Abstract
Inositol phosphates (InsP) are widely produced throughout animal and plant tissues. Diphosphoinositol pentakisphosphate (InsP7) contains an energetic pyrophosphate bond. Here, we demonstrate that disruption of InsP6K1, one of the three mammalian InsP6Ks that convert InsP6 to InsP7, confers enhanced PtdIns(3,4,5)P3-mediated membrane translocation of Akt pleckstrin homology (PH) domain and thus augments downstream PtdIns(3,4,5)P3 signaling in murine neutrophils. Consequently, these neutrophils exhibited elevated phagocytic and bactericidal capabilities and amplified NADPH oxidase-mediated superoxide production. These phenotypes were replicated in human primary neutrophils with pharmacologically inhibited InsP6Ks. By contrast, increasing intracellular InsP7 amounts blocked chemoattractant-elicited PH domain membrane translocation and dramatically suppressed PtdIns(3,4,5)P3-mediated cellular events in neutrophils. These findings establish a role for InsP7 in signal transduction and provide a mechanism for modulating PtdIns(3,4,5)P3 signaling in neutrophils.
Highly energetic pyrophosphate bonds are a fundamental component of several higher inositol polyphosphates, including diphosphoinositol-pentakisphosphate (InsP7) and bis-diphosphoinositol tetrakisphosphate (InsP8)1, 2. InsP7 arises from pyrophosphorylation of InsP6 (phytic acid), one of the most abundant inositol phosphates in mammalian cells. The enzymes that catalyze the synthesis of InsP7 from InsP6 comprise a family of inositol hexakisphosphate kinases (InsP6K) including InsP6K1, InsP6K2, and InsP6K33, 4. InsP7 and InsP8 are dynamic molecules with very rapid turnover rates 5–7. In mammalian cells, InsP7 has been implicated in several cellular functions including vesicular trafficking and exocytosis 8, 9, apoptosis 10–13, and insulin disposition14. InsP7 is a physiologic inhibitor of Akt, a serine/threonine protein kinase that regulates glucose homeostasis by inhibiting GSK3β15. InsP7 affects this pathway by potently inhibiting PDK1 phosphorylation of Akt, preventing its activation and thereby affecting insulin signaling. Akt signaling is dramatically augmented and GSK3β signaling reduced in skeletal muscle, white adipose tissue, and liver of mice with targeted deletion of InsP6K1. As a result, InsP6K1 knockout mice manifest insulin sensitivity and are resistant to obesity elicited by high-fat diet or aging15.
Although it is well documented that InsP7 can regulate a variety of cellular processes, its physiological significance and the underlying molecular mechanism are unclear. In Dictyostelium discoideum, InsP7 competes with PtdIns(3,4,5)P3 for PH domain binding and negatively regulates PtdIns(3,4,5)P3 signaling16. In these cells, chemoattractant stimulation triggers membrane translocation of many PH domain-containing proteins via specific binding to PtdIns(3,4,5)P3, which induces actin polymerization and chemotactic migration17–19. Depletion of InsP7 by deleting the gene for InsP6 kinase enhances PH domain membrane translocation and augments downstream chemotactic signaling. Membrane translocation of PH-domains was previously thought to be dependent solely upon concentrations of PtdIns(3,4,5)P3 in the membrane20. Thus, these findings not only established a fundamental role for InsP7 in cellular signal transduction pathways, but also described a novel mode of regulation for PH domain function, namely relative levels of InsP7 and PtdIns(3,4,5)P3. In the current study we further assessed the role of InsP6K1 in neutrophils. We show that InsP7, by blocking PtdIns(3,4,5)P3-mediated plasma membrane translocation of PH domain-containing proteins, negatively regulates several PtdIns(3,4,5)P3-mediated neutrophil functions including NADPH oxidase-mediated superoxide production, phagocytosis, and bacterial killing. These findings establish InsP6K1 as a physiological negative regulator in neutrophils and suggest that therapeutic interventions targeting InsP6K1 may be a promising approach to modulate neutrophil response in infectious and inflammatory diseases.
Results
InsP6K1 disruption augments PtdIns(3,4,5)P3 signaling in neutrophils
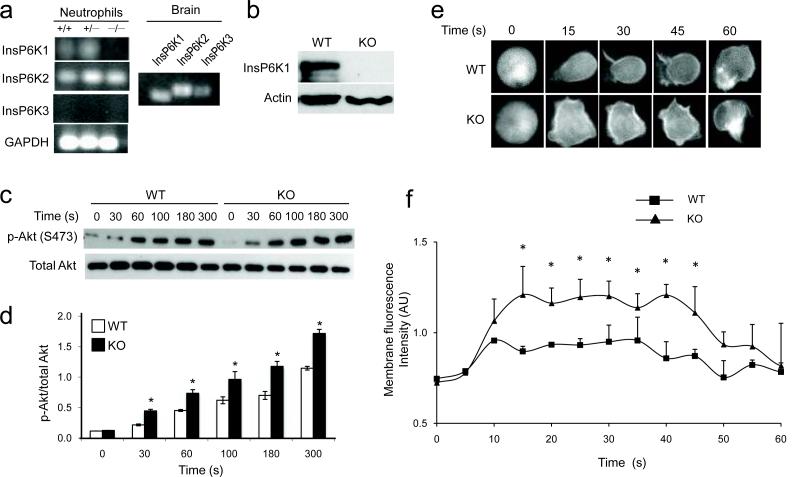
To study the cellular function of inositol pyrophosphate in neutrophils, we first determined which InsP6K isoform(s) are expressed in these cells. RT-PCR analysis revealed expression of InsP6K1 and InsP6K2, but not InsP6K3, in neutrophils (Fig. 1a). Homozygous InsP6K1-deficient mice are viable and do not display any gross physical or behavioral abnormalities14. We characterized the hematopoietic cells in these mice and found that the peripheral blood count was normal. Microscopic examination of blood smears did not show any morphological abnormality in InsP6K1-deficient neutrophils (data not shown). The depletion of InsP6K1 protein in neutrophils was confirmed by Western blot analysis as well as RTPCR (Fig. 1a, b).
Figure 1. Disruption of InsP6K1 in murine neutrophils augments PtdIns(3,4,5)P3 signaling.
(a) RTPCR analysis of expression of InsP6K isoforms in neutrophils. RNA extracted from brain was used as positive control. The results shown are representative of three experiments. (b) Immunoblot analysis of InsP6K1 protein expression of InsP6K1−/− and wild-type mouse neutrophils. The results shown are representative of three experiments. (c) Immunoblot analysis of total and phosphorylated Akt in neutrophils stimulated with 1μM fMLP for the indicated time periods. The results shown are representative of three experiments. (d) Ratios of phosphorylated Akt and total Akt in neutrophils stimulated with 1μM fMLP as quantified with NIH Image software21. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (e) Time-lapse images of murine bone marrow derived neutrophils isolated from InsP6K1−/− and wild-type mice and transfected with an Akt-PHGFP construct. Cells were stimulated with 1 μM fMLP for the indicated time. Representative fluorescence images of three experiments are shown. (f) Quantitative analysis of membrane translocation of Akt-PH-EGFP in murine bone marrow derived neutrophils stimulated with 1 μM fMLP. The average membrane fluorescence intensities (arbitrary unit, AU) were measured with ImageJ software16. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test).
We previously showed that InsP7 significantly inhibits PtdIns(3,4,5)P3-PH domain binding16. In D. discoideum, depletion of InsP7 by deleting the gene for InsP6 kinase enhances PH-domain membrane translocation and augments downstream chemotactic signaling16. To address whether mammalian InsP6K1 and its product InsP7 regulates PtdIns(3,4,5)P3 signaling in neutrophils, we first measured the activation of endogenous Akt by examining the level of Akt phosphorylation21 (Fig. 1c). Prior to chemoattractant stimulation, Akt phosphorylation was virtually undetectable in both wild-type and InsP6K1-deficient neutrophils. We observed pronounced elevation of Akt phosphorylation in response to formyl-Met-Leu-Phe (fMLP), a tripeptide widely used as a model chemoattractant in studies of neutrophil function. The level of Akt phosphorylation was significantly augmented in InsP6K1-deficient neutrophils at every time point examined, whereas the time course for the increase was not altered (Fig. 1d).
