Abstract
TSLP is a type 1 cytokine that contributes to lymphopoiesis and the development of asthma and atopic dermatitis. TSLP acts on multiple lineages, including dendritic cells (DCs), T cells, NKT cells, eosinophils, and mast cells, mediating proliferation and survival, and linking innate and adaptive immune responses. TSLP is produced by a range of cells, including epithelial cells, fibroblasts, stromal cells, and keratinocytes. DCs are important primary targets of TSLP, and we now unexpectedly demonstrate that DCs also produce TSLP in response to Toll-like receptor (TLR) stimulation and that this is augmented by IL-4. Moreover, we demonstrate that when mice are challenged with house dust-mite (HDM) extract, lung CD11c+ DCs express TSLP mRNA at an even higher level than epithelial cells. These data suggest that DCs not only respond to TSLP but also are a source of TSLP during pathogen and/or allergen encounter.
Introduction
Thymic stromal lymphopoietin (TSLP) was originally identified as a growth-promoting activity produced by mouse thymic stromal cells that supported the development of immature B cells to the B220+/IgM+ stage (1). TSLP is a type I cytokine that acts via a receptor containing the IL-7 receptor α chain (IL-7Rα) and a TSLP-specific subunit, TSLPR (2, 3), and signals via JAK1 and JAK2 to mediate the activation of STAT5A and STAT5B (4). TSLPR is most similar to the common cytokine receptor γ-chain, γc, which is a component of the receptors for IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 (5) and whose mutation results in X-linked severe combined immunodeficiency in humans (6).
TSLP has been implicated in the development of asthma (7), atopic dermatitis (8), and inflammatory arthritis (9). Moreover, it is required for normal worm expulsion during helminth infection (10) and also for the inflammatory process that promotes cancer progression and metastasis (11–13). In addition to its actions on B cells, TSLP acts on other lineages, including dendritic cells (DCs) (14), T cells (4, 15, 16), mast cells (17), NKT cells (18), and eosinophils (19). TSLP can act via DCs to regulate the activation, differentiation, and homeostasis of T cells (8), but also has direct effects on T cells, promoting their survival and proliferation in response to TCR activation (15, 16, 20). Interestingly, TSLPR KO mice have a defective allergic inflammatory response in the lung, but this can be reversed by adoptive transfer of wild type (WT) CD4+ T cells (7), underscoring a key role for the action of TSLP on these cells.
TSLP has been reported to be expressed by a range of cell types, including epithelial cells, fibroblasts, keratinocytes, IgE receptor-activated mast cells, protease-activated basophils, and human CD68+ macrophages, whereas it was not observed to be produced by other lympho-hematopoietic cells, including neutrophils, B cells, T cells, monocytes, myeloid and plasmacytoid DCs, and endothelial cells (14, 21–23). Because DCs are involved in the initiation of immune responses, we decided to re-explore the possibility that DCs might also produce TSLP. Activation of DCs via Toll-like receptors (TLRs) is known to induce the expression of MHC II, CD80, and CD86 and to induce the secretion of inflammatory cytokines and chemokines that are important for the adaptive immune response (24–27). We now show that TLR signals also induce the production of TSLP by bone marrow-derived DCs, and that this is augmented by IL-4, with myeloid DCs (mDCs) producing much higher levels of TSLP than did plasmacytoid DCs (pDCs). TSLP expression was also induced by murine splenic DCs, human monocytes and monocyte-derived DCs. Moreover, when lung cells were sorted into epithelial cells or DCs, TSLP mRNA was expressed not only by the epithelial cells but also by the DCs, and levels were enhanced when mice were challenged with house dust-mite (HDM) extract, which induces allergic inflammation in the lung (28). Our study therefore reveals that in addition to their responding to TSLP, DCs can also produce TSLP in response to pathogens/allergens, with increased production in the presence of IL-4. The production of TSLP by DCs might bypass the need for epithelial cell-derived TSLP, potentially creating an autocrine loop wherein DCs both produce and respond to TSLP, as well as serving as a source of TSLP for other immune cells.
