Abstract
Medicinal interventions of limited efficacy are currently available for the treatment of glioblastoma multiforme (GBM), the most common and lethal primary brain tumor in adults. The eosinophil is a pivotal immune cell in the pathobiology of atopic disease that is also found to accumulate in certain tumor tissues. Inverse associations between atopy and GBM risk suggest that the eosinophil may play a functional role in certain tumor immune responses. To assess the potential interactions between eosinophils and GBM, human primary blood eosinophils were cultured with two separate human GBM-derived cell lines (A172, U87-MG) or conditioned media generated in the presence or absence of TNF-α. Results revealed differential eosinophil adhesion and increased survival in response to co-culture with GBM cell lines. Eosinophil responses to GBM cell line-conditioned media included increased survival, activation, CD11b expression and S100A9 release. Addition of GM-CSF neutralizing antibodies to GBM cell cultures or conditioned media reduced eosinophil adhesion, survival and activation, linking tumor cell-derived GM-CSF to the functions of eosinophils in the tumor microenvironment. Dexamethasone, which has been reported to inhibit eosinophil recruitment and shrink GBM lesions on contrast enhanced scans, reduced the production of tumor cell-derived GM-CSF. Furthermore, culture of GBM cells in eosinophil-conditioned media increased tumor cell viability, and generation of eosinophil-conditioned media in the presence of GM-CSF enhanced the effect. These data support the idea of a paracrine loop between GM-CSF producing tumors and eosinophil-derived growth factors in tumor promotion/progression.
Keywords: eosinophils, glioblastoma, GM-CSF
INTRODUCTION
Eosinophils are terminally differentiated granulocytic innate immune cells, originally characterized by Paul Ehrlich in 1879 (1). Of note, the main component of eosinophil primary granules was first described in Charcot and Robin’s 1853 post-mortem examination of a leukemia patient (2). Subsequent findings in asthmatic sputum by Leyden in 1872 resulted in the present day nomenclature of Charcot-Leyden crystals (3) and the first insight into a potential link between eosinophils and the inflammatory responses associated with cancer and asthma.
Blood and tissue eosinophils are now extensively reported in many types of human cancers (4, 5) and are well established contributors to the pathology of asthma and allergy (6). Cancer may form in response to chronic inflammation or promote inflammation though the activation of oncogenes (7). Although the role of eosinophils in these processes is not yet clear, eosinophil recruitment to the tumor microenvironment has been indicated to occur in response to necrosis, tumor secreted interleukin-5 (IL-5), IgE antibodies, and therapeutic treatment with IL-2, IL-4, or granulocyte macrophage-colony stimulating factor (GM-CSF) (5, 8–10). In asthma, the immune response has been characterized by early phase IgE-mediated activation of mast cells, the production of pro-inflammatory cytokines (e.g.: IL-2, IL-4, IL-5, GM-CSF) and the late phase recruitment of Th2 cells and eosinophils (11). Evidence of an inverse relationship between atopic disease and the development of a particular cancer, glioblastoma (12–15), suggests that the eosinophil and/or eosinophilic mediators may play a pivotal role in an anti-cancer response.
Glioblastoma multiforme (GBM) is the most common and lethal primary brain tumor in adults despite the available cancer treatments of surgical resection, radiotherapy and chemotherapy (16). Glioblastoma tumor cells are reportedly able to evade surgical, radiotherapeutic, chemotherapeutic and immunotherapeutic interventions by respectively infiltrating into the surrounding brain tissue, down-regulating tumor suppressor proteins, up-regulating DNA repair enzymes, and producing immunosuppressive cytokines (17). Notably, enhanced glioblastoma patient survival has been correlated with tissue eosinophilia in clinical trials involving postoperative treatments with IL-2 (18, 19). In animal models, transplanted glioblastoma tumor cells expressing a high level of IL-2, IL-4 or GM-CSF displayed enhanced survival, reduced tumor growth and significant eosinophil infiltrate compared to controls (20–22).
Eosinophil recruitment has also been indicated to occur in response to developing subdural hematomas (23) necrotic tissue (24), and radiotherapy (25), conditions known to exist in human primary GBM (16, 26). In patients with allergy and asthma, eosinophil recruitment involves cytokine (IL-3, IL-5, GM-CSF) priming in the peripheral blood that sensitizes eosinophilic adhesion molecules (CD11b/CD18, CD49d/CD29) to more effectively interact with adhesion ligands (intercellular adhesion molecule (ICAM)-1, vascular adhesion molecule (VCAM)-1) on the inflamed endothelia (27). Whether similar interactions occur in eosinophil recruitment to certain tumors is unclear. Understanding the distinct tumor microenvironments that encourage eosinophil infiltration may lead to more effective treatment parameters. Therefore, the aim of the present work was to examine potential paracrine interactions between human primary eosinophils and glioblastoma cells with a particular focus on the cytokine GM-CSF.
MATERIALS AND METHODS
Isolation of peripheral blood eosinophils
Peripheral blood was obtained from human allergic patients under informed consent. The study was approved by the University of Wisconsin-Madison Center for Health Sciences Human Subjects Committee. Eosinophils were purified from heparinized blood that was diluted with Hank’s buffered salt solution (HBSS, Mediatech, Manassas, VA) without Ca2+ and centrifuged for 20 min at 700 × g over 1.090 g/ml Percoll. A granulocyte fraction was obtained after removal of the plasma, mononuclear cell band, and Percoll. Granulocytes were then subjected to red blood cell lysis via hypotonic shock, washed with 4°C HBSS supplemented with 2% new born calf serum (Life Technologies Grand Island, NY), and incubated 40 min with magnetic beads coated with anti-CD16, anti-CD14, and anti-CD3 (Miltenyi Biotechnology; Auburn, CA) prior to negative selection with an AutoMACS separator (Miltenyi Biotechnology). The recovered mixture (>97% purity, > 98% viability) was evaluated by Giemsa’s-based Diff-Quik stain (Baxter Scientific Products, McGaw Park, IL) and trypan blue exclusion respectively.
Cell lines, cell culture and reagents
The A172 and U87-MG glioblastoma cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA). The H358 non-small cell lung cancer (NSCLC) and DU145 prostate carcinoma cell lines were kindly provided by Dr. Paul Harari and Dr. Wade Bushman (University of Wisconsin, Madison, WI), respectively. Eosinophils and cell lines were cultured in DMEM (Mediatech) supplemented with 10% cosmic calf serum (CCS, Hyclone, Logan, UT), 100 U/ml penicillin/streptomycin (Mediatech) at 37°C, 5% CO2. Cells, as indicated in the manuscript, were treated with GM-CSF (R&D Systems, Minneapolis, MN), TNF-α (R&D Systems), or dexamethasone (Sigma Chemical Co., St. Louis, MO).
Enzyme-linked immunosorbent assay (ELISA)
Monoclonal anti-GM-CSF antibodies (Clone 6804, R&D Systems, 1:1000) in 0.1 M sodium carbonate buffer, pH 9.6 were coated onto 96-well EIA/RIA plates (Costar, Corning, NY). Blocking buffer containing 1% bovine serum albumin (Sigma Chemical Co.) and 0.5% Tween®20 (Fischer Scientific, Pittsburg, PA) in phosphate buffered saline (PBS) was added to wells for 2 hr. Serial dilutions of GM-CSF standard (215-GM, R&D Systems) and cell-free supernatants were aliquoted and incubated at 4°C overnight. GM-CSF was detected with biotinylated GM-CSF antibodies (Clone 3209, R&D Systems, 1:1000) and subsequent exposure to strepavidin HRP-40 (Fitzgerald Industries International, Concord, MA). A colorimetric HRP substrate tetramethylbenzidine (TMB, Biofx Laboratories, Owings Mills, MD) was used to evaluate captured HRP activity and the enzymatic reaction was stopped with 0.18 M sulfuric acid. Optical density was determined on an ELX800 Universal Microplate Reader (BioTek Instruments, Inc., Winooski, VT). Absorbance was quantified at 450 nM, using 600 nM as a reference wavelength. GM-CSF concentrations were calculated by interpolation from a standard curve and all determinations were performed in triplicate.
Flow cytometric analysis of cell surface molecules
Tumor cell lines or eosinophils were suspended in 100 µl DMEM containing 1% CCS and treated (1 µg antibody / 1×106 cells) with unconjugated mouse anti-human ICAM-1 antibodies (Clone BBIG-I1(11C81), R&D systems), FITC-conjugated CD69 antibodies (Clone FN50, BD Biosciences Pharmingen, San Jose, CA), PE-conjugated CD11b antibodies (Clone ICRF44, BD Biosciences Pharmingen), or isotype control, and incubated for 30 min at 4°C. Cells were washed with 1 ml 1% CCS DMEM. For analysis of ICAM-1, which involved unconjugated primary antibodies, phycoerythrin (PE) goat anti-mouse antibodies (Invitrogen, Eugene, OR) were used as a secondary, and incubated for 30 min at 4° in the dark. Cells were suspended in PBS, treated with propidium iodide (3µg/ml) to exclude dead cells and analyzed at 10,000 events on a FACScan flow cytometer (Becton–Dickinson, Bedford, MA) at the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Core Facility. Data were analyzed with FlowJo data analysis software (TreeStar, Ashaland, OR).
Assay of eosinophil peroxidase activity (EPO)
Eosinophil adherence was determined by measuring the EPO activity of adherent cells using a modification of the methods previously described (28–30). In triplicate, 100 µl of media (10% CCS DMEM) or glioblastoma cells (5×105/ml) was added to 96-well tissue-culture plates (Sarstedt, Newton, NC). After 24 hr incubation, 10 ng/ml TNF-α or buffer control was added to media or glioblastoma cells for an additional 24 hr. At the 48 hr time point, 100 pg/ml GM-CSF, 10 µg/ml GM-CSF neutralizing antibodies (Clone BVD2-23B6, Invitrogen) or isotype control antibodies were added for 1hr. Eosinophils were suspended in HBSS (Mediatech) and added to the plate in 10 µl aliquots for a final concentration of 1×105 eosinophils/ml. Additional eosinophils were saved on ice for use as a standard while the 96-well plate containing the samples was incubated at 37°C for 30 min. The plate was washed twice with 200 µl of HBSS and 100 µl HBSS was aliquoted to each well. An eosinophil standard was plated in triplicate (1×105 eosinophils/ml) and serially diluted in HBSS. Reaction buffer (100 µl) containing 0.1% Triton® X-100 (Sigma Chemical Co.), 50 mM Tris (pH 8), 1 mM H2O2 and 1 mM O-phenylenediamine dihydrochloride (OPD, Sigma Chemical Co.) was added to each well for an additional 30 min. The reaction was stopped with 4M H2SO4 (50 µl) and optical density (OD) was measured at 490 nm. The absorbance levels of EPO activity were used as an indirect measurement of eosinophil adherence by subtracting background values and interpolating the relative number of cells adhered from the standard curve.
