 | 6-months | 9-months | |
|---|---|---|---|---|
| EKG | X | X | X | X |
| Electrolytes | X | Monthly | ||
| Liver Function Tests | X | X | X | |
| BUN | X | X | X | X |
| Creatinine | X | X | X | X |
| CPK | X | X | X | X |
| Urinalysis | X | X | X | X |
| Pregnancy test | X | Months 3, 6, 12, 15 |
In addition to safety concerns with fluoxetine, recruitment of adolescent participants was negatively affected because of participant/family resistance to fluoxetine. Prior to initiation of recruitment, there was widespread public concern and significant media attention suggesting that that the use of antidepressants may increase the likelihood of suicidal behavior in youths.(47) Following a thorough and comprehensive review of all the available published and unpublished controlled clinical trials of antidepressants in children and adolescents, the U.S. Food and Drug Administration (FDA) issued a public warning (http://www.fda.gov/Drugs/DrugSafety/InformationbyDrugClass/UCM096273) in October 2004 about an increased risk of suicidal thoughts or behavior in children and adolescents treated with SSRI’s.(47) In 2006, an advisory committee to the FDA recommended that the agency extend the warning to include young adults up to age 25. More recently, results of a comprehensive review of pediatric trials conducted between 1988 and 2006 suggested that the benefits of antidepressant medications likely outweigh their risks to children and adolescents with major depression and anxiety disorders. The study, partially funded by NIMH, was published in the April 18, 2007, issue of the Journal of the American Medical Association (48) and was widely reported in the press shortly before recruitment to the trial was scheduled to begin (July 1, 2007). Because of these warnings the following additional risks of taking these medications were added to the consent and assent documents: :the risk of developing a serotonin syndrome in participants taking triptans (migraine medications) with an SSRI and risk of neonatal persistent pulmonary hypertension in infants born to mothers taking SSRIs after the 20th week of pregnancy. The DSMB also encouraged investigators to include a description of risks observed in animal studies of fluoxetine (including developmental effects and emotional behaviors).
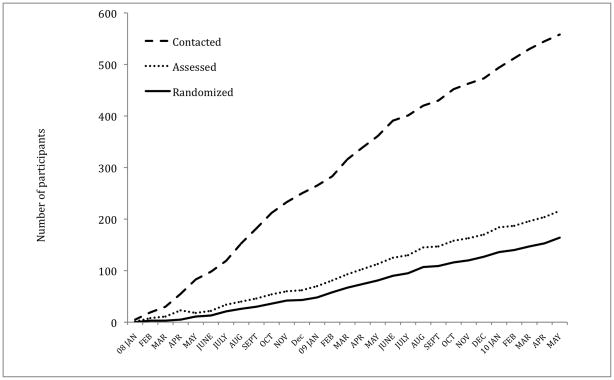
The negative media attention about antidepressant risk together with the risks detailed in the consent forms had a profound impact on early recruitment. After 6-months of recruitment (July 1, 2008), only 20 individuals had been entered to the study (See Figure 1). Forty-seven percent of individuals eligible for the study had refused fluoxetine and a further 10% were on medications that called for exclusion. It therefore became clear to the study steering committee and to the DSMB that fluoxetine was a major barrier to recruitment. Moreover, even at this early stage of the trial, 25% of participants had withdrawn from the medication arm of the study. Consequently, the PI Steering Committee decided to remove the medication arms of the study to enhance recruitment.. To address these changes, participants on medication were unblinded and given the opportunity to stop or continue fluoxetine. The majority elected to stop the medication and fluoxetine was withdrawn under supervision of the study psychiatrist at each site. Withdrawal was managed on a graduated schedule dependent on dosage and clinical response with weekly visits until withdrawal was completed and the participant was stable. Because suicidal ideation may increase during this particular phase particular attention was paid to both depression and suicidal thinking during the withdrawal period. Only 3 participants remained on fluoxetine. These participants will be included in the group of participants who were on medication at entry to the study or who were placed on medication during the trial. Hence medication status will be analyzed as a moderator of treatment outcome.
Figure 1.
Graph showing the rates of study contacts, assessment, and study entries for all sites combined. The medication arm of the study was removed (including DSMB and sites IRB approval) on January 1, 2009 and the Stanford site began recruiting at the same time.
