Abstract
Purpose
Corneal tissue engineering has attracted the attention of many researchers over the years in part due to the cornea’s avascularity and relatively, straight forward structure. However, the highly organized and structured nature of this optically clear tissue has presented a great challenge. We have previously developed a model where human corneal fibroblasts (HCFs) are stimulated by a stable Vitamin C (VitC) derivative to self-assemble an extracellular matrix (ECM). Addition of TGF-β1 enhanced the assembly of ECM; however, it was accompanied by the upregulation of specific fibrotic markers. In this study, we tested the effects of all three TGF-β isoforms (-β1, -β2 and -β3) on ECM production, as well as, expression of fibrotic markers.
Methods
HCFs were grown in four media conditions for 4 weeks: (Control) VitC only; (T1) VitC+TGF-β1; (T2) VitC+TGF-β2; or (T3) VitC+TGF-β3. Cultures were analyzed with Western Blots, TEM and indirect-immunofluorescence (IF).
Results
Compared to controls, all TGF-β isoforms stimulated matrix production by ~3 times. IF showed the presence of type III collagen and smooth muscle actin (SMA) in T1 and T2; however, T3 showed little, to no, expression. In western blots, T3 stimulated a lower type III/type I collagen ratio when compared to the other conditions. In addition, TEM indicated that T3 stimulated a higher level of matrix alignment and organization.
Conclusions
HCFs stimulated by VitC and TGF-β3 appear to generate a matrix that mimics the normal adult or developing human cornea; whereas, TGF-β1 and -β2 are driving the constructs toward a more fibrotic path.
Keywords: Cornea, Corneal Fibroblasts, Extracellular Matrix, Growth Factors, TGF-β3, fibrosis, Collagen, Vitamin C
1. Introduction
The cornea accounts for approximately two-thirds of the human eye’s total optical power. It consists of three main layers: epithelium, stroma and endothelium. The stroma makes up ~90% of the corneal thickness and is composed of extracellular matrix (ECM) and cells. The ECM is made up of exquisitely aligned and organized collagen, with type I collagen being the most abundant with its fibrillar structure conferring tensile strength to the cornea. The primary cell type that resides in the adult stroma is termed keratocytes. During development, these cells are known to secrete the precursors of all the ECM components, such as collagens, proteoglycans and other glycoproteins. Activated keratocytes or cells cultured in serum are generally termed cornel fibroblasts.
In the cornea, the corneal keratocytes or fibroblasts play a central role in mediating the corneal response to injury or refractive surgery (Jester et al. 1999; Moller-Pedersen et al. 1997; Petroll et al. 1992). In a normal unwounded cornea, the keratocytes are mainly quiescent. Following injury/wounding, the keratocytes adjacent to the wound undergo apoptosis (Helena et al. 1998; Zieske et al. 2001), while cells more distal to the wound differentiate into fibroblasts and/or myofibroblasts. The development of scar tissue is, in most cases, the end result of this healing process (Kubota and Fagerholm 1991; Suzuki et al. 2003).
The main regulators of corneal wound healing, and ultimately clarity, are growth factors, such as FGF-2 (fibroblast growth factor-2), PDGF (platelet derived growth factor), TGF-β (transforming growth factor-β), IL-1α (interleukin-1α), and IGF (insulin-like growth factor), which are known to be strongly involved in scarring and neovascularization (Guo et al. 2007; Jester and Chang 2003; Jester et al. 2002; Kane et al. 2009; Long et al. 2000; Musselmann et al. 2005; Musselmann et al. 2008). In the human eye, TGF-β is known to participate in fibrotic pathologies. TGF-β has been reported to modulate collagen synthesis and cause tissue fibrosis (Border and Noble 1994; Wahl et al. 1987; Younai et al. 1994). It is also known to increase granulation, tissue formation and breaking strength in healing dermal wounds (Mustoe et al. 1987; Sporn et al. 1983). There are three TGF-β isoforms identified, TGF-β1, -β2 and -β3 (T1, T2 and T3, respectively), and their distinct and differential roles have been shown with studies involving mouse and human embryos (Gatherer et al. 1990; Millan et al. 1991; Pelton et al. 1990; Pelton et al. 1991; Schmid et al. 1991).
We have developed a stroma-like model that consists of primary human corneal fibroblasts (HCFs) and their self-assembled matrix, which we have shown to mimic the stroma seen during development (Guo et al. 2007; Karamichos et al. 2010; Ren et al. 2008). As many as 10–12 layers of flattened cells have been observed in this model. In addition, perpendicular orientation of adjacent fibril layers are present. In this study, we investigated the role of the different TGF-β isoforms on the cells, and the synthesis and assembly of ECM. Several studies have shown the contribution of these isoforms to tissue regeneration (O'Kane and Ferguson 1997), cell differentiation (Williams et al. 2003) and embryonic development (Roberts and Sporn 1992). However, their specific role is different. T1 and T2 are responsible for fibrosis and scar formation, whereas T3 acts as an inhibitor. In our studies, T1 and T2 have been found to stimulate ECM accumulation, along with markers of fibrosis, such as type III collagen and α-smooth muscle actin (SMA). In contrast, T3 stimulates ECM without fibrotic markers. These findings correlate with studies in skin, where T3 has been found to promote scar-free healing in cutaneous wounds (Shah et al. 1995).
In our model, corneal fibroblasts can be regulated by the means of growth factors to produce a better architectural ECM without expressing fibrotic markers. The novel system here and its development can help identify the factors involved in corneal scarring and how they may be regulated. This information will be vital for sight-threatening cases.
2. Materials and methods
2.1. Primary Culture of Human Corneal Fibroblasts (HCFs)
Human corneas were obtained from the National Disease Research Interchange (NDRI; Philadelphia, PA). HCFs were isolated as previously described (Guo et al. 2007). In brief, corneal epithelium and endothelium were removed from the stroma by scraping with a razor blade. The stromal tissue was cut into small pieces (~2×2mm) and put into 6-well plates (4 or 5 pieces per well). Explants were allowed to adhere to the bottom of the wells and Eagle’s Minimum Essential Medium (EMEM: ATCC; Manassas, VA) containing 10% fetal bovine serum (FBS: Atlantic Biologicals; Lawrenceville, CA) was added. After 1–2 weeks of cultivation, the fibroblasts were passaged into a 100mm2 cell culture plate. The cells were allowed to grow to 100% confluence before being used in the culture system. All research adhered to the tenets of the Declaration of Helsinki. Passages up to number three were used throughout these experiments.
2.2. Fibroblast Assembled ECM
The HCFs were plated on transwell 6-well plates containing polycarbonate membrane inserts with 0.4µm pores (Costar; Charlotte, NC). One milliliter of the HCFs were plated per well (106 cells/ml) and cultured for 4 weeks with EMEM plus 10% FBS and 0.5mM 2-O-α-D-glucopyranosyl-L-ascorbic acid (VitC: Wako Chemicals USA, Inc.; Richmond, VA), as well as, one of the following: No Growth Factor (Control), T1, T2, or T3. T1, T2 and T3 were used at a concentration of 0.1ng/ml. Initial concentration-dependent experiments investigated cell-ECM responses using all three isoforms. Contraction was shown for all T1, T2 and T3 conditions at concentrations greater than 0.5ng/ml. At week 4, samples of the resulting constructs were collected and processed for whole mount indirect-immunofluorescence (IF), light microscopy, transmission electron microscopy (TEM), and Western Blotting (WB). All experiments were repeated at least three times.
