Abstract
In West Africa, lineage splitting between the M and S molecular forms of the major Afro-tropical malaria mosquito, Anopheles gambiae is thought to be driven by ecological divergence, occurring mainly at the larval stage. Here, we present evidences for habitat segregation between the two molecular forms in and around irrigated rice-fields located within the humid savannahs background of western Burkina Faso. Longitudinal sampling of adult mosquitoes emerging from a range of breeding sites distributed along a transect extending from the heart of the rice-fields area into the surrounding savannahs was conducted from June to November 2009. Analysis revealed that the two molecular forms and their sibling species An. arabiensis are not randomly distributed in the area. A major ecological gradient was extracted, in relation to the rice-fields perimeter. The M form was associated with larger breeding sites, which were mainly represented by rice field paddies whereas the S form and An. arabiensis were found to depend upon temporary, rain-filled breeding sites. These results support hypotheses about larval habitat segregation and confirm that both forms have different larval habitat requirement. Segregation appears clearly linked to anthropogenic permanent habitats and the community structure they support.
Keywords: Anopheles gambiae, segregation, larval habitat, hydro-periodicity, community structure, niche partitioning
Introduction
Environmental changes by habitat modification often expose populations to new ecological constraints including variations in climatic conditions, interspecies interactions, resource availability and other biotic and abiotic components of their ecosystem (Schluter, 2001). Divergent selection acts on species evolving under contrasting environments, and may ultimately result in reproductive isolation through a process known as ecological speciation (Schluter, 2009). Although demonstrating ecological speciation as a by-product of local adaptation has been proven difficult in the field (Nosil et al., 2009) there are now multiple examples of rapid speciation due to ecological diversification in the literature (see Schluter, 2001; Mallet, 2008; Nosil et al., 2009; Schluter, 2009 for reviews) and it has received experimental support in various model organisms (Rice & Hostert, 1993; Funk, 1998; Funk et al., 2002). Such process could be prompted by environmental modifications which modify ancestral habitats and offer new ecological niches to resident, as well as invasive species.
Today, human activities are the main causes of ecosystem disturbance driving species towards adaptation, or extinction (Palumbi, 2001). In West Africa, man-made environmental modifications have been suspected to be at the origin of the diversification and radiation of the major human malaria vector, the mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) through the creation of new ecological niches in marginal habitats (Coluzzi et al., 1985; Coluzzi, 1999). The spread of new agricultural practices combined with deforestation and creation of water reserves has been hypothesized to be the original environmental change prompting ecological niche specialization within An. gambiae s.s. through divergent selection in these new habitats (Coluzzi et al., 2002). Understanding vector species’ distribution and their dynamics in relation to their fluctuating environment is of paramount importance to better understand the epidemiology of the diseases these mosquitoes transmit, predict their evolution in the face of ongoing global environmental changes, and eventually improve their control (Fontenille & Simard, 2004).
Anopheles gambiae s.s. is split into two molecular forms known as ‘M’ and ‘S’, which are morphologically identical and largely sympatric throughout West Africa (della Torre et al., 2001; della Torre et al., 2005), but show widespread molecular differences throughout their genomes (Lawniczak et al., 2010; Neafsey et al., 2010; White et al., 2010). Although the two molecular forms share the same resources such as vertebrate hosts or freshwater habitats, they have been shown to diverge in some of their biological and ecological requirements (Coluzzi et al., 1985; Touré et al., 1994; Lehmann & Diabaté, 2008; Costantini et al., 2009; Simard et al., 2009). In the dry savannahs of West Africa, the S form preferentially breeds in temporary aquatic habitats and is found during the rainy season only, whereas the M form is present all year round, breeding in man-made permanent aquatic habitats (Costantini et al., 2009; Simard et al., 2009). Such differences in the spatial and temporal distribution of these vector mosquitoes have implications for the epidemiology of malaria transmission: in areas where irrigated agricultural management provides permanent breeding opportunities, malaria is potentially transmitted throughout the year. However, although this ecological segregation between breeding sites is generally accepted, a quantitative framework formalizing such observation is still lacking.
In order to better understand the ecological determinants of larval habitat segregation between the molecular forms of An. gambiae, we undertook a longitudinal entomological survey in an area of extensive rice cultivation in Southwestern Burkina Faso (West Africa), where both molecular forms of An. gambiae occur together with their sibling species, Anopheles arabiensis. The main objectives of this study were (i) to determine the spatial distribution of An. gambiae sensu lato (s.l.) mosquitoes along a lentic (e.g., still or slow-moving) freshwater gradient during the rainy season and (ii) to associate environmental parameters with mosquito occurrence in order to characterize the ecological factors explaining habitat segregation at a local geographical scale. Systematic sampling of adults emerging from a range of larval development sites allowed us to depict the distribution pattern of An. gambiae s.l. mosquitoes in and around the rice cultivation area and to identify relevant biotic and abiotic factors that shape the ecological niche of these taxa.
Materials and Methods
Study site and sampling scheme
The study was conducted in the vicinity of Bama (11°23' N, 4°24' W), a village of the Kou Valley, situated 30 km north of Bobo Dioulasso, Southwestern Burkina Faso (Figure 1). The village is surrounded by 1,200 ha of irrigated rice-fields embedded within a typical Guinean savannah background. The rainy season extends from May to October in the area, with a yearly rainfall averaging 1,200 mm (Costantini et al., 2009). The M form of An. gambiae is present throughout the year in high densities in the rice fields. In surrounding savannahs, the S form is typically found in great numbers during the rainy season, together with An. arabiensis (Diabaté et al., 2002; Baldet et al., 2003; Costantini et al., 2009; Lefèvre et al., 2009).