To further assess the effect of InsP6K1 depletion on fMLP-elicited PtdIns(3,4,5)P3 signaling, we directly measured chemoattractant-elicited PH domain translocation (Fig. 1e) by using the PH domain of Akt (PHAkt) fused with green fluorescent protein (PHAkt-GFP) as a marker22. During uniform chemoattractant treatment, PHAkt-GFP transiently translocates from cytosol to the plasma membrane23., Membrane translocation of PHAkt-GFP occurred instantaneously and peaked within 10–20 sec at a saturating concentration of fMLP (100 nM). The amount of membrane-associated PHAkt-GFP in InsP6K1-deficient neutrophils was significantly higher than in wild-type neutrophils (Figure 1F). The InsP6K1 disruption-induced elevation of Akt membrane translocation was dependent on PtdIns(3,4,5)P3 production, because the PI3K inhibitors wortmannin and LY294002 completely abolished Akt translocation (Supplementary Fig. 1a) and the subsequent activation (Supplementary Fig. 1b) of InsP6K1-deficient neutrophils. In addition, this cellular process also depended on direct binding of Akt-PH domain to PtdIns(3,4,5)P3. Two Akt-PH domain mutants that have lost the ability to bind PtdIns(3,4,5)P3, Akt-PH R25C and Akt-PH K14R24, failed to respond to chemoattractant stimulation even in InsP6K1-deficient neutrophils (Supplementary Fig. 2). The effect of InsP6K1 disruption on PtdIns(3,4,5)P3 signaling appeared to be specific. Receptor expression (Supplementary Fig. 3a), phosphorylation of several other protein kinases, such as ERK and p38 (Supplementary Fig. 3b), calcium mobilization (Supplementary Fig. 3c), and the sensitivity to chemoattractant stimulation (Supplementary Fig. 4a and Supplementary Movies 1–2) were unaltered in InsP6K1-deficient neutrophils. Collectively, these results indicate that InsP6K1 and its product InsP7 are specific negative regulators of PtdIns(3,4,5)P3 signaling in neutrophils.
Enhanced superoxide production in InsP6K1−/− neutrophils
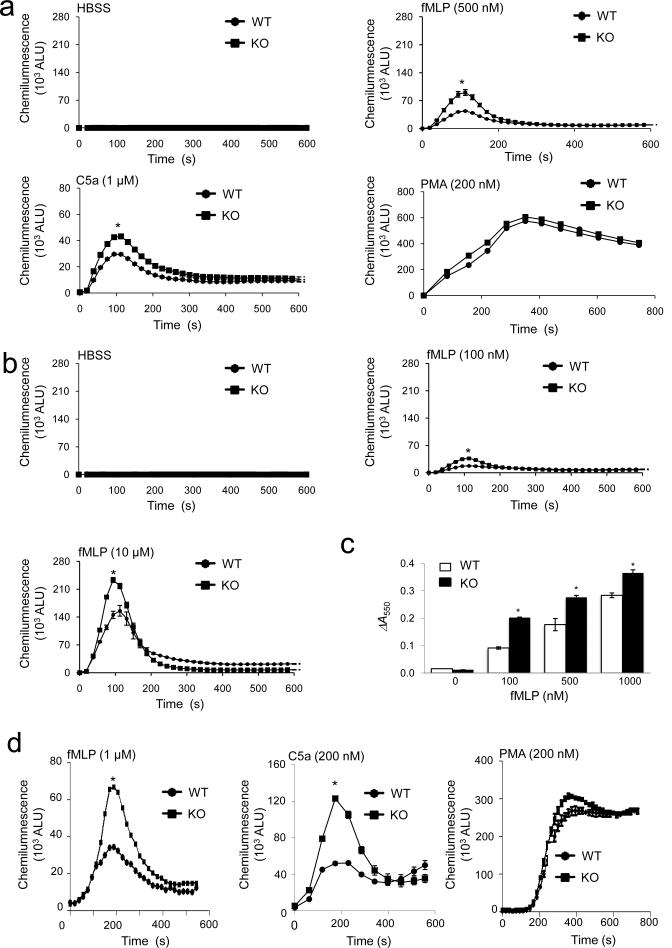
One important downstream effect of chemoattractant-induced PtdIns(3,4,5)P3 production in neutrophils is NADPH oxidase-mediated superoxide production25, 26,27, 28,29–31. Consistent with the augmented PtdIns(3,4,5)P3 signaling, InsP6K1-deficient neutrophils displayed significantly enhanced NADPH oxidase activation as assayed using an isoluminol chemiluminescence assay (Fig. 2a, b). When treated with phorbol-12-myristate-13-acetate (PMA), a PKC activator, InsP6K1-deficient neutrophils generated almost the same amount of superoxide as wild-type neutrophils, suggesting that the enhanced superoxide production in InsP6K1-deficient neutrophils is specific for receptor-mediated signals. In addition, a cytochrome-c reduction assay showed that total Reactive Oxygen Species (ROS) production was substantially enhanced in InsP6K1-deficient neutrophils in comparison to wild-type neutrophils (Fig. 2c). Isoluminol is a membrane impermeable reagent and thus can only detect ROS released to the extracellular space by the oxidase on the plasma membrane. To determine whether InsP6K1 also regulates the NADPH oxidase on the intracellular granules, endosomes and lysosomes, we used membrane-permeable luminol, which in the presence of catalase and superoxide dismutase (SOD), only measures ROS production by intracellular NADPH oxidase. Under these conditions, we observed markedly elevated ROS production in InsP6K1-deficient neutrophils (Fig. 2d). The InsP6K1 disruption-induced elevation of ROS production was abolished in neutrophils treated with wortmannin and LY294002 (Supplementary Fig. 5a). Chemoattractant-elicited ROS production is mainly mediated by G protein-coupled receptors (GPCR) and PI3Kγ. A specific PI3Kγ inhibitor (AS-252424) inhibited ROS production in both wild-type and InsP6K1-deficient neutrophils (Supplementary Fig. 5a), while a specific Akt inhibitor, Akti VIII, only partially suppressed ROS production but completely abrogated the enhancing effect on ROS production caused by InsP6K1 disruption (Supplementary Fig. 5a). These results suggest that Akt may not be the only mediator of chemoattractant-elicited ROS production, but is a key downstream target of InsP6K1. Thus, via regulating PtdIns(3,4,5)P3 signaling, InsP6K1 acts as a key regulator of superoxide production in mouse neutrophils.
Figure 2. Disruption of InsP6K1 leads to enhanced chemoattractant-elicited intracellular and extracellular superoxide production in murine neutrophils.
(a) Extracellular ROS production monitored by an isoluminol chemiluminescence assay. Bone marrow-derived neutrophils isolated from wild-type and InsP6K1-deficient mice were stimulated with indicated concentration of fMLP, C5a or PMA. Shown are arbitrary light units (ALU) of each measurement. (b) Extracellular ROS production elicited with indicated amount of fMLP. (c) Total amount of ROS production monitored by an cytochrome C reduction assay. Bone marrow neutrophils from wild-type and InsP6K1-deficient mice were incubated with cytochrome-c with or without SOD. Absorbance at 550 nm was measured 5 min after fMLP stimulation. Total amount of ROS production was calculated as the difference in absorbance between samples with and without SOD (ΔA550). (d) Intracellular ROS production monitored by an luminol chemiluminescence assay. Bone marrow-derived neutrophils isolated from wild-type and InsP6K1-deficient mice were stimulated with indicated amount of fMLP, C5a or PMA. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test).