2.3. Whole mount indirect-immunofluorescence
Constructs were collected and fixed in 4% paraformaldehyde. IF was performed as previously described (Zieske et al. 2001). Following fixation, the constructs were incubated at 4°C overnight with the primary antibody—anti-type III collagen (1:40: Southern Biotech; Birmingham, AL), or anti-SMA (1:50: Dako North America; Carpinteria, CA)—diluted in 1%BSA + 0.1%Triton-X. The constructs were then washed and incubated overnight at 4°C with the corresponding secondary antibody—donkey anti-goat IgG (1:200, type III collagen), or donkey anti-mouse IgG (1:200, SMA)—diluted in 1%BSA + 0.1%Triton-X. Phalloidin-rhodamine (Invitrogen; Carlsbad, CA), which binds to the f-actin in all cells, was also used. Constructs were counterstained with TOPRO-3 iodide (1:1000, Invitrogen), a marker of all cell nuclei. Negative controls, where the primary antibody was omitted, were run with all experiments. Constructs were washed, mounted with Vectashield Mounting Media (Vector Laboratories; Burlingame, CA), observed and photographed using a confocal TCS-SP2 Leica microscope (Leica Microsystems; Bannockburn, IL). In addition, construct thicknesses were also measured with the confocal microscope, beginning with the first cell visible at the top of the construct and the last cell visible at the bottom. Data was averaged and analyzed.
2.4. Light and Transmission Electron Microscopy
Constructs collected for TEM were fixed in ½ strength Karnovsky’s fixative (2% paraformaldehyde, 2.5% gluteraldehyde in cacodylate buffer, pH 7.4) and processed using standard procedures, as described previously (Gipson et al. 1983). Briefly, a diamond knife ultramicrotome (LKB ultramicrotome; Bromma, Sweden) was used to cut transverse to the plane of the construct. Sections were collected for both light microscopy and TEM. For light microscopy, optical thick-sections of 1–2µm were obtained and stained with phenylenediamine. These thick-sections were viewed and documented with a Nikon Eclipse E800 equipped with a SPOT camera (Nikon Instruments, Inc.; Melville, NY). For TEM, 60–90Å-sections were obtained, viewed and photographed with a Philips 410 Transmission Electron Microscope (Philips Electronics N.V.; Eindhoven, The Netherlands)
2.5. Western Blot
Constructs were flash frozen with liquid nitrogen and processed for Western blotting, as previously described (Chung et al. 1999). Briefly, tissues were homogenized and lysed. The protein was then purified and assayed by the Bradford dye-binding method (Bio-Rad; Hercules, CA). Equal amounts of total protein were loaded on a reduced 6% Tris-Glycine gel (Invitrogen). After separation, the protein was transferred to a membrane (Immobilon-P: Millipore; Billerica, MA), which was then stained with Ponceau (Sigma; St.Louis, MO) to check for transfer efficiency. Membranes were blocked with Blotto (5% milk in TBS) and then probed, as previously described (Chung et al. 1999), with the following antibodies: type I (1:2000: Abcam; Cambridge, MA) and type III (1:2000: Novus Biologicals; Littleton, CO) collagen. Results were normalized to Control (VitC only) and plotted as the fold enhancement of type III/type I collagen ratio. In addition, culture media from all the conditions were collected every week and equal volumes were loaded. Identical analysis was performed as with the tissue (see above).
2.6. 3D Software – Intensity measurements
Image Pro Plus (Image Pro Plus v.7: Media Cybernetics; Bethesda, MD) is a 2 and 3D analysis program and was used to analyze and quantitate data from the confocal z-series and TEM photomicrographs. The analyses performed for this study were as follows:
Cell Counting and Integrated Optical Density (IOD)
Image Pro Plus was used to quantitate the number of cells/construct and the intensity of antibody binding in the constructs. We used the confocal z-series in order to calculate the total binding intensity of the antibody of interest (IOD-intensity value). We also counted each cell throughout the construct, as well as the diameter of the nuclei. At least three confocal z-series were used at each condition and their average was plotted and analyzed.
Fibril diameter
At least ten random TEM photomicrographs were used for each condition, and a total of 100 fibrils were measured for their diameter. The scale bar for the TEM was used to calibrate the measurements.
2.7. Statistical Analysis
All experiments were performed in triplicates and data was averaged and analyzed for significant variations (p<0.05) using the Student’s t-test and/or Dunnett’s Multiple Comparison test.
3. Results
3.1. Cellular organization and growth factor effects
Previously, we demonstrated that cells incubated with VitC on transwell polycarbonate membranes lead to multi-layer 3D constructs. These constructs showed alternate orientations of both cells and ECM components (Guo et al. 2007; Karamichos et al. 2010). We have also demonstrated that TGF-β1 stimulates accumulation of fibrotic matrix in a time and dose dependent manner (Karamichos et al. 2010). In this current study, we extend our investigation to all three TGF-β isoforms in order to investigate their differential effect on our system. These experiments were stimulated by many reports that the three isoforms can have differing effects, even though they appear to bind and activate the same set of receptors. The three different growth factors were used at the same concentration (0.1ng/ml) to promote matrix deposition. All experiments were performed in triplicate.
Our constructs showed several cell layers and high cell alignment throughout, with the highest levels of organization observed at the top and bottom. Interestingly, cell nuclei diameters were found to vary significantly, with the nuclei of cells stimulated with any of the three isoforms being ~3-fold smaller than controls (Table 1, *p<0.001). We postulated that the smaller nuclei may be present in fibrotic cells; however, no correlation between cell shape and nuclei size was observed.
Table.
The nuclear diameter and number of cells in the constructs were measured and analyzed.
| Control | T1 | T2 | T3 | |
|---|---|---|---|---|
| Nuclear Diameter (µm) | 31.9±0.3 | *11.3±0.2 | *11.5±0.2 | *13.2±0.1 |
| Cell Number (×106) | 2.48±0.2 | *6.63±0.4 | *6.99±0.5 | *5.14±0.4 |
The nuclear diameters of the cells in the constructs of all three TGF-β isoforms (T1, T2, and T3) were significantly smaller than controls (*p<0.001). The cell number in the constructs of all three TGF-β isoforms was significantly increased compared to controls (*p<0.001).
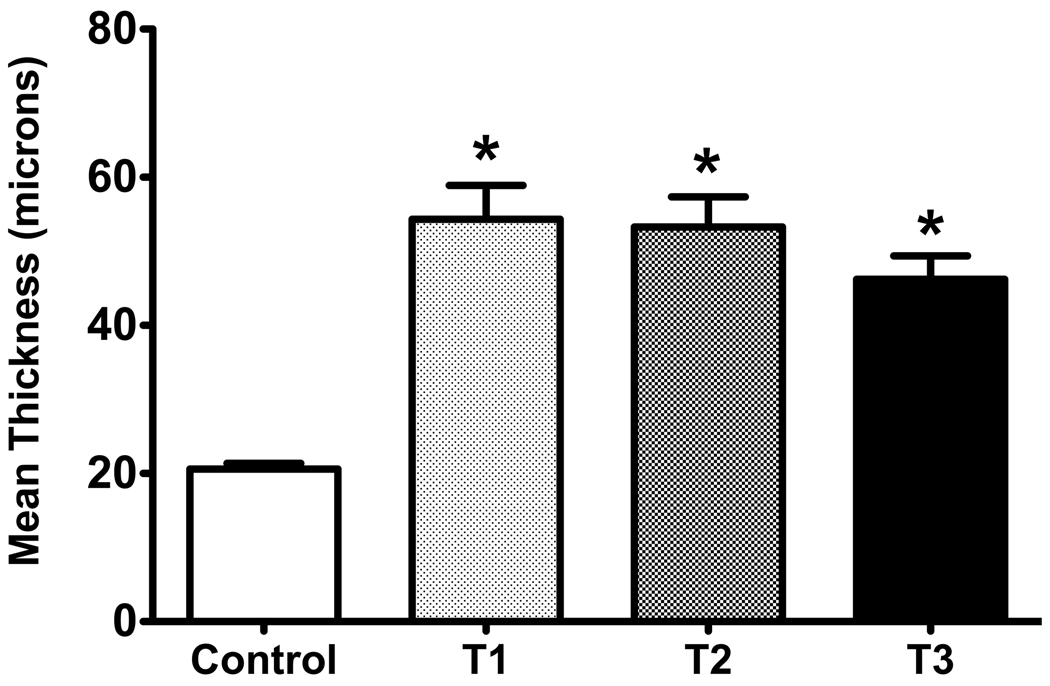
Previously, we have shown that T1 stimulated the accumulation of a fibrotic matrix. In the current study, all three isoforms, T1, T2 and T3, stimulated cell stratification and matrix component production when compared to controls. We found no significant differences in the thickness of the constructs cultured with any of the three isoforms (Figure 1); however, they were all significantly different, *p<0.05, when compared to controls (2.6-, 2.6- and 2.2-fold, respectively).