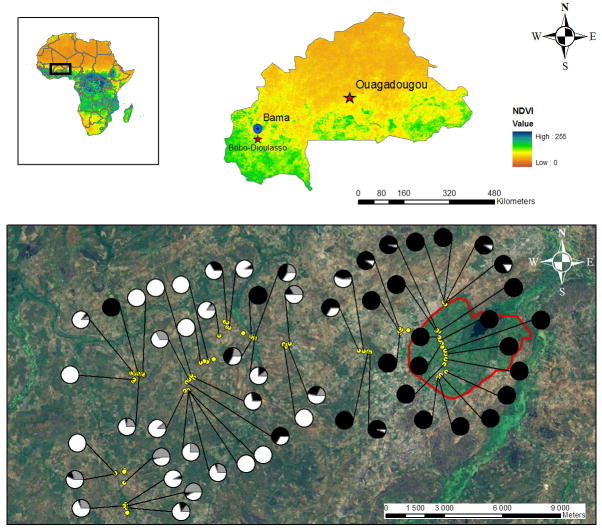
Figure 1.
Relative frequency and distribution of members of the An. gambiae complex emerging from breeding sites in the area of Bama, Western Burkina Faso.
Above: position of Burkina Faso in Africa and localization of the area of Bama (blue dot) in Burkina Faso (Source NDVI: http://free.vgt.vito.be/index.php).
Below: the area of Bama (Wellens et al., 2008) showing the rice-fields perimeter (red) and the 80 breeding sites (yellow dots) monitored throughout the 2009 rainy season. Pie charts show the relative frequencies of the members of the An. gambiae s.l. complex emerging from each larval development site (data pooled across collections dates). The M form is in black, the S form is in white and An. arabiensis is in grey.
The sampling plan was conceived from maps of the study area constructed from road maps (1:1,000,000), low altitudinal aerial photographs at 0.8m resolution (Wellens et al., 2008) and field observations. In order to explore the distribution and ecological niche requirements of both molecular forms of An. gambiae and An. arabiensis, we focused on adult mosquitoes emerging from breeding sites, as these represent the outcome of larval life, reflecting its specific selective pressures. Eighty breeding sites were chosen along a 15 km-long East-West transect ranging from the core of the rice fields perimeter to the surrounding savannah. Among these, 15 breeding site were located within the rice fields perimeter, and 65 were located in surrounding savannah (Figure 1). Each larval habitat was geo-referenced (Garmin GPS Map 60cx, Garmin International Inc., Olathe, US) and sampled every other week from June to November 2009, until all temporary larval development sites had dried out, resulting in a total of 960 samplings (i.e., 80 water collections sampled 12 times each in the course of the study).
Mosquito collection
Emergence traps covering 1m2 of ground surface were set out in larval breeding sites for twenty four hours. Fifteen aquatic habitats were sampled daily during one week to cover the whole sampling area (i.e., 80 breeding sites). The same procedure was repeated every other week. Emerging mosquitoes were collected from the trap using a “CDC backpack aspirator” (Clark et al., 1994). They were sexed and identified morphologically in the field (Gillies & De Meillon, 1968) and stored at −20°C in individual tubes until further use in the laboratory. All mosquitoes from the An. gambiae complex were identified to species and molecular form using a recently developed PCR assay (Santolamazza et al., 2008). However, when sample sizes per trap were above 20, only 20 randomly selected specimens were processed and the results were extrapolated to the remainder of the trap sample.
Breeding site identification and recording of ecological parameters
To record habitat type, every water collection was categorized as one of the following: stream pools, rice paddies, quarries and road ruts. Nominal environmental variables (biotic and abiotic) used to characterize aquatic habitats were: the presence of floating (mainly Marsilea polycarpa and Nymphea sp.) and emerging plants, algae, predators (mainly Notonectidae and Dytiscidae), Culex larvae (mainly Culex quinquefasciatus), floating debris, and turbidity (“turbid”, when the ground of the breeding site was not visible). General surroundings were characterized by three nominal variables: village, savannah and culture. All these nominal variables were represented by dummy variables (Boolean) for subsequent analyses (below). Assessment of these environmental factors were done visually and always by the same person for consistency. Continuous variables included the surface (in m2) and depth (in cm) of the habitat and distances (in m) to the nearest house and to the rice fields. The two former variables were assessed using a tape and the two later variables were estimated from a Geographical Information System (ArcGis v9.3). Collection month was represented by a series of dummy variables from June to November.
Statistical Analysis
Statistical analysis was carried out on aquatic habitats where at least one emerging adult of An. gambiae s.l. was collected. To explore whether the M and S forms of An. gambiae and their sibling species An. arabiensis were randomly distributed or ecologically structured in our study area, we performed a statistical randomization of the original occurrence data by simulating 5,000 random matrices using the computer program EcoSim (Gotelli & Entsminger, 2010). We used the C-score (Stone & Roberts, 1990), Ecosim’s default co-occurrence index that measures the average number of checkerboard units between all possible pairs of species in a co-occurrence matrix. We used EcoSim’s recommended randomization algorithm, which maintains fixed sums for rows and fixed sums for columns so that each generated matrix has the same number of species and the same number of samples as the original one (Connor & Simberloff, 1979). If a community is ecologically structured, the C-score should be greater than expected by chance (Gotelli, 2000). This algorithm has a low chance of falsely rejecting the null hypothesis (Type I error) (Gotelli, 2000). To illustrate species distribution in our study area, we performed a logistic regression model using species relative frequencies as response data and distance to the rice-fields perimeter as spatial gradient.
