Abstract
Eomesodermin (Eomes) is a transcription factor that is essential for trophoblast development. Stress stimuli activate stress-activated protein kinase (MAPK8/9) and modulate transcription factors in trophoblast stem cells (TSCs). In this study, we test the hypothesis that stress-induced Eomes upregulation and downstream trophoblast development are MAPK8/9-dependent. Immunocytochemical and immunoblot assays suggest that Eomes is induced by hyperosmolar stress in a dose- and time-dependent manner. Two MAPK8/9 inhibitors that work by different mechanisms, LJNKl1 and SP600125, block induction of Eomes protein by stress. During normal TSC differentiation, the transcription factor heart and neural crest derivatives expressed 1 (HAND1) is dependent on Eomes, and chorionic somatomammotropin hormone 1 (CSH1) expression is dependent on HAND1. Similar to Eomes, HAND1 and CSH1 induction by stress are MAPK8/9-dependent, and CSH1 is induced in nearly all stressed TSCs. CSH1 induction normally requires downregulation of the transcription factor inhibitor of differentiation 2 (ID2) as well as HAND1 upregulation. It was shown previously that hyperosmolar stress induces AMP-activated protein kinase (PRKAA1/2)-dependent ID2 loss in a MAPK8/9-independent manner. Inhibition of PRKAA1/2 with compound C and LJNKl1, more that MAPK8/9 inhibitors alone, inhibits the induction of CSH1 by stress. Taken together these data suggest that stress-induced MAPK8/9 and PRKAA1/2 regulate transcription factors Eomes/HAND1 and ID2, respectively. Together this network mediates induction of CSH1 by stress. Therefore, stress triggers a proportional increase in a normal early TSC differentiation event that could be adaptive in inducing CSH1. But the flexibility of TSCS to undergo stress-induced differentiation could lead to pathophysiological consequences if stress endured and TSC differentiation became unbalanced.
Keywords: Hyperosmolar stress, Eomesodermin, MAPK8/9 (SAPK/JNK, Trophoblast stem cells (TSC), AMP-activated protein kinase (PRKAA1/2, or AMPK), heart and neural crest derivates expressed transcription factor (HAND)1, chorionic somatomammotropin (CSH)1
Introduction
The molecular mechanisms are emerging that govern normal trophoblast stem cell (TSC) multipotency and the differentiation of the various trophoblast placental cell lineages. This has been made possible through studies of mutant mice, identification of trophoblast lineages, and markers for the lineages of the placenta (Hernandez-Verdun 1974; Simmons and Cross 2005; Simmons et al. 2007). Trophoblast specification occurs after fertilization, and differentiated lineages are induced soon after implantation.
TSCS are derived from the preimplantation blastocyst, where fibroblast growth factor 4 (FGF4) is necessary to maintain multipotency and proliferation (Chai et al. 1998; Rappolee et al. 1994). FGF4 removal in culture induces events similar to early postimplantation differentiation (Tanaka et al. 1998), yet cellular stress induces early post-implantation differentiation of cultured TSCS despite the presence of FGF4 (Liu et al. 2009). Similar to normal differentiation, the transcription factor inhibitor of differentiation 2 (ID2) is lost, but this is AMP-activated protein kinase (PRKAA1/2, or AMPK)-dependent (Rappolee et al. 2010b; Xie et al. 2010; Zhong et al. 2010). In addition to loss of differentiation-inhibiting transcription factors, a sequence of trophoblast-specific transcription factors are induced in preimplantation embryos that lead to production of chorionic somatomammotropin 1 (CSH1, or placental lactogen 1 (PL1)) soon after implantation.
During normal trophoblast development in mouse embryos, zygotic transcription factor caudal related homeobox (Cdx)2 is expressed at the 8-cell stage (Yagi et al. 2007) and is necessary for the induction of Eomes (Strumpf et al. 2005). Eomes is a T-box transcription factor (Ryan et al. 1996) and performs essential functions in trophoblast development (Russ et al. 2000). Mouse embryos lacking Eomes arrest at the blastocyst stage (Strumpf et al. 2005) and fail to express HAND1 (Russ et al. 2000). HAND1 is necessary to induce CSH1 in embryos (Riley et al. 1998; Sahgal et al. 2005) and in TSCs (Hughes et al. 2004). Loss of ID2 is necessary to mediate CSH1 production by de-repressing HAND1 (Cross et al. 2002).
Hyperosmolar stress is sufficient to induce de novo CSH1 mRNA in TSCS (Liu et al. 2009). This suggests that stress induces ID2 loss (Xie et al. 2010; Zhong et al. 2010), thereby de-repressing and activating HAND1. But, it is not known if stress induces HAND1 and which stress enzyme mediates HAND1 induction or activation.
The trophectoderm overlying the inner cell mass (ICM) retains its capacity to proliferate and expands to form the extraembryonic ectoderm and ectoplacental cone (Gardner et al. 1973), but mural trophectoderm opposite the ICM forms primary trophoblast giant cells. These giant cells produce CSH1 that is detectable in maternal blood within 36 hours of implantation (Ogren et al. 1989). CSH1 binds corpus luteal cells, inducing progesterone secretion and maintenance of the endometrial secretions that support implantation (Thordarson et al. 1997). Thus, CSH1 is important in facilitating implantation. Stress that reduces TSC proliferation should induce compensatory differentiation of TSCs (Rappolee et al. 2010b; Xie et al. 2011a) to produce sufficient CSH1.
Cellular stressors, exemplified by metabolic, malnutritional, psychological, hormonal and infectious stimuli, induce intracellular enzyme cascades that regulate TSC functions in preimplantation embryos and the early placenta (Ip and Davis 1998; Kwong et al. 2000; Xie et al. 2011a; Xie et al. 2006a; Xie et al. 2007b; Zhong et al. 2007). These stressors, including benzo(a)pyrene (Xie et al. 2010), hypoxia (Zhou et al. 2011) and hyperosmolar stimulation (Zhong et al. 2007), induce stress signaling and prioritize developmental decision-making. Decreased cell accumulation is a standard response to stress (Ip and Davis 1998; Kwong et al. 2000; Xie et al. 2006a; Xie et al. 2007b; Zhong et al. 2007) as energy is diverted from macromolecule synthesis. Stressful stimuli activate the stress-activated branch of mitogen-activated protein kinases (MAPK8/9, or stress-activated protein kinase / jun kinase SAPK/JNK) (Wang et al. 2005; Wang et al. 2009b; Xie et al. 2007a; Xie et al. 2006c; Xie et al. 2007b) and cause both decreased cell entry into S phase and apoptosis (Xie et al. 2006a; Xie et al. 2007b; Zhong et al. 2007). MAPK8/9 mediates biological changes in stressed TSCS and embryos (Liu et al. 2009; Xie et al. 2007b; Zhong et al. 2007) and this makes MAPK8/9 a candidate for mediating stress effects in TSCS.