Figure 1.
Graph of the mean thickness of the constructs treated with one of three TGF-β isoforms and control. Four conditions were examined and analyzed: (Control) VitC only, (T1) VitC + TGF-β1, (T2) VitC + TGF-β2, and (T3) VitC + TGF-β3. All three isoforms led to a significant increase in thickness as compared to the control (*p<0.05).
To determine if this increase in thickness was the result of an increase in the number of cells or an increase in the amount of matrix produced per cell, we examined the cell number. Results showed a significant increase in cell number following TGF-β stimulation (*p<0.001) compared to controls (Table 1). However, the amount of ECM produced per cell remained relatively constant under all conditions.
3.2. ECM organization
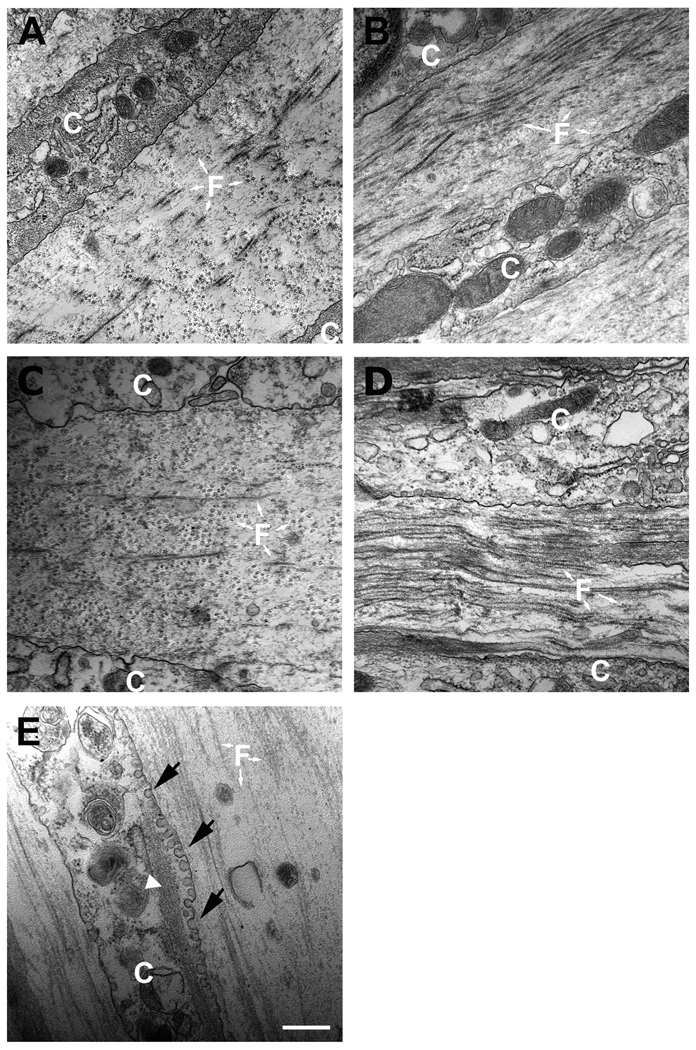
Constructs were examined at the end of 4 weeks using TEM. Representative images for each condition are shown in Figure 2. Cell-ECM interactions were observed in each of the constructs. T1 and T2 (Figs. 2B and C) showed areas of organized ECM, as well as many cells with aligned actin-like filaments, indicative of myofibroblasts (Fig. 2E, white arrowhead). T3 (Fig. 2D) showed the highest density of collagen fibrils and ECM alignment, as compared to control (Fig. 2A) and the other two TGF-β isoforms (Figs. 2B and C). It was clear that fewer cells with actin stress fibers were present, suggesting a “quieter” and less fibrotic construct (Fig. 2D). Furthermore, TEM revealed an increased number of cells with vesicles (Fig. 2E, black arrows). The vesicles appeared to correlate with the presence of actin filaments and may be another marker for myofibroblasts. Presence of vesicles has not been previously suggested as a marker of myofibroblasts. Our previous publication (Guo et al. 2007) noted the presence of these structures.
Figure 2.
TEM (31 000×) showing cell-matrix interaction and matrix condition. A) Control, B) TGF-β1 treated (T1), C) TGF-β2 treated (T2), and D) TGF-β3 treated (T3) constructs. The fibril orientation changes direction more than once in all conditions. T3 treated construct (D) shows higher density of collagen fibril and ECM alignment compared to controls, T1 and T2. T3 also appeared to show fewer cells with actin stress fibers (data not shown). E) Example of cell with vesicles (arrows) correlating with actin filaments (arrowhead). White “C” = cell and “F” = fibrils. Bars = 500nm.
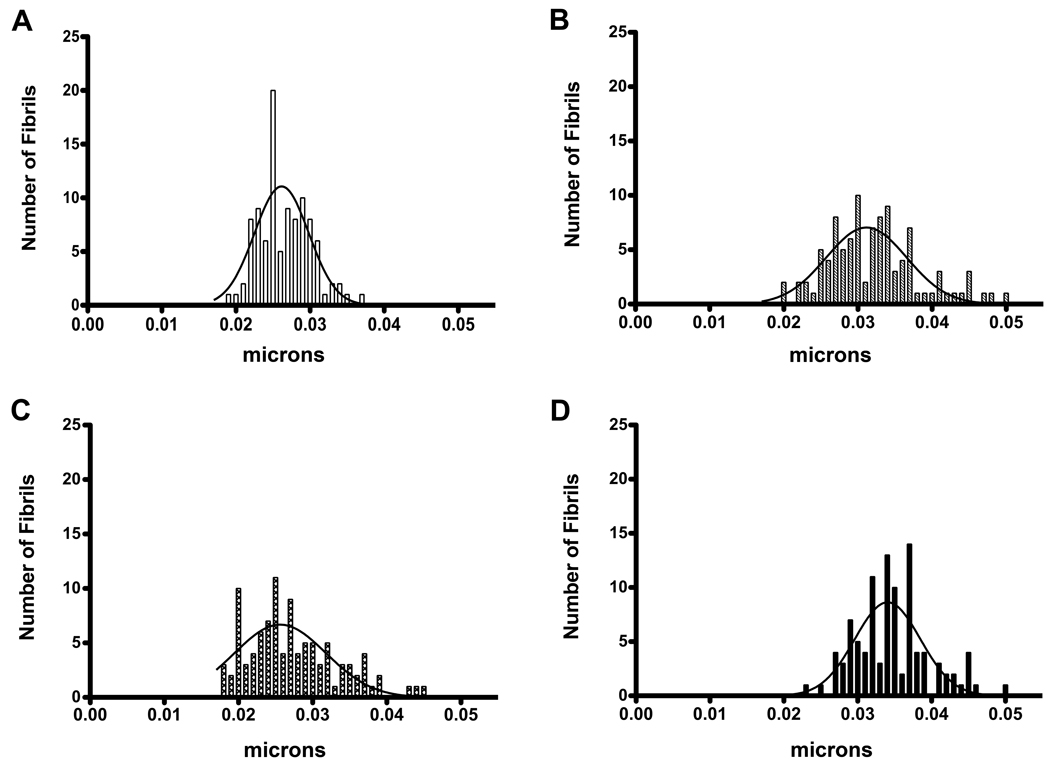
Following TEM, photomicrographs (31 000×) of the constructs were used to measure and quantify the fibril diameter for each condition. A mature human corneal stroma is comprised primarily of hydrated type I/V collagen fibrils of uniform diameter, 30–35 nm. The scale bar for the photomicrographs was used to calibrate the measurements. One hundred random fibril diameters were measured for each condition. Controls (Fig. 3A) showed a fibril diameter range of 18–35nm with the peak at 25nm. Similar peak (25nm) was found with T2 (Fig. 3C), with a slightly larger range of fibril diameters (17–46nm). T1, on the other hand, showed a peak at 30nm (Fig. 3B), and T3’s peak increased even further, 37nm (Fig. 3D). T3 had 46% of its fibrils measuring at 35nm compared to 20–35% of controls and the other two TGF-β isoforms.