Materials and Methods
Cell culture, activation and FACS analysis
Bone marrow cells from WT or Stat6 KO BALB/c mice (Jackson Laboratory) were cultured with GM-CSF (20 ng/ml, Peprotech), or FLT-3L (100 ng/ml, Peprotech) for 8 days in RPMI 1640 medium supplemented with 10% FBS, 2 mM glutamine, 100 U/ml penicillin, and streptomycin. On day 8, GM-CSF cells were stained with APC-conjugated CD11c antibodies (BD Biosciences and R & D Systems, Inc.) to assess purity and were >90% pure. Also on day 8, FLT-3L cells were sorted into CD11c+B220+ cells using a MoFlo cell sorter (Beckman Coulter). Cells were activated for varying periods of time with 10 μg/ml zymosan (Alexis Biochemicals), 1 μg/ml LPS (Sigma), or 0.1 μM CpG (Alexis Biochemicals), with or without 10 ng/ml IL-4 (Peprotech), and TSLP mRNA and protein expression was evaluated. Splenic DCs were isolated using mouse pan-DC beads (Miltenyi Biotec). These cells were 80–85% pure based on staining with CD11c and B220 (BD Biosciences) and were activated with LPS or CpG for varying time points. Human monocytes were isolated using CD14 beads (Miltenyi Biotec) and cultured with human GM-CSF (50 ng/ml) and IL-4 (10 ng/ml) (Peprotech) for 7 days. The cells were washed and then stimulated with LPS and zymosan for 4 or 16 hr to evaluate TSLP mRNA and protein expression, respectively. Monocyte and monocyte-derived DC (MDDC) purity was assessed by staining with PE-Cy7-CD14 (BD Pharmingen) and FITC-CD1c (Miltenyi Biotec). Monocyte purity was 85–90% and monocyte-derived DC purity was 85–90% after 7 days of culture in GM-CSF+IL-4. Staining with FITC-Annexin V (BD Pharmingen) and 7-AAD (Molecular Probes) was used to distinguish living and dead cells. Bronchoalveolar lavage (BAL) fluid cells were stained with FITC-conjugated Ly-6G, PE-CD11c, and APC-CD11b (BD Pharmingen and eBioscience) to distinguish neutrophils from DCs. PE-TCRβ (BD Pharmingen) was used to identify T cells.
RNA isolation and real-time PCR
RNA was extracted using Trizol reagent (Invitrogen) or RNeasy kit (Qiagen), reverse-transcribed using the Omniscript cDNA synthesis kit (Qiagen), and cytokine cDNAs identified by a fluorogenic 5′-nuclease PCR assay and an ABI Prism 7900HT sequence detection system (Perkin Elmer). One microgram of RNA was used for RT-PCR, and 0.2 μg of this product was amplified using mouse or human TSLP, TNFα, TARC, MIP-1α and IL-8 TaqMan FAM-MGB primers (Applied Biosystems), and mRNA levels measured using standard curves relative to mouse or human RPL7 mRNA. The primers used for mouse Rpl7 were 5′-TACCCAAGCGACTGGTCAGA-3′ and 5′-TGGGAGGCGTTGGTGTCT-3′ and the TaqMan FAM-TAMRA probe was 5′-TGACATGCTGGCAGAGAGGCGAGATT-3′; the primers for human RPL7 were 5′-ACGCTTTGATTGCTCGATCTC-3′ and 5′-CCTCTTTGAAGCGTTTTCCAA-3′ and the TaqMan FAM-TAMRA probe was 5′-ATACGGCATCATCTGCATGGAGGATTTGAT-3′.
HDM-induced lung inflammation and isolation of lung epithelial cells and DCs
BALB/c mice were injected intratracheally with PBS or HDM (Greer Laboratories) on days 0, 7, and 14, sacrificed on day 17, and BAL fluid was collected. 2 ml of dispase (Qiagen) was injected intratracheally. The lungs were subsequently incubated in 1 ml dispase for 45 min at room temperature, after which they were minced into small pieces in 5 ml of RPMI medium supplemented with HEPES and 1 mg/ml DNase I (Sigma), shaken for 10 min, and then cell single suspensions were prepared by passage through mesh. After lysis of red blood cells with ACK buffer, the remaining cells were stained with PE-anti-CD11c (BD Pharmingen) and Alexafluor647 anti-CD324 (Decma-1) (eBioscience), and sorted using a MoFlo cell sorter (Beckman Coulter). The purity of the cells was 85–95%. A small part of the lung was homogenized in 1 ml PBS containing protease inhibitor cocktail tablet (Roche Diagnostics), and the supernatant was used for measuring TSLP and TARC.