Immunofluorescence
Glioblastoma cells (1×106/ml) were labeled with 1 µM Cell Trace™ CFSE (C34554, Invitrogen-Molecular Probes, Eugene, OR) per manufacture instructions and plated on coverslips (5×105/ml) and incubated at 37°C. After a 24 hr incubation, 10 ng/ml TNF-α or buffer control was added to media or glioblastoma cells for an additional 24 hr at 37°C. At the 48 hr time point, 100 pg/ml GM-CSF, 10 µg/ml GM-CSF neutralizing antibodies (Clone BVD2-23B6, Invitrogen) or isotype control antibodies were added for 1hr and incubated at 37°C. Eosinophils (1×105/ml) were mono-cultured or co-cultured with glioblastoma cells and incubated 10 min at 37°C. Media was aspirated and coverslips were washed twice with 1 ml ice cold phosphate buffered saline (PBS, pH 7.4). Cells were fixed in room temperature 4% paraformaldehyde (10 min), permeabilized with PBS containing 0.1% Triton® X-100 (Sigma Chemical Co.) (10 min), washed with PBS (5 min) and Tris buffered saline containing 0.5% Tween (TBST, pH 8), (5min). Each coverslip was blocked with TBST containing 1% bovine serum albumin (Sigma Chemical Co.) and 4% normal donkey serum albumin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA). Cells were immunostained at room temperature with CD11b antibodies (Clone ICRF44, R&D Systems, 1:50, 1 hr), washed three times 10 min with TBST, and immunostained with DAPI nucleic acid stain (Invitrogen, 1:10000) and Alexa Fluor 594-labelled donkey anti-mouse antibodies (Invitrogen, 1:2000, 1hr). After washing three times for 10 min with TBST, coverslips were rinsed in distilled H2O and mounted onto slides with Prolong® Gold anti-fade reagent (Invitrogen). Glioblastoma cells and eosinophils were respectively visualized at 488 nm and 594 nm using a Nikon epi-fluorescence microscope. A total of 5 images of each coverslip were randomly taken. Eosinophils identified by red CD11b staining and blue DAPI staining from 4 different donors were counted and averaged.
Eosinophil viability assay
Human glioblastoma cells (5×104 cells/0.5 ml) were cultured above 150 µl 0.5% w/v LE analytical grade agarose (Promega, Madison, WI), 48-well plate (Sarstedt). At 24 hr, 500 µl of eosinophils (2×105 cells) was added to semi-solid glioblastoma spheres for an additional 96 hr. In some experiments, GBM cell line-conditioned media (5×105 cells/ml, 48 hr) as described previously (31) was used instead of the glioblastoma spheres. Eosinophils were also cultured alone in the presence or absence of GM-CSF (100 pg/ml) as positive and negative controls, respectively. To block GM-CSF activity, neutralizing antibodies (Clone 3209, R&D Systems or Clone BVD2-23B6, Invitrogen) or isotype control (10 µg/ml) were added in some experiments. To deplete GM-CSF, neutralizing antibodies (Clone BVD2-23B6, Invitrogen) were added to conditioned media (30 min, 4°C) followed by immunoprecipitation beads (20 µl/ml, Santa Cruz Biotechnology, Inc, 30 min, 4°C) that were previously washed with 1 ml PBS to remove contaminating azide, similar to previous study (32). At 96 hr, cells were aspirated into 1.5 ml microfuge tubes, centrifuged (400 × g, 5 min, 4°C) and decanted to 100 µl. To identify eosinophils in co-cultures with glioblastoma spheres, cells were stained with 5 µL of PE-conjugated anti-CD11b (Clone ICRF44, BD Biosciences Pharmingen) or isotype control for 30 min. Cells were washed with 1 ml of media (DMEM) containing 1% CCS, suspended in PBS, treated with propidium iodide (3 µg/ml) and analyzed at 10,000 events on a FACScan flow cytometer (Becton–Dickinson) at the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Core Facility. Data were analyzed with FlowJo data analysis software (TreeStar) as shown in a representative example (Supplementary Figure 2).
Immunoblotting
For an assessment of RAGE expression, cells were solubilized with lysis buffer (1% sodium dodecyl sulfate (SDS)), 10 mM dithiothreitol (DTT), 0.5 mM Na3VO4, 1 mM EDTA, 10% glycerol, 10 mM Tris, pH 8.0), sonicated, boiled (5 min) and 50 µg of protein as determined by Micro-BCA protein assay reagents (Thermo Scientific Pierce, Rockford, IL) was loaded onto a 10% SDS PAGE gel. For analysis of S100A9, human primary blood eosinophils (5×106/ml) were cultured in 96-well tissue-culture plates (Sarstedt), +/− 100 pg/ml GM-CSF, 10 ng/ml TNF-α or GBM cell line-conditioned media generated in the presence or absence of 10 ng/ml TNF-α. After 24 hr, total eosinophil conditioned medium from a total of 5×10^5 cells was isolated via centrifugation 400 × g, 5 min). Concentrated (10X) lysis buffer was added to cell-free conditioned media (1:10 dilution) and boiled (5 min). Total conditioned media was loaded onto a 15% SDS PAGE gel. Proteins were transferred onto 0.45 µm Immobilion-P polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA) and incubated with antibodies raised against human RAGE (R&D Systems, MAB1145, 1:500)or S100A9 (Santa Cruz Biotechnology, Santa Cruz, CA, Calgranulin B (C-19): sc-8114, 1:200). The immunoblots were washed and subsequently incubated with horseradish peroxide- (HRP−) conjugated secondary antibodies. Bound secondary antibody was visualized following incubation of the membrane with Super Signal West chemiluminescent HRP substrate (Thermo Scientific Pierce) and an Epichemi II darkroom UVP equipped with a 12-bit cooled CCD camera. Luminescence was quantified and evaluated using ImageJ software (National Institutes of Health).
MTS assay
Metabolism of MTS was used as an index of cell viability. Glioblastoma cell lines (2×104 cells/ml) were cultured in complete media in 96-well tissue-culture plates (Sarstedt) for 4 hr to allow for adherence at 37°C. Media was replaced with serum free media for 24 hr and subsequently replaced with 0.1% CCS DMEM (+/− 100 pg/ml GM-CSF) [media controls], 10% CCS DMEM [live control], 10 mM sodium azide (NaN3) in 0.1% CCS DMEM [dead control], or eosinophil-conditioned media obtained from 2 day cultures of eosinophils (2×106/ml) in 0.1% CCS DMEM (+/− 100 pg/ml GM-CSF). Glioblastoma cells were incubated with specified controls or eosinophil-conditioned media for 48 hr at 37°C. Each well was aspirated and replaced with 100 µl/well PBS plus 20 µl/well of the CellTiter 96® Non-Radioactive Cell Proliferation Assay compound involving MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt; MTS] (G1111 Promega, Madison, WI, 2 mg/ml) and an electron coupling reagent PMS [phenazine methosulfate] (P9625 Sigma Chemical Co., 0.92 mg/ml). After a 1 hr incubation at 37°C, the reduction of MTS to formazan in metabolically active cells was measured at an optical density of 490 nm. Determinations were performed in triplicate and average values were compared across samples.
Statistical analysis
Analyses in all experiments were assessed among conditions using mixed-effects ANOVA models with a fixed-effect covariate per condition and a random-effect covariate to account for within-patient correlation of measurements. A 2-sided P value of <0.05 was regarded as statistically significant. The Standard Error of the Mean (SEM) indicates inter-assay variability.
RESULTS
Characterization of glioblastoma cell lines
In allergy and asthma, the cytokine GM-CSF has been indicated to induce the sensitization of eosinophil adhesion molecules (CD11b/CD18, CD49d/CD29) to more effectively interact with their ligands (27). These ligands include ICAM-1, VCAM-1, and possibly the receptor for advanced glycation end-products (RAGE) (27, 33). To begin to understand eosinophil recruitment mechanisms in response to tumor cells, the human A172 and U87-MG GBM cells were examined for their potential production of GM-CSF and expression of adhesion molecules (ICAM-1, VCAM-1, RAGE). In Figure 1A, basal levels of GM-CSF production were not detectable with the A172 cells whereas the U87-MG cells produced more than 100 pg/ml GM-CSF during the 48 hr time span. Addition of TNF-α to the cells enhanced the production of GM-CSF by either cell line, most significantly for the U87-MG cells. Likewise, in Figure 1B, basal levels of adhesion molecules were not detectable in the A172 cells whereas ICAM-1 was significantly expressed by the U87-MG cells. Addition of TNF-α to the cells induced ICAM-1 and VCAM-1 expression in the A172 cells but only enhanced ICAM-1 expression in the U87-MG cells. In examining RAGE expression, the A172 and U87-MG cell lines were compared to known positive (DU-145 prostate carcinoma cell line) (34) and negative (H358 NSCLC cell line) (35) controls. Figure 1C illustrates that both the A172 and U87-MG cell lines express RAGE, and that the U87-MG cells display 5-fold higher levels of this molecule when compared to the A172 cells. These data suggest that in the absence of stimuli, such as TNF-α, the U87-MG cell line may be able to induce stronger eosinophil adhesion responses in culture.
Figure 1. Characterization of human A172 and U87-MG glioblastoma cell lines.
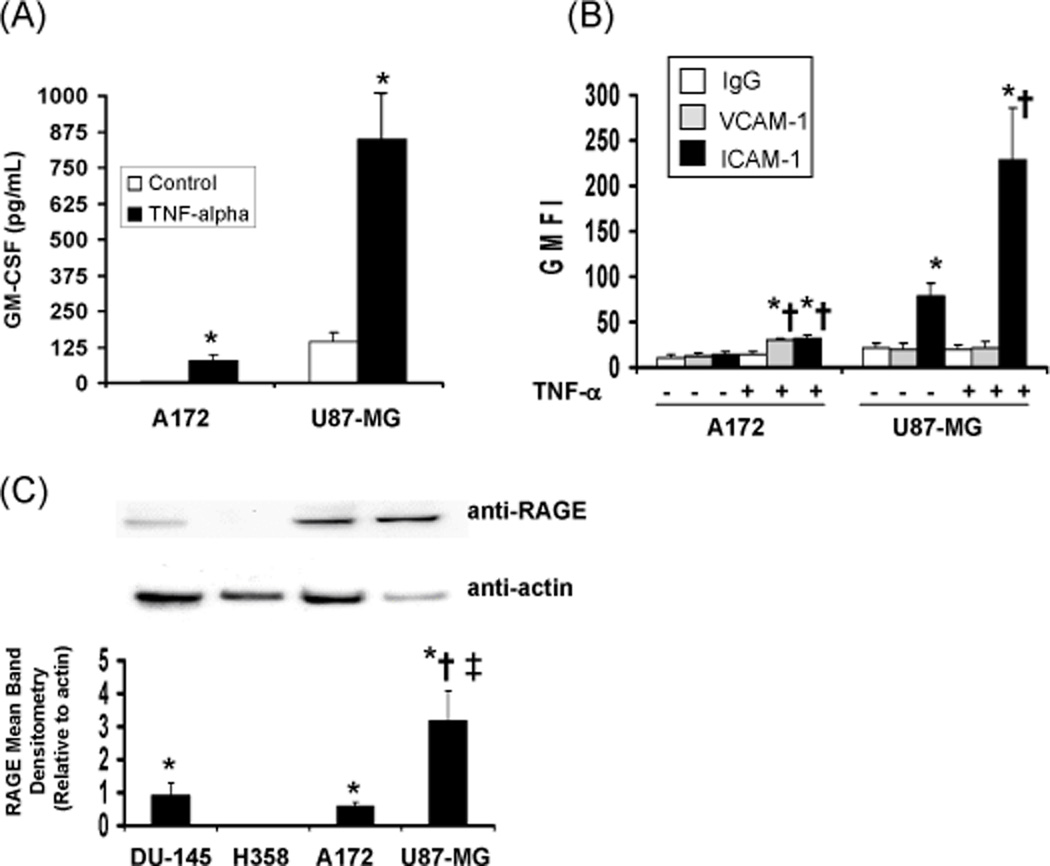
(A) GM-CSF ELISA of supernatants collected from tumor cell lines (5×105 cells/ml) plated 24 hr and treated with buffer control or 10 ng/ml TNF-α for an additional 24 hr. The mean concentration is displayed, +/−SEM, N=3, *p<0.0001 vs control; (B) Flow cytometry analysis of VCAM-1 and ICAM-1 geometric mean fluorescence intensity (GMFI) from tumor cell lines (5×105 cells/ml) plated 24 hr and treated with buffer control or 10 ng/ml TNF-α for an additional 24 hr +/−SEM, N=3, *p<0.0001 vs isotype control, †p<0.0001 vs basal expression. (C) A representative immunoblot and ImageJ assessment of GBM RAGE compared to positive (DU-145 prostate carcinoma) and negative (H358 NSCLC) controls. Data charted represent RAGE mean band densitometry relative to actin +/− SEM, N=5, *p≤0.0006 vs H358, †p=0.007 vs DU-145, ‡p=0.002 vs A172.