Family Treatment Alone vs. Multimodal, Multidisciplinary Treatment
Another significant barrier to recruitment centered on participant concerns about entering a trial where family therapy was the only treatment modality. This was particularly true at sites that had previously promoted or emphasized the availability, and importance of multi-modal, multi-disciplinary treatment for eating disorders. The study was designed to evaluate the specific role of family therapy in adolescent AN and specifically excluded the use of nutritional counseling, individual psychotherapy, and other forms of intervention. Despite ongoing, intensive efforts to recruit participants, some of the RIAN sites found that their traditional multimodal treatments were being selected instead of the research protocol by potential research participants. Ultimately, such problems led to the need to withdraw one site from participating because of failure to recruit sufficient participants. The site recruited only 3 participants in 9-months and recruitment was not enhanced after the removal of the study medication. The site was replaced with an additional intervention site to assure the study could complete recruitment in a timely fashion. The change was feasible because the new site had clinicians already trained in both therapies, as well as a team trained in the assessment procedures. Further, the site had a history of successful recruitment of adolescent AN subjects into treatment trials. The substitute site began recruiting in January 2009. Recruitment from the combined sites then attained a fairly steady pace of 7 participants/month over the next 12-months compared with 3.75 participants/month for the previous year.
Randomization Difficulties Related to Publicity About “Maudsley” Family Therapy
An unanticipated recruitment obstacle at some sites resulted from the burgeoning interest in, and publicity about, the “Maudsley Model”, or FBT. Because so little is known about effective treatments for AN and preliminary studies have supported FBT, many families were interested in receiving this treatment. While on the surface this would appear to be of substantial benefit in recruiting participants, paradoxically, some parents and participants were hesitant to enter a randomized trial with a 50% chance of not receiving FBT. This recruitment issue was further complicated by community discussion by potential research participants and their families at support groups and on web-based eating disorder chat groups (e.g., Maudsley Parents (www.maudsleyparents.org) and F.E.A.S.T (www.feast-ed.org)
Controversies Related to Weight Criteria and Use of Hospitalization for Medical Stabilization
Another issue that emerged in recruitment of participants was that many potential participants were not at a sufficiently low weight for inclusion. They met DSM-IV criteria for EDNOS as opposed to AN, even though the cut-point for entry to the study was an IBW of 87%. Although evidence suggests these patients are often as medically vulnerable as those who meet full diagnostic thresholds (49), and early intervention is likely the best approach (Hoek & Hoekan, 2003), these cases had to be excluded
Conversely, some participants presented to treatment sites meeting study weight criteria but with serious medical/physiological morbidity and illness severity warranting treatment in higher levels of care (i.e., inpatient or day-treatment). This has posed another recruitment obstacle, not only because the participant was initially ineligible to enter the trial because it was unsafe to treat the patient as an outpatient, but also because in some cases, weight gained in the higher level of care was of a magnitude (i.e., > 87% IBW) that ultimately excluded the participant from the trial. This problem is, in part, created by an incomplete understanding of the role of medical and psychiatric hospitalization for adolescent AN. While guidelines for medical safety of these patients have been published, the basis of the specific thresholds are derived from professional consensus rather than scientific study.(45, 50) This is particularly the case for weight thresholds, where medical discharge varies from 75%–100% of IBW depending on program treatment philosophy.(51)
Final Study Design
The changes outlined above resulted in a 6 sites X 2 treatments (FBT and SFT) design. This reduced the participant recruitment burden from 240 participants to 160 participants with an 88% power to detect a moderate difference between groups. A moderate effect in this case is a Cohen’s d (standardized mean difference between two groups) of 0.5. With this effect size, the probability that a randomly selected participant in one group, say FBT has an outcome clinically preferable to one from the other group, say SFT, is 63.8%.
Final recruitment results
As shown in Table 4 and Figure 1, 564 potential participants were contacted of which 216 were interviewed and 164 entered into the study in 29 months (January 1st 2008 to May 31st 2010). Reasons for exclusion are shown in Table 5. The most common reason was not meeting the weight criteria for the study (N=154) i.e. having a weight below 75% IBW or above 87% IBW. The next most common reason (N=101) was refusal to stop current treatment or refusal of family therapy.
Table 4.
Numbers of individuals contacted, screened, eligible, interviewed and randomized by site.
| Cornell | Laureate | Sheppard Pratt | Stanford | Toronto | UCSD | Washington University | Total | |
|---|---|---|---|---|---|---|---|---|
| Contacted | 77 | 30 | 106 | 81 | 79 | 118 | 73 | 564 |
| Screened | 74 | 30 | 106 | 80 | 77 | 118 | 73 | 558 |
| Eligible | 41 | 9 | 59 | 55 | 45 | 56 | 40 | 305 |
| Interview | 31 | 4 | 31 | 40 | 36 | 36 | 38 | 216 |
| Entered | 30 | 3 | 22 | 28 | 28 | 25 | 28 | 164 |
Table 5.