InsP6K inhibition in human neutrophils
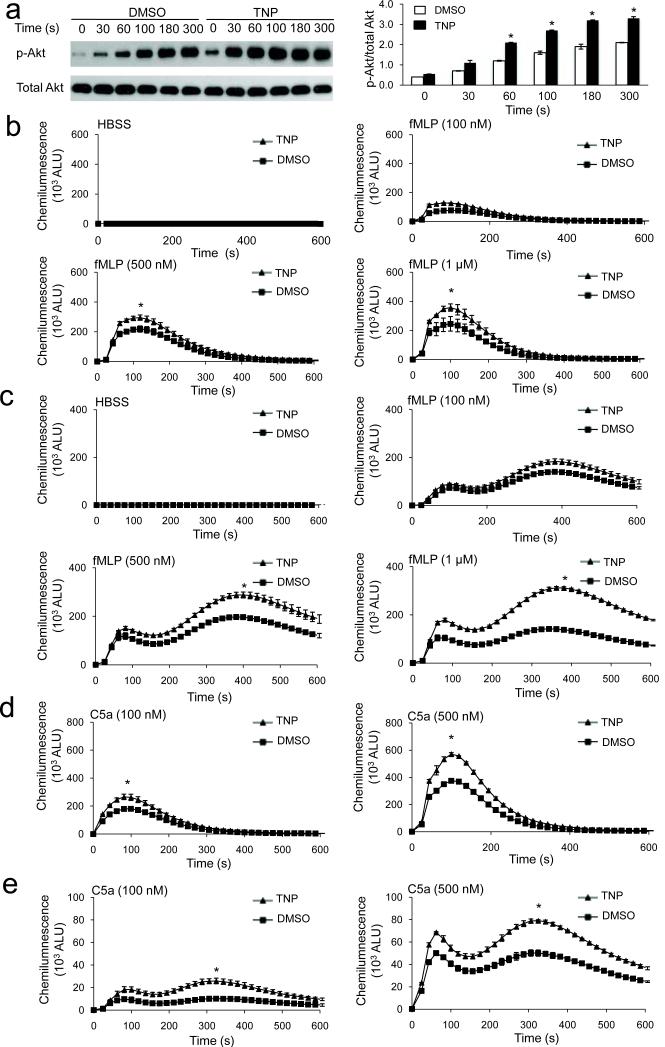
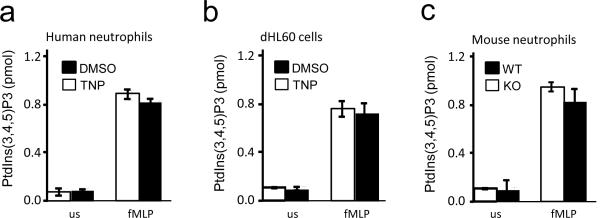
We next examined whether inhibition of InsP6 kinase can elevate PtdIns(3,4,5)P3 signaling in human primary neutrophils. N(2)-(m-(trifluoromethy)lbenzyl) N(6)-(p-nitrobenzyl)purine (TNP) is a selective inhibitor of InsP6K activity in vitro and inhibits InsP7 and InsP8 synthesis in vivo without affecting the amounts of other inositol phosphates and the activity of a large number of protein kinases 32. Human neutrophils treated with TNP exhibited significantly enhanced fMLP-elicited Akt phosphorylation, indicating that InsP6K1 negatively regulates PtdIns(3,4,5)P3 signaling in human neutrophils (Fig. 3a). Consequently, both intracellular and extracellular NADPH oxidase-mediated superoxide production were significantly elevated in human neutrophils treated with TNP (Fig. 3b,c). A distinct chemoattractant, complement fragment C5a, also induced enhanced ROS production in TNP treated neutrophils relative to control cells (Fig. 3d.e). Similar to observations in mouse neutrophils, the augmented ROS production in InsP6K1-disrupted human neutrophils was dependent on PtdIns(3,4,5)P3 generation and Akt activation (Supplementary Fig. 5a). Disruption of InsP6K1 did not directly alter the level of PtdIns(3,4,5)P3 in either unstimulated or fMLP-stimulated neutrophils (Fig. 4). These results suggest that InsP6K also plays a role in regulating PtdIns(3,4,5)P3-mediated PH-domain membrane translocation in human neutrophils.
Figure 3. Pharmacological inhibition of InsP6K activity augments PtdIns(3,4,5)P3 signaling and NADPH oxidase-mediated superoxide production in human primary neutrophils.
(a) Immunoblot analysis of total and phosphorylated Akt in TNP (10μM)-treated and untreated human neutrophils stimulated with 1μM fMLP. The results shown are representative of three experiments. Relative amounts of phosphorylated Akt were quantified with NIH Image software. (b) fMLP-elicited extracellular ROS production in DMSO and TNP-treated human neutrophils. (c) fMLP-elicited intracellular ROS production in human neutrophils. (d) C5a -elicited extracellular ROS production in human neutrophils. (e) C5a-elicited intracellular ROS production in human neutrophils. Data are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test).
Figure 4. InsP6K1 disruption does not alter PtdIns(3,4,5)P3 level in neutrophils.
(a) PtdIns(3,4,5)P3 levels in TNP (10μM)-treated and untreated human neutrophils unstimulated (us) or stimulated with 1μM fMLP for 2 min. (b) PtdIns(3,4,5)P3 levels in neutrophil-like differentiated HL60 cells (dHL60) unstimulated (us) or stimulated with 1μM fMLP for 2 min. (c) PtdIns(3,4,5)P3 levels in InsP6K1−/− and wild-type murine neutrophils unstimulated (us) or stimulated with 1μM fMLP for 2 min. Data shown are means ± SD of 3 experiments.
InsP6K overexpression suppresses PtdIns(3,4,5)P3 signaling
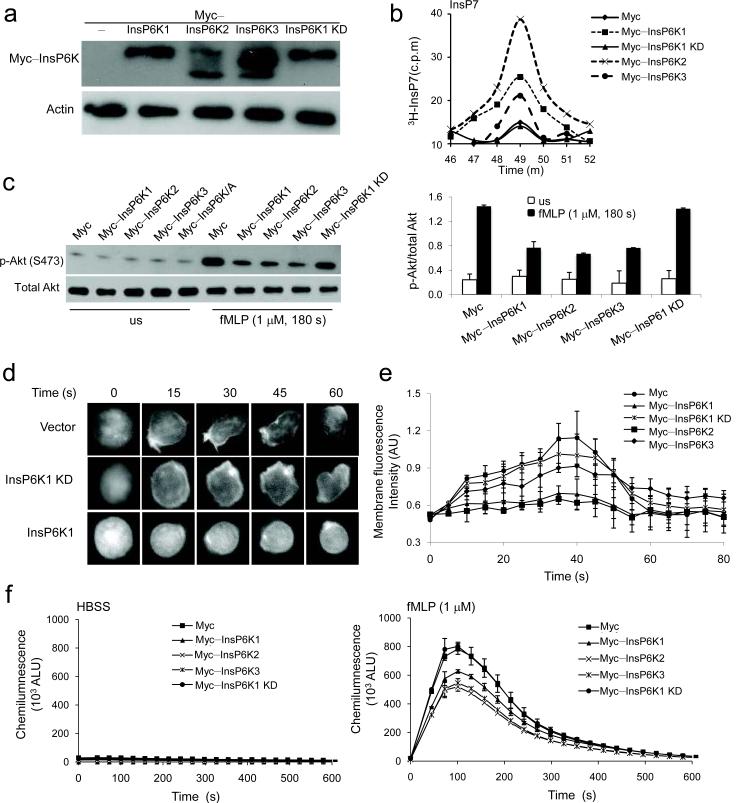
To investigate whether increasing InsP6K1 expression and the amount of cellular InsP7 can suppress PtdIns(3,4,5)P3 signaling, we used neutrophil-like differentiated HL60 cells (dHL60), in which specific genes can be easily over-expressed (Supplementary Fig. 6). We labeled endogenous inositol phosphate stores with [3H]inositol and measured the amount of inositol phosphates using HPLC. A significant increase of InsP7 was detected in HL60 cells overexpressing InsP6K1, while a control construct or a kinase-dead InsP6K1 (InsP6K1 K/A mutant) had no effect (Fig. 5a,b). Akt phosphorylation was increased in dHL60 cells stimulated with fMLP (Fig. 5c). The increase was significantly suppressed in cells overexpressing InsP6K1, but not a kinase-dead InsP6K1 (InsP6K1 K/A mutant, InsP6K1 KD), implying that the InsP6K1-mediated conversion from InsP6 to InsP7 is essential for the suppression of PtdIns(3,4,5)P3 signaling. In addition, overexpression of InsP6K1 in dHL60 cells resulted in lower membrane translocation of PHAkt-GFP than in control cells (Fig. 5d,e). As a result, NADPH oxidase-mediated ROS production declined in dHL60 cells overexpressing InsP6K1 (Fig. 5f). The suppression of ROS production was depended on the kinase activity of InsP6K, because the overexpression of the InsP6K1 K/A mutant failed to elicit the same effect (Fig. 5f). Overexpression of InsP6K2 and InsP6K3 increased InsP7 level in dHL60 cells as well and consequently suppressed PtdIns(3,4,5)P3 signaling in these cells (Fig. 5a–f). We have previously shown that InsP7, the product of InsP6K1, directly binds Akt-PH domain and consequently inhibits PtdIns(3,4,5)P3-PH domain binding16. Together with the inability of the kinase-dead InsP6K1 to suppress PtdIns(3,4,5)P3 signaling in dHL60 cells, these observations indicate that the inhibitory effect of InsP6K1 on PtdIns(3,4,5)P3 signaling and NADPH oxidase-mediated oxidative burst is mediated by its metabolic product, InsP7.
Figure 5. Overexpression of InsP6Ks suppresses PtdIns(3,4,5)P3 signaling in neutrophil-like dHL60 cells.