Figure 3.
Histograms demonstrating the distribution of fibril diameters. A) Control, B) TGF-β1 treated (T1), C) TGF-β2 treated (T2), and D) TGF-β3 treated (T3) constructs. Control fibril diameters ranged from 18 to 35nm with a peak at 25nm. T2 also peaked at 25nm, but had a slightly larger range (17–46nm). T1’s range was similar to T2 (20–50nm), with a peak of 30nm. T3, however, peaked at 37nm with 46% of its fibrils measuring 30–35nm in diameter, compared to 20–35% for Control, T1 and T2. A mature human corneal stroma has fibrils of 30–35nm.
3.3. HCF response to growth factors – IF
To assess cellular responses to growth factors, media was supplemented with 10% FBS, VitC and one of three TGF-β isoforms. Controls were cultured in 10% FBS and VitC only, no growth factors. The final concentration of the growth factors was determined from a preliminary dose-dependent study (data not shown) and represents the common dose for all growth factors for maximum response without contraction of the construct.
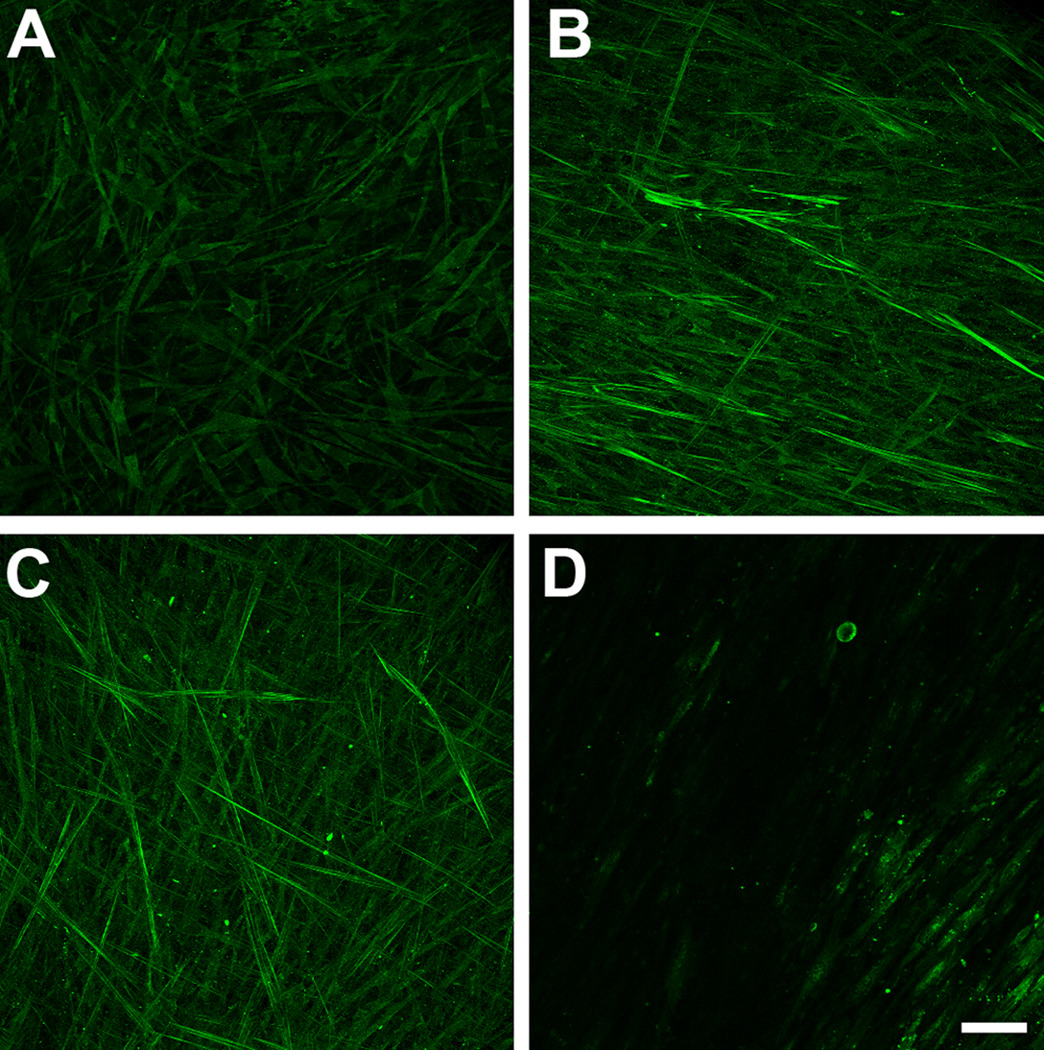
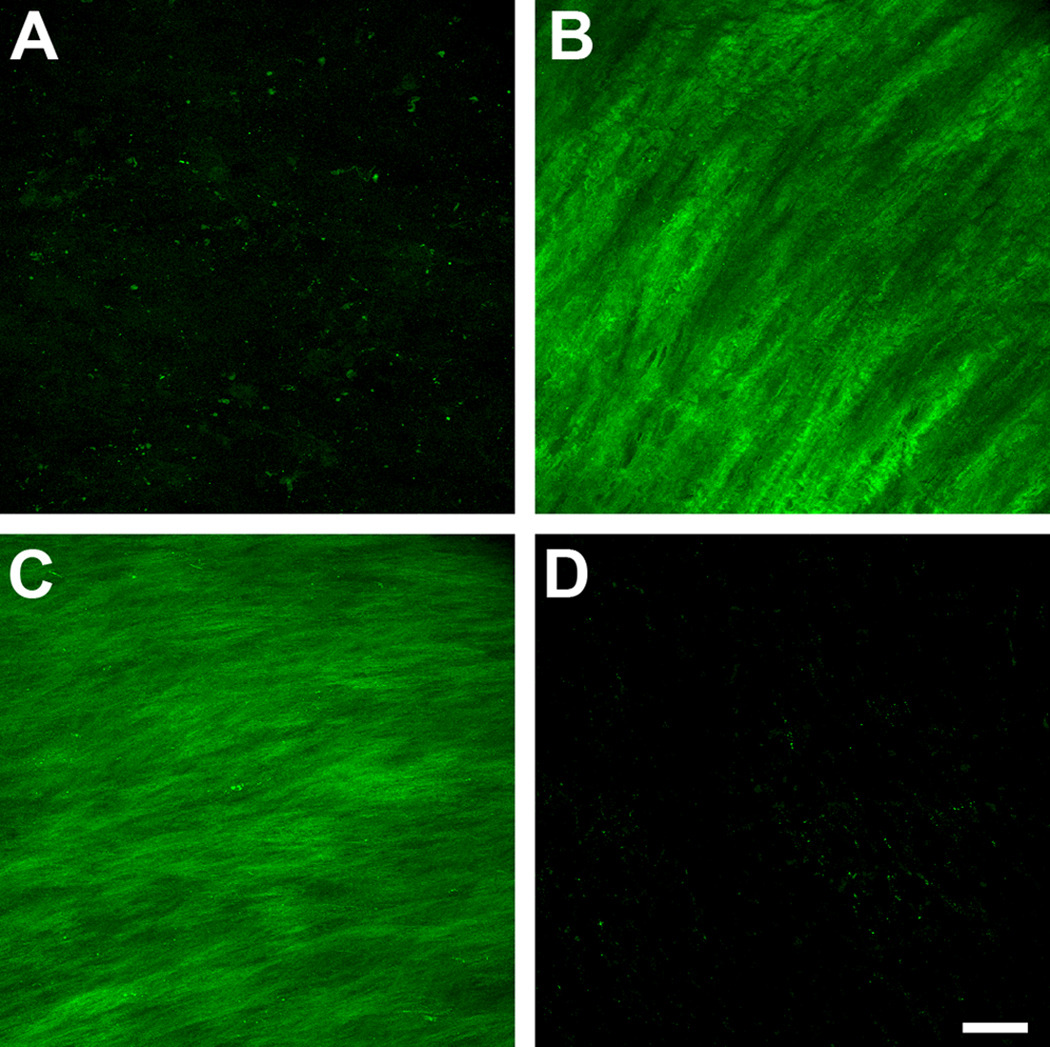
We investigated the presence and expression of specific fibrotic markers and how these may be regulated when the cells were stimulated by the three isoforms. In agreement with our previous work (Karamichos et al. 2010), few, if any myofibroblasts were present in control constructs (Fig. 4A); however, the addition of both T1 and T2 led to a clear increase of positive SMA cells (Fig. 4B and C, respectively). These cells appeared throughout the construct with a high degree of alignment and a preference towards the top and bottom of the construct. T3 on the other hand, showed minimal, if any, expression of SMA-positive cells, indicating a less fibrotic effect (Fig. 4D).