ELISA
1 or 2 × 106 mouse DCs were cultured with medium alone or medium containing LPS, CpG, or zymosan ± IL-4 for 16 hr. BAL fluid, lung supernatant, and in vitro culture supernatants were collected, and the concentration of TSLP, TARC and MIP-1α was determined using an ELISA kit (R & D Systems and Antigenix).
Western blot
6–10 × 106 freshly isolated monocytes or 7 day old MDDCs were stimulated with LPS or zymosan for 12hr + 4hr with Golgi plug (Sigma) (total stimulation time = 16 hours). The cells were then lysed and subject to western blot analysis with sheep anti-human TSLP primary antibody for TSLP detection (R & D systems). As a loading control, the membrane was stripped to check for expression of β-actin (Sigma).
Statistics
Results are expressed as mean ± S.D. P values < 0.05, as determined by the Student’s t test by Prism 4 software, were considered to be significant. * denotes p < 0.05 and ** denotes p < 0.01 relative to the corresponding untreated samples.
RESULTS
Mouse bone marrow-derived and splenic DCs express TSLP and this expression is augmented by IL-4
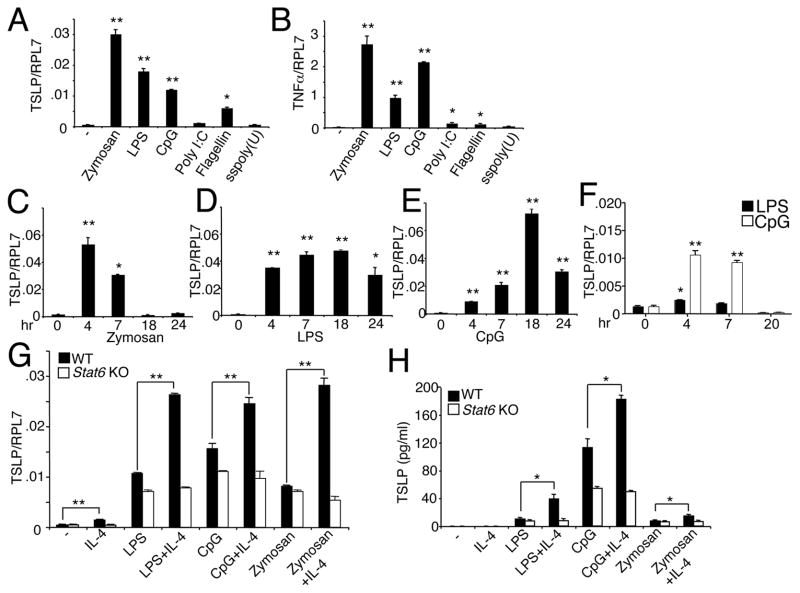
To determine whether DCs can produce TSLP, we first analyzed GM-CSF-induced mouse bone marrow derived DCs. Treatment of these cells with the TLR ligands zymosan, LPS, and CpG, which signal via TLR2/6, TLR4, and TLR9, respectively, each significantly induced not only TSLP mRNA (Fig. 1A); as expected, these stimuli also significantly induced TNFα mRNA (Fig. 1B), analogous to a previous report (27). Poly I:C, flagellin, and single-stranded poly (U), which signal via TLR3, TLR5, and TLR7, respectively, had weaker effects on both TSLP (Fig. 1A) and TNFα (Fig. 1B) induction, with only flagellin significantly inducing TSLP mRNA, albeit more modestly than zymosan, LPS, and CpG (Fig. 1A). Zymosan (Fig. 1C), LPS (Fig. 1D), and CpG (Fig. 1E) each induced TSLP mRNA within 4 hr, but these agents differ in their kinetics, with peak TSLP mRNA expression occurring earliest in response to zymosan. In addition to the production of TSLP mRNA by bone marrow-derived DCs, TSLP mRNA was also produced by splenic DCs (Fig. 1F). Because IL-4 has been reported to augment TSLP production by keratinocytes, fibroblasts, and epithelial cells when combined with TNFα, IL-1β, or poly I:C (29, 30), we evaluated the effect of IL-4 on TSLP expression. IL-4 augmented zymosan-, LPS-, and CpG-induced TSLP mRNA expression (Fig. 1G) and protein secretion (Fig. 1H) from bone marrow-derived DCs. Consistent with a key role for STAT6 in IL-4 signaling, IL-4 did not induce TSLP mRNA (Fig. 1G) or protein (Fig. 1H) in bone marrow-derived Stat6−/− DCs stimulated with LPS, CpG, or zymosan. This defect did not result from diminished viability of the cells in the absence of STAT6 (data not shown). Together, these results demonstrate that TSLP expression by DCs is induced by TLR stimulation and suggest that during pathogen/allergen exposure, the recruitment of Th2 cells that produce IL-4 may amplify allergic inflammatory responses by augmenting production of TSLP by DCs.