The relationships between the species pattern and the environmental variables measured in the field were analysed using the multivariate CANOCO v.4.5 procedure and the results were visualised with the extension CanoDraw for Windows (Ter Braak & Smilaurer, 2002). This multivariate method allows the identification of a subset of environmental parameters that best correlate to the biotic structure and may thus be assumed to strongly affect the species pattern. Nominal variables were defined by series of indicators or dummy variables (one per category). A dummy variable takes the value of 1 when the sample belongs to a given category and 0 otherwise. Prior to analysis, species data were log-transformed. Principal Component Analysis (PCA) was first used to describe the general patterns of variance in species data. This analysis did not include any explanatory variable but allowed their inclusion as passive variables. The distribution of the variability in species was visually checked to assess the existence of any pattern. Then, inferential analyses were completed using Redundancy Analysis (RDA), which was used to estimate how much variation in the species data was attributable to environmental variables. The forward-selection option was used to determine the minimal set of environmental parameters that could explain the largest amount of variation in species data. At each step, the statistical significance of the environmental variable added in the course of the forward selection was tested by means of unrestricted Monte Carlo permutation test (n=499). Then, a partial RDA analysis was performed with spatial and seasonal effects as covariates in order to identify other significant environmental factors that could be hidden by space and time effects.
Results
Species co-occurrence
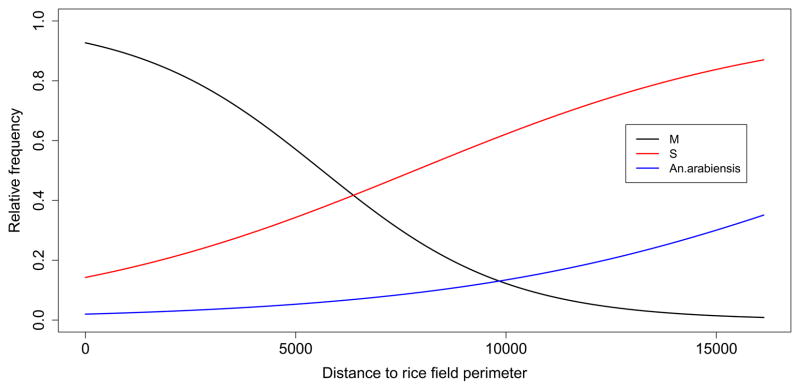
Among 960 traps settled during the survey, 125 were positive for emerging An. gambiae s.l. mosquitoes. Some of the putative larval habitats covered were negative all throughout the survey. In total, 1,364 An. gambiae s.l. were collected (591 males and 773 females) and 826 were identified by PCR (out of 828 specimens tested, two of which repeatedly failed to amplify). Overall, the M form accounted for 31% of the specimens collected, 55% were S form and 14% were An. arabiensis. One putative hybrid specimen between the M form and An. arabiensis was recorded and no MS hybrid pattern was observed. Species co-occurrence analysis indicated significant differences between the C-scores (C-scoreObs>C-scoreEst, P=0.042) suggesting that members of the An. gambiae complex were not randomly distributed in the area. Logistic plot using distance to the rice-fields perimeter as a spatial explanatory factor showed that the three taxa were distributed in relation to the distance to the rice fields (Figure 2). The M form was mainly found within the irrigated perimeter where it represented 99% of emerging mosquitoes, whereas the S form and An. arabiensis were found in the surrounding areas.
Figure 2.
Logistic plot of relative frequencies of the M and S form of An. gambiae and An. arabiensis in relation to distance to the rice-fields perimeter.
Gradient analysis
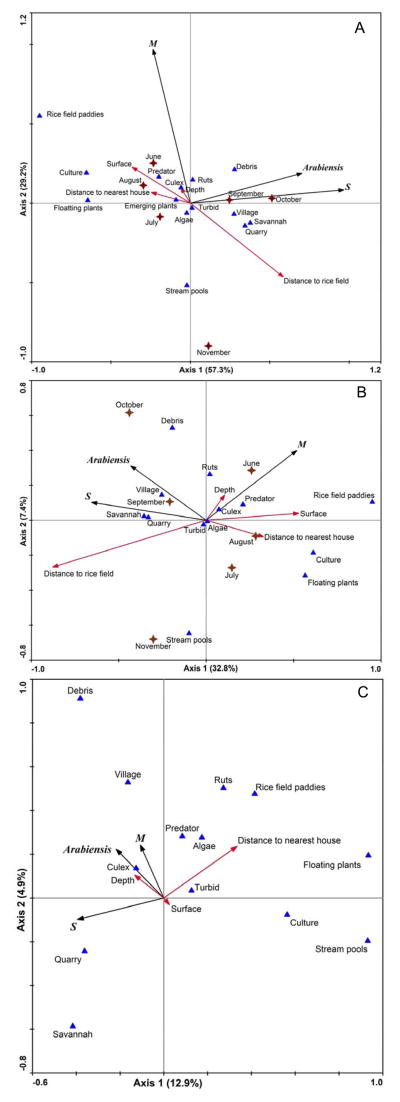
Principal component analysis was used to analyse the distribution and mutual relationships between taxa and samples. The graphic representation showed association between An. gambiae S form and An. arabiensis suggesting that these species had similar ecological requirements (Figure 3A). This group mapped together with quarries on the right side of the first axis, and is located far away from the rice fields in savannah and village areas. This is also the area of the graph where the last months of the rainy season, September and October are found. The M form was independent of the first axis and mainly correlated with the second axis. It mapped in the upper part of the ordination diagram, which represents complex habitats such as wide rice field paddies with predators and Culex larvae. The first two axes of the ordination diagram explained 86.5% of the variability in species data.