Reasons for exclusion from the study
| Cornell University | Laureate | Sheppard-Pratt | Stanford University | Toronto | UCSD | Washington University | Total | |
|---|---|---|---|---|---|---|---|---|
| INELIGIBLE BY STUDY PROTOCOL | ||||||||
| Does not meet weight criteria | 16 | 10 | 29 | 16 | 19 | 39 | 25 | 154 |
| Does not want to discontinue present treatment | 14 | 3 | 0 | 2 | 2 | 4 | 8 | 33 |
| Out of age range | 5 | 2 | 0 | 2 | 5 | 7 | 2 | 23 |
| On prohibited medications | 2 | 2 | 9 | 1 | 3 | 2 | 1 | 20 |
| Barriers (no insurance, transportation, language) | 0 | 1 | 1 | 0 | 1 | 6 | 5 | 14 |
| Medical or psychiatric exclusion | 1 | 1 | 2 | 1 | 3 | 3 | 0 | 11 |
| Previous family therapy | 0 | 0 | 4 | 1 | 1 | 1 | 0 | 7 |
| Not able to fulfill time requirements | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 6 |
| Parents excluded | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 5 |
| Other | 0 | 2 | 0 | 0 | 1 | 2 | 0 | 5 |
| Substance dependence | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INELIGIBLE BY SUBJECT/FAMILY DECISION | ||||||||
| Prefer other treatment /Refuse family therapy | 11 | 2 | 15 | 19 | 6 | 12 | 3 | 68 |
| Lost contact | 0 | 1 | 10 | 2 | 3 | 5 | 1 | 22 |
| Refuses medication | 2 | 3 | 7 | 0 | 2 | 2 | 1 | 17 |
| Out of Area | 0 | 0 | 1 | 2 | 3 | 10 | 1 | 17 |
| Other | 4 | 0 | 4 | 1 | 3 | 0 | 0 | 12 |
| Did not show for interview | 0 | 0 | 2 | 3 | 0 | 3 | 0 | 8 |
| Refuses to participate in randomization process | 0 | 0 | 1 | 1 | 1 | 2 | 0 | 5 |
| Did not sign consent | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
Forty-nine participants were entered during the first year of recruitment and 115 after January 1st 2009. As can be seen in Figure 1, the ratio of initial contacts to entries increased over the course of the study. However, the ratio of those assessed to those entered changed much less, suggesting that over the course of the study a larger number of unsuitable potential participants were referred. This increases the workload as recruitment progresses. As shown in Table 1, the ratios of entered participants to contacts ranged from 10% at Laureate the least efficient, to 39% at Cornell the most efficient. Only one site did not have an inpatient unit from which the bulk of cases were recruited namely, UCSD. Hence, it took the most contacts (118) to enter 25 participants because this site relied on recruitment from the community. However, Cornell maintained its efficient ration despite the fact its inpatient unit was closed for renovations for 1 year. Recruitment rates also varied considerably with Stanford recruiting at the rate of 1.6 participants/month compared with an average of 0.9 participants/month at the other sites (excluding the Laureate site). These data illustrate that multisite studies may recruit from different settings and emphasize the importance of careful delineation of “caseness” in the context of an RCT in order to assure that similar participants are included across the sites.
Discussion
Previous treatment studies of AN have focused on a number of problems, the most daunting of which is the difficulty in recruiting sufficient participants although this difficulty appears less problematic in adolescents than adults.(7, 8, 30, 52, 53) In a multisite trial enrolling a majority of adults it appeared that the pool of potential participants began to shrink after 2 years and was largely drained within 4 years.(8) In retrospect this is not surprising because the adult pool is refilled slowly as those who have failed treatment in adolescence come into the pool. Many of these individuals are unwilling to seek treatment. The adolescent pool on the other hand is continually refilled with new cases and adolescents are essentially unable to refuse treatment.
In the RIAN study the numbers entered varied between sites as did the entry rates and one site was discontinued because of a low entry rate. This raises a difficult problem for future research. Because AN has a relatively low incidence many studies will have to rely on a multisite design. For this reason it will be necessary to identify sites in the U.S. capable of recruiting sufficient participants to engage in such trials. Our study makes it clear that forecasting recruitment rates is difficult unless there is prior experience in each of the sites with a similar trial. In the RIAN trial none of the original sites (all but Stanford) had experience in recruiting adolescents with AN to treatment studies although some sites had experience recruiting adults with AN. The original recruitment rate was set at approximately 2 participants/month per site based on the experience of two sites in a previous multisite study.(30, 54) However, the recruitment rate for the RIAN trial averaged 0.9/month per site with considerable variability between sites. With medication the rate was slower averaging 0.6/month per site. The rate for adult AN trials will likely be considerably less. This raises the question whether future multisite studies should include more sites than appear to be needed in order to ensure successful recruitment. In the RIAN trial it was fortuitous that another site (Stanford) with therapists trained in each modality and with prior experience in recruitment of adolescents with AN was available to replace the site with recruitment difficulties.