Eosinophil adherence as measured by EPO activity in response to glioblastoma co-cultures
To assess whether the U87-MG cell line induces preferential eosinophil adhesion compared to the A172 cell line, EPO assays were performed as an indirect measurement of adherence. Figure 2A displays data revealing that eosinophil adherence to both tumor cell lines was not significantly different than the media control. These data indicate that eosinophils do not preferentially adhere in response to co-culture with the U87-MG cells compared to the A172 cells in a 30 min time frame. Pre-treatment of the cell lines with TNF-α for 24 hr enhanced eosinophil adhesion to the plate and to the A172 cells but not to the U87-MG cells. Pre-incubation (1 hr) with GM-CSF neutralizing antibodies reduced adhesion, most significantly for the GM-CSF control and for the A172 cells stimulated with TNF-α. These results indicate that eosinophils are more adhesive in the presence of the TNF-α treated A172 cells, which produce lower levels of the cytokine GM-CSF compared to U87-MG cells (Figure 1A).
Figure 2. Human blood eosinophil adherence as measured by EPO activity and immunofluorescence.
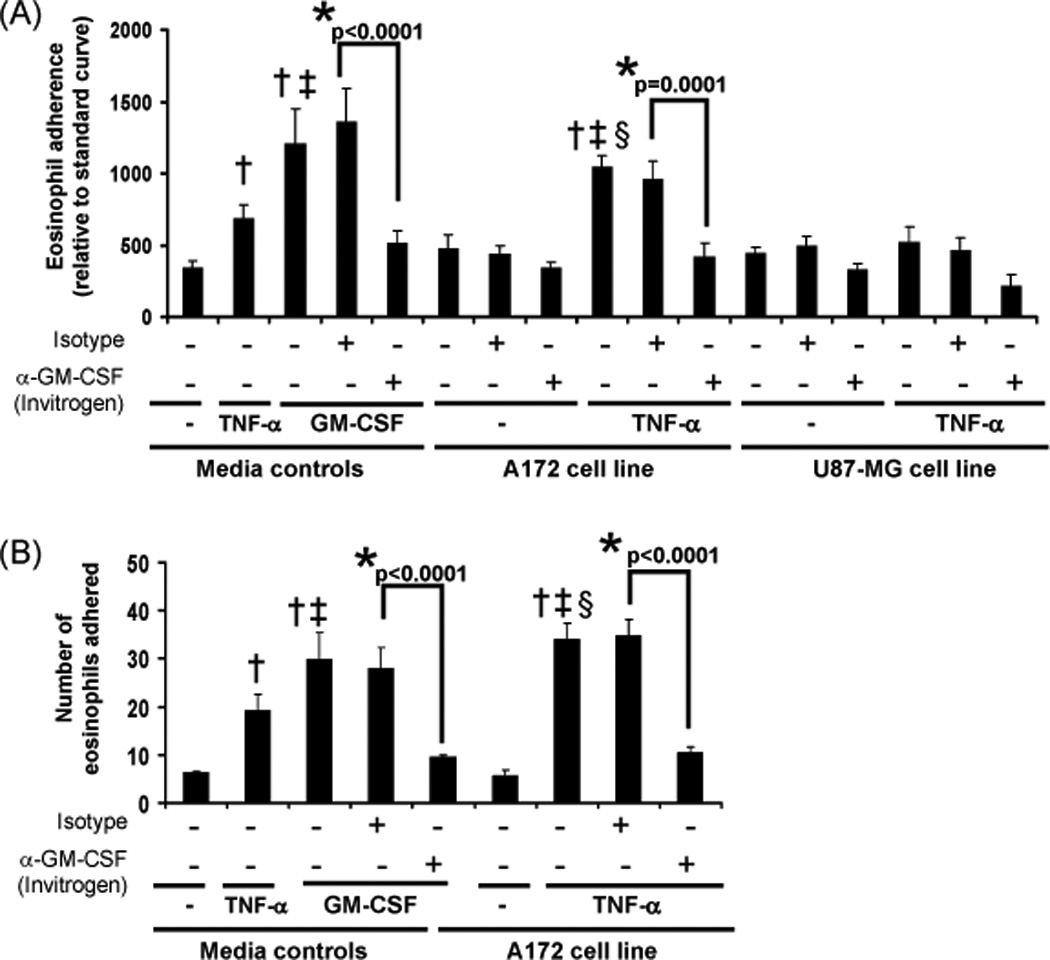
(A) Media controls (buffer, 100 pg/ml GM-CSF, 10 ng/ml TNF-α) or tumor cell lines stimulated (24 hr) with buffer control or 10 ng/ml TNF-α were treated with an isotype control or GM-CSF neutralizing antibody (1 hr) prior to adding eosinophils. An EPO assay was performed after 30 min incubation with eosinophils as described in the Materials and Methods. Data represent the average relative number of eosinophils adhered, +/− SEM, N=5, *p≤0.0001 vs respective isotype control, †p≤0.009 vs media control, ‡p≤0.005 vs TNF media control, §p<0.0001 vs A172 cell line control. (B) Media controls (buffer, 100 pg/ml GM-CSF, 10 ng/ml TNF-α) or tumor cell line cultures stimulated (24 hr) with buffer control or 10 ng/ml TNF-α were treated with an isotype control or GM-CSF neutralizing antibody (1 hr) prior to adding eosinophils. After a 10 min incubation with eosinophils, the cells were washed fixed and stained as described in the Materials and Methods. Data represent the average number of eosinophils adhered, +/− SEM, N=4, *p≤0.0001 vs respective isotype control, †p≤0.0006 vs media control, ‡p≤0.004 vs TNF media control, §p<0.0001vs A172 cell line control.
Immunofluorescence of eosinophil adherence in co-cultures
To further assess the adhesive functions of eosinophils in co-cultures with glioblastoma cell lines, immunofluorescence assays were performed. Similar to the results of the EPO assay, the TNF-α-treated A172 cell line appeared to induce eosinophil adherence after 10 min culture and immunofluorescent microscopy examination (Figure 2B). Addition of GM-CSF neutralizating antibodies (Clone BVD2-23B6) to TNF-α-treated A172 cell line cultures 1 hr prior to the addition of eosinophils mitigated the adhesive response (Figure 2B). Significant eosinophil adherence was not identified in control A172 cultures (Figure 2B) or U87-MG cultures generated in the presence or absence of TNF-α (data not shown).
Eosinophil viability in response to glioblastoma spheroid co-culture
Tumor cell lines suspended above agar are known to form spheroids, involving a diverse population of quiescent, hypoxic, and necrotic cells similar to human tumors and equally resistant to radiation and experimental drugs (36, 37). Spheroids in culture with immune cells have previously been used to model the tumor microenvironment and examine potential immunotherapeutic treatments (38). A previous study has also identified enhanced eosinophil viability in co-cultures of human biologically active GBM explants and autologous peripheral blood leukocytes (39). To examine if growing tumor spheroids affect eosinophil viability, eosinophils were co-cultured with GBM spheroids for 4 days. Tumor cell lines were evaluated for CD11b expression via flow cytometry analysis and determined to be negative for this cell adhesion molecule (data not shown). The expression of CD11b has been previously characterized on eosinophils (40) and was therefore used as a positive selection marker in identifying eosinophils in co-cultures. As a positive indicator of viability, GM-CSF was added to eosinophil mono-cultures similar to our previous study (41). Viability was determined by the absence of propidium iodide stain, revealing increased eosinophil viability from 4 different patients in the presence of GM-CSF or GBM spheroids (Figure 3A). These data were confirmed with 3 additional patients via analysis of live cells using a trypan blue exclusion assay (Figure 3B). Concomitant examination of 4 day culture supernatants by ELISA revealed the presence of GM-CSF. Of note, as shown in Figure 3C, we observed that the diminished levels of GM-CSF found in the positive control could still maintain eosinophil viability. Significant production of GM-CSF in U87-MG cell cultures did not further alter eosinophil viability compared to the positive control or the observations associated with the A172 cell cultures. These results suggest that the very low levels of GM-CSF in the A172 cell cultures are sufficient for maximum viability and/or that another factor(s) may contribute to eosinophil viability in the A172 cell cultures.
Figure 3. Human blood eosinophil viability in response to co-culture with A172 and U87-MG spheroids.