In this study we test the hypothesis that Eomes, HAND1 and CSH1 are upregulated by stress in the TSCS, and test if this is MAPK8/9-dependent. We used CSH1 as a metric to report the activity and induction of early anti-luteolytic hormone (like human chorionic gonadotropin (hCG) in humans) by stress. The importance of this study is that understanding stress effects on normal and pathophysiologic development may allow insight into how stress causes short-term lethal and long-term sublethal placental consequences.
Results
Hyperosmolar stress induced Eomes in a dose- and time-dependent manner
We previously found that hyperosmolar stress doses that activated the highest MAPK8/9 levels (Zhong et al. 2007) also induced TSC differentiation and CSH1 expression (Liu et al. 2009). Eomes is necessary to produce HAND1, which is necessary to induce CSH1 (Strumpf et al. 2005). Thus we tested the hypothesis that stress induces Eomes and HAND1, thereby leading to CSH1 production.
We tested if sorbitol hyperosmolar stress induced Eomes in a time-dependent manner and whether or not MAPK8/9-mediated Eomes upregulation. We used two mechanistically and structurally unrelated MAPK8/9 inhibitors, LJNKl1 (Tat-JIP1) and SP600125 (anthrapyrazolone), to address specificity of the effects. Eomes immunostaining in TSCs after 400mM sorbitol for 1hr was significantly higher than at time 0 (Figure 1, ANOVA with Duncan post hoc test, p<0.03). We chose 1hr because our laboratory has previously shown that peak phosphorylated MAPK8/9 was induced by 400mM sorbitol at 0.5hr–2hr (Zhong et al. 2007). Eomes induction was significantly reduced in TSCs inhibited with LJNKI1 after 1hr of 400mM sorbitol (Figure 1, ANOVA, with Duncan post hoc test, p<0.004); SP600125 had similar inhibitory effects (data not shown). The presence of inhibitors did not shift the Eomes response from baseline as signal was not significantly different from time 0 (ANOVA, p>0.48). LJNKI1 seems to have no effect on Eomes expression in unstressed TSC (Figure 1). Consistent with the nuclear function of Eomes, 1hr of 200mM sorbitol induced increased nuclear localization of Eomes (Supplemental Figure 1).
Figure 1.
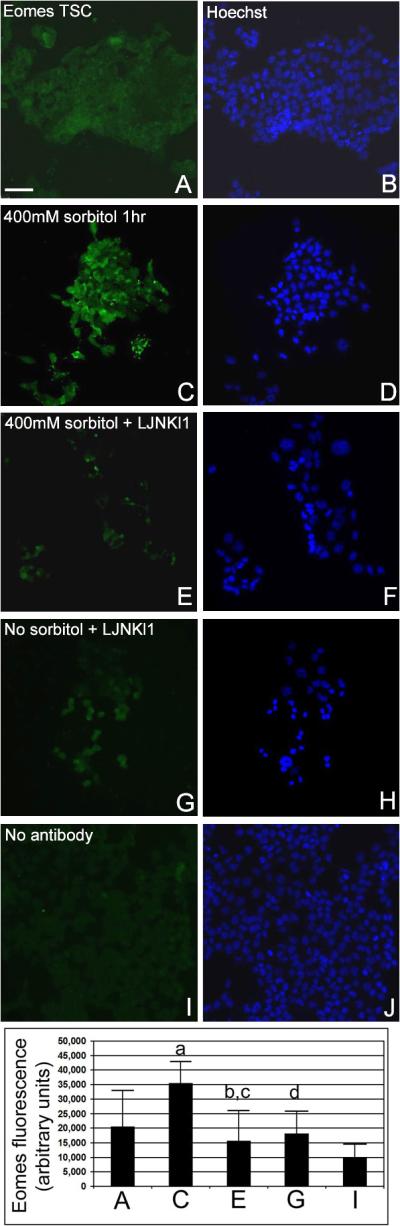
Hyperosmolar sorbitol induces Eomes in TSCs in a MAPK8/9-dependent (MAPK8/9-inhibitor sensitive) manner. TSCs were incubated with 400mM sorbitol for 0–1hr ± MAPK8/9 inhibitors LJNKl1 or SP600125 (data not shown), fixed, and stained for Eomes. TSCs were cultured for 1hr in media alone (A, B), with 400mM sorbitol (C, D), with 400mM sorbitol and MAPK8/9 inhibitor LJNKl1 (E, F), or LJNKl1 alone (G, H), and stained for Eomes. TSCs were incubate with 400mM sorbitol but developed without anti-Eomes antibody. The micrographs in (B, D, F, H, and J) are Hoechst-stained nuclei corresponding to the cells in (A, C, E, G, and I), respectively. The histogram shows mean fluorescence intensity of 6 replicates of the panels shown in A–I as indicated. Error flags are SD of the means for the 6 replicates. Statistical analysis by ANOVA, with Duncan post hoc test, compares the following pairs; (a) C to A (p=0.03), (b) E to C (p=0.004), (c) E to A (p=0.48), and (d) G to A (p=0.7).
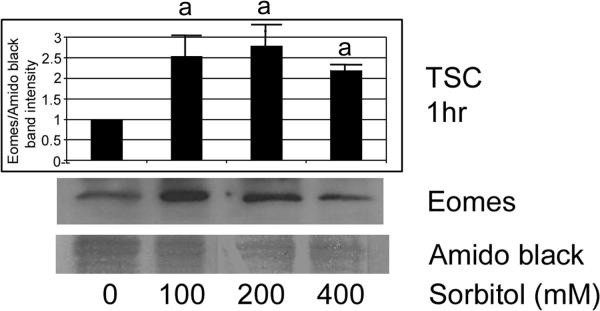
Our previous studies suggested that higher doses of stress induce stress enzymes that mediate developmental functions (Rappolee et al. 2010b; Zhong et al. 2010). Therefore, we performed immunoblots to determine if hyperosmolar stress induces Eomes in a dose-dependent manner (Figure 2). TSCS were incubated with different doses of sorbitol (0–400mM) for 1hr, and Eomes protein level was examined by immunoblot. After treatments, equal protein amounts were size-fractionated by SDS-PAGE and blotted using Eomes and amido black. There was a significant induction of Eomes at all sorbitol dose levels (ANOVA, with Dunnett t test, p<0.05). The Eomes band was at the correct size of 72 kDa, with a band at 60 kDa previously detected in somatic cells (Bjornson et al. 2005).
Figure 2.
Hyperosmolar stress of TSCs induces Eomes in a dose-dependent manner. TSCs were incubated with the sorbitol doses shown for 1hr, and Eomes protein level was examined by immunoblot. After treatments, equal amounts of protein were examined after size fractionation by SDS-PAGE and blotting using Eomes and amido black. Histogram shows the ratio of Eomes/Amido black intensity). Error flags are the standard deviations from three experiments. (a) denotes that Eomes is induced to significantly higher levels (ANOVA, with Dunnett t test, p<0.05) than unstressed TSCs at all sorbitol doses (p=0.01).