Figure 1.
DCs express TSLP in response to TLR stimulation and IL-4 can augment this induction. (A and B) Bone marrow derived DCs were stimulated for 4 hr with various TLR ligands, and TSLP (A) and TNFα (B) mRNA levels relative to RPL7 were determined. (C–E) Bone marrow derived DCs were stimulated with zymosan (C), LPS (D), or CpG (E) for varying time points and TSLP mRNA expression was assessed. (F) Purified splenic DCs were activated with LPS and CpG for varying time points and TSLP mRNA expression was assessed. The data are representative of 3 independent experiments. (G and H) IL-4 augments TSLP mRNA and protein production in bone marrow-derived DCs. On day 8, WT or Stat6 KO DCs were activated with zymosan, LPS, or CpG, without or with IL-4 for 4 or 16 hr and mRNA and supernatant harvested for TSLP RT-PCR (G) and ELISA (H), respectively. The data are representative of 3 independent experiments. *, p < 0.05 and **, p < 0.01 as compared to untreated samples (A–F) or as indicated (G,H).
TSLP production by myeloid DCs but not by plasmacytoid DCs
To further explore the range of DCs that can produce TSLP, we cultured bone marrow cells with either GM-CSF for 8–12 days to produce myeloid DCs (mDCs) (31) or with FLT-3L to generate plasmacytoid DCs (pDCs) (32). In response to LPS or CpG, TSLP mRNA expression was potently induced in mDCs, but little if any TSLP was induced in pDCs (Fig. 2A). In contrast, expression of MIP-1α, a chemokine known to be produced by both mDCs and pDCs (33), was increased in both populations of DCs (Fig. 2B). Correspondingly, LPS and CpG induced TSLP protein secretion by mDCs but not pDCs (Fig. 2C), whereas MIP-1α protein was secreted by both cell types (Fig. 2D). The detection of TSLP protein in the supernatant reflected secretion rather than release from dying cells, as LPS and CpG did not diminish the viability of the different DC populations (Fig. 2E). Thus, mDCs but not pDCs are producers of TSLP.
Figure 2.
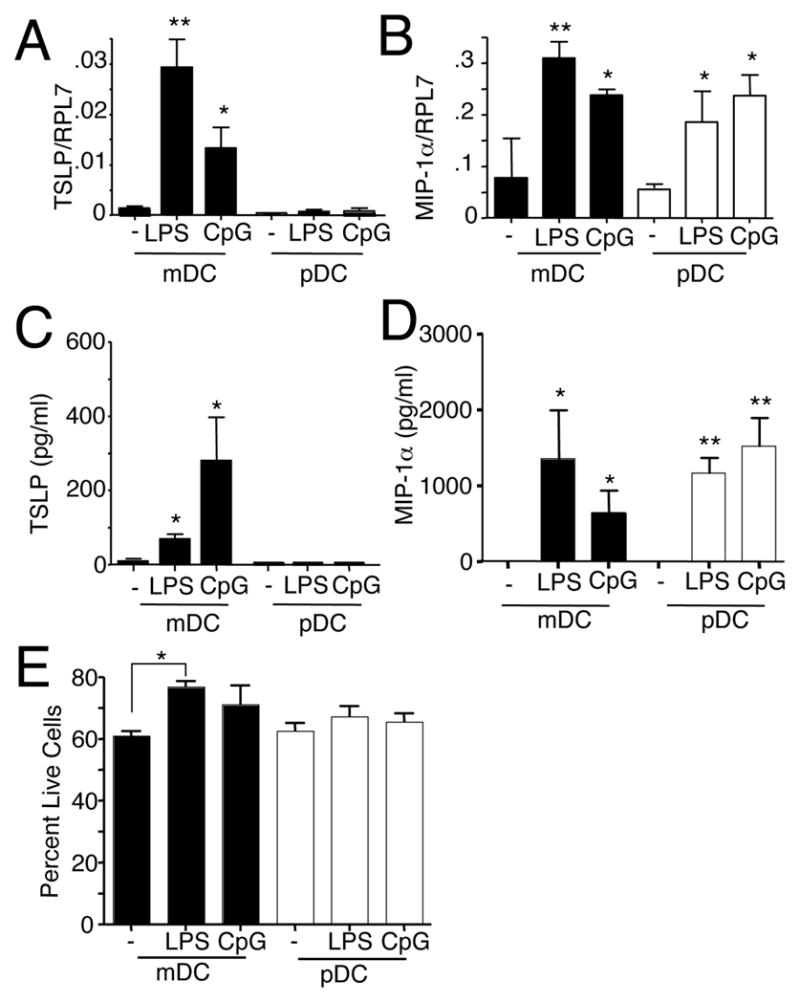