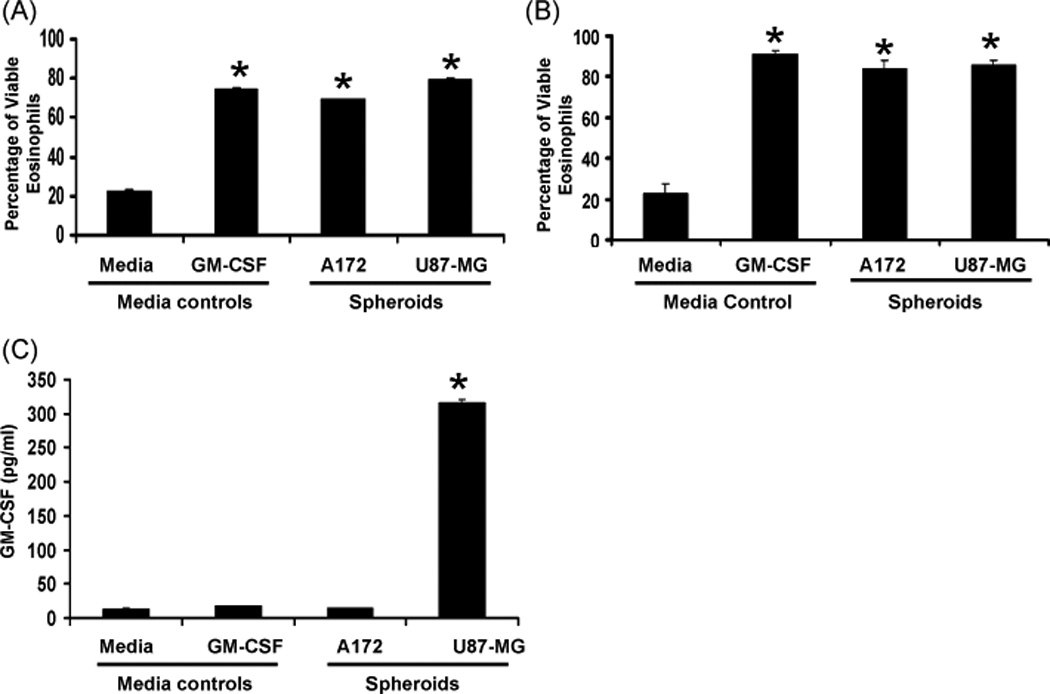
Human blood eosinophils (2×105/ml) were cultured above 0.5% w/v agarose, 48-well plate, +/− 100 pg/ml GM-CSF or glioblastoma cells (5×104/ml) for 4 days. (A) Cells were positively selected for CD11b expression and negatively selected for propidium iodide stain. The percent average viability is displayed, +/− SEM, N=4, *p<0.0001 vs media control. (B) Cells cultures were resuspended in 50 µl media and diluted 1:2 in trypan blue. Cells were identified as viable via trypan blue exclusion. The percent average viability is displayed, +/− SEM, N=3, *p<0.0001 vs media control (C) Supernatants from 4 day cultures were assessed for the presence of GM-CSF via ELISA (as detailed in Materials and Methods). The mean concentration is displayed, +/− SEM, N=3, *p<0.0001 vs all other samples.
Eosinophil viability in response to GBM cell line-conditioned media
Soluble factors derived from tumor cells have been found to affect the activation of human monocytic cells (31). To test the idea that soluble factors produced by tumor cells induced eosinophil survival as suggested by the data in Figure 3, eosinophils were cultured in GBM cell line-conditioned media for 4 days and analyzed by flow cytometry. As shown in Figure 4A, GBM cell line-conditioned media is also able to induce the survival of eosinophils from 5 different patients, similar to the GM-CSF positive control, comparable to spheroid co-culture (Figure 3A and 3B), and despite differential production levels of GM-CSF between tumor cell lines (Figure 3C).
Figure 4. Human blood eosinophil viability in response to GBM cell line-conditioned media.
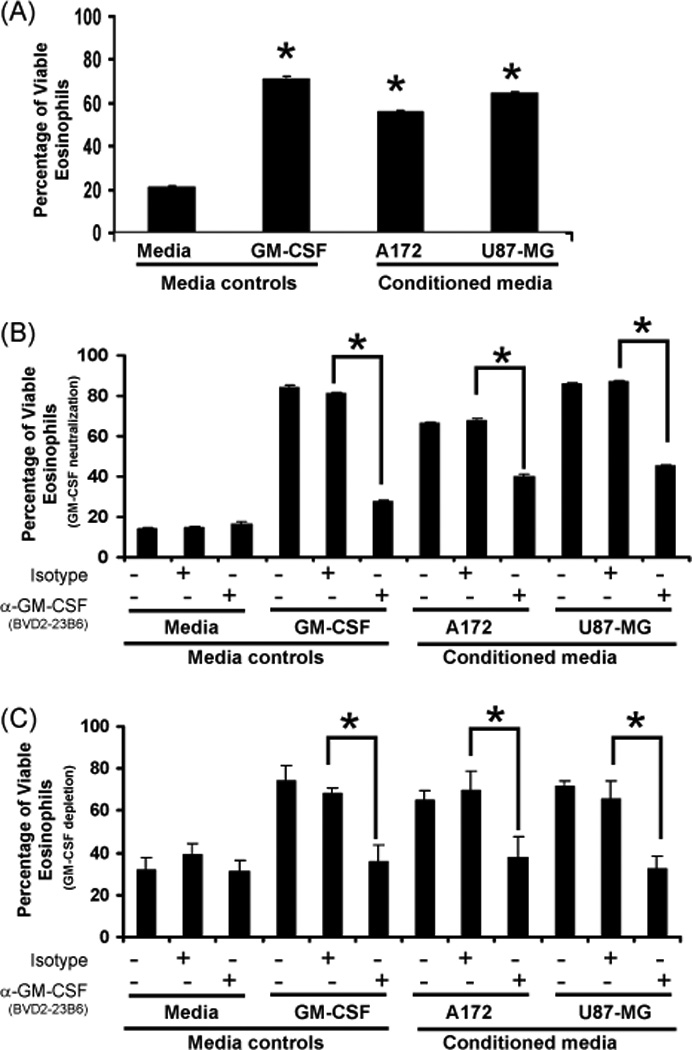
Human blood eosinophils (2×105/ml) were cultured above 0.5% w/v agarose, 48-well plate, +/− 100 pg/ml GM-CSF or GBM cell line-conditioned media for 4 days. Cells were gated (as shown in Supplementary Figure 2) and negatively selected for propidium iodide stain. (A) The percent average viability in response to media controls or conditioned media is displayed, N=5, *p≤0.001 vs media control. (B) The percent average viability in response to co-culture with anti-GM-CSF (Clone BVD2-23B6) or isotype antibodies is displayed, N=4, *p≤0.0001 vs media control. (C) The percent average viability in response GM-CSF cytokine depletion (Clone BVD2-23B6) or isotype controls is displayed, N=3, *p≤0.0001 vs media control.
The effect of GM-CSF neutralization on GBM cell line-conditioned media-induced eosinophil viability
To assess if the soluble factor involved in GBM cell line-conditioned media-induced viability is GM-CSF, neutralizing antibodies from two separate vendors were added to 4 day cultures. As shown in Figure 4B, GBM cell line-conditioned media-induced eosinophil viability from 4 different patients is reduced in the presence of GM-CSF neutralizing (Clone BVD2-23B6) antibodies but not in the presence of the isotype controls. Additional viability analyses of human blood eosinophils from 3 different patients following GM-CSF neutralization using an antibody from an alternative source (Clone 3209) confirmed these results (Supplementary Figure 3). These combined data suggest that low levels of GM-CSF, produced by tumor cell lines or eosinophils in response to the conditioned media, are able to enhance eosinophil survival.
The effect of GM-CSF depletion on GBM cell line-conditioned media-induced eosinophil viability
Because eosinophils have been suggested to produce GM-CSF in response to various stimuli (42, 43), GM-CSF cytokine depletion of tumor cell-conditioned media was performed prior to culturing eosinophils. As shown in Figure 4C, GBM cell line-conditioned media-induced eosinophil viability from 4 different patients is reduced after GM-CSF cytokine depletion but not in response to the isotype controls. These data suggest that GM-CSF mediated viability responses are a function of tumor-derived GM-CSF.
Eosinophil CD69 expression in response to GBM cell line-conditioned media
A strong inducer of eosinophil activation is GM-CSF as exhibited by CD69 expression (44). To examine if GBM cell line-conditioned media also induces CD69 expression, eosinophils were cultured with GBM cell line-conditioned media for 3 hr prior to analysis via flow cytometry. Because previous research has indicated that TNF-α induces GM-CSF production by GBM cells (45), 10 ng/ml TNF-α was added to selected GBM cultures 24 hr prior to harvesting the GBM cell line-conditioned medias. As shown in Figure 5A, significant eosinophil CD69 expression from 6 different patients occurred in response to U87-MG but not A172 cell-conditioned media compared to media alone and the GM-CSF media control. Addition of TNF-α during the generation of GBM cell line-conditioned media induced CD69 expression in A172 cultures and enhanced the expression in U87-MG cultures compared to TNF-α and respective GBM cell line-conditioned media alone. The absence of CD69 expression in the presence of A172 cell-conditioned media may be a response to the lower basal levels of GM-CSF produced by these cells compared to the U87-MG (Figure 1A).
Figure 5. Human blood eosinophil CD69 expression in response to GBM cell line-conditioned media.
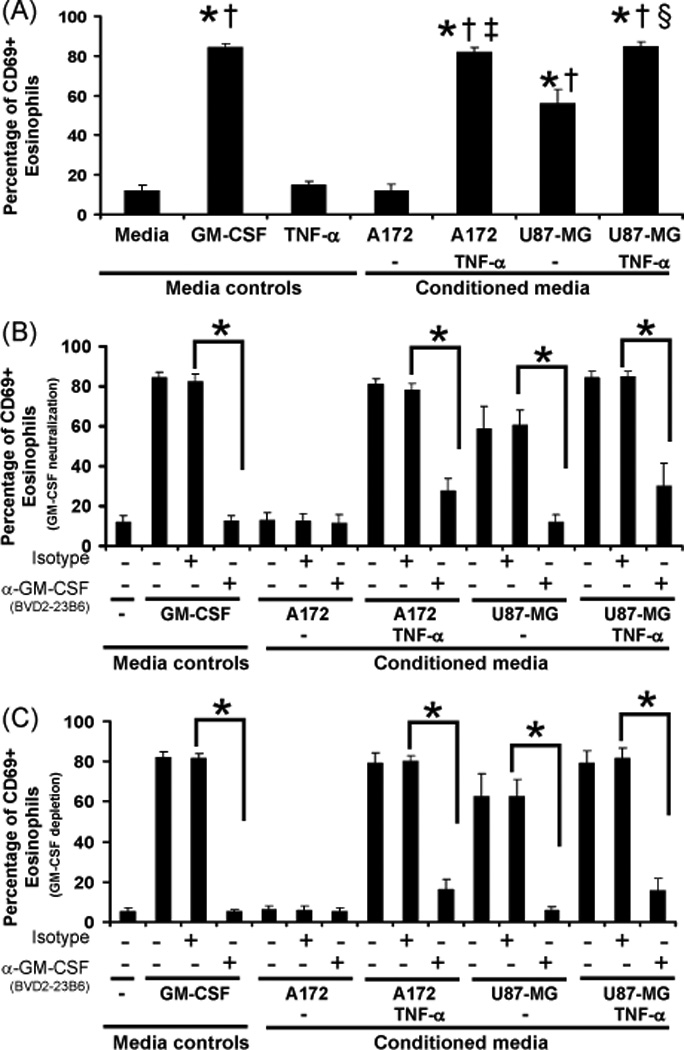
Human blood eosinophils (2×105/ml) were cultured above 0.5% w/v agarose, 48-well plate, +/− 100 pg/ml GM-CSF or GBM cell line-conditioned media generated in the presence or absence of 10 ng/ml TNF-α. In some wells, 10 µg/ml anti-GM-CSF (Clone BVD2-23B6) or isotype control antibodies were added. Cultures were maintained for 3 hr. Cells were gated (as shown in Supplementary Figure 4), negatively selected for propidium iodide stain, and positively selected for CD69 expression. (A) The percent average CD69 expression in response to media controls or conditioned media is displayed, N=5, +/− SEM, *p<0.0001 vs media, †p<0.0001 vs TNF-α, ‡p<0.0001 vs A172, §p<0.0001 vs U87-MG. (B) The percent average CD69 expression in response to co-culture with anti-GM-CSF (Clone BVD2-23B6) or isotype antibodies is displayed, N=3, +/− SEM, *p<0.0001. (C) The percent average CD69 expression in response GM-CSF cytokine depletion (Clone BVD2-23B6) or isotype controls is displayed, N=4, +/− SEM, *p<0.0001.