Figure 4.
Maximum projection confocal images of indirect immunofluorescence with α-smooth muscle-actin (SMA) on full-thickness constructs at 4 weeks. A) Control, B) TGF-β1 treated (T1), C) TGF-β2 treated (T2), and D) TGF-β3 treated (T3) constructs. Compared to controls, the number of cells expressing SMA stress fibrils increased considerably when T1 or T2 were introduced to the system. T3 treated constructs, however, showed minimal, if any expression of SMA. T1 and T2 appeared to stimulate fibrosis, while T3 did not. Bar = 50 microns.
Also, as seen in Figure 5, moderate amounts of type III collagen was found in control (Fig. 5A); whereas, T1 and T2 treated cells showed high amounts of type III collagen, with a high degree of alignment (Fig. 5B and C, respectively). Type III collagen is another fibrotic marker, and as with SMA, when T3 was used to stimulate the cells, its expression was down to a minimum, if any (Fig. 5D).
Figure 5.
Maximum projection confocal images of indirect immunofluorescence with type III collagen on full-thickness constructs at 4 weeks. A) Control, B) TGF-β1 treated (T1), C) TGF-β2 treated (T2), and D) TGF-β3 treated (T3) constructs. Compared to controls, the expression of type III collagen was increased considerably when T1 or T2 were introduced to the system. T3 treated constructs, however, showed minimal, if any expression of type III collagen. T1 and T2 appeared to stimulate fibrosis, while the T3 did not. Bar = 50 microns.
Our data shows that with the addition of T3, specific fibrotic markers were not upregulated, as seen with the other isoforms. This data is supported by the observations made using TEM, as well as the quantification of fibril diameters, shown above.
3.4. Evaluation of integrated optical density
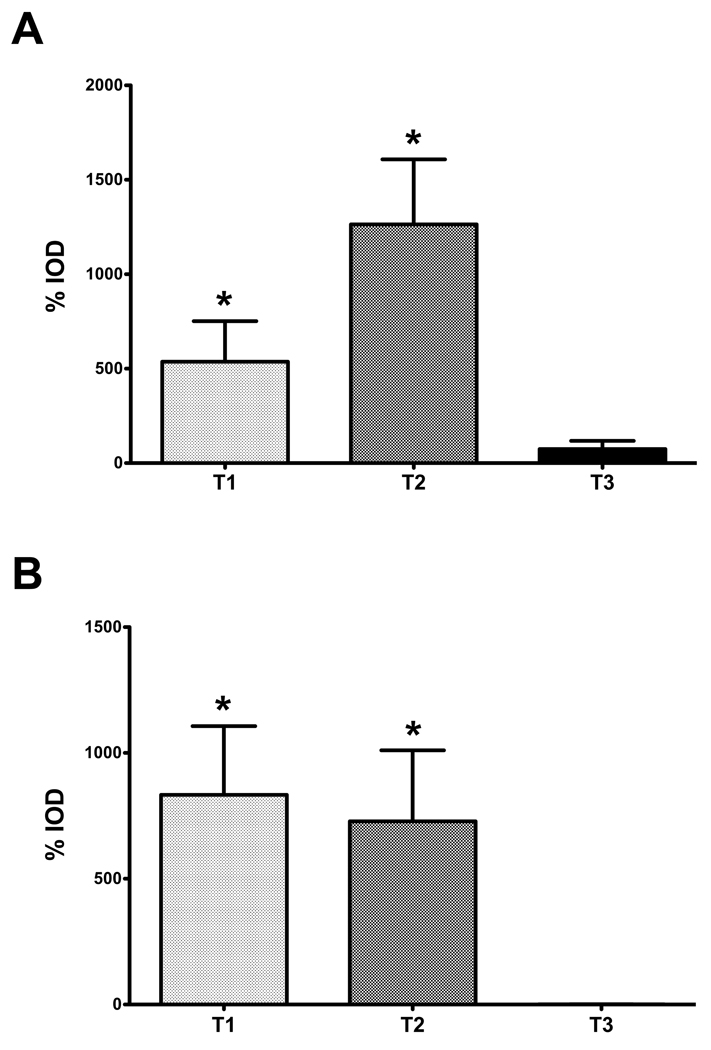
In order to quantitate the amount of antibody binding present in our 3D constructs, we analyzed our IF data using the Image Pro Plus Software. With this software we were able to calculate and compare the integrated optical density (IOD), which is the total amount of antibody present within the area of interest, which, in our study, are the 3D constructs. Results were in agreement with our IF observations, showing significant increase of the IOD when cells were treated with T1 and T2 (*p<0.05), and an IOD of almost zero when T3 was present. As shown in Figure 6, we plotted the percent (%) increase of IOD for all conditions (T1, T2 and T3) compared to controls for both SMA (Fig. 6A) and type III collagen (Fig. 6B). Both T1 and T2 showed enormous % IOD increase of SMA (500 and 1200, respectively) and type III collagen (800 and 700, respectively). This is in agreement with our data, as well as previous work, that these isoforms lead to myofibroblast formation and fibrotic ECM. In contrast, T3 was higher than controls; however, the % IOD was only 1 and 74% for type III collagen and SMA, respectively.
Figure 6.
Graph of the percent (%) integrated optical density (IOD) of SMA and type III collagen in the constructs. Four conditions were examined and analyzed after indirect immunofluorescence was performed with either SMA or type III collagen: (Control) VitC only, (T1) VitC + TGF-β1, (T2) VitC + TGF-β2, and (T3) VitC + TGF-β3. Data was plotted as the % increase of IOD for all conditions (T1, T2 and T3) compared to controls for both smooth muscle actin (A) and type III collagen (B). (A) %IOD for SMA expression significantly increased (*p<0.05) when cells were treated with T1 and T2. (B) Similar results were shown for type III collagen, with *p<0.05 for both T1 and T2. T3, however, had a %IOD of almost zero for both SMA (A) and type III collagen (B).