Figure 3.
Multifactorial analysis of the distribution of An. gambiae complex mosquitoes in relation to environmental variables. Principal component analysis (PCA) diagram is shown in A with environmental variables passively plotted. Ordination diagram from redundancy analysis (RDA) is shown in B and species-environment biplot from partial redundancy analysis (RDA) in C. For each graph the proportion of total variance in species data explained by each axis is given in brackets. Blue triangles represent nominal environmental factors and red arrows represent continuous ones. Mosquito taxa are represented by black arrows and collection months by brown crosses.
Redundancy analysis showed that mosquito taxa segregated mainly along the first two axes which accounted for 32.8% and 7.4% of the variance in species data, respectively, and 73.6% and 16.7% of the species-environment correlation, respectively (Figure 3B). The correlation between species and environmental variables along these axes was high (r2=0.78) for the first axis and moderate (r2=0.49) for the second axis. The first axis defined a spatial gradient where species segregated from the core of the rice field perimeters to the surrounding villages and savannah areas. This gradient supports distinct ecosystems. Positive values of the axis represented complex and permanent habitats constituted of rice fields with a large surface colonized by floating plants, predators and found far from human dwellings. Negative values represented sites found far from the rice-fields perimeter in surrounding savannah and villages where mosquitoes exploit quarries. The second axis was influenced by stream pools and floating plants on negative values and by debris on positive ones. The M form was associated with the early months of the rainy season from June to August whereas the S form and An. arabiensis were associated with the late months, September and October.
Six variables were significantly associated with mosquitoes distribution: distance to the rice-fields perimeter (P=0.002), floating plants (P=0.002), culture (P=0.048), distance to the nearest house (P=0.012), rice field habitat (P=0.046) and the month of October (P=0.006). The rice field habitat, presence of floating plants, culture and distance to the nearest house were the best predictors of the M form larval habitat, whereas distance to the rice-fields perimeter and late collection month (October) appeared to be predictors for the S form and An. arabiensis.
Partial RDA (collection month and distance to the rice fields considered as covariates) highlighted the additional effects of other explanatory variables potentially hidden by the main effects of distance to the rice fields and collection month. Analyses showed that mosquito taxa segregated along the first two axes which accounted for 12.9% and 4.9% of the variance in species data, respectively, and for 68.5% and 25.8% of the species-environment correlation, respectively (Figure 3C). This strong reduction in the proportion of total variance explained by the environmental factors highlights the major effects of collection month and distance to the rice fields on mosquitoes distribution in our study area. The correlation between species and environmental variables along the first and second axes was moderate (r2=0.47 and r2=0.46, respectively). Again, the first axis reflects a habitat complexity gradient. Positive values represent complex aquatic ecosystems, constituted by rice fields and stream pools which support high densities of predators, algae and floating plants and are found far from human dwellings. Negative values represent quarries found in savannah areas which seem to be less complex habitats with regard to the communities they support. The second axis could be considered as an anthropogenic gradient. Positive values represented environmental variables that characterize habitat degradation, such as the presence of debris, villages and ruts. Negative values represent more rural and feral savannah areas. The M form and An. arabiensis clustered along positive values of the second axis, which suggests that both taxa are more adapted to disturbed habitats unlike the S form, which appears to be a savannah mosquito exploiting habitats with simple community structures. Two significant variables explained taxa distribution: floating plants (P=0.004) and culture (P=0.034), mainly negatively correlated to the S form.
Discussion
According to Hutchinson (1978), in order to coexist, two species must have some differences in their ecological niche requirements or in some aspect of their life histories. Our survey showed that, at a microgeographic scale, the three sympatric taxa of the Anopheles gambiae complex are not randomly distributed among aquatic habitats and that distance to the rice-fields perimeter appears as a structuring abiotic factor shaping their ecological niches. Furthermore, multifactorial analyses showed that the complexity of communities’ arrangements in aquatic ecosystems is a key ecological factor modulating niche partitioning between these taxa.
Because community structure across freshwater habitat gradients is known to depend mainly upon physical factors such as pond drying and biotic effects mediated by ecological interactions such as predation and competition (Wellborn et al., 1996), our study was based on the collection of mosquitoes emerging from larval development sites. Emergence is the endpoint of mosquito larval development. Our approach therefore has the advantage to provide a picture of the mosquito population that successfully develops and emerges under different and specific selection pressures occurring in various larval habitats. The relevance of this sampling method is further reinforced by recent studies demonstrating different levels of susceptibility to predation between molecular forms of An. gambiae (Diabaté et al., 2008; Gimonneau et al., 2010). One drawback however, is that sample sizes are lower than when larval immature specimens are directly collected in the breeding sites, both in terms of the number of positive collections (i.e., the number of collections where at least one adult An. gambiae specimen is present) as well as in the number of specimens collected per sampling unit. This obviously prompts for increased sampling effort in order to gain statistical power. However, the fact that only 125 out of 960 traps (i.e., 13%) were positive for emerging An. gambiae s.l. mosquitoes is unlikely to have biased our results and inferences, because all taxa should be exposed to the same probability of sampling upon emergence. Variations in eggs hatching time and larval development time within and between species and molecular forms in the An. gambiae complex as well as across water types (Diabaté et al., 2005; Yaro et al., 2006; Diabaté et al., 2008) result in the distribution of emerging mosquitoes across several days, ensuring representative sampling of emerging mosquitoes throughout a 24h period. PCA identified a pattern of association between the S form and An. arabiensis, whereas the M form was independent from this group. A high proportion of species variance was explained along the first two axes (86.5%). The S form and An. arabiensis clustered on the first axis, indicating that these taxa seem to segregate in relation to distance to the rice-fields perimeter. Conversely, the M form was mainly associated with the second axis which negatively correlated with distance to the rice fields and, as a consequence, positively correlated with rice-fields habitat. This suggests that the spatial pattern represented by the distance to the rice-fields perimeter acts as a major environmental factor in structuring the ecological niches of members of the An. gambiae complex.