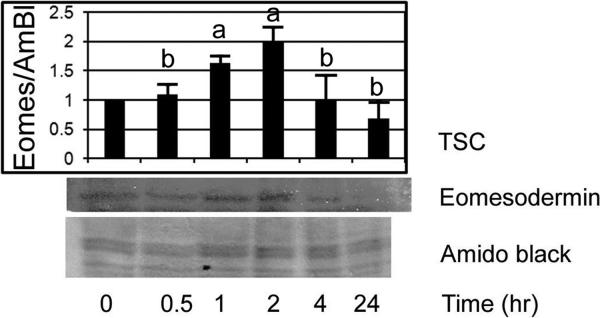
We performed immunoblots to confirm that Eomes was induced by stress in a time-dependent manner (Figure 3). Sorbitol induces an early peak of Eomes at 60–120min that subsides by 4hr, and returned to below baseline at 24hr. Eomes induction was significantly elevated between 60 and 120min and compared with unstressed TSCS at 0min, 30min, 4hr and 24h (ANOVA, with Dunnett t test, p<0.001), but Eomes is not significantly different in the sorbitol-treated group at 4hr or 24hr compared to unstressed TSCs at 0min (post hoc Dunnett t test).
Figure 3.
Sorbitol induces an early peak of Eomes at 60 to 120 minutes that subsides by 4hr and returned to below baseline at 24hr. TSCs were cultured in 200mM Sorbitol for 0–24hr, lysed, and fractionated by SDS-PAGE, and immunoblotted for Eomes or stained by amido black as a loading control. In the histogram, error flags show the standard deviation for three experiments. Overall ANOVA shows significant Eomes induction for all Sorbitol durations between 60 and 120min when compared with unstressed TSCs at 0min, 30mins, 4hr and 24hr. Post hoc Dunnett t test shows Eomes is induced significantly at 60min–120min (a) (p<0.001), but that Eomes is not significantly different in the Sorbitol-treated group at 4hr. Post hoc Dunnett t test shows no difference for Eomes when TSCs are stimulated by sorbitol for 24hr (b) compared with unstressed TSCs at 0min.
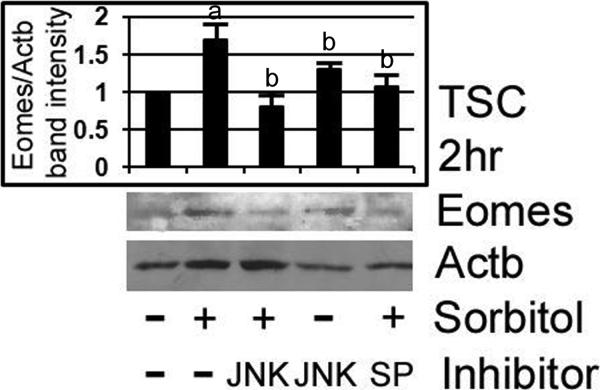
We confirmed by immunoblot that stress-induced Eomes is MAPK8/9-dependent. TSCs were stressed with 400mM sorbitol for 2hr with or without LJNKI1 or SP600125, and assayed for Eomes by immunoblot. The TSCs were preincubated with inhibitors for 3hr before stress was added, and both MAPK8/9 inhibitors blocked Eomes induction (Figure 4). Peak Eomes induction at 1hr was preceded by MAPK8/9 induction at 0.5hr (Zhong et al. 2007), and MAPK8/9 inhibitors suppressed Eomes induction to levels not significantly different than unstressed TSCs (ANOVA, p>0.55).
Figure 4.
Hyperosmolar stress-induced Eomes expression in TSCs is MAPK8/9 dependent, as shown by inhibition by two MAPK8/9 inhibitors, LJNKI1 and SP600125. TSCs were stressed with 400mM sorbitol for 3hr with or without LJNKI1 or SP600125, and assayed for Eomes by immunoblot. After treatments, equal amounts of protein were examined after size fractionation by SDS-PAGE and blotting using Eomes and ACTB antibody. Histogram shows the ratio of Eomes/ACTB band intensity). Error flags are the standard deviations from three experiments. (a) shows that Eomes is significantly increased by stress (Post hoc Dunnett t test, p<0.001), and (b) shows that MAPK8/9 inhibitors alone or MAPK inhibitors in the presence of sorbitol are not significantly different than Eomes in unstressed cells (ANOVA, p>0.55)
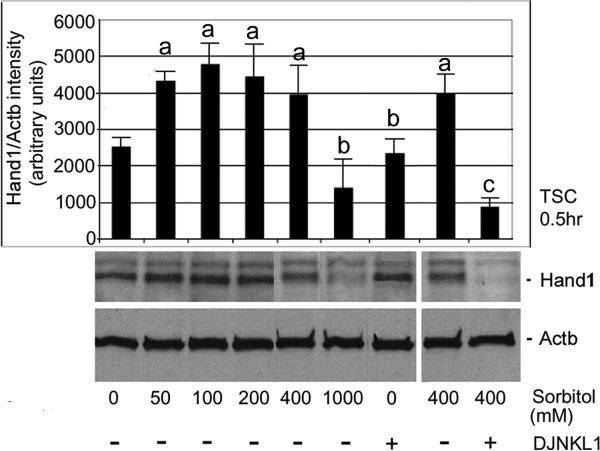
Next, we show that stress due to sorbitol increases HAND1 and maintains it in a MAPK8/9-dependent way. Hyperosmolar stress regulates HAND1 protein expression in a dose-dependent manner. TSCs were stressed with increasing sorbitol (0, 50, 100, 200, 400, and 1,000 mM) for 0.5hr, and HAND1 levels were examined using Western blot. After treatments, equal amounts of protein were examined by Western blots using HAND1 and Actb antibody. Overall ANOVA shows significant HAND1 induction for all Sorbitol concentrations (except 1000 mM sorbitol) compared with unstressed TSCs (Figure 5, ANOVA, with Dunnett t test, p<0.001). 1000 mM sorbitol represents a toxic dose, characterized by decreased cell accumulation and rapid apoptosis, hence the comparatively low HAND1 induction at that dose. Correspondingly, phosphorylated MAPK8/9 activation is also lower at 1,000 mM than at 400 mM sorbitol after 30min (Zhong et al. 2007), although total MAPK8/9 is constant. We also show that induction of HAND1 is MAPK8/9-dependent as it is inhibited by LJNKl1 (Figure 5) and SP600125 (data not shown).
Figure 5.
Stress due to sorbitol increases Hand1 and maintains it in a MAPK8/9-dependent way. TSCs were incubated with increasing sorbitol (0, 50, 100, 200, 400, and 1,000mM) for 0.5h, and Hand1 levels were examined using Western blot. After treatments, equal amounts of protein were examined with immunoblots using HAND1 and Actb antibody (histogram shows the ratio of Hand1/Actb band intensity). (a) shows a significant increase in HAND1 compared with unstressed TSCs (ANOVA, with Dunnett t test, p<0.001); (b) shows a sorbitol dose with not significant HAND1 increase, and no effect of MAPK8/9 inhibitor on HAND1 in unstressed TSCs (ANOVA, p>0.6); and (c) shows a significant decrease in HAND1 when MAPK8/9 was inhibited in stressed TSCs (ANOVA, with Dunnett t test, p<0.001).