3.5. Protein analysis
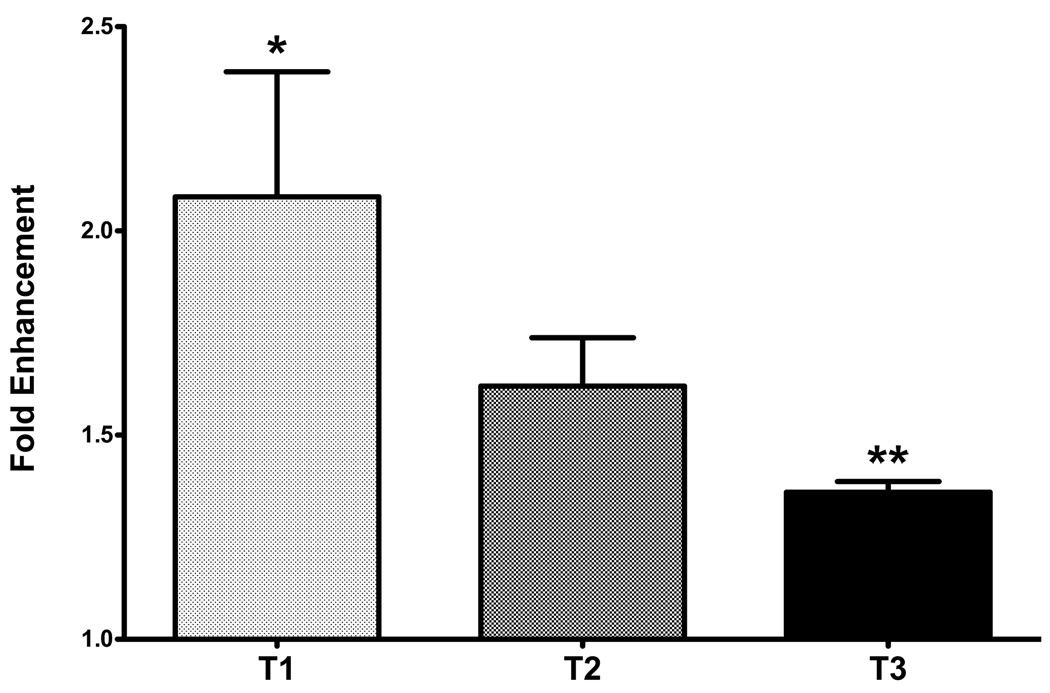
The constructs were examined for the presence of types I and III collagens. Results were normalized to controls and plotted as the fold enhancement of type III/type I collagen ratio. As shown in Figure 7, cultures treated with T1 showed a significant increase of 2.1-fold (p<0.5), as compared to controls. This indicated that the constructs treated with T1 were progressively becoming fibrotic, which agrees with our IF and TEM data, shown above. T3, on the other hand, was confirmed for its non-fibrotic effects on our model by resulting only in a 1.4-fold increase, which was not statistically significantly from controls. Furthermore, T3 was found to be statistically different from T1 (p<0.05), supporting the data shown here about T3’s non-fibrotic effects. Although IF and TEM data for constructs treated with T2 indicated that the constructs were becoming more fibrotic, the increase in the typeIII/type I collagen ratio was not significant. Interestingly, when we analyzed the amounts of type I and III collagen secreted in the culture media, T3 showed a reduction of ~2-fold when compared to the other two isoforms (data not shown). This indicates that more of the collagen secreted by the cells treated with T3 gets deposited in the ECM compared to the other two isoforms.
Figure 7.
Graph of the fold enhancement of the regulation of type III/type I collagen ratio following TGF-β1 (T1), -β2 (T2) or -β3 (T3) stimulation. Data is normalized to control. T1 showed a significant increase as compared with control (*p<0.05). T3 showed a slight, but not significant, increase as compared to control; however, T3 decreased significantly as compared with T1 (**p<0.05).
4. Discussion
The concept of “curing” corneal opacity by replacement with a clear substitute has been discussed in published form for over 200 years, with the first suggestion being to transplant a piece of quartz to repair the cornea. In the ensuing years, many advances have been made in corneal transplantation, including the use of cadaver corneas. However, since there has been a constant shortage of donor corneas worldwide, and since certain types of injuries and diseases are not amenable to transplantation, many efforts have been made to find a corneal replacement. Over the past 50–60 years, several investigations have been made into the use of plastics to develop an artificial cornea, also termed keratoprosthesis. These keratoprosthesis have enjoyed some success, with the Boston keratoprosthesis being placed in over 1000 patients in 2009 (Gomaa et al. 2010). Another device that initially gave promising results is the AlphaCor (Bleckmann and Holak 2006; Hicks et al. 2006), although recent reports have been less encouraging (Chalam et al. 2007; Holak et al. 2009). As an alternative to the use of plastics, several investigations have been made to engineer an artificial cornea using natural compounds, such as collagens, cells and cell lines (Alaminos et al. 2006; Fagerholm et al. 2009; Gonzalez-Andrades et al. 2009; Huang and Li 2007; Mi et al. 2010; Schneider et al. 1999; Vrana et al. 2008). The goal of these studies is to develop a synthetic cornea that mimics the native cornea and also integrates into the human eye.
Minami et al. (Minami et al. 1993) were one of the initial studies trying to reconstruct a cornea in vitro. They developed all three layers using primary bovine corneal cells and tested their system in animals. Zieske et al. (Zieske et al. 1994) also developed a similar model using primary rabbit epithelial and stromal cells and immortalized mouse endothelial cells. This system was remarkable for developing a continuous basement membrane in culture. Griffith et al. (Griffith et al. 1999) has more recently published the development of a full three-layer cornea-like construct using human corneal epithelial, endothelial and keratocyte cell lines in a type I collagen/chondroitin sulphate substrate. Li et al. (Li et al. 2003) evaluated the use of hydrogels (a collagen-copolymer) and reported successful growth of epithelium and stromal cells. The constructs had good clarity and were strong enough to be sutured. Most recently, Proulx et al. (Proulx et al. 2010) reported a three-layer substitute, which used human cells and exhibited excellent epithelial and endothelial morphology, as well as an intact basement membrane. Other studies have also used human cells, combined with immortalized cell lines, in order to reconstruct an artificial three-layer construct (Reichl and Muller-Goymann 2003; Vrana et al. 2008) with promising results.
Even though these approaches obtain clear strong constructs, they are still somewhat limited by the lack of a cornea-like ECM. The partial corneal construct approach has become of great interest since it has the advantage of 1) targeting the area or layer that has been damaged or injured, and 2) can concentrate more on the optimum growth conditions of this layer.
This current study focuses on the stromal region and using a cell-based approach involving the stimulation of corneal cells to secrete and assemble their own ECM. Guo et al. (Guo et al. 2007) used primary human corneal fibroblasts to self-assemble a stromal substitute by stimulating them with a stable form of Vitamin C (VitC). Specific ECM macromolecules were identified, and collagen fibril alignment, along with changes in orientation of the fibrils, similar to that seen in vivo, was documented (Guo et al. 2007). More recently, our group showed the effect of T1 on the development of these 3D constructs (Karamichos et al. 2010). T1 stimulated the accumulation of enhanced amounts of matrix; however, the limitation and potential problem for this model is the accompanying enhanced presence of myofibroblasts and fibrotic markers. The presence of fibrotic markers is associated with corneal opacity, making it unclear if this model would be useful for corneal transplantation. In an unpublished study, the constructs without T1 have been transplanted in vivo into the corneas of Balb/C mice, and have been accepted by the host cornea. However, the long-term survival, as well as mechanical integrity, has yet to be tested.
The cell-based stromal constructs developed here were stimulated with one of three TGF-β isoforms, and the matrix secretion increased dramatically. We showed striking differences between the three isoforms using a variety of techniques, such as IF, TEM and 3D analysis. Our data shows a novel way to control specific fibrotic markers using T3, as well as the improvement on collagen density, cell proliferation and collagen fibril diameter, which mimic corneal development. The main conclusion from this current study is that stimulation with T3 leads to a construct that has non-fibrotic characteristics, as well as ECM properties that closely mimic in vivo corneal stroma. Type III collagen and SMA were elevated in cultures stimulated by T1 or T2, and suppressed by T3, while ECM deposition remained unchanged (ie. construct thickness). In an adult cornea Type III collagen levels are very low; however, following wounding, these levels are elevated. Our findings correlate well with studies in skin, where T3 was found to promote scar-free healing in cutaneous wounds. Occleston et al. (Occleston et al. 2008) have demonstrated the benefits of exogenous addition of TGF-β3 to cutaneous wounds. The study showed not only improvement on the neodermis architecture, but also safe use in humans. This could potentially lead to the treatment of cornea wounds as well.
In both healthy and injured human corneas, all three TGF-β isoforms have been observed (Carrington et al. 2006; Tseng et al. 1999), indicating that their regulation is vital for both the development and wound healing of the cornea. In a cutaneous wound model, Shah and coworkers (Shah et al. 1995) showed that an increase in T3 levels relative to T1/T2 results in a scarfree phenotype. The ability of all the three isoforms to stimulate cells to synthesize type I collagen was shown; however, only T3 inhibited the secretion and deposition of type III collagen, which is well known for its fibrotic appearance. This agrees with our findings reported here, suggesting that T3 has similar effects in the cornea.
