Abstract
Purpose
Photon radiotherapy has been standard adjuvant treatment for stage I seminoma. Single dose carboplatin and observation have emerged as alternative options due to concerns of acute toxicities and secondary malignancies from radiation. In this IRB-approved study, we compare photon and proton radiotherapy for stage I seminoma and predict rates of excess secondary malignancies for both treatment modalities.
Methods and Material
CT images from 10 consecutive patients with stage I seminoma were used to quantify dosimetric differences between photon and proton therapy. Structures reported to be at increased risk for secondary malignancies and in-field critical structures were contoured. Reported models of organ-specific radiation-induced cancer incidence rates based on organ equivalent dose were used to determine the excess absolute risk of secondary malignancies. Calculated values were compared with tumor registry reports of excess secondary malignancies among testicular cancer survivors.
Results
Photon and proton plans provided comparable target volume coverage. Proton plans delivered significantly lower mean doses to all examined normal tissues except the kidneys. The greatest absolute reduction in mean dose was observed for the stomach (119cGy vs. 768cGy, p<0.0001). Significantly more excess secondary cancers per 10,000 patients/yr were predicted with photons compared with protons for the stomach (4.11; 95% CI=3.22–5.01), large bowel (0.81; CI=0.39–1.01), and bladder (0.03; CI=0.01–0.58), while no difference was demonstrated for the pancreas (0.02; CI=−0.01–0.06).
Conclusions
For patients with stage I seminoma, proton therapy reduced the predicted secondary cancer risk compared with photon therapy. We predict a reduction of one additional secondary cancer for every 50 patients with a life expectancy of 40 years from the time of radiation treated with protons instead of photons. Protons also allowed significant sparing of most critical structures examined and warrant further study for patients with seminoma to decrease radiation-induced toxicity.
Keywords: seminoma, secondary malignancies, proton therapy, dosimetry, para-aortic
INTRODUCTION
Testicular cancers are the most common solid malignancies among men aged 20–35 years. In 2009, there were a projected 8,400 new cases of testicular malignancies in the Unites States, with 380 deaths (1). Most of these malignancies represent primary germ cell tumors, with pure seminoma comprising 60% of these tumors. Approximately 80% of patients diagnosed with seminoma have stage I disease (2).
The standard initial treatment for stage I seminoma is radical inguinal orchiectomy. Since the mid-20th century, photon external beam radiotherapy has been standard adjuvant treatment. Patients receiving radiotherapy achieve cause-specific survival rates approaching 100% and long-term relapse-free survival rates exceeding 95%, with virtually no relapses within the radiation portal (3–4).
However, significant treatment-related morbidities following radiotherapy have been reported. Although acute toxicities are generally mild and self-limiting, patients are at an increased risk for late gonadal toxicity (5) and cardiovascular disease (6–7), particularly among patients receiving prophylactic mediastinal irradiation [PMI] (6). Studies have also revealed increases in contralateral testicular germ cell tumors in the first decade following radiotherapy (8–9) and increases in non-germ cell malignancies after 10–35 years (6–7,10–12). In the study assessing the largest population of seminoma patients for the development of secondary cancers, 40,576 patients with first primary cancers of the testis between 1943–2001 who survived at least one year were evaluated (10). Patients treated with adjuvant radiotherapy alone had a significantly increased risk of solid cancers (relative risk [RR]=2.0, 95% CI=1.9–2.2), with the risk highest in patients treated at younger ages. Among organs in the standard para-aortic field radiation portal, secondary cancer rates were elevated for the stomach (RR=4.1, CI=3.2–5.2), large bowel (RR=1.9, CI=1.5–2.5), pancreas (RR=3.8, CI=2.7–5.0), and bladder (RR=2.7, CI=2.1–3.3). These risks were slightly higher for patients with seminoma than nonseminoma malignancies. As seminoma largely affects younger patients and cure rates are excellent, second primary cancers are a leading cause of death among testicular cancer survivors (6–7,12).
Attempting to decrease radiation-associated treatment morbidities and secondary malignancies, studies have investigated reducing the adjuvant radiotherapy dose and treatment volume. The United Kingdom Medical Research Council (MRC) randomized 625 patients with stage I seminoma to 20Gy or 30Gy in 200cGy fractions following orchiectomy. Rates of acute toxicities were lower among patients receiving 20Gy, with no difference in relapse-free survival or overall survival (3). With the recognition that PMI increases cardiac mortality (6), treatment to the mediastinum was largely abandoned by the mid-1980’s. Following Royal Marsden Hospital reporting no difference in relapse patterns for patients with scrotal violations, and following the International Consensus Conference in Leeds in 1989, radiotherapy to the ipsilateral groin and scrotum is typically avoided (13). Following an MRC randomized trial of 478 patients with stage I seminoma demonstrating no improvement in overall survival or relapse-free survival in patients treated to the ipsilateral pelvis, radiotherapy to the para-aortic region alone has become an acceptable target volume for patients with undisturbed lymphatic drainage (4).