(a) Expression of Myc-tagged InsP6K1, InsP6K2, InsP6K3, and the kinase dead form InsP6K1 (InsP6K1–KD) mutant in dHL60 cells transfected with indicated construct. The blot shown is representative of three experiments. (b) The amounts of InsP7 in cells transfected with indicated constructs as analyzed by HLPC analysis. Inositol phosphates were identified by their co-elution with the standards of H3-inositol phosphates. The data were normalized to the total amount of protein extracted from the same samples. Shown are the peaks of InsP7. The results shown are representative of three experiments. (c) Immunoblot analysis of total and phosphorylated Akt in dHL60 cells unstimulated (us) or stimulated with 1μM fMLP for 3 min. The results shown are representative of three experiments. Relative amounts of phosphorylated Akt were quantified using NIH Image. Data are mean ± SD of 3 independent experiments. (d) fMLP-elicited membrane translocation of Akt-PH-GFP in dHL60 cells over-expressing InsP6Ks. HL60 cells were co-transfected with the Akt-PH-GFP construct and indicated construct. The results shown are representative of three experiments. (e) The average membrane fluorescence intensities in Fig 5c as measured with ImageJ software. Data are mean ± SD of 3 independent experiments. (f) fMLP-elicited ROS production in dHL60 cells over-expressing InsP6Ks. dHL60 cells transfected with indicated construct were uniformly stimulated with 1μM fMLP and extracellular ROS production was measured as described in Fig. 2. Data are mean ± SD of 3 independent experiments.
InsP7 inhibits superoxide production in a cell free system
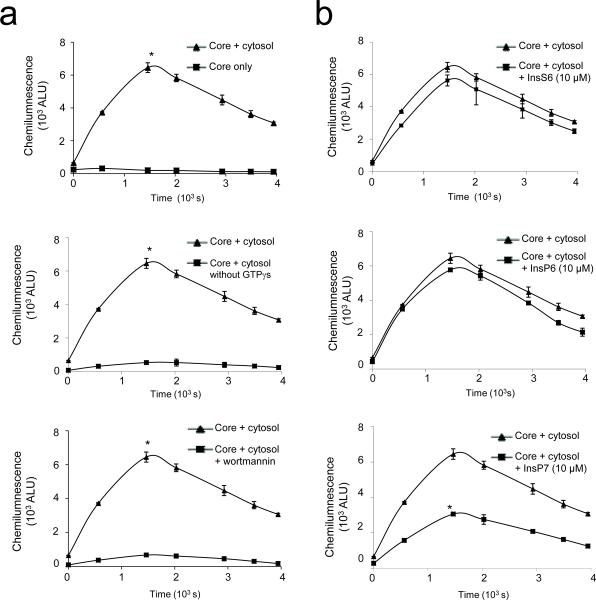
Given that InsP7 is a highly hydrophilic molecule and cannot passively cross the plasma membrane, the intracellular level of InsP7 cannot be raised by addition to the culture medium. To circumvent this prohibitive property of InsP7 and assess its intracellular functions directly, we took advantage of an established cell-free system for NADPH oxidase activation33 which reconstitutes intracellular NADPH oxidase activity in Streptolysin-O (SLO) permeabilized neutrophils. Pore formation by SLO is restricted to the plasma membrane and keeps intracellular membranes intact. Thus, this system faithfully recapitulates the assembly of the NADPH oxidase within intracellular compartments. The reconstitution was achieved using cytosol-depleted SLO-permeabilized PMNs, cytosol, NADPH, ATP, PMA and GTPγS, which activates the GPCR in the absence of receptor activation. Similar to NADPH oxidase-mediated ROS production by intact neutrophils, NADPH oxidase reconstitution in this ex vivo system depends upon GPCR and PI3-K activity, because an inhibitor of PI3K, wortmannin, dramatically suppressed GTPγS-induced ROS production (Fig. 6a). Addition of exogenous InsP7 to the reaction reduced ROS production, while InsP6 and InsS6 were essentially ineffective (Fig. 6b). Collectively, these results demonstrate that InsP7 directly inhibits PtdIns(3,4,5)P3 signaling and NADPH oxidase activity in neutrophils.
Figure 6. InsP7 inhibits superoxide production in a cell free reconstitution assay.
(a) Reconstitution reactions performed using permeabilized PMN cores and mock-depleted cytosol supplied with ATP, creatine kinase, PMA and NADPH in the presence or absence of GTPγS or wortmannin. (b) GTPγS-induced superoxide production in the presence of InsP7, InsP6, or InsS6. The reaction mixture were incubated for 10 min at 37°C before the assay. Kinetics of NADPH oxidase activation was monitored by luminol-dependent chemiluminescence. Data are means ± SD of 3 independent experiments. *p < 0.01 (Student's t test).
Chemoattractant stimulation reduces InsP7 in neutrophils
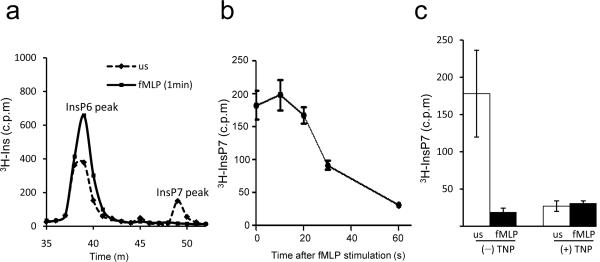
The levels of intracellular signaling molecules are often tightly regulated. Thus we explored whether chemoattractant stimulation alters endogenous InsP7 amounts in dHL60 neutrophils. These cells express a substantial amount of InsP7 (Fig. 7a). fMLP exposure induced a pronounced and rapid reduction of InsP7, which decreased by more than 80% within 1 min of fMLP stimulation (Fig. 7b). The degree of down-regulation induced by fMLP was similar to that induced by the InsP6K inhibitor, TNP (Fig. 7c). These results indicates that more than half of the original amounts of InsP7 were still present in the cells at the time of peak Akt-PH domain membrane translocation, which happens at about 30 sec following stimulation (Fig. 5e and Fig. 7b). These observations suggest that InsP7 may represent a mechanism for controlling optimal Akt activation. The high amounts of InsP7 in unstimulated dHL60 cells may be important to prevent neutrophil hyperactivation, while the reduction in InsP7 following chemoattractant stimulation may be necessary to allow sustained Akt signaling in stimulated cells.
Figure 7. Chemoattractant stimulation rapidly reduces InsP7 levels in neutrophils.
(a) InsP7 level in dHL60 cells unstimulated (us) or stimulated with 1μM fMLP for 1 min. The data were normalized to the total amount of protein extracted from the same samples. Shown are the peaks of InsP6 and InsP7. (b) InsP7 level in dHL60 cells stimulated with 1μM fMLP for indicated time periods. Data shown are mean ± SD of 3 experiments. (c) Production of InsP7 in TNP-treated dHL60 cells. Differentiated HL60 cells were treated with TNP (10μM) or DMSO at 37 °C for 30 min and stimulated with/without 1μM fMLP for 1min. Data shown are mean ± SD of 3 experiments.
Augmented bacterial killing in InsP6K1−/− mice
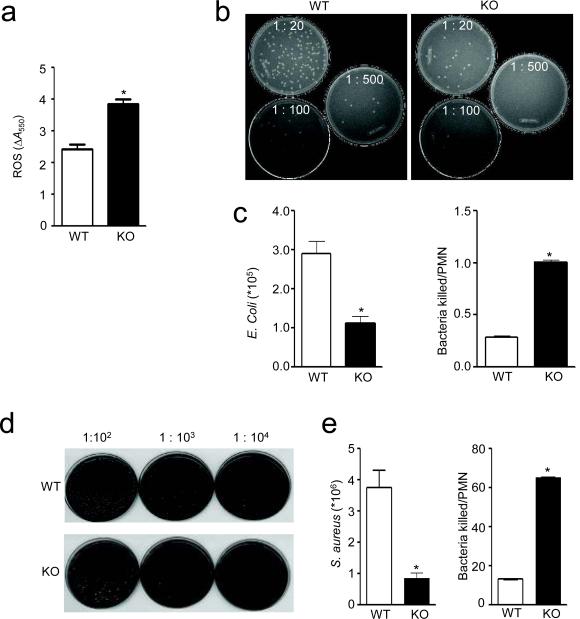
Next we used a murine acute peritoneal inflammation (peritonitis) model 22, 34 to examine neutrophil-mediated bacterial killing in InsP6K1-deficient mice in vivo. Mice were challenged by intraperitoneal injection of E.coli. We detected significantly increased amount of ROS in the peritoneal lavage of the InsP6K1-deficinet mice compared to the wild-type mice (Fig. 8a). To assess the neutrophil bacterial killing capability following bacterial challenge, we explored the survival rate of intraperitoneally injected live E.coli (Fig. 8b,c). Due to cell proliferation, the number of bacteria gradually increased after the initial injection. When a significant number of neutrophils accumulated in the peritoneal cavity (4 hrs after the injection), the number of bacteria stopped increasing and started to decline, reflecting the bacterial-killing capability of neutrophils (data not shown). We detected fewer bacteria in inflamed InsP6K1-deficient mice, suggesting enhanced bacteria-killing capability (Fig. 8c). We obtained similar results in a peritonitis model with another bacterial pathogen, Streptococcus aureus (Fig. 8d,e). To eliminate any effect caused by neutrophil recruitment, we normalized the numbers of live bacteria to the amount of neutrophils recruited to the inflamed peritoneal cavity (Fig. 8c,e). Because the assessment of live bacterial numbers was done at a relatively early stage of the infection (4 hr after bacteria injection), prior to recruitment of inflammatory macrophages and lymphocytes, the elevated bacterial-killing capability observed in InsP6K1-deficient mice most likely results from the enhanced PtdIns(3,4,5)P3 signaling in InsP6K1-deficient neutrophils.