A further problem noted in many AN studies is a large dropout rate (8, 55), sometimes close to half the participants in adult trials.(56) This raises the question at which level of dropout has the initial randomization been lost making the study impossible to analyze.(10, 57–59) Moreover, it is possible that there will be an interaction between participant characteristics and treatment type for dropouts. Again, this problem appears less severe in adolescents. However it is clearly important to put into place measures to ameliorate treatment and study dropout rates.
Although studies of medications are potentially important, adults with AN often refuse them for fear of weight gain and other undesirable side effects,(8, 56). Parents of younger patients appear to be reluctant to experiment with the use of medications at least without more specific preliminary support for their effectiveness.(60–62) Treatment studies employing medications are likely to be hampered by these limitations, as was the case in the RIAN trial, resulting in a major redesign of the study. A previous controlled study of olanzapine was abandoned because only 7 of 27 (ratio=0.26) eligible patients were enrolled due to fears concerning the medication and reluctance to consider medication as a treatment option.(62) This rate is about one half of that achieved in the RIAN trial (ratio=0.54). These figures suggest that it would have taken 6-years for the RIAN sites to recruit 240 participants for a medication study. Hence, 12 sites would have been needed to complete recruitment in a reasonable time. Our experience highlights the need for researchers planning a study of this type to consider clinical epidemiology and process to avoid repeating mistakes from previous studies.
Although the participant safety issues were resolved for this trial, they form an important precedent for other trials. Safety procedures for adolescent AN treatment studies should include the following. First, monitoring of physiologic variables should be done within the treatment sites allowing results to be rapidly reviewed and decisions regarding hospitalization to be made by study personnel. Second, in this study we elected to continue brief psychiatric interviews on a regular basis to monitor vital signs, depression, and suicidality, even though medication had been discontinued. Regular pediatric care continued outside the trial. Whether or not to bring such pediatric care within the site is a difficult decision to make. Obviously such a decision would increase trial costs. Most sites in the RIAN trial elected to use a small group of pediatricians. These were pediatricians that were often affiliated with the treatment site allowing for close communication between the pediatrician and the trial personnel. This may be the ideal arrangement, but it would restrict studies to a relatively few comprehensive treatment centers. Similar arrangements concerning physiologic monitoring and medical surveillance might be considered for treatment trials of adults with AN. The requirement regarding adequate resources should hospitalization be needed also appears important because it allows for continuity of care as well as enhanced participant safety.
Although medical monitoring of adolescents with AN is crucial to providing safety in an outpatient clinical trial, the lack of clear medical guidance on the necessary procedures may lead to undue burden and discomfort for patients. For example, although guided by clinical knowledge, there was no research basis supporting the frequency of tests decided on for this study, and the frequencies chosen probably diverge from the practice of many pediatricians providing medical monitoring for cases of adolescent AN. Should the frequency of testing be the same for all participants or should the frequency vary depending on the clinical status of the participant? Some form of algorithm governing the frequency of testing in light of the patient’s progress toward recovery might be considered in future studies. Moreover, it is unclear which tests provide the most information regarding the physiological stability of adolescents with AN.(49) Further research on this, and other aspects of medical safety is needed.
The need to make changes in study design in response to these challenges had significant impacts on study progress. As a result of delays and study changes, recruitment began 6 months later than expected. This hiatus made it more difficult for therapists to maintain mastery of treatments, disrupted assessment procedures, and delayed data entry and protocol finalization. We utilized this time to provide therapists with extra training and supervision both at individual sites and across sites. Nonetheless, each of the factors independently had the potential to cause major problems, but taken together, they significantly challenged the ability of the PIs to complete the study. Removal of the medication arms of the trial led to faster recruitment, however by the time the decision was made, agreed on by the DSMB, and the new design approved by each site based IRB, more recruitment time was lost. Recruitment difficulties also led to the replacement of one site that was not able to recruit at a sufficient rate. Again it took several months to detect this problem and to start-up a new site. These experiences highlight the need when designing such studies to address the possibilities of such delays and anticipate that procedural changes require careful review and take time.
Although it is promising that NIH is providing much needed stimulus for the study of treatments for AN the reality is that it is a difficult illness to study. Especially careful consideration of experience in recruitment, retention, and experience with treating participants with AN is warranted. In addition, dilemmas about competing philosophies of treatment and competing treatment modalities should be addressed in assessing the feasibility of a particular setting. For both adults and children with AN medication trials are particularly challenging. To date, studies mostly document the failure of acceptability of this treatment modality, rather than efficacy data.