The effect of GM-CSF neutralization on GBM cell line-conditioned media-induced eosinophil CD69 expression
To determine if tumor-derived GM-CSF induces eosinophil CD69 expression, neutralizing antibodies to GM-CSF were added to eosinophils cultured in GBM cell line-conditioned media and compared to a GM-CSF positive control. As shown in Figure 5B, eosinophil CD69 expression from 4 different patients is reduced in the presence of GM-CSF neutralizing antibodies with significant reductions found in TNF-α generated conditioned media, the U87-MG-conditioned media alone and the GM-CSF control.
The effect of GM-CSF depletion on GBM cell line-conditioned media-induced eosinophil CD69 expression
Because eosinophils have been indicated to produce GM-CSF in response to various stimuli (42, 43), GM-CSF cytokine depletion of tumor cell-conditioned media was performed prior to culturing eosinophils. As shown in Figure 5C, GBM cell line-conditioned media-induced eosinophil CD69 expression from 3 different patients is reduced after GM-CSF cytokine depletion but not in response to the isotype controls, similar to Figure 5B. These data suggest that GM-CSF induced CD69 expression is a function of tumor cell-derived GM-CSF and not eosinophil autocrine activity.
GBM cell line-conditioned media-induced eosinophil CD11b expression
The eosinophil adhesion molecule CD11b is known to be a responsive to GM-CSF (27). To test whether GBM cell line-conditioned media affects eosinophil CD11b expression, eosinophils were cultured with GBM cell line-conditioned media for 24 hr prior to analysis via flow cytometry. As shown in Figure 6, significant eosinophil CD11b expression from 3 different patients occurred in response to U87-MG but not A172 cell-conditioned media. Addition of TNF-α during the generation of GBM cell line-conditioned media induced significant CD11b expression in A172 cell cultures and maintained U87-MG CD11b expression compared to media alone, GM-CSF and TNF-α treatments.
Figure 6. Human blood eosinophil CD11b expression in response to GBM cell line-conditioned media.
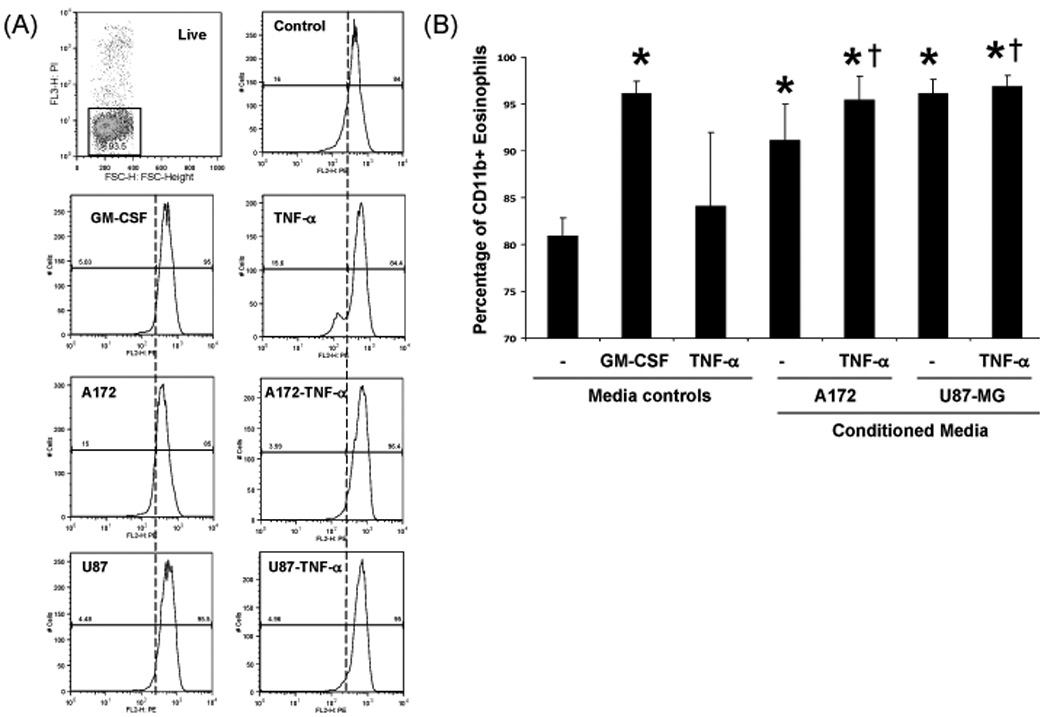
Human blood eosinophils (2×105/ml) were cultured above 0.5% w/v agarose, 48-well plate, +/− 100 pg/ml GM-CSF, 10 ng/ml TNF-α, or GBM cell line-conditioned media generated in the presence or absence of 10 ng/ml TNF-α. Cultures were maintained for 24 hr. (A) Cells were negatively selected for propidium iodide stain and positively selected for CD11b expression. (B) The percent average CD11b expression in response to media controls or conditioned media is displayed, N=3, +/− SEM, *p≤0.01 vs control, †p<0.03 vs TNF-α.
GBM cell line-conditioned media-induced eosinophil S100A9 release
Induction of CD11b is reportedly dependent upon the presence of a cytplasmic calcium binding protein, S100A9 (46). Release of S100A9 in the tumor microenvironment may be tumoristatic or tumorigenic depending on the concentration of S100A9 and tumor type (47, 48). To examine if eosinophils produce S100A9, eosinophils were cultured with GBM cell line-conditioned media for 24 hr and the cell-free conditioned media was examined for S100A9 expression via immunoblot. As shown in Figure 7, S100A9 was identified in co-cultures involving media controls and GBM cell line-conditioned media generated in the presence or absence of TNF-α from 3 different patients.
Figure 7. Human blood eosinophil-associated S100A9 release in response to GBM cell line-conditioned media.
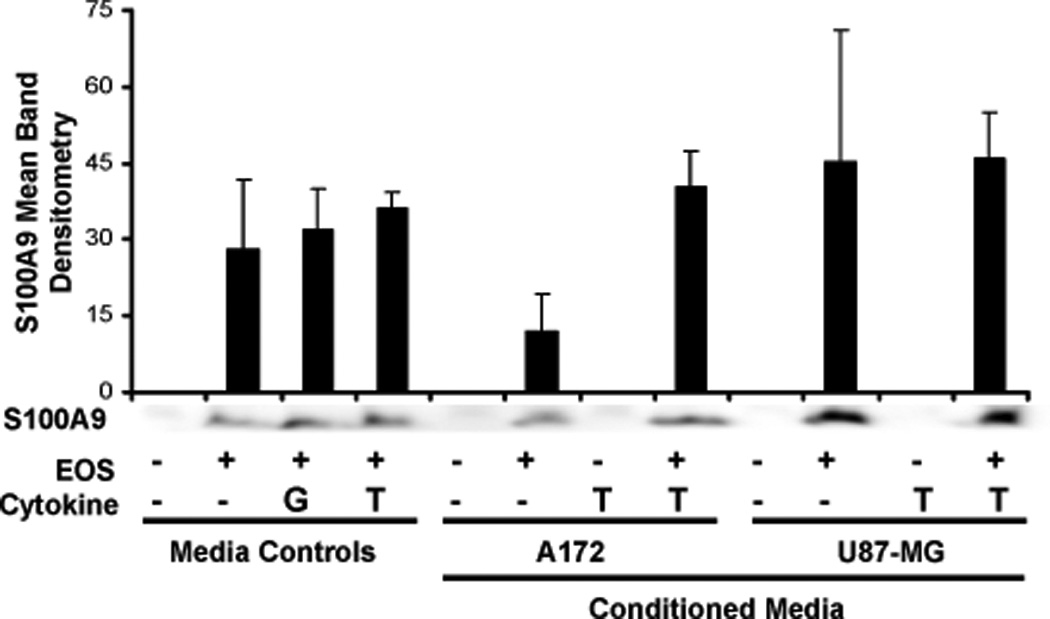
Human blood eosinophils (5×106/ml) were cultured with media +/− 100 pg/ml GM-CSF (G), 10 ng/ml TNF-α (T) or GBM cell line-conditioned media generated in the presence or absence of 10 ng/ml TNF-α for 24 hr. Total eosinophil cell-free conditioned media or media controls were loaded onto a 15% gel. A representative immunoblot and ImageJ assessment of S100A9 are displayed, N=3, +/−SEM.
The effect of dexamethasone on GM-CSF production by tumor cell lines
Eosinophil recruitment to the lung is reduced by dexamethasone (49), a common corticosteroid administered to GBM patients with peritumoral edema (50). Dexamethasone is also indicated to inhibit the release of GM-CSF from human primary T cells (51), suggesting that similar responses may occur in tumor cell lines known to produce GM-CSF. To test whether dexamethasone affects GBM cell line GM-CSF production, 5×105 cells/ml were plated for 24 hr prior to the addition of vehicle control, 10 ng/ml TNF-α, and increasing concentrations of dexamethasone, followed by an additional 24 hr incubation. As shown in Figure 8, TNF-α-induced, but not basal, GM-CSF production by the GBM tumor cell lines is significantly reduced by dexamethasone. Tumor cell line viability was not inhibited by dexamethasone or TNF-α treatments (Supplementary Figure 5).
Figure 8. A172 and U87-MG cell line GM-CSF production in response to dexamethasone.