Despite reductions in radiation doses and fields, concerns of late toxicities and secondary malignancies persist. Proton therapy may provide equivalent rates of disease control, while improving the toxicity profile of photon therapy. Protons allow energy deposition at a specific depth known as the Bragg peak, with rapid energy falloff beyond this point (14). Therefore, protons can allow normal tissues distal to the target volume to be spared.
To date, there is no published data directly comparing different types of ionizing radiotherapy for treatment of stage I seminoma, and no data exist predicting the risk of secondary malignancies from proton therapy in this patient population. In this study, we compare dose volume histograms (DVHs) of target volumes and normal tissue structures in photon-based versus proton-based plans in patients with stage I seminoma, and we determine the excess absolute risk (EAR) of secondary malignancies for photon and proton plans.
MATERIALS AND METHODS
Ten consecutive patients with stage I seminoma treated with radiotherapy at Walter Reed Army Medical Center (WRAMC) who had CT simulation images include the entire bladder were assessed in the present study. This study was approved by the WRAMC Institutional Review Board. All patients underwent radical orchiectomy and received adjuvant fractionated 2D radiotherapy with megavoltage photons to the para-aortic lymph node region from 6/2006–9/2008. These CT images were used to quantify dosimetric differences between photon and proton radiotherapy. CT data were acquired with slice thicknesses of 3mm. CT images were imported into a commercial treatment planning system (Eclipse, Varian Medical Systems, Palo Alto, CA) to define target and nontarget structures.
Planning target volumes (PTVs) and adjacent organs at risk (OARs) were delineated on simulation CT images. Organs in the treatment field previously reported to be at increased risk to develop secondary malignancies (10), including bladder, stomach, pancreas and large bowel, were contoured. In-field critical structures, including liver and kidneys, were also contoured. All contours were performed by a single radiation oncologist (CS) and reviewed by two additional radiation oncologists (JO’C, WO). Target and nontarget structure sets for a given patient CT image set were held constant for all treatment plans.
Two plans were generated for each patient (n=20 plans). For photon plans, patients were treated with an AP-PA technique, whereas only a PA field was used for proton plans. For photon plans, patients were treated with a standard 2D rectangular treatment field, with field borders defined by the T10-T11 intervertebral space cranially, L5-S1 intervertebral space caudally, and 2.0cm laterally beyond the lateral edge of the vertebral bodies, bilaterally.
Because all patients had node-negative disease, no Gross Tumor Volume was utilized. For the proton therapy para-aortic nodal Clinical Target Volume (CTVproton), defined as regions of potential microscopic disease, the aorta and common iliac vessels from the mid-T11 vertebral body cranially to the caudal third of the L5 vertebral body caudally were contoured together as a single structure. These vessels served as surrogates for para-aortic lymph node positioning and represented the region at risk for para-aortic lymph node metastasis. In patients with bifurcation of the aorta above the caudal third of L5, contours from both the right and left iliac branches were included. The cranial and caudal extents of vessel contours were derived to allow the cranial and caudal extents of irradiation volumes to be equal for photon and proton plans. A radial expansion of 1.3cm was added to this structure to comprise CTVproton. Bone was excluded from CTVproton.
To account for setup variation and organ and patient motion, a radial expansion of 0.7cm was added to CTVproton to define PTVproton. To account for proton beam properties and range uncertainties, based on Moyer’s formula applied to the average range values of the study population, proton beam range compensators were designed to provide 0.6cm proximal and 0.9cm distal margins relative to PTVproton, and blocking was designed to create a 1.3cm lateral margin relative to PTVproton.
Plans were calculated to deliver 25.5Gy with photons or 25.5 cobalt Gray equivalents (CGE) with protons over 17 fractions, with proton doses corrected with the accepted relative biologic effectiveness value of 1.1 (15). All plans were optimized to provide optimal target volume coverage and dose homogeneity throughout the target volumes. DVHs of target volumes and OARs were generated to compare doses to tumor volumes and normal structures.
Previously reported models of organ-specific radiation-induced cancer incidence rates based on organ equivalent dose (OED) were used to determine EAR of secondary malignancies for photon and proton plans. OED is a tool to describe radiation-induced malignancies for nonuniform dose distributions. The organ-specific cancer incidence rate was calculated according to , where I0org is the organ-specific cancer incidence rate for a low dose (EAR per 10,000 patients/yr/Gy), D is the total dose administered, and αorg is an organ-specific cell sterilization parameter. OED for radiation-induced cancer was calculated according to , where the sum is taken over N dose calculation points. This approach has been previously described in detail by Schneider, et al. (16–18). Calculated predicted values of secondary malignancies were compared with tumor registry population-based reports of EAR of second solid cancers among testicular cancer survivors (10).