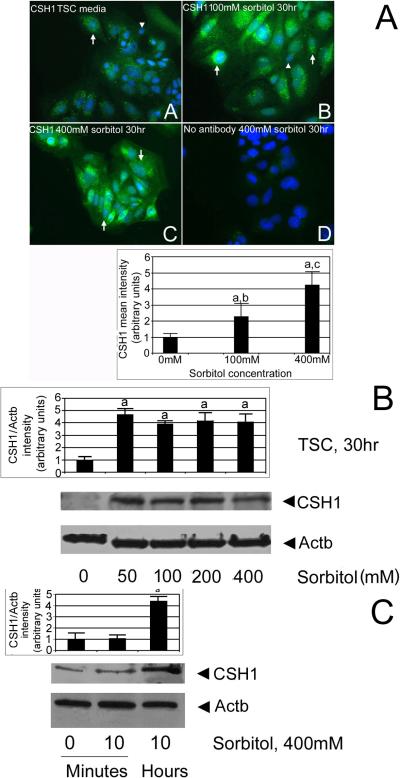
We tested if CSH1 is induced by hyperosmolar stress in a dose-dependent manner and whether or not stress affected subpopulations of TSCs. TSCs were cultured in 0, 100, or 400 mM sorbitol for 30hr, and then fixed and stained for CSH1 by immunocytochemical means. CSH1 protein is significantly induced in nearly all cultured TSCs by 100 or 400 mM sorbitol (Figure 6A, ANOVA, with Dunnett t test, p<0.05). TSCs were probed without primary antibody or with non-immune IgG (not shown), and showed little non-specific staining. Similar to the HAND1 dose response (Figure 5), 50–400 mM sorbitol induced significant but similar amounts of CSH1 (Figure 6B, ANOVA, with Dunnett t test, p<0.01). Significant CSH1 was induced by 10hr (Figure 6C, ANOVA, with Dunnett t test, p<0.01).
Figure 6.
CSH1 protein is induced in nearly all stressed TSCs in a time- and dose-dependent manner. (A) CSH1 protein is induced in cultured TSCs by 100 or 400mM sorbitol. TSCs were cultured in 0 (A) 100mM (B), or 400mM (C) sorbitol for 30hr and then fixed and stained for CSH1 using indirect immunocytochemistry. In (D), 400mM sorbitol-treated TSCs were probed without primary antibody. Triplicate biological experiments indicate that (a) stress at 100 or 400mM sorbitol induced significant amounts of CSH1 (post hoc Dunnett t test shows p<0.01), (b, c) 100 and 400 were significantly different than each other (post hoc Dunnett t test shows p<0.01). (B). CSH1 protein is induced in cultured TSCs by 50–400mM sorbitol after 30hr. TSCs were cultured in 0, 50, 100, 200, or 400mM sorbitol for 30hr and lysates were fractionated by SDS-PAGE, blotted, and stained for CSH1 and Actb. In (D), 400mM sorbitol treated TSCs were probed without primary antibody. Triplicate biological experiments indicate that stress induced significant amounts of CSH1 (a) at 50–400mM sorbitol (post hoc Dunnett t test shows p<0.01). (C) CSH1 protein is induced in cultured TSC by 10hr, but not by 10minutes. TSCs were cultured in 400mM sorbitol for 10minute or 10hr, and lysates were fractionated by SDS-PAGE, blotted, and stained for CSH1 and Actb. Triplicate biological experiments indicate that stress induced significant amounts of CSH1 (a) at 10hr post hoc Dunnett t test shows p<0.01). In all panels, flags show standard error mean across replicates.
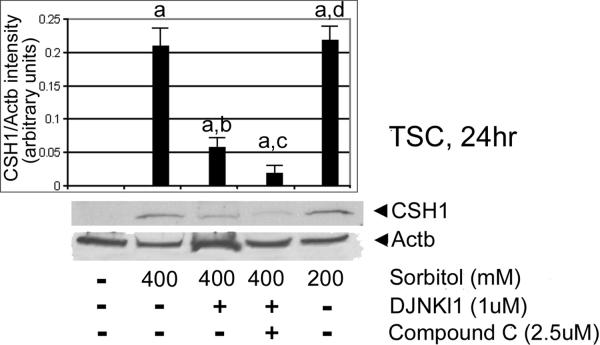
We next tested for MAPK8/9-dependence of CSH1 induction. TSCs were cultured in 200 or 400 mM sorbitol with or without DJNKI1 (inhibitor of MAPK8/9), or DJNKl1 and compound C (PRKAA1/2 [AMPK] inhibitor) for 30hr. After treatments, immunoblots were tested with CSH1 and ActB antibody. CSH1 induction was largely MAPK8/9-dependent (ANOVA, with Dunnett t test, p<0.001), with a smaller but significant (p<0.05) contribution from PRKAA1/2 (Figure 7.
Figure 7.
CSH1 induction in TSCs is largely MAPK8/9-dependent. TSCs were cultured in 200 or 400mM sorbitol with or without DJNKI1 (inhibitor of MAPK8/9), or DJNKl1 and compound C (PRKAA1/2 [AMPK] inhibitor) for 24hr. After treatments, equal amounts of protein were examined with Western blots using CSH1 and Actb antibody. Triplicate biological experiments, with error flags showing standard error means, indicate that stress induced significant amounts of CSH1 (a), but that DJNKl1 diminished CSH1 induction (b), DJNKl1 and compound C diminished CSH1 induction more than DJNKl1 alone (c) (all by post hoc Dunnett t test shows (p<0.01), and 200Mm induction was not significantly different than 400mM sorbitol (d)(p>0.4)
Discussion
Stress induces Eomes expression in a time-, dose-, and MAPK8/9-dependent manner in TSCs Since Eomes is expressed in the trophectoderm of the blastocyst, the extra-embryonic ectoderm of the early postimplantation embryo (Russ et al. 2000), and placental chorion and labyrinth (Simmons and Cross 2005), stress may have effects on Eomes function during these periods.