Acknowledgments
Supported by grants: 1 U01 MH 076290, MH 076287, K24 MH-074467 (Stanford); 1U01 MH 076254 (Sheppard-Pratt) 1 U01 MH 076253 (Toronto) 1 U01 MH 076251 (Cornell), 1U01 MH 076253 (UCSD) 1 U01 MH 076250 (Laureate) 1 U01 MH 076255 (Washington University-St Louis)5K24 MH70446 (Washington University-St. Louis)
Footnotes
FINANCIAL DISCLOSURES:
The authors have the following financial disclosures: Drs. Lock and Agras receive royalties from Guilford Press for books related to Family-Based Treatment. Dr. Lock provides training and consultation for the Training Institute for Child and Adolescent Eating Disorders. Dr. Kaye is a consultant for the Denver Eating Disorder Center and receives grant funding from Astra Zeneca Pharmaceuticals. Drs. Halmi, Brandt, Wilfley, Woodside and Johnson report no financial disclosures.
References
- 1.Hoek H, Hoeken Dv. Review of prevalence and incidence of eating disorders. Int J Eat Disord. 2003;34:383–396. doi: 10.1002/eat.10222. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF. Mortality in anorexia nervosa. American Journal of Psychiatry. 1995;152:1073–1074. doi: 10.1176/ajp.152.7.1073. [DOI] [PubMed] [Google Scholar]
- 3.Crow S, Peterson C, Swanson S, Raymond N, Specker S, Eckert ED, Mitchell J. Increased mortality in bulimia nervosa dn other eating disorders. Am J Psychiatry. 2009;166:1342–1346. doi: 10.1176/appi.ajp.2009.09020247. [DOI] [PubMed] [Google Scholar]
- 4.Bulik CM, Berkman N, Kimberly A, Brownly JS, JA, Lohr K. Anorexia nervosa: a systematic review of randomized clinical trials. Int J Eat Disord. 2007;40:310–320. doi: 10.1002/eat.20367. [DOI] [PubMed] [Google Scholar]
- 5.Agras WS, Brandt H, Bulik CM, Dolan-Sewell R, Fairburn CG, Halmi CA, Herzog DB, Jimerson D, Kaplan AS, Kaye WH, Le Grange D, Lock J, Mitchell J, Rudorfer M, Street L, Streigel-Moore R, Vitousek K, Walsh BT, Wilfley D. Report of the National Institutes of Health Workshop on Overcoming Barriers to Treatment Research in Anorexia Nervosa. Int J Eat Disord. 2004;35:509–521. doi: 10.1002/eat.10261. [DOI] [PubMed] [Google Scholar]
- 6.Robin A, Siegal P, Moye A, Gilroy M, Dennis A, Sikand A. A controlled comparison of family versus individual therapy for adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatry. 1999;38(12):1482–1489. doi: 10.1097/00004583-199912000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Halmi CA. The perplexities of conducting randomized, double-blind, placebo-controlled treatment trials in anorexia nervosa patients. American Journal of Psychiatry. 2008;165:1227–1228. doi: 10.1176/appi.ajp.2008.08060957. [DOI] [PubMed] [Google Scholar]
- 8.Halmi CA, Agras WS, Crow SJ, Mitchell J, Wilson GT, Bryson S, Kraemer H. Predictors of treatment acceptance and completion in anorexia nervosa: implications for future study designs. Arch Gen Psychiatry. 2005;(62):776–781. doi: 10.1001/archpsyc.62.7.776. [DOI] [PubMed] [Google Scholar]
- 9.Steinhausen H. Outcome of eating disorders. Child and Adolescent Psychiatric Clinics of North America. 2009;18:225–242. doi: 10.1016/j.chc.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 10.Lock J, Couturier J, Bryson S, Agras WS. Predictors of dropout and remission in family therapy for adolescent anorexia nervosa in a randomized clinical trial. Int J Eat Disord. 2006;39:639–647. doi: 10.1002/eat.20328. [DOI] [PubMed] [Google Scholar]
- 11.Eisler I, Dare C, Hodes M, Russell G, Dodge E, Le Grange D. Family therapy for adolescent anorexia nervosa: the results of a controlled comparison of two family interventions. J Child Psychol Psychiatry. 2000;41(6):727–36. [PubMed] [Google Scholar]
- 12.Le Grange D, Eisler I, Dare C, Russell G. Evaluation of family treatments in adolescent anorexia nervosa: a pilot study. Int J Eat Disord. 1992;12(4):347–357. [Google Scholar]
- 13.Russell GF, Szmukler GI, Dare C, Eisler I. An evaluation of family therapy in anorexia nervosa and bulimia nervosa. Arch Gen Psychiatry. 1987;44(12):1047–56. doi: 10.1001/archpsyc.1987.01800240021004. [DOI] [PubMed] [Google Scholar]