Statistical analysis was performed using Microsoft Excel for Windows (Microsoft Office Excel 2003). Paired t-test was used to evaluate differences between pairwise comparisons. A two-tailed p-value was utilized, with statistical significance defined as p≤0.05.
RESULTS
Among the study population, the mean age was 31 years (range 22–48 years) (Table 1). Patients had pT1 (eight patients) or pT2 (two patients) disease [AJCC, 6th Ed.]. Seven patients had right-sided primary testicular seminomas, whereas three had left-sided tumors.
Table 1.
Patient Characteristics
| Patient Number | Patient Age (Years) | T Stage* | Side of Primary Tumor |
|---|---|---|---|
| 1 | 31 | pT1 | Right |
| 2 | 35 | pT2 | Left |
| 3 | 34 | pT1 | Right |
| 4 | 31 | pT1 | Right |
| 5 | 25 | pT1 | Left |
| 6 | 28 | pT1 | Left |
| 7 | 22 | pT1 | Right |
| 8 | 48 | pT1 | Right |
| 9 | 23 | pT2 | Right |
| 10 | 28 | pT1 | Right |
AJCC, 6th Ed.
All photon and proton plans provided acceptable and comparable target volume coverage. Although dose distributions for proton plans were typically more homogenous throughout the target volumes than photon plans, this difference did not appear clinically significant. Furthermore, no target or nontarget volume received >114% of the prescribed dose in any photon or proton plan.
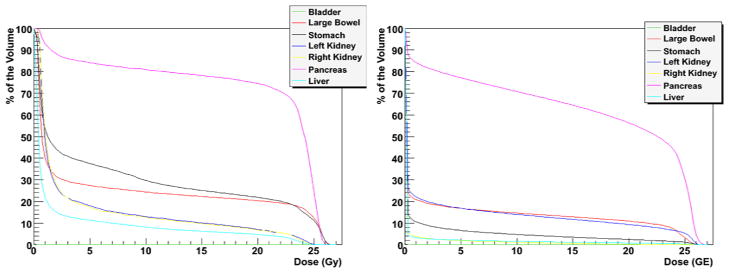
Overall, proton plans had superior dose conformality, with significant sparing of most normal tissues examined (Figure 1). Among OARs examined, the largest absolute difference in mean dose between proton and photon plans was observed for the stomach (Table 2). Compared with photons, protons significantly reduced the mean dose to the stomach (119cGy vs. 768cGy, p<0.0001) (Figure 2). Protons also achieved significant reductions in mean doses to the pancreas (1697cGy vs. 1991cGy, p=0.0002), large bowel (352cGy vs. 651cGy, p=0.0015), and liver (33cGy vs. 313cGy, p=0.0006). The average maximum point dose to the large bowel was also lower with protons (2618cGy vs. 2732cGy, p=0.0096). The maximum doses to the liver (2141cGy vs. 2591cGy, p=0.0689) and stomach (2147cGy vs. 2678cGy, p=0.0791) trended lower with protons, while no difference was observed for the pancreas (2634cGy vs. 2657cGy, p=0.4072). Both the mean (0cGy vs. 1cGy, p=0.0304) and maximum (11cGy vs. 51cGy, p=0.0071) doses to the bladder were lower with proton therapy, although these doses were not clinically significant. There were no differences in mean or maximum doses received by the kidneys between the two treatment modalities.
Figure 1.
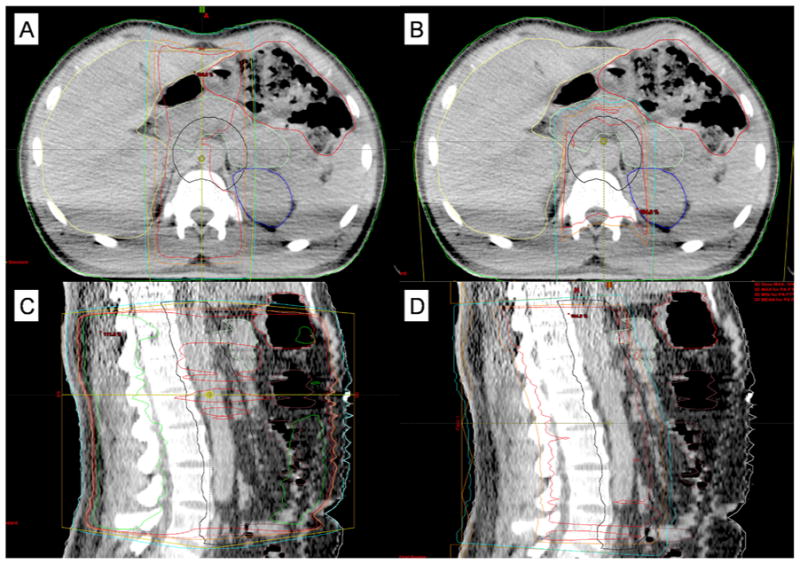
Representative treatment planning images in axial planes for A) photon therapy and B) proton therapy, as well as sagittal planes for C) photon therapy and D) proton therapy.