This result was confirmed by redundancy analysis. Distance to the rice-fields perimeter was the most significant factor along which species segregated. Other significant environmental factors representative of the irrigated perimeter were highlighted by RDA such as the presence of floating plants, distance to the nearest house, culture and rice-fields habitat. These key environmental parameters were all associated with the presence of the M form. The significant association with floating plants (mainly Marsilea polycarpa and Nymphea sp.) is certainly the most important ecological factor as it could be considered as a bio-marker of habitat stability and more generally, of habitat complexity because plants offer refuges allowing many species to escape predation (Sih, 1987). Habitat complexity can be illustrated by the presence of predators (mainly Notonectidae and Dytiscidae) and Culex larvae (mainly Culex quinquefasciatus) which were found in high densities in the rice paddies. The high diversity found in this kind of habitat (Roger, 1996; Bambaradeniya et al., 2004) favours and sustains, in turn, complex trophic relationships and multiple biological interactions between species (Menge & Sutherland, 1976).
Temporal pattern was significant in October and associated with the presence of the S form and An. arabiensis which were found to exploit the same breeding sites at the end of the rainy season. This reflects a population turn-over during the late rainy season (Diabaté et al., 2002), where 51% of all An. arabiensis specimens were collected in October. This also suggests ecological niche overlap driven by the scarcity of temporary breeding sites available for females’ oviposition in savannah areas at the end of the rainy season. Although the M form was present all through the year in the locality of Bama (Diabaté et al., 2002; Baldet et al., 2003), this mosquito was only associated with the early month (June to August) of the rainy season. This pattern may be linked to the rice cultivation cycle, where rice field paddies are more suitable for An. gambiae development during the early phases of crop cultivation (Baldet et al., 2003).
The ordination diagram from RDA also shows that the S form and An. arabiensis tend to exploit temporary pools in savannah areas and villages found far away from the rice-fields perimeter. Their preferred habitat types are generally vegetation free (73% without floating plants for the S form and 85% for An. arabiensis) indicating that these taxa exploit simple rain-dependant temporary aquatic habitats. This is consistent with previous observations in Mali, where species’ ecological niches seem to segregate according to habitat type and temporality (Edillo et al., 2002). Here, our results identified distance to the rice-fields perimeter as the main physical factor modulating distribution of species and molecular forms. However, this dimension also has an ecological value, because these taxa segregated through a habitat complexity gradient supported by hydro-periodicity of the larval habitat. As a result, distance to the rice fields perimeter represents an ecological cline where a range of breeding sites were represented by permanent and complex aquatic habitat at one end and by temporary and simpler habitat to the other end. This ecological gradient which extends from permanent to temporary freshwater habitats has long been recognized critical in shaping aquatic communities structure in relation to the ecological interactions they support (Schneider & Frost, 1996; Wellborn et al., 1996).
As a result, the rice-fields perimeter appears to be the core habitat of the M form from which it successfully emerges and spreads into the surrounding savannahs and villages where the S form and An. arabiensis are thriving. This result confirms previous studies showing that the M form outcompetes the S form in permanent aquatic habitats (Diabaté et al., 2008). Predation pressure is more important in permanent habitats than in temporary ones (Sunahara et al., 2002; Diabaté et al., 2008) and was clearly identified as a biotic process that contributes to the ecological niche segregation between forms (Gimonneau et al., 2010). However, we could expect that An. arabiensis and the M form share a part of their larval niche requirements as in East Africa, where the M form is absent, An. arabiensis exploits rice paddies (Muturi et al., 2008). These differences in species distribution between East and West Africa probably reflect different evolutionary paths mediated by species interactions in these different geographical contexts. In West Africa, interaction with the M form in permanent habitats may have led to ecological niche displacement for An. arabiensis. Although competitive exclusion between these species has never been documented, this observation suggests that the M form may be more competitive than its sibling species in this kind of habitat in West Africa. It is noticeable indeed, that An. arabiensis -- as well as the S form of An. gambiae -- populations from West and East Africa (i.e, on both sides of the Great Rift Valley) are equipped with different chromosomal inversions arrangements, and might therefore represent distinct ecotypes populating distinct ecological niches in West and East Africa (Petrarca et al., 2000; Coluzzi et al., 2002; Pombi et al., 2008).
However, the M form and An. arabiensis seem well adapted to anthropogenic contexts as they segregated together along a human disturbance gradient in partial RDA. In East and West Africa, studies have shown that An. arabiensis can breed in rice paddies (Muturi et al., 2008) and urban areas (Robert et al., 1998; Jacob et al., 2003; Machault et al., 2009). In Burkina Faso, it was recently shown that both species could be found in peri-urban and urban areas in polluted larval habitats (Fournet et al., 2010). Moreover, the association with Culex larvae which are commonly found in highly polluted aquatic habitats (Subra, 1981) reflects the anthropogenic nature of these breeding sites. These findings are in agreement with our results and suggest that An. gambiae M form and An. arabiensis are adapting to anthropogenic contexts. On the opposite, the S form appears to be a more feral mosquito mainly found in savannah areas and able to exploit quarries.
