Abstract
Objective
The purpose of this study was to extend our earlier work to determine the extent to which cardiorespiratory fitness is associated with the frequency of memory problems via its effects on the hippocampus and spatial working memory. We hypothesized that age, sex, education, body composition, and physical activity were direct determinants of fitness which, in turn, influenced frequency of forgetting indirectly through hippocampal volume and spatial working memory.
Method
We conducted assessments of hippocampal volume, spatial working memory, frequency of forgetting, BMI, physical activity, demographic characteristics, and cardiorespiratory fitness in 158 older adults (M age = 66.49). Path analyses within a covariance modeling framework were used to examine relationships among these constructs.
Results
Sex, age, BMI, and education were all significant determinants of cardiorespiratory fitness. The hypothesized path models testing the effects of fitness on frequency of forgetting through hippocampal volume and accuracy and speed of spatial working memory all fit the data well.
Conclusions
Our findings suggest that older adults with higher levels of fitness show greater preservation of hippocampal volume which, in turn, is associated with more accurate and faster spatial memory and fewer episodes of forgetting. Given the proportion of older adults reporting memory problems, it is necessary to determine whether improvements in fitness brought about by physical activity interventions can result in subsequent attenuation of memory problems or potentially improvements in memory.
Keywords: Frequency of Forgetting, Hippocampus Volume, Cardiorespiratory Fitness, Spatial Memory, Older Adults
The forthcoming decades will witness a dramatic increase in the population of older adults in the United States, resulting in an increased prevalence of individuals at risk for, and living with, chronic disease and functional disability (CDC, 2008). To some extent, preventive health behaviors such as a healthy diet and regular physical activity can reduce these risks. In particular, physical activity can enhance cardiorespiratory fitness, which is associated with a decreased risk of all-cause mortality and chronic illnesses, especially cardiovascular disease (Blair et al., 1996; Carnethon, Gulati, & Greenland, 2005). Importantly, cross-sectional studies and randomized clinical trials suggest that cardiorespiratory fitness is associated with brain structure and function. For example, Colcombe, et al. (2003) reported loss of tissue densities in the frontal, parietal, and temporal cortices due to aging were substantially reduced in adults with higher levels of cardiorespiratory fitness. Additionally, improvements in fitness brought about by exercise training have been associated with improvements in processing speed and executive function (Kramer et al., 1999). Colcombe et al. (2004) also demonstrated that aerobically trained older adults had greater task-related activity in regions of the prefrontal and parietal cortices that are involved in selective attention and inhibitory functioning.
The aging process has well-documented effects on brain structure, in particular, the hippocampal region (Raz & Rodrigue, 2006). Longitudinal studies suggest that hippocampal volume shrinks at a median rate of 1.23% per year (Raz, 2004) and this rate is twice as great in healthy older adults than younger adults (Raz et al., 2004). Compromised hippocampal volume has, in turn, been associated with poor spatial working memory in humans (Kelley et al., 1998; Maguire, Frackowiak, & Frith, 1997) and rodents (Seamans, Floresco, & Phillips, 1998). Importantly, spatial working memory tasks are often composed of cognitive skills that are largely dependent on a fully functioning hippocampus (Lee, Jerman, & Kesner, 2005). Whether higher levels of cardiorespiratory fitness play a protective role in the preservation of hippocampal volume is an important research question, given that fitness is modifiable with increased exercise participation. This relationship has been studied in animals and this literature provides evidence that exercise facilitates hippocampal neurogenesis (Pereira et al., 2007; van Praag, Kempermann, & Gage, 1999; van Praag, Shubert, Zhao, & Gage, 2005), which has been associated with learning and memory (Leuner, Waddell, Gould, & Shors, 2006). In a recent report, Erickson and his colleagues (2009) suggested that higher levels of aerobic fitness were associated with greater hippocampal volume in a large sample of older adults. In turn, greater volume was associated with better performance on a spatial memory task. In these analyses, Erickson et al. statistically controlled for the potential confounding influence of demographic characteristics such as age, sex, and education. However, a number of other factors associated with lifestyle that have previously been associated with cognitive function, including physical activity (Weuve et al., 2004) and body composition (Gunstad et al., 2007), were not considered. However, these latter factors, coupled with age, education and sex represent legitimate foundational elements of fitness, rather than being treated as simply noise in the system (i.e., covariates).