Table 2.
Comparison of Dose (cGy) to Normal Tissues Between Photon and Proton Treatment Plans
| Bladder | Large Bowel | Liver | Pancreas | Stomach | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Mean | Max† | Mean | Max | Mean | Max | Mean | Max | Mean | Max |
| Photon | 1 | 51 | 651 | 2732 | 313 | 2591 | 1991 | 2657 | 768 | 2678 |
| Proton | 0 | 11 | 352 | 2618 | 33 | 2141 | 1697 | 2634 | 119 | 2147 |
| P Value | 0.0304 | 0.0071 | 0.0015 | 0.0096 | 0.0006 | 0.0689 | 0.0002 | 0.4072 | <0.0001 | 0.0791 |
Max = average maximum point dose
Figure 2.
Comparison of mean dose-volume histograms of photon (left) and proton (right) radiation treatment plans.
Previously reported tumor registry population-based studies of EAR of second solid cancers revealed that for patients diagnosed with seminoma at age 35 years, the cumulative risk of solid cancer 40 years later was 36%, compared with 23% for the general population (10). Among 9,551 testicular cancer 10-year survivors reported to cancer registries from Denmark, Finland, and Norway and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program, we estimated 8,986 patients received radiotherapy alone based on ratios of relative risks and reported numbers of second solid tumors for patients treated with radiotherapy alone, chemotherapy alone, and radiotherapy and chemotherapy. Using models of organ-specific radiation-induced cancer incidence rates to determine the EAR of secondary malignancies, we predict 6.94 excess bladder, large bowel, pancreas, and stomach secondary malignancies per 10,000 patients/yr from photon radiotherapy to the para-aortic region (Table 3). Based on this calculation, for a population of 10,000 patients followed for 40 years after photon therapy, we predict 278 radiation-induced in-field excess solid secondary malignancies. For a population of 8,986 patients, therefore, we predict 249 such secondary malignancies. This prediction compares very favorably with the 246 in-field excess solid secondary malignancies reported among the actual 8,986 patient cohort receiving photon radiotherapy described by Travis, et al. (10).
Table 3.
Predicted Secondary Malignancies Per 10,000 Patients/Year
| Bladder | Large Bowel | Pancreas | Stomach | In-field Totals | |
|---|---|---|---|---|---|
| Photon Therapy | 0.03 | 1.12 | 0.58 | 5.21 | 6.94 |
| Proton Therapy | 0.00 | 0.31 | 0.56 | 1.10 | 1.97 |
| Excess Malignancies from Photons | 0.03 | 0.81 | 0.02 | 4.11 | 4.97 |
| 95% Confidence Interval* | 0.01 to 0.58 | 0.39 to 1.01 | −0.01 to 0.06 | 3.22 to 5.01 | 3.99 to 5.97 |
Confidence Interval is expressed as the range of predicted excess secondary malignancies per 10,000 patients/year from photon radiotherapy, when compared with proton radiotherapy. The null value is defined as 0, with statistical significance achieved for all ranges not overlapping 0.
Significantly more excess secondary malignancies per 10,000 patients/yr were predicted with photons than protons. When assessing OARs in the treatment field previously reported to be at increased risk to develop secondary malignancies, higher rates of secondary cancers with photons were predicted for the stomach (5.21 vs. 1.10, 95% CI=3.22–5.01), large bowel (1.12 vs. 0.31, CI=0.39–1.01), and bladder (0.03 vs. 0.00, CI=0.01–0.58), whereas no difference was predicted for the pancreas (0.58 vs. 0.56, CI=−0.01–0.06). In total, we predicted 4.97 (CI=3.99–5.97) excess secondary malignancies per 10,000 patients/yr from photon therapy compared with proton therapy. For patients with life expectancies of 40 years from the time of radiotherapy treated with photons instead of protons, 198.80 excess secondary malignancies per 10,000 patients are predicted. Therefore, approximately 1 excess secondary malignancy per 50 patients would be avoided by treating with protons instead of photons.