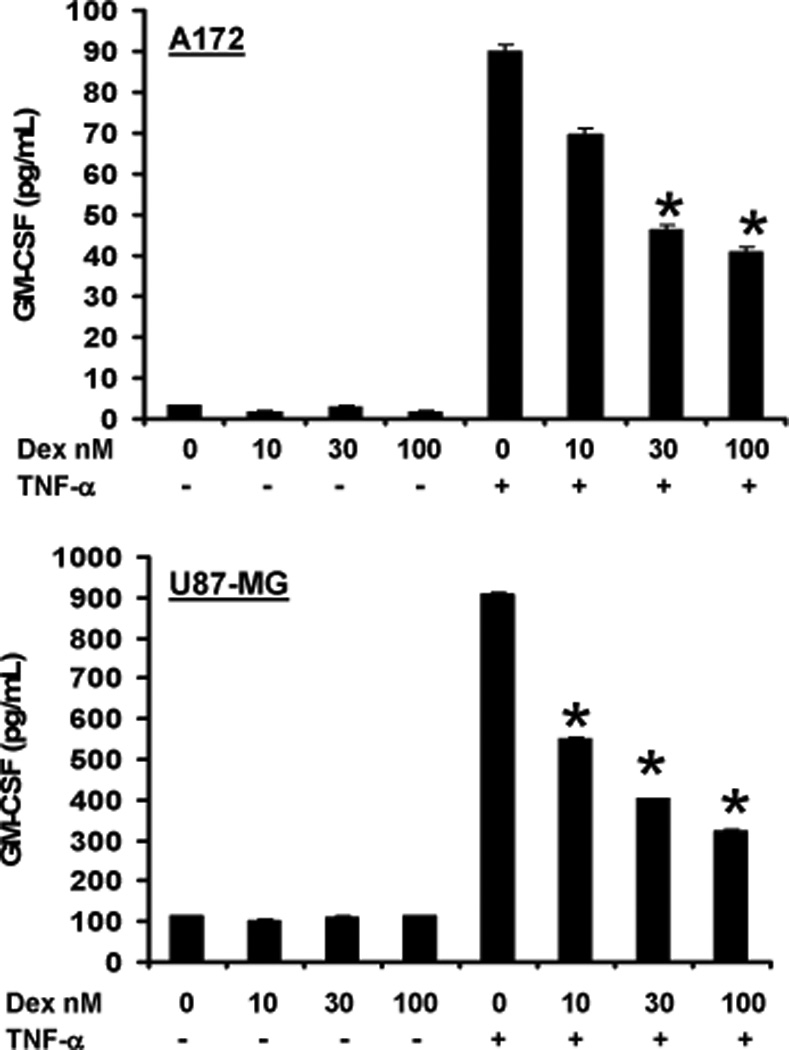
Glioblastoma cells (5×105/ml) were treated with increasing concentrations of dexamethasone (Dex) +/− 10 ng/ml TNF-α, 96-well plate, 24 hr. Culture supernatant fluids were examined in triplicate for GM-CSF via ELISA. The mean concentration is displayed, +/− SEM, N=5, *p≤0.007 vs TNF treatment alone.
Tumor cell line growth in response to eosinophil-conditioned media
Eosinophil-conditioned media generated in the presence or absence of GM-CSF has been shown to enhance endothelial cell proliferation (52). To assess whether soluble factors produced by eosinophils affect glioblastoma cell growth, cell lines were cultured with eosinophil-conditioned media and subjected to an MTS assay. As shown in Figure 9, soluble factors from eosinophils significantly induced glioblastoma cell growth compared to controls. These conditions resulted in 1.7 and 1.3 fold increases in respective A172 and U87-MG viability over the media control. Generation of eosinophil-conditioned media in the presence of 100 pg/ml GM-CSF enhanced the effect with 2.1 (A172) and 1.6 (U87-MG) fold increases in viability over the GM-CSF media control.
Figure 9. A172 and U87-MG cell line proliferation in response to human blood eosinophil-conditioned media.
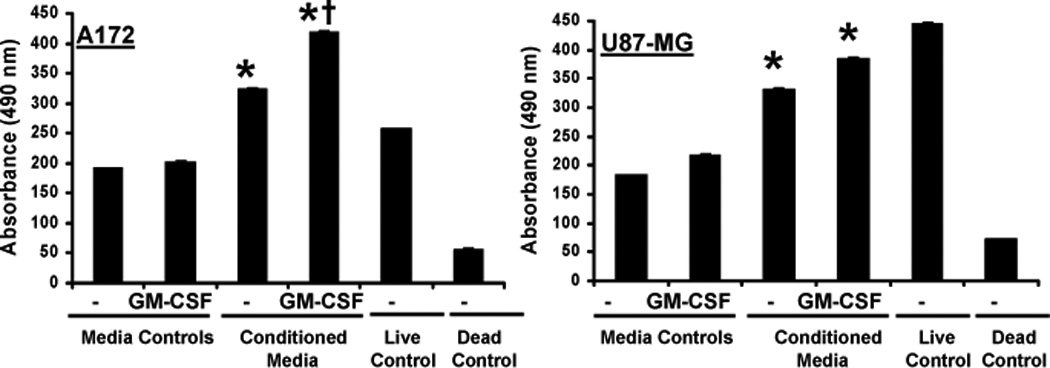
Human blood eosinophil-conditioned media was generated by culturing 2×106 cells/ml in 0.1% CCS DMEM +/−100 pg/ml GM-CSF, 24-well plate, 48 hr. Serum starved tumor cell lines (2×104/ml) were cultured in 100 µl of eosinophil-conditioned media, media controls, 10% DMEM (live control), 10mM sodium azide (dead control), 96-well plate, 48 hr. Cell proliferation was assessed via MTS assay. Average absorbance values are displayed, +/− SEM, N≥4, *p≤0.002 vs respective media control; †p=0.0004 vs conditioned media alone.
DISCUSSION
The reported inverse correlation between atopic disease and glioblastoma risk (12–15) suggests that an immune cell pivotal to the allergic response, such as the eosinophil (6), may also function in an anti-cancer response. Because cytokines such as GM-CSF and adhesion molecules (CD11b/CD18, CD49d/CD29) function in the recruitment of eosinophils in atopic disease (27), similar mechanisms may be essential to the migration of eosinophils into tumor tissue. Examination of glioblastoma cell lines indicated that the U87-MG but not the A172 cell line expressed significant basal levels of GM-CSF, ICAM-1, and higher levels of RAGE (Figure 1). These data suggest that CD11b-expressing eosinophils (Figure 6) may be more inclined to interact with the U87-MG compared to the A172 cell line. Assessment of eosinophil viability (Figure 3A and 3B) revealed increased eosinophil survival in co-cultures compared to controls, irrespective of the glioblastoma cell type.
To determine if the enhanced eosinophil viability in glioblastoma co-cultures was a product of interactions between the cell types or soluble factors within the media, GBM cell line-conditioned media was generated for culture with eosinophils. Assessment of eosinophil viability in response to GBM cell line-conditioned media revealed enhanced eosinophil survival (Figure 4A) that was comparable to cell co-culture (Figure 3A and 3B) and reduced in the presence of GM-CSF neutralizing antibodies (Figure 4B and Supplementary Figure 3) or in response to cytokine depletion (Figure 4C). These data indicate tumor cell-derived GM-CSF from either the A172 or U87-MG cell line enhanced eosinophil viability.
Because treatment of GBM cell lines with TNF-α led to increased tumor cell-derived GM-CSF production (Figures 1A and 8), additional studies were performed assessing possible differential TNF-α-induced responses. Stimulating A172 cells with TNF-α and inclusion of TNF-α in the generation of A172-conditioned media significantly enhanced respective eosinophil adhesion (Figure 2A and 2B) and CD69 expression (Figure 5) that was mitigated by the addition of GM-CSF neutralizing antibodies (Figure 5B) or GM-CSF cytokine depletion (Figure 5C). Similar treatment of U87-MG cells with TNF-α did not yield enhanced adhesion (Figure 2) but did enhance CD69 expression (Figure 5) in a GM-CSF-dependent manner. In the absence of TNF-α, only the U87-MG cell line induced CD69 activation that was also reduced by the addition of GM-CSF neutralizing antibodies (Figures 5). Interestingly, eosinophils were found to co-localize with either A172 or U87-MG spheriods with enhanced eosinophil clustering and recruitment noted in response to increased incubation and/or TNF-α treatment (Supplementary Figure 1). These differential functions may be in response to the levels of GM-CSF produced by tumor cells (Figures 1A and 8), production of the adhesion associated molecule S100A9 by eosinophils (53, 54) (Figure 7), changes in eosinophil CD11b expression (Figure 6), culture conditions (Figure 2 and Supplementary Figure 1), differential expression of tumor cell adhesion ligands (Figure 1B) and extracellular matrix proteins (55) or additional cytokines (IL-1β, TGF-β) produced by U87-MG but not A172 cells (56).
Co-expression of GM-CSF and its receptor have been found exclusively in cultures derived from grade IV astrocytomas (GBM) but not in lower grades or normal brain tissue (57). Because dexamethasone is a potent inhibitor of GM-CSF (51) and a common corticosteroid administered to GBM patients with peritumoral edema (50), examination of tumor cell line GM-CSF production in the presence of dexamethasone was assessed. In Figure 8, TNF-α-induced but not basal GM-CSF production was significantly inhibited by dexamethasone. In Supplementary Figure 5, tumor cell proliferation was not reduced but enhanced by dexamethasone, similar to previous assessments of glioma cell lines (58) and at concentrations within the physiological range of previously assessed human brain tissue biopsy specimens (59). Dexamethasone has also been reported to reduce eosinophil recruitment (49), induce eosinophil apoptosis (60) and inhibit eosinophil TNF-α-induced GM-CSF production (42). Focal expression of TNF-α has been identified in infiltrating leukocytes in GBM tumors (61). Dexamethasone therapy (16mg/day) in some GBM cases reduced the imaging of lesions on contrast-enhanced scans (62, 63), possibly by inhibiting immune cell recruitment via reduced capillary permeability at the brain-tumor barrier (64) inducing eosinophil apoptosis (60), and/or reducing localized GM-CSF production (Figure 8), (42). Thus, the efficacy of dexamethasone treatment may be a factor of the tumor microenvironment and whether the established immune response is tumorigenic or immunogenic.
In examining the eosinophil immune response, GBM cell lines were cultured with eosinophil-conditioned media and assessed for proliferation via MTS assay. As shown in Figure 9, eosinophil-conditioned media, generated in the presence or absence of GM-CSF, enhanced glioblastoma cell growth compared to respective controls. These data are supported by examining A172 carboxyfluorescein succinimidyl ester (CFSE) staining (Supplementary Figure 6). After 48 hr culture with eosinophil-conditioned media, generated in the presence or absence of GM-CSF, the A172 cells exhibited reduced CFSE expression compared to media controls, suggesting increased proliferation (p ≤ 0.02). Eosinophils stimulated with GM-CSF are known to produce amphiregulin and transforming growth factor-alpha (TGF-α), ligands highly implicated in tumor promotion via a common receptor, epidermal growth factor receptor (EGFR) (65–67). In primary GBM, amplification of the EGFR gene and subsequent over-expression of EGFR protein is the most common genetic alteration (68). Release of S100A9 (Figure 7) was identified in response to GBM cell line-conditioned media, and interestingly, this protein is also increased in response to radiochemotherapy in GBM studies (69). The present work is consistent with the idea that S100A9 may interact with GBM-associated RAGE (Figure 1C) in tumor progression (48). In addition, eosinophils have been shown to produce vascular endothelial growth factor (VEGF) in response to GM-CSF treatment. VEGF is an established factor in GBM pathology and progression (70). Because GBM tumors are known to produce GM-CSF (57, 71), a paracrine loop may develop where eosinophils promote GBM development by producing amphiregulin, TGF-α, S100A9, and VEGF in response to GBM-derived GM-CSF.
In summary, we have shown that eosinophils are more viable and activated in the presence of GBM tumor cell lines or GBM cell line-conditioned media. These eosinophil responses are, in part, regulated by tumor cell-derived GM-CSF as indicated by neutralization and cytokine depletion experiments. Eosinophils, in the presence or absence of GM-CSF, produced growth factors essential to tumor cell viability, indicating a potential cooperative function of eosinophils in tumor promotion/progression. Thus, the inverse correlations reported between atopic disease and GBM risk cannot be attributed to the functional responses of eosinophils alone. The enhanced GBM production of GM-CSF and expression of adhesion ligands (ICAM-1, VCAM-1) in the presence of TNF-α suggest a synergy with microglial cells in the recruitment and activation of eosinophils. These findings offer insight into GBM and other GM-CSF secreting tumors (colon (72), prostate (73) and skin (74)) known to recruit eosinophils (5, 24, 75) and emphasize the need to understand the immunological networks within the tumor microenvironment in developing more effective immunotherapeutic protocols.