GM-CSF- but not FLT-3L-derived DCs produce TSLP. (A and B) RT-PCR was used to measure TSLP (A) and MIP-1α (B) mRNA levels relative to RPL7 control mRNA in bone marrow derived DCs that were activated with LPS or CpG for 4 hr. mDCs were induced by culturing for 8 days in GM-CSF and pDCs were induced by culturing with FLT-3L for 8 days and then isolated CD11c+/B220+ cells by cell sorting. (C and D) TSLP and MIP-1α protein levels, as measured by ELISA, after 16 hr of LPS or CpG stimulation of DCs that had been differentiated. (E) Percent live cells after 16 hr stimulation, as measured by Annexin V and 7-AAD staining. The data are from 3 independent experiments for TSLP and 2 independent experiments for MIP-1α. *, p < 0.05 and **, p < 0.01 as compared to untreated samples.
Human peripheral blood monocytes and monocyte-derived DCs also express TSLP
Monocytes from human blood develop into DCs when cultured with GM-CSF + IL-4, and these monocyte-derived DCs (MMDCs) can function as potent APCs (34, 35). We therefore used freshly-isolated human monocytes (Fig. 3A) and MDDCs (Fig. 3B) and found that TSLP mRNA was induced in both populations by either LPS or zymosan (Fig. 3C). As expected, as a control, IL-8 mRNA expression was also induced in both populations, but the basal as well as LPS- and zymosan-induced levels in MDDCs were lower than in monocytes (Fig. 3D). Western blot analysis of monocytes and MDDC revealed upregulation of TSLP protein upon LPS and zymosan stimulation (Fig. 3E). Hence human monocytes and MDDC also express TSLP.
Figure 3.
Human peripheral blood monocytes and monocyte-derived DCs produce TSLP and IL-8 mRNA in response to LPS and zymosan. Freshly isolated monocytes (CD14high CD1c−)(A) and monocyte-derived DCs (MDDC) (CD14lowCD1c+) that had been grown for 7 days with GM-CSF + IL-4 (B) were stimulated with LPS and zymosan, and TSLP (C) and IL-8 (D) mRNA levels were then determined by RT-PCR. The data are representative of 3 independent experiments. (E) Freshly isolated monocytes and 7 day GM-CSF+IL-4 derived MDDC were either left unstimulated or stimulated with LPS and zymosan and western blot analysis performed. Data are from 1 out of 2 independent experiments. *, p < 0.05 and **, p < 0.01 as compared to untreated control.