The fact that most species living in temporary habitat are absent from permanent ones (Wiggins et al., 1980) suggests that some factors such as predation and/or competition may prevent species from occupying habitats that are apparently suitable to their development. Susceptibility to predation has been shown to be one of the main forces structuring species among aquatic habitats (Wellborn et al., 1996). The M form, which is totally dependent on permanent habitats during the dry season, was shown to express predator-avoidance behaviour in predator-rich habitats (Gimonneau et al., 2010). On the other hand, more rapid larval development, as observed in the S form (Diabaté et al., 2005; Diabaté et al., 2008; Lehmann & Diabaté, 2008) represents a better adaptation to fluctuating habitats with a high risk of desiccation. Consequently, fitness success at one end of the gradient implies lower performance at other end of the gradient, which results in the observed distribution pattern for members of the An. gambiae complex.
This study allowed us to better understand the ecological requirements of sympatric taxa of the An. gambiae complex unevenly distributed among aquatic habitats in a savannah area of South Burkina Faso. Species and molecular forms sharply segregated along a gradient ranging from “permanent-anthropic” habitats, exploited by the M form, to “temporary-natural” habitats where the S form and An. arabiensis are found. Moreover, An. arabiensis is more associated with village areas suggesting that, as the M form, they are more adapted to anthropogenic conditions. Consequently, larval ecology of this species complex appears clearly linked to habitat hydro-periodicity and to the ecological communities they support in relation to anthropogenic activities. In the actual context of increasing food self-sufficiency in West Africa leading to dam building in rural and peri-urban areas for irrigated agriculture, understanding vector species distribution gains value for preventing malaria transmission in these new modified environments.
Acknowledgments
We thank Baudoin Dabiré, Boubacar Nikiema, Hervé Somda, and Pascal Yéyé for excellent assistance in field and laboratory work. We also thank Phillipe Cecchi, Carlo Costantini and Jérémy Bouyer for constructive discussions and Joost Wellens for GIS assistance. This work was supported in parts by a NIH grant R01-A1063508 to N.J. Besansky (Notre Dame University, USA) and by the French National Research Agency under the reference ANR-08-MIEN-006 to FS. GG was supported by a PhD fellowship from the Fondation pour la Recherche Médicale (FRM).
References
- Baldet T, Diabaté A, Guiguemde TR. Malaria transmission in 1999 in the rice field area of the Kou Valley (Bama), (Burkina Faso) Santé. 2003;13:55–60. [PubMed] [Google Scholar]
- Bambaradeniya CNB, Edirisinghe JP, De Silva DN, Gunatilleke CVS, Ranawana KB, Wijekoon S. Biodiversity associated with an irrigated rice agro-ecosystem in Sri Lanka. Biodiversity and Conservation. 2004;13:1715–1753. [Google Scholar]
- Clark GG, Seda H, Gubler DJ. Use of the Cdc Backpack Aspirator for Surveillance of Aedes aegypti in San-Juan, Puerto-Rico. Journal of the American Mosquito Control Association. 1994;10:119–124. [PubMed] [Google Scholar]
- Coluzzi M. The clay feet of the malaria giant and its African roots: Hypotheses and inferences about origin, spread and control of Plasmodium falciparum. Parassitologia (Rome) 1999;41:277–283. [PubMed] [Google Scholar]
- Coluzzi M, Petrarca V, Di deco MA. Chromosomal inversion intergradation and incipient speciation in Anopheles gambiae. Bollettino Di Zoologia. 1985;52:45–63. [Google Scholar]
- Coluzzi M, Sabatini A, della Torre A, Di Deco MA, Petrarca V. A Polytene Chromosome Analysis of the Anopheles gambiae Species Complex. Science. 2002;298:1415–1418. doi: 10.1126/science.1077769. [DOI] [PubMed] [Google Scholar]
- Connor EF, Simberloff D. The Assembly of Species Communities: Chance or Competition. Ecology. 1979;60:1132–1140. [Google Scholar]
- Costantini C, Ayala D, Guelbeogo W, Pombi M, Some C, Bassole I, et al. Living at the edge: biogeographic patterns of habitat segregation conform to speciation by niche expansion in Anopheles gambiae. BMC Ecology. 2009;9:16. doi: 10.1186/1472-6785-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- della Torre A, Fanello C, Akogbeto M, Dossouyovo J, Favia G, Petrarca V, et al. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Molecular Biology. 2001;10:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- della Torre A, Tu Z, Petrarca V. On the distribution and genetic differentiation of Anopheles gambiae s.s. molecular forms. Insect Biochemistry and Molecular Biology. 2005;35:755–759. doi: 10.1016/j.ibmb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Diabaté A, Baldet T, Chandre F, Akoobeto M, Guiguemde TR, Darriet F, et al. The role of agricultural use of insecticides in resistance to pyrethroids in Anopheles gambiae s.l. in Burkina Faso. Am J Trop Med Hyg. 2002;67:617–622. doi: 10.4269/ajtmh.2002.67.617. [DOI] [PubMed] [Google Scholar]