Supplementary Material
Acknowledgments
The authors wish to thank Greg J. Wiepz, Ph.D. for technical advice and manuscript review, Kathleen Schell, M.S. and the University of Wisconsin Comprehensive Cancer Center Flow Cytometry Core Facility for flow cytometry support and Beth Schwantes, B.S. for overseeing the eosinophil purification process.
This work was supported by NIH grants HL088594, HL56396, AI070503 and HL069116 (to PJB) as well as by the institutional grant 1UL1RR025011 from the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources, National Institutes of Health.
Abbreviations used in this paper
- GBM
glioblastoma multiforme
REFERENCES
- 1.Giembycz MA, Lindsay MA. Pharmacology of the eosinophil. Pharmacol Rev. 1999;51:213. [PubMed] [Google Scholar]
- 2.Charcot JM, Robin C. Observation de Leucocythemia. C. R. Mem. Soc. Biol. 1853;5:44. [Google Scholar]
- 3.Leyden E. Zur Kenntniss des bronchial asthma. Arch. Pathol. Anat. 1872;54:324. [Google Scholar]
- 4.Lowe D, Jorizzo J, Hutt MS. Tumour-associated eosinophilia: a review. J Clin Pathol. 1981;34:1343. doi: 10.1136/jcp.34.12.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samoszuk M. Eosinophils and human cancer. Histol Histopathol. 1997;12:807. [PubMed] [Google Scholar]
- 6.Denburg JA, Keith PK. Eosinophil progenitors in airway diseases: clinical implications. Chest. 2008;134:1037. doi: 10.1378/chest.08-0485. [DOI] [PubMed] [Google Scholar]
- 7.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 8.Pandit R, Scholnik A, Wulfekuhler L, Dimitrov N. Non-small-cell lung cancer associated with excessive eosinophilia and secretion of interleukin-5 as a paraneoplastic syndrome. Am J Hematol. 2007;82:234. doi: 10.1002/ajh.20789. [DOI] [PubMed] [Google Scholar]
- 9.Jensen-Jarolim E, Achatz G, Turner MC, Karagiannis S, Legrand F, Capron M, Penichet ML, Rodriguez JA, Siccardi AG, Vangelista L, Riemer AB, Gould H. AllergoOncology: the role of IgE-mediated allergy in cancer. Allergy. 2008;63:1255. doi: 10.1111/j.1398-9995.2008.01768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotfi R, Lee JJ, Lotze MT. Eosinophilic granulocytes and damage-associated molecular pattern molecules (DAMPs): role in the inflammatory response within tumors. J Immunother. 2007;30:16. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 11.Trivedi SG, Lloyd CM. Eosinophils in the pathogenesis of allergic airways disease. Cell Mol Life Sci. 2007;64:1269. doi: 10.1007/s00018-007-6527-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiemels JL, Wiencke JK, Sison JD, Miike R, McMillan A, Wrensch M. History of allergies among adults with glioma and controls. Int J Cancer. 2002;98:609. doi: 10.1002/ijc.10239. [DOI] [PubMed] [Google Scholar]
- 13.Wiemels JL, Wiencke JK, Patoka J, Moghadassi M, Chew T, McMillan A, Miike R, Barger G, Wrensch M. Reduced immunoglobulin E and allergy among adults with glioma compared with controls. Cancer Res. 2004;64:8468. doi: 10.1158/0008-5472.CAN-04-1706. [DOI] [PubMed] [Google Scholar]
- 14.Linos E, Raine T, Alonso A, Michaud D. Atopy and risk of brain tumors: a meta-analysis. J Natl Cancer Inst. 2007;99:1544. doi: 10.1093/jnci/djm170. [DOI] [PubMed] [Google Scholar]
- 15.Wigertz A, Lonn S, Schwartzbaum J, Hall P, Auvinen A, Christensen HC, Johansen C, Klaeboe L, Salminen T, Schoemaker MJ, Swerdlow AJ, Tynes T, Feychting M. Allergic conditions and brain tumor risk. Am J Epidemiol. 2007;166:941. doi: 10.1093/aje/kwm203. [DOI] [PubMed] [Google Scholar]
- 16.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11:152. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 17.Gomez GG, Kruse CA. Mechanisms of malignant glioma immune resistance and sources of immunosuppression. Gene Ther Mol Biol. 2006;10:133. [PMC free article] [PubMed] [Google Scholar]
- 18.Hayes RL, Koslow M, Hiesiger EM, Hymes KB, Hochster HS, Moore EJ, Pierz DM, Chen DK, Budzilovich GN, Ransohoff J. Improved long term survival after intracavitary interleukin-2 and lymphokine-activated killer cells for adults with recurrent malignant glioma. Cancer. 1995;76:840. doi: 10.1002/1097-0142(19950901)76:5<840::aid-cncr2820760519>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 19.Hayes RL, Arbit E, Odaimi M, Pannullo S, Scheff R, Kravchinskiy D, Zaroulis C. Adoptive cellular immunotherapy for the treatment of malignant gliomas. Crit Rev Oncol Hematol. 2001;39:31. doi: 10.1016/s1040-8428(01)00122-6. [DOI] [PubMed] [Google Scholar]
- 20.Yu JS, Wei MX, Chiocca EA, Martuza RL, Tepper RI. Treatment of glioma by engineered interleukin 4-secreting cells. Cancer Res. 1993;53:3125. [PubMed] [Google Scholar]
- 21.Tseng SH, Hwang LH, Lin SM. Induction of antitumor immunity by intracerebrally implanted rat C6 glioma cells genetically engineered to secrete cytokines. J Immunother. 1997;20:334. doi: 10.1097/00002371-199709000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Herrlinger U, Kramm CM, Johnston KM, Louis DN, Finkelstein D, Reznikoff G, Dranoff G, Breakefield XO, Yu JS. Vaccination for experimental gliomas using GM-CSF-transduced glioma cells. Cancer Gene Ther. 1997;4:345. [PubMed] [Google Scholar]
- 23.Golden J, Frim DM, Chapman PH, Vonsattel JP. Marked tissue eosinophilia within organizing chronic subdural hematoma membranes. Clin Neuropathol. 1994;13:12. [PubMed] [Google Scholar]
- 24.Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, Dimina D, Ochkur SI, O'Neill K, Colbert D, Lombari TR, Constant S, McGarry MP, Lee JJ, Lee NA. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131. doi: 10.1189/jlb.0106027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muggia FM, Ghossein NA, Wohl H. Eosinophilia following radiation therapy. Oncology. 1973;27:118. doi: 10.1159/000224727. [DOI] [PubMed] [Google Scholar]
- 26.Lee EL, Armstrong TS. Increased intracranial pressure. Clin J Oncol Nurs. 2008;12:37. doi: 10.1188/08.CJON.37-41. [DOI] [PubMed] [Google Scholar]
- 27.Lampinen M, Carlson M, Hakansson LD, Venge P. Cytokine-regulated accumulation of eosinophils in inflammatory disease. Allergy. 2004;59:793. doi: 10.1111/j.1398-9995.2004.00469.x. [DOI] [PubMed] [Google Scholar]
- 28.Strath M, Warren DJ, Sanderson CJ. Detection of eosinophils using an eosinophil peroxidase assay. Its use as an assay for eosinophil differentiation factors. J Immunol Methods. 1985;83:209. doi: 10.1016/0022-1759(85)90242-x. [DOI] [PubMed] [Google Scholar]
- 29.Nagata M, Yamamoto H, Shibasaki M, Sakamoto Y, Matsuo H. Hydrogen peroxide augments eosinophil adhesion via beta2 integrin. Immunology. 2000;101:412. doi: 10.1046/j.1365-2567.2000.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bates ME, Sedgwick JB, Zhu Y, Liu LY, Heuser RG, Jarjour NN, Kita H, Bertics PJ. Human airway eosinophils respond to chemoattractants with greater eosinophil-derived neurotoxin release, adherence to fibronectin, and activation of the Ras-ERK pathway when compared with blood eosinophils. J Immunol. 184:7125. doi: 10.4049/jimmunol.0900634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuang DM, Wu Y, Chen N, Cheng J, Zhuang SM, Zheng L. Tumor-derived hyaluronan induces formation of immunosuppressive macrophages through transient early activation of monocytes. Blood. 2007;110:587. doi: 10.1182/blood-2007-01-068031. [DOI] [PubMed] [Google Scholar]
- 32.Conboy IM, DeKruyff RH, Tate KM, Cao ZA, Moore TA, Umetsu DT, Jones PP. Novel genetic regulation of T helper 1 (Th1)/Th2 cytokine production and encephalitogenicity in inbred mouse strains. J Exp Med. 1997;185:439. doi: 10.1084/jem.185.3.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chavakis T, Bierhaus A, Al-Fakhri N, Schneider D, Witte S, Linn T, Nagashima M, Morser J, Arnold B, Preissner KT, Nawroth PP. The pattern recognition receptor (RAGE) is a counterreceptor for leukocyte integrins: a novel pathway for inflammatory cell recruitment. J Exp Med. 2003;198:1507. doi: 10.1084/jem.20030800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiguro H, Nakaigawa N, Miyoshi Y, Fujinami K, Kubota Y, Uemura H. Receptor for advanced glycation end products (RAGE) and its ligand, amphoterin are overexpressed and associated with prostate cancer development. Prostate. 2005;64:92. doi: 10.1002/pros.20219. [DOI] [PubMed] [Google Scholar]
- 35.Bartling B, Hofmann HS, Weigle B, Silber RE, Simm A. Down-regulation of the receptor for advanced glycation end-products (RAGE) supports non-small cell lung carcinoma. Carcinogenesis. 2005;26:293. doi: 10.1093/carcin/bgh333. [DOI] [PubMed] [Google Scholar]
- 36.Khaitan D, Chandna S, Arya MB, Dwarakanath BS. Establishment and characterization of multicellular spheroids from a human glioma cell line; Implications for tumor therapy. J Transl Med. 2006;4:12. doi: 10.1186/1479-5876-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desoize B, Jardillier J. Multicellular resistance: a paradigm for clinical resistance? Crit Rev Oncol Hematol. 2000;36:193. doi: 10.1016/s1040-8428(00)00086-x. [DOI] [PubMed] [Google Scholar]
- 38.Gottfried E, Kunz-Schughart LA, Andreesen R, Kreutz M. Brave little world: spheroids as an in vitro model to study tumor-immune-cell interactions. Cell Cycle. 2006;5:691. doi: 10.4161/cc.5.7.2624. [DOI] [PubMed] [Google Scholar]
- 39.Ciembroniewicz J, Kolar O. Eosinophilic response in glioblastoma tissue culture after addition of autologous lymphocytes. Science. 1967;157:1054. doi: 10.1126/science.157.3792.1054. [DOI] [PubMed] [Google Scholar]