- 14.Robin A, Siegal P, Koepke T, Moye A, Tice S. Family therapy versus individual therapy for adolescent females with anorexia nervosa. J Dev Behav Pediatr. 1994;15(2):111–116. [PubMed] [Google Scholar]
- 15.Dare C, Eisler I. Family therapy for anorexia nervosa. In: Garner DM, Garfinkel P, editors. Handbook of Treatment for Eating Disorders. New York: Guilford Press; 1997. pp. 307–324. [Google Scholar]
- 16.Pote H, Stratton P, Cottrell D, Boston P, Shapiro D. Systemic family therapy manual therapist adherenceprotocol. University of Leeds, The Leeds Family Therapy and Research Centre; 2001. p. 2001. [Google Scholar]
- 17.Fairburn CG, Cooper J, Doll H, Welch S. Risk factors for anorexia nervosa: three integrated case-control comparisons. Arch Gen Psychiatry. 1999;56:468–76. doi: 10.1001/archpsyc.56.5.468. [DOI] [PubMed] [Google Scholar]
- 18.Anderluch M, Tchanturia K, Rabe-Hesketh S, Treasure JL. Childhood obsessive compulsive personality traits in adult women with eating disorders: Defining a broader eating disorder phenotype. Am J Psychiatry. 2003;160:242–247. doi: 10.1176/appi.ajp.160.2.242. [DOI] [PubMed] [Google Scholar]
- 19.Deep A, Nagy L, Welzin T, Roa R, WHK Premorbid onset of psychopathology in long-term recovered anorexia nervosa. Int J Eat Disord. 1995;17:291–97. [PubMed] [Google Scholar]
- 20.Bulik CM, Sullivan PF, Fear J, Joyce PR. Eating disorders and antecedent anxiety disorders: a controlled study. Acta Psychiatrica Scandinavica. 1997;96:101–107. doi: 10.1111/j.1600-0447.1997.tb09913.x. [DOI] [PubMed] [Google Scholar]
- 21.Braun D, Sunday S, Halmi CA. Psychiatric co-morbidity in patients with eating disorders. Psychological Medicine. 1994;24:859–67. doi: 10.1017/s0033291700028956. [DOI] [PubMed] [Google Scholar]
- 22.Halmi CA, Eckert ED, Marchi M, Sampugnaro V, Apple R, Cohen J. Co-morbidity of psychiatric diagnoses in anorexia nervosa. Arch Gen Psychiatry. 1991;48:712–718. doi: 10.1001/archpsyc.1991.01810320036006. [DOI] [PubMed] [Google Scholar]
- 23.Silberg J, Bulik CM. Developmental association between eating disorder symptomss and symptoms of depression and anxiety in juvenile twin girls. submitted. [DOI] [PubMed] [Google Scholar]
- 24.Costello A, editor. Epidemiology in Anxiety Disorders in Children and Adolescents. New York: Guilford Press; 1995. [Google Scholar]
- 25.Srinivasagam N, WHK, Plotnicov K, Greeno C, Weltzin T, Rao R. Persistent perfectionism, symmetry, and exactness after long-term recovery from anorexia nervosa. Am J Psychiatry. 1995;152:1630–4. doi: 10.1176/ajp.152.11.1630. [DOI] [PubMed] [Google Scholar]
- 26.Kaye WH, Greeno C, Moss H, Fernstrom J, Fernstrom M, Lilenfeld L, Weltzin T, Mann J. Alterations in serotonin activity and psychiatric symptoms after recovery from bulimia nervosa. Arch Gen Psychiatry. 1998;(55):927–935. doi: 10.1001/archpsyc.55.10.927. [DOI] [PubMed] [Google Scholar]
- 27.Casper R, Hedeker D, McClough J. Personality dimensions in eating disorders and their relevance for subtyping. J Am Acad Child Adolesc Psychiatry. 1992;31:830–840. doi: 10.1097/00004583-199209000-00008. [DOI] [PubMed] [Google Scholar]
- 28.Godart NT, Flament MF, Lecrubier Y, Jeammet P. Anxiety disorders in anorexia nervosa and bulimia nervosa: comorbidity and chronology of appearance. European Psychiatry. 2000;15:38–45. doi: 10.1016/s0924-9338(00)00212-1. [DOI] [PubMed] [Google Scholar]
- 29.Godart N, Flament M, Perdereau F, Jeammet P. Comorbidity between eating disorders and anxiety disorders: A review. Int J Eat Disord. 2002:32. doi: 10.1002/eat.10096. [DOI] [PubMed] [Google Scholar]
- 30.Lock J, Agras WS, Bryson S, Kraemer H. A comparison of short-and long-term family therapy for adolescent anorexia nervosa. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:632–639. doi: 10.1097/01.chi.0000161647.82775.0a. [DOI] [PubMed] [Google Scholar]