Stress also induces HAND1 and CSH1 protein in a time-, dose-, and MAPK8/9-dependent manner. CSH1 mRNA induction by stress (Liu et al. 2009) suggests that PRKAA1/2-dependent loss of ID2 (Xie et al. 2010; Zhong et al. 2010) must be complemented by stress-induced upregulation of HAND1. Both events are known to be required during normal TSC differentiation to produce CSH1 (Rappolee et al. 2010b; Xie et al. 2011a). Since HAND1 requires Eomes during normal development (Strumpf et al. 2005), stress-induced Eomes is a precursor to HAND1 induction. It is likely that MAPK8/9 directly induces and preserves HAND1 protein as MAPK8/9 activity leads to increases in HAND1 mRNA (Abell et al. 2009; Xie et al. 2011b). Recently, it was shown that MEKK4−/− null mutant TSCs have delayed CSH1 induction when TSC differentiation is mediated by FGF4 removal (Abell et al. 2009). In this model, however, p38MAPK (MAPK11) inhibitors blocked CSH1 induction in wild type TSCs when FGF4 is removed, but MAPK8/9 inhibitors did not. The data here suggest that stress-induced Eomes may be a necessary precursor to stress-induced HAND1 and CSH1 when TSCs are stressed in the presence of FGF4. Thus, MAPK8/9 may mediate differentiation of stressed TSCs and MAPK11 may mediate differentiation with lower levels of stress intrinsic to any culture model.
Stress-sensing enzymes may play a role in normal and stressed preimplantation development. MAPK8/9 are expressed in mouse and human placental cells (Zhong et al. 2004), and are activated by multiple cellular stress types in somatic cells (Gillis et al. 2001; Kyriakis et al. 1995; Tibbles and Woodgett 1999; Woodgett et al. 1996a; Woodgett et al. 1996b) and in preimplantation embryos and their constituent TSCs (Wang et al. 2005; Wang et al. 2009a; Xie et al. 2008; Xie et al. 2007a; Xie et al. 2006c; Xie et al. 2007b; Zhong et al. 2007). MAPK8/9 is a p46/p54, single subunit protein kinase that activates c-Jun on ser63/ser73 (Hibi et al. 1993) (Pulverer et al. 1991). MAPK8/9 is phosphorylated and localized to the nucleus in mouse preimplantation embryos and TSCs (Xie et al. 2007b; Zhong et al. 2007), and human first trimester placental (Xie et al. 2006a) cell lines. The magnitude of phosphorylation induced by seven embryo culture media is inversely proportional to rates of embryo development (Wang et al. 2005). MAPK8/9 may also be responsible for cell cycle arrest and apoptosis in placental lineage cells during implantation. Phosphorylated MAPK8/9 controls slower growth of TSCs by mediating 100% of increased cell arrest and 70% of increased apoptosis (Zhong et al. 2007). MAPK8/9 is induced by 400 mM sorbitol, peaked at 0.5–4hr, and largely subsided by 12hr. Given that MAPK8/9 has a rapid peak at 0.5hr, and biological effects are often studied at 24hr, we used these time points to study the time- and dose-dependent effect of hyperosmolar stress on Eomes.
To avoid problems with interpreting the specificity of MAPK8/9 inhibitors, we used SP600125 and LJNKl1 (Bennett et al. 2001), two mechanistically and structurally unrelated inhibitors. As these two MAPK8/9 inhibitors inhibited stress-induced Eomes expression in TSCs, it is unlikely that separate nonspecific effects mediated this inhibition. Thus MAPK8/9 regulates transient Eomes induction and long term upregulation of HAND1 and CSH1.
Embryo loss is greatest in the peri-implantation period (Cross et al. 1994) and embryos are also susceptible to sublethal in vitro and in vivo stress effects, leading to post-natal consequences such as hypertension (Kwong et al. 2000) and learning disabilities (Ecker et al. 2004). MAPK8/9 is activated during assisted reproductive technology (ART), embryo culture (Xie et al. 2006a) and embryo handling (Xie et al. 2007a). This activation is associated with stress levels that decrease proliferation and increases apoptosis. Therefore, sustained stress leading to loss of cell accumulation also leads to the molecular preparation for differentiation. Interestingly, the stress of IVF embryo culture can lead to changes in placental hormone production in the first trimester (Johnson et al. 1993), consistent with a role of stress enzyme effects in aberrant differentiation.
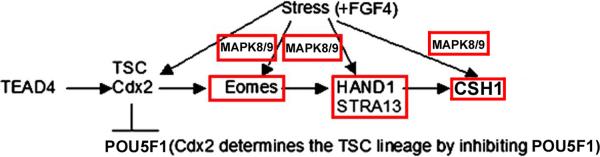
Although CSH1 mRNA arises 5 days after FGF4 removal (Hughes et al. 2004), our data (Liu et al. 2009) suggests that its expression is accelerated during stressed differentiation, because CSH1 mRNA (and protein) is induced 6–24hr after 400mM sorbitol addition. Here we show that CSH1 protein is detected in lysates at least as early as 10hr into 400mM sorbitol exposure. CSH1 increase is dependent upon HAND1, which in turn is dependent on Eomes, but Eomes does not persist in the differentiated TGCs as HAND1 must. Stress-induced Eomes decreases after 2hr, and by 24hr is less than in unstressed TSCs in which CSH1 synthesis is induced (Figure 3). Therefore, differentiation may emulate the organismal survival response by TSCs as a model for the stressed implanting embryo. At high stress doses that kills most TSCs, almost all surviving, stressed TSCs differentiate as part of an organismal survival strategy (Rappolee et al. 2010a; Xie et al. 2011a). Eomes and HAND1 transcription factors and CSH1 protein are all induced by stress in a MAPK8/9-dependent manner that contributes to survival (Figure 8).
Figure 8.
Eomes, Hand1 and CSH1 transcription factor proteins are induced by stress in a MAPK8/9-dependent manner. Four transcription factors work in sequence in the peri-implantation embryo to first determine (e.g. TEAD4, CDX2) and then differentiation placental stem cells. Eomes is required for Hand1 during normal development, and Hand1 is required for CSH1 and the terminally differentiated state.
Conclusions
The predominant cell type in the implanting blastocyst is the TSCs, and a subpopulation must differentiate after implantation to produce the first placental hormone. CSH1 mediates the antiluteolytic response needed for survival of the conceptus. Stress causes a slower cell cycle and apoptosis in preimplantation mouse TSCs. Therefore, proliferation and differentiation defects in cytotrophoblasts early after implantation may lead to diseases of placental insufficiency such as preeclampsia.
Immunocytochemistry and immunoblot suggest that transient increases in Eomes are induced by hyperosmolar stress in a dose- and time-dependent manner. Two MAPK8/9 inhibitors block induction of Eomes by stress. MAPK8/9 is also necessary to induce and maintain HAND1 under stress, and both MAPK8/9 and PRKAA1/2 (AMPK) contribute to CSH1 production from 10–30hr of stress. In contrast to Eomes, the induction of HAND1-CSH1 is permanent, not transient. MAPK8/9 appears to mediate prioritized differentiation to produce CSH1, and the first lineage arising from TSCs after implantation while other studies suggest that MAPK8/9 suppresses other TSC lineages arising after implantation.