- 40.in 't Veen JC, Grootendorst DC, Bel EH, Smits HH, Van Der Keur M, Sterk PJ, Hiemstra PS. CD11b and L-selectin expression on eosinophils and neutrophils in blood and induced sputum of patients with asthma compared with normal subjects. Clin Exp Allergy. 1998;28:606. doi: 10.1046/j.1365-2222.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- 41.Kelly EA, Koziol-White CJ, Clay KJ, Kelly EA, Kelly EA, Koziol-White CJ, Clay KJ, Liu LY, Bates ME, Bertics PJ, Jarjour NN. Potential contribution of IL-7 to allergen-induced eosinophilic airway inflammation in asthma. J Immunol. 2009;182:1404. doi: 10.4049/jimmunol.182.3.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Uings I, Puxeddu I, Temkin V, Smith SJ, Fattah D, Ray KP, Levi-Schaffer F. Effects of dexamethasone on TNF-alpha-induced release of cytokines from purified human blood eosinophils. Clin Mol Allergy. 2005;3:5. doi: 10.1186/1476-7961-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kobayashi T, Kouzaki H, Kita H. Human eosinophils recognize endogenous danger signal crystalline uric acid and produce proinflammatory cytokines mediated by autocrine ATP. J Immunol. 184:6350. doi: 10.4049/jimmunol.0902673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ziegler SF, Ramsdell F, Alderson MR. The activation antigen CD69. Stem Cells. 1994;12:456. doi: 10.1002/stem.5530120502. [DOI] [PubMed] [Google Scholar]
- 45.Frei K, Piani D, Malipiero UV, Van Meir E, de Tribolet N, Fontana A. Granulocyte-macrophage colony-stimulating factor (GM-CSF) production by glioblastoma cells. Despite the presence of inducing signals GM-CSF is not expressed in vivo. J Immunol. 1992;148:3140. [PubMed] [Google Scholar]
- 46.Manitz MP, Horst B, Seeliger S, Strey A, Skryabin BV, Gunzer M, Frings W, Schonlau F, Roth J, Sorg C, Nacken W. Loss of S100A9 (MRP14) results in reduced interleukin-8-induced CD11b surface expression, a polarized microfilament system, and diminished responsiveness to chemoattractants in vitro. Mol Cell Biol. 2003;23:1034. doi: 10.1128/MCB.23.3.1034-1043.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salama I, Malone PS, Mihaimeed F, Jones JL. A review of the S100 proteins in cancer. Eur J Surg Oncol. 2008;34:357. doi: 10.1016/j.ejso.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 48.Ghavami S, Chitayat S, Hashemi M, Eshraghi M, Chazin WJ, Halayko AJ, Kerkhoff C. S100A8/A9: a Janus-faced molecule in cancer therapy and tumorgenesis. Eur J Pharmacol. 2009;625:73. doi: 10.1016/j.ejphar.2009.08.044. [DOI] [PubMed] [Google Scholar]
- 49.Ito A, Miyake M, Morishita M, Ito K, Torii S, Sakamoto T. Dexamethasone reduces lung eosinophilia, and VCAM-1 and ICAM-1 expression induced by Sephadex beads in rats. Eur J Pharmacol. 2003;468:59. doi: 10.1016/s0014-2999(03)01640-6. [DOI] [PubMed] [Google Scholar]
- 50.McClelland S, 3rd, Long DM. Genesis of the use of corticosteroids in the treatment and prevention of brain edema. Neurosurgery. 2008;62:965. doi: 10.1227/01.neu.0000318183.25783.77. [DOI] [PubMed] [Google Scholar]
- 51.Bergmann MW, Staples KJ, Smith SJ, Barnes PJ, Newton R. Glucocorticoid inhibition of granulocyte macrophage-colony-stimulating factor from T cells is independent of control by nuclear factor-kappaB and conserved lymphokine element 0. Am J Respir Cell Mol Biol. 2004;30:555. doi: 10.1165/rcmb.2003-0295OC. [DOI] [PubMed] [Google Scholar]
- 52.Puxeddu I, Alian A, Piliponsky AM, Ribatti D, Panet A, Levi-Schaffer F. Human peripheral blood eosinophils induce angiogenesis. Int J Biochem Cell Biol. 2005;37:628. doi: 10.1016/j.biocel.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 53.Curran C, S, Bertics P. Human eosinophils express RAGE, produce RAGE ligands, exhibit PKC-delta phosphorylation and enhanced viability in response to the RAGE ligand, S100B. 2011 doi: 10.1093/intimm/dxr083. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Anceriz N, Vandal K, Tessier PA. S100A9 mediates neutrophil adhesion to fibronectin through activation of beta2 integrins. Biochem Biophys Res Commun. 2007;354:84. doi: 10.1016/j.bbrc.2006.12.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zamecnik J, Vargova L, Homola A, Kodet R, Sykova E. Extracellular matrix glycoproteins and diffusion barriers in human astrocytic tumours. Neuropathol Appl Neurobiol. 2004;30:338. doi: 10.1046/j.0305-1846.2003.00541.x. [DOI] [PubMed] [Google Scholar]
- 56.Chen TC, Hinton DR, Apuzzo ML, Hofman FM. Differential effects of tumor necrosis factor-alpha on proliferation, cell surface antigen expression, and cytokine interactions in malignant gliomas. Neurosurgery. 1993;32:85. doi: 10.1227/00006123-199301000-00013. [DOI] [PubMed] [Google Scholar]
- 57.Mueller MM, Herold-Mende CC, Riede D, Lange M, Steiner HH, Fusenig NE. Autocrine growth regulation by granulocyte colony-stimulating factor and granulocyte macrophage colony-stimulating factor in human gliomas with tumor progression. Am J Pathol. 1999;155:1557. doi: 10.1016/S0002-9440(10)65472-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Langeveld CH, van Waas MP, Stoof JC, Sutanto W, de Kloet ER, Wolbers JG, Heimans JJ. Implication of glucocorticoid receptors in the stimulation of human glioma cell proliferation by dexamethasone. J Neurosci Res. 1992;31:524. doi: 10.1002/jnr.490310316. [DOI] [PubMed] [Google Scholar]
- 59.Nestler U, Winking M, Boker DK. The tissue level of dexamethasone in human brain tumors is about 1000 times lower than the cytotoxic concentration in cell culture. Neurol Res. 2002;24:479. doi: 10.1179/016164102101200203. [DOI] [PubMed] [Google Scholar]
- 60.Druilhe A, Cai Z, Haile S, Chouaib S, Pretolani M. Fas-mediated apoptosis in cultured human eosinophils. Blood. 1996;87:2822. [PubMed] [Google Scholar]
- 61.Roessler K, Suchanek G, Breitschopf H, Kitz K, Matula C, Lassmann H, Koos WT. Detection of tumor necrosis factor-alpha protein and messenger RNA in human glial brain tumors: comparison of immunohistochemistry with in situ hybridization using molecular probes. J Neurosurg. 1995;83:291. doi: 10.3171/jns.1995.83.2.0291. [DOI] [PubMed] [Google Scholar]
- 62.Goh JJ, See SJ, Ang E, Ng WH. Vanishing glioblastoma after corticosteroid therapy. J Clin Neurosci. 2009;16:1226. doi: 10.1016/j.jocn.2008.10.029. [DOI] [PubMed] [Google Scholar]
- 63.Hasegawa H, Pal D, Ramirez R, Ismail A, Marks P. Glioblastoma multiforme fades on CT imaging after dexamethasone therapy. J Clin Neurosci. 2009 doi: 10.1016/j.jocn.2009.02.024. [DOI] [PubMed] [Google Scholar]
- 64.Gu YT, Qin LJ, Qin X, Xu F. The molecular mechanism of dexamethasone-mediated effect on the blood-brain tumor barrier permeability in a rat brain tumor model. Neurosci Lett. 2009;452:114. doi: 10.1016/j.neulet.2008.12.047. [DOI] [PubMed] [Google Scholar]
- 65.Walz TM, Nishikawa BK, Malm C, Briheim K, Wasteson A. Transforming growth factor alpha expression in normal human blood eosinophils: differential regulation by granulocyte-macrophage colony-stimulating factor and interleukin-3. Leukemia. 1994;8:612. [PubMed] [Google Scholar]
- 66.Matsumoto K, Fukuda S, Nakamura Y, Saito H. Amphiregulin production by human eosinophils. Int Arch Allergy Immunol. 2009;149 Suppl 1:39. doi: 10.1159/000210652. [DOI] [PubMed] [Google Scholar]
- 67.Zandi R, Larsen AB, Andersen P, Stockhausen MT, Poulsen HS. Mechanisms for oncogenic activation of the epidermal growth factor receptor. Cell Signal. 2007;19:2013. doi: 10.1016/j.cellsig.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 68.Gan HK, Kaye AH, Luwor RB. The EGFRvIII variant in glioblastoma multiforme. J Clin Neurosci. 2009;16:748. doi: 10.1016/j.jocn.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 69.Deininger MH, Pater S, Strik H, Meyermann R. Macrophage/microglial cell subpopulations in glioblastoma multiforme relapses are differentially altered by radiochemotherapy. J Neurooncol. 2001;55:141. doi: 10.1023/a:1013805915224. [DOI] [PubMed] [Google Scholar]
- 70.Yamahara T, Numa Y, Oishi T, Kawaguchi T, Seno T, Asai A, Kawamoto K. Morphological and flow cytometric analysis of cell infiltration in glioblastoma: a comparison of autopsy brain and neuroimaging. Brain Tumor Pathol. 27:81. doi: 10.1007/s10014-010-0275-7. [DOI] [PubMed] [Google Scholar]
- 71.Li JJ, Dickson D, Hof PR, Vlassara H. Receptors for advanced glycosylation endproducts in human brain: role in brain homeostasis. Mol Med. 1998;4:46. [PMC free article] [PubMed] [Google Scholar]
- 72.Trutmann M, Terracciano L, Noppen C, Kloth J, Kaspar M, Peterli R, Tondelli P, Schaeffer C, Zajac P, Heberer M, Spagnoli GC. GM-CSF gene expression and protein production in human colorectal cancer cell lines and clinical tumor specimens. Int J Cancer. 1998;77:378. doi: 10.1002/(sici)1097-0215(19980729)77:3<378::aid-ijc12>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 73.Lang SH, Miller WR, Duncan W, Habib FK. Production and response of human prostate cancer cell lines to granulocyte macrophage-colony stimulating factor. Int J Cancer. 1994;59:235. doi: 10.1002/ijc.2910590216. [DOI] [PubMed] [Google Scholar]
- 74.Bennicelli JL, Guerry Dt. Production of multiple cytokines by cultured human melanomas. Exp Dermatol. 1993;2:186. doi: 10.1111/j.1600-0625.1993.tb00031.x. [DOI] [PubMed] [Google Scholar]
- 75.Luna-More S, Florez P, Ayala A, Diaz F, Santos A. Neutral and acid mucins and eosinophil and argyrophil crystalloids in carcinoma and atypical adenomatous hyperplasia of the prostate. Pathol Res Pract. 1997;193:291. doi: 10.1016/S0344-0338(97)80006-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