DISCUSSION
This study demonstrated that adjuvant proton radiotherapy, when compared with photon radiotherapy, significantly reduced the predicted secondary cancer risk for patients with stage I seminoma. This reduction was greatest for radiation-induced gastric and large bowel malignancies. A reduction of one additional secondary cancer for every 50 patients with life expectancies of 40 years from the time of therapy treated with protons instead of photons was predicted. A high degree of dose homogeneity was achieved for all plans, and there was no significant difference in target volume coverage between photon and proton plans. Proton therapy in this study achieved superior normal tissue sparing and significantly reduced the mean doses delivered to the stomach, pancreas, large bowel, liver, and bladder. Additionally, the maximum doses delivered with protons were significantly lower to the large bowel and bladder and trended lower to the liver and stomach.
Over the past two decades, despite reductions in radiation doses and treatment fields, due to concerns of late complications, many practitioners have increasingly offered surveillance or single-dose carboplatin as alternative adjuvant courses for stage I seminoma. Approximately 15–20% of unselected patients with stage I seminoma who undergo surveillance following orchiectomy develop disease recurrence (19). As greater than 80% of relapses occur in para-aortic lymph nodes, deferring immediate adjuvant therapy and administering chemotherapy or radiotherapy upon relapse allows for successful salvage in most patients (19). However, treatment of patients that relapse is usually more intensive and associated with increased morbidity (20). More recent risk-adapted strategies recommended surveillance only for low-risk patients committed to long-term follow-up with pT1-T2 histologies, lack of rete testis invasion, and tumors less than 4cm (19–21).
Over the past decade, there has been increasing interest in adjuvant short-course carboplatin. Reports from phase II trials and single-institution retrospective experiences indicate 5-year recurrence rates under 5% with adjuvant carboplatin (20,22). An MRC and European Organization for Research and Treatment of Cancer trial randomized 1,447 patients with stage I seminoma to one course of carboplatin or adjuvant radiotherapy to 20–30Gy in 200cGy daily fractions primarily to the para-aortic region (8–9). At a median follow-up of 6.5 years, there was no difference in 5-year relapse-free survival rates between carboplatin and radiotherapy (95% vs. 96%, 90% CI=0.83–1.89). While patients receiving carboplatin had higher rates of hematologic toxicities, patients receiving radiation had more dyspepsia and a trend towards increased acute lethargy and time off of work. Additionally, fewer new contralateral testicular germ cell cancers were observed with carboplatin (0.4% vs. 1.7%, p=0.03). Despite these promising findings, with more limited long-term data than adjuvant radiotherapy, carboplatin is associated with an uncertain frequency of late relapses and a need for more rigorous CT surveillance with abdominal and pelvic CT imaging at every visit for up to 10 years (20–21). The radiation exposure from this more frequent CT surveillance may result in an increase in secondary malignancies (23).
Carboplatin also has potential risks of acute nephrotoxicity, ototoxicity, neurotoxicity, and gonadal damage, as well as long-term cardiac disease and secondary malignancies (10). Population-based cancer registry studies of patients primarily treated with combination chemotherapy reveal an increased risk of secondary solid malignancies highest among patients receiving both chemotherapy and radiotherapy (RR=2.9, 95% CI=1.9–4.2), with similar risks for patients treated with radiotherapy alone (RR=2.0, CI=1.9–2.2) and adjuvant chemotherapy alone (RR=1.8, CI=1.3–2.5) (10). Several studies also report increased risks of secondary leukemias and myelodysplastic syndrome following chemotherapy for testicular cancer (24–25). Longer follow-up from prospective trials is needed to determine the risk of secondary cancers from single-agent carboplatin.
Although no clinical trials or published data exist assessing proton therapy to treat patients with testicular malignancies, protons may represent a viable alternative to photon radiotherapy, surveillance, or carboplatin for stage I seminoma. The dosimetric advantages of protons demonstrated in this study might improve the therapeutic ratio for stage I patients. Based on historical dose-response relationships, with significantly lower radiation doses to several OARs demonstrated in this study, patients treated with protons may have improved quality of life and reduced rates of acute toxicities previously reported in seminoma patients receiving adjuvant radiotherapy, including nausea, lethargy, and delay in return to work. Longitudinal studies examining normal tissue toxicities from photon and proton radiotherapy are needed to confirm the clinical significance of our findings.
While this study evaluated radiotherapy as the primary inducer of secondary cancers, patients with testicular malignancies, particularly seminomas, are at increased risk for developing subsequent cancers, regardless of their testicular cancer treatment course. Patients with testicular malignancies undergoing orchiectomy alone still have an increased risk of developing a second cancer compared with the general public (26), likely from increased genetic susceptibility for extragonadal secondary tumors in this patient population (27). By comparing one type of radiation therapy to another and reporting findings as an absolute excess risk of secondary malignancies for one radiation treatment type over another, we have attempted to control for this increased genetic susceptibility.