Figure 8. InsP6K1 knockout mice have enhanced in vivo bacterial killing capability.
(a) ROS accumulation in the inflamed peritoneal cavity of wild-type and InsP6K-deficient mice as monitored by the cytochrome C reduction assay. Total amount of ROS was calculated as the difference in absorbance between samples with and without SOD (ΔA550). (b) In vivo killing of E.coli by wild-type and InsP6K-deficient mice as reflected by bacteria colony forming units. Images of representative culture plates are shown. (c) Total numbers of survived E.coli and E.coli killed relative to the number of recruited neutrophils. (d) In vivo killing of S. aureus by wild-type and InsP6K-deficient mice as reflected by bacteria colony forming units. Images of representative culture plates are shown. (e) Total numbers of survived S. aureus and S. aureus killed relative to the number of recruited neutrophils. Data are means ± SD of 3 independent experiments. *p < 0.01 by Student's t test.
InsP6K1 does not regulate neutrophil trafficking and survival
Neutrophil accumulation at inflammatory sites is an essential component of innate immunity and is required for the host's ability to kill invading pathogens. In the peritonitis model described above, we observed attenuated peritoneal neutrophil accumulation in the InsP6K1-deficient mice (Supplementary Fig. 7). This may be due to the fast clearance of bacteria and accelerated resolution of inflammation in these mice. Alternatively, it could be the result of the elevated ROS level in peritoneal cavity. ROS can induce deactivation of proinflammatory chemokines such as C5a, fMLP35, LTB436, and IL837, leading to reduced neutrophil recruitment. Another potential cause might be the alteration of neutrophil trafficking from circulation to the inflamed peritoneal cavity. PtdIns(3,4,5)P3 signaling was implicated in several cellular processes related to neutrophil trafficking, particularly adhesion and chemotaxis18, 38–40. However, elevation of PtdIns(3,4,5)P3 signaling by disruption of InsP6K1 failed to further augment cell adhesion, directionality, and migration speed in mouse neutrophils (Supplementary Fig. 8 and Supplementary Movies 3a–6). In addition, in an in vivo adoptive transfer assay, we detected similar recruitment of InsP6K1-deficient neutrophils and wild-type neutrophils to the inflamed peritoneal cavity (Supplementary Fig. 9). Lastly, neutrophil accumulation could be a result of accelerated neutrophil death. However, we examined neutrophil spontaneous death using an in vitro assay and found no significant difference between wild-type and InsP6K1−/− neutrophils (Supplementary Fig. 10). Taken together, These results demonstrate that the attenuated peritoneal neutrophil accumulation and enhanced bacteria-killing capability observed in InsP6K1-deficient mice are not due to altered neutrophil recruitment.
Enhanced phagocytosis in InsP6K1−/− neutrophils
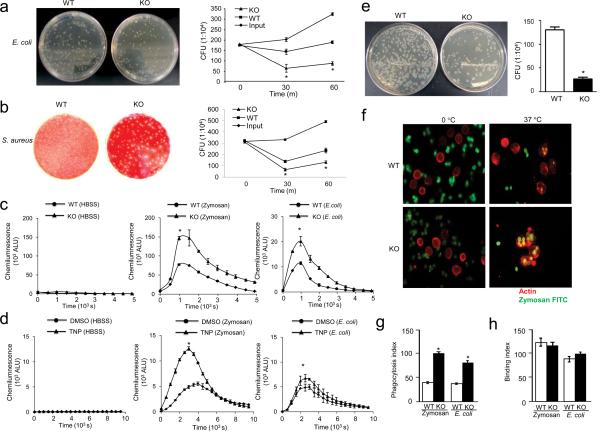
To address if InsP6K1 deletion enhances the intrinsic bacteria-killing capability of neutrophils, we conducted an in vitro bacterial killing assay using purified wild-type and InsP6K1-deficient neutrophils. The capability of InsP6K1-deficient neutrophils to kill E.coli was 140% higher at 30 min and 125% higher at 1 hr post-infection, compared with wild-type neutrophils (Fig. 9a). Similar results were observed following incubation with Streptococcus aureus (Fig. 9b). Because elevated bacterial killing could be due to enhanced superoxide production in the phagosomes, we investigated if InsP6K1 deletion, in addition to augmenting chemoattractant-elicited ROS production, influenced phagocytosis-induced ROS generation. Relative to wild-type neutrophils, InsP6K1-deficient neutrophils were 100% and 80% more efficient in phagocytosis-associated ROS production in response to Zymosan and E.coli stimulation, respectively (Fig. 9c). Similar results were observed in neutrophil-like differentiated HL60 cells treated with the InsP6K inhibitor TNP (Fig. 9d). We further investigated the intracellular bactericidal activity of InsP6K1-deficient neutrophils using a gentamicin protection assay. Gentamicin cannot penetrate eukaryotic cells and thus cannot kill internalized intracellular bacteria. Viable intracellular bacteria were quantified by subsequent plating of the lysed samples onto agar plates. Consistent with an elevated superoxide production in the phagosome, InsP6K1-deficient neutrophils killed engulfed bacteria more efficiently than wild-type neutrophils (Fig. 9e).
Figure 9. InsP6K1 null neutrophils exhibit augmented in vitro bacterial killing capability with enhanced phagocytosis and ROS production.
(a) In vitro killing of E coli by wild-type and InsP6K-deficient mouse neutrophils. Diluted aliquots were spread on agar plates and incubated overnight at 37°C. In vitro bacterial killing capabilities were reflected by the decrease of bacteria colony forming units (CFU) after incubation with neutrophils. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (b) In vitro killing of S. aureus by wild-type and InsP6K-deficient mouse neutrophils. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (c) Superoxide production by wild-type and InsP6K-deficient mouse neutrophils in response to Zymosan or E.coli. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (d) Phagocytosis-associated ROS production by TNP-treated (10 µM, 2 hours) and untreated dHL60 cells. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (e) In vitro killing of internalized E.coli by wild-type and InsP6K-deficient mouse neutrophils as assessed using a gentamicin protection assay. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (f) Phagocytosis fluorescein-conjugated zymosan particles by wild-type and InsP6K-deficient mouse neutrophils. The results shown are representative of three experiments. (g) Phagocytosis index as expressed by the number of bioparticles engulfed by 100 neutrophils. More than 200 neutrophils were counted in each group. Results are the means (±SD) of three independent experiments. *p < 0.01 (Student's t test). (h) Binding index as expressed by the number of bioparticles bound by 100 neutrophils. Results are the means (±SD) of three independent experiments.
Augmented phagocytosis may also explain the enhanced bacterial-killing observed in InsP6K1-deficient mice. To test this, we quantified the number of zymosan (Saccharomyces cerevisiae) bioparticles engulfed by each mouse neutrophil via an in vitro phagocytosis assay (Fig. 9f). The phagocytic index of wild-type neutrophils was 47, meaning that an average of 47 mouse-serum-opsonized fluorescein-conjugated zymosan particles were engulfed by 100 neutrophils after 1 hr incubation at 37°C. InsP6K1-deficient neutrophils had an average phagocytic index of 100 (Fig. 9g). A similar effect was detected in in vitro phagocytosis assays with bacteria bioparticles. The phagocytosis index of InsP6K1−/− neutrophils was 120% higher than that of wild-type neutrophils (Fig. 9g). The augmented phagocytosis was likely a result of enhanced engulfment, because there was no difference between wild-type and InsP6K1-null neutrophils in the initial binding to Zymosan or E.coli particles (Fig. 9h). Taken together, these findings demonstrate that augmenting PtdIns(3,4,5)P3 signaling by InsP6K1 disruption leads to enhanced phagocytosis and antimicrobial defense in InsP6K1 knockout mice.