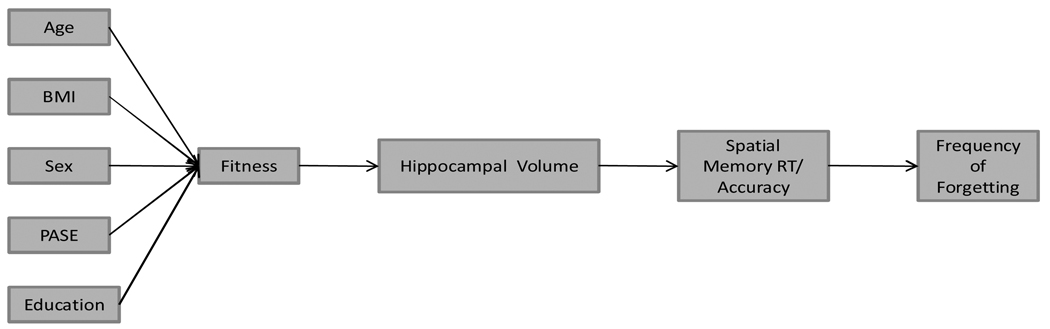
Given that the prevalence of memory problems in older adults range from 25–60% (De Jager, Blackwell, Budge, & Sahakian, 2005; Jonker, Geerlings, & Schmaud, 2000), understanding the biological, behavioral, and physiological factors that may influence these problems is an important public health issue. This is particularly the case if some of these factors are modifiable through lifestyle change. Given that self-appraisals of memory have been associated with subsequent cognitive decline in older adults (Schofield et al., 1997) and with brain function (Small, Larue, Komo, Kaplan, & Mandelkern, 1995), we were interested in further examining the association between cardiorespiratory fitness and self-appraisals of memory (i.e., frequency of forgetting). To do so, we significantly extend the data published by Erickson et al. (2009) in the following manner. First, we treated sex, age, education, physical activity, and body composition (i.e., body mass index; BMI) as determinants of fitness. Second, based upon the animal and human literature that indicates a well-functioning hippocampus is associated with spatial working memory and that exercise-induced fitness is associated with hippocampal preservation, we hypothesized that the relationship between fitness and memory self-appraisals (frequency of forgetting) would be mediated first by hippocampal volume and, in turn, by spatial working memory. Third, we use path analysis within a covariance modeling framework to test the hypothesized models. Fourth, in examining relationships among the hippocampus, spatial working memory, and frequency of forgetting, we used sex, age, and education as covariates. Finally, because hypertension and cardiovascular risk factors are associated with hippocampal atrophy (Raz & Rodrigue, 2006) and depression is correlated with more frequent memory complaints (Zelinski & Gilewski, 2004), we used these factors as covariates in preliminary model testing. This allowed for a very stringent test of the following two path models. In both, we hypothesized that being male, younger, more active, better educated, and having lower BMI would be associated with greater levels of fitness. In addition, being fitter was hypothesized to be associated with greater hippocampal volume which would, in turn, be associated with better spatial memory performance (i.e. in Model 1, faster reaction time and in Model 2, better accuracy). Finally, we hypothesized that better spatial memory would be associated with less frequent episodes of forgetting. The hypothesized model is shown in Figure 1.
Figure 1.
Hypothesized Path Model
Notes: BMI = Body Mass Index; PASE = Physical Activity Scale for the Elderly
Methods
Recruitment and Participant Characteristics
One-hundred and fifty eight older adults (105 female; 53 male) were recruited for study participation via local media (e.g., newspaper announcement, radio and television public service announcements, and posting of flyers in the community). Participants were required to be between 60 and 80 years of age, not currently physically active, right handed, have at least 20/40 vision in both eyes (or corrected equivalent), and have no chronic inflammatory diseases or surgically implanted devices. Joint replacements located sufficiently far from the head and thereby unlikely to interfere with the MRI were acceptable. Not currently active was defined as engaging in 20 minutes of aerobic activity on two or fewer days per week in the past six months. Other exclusionary criteria included claustrophobia, history of stroke, or depression as classified by the 5-item Geriatric Depression Scale (Hoyl et al., 1999). Additionally, we screened for cognitive status. We initially used a score below 51 on the modified Mini-Mental Status Examination (mMMSE; Stern, Sano et al. 1987) as a screening criteria but retained several individuals with a slightly lower score whose performance on battery of neuropsychological tests gave no indication of reduced cognitive status. Prior to all testing, all participants were cleared for participation by their personal physician and completed an informed consent form previously approved by the university institutional review board. Table 1 details sample statistics for demographics and primary study variables.