- 31.Walkup J, Albano A, Piacentini J, Birmhaher B, Compton S, Sherrill J, Ginsberg G, Rynn M, McCracken J, Waslick B, Iyengar S, March J, Kendall P. Cognitive Behavioral Therapy, sertraline, or a combination in childhood anxiety. New England Journal of Medicine. 2008;359:2753–2766. doi: 10.1056/NEJMoa0804633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Team POSP. Cognitive-behavior therapy, sertraline, and their combination for children and adolescents with obsessive-compulsive disorder: the Pediatric OCD Treatment Study (POTS) randomized controlled trial. JAMA. 2004;292:1969–76. doi: 10.1001/jama.292.16.1969. [DOI] [PubMed] [Google Scholar]
- 33.Couturier J, Lock J. Review of Medication Use for Children and Adolescents with Eating Disorders. Journal of the Canadian Academy of Child and Adolescent Psychiatry. 2007;16:173–176. [PMC free article] [PubMed] [Google Scholar]
- 34.Kaye WH. An open trial of fluoxetine in patients with anorexia nervosa. Journal of Clinical Psychiatry. 1991;52:464–471. [PubMed] [Google Scholar]
- 35.Kaye WH, Nagata T, Weltzin T, Hsu B, Sokol M, McConaha C, Plotnicov K, Weise J. Double-blind placebo controlled administration of fluoxetine in restricting and restricting-purging type anorexia nervosa. Biological Psychiatry. 2001;49:644–52. doi: 10.1016/s0006-3223(00)01013-1. [DOI] [PubMed] [Google Scholar]
- 36.Hebebrand J, Himmelmann G, Hesecker H, Schaefer H, Remschmidt H. The use of percentiles for the body mass index in anorexia nervosa: diagnostic, epidemiological, and therapeutic considerations. Int J Eat Disord. 1996;19:359–369. doi: 10.1002/(SICI)1098-108X(199605)19:4<359::AID-EAT4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 37.Hebebrand J, Wehmeier P, Remschmidt H. Weight criteria for diagnosis of anorexia nervosa. Am J Psychiatry. 2000;157:6. doi: 10.1176/appi.ajp.157.6.1024. [DOI] [PubMed] [Google Scholar]
- 38.Hebebrand J, Casper R, Treasure JL, Schweiger U. The need to revise the diagnostic criteria for anorexia nervosa. J Neural Transmission. 2004;111:827–840. doi: 10.1007/s00702-004-0136-9. [DOI] [PubMed] [Google Scholar]
- 39.Roberto C, Steinglass J, Walsh BT. The clinical significance of amenorrhea as a diagnostic criterion for anorexia nervosa. Int J Eat Disord. 2008;41:559–563. doi: 10.1002/eat.20534. [DOI] [PubMed] [Google Scholar]
- 40.Lock J, Le Grange D, Agras WS, Bryson S, Jo B. A randomized clinical trial comparing family based treatment to adolescent focused individual therapy for adolescents with anorexia nervosa. Archives of General Psychiatry. 2010;67:1025–1032. doi: 10.1001/archgenpsychiatry.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agras WS, Walsh BT, Fairburn CG, Wilson GT, Kraemer HC. A multicenter comparison of cognitive-behavioral therapy and interpersonal psychotherapy for bulimia nervosa. Archives of General Psychiatry. 2000;57:459–466. doi: 10.1001/archpsyc.57.5.459. [DOI] [PubMed] [Google Scholar]
- 42.Lock J, Le Grange D, Agras WS, Dare C. Treatment manual for anorexia nervosa: A family-based approach. New York: Guilford Publications, Inc; 2001. [Google Scholar]
- 43.Pote H, Stratton P, Cottrell D, Shapiro D, Boston P. Systemic family therapy can be manualized: research process and findings. Journal of Family Therapy. 2003;25:236–262. [Google Scholar]
- 44.Pote H, Stratton P, Cottrell D, Boston P, Shapiro D. Systemic family therapy manual. University of Leeds: the Family Therapy Research Center; 2001. p. 2001. [Google Scholar]
- 45.Golden N, Katzman D, Kreipe R, Stevens S, Sawyer S, Rees J, Nicholls D, Rome E. Eating disorders in adolescents: position paper of the Society for Adolescent Medicine:Medical Indications for Hospitalization in an Adolescent with an Eating Disorder. J Adolesc Health. 2003;33:496–503. doi: 10.1016/s1054-139x(03)00326-4. [DOI] [PubMed] [Google Scholar]
- 46.Lock J. How clinical pathways can be useful: An example of a clinical pathway for the treatment of Anorexia Nervosa in adolescents. Clinical ChildPsychology and Psychiatry. 1999;4:331–340. [Google Scholar]