Discussion
Here we describe that InsP7, which is synthesized by InsP6K, competes with Akt PH-domain for the binding of PtdIns(3,4,5)P3 and thus negatively regulates PtdIns(3,4,5)P3-mediated cellular functions in neutrophils. Disruption of InsP6K1, one of the three mammalian InsP6 kinases that convert InsP6 to InsP7, resulted in enhanced PtdIns(3,4,5)P3 signaling in murine neutrophils. InsP6K1-deficient neutrophils exhibited elevated phagocytic and bactericidal capabilities as well as amplified NADPH oxidase-mediated superoxide production.
InsP7 contains a high energy pyrophosphate and has been implicated in a variety of cellular activities, but its signaling pathways have been obscure. Our observation that InsP7 is a physiologic regulator of interaction between Akt PH domain and PtdIns(3,4,5)P3 may be indicative of a general phenomenon. InsP7 functions as a PtdIns(3,4,5)P3-binding competitor for several other PH-domain containing proteins, such as PIKE and Tiam16. InsP7 can also bind and regulate the function of proteins without a PH domain. InsP7 directly binds cytosolic cyclin-dependent kinase (CDK)-CDK inhibitor (CKI) complex, required for phosphate homeostasis in yeast41. Additionally, it binds to clathrin-associated proteins such as AP2 and AP18042, 43. Binding of InsP7 to AP180 negatively regulates clathrin cage assembly activity43, 44. InsP7 can also serve as a phosphate donor, in a nonenzymatic fashion45, 46. Inositol pyrophosphates can only transfer their high-energy β-phosphate moiety to pre-phosphorylated serine residues to generate pyrophosphoserine45, 46. InsP7-mediated protein phosphorylation occurs predominantly at a region containing extensive stretches of serine residues surrounded by acidic amino acids45, 46. Such sequence was not found in Akt, which does not seem to be a target protein45, 46. Thus pyrophosphorylation is unlikely involved in the regulation of Akt by InsP7.
Activation of neutrophils at inflammatory sites is an essential component of the innate immune response. However, hyper-activation of neutrophils can also damage surrounding tissues via the release of toxic reactive oxygen species and granule enzymes such as proteases, causing acute inflammation. Thus, neutrophil activity needs to be carefully controlled by both positive and negative regulators. InsP7 becomes a putative candidate for such homeostatic regulation. The amount of InsP7 is tightly regulated in neutrophils. A substantial amount of InsP7 exists in unstimulated cells, preventing neutrophil hyperactivation and ensuring optimal cellular inflammatory response. InsP7 is rapidly reduced upon chemoattractant stimulation, allowing the induction of sustained PtdIns(3,4,5)P3 signals in responding neutrophils. InsP7 negatively regulates Akt signaling in glucose homeostasis and protein translation15 but, unlike the observations in the present study, where chemoattractant inhibits InsP7 formation, growth factors stimulate InsP7 generation. Therefore, although the inhibition of Akt signaling by InsP7 may be a general phenomenon in cellular signal transduction, the mechanism of its regulation as well as the resulting physiological consequences can be considerably different in different cell systems. Currently, the mechanisms by which InsP7 production is suppressed in chemoattractant stimulated neutrophils are largely unknown. It likely involves activation of inositol pyrophosphate phosphatase and/or deactivation of InsP6 kinase.
PtdIns(3,4,5)P3 signaling was implicated in several cellular processes related to neutrophil trafficking, particularly adhesion and chemotactic migration18, 38–40. Similar results were also reported in other cell types such as mast cells, in which PI3K pathway plays an important role in integrin-mediated cell adhesion and migration47. Nevertheless, InsP6K1 disruption failed to further augment cell adhesion, directionality, and migration speed in neutrophils. These results are somewhat different from the migration phenotypes observed in PTEN-deficient neutrophils, which also exhibit markedly enhanced PtdIns(3,4,5)P3 signaling21. Although the overall chemotactic migration is relatively normal, PTEN disruption results in mildly impaired directionality, enhanced sensitivity to chemoattractant stimulation and slightly increased migration speed21. The distinct effects are likely caused by different temporal and spatial regulation of PTEN and InsP6K1 in neutrophils. PTEN activity is increased and its subcellular localization is altered after chemoattractant stimulation48. On the contrary, InsP7 level is high in unstimulated neutrophils and is significantly reduced after chemoattractant stimulation. In addition, the mechanisms by which PTEN and InsP6K1 regulate PtdIns(3,4,5)P3 signaling are different. PTEN regulates the amount of PtdIns(3,4,5)P3 and controls neutrophil function via several downstream pathways. By contrast, InsP6K1 deletion does not alter the amount of PtdIns(3,4,5)P3 in the cell and its effect is limited to inhibition of Akt.
Although increased PtdIns(3,4,5)P3 signaling following InsP6K1 deletion directly improved the phagocytic and bactericidal capability of neutrophils, we cannot completely rule out that other cell types, such as macrophages, also account for the improved bacterial killing in the peritoneum of InsP6K1−/− mice. The enhanced bacterial killing in the InsP6K1−/− mice is associated with attenuated peritoneal neutrophil accumulation. It is unlikely that this effect is caused by accelerated neutrophil death, because we measured the neutrophil numbers at 4 hr after the induction of peritonitis, when neutrophil death has not yet occurred. We also directly examined neutrophil spontaneous death using an in vitro assay and found no significant difference between wild-type and InsP6K1−/− neutrophils. Additionally, using an adoptive transfer assay, we revealed that disruption of InsP6K1 does not affect neutrophil migration to sites of inflammation. Thus, the reduced neutrophil accumulation is likely an outcome of augmented bacteria clearance and accelerated resolution of inflammation. Alternatively, it can be due to the elevated ROS level in the inflamed peritoneal cavity of InsP6K1-deficient mice. ROS are able to deactivate proinflammatory chemokines such as C5a, fMLP35, LTB436, and IL837, leading to reduced neutrophil recruitment.
Our findings indicate that higher inositol phosphates are significant players in a variety of cellular functions and can regulate signal transduction in a ubiquitous fashion akin to well-described signaling effectors such as kinases and phosphatases. The fairly ubiquitous expression of InsP6K1 suggests it may also regulate other blood cells such as lymphocytes and macrophages. These findings suggest that InsP6K1 and its phosphorylation product InsP7 may be promising therapeutic targets for modulating immune cell functions in various infectious and inflammatory diseases.
Online Methods
Mice
InsP6K1 knockout mice were generated as previously described1. Corresponding wild-type littermates were used as paired controls for InsP6K1 knockout mice. Mice aged 8–14 weeks were used in this study. All procedures involving mice were approved and monitored by the Children's Hospital Institutional Animal Care and Use Committee.
Neutrophil purification and functional assays
Human blood neutrophil purification and western blotting were performed as described previously21, 49, 50. EZ-taxiscan chemotaxis Assay, analysis of cell tracks and morphology, bacterial killing assays, measurement of calcium signaling, and other related assays were described in the “Supplementary Methods” section.
The expression of InsP6K isoforms
RT-PCR was performed to confirm successful disruption of the InsP6K1 transcript in the knockout mice and to assess expression of InsP6K isoforms in murine neutrophils. Total RNA was prepared from neutrophils or brain tissues of wild-type and InsP6K1 knockout mice using TRIZOL reagent (Invitrogen). cDNA was then prepared with a iScript cDNA synthesis kit (Bio-Rad) and PCR was performed using a SSO Fast EvaGreen supermix. Primers with specificity for murine InsP6K1, InsP6K2, and InsP6K3 were validated using brain tissue from wild-type mice, a region previously identified to express all three isoforms. GAPDH primers were used as a positive control to assess the quality and quantity of cDNA and PCR reactions. Agarose gel electrophoresis (1%) was used to visualize expression of reverse transcripts. InsP6K1 protein was detected by wetern blotting with specific InsP6K1 antibody (GeneTex Inc.).
Analysis of Inositol Phosphates in HL60 Cells
Human premyelocytic leukemia HL-60 cells were cultured in RPMI-1640 medium supplemented with 10% FBS and 4 mM glutamine in the presence of [3H]-inositol (20μCi/ml). Cells were plated at a density of 1×106 cells/ml in 4ml of medium, adding 1 volume of fresh medium containing [3H]-inositol every 2 days and cultured for another 6 days. Cells were harvested for transfection of different InsP6K constructs using the Amaxa nucleofector device (20 million cell in 200μl of transfection reagent, 30μg of plasmid, program T019). After 3 h of transfection, the inositol phosphates were extracted and analyzed by HPLC as previously described50.