One of the puzzling questions regarding TGF-β signaling is how do the three isoforms stimulate different responses? TGF-β functions by signaling through an intracellular Smad pathway (Yao et al. 2010) or a non-Smad pathway. Despite the fact that the three isoforms have 70–80% sequence homology (Hao et al. 2008) and share most cell-surface receptors, they have been shown to possess distinct roles in wound healing. Propagation of TGF-β signals is vital for understanding differences and similarities of these isoforms. Briefly, phosphorylation of intracellular Smad proteins follows activation of Type I and II receptors. The active form of TGF-β engages to TGF- βRII. T1 and T3 bind to TGF-βRII with high affinity; however, T2 may only bind to this receptor in the presence of TGF-βRIII, a membrane bound betaglycan (Li et al. 2006). It has been speculated that T3 stimulates the activation of different signaling molecules than T1 or T2; however, this is yet to be fully documented. In our current study, we have demonstrated the non-fibrotic effects of T3, which suggests a differential T3 function stimulating a signal transduction pathway to inhibit the synthesis of type III collagen. Examining these pathways will be the subject of future experimentation.
In tissue engineering, the development of this model holds great potential benefits. Firstly, T3 may be a therapeutic agent of corneal repair, especially when no harmful effect is known to be caused by it on corneal re-epitheliazation (Carrington et al. 2006). Secondly, the use of this natural cell-assembled construct means that from one cadaver eye we can get enough cells for the equivalent of over 100 transplanted corneas. It is also conceivable that the patient’s own cells could be used to generate the replacement matrix.
Acknowledgments
Supported by: NIH/NEI Grant No. 1R21EY018939-01
Footnotes
Conflicts of Interest: None (all authors)
Ethics: This research has been reviewed by the institute’s internal review board (IRB) for the use of human tissue. The IRB approved the research and the research falls under exemption 4 for the NIH guidelines. All research adhered to the tenets of the Declaration of Helsinki.
References
- Alaminos M, Del Carmen Sanchez-Quevedo M, Munoz-Avila JI, et al. Construction of a complete rabbit cornea substitute using a fibrin-agarose scaffold. Invest Ophthalmol Vis Sci. 2006;47(8):3311–3317. doi: 10.1167/iovs.05-1647. [DOI] [PubMed] [Google Scholar]
- Bleckmann H, Holak S. Preliminary results after implantation of four AlphaCor artificial corneas. Graefes Arch Clin Exp Ophthalmol. 2006;244(4):502–506. doi: 10.1007/s00417-005-0068-6. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331(19):1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Carrington LM, Albon J, Anderson I, et al. Differential regulation of key stages in early corneal wound healing by TGF-beta isoforms and their inhibitors. Invest Ophthalmol Vis Sci. 2006;47(5):1886–1894. doi: 10.1167/iovs.05-0635. [DOI] [PubMed] [Google Scholar]
- Chalam KV, Chokshi A, Agarwal S, et al. Complications of AlphaCor keratoprosthesis: a clinicopathologic report. Cornea. 2007;26(10):1258–1260. doi: 10.1097/ICO.0b013e31813e0bd8. [DOI] [PubMed] [Google Scholar]
- Chung EH, Hutcheon AEK, Joyce NC, et al. Synchronization of the G1/S transition in response to corneal debridement. Invest Ophthalmol Vis Sci. 1999;40(9):1952–1958. [PubMed] [Google Scholar]
- Fagerholm P, Lagali NS, Carlsson DJ, et al. Corneal regeneration following implantation of a biomimetic tissue-engineered substitute. Clin Transl Sci. 2009;2(2):162–164. doi: 10.1111/j.1752-8062.2008.00083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatherer D, Ten Dijke P, Baird DT, et al. Expression of TGF-beta isoforms during first trimester human embryogenesis. Development. 1990;110(2):445–460. doi: 10.1242/dev.110.2.445. [DOI] [PubMed] [Google Scholar]
- Gipson IK, Grill SM, Spurr SJ, et al. Hemidesmosome formation in vitro. J Cell Biol. 1983;97(3):849–857. doi: 10.1083/jcb.97.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa A, Comyn O, Liu C. Keratoprostheses in clinical practice - a review. Clin Experiment Ophthalmol. 2010;38(2):211–224. doi: 10.1111/j.1442-9071.2010.02231.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Andrades M, Garzon I, Gascon MI, et al. Sequential development of intercellular junctions in bioengineered human corneas. J Tissue Eng Regen Med. 2009;3(6):442–449. doi: 10.1002/term.178. [DOI] [PubMed] [Google Scholar]
- Griffith M, Osborne R, Munger R, et al. Functional human corneal equivalents constructed from cell lines. Science. 1999;286(5447):2169–2172. doi: 10.1126/science.286.5447.2169. [DOI] [PubMed] [Google Scholar]
- Guo X, Hutcheon AEK, Melotti SA, et al. Morphologic characterization of organized extracellular matrix deposition by ascorbic acid-stimulated human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2007;48(9):4050–4060. doi: 10.1167/iovs.06-1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Varshney RR, Wang DA. TGF-beta3: A promising growth factor in engineered organogenesis. Expert Opin Biol Ther. 2008;8(10):1485–1493. doi: 10.1517/14712598.8.10.1485. [DOI] [PubMed] [Google Scholar]
- Helena MC, Baerveldt F, Kim WJ, et al. Keratocyte apoptosis after corneal surgery. Invest Ophthalmol Vis Sci. 1998;39(2):276–283. [PubMed] [Google Scholar]
- Hicks CR, Crawford GJ, Dart JK, et al. AlphaCor: Clinical outcomes. Cornea. 2006;25(9):1034–1042. doi: 10.1097/01.ico.0000229982.23334.6b. [DOI] [PubMed] [Google Scholar]
- Holak SA, Holak HM, Bleckmann H. AlphaCor keratoprosthesis: postoperative development of six patients. Graefes Arch Clin Exp Ophthalmol. 2009;247(4):535–539. doi: 10.1007/s00417-008-0964-7. [DOI] [PubMed] [Google Scholar]
- Huang YX, Li QH. An active artificial cornea with the function of inducing new corneal tissue generation in vivo-a new approach to corneal tissue engineering. Biomed Mater. 2007;2(3):S121–S125. doi: 10.1088/1748-6041/2/3/S07. [DOI] [PubMed] [Google Scholar]
- Jester JV, Chang J-H. Modulation of cultured corneal keratocyte phenotype by growth factors/cytokines control in vitro contractility and extracellular matrix contraction. Exp Eye Res. 2003;77(5):581–592. doi: 10.1016/s0014-4835(03)00188-x. [DOI] [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, et al. TGFbeta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGFbeta, PDGF and integrin signaling. Exp Eye Res. 2002;75(6):645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Cavanagh HD. Corneal stromal wound healing in refractive surgery: the role of the myofibroblast. Prog Retinal Eye Res. 1999;18(3):311–356. doi: 10.1016/s1350-9462(98)00021-4. [DOI] [PubMed] [Google Scholar]
- Kane BP, Jester JV, Huang J, et al. IGF-II and collagen expression by keratocytes during postnatal development. Exp Eye Res. 2009;89(2):218–223. doi: 10.1016/j.exer.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamichos D, Guo XQ, Hutcheon AEK, et al. Human corneal fibrosis: an in vitro model. Invest Ophthalmol Vis Sci. 2010;51(3):1382–1388. doi: 10.1167/iovs.09-3860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota M, Fagerholm P. Corneal alkali burn in the rabbit. Waterbalance, healing and transparency. Acta Ophthalmol (Copenh) 1991;69(5):635–640. doi: 10.1111/j.1755-3768.1991.tb04852.x. [DOI] [PubMed] [Google Scholar]
- Li F, Carlsson D, Lohmann C, et al. Cellular and nerve regeneration within a biosynthetic extracellular matrix for corneal transplantation. Proc Natl Acad Sci U S A. 2003;100(26):15346–15351. doi: 10.1073/pnas.2536767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WY, Huang EY, Dudas M, et al. Transforming growth factor-beta3 affects plasminogen activator inhibitor-1 expression in fetal mice and modulates fibroblast-mediated collagen gel contraction. Wound Repair Regen. 2006;14(5):516–525. doi: 10.1111/j.1743-6109.2006.00158.x. [DOI] [PubMed] [Google Scholar]