Materials and Methods
Materials
Eomes (PAB-11141) antibodies were obtained from Orbigen (San Diego, CA). HAND1 (SC9413) antibodies were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA) while CSH1 (AB1288) antibodies were from Chemicon (Temecula, CA). Anti-rabbit HRP-conjugated antibody was purchased from Zymed Laboratories (San Francisco, CA). The specific inhibitors for MAPK8/9 (LJNKI1 and DJNKI1) were from Alexis (San Diego, CA) while the chemical anthrapyrazolone (MAPK8/9 inhibitor SP600125) (Bennett et al. 2001; Xie et al. 2006b; Zhong et al. 2007) was purchased from Calbiochem-EMD (San Diego, CA). Compound C (C#171261) and AraA were purchased from Alexis/Calbiochem (San Diego, CA). Fetal bovine serum and RPMI1640 were from Gibco (Grand Island, NY). BCA protein Assay kit was from Pierce (Rockford, IL). Hoechst 33258 stain was from Sigma Chemical Co. (St Louis, MO). Amido black was from eBioscience, Inc. (San Diego, CA). The primary antibody for actin (CS4967) was from Cell signaling (Danvers, MA). Enhanced chemiluminescence (ECL) assay and Hybond nitrocellulose membranes were from Amersham Bioscience (Aylesbury, UK).
Cell lines and culture conditions
The mouse TSCs were obtained from Dr. Rossant (Lunenfeld Research Institute, Ontario, Canada) and cultured as described previously (Xie et al. 2005a), (Xie et al. 2005b). Sorbitol was used as stressor because it activates stress enzyme responses in all stem cells and embryos, and is a standard stressor for enzymologists (Rappolee 2007). Sorbitol (≤400mM) was used to produce hyperosmolar stress, as is commonly used in somatic cells (Ip and Davis 1998; Zhong et al. 2007). TSCs were cultured until 70–80% confluent, and then subject to hyperosmolar stress using sorbitol in the presence or absence of MAPK8/9 (LJNKI1 and SP600125) and PRKAA1/2 (Compound C and AraA) inhibitors. The inhibitors were preincubated with TSCs for 3hr before stress was added to inhibit the MAPK8/9 PRKAA1/2 pools and during stress. Sorbitol at 400mM was chosen because it induces peak MAPK8/9 and PRKAA1/2 activation in TSCs (Xie et al. 2007b; Zhong et al. 2010; Zhong et al. 2007).
Image J software was used to quantitate fluorescence by immunocytochemical means, or to measure band signal intensity during Western blot analysis as done previously (Zhong et al. 2010).
Immunocytochemistry
For immunofluorescence, TSCs on coverslips were treated with 0–400mM sorbitol for 1–24hr with or without LJNKl1 or SP600215. TSCs were stained with primary Eomes or CSH1 antibody (diluted at 1:100 – 500), and by secondary antibody (polyclonal HRP antibody diluted at 1:20000) as described previously (Liu et al. 2004; Xie et al. 2005b; Xie et al. 2005c). No first antibody and non-immune IgG specificity controls yielded similar low backgrounds.
Western blot
Sodium dodecyl sulfate-polyacrylamide gel elecrophoresis (SDS-PAGE) and immunoblot were performed as previously described (Wang et al. 2004; Xie et al. 2005b). For Eomes, Hand1, and CSH1 immunoblots no first antibody controls were performed and yielded no specific bands.
Statistical analysis
The data in this study represent at least three independent biological experiments and indicated as mean+/−s.e.m. The statistical significance of differences between treated samples was analyzed by One way analysis of variance (ANOVA) for continuous data with more than two groups (SPSS 11.0). Dunnett t test, Duncan, and Student-Newman-Keuls post hoc tests were used to analyze significance of pairwise comparisons. Groups were considered to be significantly different if p<0.05.
Supplementary Material
Increased nuclear Eomes is induced in cultured TSCs after stimulation with 200 mM sorbitol for 1hr. TSCs were cultured in 0 or 200mM sorbitol for 1hr, then fixed and stained for Eomes using indirect immunocytochemistry. (A, C) show Hoechst stained nuclei, (B, D) show Eomes, 10 and (C, F) show merge Hoechst stained nuclei and Eomes immunostaining for 1hr in 200mM sorbitol and unstressed for each pair, respectively.
Acknowledgements
We thank Mike Kruger for advice on statistical analysis. We are also indebted to Dr. Michael Diamond for helpful discussion and criticisms of the manuscript.
This was supported by a grant to DAR from NICHD, NIH, (R01 HD40972A).
Abbreviations
- CSH1
chorionic somatomammotropin (also, placental lactogen 1 (PL1))
- Eomes
eomesodermin
- FGF4
fibroblast growth factor 4
- HAND1
heart and neural crest derivatives 1
- Id2
inhibitor of differentiation 2
- LJNKl1
Tat-JIP1 (SAPK inhibitor)
- MAPK8/9
mitogen activated protein kinase 8/9 (also, stress activated protein kinase/jun kinase (SAPK/JNK)
- PRKAA1/2
AMP-activated protein kinase (also AMPK)
- SP600125
anthrapyrazolone (SAPK inhibitor)
- TSC
trophoblast stem cell
References
- Abell AN, Granger DA, Johnson NL, Vincent-Jordan N, Dibble CF, Johnson GL. Trophoblast stem cell maintenance by fibroblast growth factor 4 requires MEKK4 activation of Jun N-terminal kinase. Mol Cell Biol. 2009;29(10):2748–2761. doi: 10.1128/MCB.01391-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98(24):13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornson CR, Griffin KJ, Farr GH, 3rd, Terashima A, Himeda C, Kikuchi Y, Kimelman D. Eomesodermin is a localized maternal determinant required for endoderm induction in zebrafish. Dev Cell. 2005;9(4):523–533. doi: 10.1016/j.devcel.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Chai N, Patel Y, Jacobson K, McMahon J, McMahon A, Rappolee DA. FGF is an essential regulator of the fifth cell division in preimplantation mouse embryos. Dev Biol. 1998;198(1):105–115. doi: 10.1006/dbio.1997.8858. [DOI] [PubMed] [Google Scholar]
- Cross JC, Anson-Cartwright L, Scott IC. Transcription factors underlying the development and endocrine functions of the placenta. Recent Prog Horm Res. 2002;57:221–234. doi: 10.1210/rp.57.1.221. [DOI] [PubMed] [Google Scholar]
- Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266(5190):1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci U S A. 2004;101(6):1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner RL, Papaioannou VE, Barton SC. Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass. J Embryol Exp Morphol. 1973;30(3):561–572. [PubMed] [Google Scholar]