The predicted rates of secondary malignancies calculated in this study were based on previously reported models of organ-specific radiation-induced cancer incidence rates based on OED. The effect of radiotherapy on secondary cancer risk for stage I seminoma patients has previously been assessed using OED models (27). In that dosimetric study, AP-PA treatment plans with 6MV photons were predicted to result in a 20.8–23.3% risk of secondary cancers for a 75-year-old patient treated with radiotherapy to the para-aortic region at age 35, compared with a 19.8% risk for the general population.
As CT simulation images used in this study were from patients previously treated with radiotherapy to the para-aortic region alone, lack of images encompassing the entire scrotum and total lung volume limits our ability to predict second cancer rates of these organs. Of the lung volume imaged, however, the mean lung volume irradiation was significantly lower with protons than photons (p<0.01).
The model used for predicting secondary cancer risk does not allow for predictions of radiation-induced leukemia risk. While a significant risk of treatment-induced leukemia has been reported following adjuvant chemotherapy (RR 5.0), seminoma patients have a nonsignificant increased risk of developing leukemia following radiotherapy (RR 3.1), and this risk is lowest among patients treated after 1974 and whose radiotherapy was limited to abdominal and pelvic fields. This risk of leukemia is significantly lower than the risk of radiation-induced solid tumor induction (10,24).
The significant risk reduction in secondary malignancies predicted with proton radiotherapy must be measured relative to potential risks for neutron contamination that may decrease the magnitude of benefit of proton radiotherapy in preventing secondary malignancies. However, this study demonstrated a 2% reduction in risk of gastric malignancies for patients with life expectancies of 40 years from the time of proton radiotherapy compared with photon radiotherapy. Previous studies have demonstrated the lifetime attributable risk of second cancers from neutron dose from proton radiotherapy to be approximately 0.2% (28), an order of magnitude lower than the benefit predicted in this study from proton radiotherapy. To further minimize the risk of neutron contamination, this study employed scanned proton therapy, which is associated with an out-of-field neutron equivalent dose an order of magnitude lower than for passive scattered proton therapy (29).
Although OED may better take into account inhomogeneities of clinical dose distributions in organs of interest, this model, which employs a linear-exponential function, may be subject to inherent errors due to lack of accuracy in dose reconstruction for patients treated in the more distant past that contributed to the development of the model (17). Furthermore, although prevailing dose-response paradigms for secondary cancer induction include linear, linear-exponential, and plateau models, the true risk of radiation-induced cancers may not perfectly fit any such model and may lie between linear and linear-exponential models (27). Therefore, the accuracy of any estimate of radiation-induced oncogenesis may be limited. However, the linear-exponential model was employed in this study to provide a conservative estimate of secondary cancer risk, as the predicted risk with this model is lower at 25.5Gy than with the other models (30). Furthermore, predicted secondary malignancies calculated in this study were very similar to the actual EAR of second solid cancers among testicular cancer survivors based on population-based reports (10). Longitudinal studies are needed to validate the predictions from these models.
Various methods were used to minimize bias in the present study. Ten consecutive patients with stage I seminoma were included to minimize sampling bias. All contours were performed by a single radiation oncologist and reviewed by two additional radiation oncologists. Standardized target volume expansions were employed for all plans. Beam range compensators and blocking relative to PTVproton to account for beam properties and range uncertainties for proton plans were employed for all plans and not optimized on a patient-by-patient basis. There was a high correlation between PTVproton volumes irradiated and the superior, inferior, and lateral extents of photon 2D volumes irradiated. As the aorta and common iliac vessels were used as surrogates for para-aortic lymph node positioning, there was less correlation in a few patients with more lateralized vasculature. In these patients, the widths of irradiation volumes with proton plans was similar to that for photon plans, but the centers of the fields were shifted laterally in proportion to the displacement of the vessels from the midline, particularly in the cranial-most portions of proton target volumes. In no patient was this shift greater than 2.7cm from the midline. As the level of bifurcation of the aorta and location of the aorta and common iliac vessels relative to the midline are patient dependent, consideration should be given to design treatment to accommodate anatomic features of individual patients when using vasculature as lymph node surrogates.
Furthermore, the volumes treated with proton therapy in this study corresponded well with the distribution of nodal spread from testicular malignancies based on historical reports of lymphangiographies and lymph node dissections (31–32). Additionally, no significant differences in target volume coverage were demonstrated between photon or proton plans, thus minimizing bias when comparing dosimetry between plans. Despite these measures, as with any retrospective study (33), it is possible that bias existed in the current study that may have favored a certain treatment strategy.
CONCLUSIONS
When compared with photon radiotherapy, proton radiotherapy reduced the mean doses delivered to most normal organs adjacent to the radiation treatment field for patients with stage I seminoma. Proton therapy also reduced the predicted incidence of radiation-induced secondary malignancies. These findings serve as a basis for pursuing a feasibility and Phase II study anticipated to open at the University of Pennsylvania Roberts Proton Facility in 2010 that will assess adjuvant proton radiotherapy for the treatment of patients with stage I seminoma.