Lung epithelial cells and DCs produce TSLP in response to house dust mite challenge in vivo
Above, we examined the ability of different DC populations to produce TSLP after being challenged in vitro. We next used intra-tracheally-administered house dust-mite (HDM) extract as an in vivo stimulus (see Materials and Methods). As expected, HDM induced an inflammatory response, with increased cells in the BAL fluid (Fig. 4A), with a higher percentage of neutrophils (Fig. 4B) and T cells (Fig. 4C) but lower percentage of DCs (Fig. 4D) in the BAL fluid of HDM-challenged mice. Both TARC (Fig. 4E), a chemokine responsible for recruiting Th2 cells (36) and TSLP (Fig. 4F) were significantly higher in the lung after HDM challenge. To assess the source of production of TARC and TSLP, we isolated epithelial cells and DCs by cell sorting. Interestingly, basal levels of TSLP (Fig. 4G) and TARC (Fig. 4H) mRNAs were more highly expressed in the CD324+CD11c+ DCs than in the CD324+CD11c− epithelial cells, and HDM further induced mRNA levels of TSLP (Fig. 4G) and TARC (Fig. 4H) in both populations of cells. As expected, mRNA encoding pulmonary surfactant-associated protein B (SFTPB) was only expressed by the epithelial cells (Fig. 4I), confirming that the effects we observed did not result from contamination of DCs by epithelial cells. These results establish that HDM-mediated lung inflammation is associated with the production of TSLP by lung DCs as well as by primary epithelial cells.
Figure 4.
Lung DCs produce TSLP upon HDM challenge. BALB/c mice were injected with HDM as described in the Materials and Methods. (A–D) BAL fluid cellularity was determined (A), and the percentages of neutrophils (B), T cells (C), and DCs (D) were determined by flow cytometric staining with Ly-6G/CD11b (B), TCRβ (C) CD11c/CD11b (D). The data are combined from 5 independent experiments with 3–5 mice in each group yielding a total of 18 mice. (E and F), TARC (E) and TSLP (F) protein levels were measured by ELISA in lung homogenate. Data are from 1 of 3 similar experiments, each of which had 3–5 mice in each group. (G–I) TSLP and TARC mRNA levels are induced by HDM in lung DCs. CD324+CD11c− (epithelial cell) and CD324+CD11c+ (dendritic cell) populations of total pooled cells from 5 mice were isolated by cell sorting from PBS or HDM-treated mice, mRNA was isolated and RT-PCR performed for TSLP, TARC and Pulmonary surfactant-associated protein B (SFTPB). Data are expressed as the ratio of TARC, TSLP, or SFTPB mRNA to that of the RPL7 housekeeping gene and are from 3 independent experiments. *, p < 0.05 and **, p < 0.01 for HDM vs. PBS.
DISCUSSION
In this study, we have demonstrated that multiple activators of TLRs, including zymosan, LPS, and CpG, could each induce expression of TSLP in mouse bone marrow-derived DCs and splenic DCs. Interestingly, TLR-induced TSLP production by bone marrow-derived DCs was augmented by IL-4; as anticipated, this was via a STAT6-dependent mechanism. Increased TSLP expression was also observed in both freshly isolated human monocytes and MDDCs. The production of TSLP by DCs suggests possible autocrine effects on DCs; moreover, DCs may serve as a source of TSLP for other responsive lineages, such as CD4+ T cells, to promote TSLP-dependent allergic inflammatory responses such as asthma and atopic dermatitis. The production of TSLP by DCs was not anticipated as DCs have been viewed only as critical targets for this cytokine. DCs produce pro-inflammatory cytokines including TNFα, IL-1β, and IL-6 upon TLR ligation (27). Therefore, we hypothesized that TSLP would also be produced by DCs resident in the gut, lung, and skin, organs that have proximity to the external environment upon TLR ligation by allergens/pathogens. In this regard, TLR pathways have been implicated in the pathogenesis of asthma (28, 30), but a link between these pathways and the production of TSLP by DCs has not been reported. Our data that lung CD324+CD11c+ DCs induced TSLP mRNA upon HDM challenge suggest that production of TSLP by DCs could play a role in the development of asthma, with implications for other DC-dependent inflammatory diseases as well.
In summary, TSLP was originally reported to be produced by epithelial cells and keratinocytes and to act on dendritic cells, which in turn can activate T cells, thereby helping to bridge innate and adaptive immune response (8). We previously showed that functional receptors for TSLP are also expressed on T cells and other lineages and that TSLPR+ T cells are critical for ovalbumin-induced lung inflammation (7). Moreover, TSLP promotes T cell survival (4, 20). We now show that DCs not only respond to TSLP but also produce this cytokine. The production of TSLP by DCs may thus allow the direct activation of the adaptive immune response, complementing and possibly circumventing the requirement for epithelial cell/keratinocyte-derived TSLP in the activation of T cells and promoting T-cell dependent immune responses.