- Gillis D, Shrode LD, Krump E, Howard CM, Rubie EA, Tibbles LA, Woodgett J, Grinstein S. Osmotic stimulation of the Na+/H+ exchanger NHE1: relationship to the activation of three MAPK pathways. J Membr Biol. 2001;181(3):205–214. doi: 10.1007/s00232-001-0023-3. [DOI] [PubMed] [Google Scholar]
- Hernandez-Verdun D. Morphogenesis of the syncytium in the mouse placenta. Ultrastructural study. Cell Tissue Res. 1974;148(3):381–396. doi: 10.1007/BF00224265. [DOI] [PubMed] [Google Scholar]
- Hibi M, Lin A, Smeal T, Minden A, Karin M. Identification of an oncoprotein- and UV-responsive protein kinase that binds and potentiates the c-Jun activation domain. Genes Dev. 1993;7(11):2135–2148. doi: 10.1101/gad.7.11.2135. [DOI] [PubMed] [Google Scholar]
- Hughes M, Dobric N, Scott IC, Su L, Starovic M, St-Pierre B, Egan SE, Kingdom JC, Cross JC. The Hand1, Stra13 and Gcm1 transcription factors override FGF signaling to promote terminal differentiation of trophoblast stem cells. Dev Biol. 2004;271(1):26–37. doi: 10.1016/j.ydbio.2004.03.029. [DOI] [PubMed] [Google Scholar]
- Ip YT, Davis RJ. Signal transduction by the c-Jun N-terminal kinase (JNK)--from inflammation to development. Curr Opin Cell Biol. 1998;10(2):205–219. doi: 10.1016/s0955-0674(98)80143-9. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Riddle AF, Grudzinskas JG, Sharma V, Campbell S, Collins WP, Lightman SL, Mason B, Nicolaides KH. Endocrinology of in-vitro fertilization pregnancies during the first trimester. Hum Reprod. 1993;8(2):316–322. doi: 10.1093/oxfordjournals.humrep.a138043. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127(19):4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Woodgett JR, Avruch J. The stress-activated protein kinases. A novel ERK subfamily responsive to cellular stress and inflammatory cytokines. Ann N Y Acad Sci. 1995;766:303–319. doi: 10.1111/j.1749-6632.1995.tb26683.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Xu W, Sun T, Wang F, Puscheck E, Brigstock D, Wang QT, Davis R, Rappolee DA. Hyperosmolar stress induces global mRNA responses in placental trophoblast stem cells that emulate early post-implantation differentiation. Placenta. 2009;30(1):66–73. doi: 10.1016/j.placenta.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren L, Southard JN, Colosi P, Linzer DI, Talamantes F. Mouse placental lactogen-I: RIA and gestational profile in maternal serum. Endocrinology. 1989;125(5):2253–2257. doi: 10.1210/endo-125-5-2253. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, Kyriakis JM, Avruch J, Nikolakaki E, Woodgett JR. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Rappolee D, Awonuga A, Puscheck E, Zhou S, Xie X. Benzopyrene and experimental stressors cause differentiation in placental trophoblast stem cells. Systems Biology in Reproductive Medicine. 2010a doi: 10.3109/19396360903431638. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA. Impact of transient stress and stress enzymes on development. Dev Biol. 2007;304(1):1–8. doi: 10.1016/j.ydbio.2006.12.032. [DOI] [PubMed] [Google Scholar]
- Rappolee DA, Awonuga AO, Puscheck EE, Zhou S, Xie Y. Benzopyrene and experimental stressors cause compensatory differentiation in placental trophoblast stem cells. Syst Biol Reprod Med. 2010b;56(2):168–183. doi: 10.3109/19396360903431638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee DA, Basilico C, Patel Y, Werb Z. Expression and function of FGF-4 in peri-implantation development in mouse embryos. Development. 1994;120(8):2259–2269. doi: 10.1242/dev.120.8.2259. [DOI] [PubMed] [Google Scholar]
- Riley P, Anson-Cartwright L, Cross JC. The Hand1 bHLH transcription factor is essential for placentation and cardiac morphogenesis. Nat Genet. 1998;18(3):271–275. doi: 10.1038/ng0398-271. [DOI] [PubMed] [Google Scholar]
- Russ AP, Wattler S, Colledge WH, Aparicio SA, Carlton MB, Pearce JJ, Barton SC, Surani MA, Ryan K, Nehls MC, Wilson V, Evans MJ. Eomesodermin is required for mouse trophoblast development and mesoderm formation. Nature. 2000;404(6773):95–99. doi: 10.1038/35003601. [DOI] [PubMed] [Google Scholar]
- Ryan K, Garrett N, Mitchell A, Gurdon JB. Eomesodermin, a key early gene in Xenopus mesoderm differentiation. Cell. 1996;87(6):989–1000. doi: 10.1016/s0092-8674(00)81794-8. [DOI] [PubMed] [Google Scholar]
- Sahgal N, Canham LN, Konno T, Wolfe MW, Soares MJ. Modulation of trophoblast stem cell and giant cell phenotypes: analyses using the Rcho-1 cell model. Differentiation. 2005;73(9–10):452–462. doi: 10.1111/j.1432-0436.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Cross JC. Determinants of trophoblast lineage and cell subtype specification in the mouse placenta. Dev Biol. 2005;284(1):12–24. doi: 10.1016/j.ydbio.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Simmons DG, Fortier AL, Cross JC. Diverse subtypes and developmental origins of trophoblast giant cells in the mouse placenta. Dev Biol. 2007;304(2):567–578. doi: 10.1016/j.ydbio.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Strumpf D, Mao CA, Yamanaka Y, Ralston A, Chawengsaksophak K, Beck F, Rossant J. Cdx2 is required for correct cell fate specification and differentiation of trophectoderm in the mouse blastocyst. Development. 2005;132(9):2093–2102. doi: 10.1242/dev.01801. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Kunath T, Hadjantonakis AK, Nagy A, Rossant J. Promotion of trophoblast stem cell proliferation by FGF4. Science. 1998;282(5396):2072–2075. doi: 10.1126/science.282.5396.2072. [DOI] [PubMed] [Google Scholar]
- Thordarson G, Galosy S, Gudmundsson GO, Newcomer B, Sridaran R, Talamantes F. Interaction of mouse placental lactogens and androgens in regulating progesterone release in cultured mouse luteal cells. Endocrinology. 1997;138(8):3236–3241. doi: 10.1210/endo.138.8.5309. [DOI] [PubMed] [Google Scholar]
- Tibbles LA, Woodgett JR. The stress-activated protein kinase pathways. Cell Mol Life Sci. 1999;55(10):1230–1254. doi: 10.1007/s000180050369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Puscheck EE, Lewis JJ, Trostinskaia AB, Wang F, Rappolee DA. Increases in phosphorylation of SAPK/JNK and p38MAPK correlate negatively with mouse embryo development after culture in different media. Fertil Steril. 2005;83(Suppl 1):1144–1154. doi: 10.1016/j.fertnstert.2004.08.038. [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang F, Sun T, Trostinskaia A, Wygle D, Puscheck E, Rappolee DA. Entire mitogen activated protein kinase (MAPK) pathway is present in preimplantation mouse embryos. Dev Dyn. 2004;231(1):72–87. doi: 10.1002/dvdy.20114. [DOI] [PubMed] [Google Scholar]