- Long CJ, Roth MR, Tasheva ES, et al. Fibroblast growth factor-2 promotes keratan sulfate proteoglycan expression by keratocytes in vitro. J Biol Chem. 2000;275(18):13918–13923. doi: 10.1074/jbc.275.18.13918. [DOI] [PubMed] [Google Scholar]
- Mi S, Chen B, Wright B, et al. Plastic compression of a collagen gel forms a much improved scaffold for ocular surface tissue engineering over conventional collagen gels. J Biomed Mater Res A. 2010;95(2):447–453. doi: 10.1002/jbm.a.32861. [DOI] [PubMed] [Google Scholar]
- Millan FA, Denhez F, Kondaiah P, et al. Embryonic gene expression patterns of TGF beta 1, beta 2 and beta 3 suggest different developmental functions in vivo. Development. 1991;111(1):131–143. doi: 10.1242/dev.111.1.131. [DOI] [PubMed] [Google Scholar]
- Minami Y, Sugihara H, Oono S. Reconstruction of cornea in three-dimensional collagen gel matrix culture. Invest Ophthalmol Vis Sci. 1993;34(7):2316–2324. [PubMed] [Google Scholar]
- Moller-Pedersen T, Vogel MD, Li H, et al. Quantification of stromal thinning, epithelial thickness, and corneal haze following photorefractive keratectomy using in vivo confocal microscopy. Ophthalmol. 1997;104(3):360–368. doi: 10.1016/s0161-6420(97)30307-8. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Alexandrou B, Kane B, et al. Maintenance of the keratocyte phenotype during cell proliferation stimulated by insulin. J Biol Chem. 2005;280(38):32634–32639. doi: 10.1074/jbc.M504724200. [DOI] [PubMed] [Google Scholar]
- Musselmann K, Kane BP, Alexandrou B, et al. IGF-II is present in bovine corneal stroma and activates keratocytes to proliferate in vitro. Exp Eye Res. 2008;86(3):506–511. doi: 10.1016/j.exer.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustoe TA, Pierce GF, Thomason A, et al. Accelerated healing of incisional wounds in rats induced by transforming growth factor-beta. Science. 1987;237(4820):1333–1336. doi: 10.1126/science.2442813. [DOI] [PubMed] [Google Scholar]
- O'Kane S, Ferguson MW. Transforming growth factor beta s and wound healing. Int J Biochem Cell Biol. 1997;29(1):63–78. doi: 10.1016/s1357-2725(96)00120-3. [DOI] [PubMed] [Google Scholar]
- Occleston NL, Laverty HG, O'Kane S, et al. Prevention and reduction of scarring in the skin by Transforming Growth Factor beta 3 (TGFbeta3): from laboratory discovery to clinical pharmaceutical. J Biomater Sci Polym Ed. 2008;19(8):1047–1063. doi: 10.1163/156856208784909345. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Dickinson ME, Moses HL, et al. In situ hybridization analysis of TGF beta 3 RNA expression during mouse development: comparative studies with TGF beta 1 and beta 2. Development. 1990;110(2):609–620. doi: 10.1242/dev.110.2.609. [DOI] [PubMed] [Google Scholar]
- Pelton RW, Saxena B, Jones M, et al. Immunohistochemical localization of TGF beta 1, TGF beta 2, and TGF beta 3 in the mouse embryo: expression patterns suggest multiple roles during embryonic development. J Cell Biol. 1991;115(4):1091–1105. doi: 10.1083/jcb.115.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroll WM, New K, Sachdev M, et al. Radial Keratotomy III. Relationship between wound gape and corneal curvature in primate eyes. Invest. Ophthal. Vis. Sci. 1992;33(12):3283–3291. [PubMed] [Google Scholar]
- Proulx S, d'Arc Uwamaliya J, Carrier P, et al. Reconstruction of a human cornea by the self-assembly approach of tissue engineering using the three native cell types. Mol Vis. 2010;16:2192–2201. [PMC free article] [PubMed] [Google Scholar]
- Reichl S, Muller-Goymann CC. The use of a porcine organotypic cornea construct for permeation studies from formulations containing befunolol hydrochloride. Int J Pharm. 2003;250(1):191–201. doi: 10.1016/s0378-5173(02)00541-0. [DOI] [PubMed] [Google Scholar]
- Ren R, Hutcheon AEK, Guo XQ, et al. Human primary corneal fibroblasts synthesize and deposit proteoglycans in long-term 3-D cultures. Dev Dyn. 2008;237(10):2705–2715. doi: 10.1002/dvdy.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts AB, Sporn MB. Differential expression of the TGF-beta isoforms in embryogenesis suggests specific roles in developing and adult tissues. Mol Reprod Dev. 1992;32(2):91–98. doi: 10.1002/mrd.1080320203. [DOI] [PubMed] [Google Scholar]
- Schmid P, Cox D, Bilbe G, et al. Differential expression of TGF beta 1, beta 2 and beta 3 genes during mouse embryogenesis. Development. 1991;111(1):117–130. doi: 10.1242/dev.111.1.117. [DOI] [PubMed] [Google Scholar]
- Schneider AI, Maier-Reif K, Graeve T. Constructing an in vitro cornea from cultures of the three specific corneal cell types. In Vitro Cell Dev Biol Anim. 1999;35(9):515–526. doi: 10.1007/s11626-999-0062-0. [DOI] [PubMed] [Google Scholar]
- Shah M, Foreman DM, Ferguson MW. Neutralisation of TGF-beta 1 and TGF-beta 2 or exogenous addition of TGF-beta 3 to cutaneous rat wounds reduces scarring. J Cell Sci. 1995;108(Pt 3):985–1002. doi: 10.1242/jcs.108.3.985. [DOI] [PubMed] [Google Scholar]
- Sporn MB, Roberts AB, Shull JH, et al. Polypeptide transforming growth factors isolated from bovine sources and used for wound healing in vivo. Science. 1983;219(4590):1329–1331. doi: 10.1126/science.6572416. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Saito J, Yanai R, et al. Cell-matrix and cell-cell interactions during corneal epithelial wound healing. Prog Retin Eye Res. 2003;22(2):113–133. doi: 10.1016/s1350-9462(02)00042-3. [DOI] [PubMed] [Google Scholar]
- Tseng SC, Li DQ, Ma X. Suppression of transforming growth factor-beta isoforms, TGF-beta receptor type II, and myofibroblast differentiation in cultured human corneal and limbal fibroblasts by amniotic membrane matrix. J Cell Physiol. 1999;179(3):325–335. doi: 10.1002/(SICI)1097-4652(199906)179:3<325::AID-JCP10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Vrana NE, Builles N, Justin V, et al. Development of a reconstructed cornea from collagen-chondroitin sulfate foams and human cell cultures. Invest Ophthalmol Vis Sci. 2008;49(12):5325–5331. doi: 10.1167/iovs.07-1599. [DOI] [PubMed] [Google Scholar]
- Wahl SM, Hunt DA, Wakefield LM, et al. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci U S A. 1987;84(16):5788–5792. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CG, Kim TK, Taboas A, et al. In vitro chondrogenesis of bone marrow-derived mesenchymal stem cells in a photopolymerizing hydrogel. Tissue Eng. 2003;9(4):679–688. doi: 10.1089/107632703768247377. [DOI] [PubMed] [Google Scholar]
- Yao Y, Zhang F, Zhou R, et al. Continuous supply of TGFbeta3 via adenoviral vector promotes type I collagen and viability of fibroblasts in alginate hydrogel. J Tissue Eng Regen Med. 2010;4(7):497–504. doi: 10.1002/term.263. [DOI] [PubMed] [Google Scholar]
- Younai S, Nichter LS, Wellisz T, et al. Modulation of collagen synthesis by transforming growth factor-beta in keloid and hypertrophic scar fibroblasts. Ann Plast Surg. 1994;33(2):148–151. doi: 10.1097/00000637-199408000-00005. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Guimaraes SR, Hutcheon AEK. Kinetics of keratocyte proliferation in response to epithelial debridement. Exp Eye Res. 2001;72(1):33–39. doi: 10.1006/exer.2000.0926. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Mason VS, Wasson ME, et al. Basement membrane assembly and differentiation of cultured corneal cells: importance of culture environment and endothelial cell interaction. Exp Cell Res. 1994;214(2):621–633. doi: 10.1006/excr.1994.1300. [DOI] [PubMed] [Google Scholar]