- Diabaté A, Dabire R, Heidenberger K, Crawford J, Lamp W, Culler L, et al. Evidence for divergent selection between the molecular forms of Anopheles gambiae: role of predation. BMC Evolutionary Biology. 2008;8:5. doi: 10.1186/1471-2148-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabaté A, Dabiré RK, Kim EH, Dalton R, Millogo N, Baldet T, et al. Larval Development of the Molecular Forms of Anopheles gambiae (Diptera: Culicidae) in Different Habitats: A Transplantation Experiment. J Med Entomol. 2005;42:548–553. doi: 10.1093/jmedent/42.4.548. [DOI] [PubMed] [Google Scholar]
- Edillo FE, Toure YT, Lanzaro GC, Dolo G, Taylor CE. Spatial and habitat distribution of Anopheles gambiae and Anopheles arabiensis (Diptera : Culicidae) in Banambani Village, Mali. Journal of Medical Entomology. 2002;39:70–77. doi: 10.1603/0022-2585-39.1.70. [DOI] [PubMed] [Google Scholar]
- Fontenille D, Simard F. Unravelling complexities in human malaria transmission dynamics in Africa through a comprehensive knowledge of vector populations. Comparative Immunology Microbiology and Infectious Diseases. 2004;27:357–375. doi: 10.1016/j.cimid.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Fournet F, Cussac M, Ouari A, Meyer PE, Toe H, Gouagna LC, et al. Diversity in anopheline larval habitats and adult composition during the dry and wet seasons in Ouagadougou (Burkina Faso) Malaria Journal. 2010;9:78. doi: 10.1186/1475-2875-9-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk DJ. Isolating a role for natural selection in speciation: Host adaptation and sexual isolation in Neochlamisus bebbianae leaf beetles. Evolution. 1998;52:1744–1759. doi: 10.1111/j.1558-5646.1998.tb02254.x. [DOI] [PubMed] [Google Scholar]
- Funk DJ, Filchak KE, Feder JL. Herbivorous insects: model systems for the comparative study of speciation ecology. Genetica. 2002;116:251–267. [PubMed] [Google Scholar]
- Gillies MT, De Meillon B. The Anophelinae of Africa South of the Sahara (Ethiopian zoogeographical region) The South African Institute for Medical Research; Johannesburg: 1968. [Google Scholar]
- Gimonneau G, Bouyer J, Morand S, Besansky NJ, Diabate A, Simard F. A behavioral mechanism underlying ecological divergence in the malaria mosquito Anopheles gambiae. Behav Ecol. 2010;21:1087–1092. doi: 10.1093/beheco/arq114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotelli NJ. Null model analysis of species co-occurrence patterns. Ecology. 2000;81:2606–2621. [Google Scholar]
- Gotelli NJ, Entsminger GL. EcoSim: Null models software for ecology. Version 7. Acquired Intelligence Inc. & Kesey-Bear; Jericho, VT 05465: 2010. http://garyentsminger.com/ecosim.htm. [Google Scholar]
- Hutchinson EG. What is a niche? In: Hutchinson EG, editor. An introduction to population ecology. Yale University Press; London: 1978. pp. 152–219. [Google Scholar]
- Jacob BG, Regens JL, Mbogo CM, Githeko AK, Keating J, Swalm CM, et al. Occurrence and distribution of Anopheles (Diptera : Culicidae) larval habitats on land cover change sites in urban Kisumu and urban Malindi, Kenya. Journal of Medical Entomology. 2003;40:777–784. doi: 10.1603/0022-2585-40.6.777. [DOI] [PubMed] [Google Scholar]
- Lawniczak MKN, Emrich SJ, Holloway AK, Regier AP, Olson M, White B, et al. Widespread Divergence Between Incipient Anopheles gambiae Species Revealed by Whole Genome Sequences. Science. 2010;330:512–514. doi: 10.1126/science.1195755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefévre T, Gouagna LC, Dabiré KR, Elguero E, Fontenille D, Renaud F, et al. Beyond Nature and Nurture: Phenotypic Plasticity in Blood-Feeding Behavior of Anopheles gambiae s.s. When Humans Are Not Readily Accessible. American Journal of Tropical Medicine and Hygiene. 2009;81:1023–1029. doi: 10.4269/ajtmh.2009.09-0124. [DOI] [PubMed] [Google Scholar]
- Lehmann T, Diabaté A. The molecular forms of Anopheles gambiae: A phenotypic perspective. Infection Genetics and Evolution. 2008;8:737–746. doi: 10.1016/j.meegid.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machault V, Gadiaga L, Vignolles C, Jarjaval F, Bouzid S, Sokhna C, et al. Highly focused anopheline breeding sites and malaria transmission in Dakar. Malaria Journal. 2009;8:138. doi: 10.1186/1475-2875-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallet J. Hybridization, ecological races and the nature of species: empirical evidence for the ease of speciation. Philosophical Transactions of the Royal Society B-Biological Sciences. 2008;363:2971–2986. doi: 10.1098/rstb.2008.0081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menge BA, Sutherland JP. Species Diversity Gradients: Synthesis of Roles of Predation, Competition, and Temporal Heterogeneity. American Naturalist. 1976;110:351–369. [Google Scholar]
- Muturi EJ, Muriu S, Shililu J, Mwangangi J, Jacob BG, Mbogo C, et al. Effect of rice cultivation on malaria transmission in central Kenya. American Journal of Tropical Medicine and Hygiene. 2008;78:270–275. [PubMed] [Google Scholar]