- Wang Y, Xie Y, Wygle D, Shen HH, Puscheck EE, Rappolee DA. A Major Effect of Simulated Microgravity on Several Stages of Preimplantation Mouse Development Is Lethality Associated With Elevated Phosphorylated SAPK/JNK. Reprod Sci. 2009a;16(10):947–959. doi: 10.1177/1933719109337544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie Y, Wygle D, Shen HH, Puscheck EE, Rappolee DA. A major effect of simulated microgravity on several stages of preimplantation mouse development is lethality associated with elevated phosphorylated SAPK/JNK. Reprod Sci. 2009b;16(10):947–959. doi: 10.1177/1933719109337544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodgett JR, Avruch J, Kyriakis J. The stress activated protein kinase pathway. Cancer Surv. 1996a;27:127–138. [PubMed] [Google Scholar]
- Woodgett JR, Kyriakis JM, Avruch J, Zon LI, Zanke B, Templeton DJ. Reconstitution of novel signalling cascades responding to cellular stresses. Philos Trans R Soc Lond B Biol Sci. 1996b;351(1336):135–141. doi: 10.1098/rstb.1996.0009. discussion 142. [DOI] [PubMed] [Google Scholar]
- Xie Y, Abdallah ME, Awonuga AO, Slater JA, Puscheck EE, Rappolee DA. Benzo(a)pyrene causes PRKAA1/2-dependent ID2 loss in trophoblast stem cells. Mol Reprod Dev. 2010;77(6):533–539. doi: 10.1002/mrd.21178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y, Awonuga A, Zhou S, Puscheck EE, Rappolee DA. Interpreting the stress response of early mammalian embryos and their stem cells. International Review of Cell & Molecular Biology. 2011a;287:43–95. doi: 10.1016/B978-0-12-386043-9.00002-5. [DOI] [PubMed] [Google Scholar]
- Xie Y, Liu J, Proteasa S, Proteasa G, Zhong W, Wang Y, Wang F, Puscheck EE, Rappolee DA. Transient stress and stress enzyme responses have practical impacts on parameters of embryo development, from IVF to directed differentiation of stem cells. Mol Reprod Dev. 2008;75(4):689–697. doi: 10.1002/mrd.20787. [DOI] [PubMed] [Google Scholar]
- Xie Y, Puscheck EE, Rappolee DA. Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced by the media. Mol Hum Reprod. 2006a;12(4):217–224. doi: 10.1093/molehr/gal021. [DOI] [PubMed] [Google Scholar]
- Xie Y, Puscheck EE, Rappolee DA. Effects of SAPK/JNK inhibitors on preimplantation mouse embryo development are influenced greatly by the amount of stress induced by the media. Mol Hum Reprod. 2006b;12(4):217–224. doi: 10.1093/molehr/gal021. [DOI] [PubMed] [Google Scholar]
- Xie Y, Sun T, Wang QT, Wang Y, Wang F, Puscheck E, Rappolee DA. Acquisition of essential somatic cell cycle regulatory protein expression and implied activity occurs at the second to third cell division in mouse preimplantation embryos. FEBS Lett. 2005a;579(2):398–408. doi: 10.1016/j.febslet.2004.10.109. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Puscheck EE, Rappolee DA. Pipetting causes shear stress and elevation of phosphorylated stress-activated protein kinase/jun kinase in preimplantation embryos. Mol Reprod Dev. 2007a;74(10):1287–1294. doi: 10.1002/mrd.20563. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang F, Zhong W, Puscheck E, Shen H, Rappolee DA. Shear stress induces preimplantation embryo death that is delayed by the zona pellucida and associated with stress-activated protein kinase-mediated apoptosis. Biol Reprod. 2006c;75(1):45–55. doi: 10.1095/biolreprod.105.049791. [DOI] [PubMed] [Google Scholar]
- Xie Y, Wang Y, Sun T, Wang F, Trostinskaia A, Puscheck E, Rappolee DA. Six post-implantation lethal knockouts of genes for lipophilic MAPK pathway proteins are expressed in preimplantation mouse embryos and trophoblast stem cells. Mol Reprod Dev. 2005b;71(1):1–11. doi: 10.1002/mrd.20116. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhong W, Wang Y, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Using hyperosmolar stress to measure biologic and stress-activated protein kinase responses in preimplantation embryos. Mol Hum Reprod. 2007b;13(7):473–481. doi: 10.1093/molehr/gam027. [DOI] [PubMed] [Google Scholar]
- Xie Y, Zhou S, Abdallah M, Puscheck E, Rappolee D. Relative strengths of O2 versus FGF4 signaling and the role of SAPK in trophoblast stem cell responses; pluripotency, lineage choice during differentiation, mitochondrial function, and invasion. PNAS. 2011b In preparation. [Google Scholar]
- Yagi R, Kohn MJ, Karavanova I, Kaneko KJ, Vullhorst D, DePamphilis ML, Buonanno A. Transcription factor TEAD4 specifies the trophectoderm lineage at the beginning of mammalian development. Development. 2007;134(21):3827–3836. doi: 10.1242/dev.010223. [DOI] [PubMed] [Google Scholar]
- Zhong W, Sun T, Wang QT, Wang Y, Xie Y, Johnson A, Leach R, Puscheck EE, Rappolee DA. SAPKgamma/JNK1 and SAPKalpha/JNK2 mRNA transcripts are expressed in early gestation human placenta and mouse eggs, preimplantation embryos, and trophoblast stem cells. Fertil Steril. 2004;82(Suppl 3):1140–1148. doi: 10.1016/j.fertnstert.2004.06.014. [DOI] [PubMed] [Google Scholar]
- Zhong W, Xie Y, Abdallah M, Awonuga AO, Slater JA, Sipahi L, Puscheck EE, Rappolee DA. Cellular stress causes reversible, PRKAA1/2-, and proteasome-dependent ID2 protein loss in trophoblast stem cells. Reproduction. 2010;140(6):921–930. doi: 10.1530/REP-10-0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong W, Xie Y, Wang Y, Lewis J, Trostinskaia A, Wang F, Puscheck EE, Rappolee DA. Use of hyperosmolar stress to measure stress-activated protein kinase activation and function in human HTR cells and mouse trophoblast stem cells. Reprod Sci. 2007;14(6):534–547. doi: 10.1177/1933719107307182. [DOI] [PubMed] [Google Scholar]
- Zhou S, Xie Y, Puscheck EE, Rappolee DA. Oxygen levels that optimize TSC culture are identified by maximizing growth rates and minimizing stress. Placenta. 2011 doi: 10.1016/j.placenta.2011.03.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Increased nuclear Eomes is induced in cultured TSCs after stimulation with 200 mM sorbitol for 1hr. TSCs were cultured in 0 or 200mM sorbitol for 1hr, then fixed and stained for Eomes using indirect immunocytochemistry. (A, C) show Hoechst stained nuclei, (B, D) show Eomes, 10 and (C, F) show merge Hoechst stained nuclei and Eomes immunostaining for 1hr in 200mM sorbitol and unstressed for each pair, respectively.