Acknowledgments
This work was supported by the U.S. Army Medical Research and Materiel Command under Contract Agreement No. DAMD17-W81XWH-04-2-0022. Views expressed in this manuscript are those of the authors and do not reflect official policies of the U.S. Government or Departments of Army, Navy, or Defense.
Financial Support: none
Footnotes
Conflict of Interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Sokoloff MH, Joyce GF, Wise M. Testis cancer. J Urol. 2007;177(6):2030–41. doi: 10.1016/j.juro.2007.01.127. [DOI] [PubMed] [Google Scholar]
- 3.Jones WG, Fossa SD, Mead GM, et al. Randomized trial of 30 versus 20 Gy in the adjuvant treatment of stage I Testicular Seminoma: a report on Medical Research Council Trial TE18, European Organisation for the Research and Treatment of Cancer Trial 30942 (ISRCTN18525328) J Clin Oncol. 2005;23(6):1200–8. doi: 10.1200/JCO.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Fosså SD, Horwich A, Russell JM, et al. Optimal planning target volume for stage I testicular seminoma: A Medical Research Council randomized trial. Medical Research Council Testicular Tumor Working Group. J Clin Oncol. 1999;17(4):1146. doi: 10.1200/JCO.1999.17.4.1146. [DOI] [PubMed] [Google Scholar]
- 5.Bieri S, Rouzaud M, Miralbell R. Seminoma of the testis: is scrotal shielding necessary when radiotherapy is limited to the para-aortic nodes? Radiother Oncol. 1999;50(3):349–53. doi: 10.1016/s0167-8140(99)00023-7. [DOI] [PubMed] [Google Scholar]
- 6.Zagars GK, Ballo MT, Lee AK, et al. Mortality after cure of testicular seminoma. J Clin Oncol. 2004;22(4):640–7. doi: 10.1200/JCO.2004.05.205. [DOI] [PubMed] [Google Scholar]
- 7.Hanks GE, Peters T, Owen J. Seminoma of the testis: long-term beneficial and deleterious results of radiation. Int J Radiat Oncol Biol Phys. 1992;24(5):913–9. doi: 10.1016/0360-3016(92)90475-w. [DOI] [PubMed] [Google Scholar]
- 8.Oliver RT, Mead GM, Fogarty PJ, et al. Radiotherapy versus carboplatin for stage I seminoma: Updated analysis of the MRC/EORTC randomized trial (ISRCTN27163214) J Clin Oncol. 2008;26(Suppl 15):A-1. [Google Scholar]
- 9.Oliver RT, Mason MD, Mead GM, et al. Radiotherapy versus single-dose carboplatin in adjuvant treatment of stage I seminoma: a randomised trial. Lancet. 2005;366(9482):293–300. doi: 10.1016/S0140-6736(05)66984-X. [DOI] [PubMed] [Google Scholar]
- 10.Travis LB, Fosså SD, Schonfeld SJ, et al. Second cancers among 40,576 testicular cancer patients: focus on long-term survivors. J Natl Cancer Inst. 2005;97(18):1354–65. doi: 10.1093/jnci/dji278. [DOI] [PubMed] [Google Scholar]
- 11.Robinson D, Moller H, Horwich A. Mortality and incidence of second cancers following treatment for testicular cancer. Br J Cancer. 2007;96(3):529–33. doi: 10.1038/sj.bjc.6603589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schairer C, Hisada M, Chen BE, et al. Comparative mortality for 621 second cancers in 29356 testicular cancer survivors and 12420 matched first cancers. J Natl Cancer Inst. 2007;99(16):1248–56. doi: 10.1093/jnci/djm081. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy CL, Hendry WF, Peckham MJ. The significance of scrotal interference in stage I testicular cancer managed by orchiectomy and surveillance. Br J Urol. 1986;58(6):705–8. doi: 10.1111/j.1464-410x.1986.tb05917.x. [DOI] [PubMed] [Google Scholar]
- 14.Suit H, Goldberg S, Niemierko A, et al. Proton beams to replace photon beams in radical dose treatments. Acta Oncol. 2003;42(8):800–8. doi: 10.1080/02841860310017676. [DOI] [PubMed] [Google Scholar]
- 15.Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys. 2002;53(2):407–21. doi: 10.1016/s0360-3016(02)02754-2. [DOI] [PubMed] [Google Scholar]
- 16.Schneider U, Kaser-Hotz B. A simple dose-response relationship for modeling secondary cancer incidence after radiotherapy. Z Med Phys. 2005;15(1):31–7. doi: 10.1078/0939-3889-00242. [DOI] [PubMed] [Google Scholar]
- 17.Schneider U, Zwahlen D, Ross D, et al. Estimation of radiation-induced cancer from three-dimensional dose distributions: Concept of organ equivalent dose. Int J Radiat Oncol Biol Phys. 2005;61(5):1510–5. doi: 10.1016/j.ijrobp.2004.12.040. [DOI] [PubMed] [Google Scholar]