Acknowledgments
We thank Dr. Jian-Xin Lin for critical comments.
Footnotes
This work was supported by the Intramural Research Program, National Heart, Lung, and Blood Institute, NIH.
M. Kashyap, Y. Rochman, R. Spolski designed and performed experiments, analyzed data, and wrote the paper, L. Samsel performed experiments, W.J. Leonard designed experiments, analyzed data, and wrote the paper.
References
- 1.Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol. 1994;22:321–328. [PubMed] [Google Scholar]
- 2.Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, Ziegler SF, Leonard WJ, Lodish HF. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol. 2000;1:59–64. doi: 10.1038/76923. [DOI] [PubMed] [Google Scholar]
- 3.Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, Largaespada DA, Copeland NG, Jenkins NA, Farr AG, Ziegler SF, Morrissey PJ, Paxton R, Sims JE. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: Formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med. 2000;192:659–670. doi: 10.1084/jem.192.5.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rochman Y, Kashyap M, Robinson GW, Sakamoto K, Gomez-Rodriguez J, Wagner KU, Leonard WJ. Thymic stromal lymphopoietin-mediated STAT5 phosphorylation via kinases JAK1 and JAK2 reveals a key difference from IL-7-induced signaling. Proc Natl Acad Sci U S A. 2010;107:19455–19460. doi: 10.1073/pnas.1008271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. doi: 10.1038/35105066. [DOI] [PubMed] [Google Scholar]
- 6.Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. doi: 10.1016/0092-8674(93)90167-o. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med. 2005;202:829–839. doi: 10.1084/jem.20050199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ziegler SF, Liu YJ. Thymic stromal lymphopoietin in normal and pathogenic T cell development and function. Nat Immunol. 2006;7:709–714. doi: 10.1038/ni1360. [DOI] [PubMed] [Google Scholar]
- 9.Koyama K, Ozawa T, Hatsushika K, Ando T, Takano S, Wako M, Suenaga F, Ohnuma Y, Ohba T, Katoh R, Sugiyama H, Hamada Y, Ogawa H, Okumura K, Nakao A. A possible role for TSLP in inflammatory arthritis. Biochem Biophys Res Commun. 2007;357:99–104. doi: 10.1016/j.bbrc.2007.03.081. [DOI] [PubMed] [Google Scholar]
- 10.Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, Artis D. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, Braga M, Di Carlo V, Doglioni C, Protti MP. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208:469–478. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, Gress RE, Hesdorffer C, Leonard WJ, Biragyn A. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol. 2011;186:5656–5662. doi: 10.4049/jimmunol.1100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, Gallegos M, Burton EC, Savino D, Hori T, Tanaka Y, Zurawski S, Zurawski G, Bover L, Liu YJ, Banchereau J, Palucka AK. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med. 2011;208:479–490. doi: 10.1084/jem.20102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, Gilliet M, Ho S, Antonenko S, Lauerma A, Smith K, Gorman D, Zurawski S, Abrams J, Menon S, McClanahan T, de Waal-Malefyt Rd R, Bazan F, Kastelein RA, Liu YJ. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 15.Al-Shami A, Spolski R, Kelly J, Fry T, Schwartzberg PL, Pandey A, Mackall CL, Leonard WJ. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rochman I, Watanabe N, Arima K, Liu YJ, Leonard WJ. Cutting edge: direct action of thymic stromal lymphopoietin on activated human CD4+ T cells. J Immunol. 2007;178:6720–6724. doi: 10.4049/jimmunol.178.11.6720. [DOI] [PubMed] [Google Scholar]
- 17.Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, Paquette N, Ziegler SF, Sarfati M, Delespesse G. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med. 2007;204:253–258. doi: 10.1084/jem.20062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol. 2007;144:305–314. doi: 10.1159/000106319. [DOI] [PubMed] [Google Scholar]
- 19.Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol. 2009;43:305–315. doi: 10.1165/rcmb.2009-0168OC. [DOI] [PubMed] [Google Scholar]