- 47.Lock J, Walker L, Rickert V, Katzman D. Suicidality in adolescents being treated with antidepressant medications and the black box label: Position paper of the Society of Adolescent Medicine. Journal of Adolecent Health. 2005;36:92–93. doi: 10.1016/j.jadohealth.2004.11.125. [DOI] [PubMed] [Google Scholar]
- 48.Bridge JA, Iyengar S, Salary CB, et al. Clinical response and risk for reported suicidal ideation and suicide attempts in pediatric antidepressant treatment: a meta-analysis of randomized controlled trials. JAMA. 2007;297:1683–1696. doi: 10.1001/jama.297.15.1683. [DOI] [PubMed] [Google Scholar]
- 49.Peebles R, Hardy K, Wilson J, Lock JPip. Eating Disorders Not Otherwise Specified: Are Diagnostic Criteria for Eating Disorders Markers of Medical Severity? Pediatrics. doi: 10.1542/peds.2008-1777. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pediatrics AAo. Policy Statement: Identifying and treating eating disorders. Pediatrics. 2003;111:204–211. doi: 10.1542/peds.111.1.204. [DOI] [PubMed] [Google Scholar]
- 51.Baran S, Weltzin T, Kaye WH. Low discharge weight and outcome in anorexia nervosa. Am J Psychiatry. 1995;152:1070–1072. doi: 10.1176/ajp.152.7.1070. [DOI] [PubMed] [Google Scholar]
- 52.Gowers S, Clark A, Roberts C, Griffiths A, Edwards V, Bryan C, Smethhurst N, Byford S, Barrett B. Clinical effectiveness of treatments for anorexia nervosa in adolescents. Br J Psychiatry. 2007;191:427–435. doi: 10.1192/bjp.bp.107.036764. [DOI] [PubMed] [Google Scholar]
- 53.Le Grange D, Crosby R, Rathouz P, Leventhal B. A randomized controlled comparison of family-based treatment and supportive psychotherapy for adolescent bulimia nervosa. Archives of General Psychiatry. 2007;64:1049–56. doi: 10.1001/archpsyc.64.9.1049. [DOI] [PubMed] [Google Scholar]
- 54.Lock J, Le Grange D, Agras WS, Bryson S, Jo B. A randomized clinical trial comparing family based treatment to adolescent focused individual therapy for adolescentswith anorexia nervosa. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2010.128. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pike K, Walsh BT, Vitousek K, Wilson GT, Bauer J. Cognitive-Behavioral Therapy in the Posthospitalization Treatment of Anorexia Nervosa. American Journal of Psychiatry. 2004;160:2046–2049. doi: 10.1176/appi.ajp.160.11.2046. [DOI] [PubMed] [Google Scholar]
- 56.Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter J, Pike K, Devlin MJ, Woodside B, Roberto L, Rockert W. Fluoxetine after weight restoration in anorexia nervosa: a randomized clinical trial. JAMA. 2006;295:2605–2612. doi: 10.1001/jama.295.22.2605. [DOI] [PubMed] [Google Scholar]
- 57.Kraemer H, Thienemann S. How Many Subjects? Statistical Power Analysis in Research. Newbury Park, California: Sage Publications, Inc; 1987. [Google Scholar]
- 58.Graham W, Donaldson S. Evaluating interventions with differential attrition: the importance of nonresponse mechanisms and use of follow-up data. Journal of Applied Psychology. 1993;78:119–128. doi: 10.1037/0021-9010.78.1.119. [DOI] [PubMed] [Google Scholar]
- 59.Little R, Rubin D. Statistical Analysis with Missing Data. New York: John Wiley; 2002. [Google Scholar]
- 60.Boachie A, Goldfield G, Spettigue W. Olanzapine use as an adjuctive treatment for hospitalized children with anorexia nerovsa: case reports. Int J Eat Disord. 2003;33:98–103. doi: 10.1002/eat.10115. [DOI] [PubMed] [Google Scholar]
- 61.Mehler C, Wewetzer C, Schulze U, Warnke A, Theisen F, Dittman R. Olanzapine in children and adolescents with chronic anorexia nervosa: A study of five cases. European Child and Adolescent Psychiatry. 2001;10:151–157. doi: 10.1007/s007870170039. [DOI] [PubMed] [Google Scholar]
- 62.Norris R, Spettigue W, Buchholz A, Henderson K, Obeid N. Factors influencing research drug trials in adolescents with anorexia nervosa. Eating Disorders. 2010;18:210–217. doi: 10.1080/10640261003719468. [DOI] [PubMed] [Google Scholar]