Gene Transfer (nucleofection) and PH domain membrane translocation assay
To overexpress PHAkt-GFP in mouse neutrophils, 3×106 mouse neutrophils were transfected with 2.0 μg of PHAkt-GFP DNA using the Amaxa nucleofector device in accordance with the manufacturer's protocol. To measure Akt PH domain membrane translocation following InsP6K1 overexpression, 6-day differentiated HL60 cells were transfected with a mixture of Myc-InsP6K1 plasmid (or Myc vector alone as control) (4 μg) and PHAkt-GFP (1 μg). Mouse neutrophils and HL60 cells were incubated for 6 hrs and 2 hrs post-transfection, respectively, washed once with HBSS, and re-suspended at 1×106/ml. Cells were allowed to settle for 3–4 min on Lab-Tek chambered cover glass. Membrane translocation of PHAkt -GFP was visualized by time-lapse imaging. Images were captured on an Olympus IX-71 microscope with a 40× oil immersion objective for 5 min at 5 second intervals. The cells were stimulated with 10× concentrated fMLP after a few initial image captures. The average membrane fluorescence intensities were measured with ImageJ software as previously described16.
NADPH oxidase reconstitution assay
Reconstitution assay using permeabilized neutrophils and neutrophil cytosol was essentially conducted as previously described with some modifications33. For cytosol preparation, human blood neutrophils (5×108 in 5ml) were suspended in RB buffer (10 mM PIPES, pH 7.3, with KOH, 100 mM K+, 3 mM Na+, 3.5 mM Mg2+) containing 0.3 mM EGTA and 5.6 mM di-isopropyl fluorophosphate and lysed by pressurization to 400 p.s.i. for 20 min at 0 °C prior to release. The cavitate was centrifuged at 2500g for 10 min at 4 °C followed by centrifugation of the resulting supernatant at 100,000g for 1 h at 4 °C. The high speed supernatant was flash-frozen as aliquots in liquid N2 and stored at −80 °C until use. For preparation of permeabilized cores, human neutrophils (3×106) were incubated in 300 μl of RB-EBL buffer containing 250U/ml of reduced streptolysin-O for 10 min at 4 °C. Cores were recovered by centrifugation at 280g for 10 min, resuspended in 300μl of RB-EBL and reconstituted within 60 min. Reconstitution reactions contained 8×104 freshly prepared neutrophil cores in 100 μl of RB-EBL buffer plus 60μl of cytosol, 4 mM ATP, 400 μM GTP, 100 ng/ml PMA, 10 mM creatine phosphate, and 25 μg/ml creatine kinase. Reactions were pre-incubated at 37 °C for 15 min to induce permeabilization, followed by addition of 400 μM NADPH and 600μM GTP-γS. Wortmannin (100μM) or InsP7 was included in the 10 min pre-incubation as indicated.
Peritonitis model and neutrophil adoptive transfer
InsP6K1 null and wild-type mice were intraperitoneally injected with either 2×106 of E.coli (strain 19138; ATCC, Manassas, VA) in 0.9% saline, 1×108 S. aureus in 0.9% saline, or saline only. Four hours after injection, mice were sacrificed and peritoneal exudates were harvested in 3 successive washes with 3 ml of PBS with 5 mM EDTA each. Total neutrophils recruited were quantified by flow cytometric analysis of Gr-1+ positive cells. To calculate the number of live bacteria that remained in the exudate, diluted exudate was plated on LB agar plates for E.coli or TSAII plates for S. aureus and colony formation was assessed. The efficiency of bacterial killing by recruited neutrophils was quantified as the number of killed bacteria divided by the number of recruited neutrophils. The neutrophil adoptive transfer was performed as previously described50. Wild type and InsP6K1-null neutrophils were labeled with 5- (and -6)-carboxyfluorescein diacetate succinimidyl esters (CFSE) or 5- (and -6)-chloromethyl SNARF-1 acetate (SNARF-1). Labeled cells were mixed 1:1 and then injected intravenously into wild-type recipient mice that were challenge with 1 ml of 3% thioglycollate for 2.5 hr. The amount of adoptively transferred neutrophils recruited to the peritoneal cavity was analyzed 1.5 hr after the injection. Relative recruitment of neutrophils was calculated as the ratio of indicated populations in the peritoneal cavity (Supplementary Fig. 9).
Statistical Analysis
Analysis of statistical significance for indicated data sets was performed using the Student's t test function within Microsoft Excel (Microsoft, Redmond, OR, USA).
Supplementary Material
Acknowledgments
The authors thank Leslie Silberstein, John Manis, and Li Chai for helpful discussions. H. Luo is supported by NIH grants HL085100, AI076471, HL092020, and GM076084.
Reference
- 1.Shears SB. Diphosphoinositol polyphosphates: metabolic messengers? Mol Pharmacol. 2009;76:236–252. doi: 10.1124/mol.109.055897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stephens L, et al. The detection, purification, structural characterization, and metabolism of diphosphoinositol pentakisphosphate(s) and bisdiphosphoinositol tetrakisphosphate(s) J Biol Chem. 1993;268:4009–4015. [PubMed] [Google Scholar]
- 3.Shears SB. How versatile are inositol phosphate kinases? Biochem J. 2004;377:265–280. doi: 10.1042/BJ20031428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burton A, Hu X, Saiardi A. Are inositol pyrophosphates signalling molecules? J Cell Physiol. 2009;220:8–15. doi: 10.1002/jcp.21763. [DOI] [PubMed] [Google Scholar]
- 5.Menniti FS, Miller RN, Putney JW, Jr., Shears SB. Turnover of inositol polyphosphate pyrophosphates in pancreatoma cells. J Biol Chem. 1993;268:3850–3856. [PubMed] [Google Scholar]
- 6.Glennon MC, Shears SB. Turnover of inositol pentakisphosphates, inositol hexakisphosphate and diphosphoinositol polyphosphates in primary cultured hepatocytes. Biochem J. 1993;293:583–590. doi: 10.1042/bj2930583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Safrany ST, Shears SB. Turnover of bis-diphosphoinositol tetrakisphosphate in a smooth muscle cell line is regulated by beta2-adrenergic receptors through a cAMP- mediated, A-kinase-independent mechanism. Embo J. 1998;17:1710–1716. doi: 10.1093/emboj/17.6.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Illies C, et al. Requirement of inositol pyrophosphates for full exocytotic capacity in pancreatic beta cells. Science. 2007;318:1299–1302. doi: 10.1126/science.1146824. [DOI] [PubMed] [Google Scholar]
- 9.Luo HR, et al. GRAB: a physiologic guanine nucleotide exchange factor for Rab3A, which interacts with inositol hexakisphosphate kinase. Neuron. 2001;31:439–451. doi: 10.1016/s0896-6273(01)00384-1. [DOI] [PubMed] [Google Scholar]
- 10.Morrison BH, Bauer JA, Kalvakolanu DV, Lindner DJ. Inositol hexakisphosphate kinase 2 mediates growth suppressive and apoptotic effects of interferon-beta in ovarian carcinoma cells. J Biol Chem. 2001;276:24965–24970. doi: 10.1074/jbc.M101161200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison BH, et al. Inositol hexakisphosphate kinase 2 sensitizes ovarian carcinoma cells to multiple cancer therapeutics. Oncogene. 2002;21:1882–1889. doi: 10.1038/sj/onc/1205265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata E, et al. Inositol hexakisphosphate kinase-2, a physiologic mediator of cell death. J Biol Chem. 2005;280:1634–1640. doi: 10.1074/jbc.M409416200. [DOI] [PubMed] [Google Scholar]
- 13.Chakraborty A, et al. HSP90 regulates cell survival via inositol hexakisphosphate kinase-2. Proc Natl Acad Sci U S A. 2008;105:1134–1139. doi: 10.1073/pnas.0711168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bhandari R, Juluri KR, Resnick AC, Snyder SH. Gene deletion of inositol hexakisphosphate kinase 1 reveals inositol pyrophosphate regulation of insulin secretion, growth, and spermiogenesis. Proc Natl Acad Sci U S A. 2008;105:2349–2353. doi: 10.1073/pnas.0712227105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chakraborty A, et al. Inositol Pyrophosphates Inhibit Akt Signaling, Thereby Regulating Insulin Sensitivity and Weight Gain. Cell. 2010;143:897–910. doi: 10.1016/j.cell.2010.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo HR, et al. Inositol pyrophosphates mediate chemotaxis in Dictyostelium via pleckstrin homology domain-PtdIns(3,4,5)P3 interactions. Cell. 2003;114:559–572. doi: 10.1016/s0092-8674(03)00640-8. [DOI] [PubMed] [Google Scholar]