- Neafsey DE, Lawniczak MKN, Park DJ, Redmond SN, Coulibaly MB, Traore SF, et al. SNP Genotyping Defines Complex Gene-Flow Boundaries Among African Malaria Vector Mosquitoes. Science. 2010;330:514–517. doi: 10.1126/science.1193036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosil P, Harmon LJ, Seehausen O. Ecological explanations for (incomplete) speciation. Trends in Ecology & Evolution. 2009;24:145–156. doi: 10.1016/j.tree.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Palumbi SR. Evolution - Humans as the world's greatest evolutionary force. Science. 2001;293:1786–1790. doi: 10.1126/science.293.5536.1786. [DOI] [PubMed] [Google Scholar]
- Petrarca V, Nugud AD, Ahmed MAE, Haridi AM, Di Deco MA, Coluzzi M. Cytogenetics of the Anopheles gambiae complex in Sudan, with special reference to An. arabiensis: relationships with East and West African populations. Medical and Veterinary Entomology. 2000;14:149–164. doi: 10.1046/j.1365-2915.2000.00231.x. [DOI] [PubMed] [Google Scholar]
- Pombi M, Caputo B, Simard F, Di Deco M, Coluzzi M, Torre A, et al. Chromosomal plasticity and evolutionary potential in the malaria vector Anopheles gambiae sensu stricto: insights from three decades of rare paracentric inversions. BMC Evolutionary Biology. 2008;8:309. doi: 10.1186/1471-2148-8-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR, Hostert EE. Laboratory Experiments on Speciation - What Have We Learned in 40 Years. Evolution. 1993;47:1637–1653. doi: 10.1111/j.1558-5646.1993.tb01257.x. [DOI] [PubMed] [Google Scholar]
- Robert V, Awono-Ambene HP, Thioulouse J. Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera : Culcidae) in market-garden wells in urban Dakar, Senegal. Journal of Medical Entomology. 1998;35:948–955. doi: 10.1093/jmedent/35.6.948. [DOI] [PubMed] [Google Scholar]
- Roger PA. Biology and management of the floodwater ecosystem in ricefields. ORSTOM; Paris: 1996. [Google Scholar]
- Santolamazza F, Mancini E, Simard F, Qi Y, Tu Z, della Torre A. Insertion polymorphisms of SINE200 retrotransposons within speciation islands of Anopheles gambiae molecular forms. Malaria Journal. 2008;7:163. doi: 10.1186/1475-2875-7-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter D. Ecology and the origin of species. Trends in Ecology & Evolution. 2001;16:372–380. doi: 10.1016/s0169-5347(01)02198-x. [DOI] [PubMed] [Google Scholar]
- Schluter D. Evidence for Ecological Speciation and Its Alternative. Science. 2009;323:737–741. doi: 10.1126/science.1160006. [DOI] [PubMed] [Google Scholar]
- Schneider DW, Frost TM. Habitat duration and community structure in temporary ponds. Journal of the North American Benthological Society. 1996;15:64–86. [Google Scholar]
- Sih A. Prey Refuges and Predator Prey Stability. Theoretical Population Biology. 1987;31:1–12. [Google Scholar]
- Simard F, Ayala D, Kamdem G, Pombi M, Etouna J, Ose K, et al. Ecological niche partitioning between Anopheles gambiae molecular forms in Cameroon: the ecological side of speciation. BMC Ecology. 2009;9:17. doi: 10.1186/1472-6785-9-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L, Roberts A. The Checkerboard Score and Species Distributions. Oecologia. 1990;85:74–79. doi: 10.1007/BF00317345. [DOI] [PubMed] [Google Scholar]
- Subra R. Biology and Control of Culex pipiens quinquefasciatus Say, 1823 (Diptera, Culicidae) with Special Reference to Africa. Insect Science and Its Application. 1981;1:319–338. [Google Scholar]
- Sunahara T, Ishizaka K, Mogi M. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. Journal of Vector Ecology. 2002;27:8–20. [PubMed] [Google Scholar]
- Ter Braak CJF, Smilaurer P. CANOCO Reference Manual and Cano Draw for Windows user's Guide: software for canonical Community ordination (version 4.5) Microcomputer Power; New York: 2002. [Google Scholar]
- Touré YT, Petrarca V, Traore SF, Coulibaly A, Maiga HM, Sankare O, et al. Ecological genetic studies in the chromosomal form Mopti of Anopheles gambiae s-str in Mali, West-Africa. Genetica. 1994;94:213–223. doi: 10.1007/BF01443435. [DOI] [PubMed] [Google Scholar]
- Wellborn GA, Skelly DK, Werner EE. Mechanisms creating community structure across a freshwater habitat gradient. Annual Review of Ecology and Systematics. 1996;27:337–363. [Google Scholar]
- Wellens J, Diallo M, Dakouré D, Compaoré NF, Denis A, Tychon B. Utilisation des prises de vue aérienne à basse altitude pour le suivi des activités hydro-agricoles – Cas du Bassin du Kou. Proceedings of the Salon Africain d’Irrigation et Drainage; 2008. p. 8. [Google Scholar]
- White BJ, Cheng CD, Simard F, Costantini C, Besansky NJ. Genetic association of physically unlinked islands of genomic divergence in incipient species of Anopheles gambiae. Molecular Ecology. 2010;19:925–939. doi: 10.1111/j.1365-294X.2010.04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins GB, Mackay RJ, Smith IM. Evolutionary and ecological strategies of animals in annual temporary ponds. Arch Hydrobiol Suppl. 1980;58:97–206. [Google Scholar]
- Yaro AS, Dao A, Adamou A, Crawford JE, Ribeiro JMC, Gwadz R, et al. Anopheles gambiae Malaria Journal. 2006. The distribution of hatching time; p. 5. [DOI] [PMC free article] [PubMed] [Google Scholar]