- 18.Schneider U, Lomax A, Besserer J, et al. The impact of dose escalation on secondary cancer risk after radiotherapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2007;68(3):892–7. doi: 10.1016/j.ijrobp.2007.02.029. [DOI] [PubMed] [Google Scholar]
- 19.Warde P, Specht L, Horwich A, et al. Prognostic factors for relapse in stage I seminoma managed by surveillance: a pooled analysis. J Clin Oncol. 2002;20(22):4448–52. doi: 10.1200/JCO.2002.01.038. [DOI] [PubMed] [Google Scholar]
- 20.Krege S, Beyer J, Souchon R, et al. European consensus conference on diagnosis and treatment of germ cell cancer: a report of the second meeting of the European Germ Cell Cancer Consensus group (EGCCCG): part I. Eur Urol. 2008;53(3):478–96. doi: 10.1016/j.eururo.2007.12.024. [DOI] [PubMed] [Google Scholar]
- 21.The NCCN Clinical Practice Guidelines in Oncology™ Testicular Cancer (Version 1.2010) National Comprehensive Cancer Network, Inc; Aug 28, 2009. [Accessed [February 22, 2010]]. Available at: NCCN.org. [Google Scholar]
- 22.Aparicio J, García del Muro X, Maroto P, et al. Multicenter study evaluating a dual policy of postorchiectomy surveillance and selective adjuvant single-agent carboplatin for patients with clinical stage I seminoma. Ann Oncol. 2003;14(6):867–72. doi: 10.1093/annonc/mdg241. [DOI] [PubMed] [Google Scholar]
- 23.Tarin TV, Sonn G, Shinghal R. Estimating the risk of cancer associated with imaging related radiation during surveillance for stage I testicular cancer using computerized tomography. J Urol. 2009;181(2):627–32. doi: 10.1016/j.juro.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Travis LB, Andersson M, Gospodarowicz M, et al. Treatment-associated leukemia following testicular cancer. J Natl Cancer Inst. 2000;92(14):1165–71. doi: 10.1093/jnci/92.14.1165. [DOI] [PubMed] [Google Scholar]
- 25.Howard R, Gilbert E, Lynch CF, et al. Risk of leukemia among survivors of testicular cancer: a population-based study of 42,722 patients. Ann Epidemiol. 2008;18(5):416–21. doi: 10.1016/j.annepidem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Travis LB, Curtis RE, Hankey BF. Second malignancies after testicular cancer. J Clin Oncol. 1995;13(2):533–4. doi: 10.1200/JCO.1995.13.2.533. [DOI] [PubMed] [Google Scholar]
- 27.Zwahlen DR, Martin JM, Millar JL, et al. Effect of radiotherapy volume and dose on secondary cancer risk in stage I testicular seminoma. Int J Radiat Oncol Biol Phys. 2008;70(3):853–8. doi: 10.1016/j.ijrobp.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 28.Zacharatou Jarlskog C, Paganetti H. Risk of developing second cancer from neutron dose in proton therapy as function of field characteristics, organ, and patient age. Int J Radiat Oncol Biol Phys. 2008;72(1):228–35. doi: 10.1016/j.ijrobp.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 29.Athar BS, Bednarz B, Seco J, et al. Comparison of out-of-field photon doses in 6 MV IMRT and neutron doses in proton therapy for adult and pediatric patients. Phys Med Biol. 2010;55(10):2879–91. doi: 10.1088/0031-9155/55/10/006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schneider U, Lomax A, Pemler P, et al. The impact of IMRT and proton radiotherapy on secondary cancer incidence. Strahlenther Onkol. 2006;182(11):647–52. doi: 10.1007/s00066-006-1534-8. [DOI] [PubMed] [Google Scholar]
- 31.Donohue JP, Zachary JM, Maynard BR. Distribution of nodal metastases in nonseminomatous testis cancer. J Urol. 1982;128(2):315–20. doi: 10.1016/s0022-5347(17)52904-3. [DOI] [PubMed] [Google Scholar]
- 32.Gagnon JH, Mount BM, Khonsari H, et al. Lymphography in germinal tumours of the testis. Br J Urol. 1972;44(2):136–42. doi: 10.1111/j.1464-410x.1972.tb10055.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown ML, Gersh BJ, Holmes DR, et al. From randomized trials to registry studies: translating data into clinical information. Nat Clin Pract Cardiovasc Med. 2008;5(10):613–20. doi: 10.1038/ncpcardio1307. [DOI] [PubMed] [Google Scholar]