- 17.Iijima M, Huang YE, Devreotes P. Temporal and spatial regulation of chemotaxis. Dev Cell. 2002;3:469–478. doi: 10.1016/s1534-5807(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 18.Stephens L, Ellson C, Hawkins P. Roles of PI3Ks in leukocyte chemotaxis and phagocytosis. Curr Opin Cell Biol. 2002;14:203–213. doi: 10.1016/s0955-0674(02)00311-3. [DOI] [PubMed] [Google Scholar]
- 19.Ridley AJ, et al. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 20.Lemmon MA. Phosphoinositide recognition domains. Traffic. 2003;4:201–213. doi: 10.1034/j.1600-0854.2004.00071.x. [DOI] [PubMed] [Google Scholar]
- 21.Subramanian KK, et al. Tumor suppressor PTEN is a physiologic suppressor of chemoattractant-mediated neutrophil functions. Blood. 2007;109:4028–4037. doi: 10.1182/blood-2006-10-055319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jia Y, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates PtdIns(3,4,5)P3 signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parent CA, Blacklock BJ, Froehlich WM, Murphy DB, Devreotes PN. G protein signaling events are activated at the leading edge of chemotactic cells. Cell. 1998;95:81–91. doi: 10.1016/s0092-8674(00)81784-5. [DOI] [PubMed] [Google Scholar]
- 24.Thomas CC, Deak M, Alessi DR, van Aalten DM. High-resolution structure of the pleckstrin homology domain of protein kinase b/akt bound to phosphatidylinositol (3,4,5)-trisphosphate. Curr Biol. 2002;12:1256–1262. doi: 10.1016/s0960-9822(02)00972-7. [DOI] [PubMed] [Google Scholar]
- 25.Dinauer MC. Chronic granulomatous disease and other disorders of phagocyte function. Hematology Am Soc Hematol Educ Program. 2005:89–95. doi: 10.1182/asheducation-2005.1.89. [DOI] [PubMed] [Google Scholar]
- 26.Matute JD, Arias AA, Dinauer MC, Patino PJ. p40phox: the last NADPH oxidase subunit. Blood Cells Mol Dis. 2005;35:291–302. doi: 10.1016/j.bcmd.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 27.Dahlgren C, Johansson A, Orselius K. Difference in hydrogen peroxide release between human neutrophils and neutrophil cytoplasts following calcium ionophore activation. A role of the subcellular granule in activation of the NADPH-oxidase in human neutrophils? Biochim Biophys Acta. 1989;1010:41–48. doi: 10.1016/0167-4889(89)90182-1. [DOI] [PubMed] [Google Scholar]
- 28.Dinauer MC, Orkin SH. Chronic granulomatous disease. Annu Rev Med. 1992;43:117–124. doi: 10.1146/annurev.me.43.020192.001001. [DOI] [PubMed] [Google Scholar]
- 29.Parekh DB, Ziegler W, Parker PJ. Multiple pathways control protein kinase C phosphorylation. Embo J. 2000;19:496–503. doi: 10.1093/emboj/19.4.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baumer AT, et al. Phosphatidylinositol 3-kinase-dependent membrane recruitment of Rac-1 and p47phox is critical for alpha-platelet-derived growth factor receptor-induced production of reactive oxygen species. J Biol Chem. 2008;283:7864–7876. doi: 10.1074/jbc.M704997200. [DOI] [PubMed] [Google Scholar]
- 31.Chen Q, et al. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 2003;170:5302–5308. doi: 10.4049/jimmunol.170.10.5302. [DOI] [PubMed] [Google Scholar]
- 32.Padmanabhan U, Dollins DE, Fridy PC, York JD, Downes CP. Characterization of a selective inhibitor of inositol hexakisphosphate kinases: use in defining biological roles and metabolic relationships of inositol pyrophosphates. J Biol Chem. 2009;284:10571–10582. doi: 10.1074/jbc.M900752200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brown GE, Stewart MQ, Liu H, Ha VL, Yaffe MB. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC delta in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 34.Li Y, et al. Targeted deletion of tumor suppressor PTEN augments neutrophil function and enhances host defense in neutropenia-associated pneumonia. Blood. 2009;113:4930–4941. doi: 10.1182/blood-2008-06-161414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark RA, Klebanoff SJ. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979;64:913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segal BH, Kuhns DB, Ding L, Gallin JI, Holland SM. Thioglycollate peritonitis in mice lacking C5, 5-lipoxygenase, or p47(phox): complement, leukotrienes, and reactive oxidants in acute inflammation. J Leukoc Biol. 2002;71:410–416. [PubMed] [Google Scholar]
- 37.Lekstrom-Himes JA, Kuhns DB, Alvord WG, Gallin JI. Inhibition of human neutrophil IL-8 production by hydrogen peroxide and dysregulation in chronic granulomatous disease. J Immunol. 2005;174:411–417. doi: 10.4049/jimmunol.174.1.411. [DOI] [PubMed] [Google Scholar]
- 38.Heit B, et al. PTEN functions to `prioritize' chemotactic cues and prevent `distraction' in migrating neutrophils. Nat Immunol. 2008;9:743–752. doi: 10.1038/ni.1623. [DOI] [PubMed] [Google Scholar]
- 39.Liu L, Puri KD, Penninger JM, Kubes P. Leukocyte PI3Kgamma and PI3Kdelta have temporally distinct roles for leukocyte recruitment in vivo. Blood. 2007;110:1191–1198. doi: 10.1182/blood-2006-11-060103. [DOI] [PubMed] [Google Scholar]
- 40.Ferguson GJ, et al. PI(3)Kgamma has an important context-dependent role in neutrophil chemokinesis. Nat Cell Biol. 2007;9:86–91. doi: 10.1038/ncb1517. [DOI] [PubMed] [Google Scholar]
- 41.Lee YS, Mulugu S, York JD, O'Shea EK. Regulation of a cyclin-CDK-CDK inhibitor complex by inositol pyrophosphates. Science. 2007;316:109–112. doi: 10.1126/science.1139080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voglmaier SM, et al. Inositol hexakisphosphate receptor identified as the clathrin assembly protein AP-2. Biochem Biophys Res Commun. 1992;187:158–163. doi: 10.1016/s0006-291x(05)81473-1. [DOI] [PubMed] [Google Scholar]
- 43.Norris FA, Ungewickell E, Majerus PW. Inositol hexakisphosphate binds to clathrin assembly protein 3 (AP- 3/AP180) and inhibits clathrin cage assembly in vitro. J Biol Chem. 1995;270:214–217. doi: 10.1074/jbc.270.1.214. [DOI] [PubMed] [Google Scholar]
- 44.Ye W, Ali N, Bembenek ME, Shears SB, Lafer EM. Inhibition of clathrin assembly by high affinity binding of specific inositol polyphosphates to the synapse-specific clathrin assembly protein AP-3. J Biol Chem. 1995;270:1564–1568. [PubMed] [Google Scholar]
- 45.Saiardi A, Bhandari R, Resnick AC, Snowman AM, Snyder SH. Phosphorylation of proteins by inositol pyrophosphates. Science. 2004;306:2101–2105. doi: 10.1126/science.1103344. [DOI] [PubMed] [Google Scholar]
- 46.Bhandari R, et al. Protein pyrophosphorylation by inositol pyrophosphates is a posttranslational event. Proc Natl Acad Sci USA. 2007;104:15305–15310. doi: 10.1073/pnas.0707338104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan BL, et al. Genetic evidence for convergence of c-Kit- and alpha4 integrin-mediated signals on class IA PI-3kinase and the Rac pathway in regulating integrin-directed migration in mast cells. Blood. 2003;101:4725–4732. doi: 10.1182/blood-2002-08-2521. [DOI] [PubMed] [Google Scholar]
- 48.Li Z, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 49.Zhu D, et al. Deactivation of phosphatidylinositol 3,4,5-trisphosphate/Akt signaling mediates neutrophil spontaneous death. Proc Natl Acad Sci USA. 2006;103:14836–14841. doi: 10.1073/pnas.0605722103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia Y, et al. Inositol 1,3,4,5-tetrakisphosphate negatively regulates phosphatidylinositol-3,4,5-trisphosphate signaling in neutrophils. Immunity. 2007;27:453–467. doi: 10.1016/j.immuni.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.