- 20.Rochman Y, Leonard WJ. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J Immunol. 2008;181:7699–7705. doi: 10.4049/jimmunol.181.11.7699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol. 2008;9:310–318. doi: 10.1038/ni1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, Zhang G, Gu S, Gao Z, Shamji B, Edwards MJ, Lee TH, Corrigan CJ. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol. 2008;181:2790–2798. doi: 10.4049/jimmunol.181.4.2790. [DOI] [PubMed] [Google Scholar]
- 23.Corrigan CJ, Jayaratnam A, Wang Y, Liu Y, de Waal Malefyt R, Meng Q, Kay AB, Phipps S, Lee TH, Ying S. Early production of thymic stromal lymphopoietin precedes infiltration of dendritic cells expressing its receptor in allergen-induced late phase cutaneous responses in atopic subjects. Allergy. 2009;64:1014–1022. doi: 10.1111/j.1398-9995.2009.01947.x. [DOI] [PubMed] [Google Scholar]
- 24.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K. Immunobiology of dendritic cells. Annu Rev Immunol. 2000;18:767–811. doi: 10.1146/annurev.immunol.18.1.767. [DOI] [PubMed] [Google Scholar]
- 25.Mellman I, Steinman RM. Dendritic cells: specialized and regulated antigen processing machines. Cell. 2001;106:255–258. doi: 10.1016/s0092-8674(01)00449-4. [DOI] [PubMed] [Google Scholar]
- 26.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 27.Dearman RJ, Cumberbatch M, Maxwell G, Basketter DA, Kimber I. Toll-like receptor ligand activation of murine bone marrow-derived dendritic cells. Immunology. 2009;126:475–484. doi: 10.1111/j.1365-2567.2008.02922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via Toll-like receptor 4 triggering of airway structural cells. Nat Med. 2009;15:410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bogiatzi SI, Fernandez I, Bichet JC, Marloie-Provost MA, Volpe E, Sastre X, Soumelis V. Cutting Edge: Proinflammatory and Th2 cytokines synergize to induce thymic stromal lymphopoietin production by human skin keratinocytes. J Immunol. 2007;178:3373–3377. doi: 10.4049/jimmunol.178.6.3373. [DOI] [PubMed] [Google Scholar]
- 30.Kato A, Favoreto S, Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol. 2007;179:1080–1087. doi: 10.4049/jimmunol.179.2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Inaba K, Swiggard WJ, Steinman RM, Romani N, Schuler G, Brinster C. Isolation of dendritic cells. Curr Protoc Immunol Chapter. 2009;3(Unit 3):7. doi: 10.1002/0471142735.im0307s86. [DOI] [PubMed] [Google Scholar]
- 32.Brawand P, Fitzpatrick DR, Greenfield BW, Brasel K, Maliszewski CR, De Smedt T. Murine plasmacytoid pre-dendritic cells generated from Flt3 ligand-supplemented bone marrow cultures are immature APCs. J Immunol. 2002;169:6711–6719. doi: 10.4049/jimmunol.169.12.6711. [DOI] [PubMed] [Google Scholar]
- 33.Proietto AI, O’Keeffe M, Gartlan K, Wright MD, Shortman K, Wu L, Lahoud MH. Differential production of inflammatory chemokines by murine dendritic cell subsets. Immunobiology. 2004;209:163–172. doi: 10.1016/j.imbio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 34.Jefford M, Schnurr M, Toy T, Masterman KA, Shin A, Beecroft T, Tai TY, Shortman K, Shackleton M, Davis ID, Parente P, Luft T, Chen W, Cebon J, Maraskovsky E. Functional comparison of DCs generated in vivo with Flt3 ligand or in vitro from blood monocytes: differential regulation of function by specific classes of physiologic stimuli. Blood. 2003;102:1753–1763. doi: 10.1182/blood-2002-12-3854. [DOI] [PubMed] [Google Scholar]
- 35.Pickl WF, Majdic O, Kohl P, Stockl J, Riedl E, Scheinecker C, Bello-Fernandez C, Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–3859. [PubMed] [Google Scholar]
- 36.Imai T, Nagira M, Takagi S, Kakizaki M, Nishimura M, Wang J, Gray PW, Matsushima K, Yoshie O. Selective recruitment of CCR4-bearing Th2 cells toward antigen-presenting cells by the CC chemokines thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Immunol. 1999;11:81–88. doi: 10.1093/intimm/11.1.81. [DOI] [PubMed] [Google Scholar]