Table 1.
Demographic characteristics of study participants
| Men (n= 53) | Women (n= 105) | Sample (n= 158) | ||||
|---|---|---|---|---|---|---|
| M (SD) or % | Min-Max | M (SD) or % | Min-Max | M (SD) or % | Min-Max | |
| Age | 66.22(5.91) | 59–80 | 66.62(5.59) | 59–81 | 66.48(5.69) | 59–81 |
| Race | ||||||
| Asian | --- | --- | 1.90% | --- | 1.30% | --- |
| Black | 5.70% | --- | 9.50% | --- | 8.20% | --- |
| White | 94.30% | --- | 88.60% | --- | 90.50% | --- |
| Married | 75.50% | --- | 48.60% | --- | 57.60% | --- |
| 1–3 years of college or more | 82.90% | 9th grade- Ph.D or equivalent | 79.00% | 12th grade- Ph.D or equivalent | 80.40% | 9th grade- Ph.D or equivalent |
| GDS | .47(.64) | 0–3 | .35(.62) | 0–3 | .39(.63) | 0–3 |
| mMMSE | 54.66(2.53) | 46–57 | 54.41(2.25) | 47–57 | 54.50(2.35) | 46–57 |
| BMI | 29.34(4.81) | 23.20–38.65 | 28.85(4.92) | 18.87–42.58 | 29.02(4.40) | 18.87–42.58 |
| PASE | 138.59(64.38) | 27.18–311.00 | 123.35(63.59) | 25.00–311.71 | 128.46(64.06) | 25.00–311.71 |
| VO2 (mL/kg) | 24.20(4.81) | 12.90–34.70 | 19.27(3.68) | 12.90–29.40 | 20.92(4.70) | 12.90–34.70 |
| Hip. Vol. (cm3) | 5.15(.82) | 3.32–7.18 | 4.71(.59) | 3.62–6.54 | 4.86(.70) | 3.32–7.18 |
| SpWM RT (ms) | 874.97(153.10) | 545.24–1204.58 | 989.17(170.86) | 636.38–1531.70 | 950.85(173.28) | 545.24–1531.70 |
| SpWM Acc | 83.7%(10.94%) | 43.00%–97.00% | 79.97%(14.31%) | 34.00%–99.00% | 81.25%(13.37%) | 34.00%–99.00% |
| F of F | 48.66(8.62) | 27.00–63.00 | 48.22(9.45) | 25.00–67.00 | 48.37(9.15) | 25.00–67.00 |
Notes: Women coded as 1 and Men as 2; BMI= Body Mass Index; PASE= Physical Activity Scale for the Elderly; GDS= Geriatric Depression; Scale VO2= Cardiorespiratory fitness; Hip. Vol.= Hippocampus Volume; mMMSE = Modified Mini-Mental Status SpWM RT= Spatial working memory reaction time; SpWM Acc= Spatial working memory accuracy; F of F= Frequency of forgetting.
Measures
Demographic Characteristics and Health Conditions
Participants completed a short questionnaire assessing basic demographic characteristics (i.e., sex, age, education, marital status, race) and self-reported health condition. From the latter, a measure of cardiovascular history was constructed. Conditions such as being diagnosed with coronary artery disease, peripheral vascular disease, experiencing shortness of breath, heart rhythm disorders, high cholesterol levels, and diabetes were checked as yes/no and all positive responses totaled to provide a measure of cardiovascular health. Presence of hypertension was indicated as a yes or no response to the question of whether the participant had high blood pressure. Unfortunately, we have no evidence as to whether participants’ blood pressure was controlled or not.
Body Mass Index (BMI)
Height and weight were measured using a Seca electronic scale and stadiometer (Model 763 1321139). Participants were measured while wearing light clothing and without shoes. BMI was calculated using the standard formula of weight (kg) / [height (m)]2.
Physical Activity
Physical activity was assessed with the Physical Activity Scale for the Elderly (PASE; Washburn, Smith, Jette, & Janney, 1993). The PASE is a 10-item measure that combines activities from several domains, including leisure, household, and occupational activities, to assess physical activity in older adults. We calculated a PASE total score by multiplying the total number of hours per week respondents spent participating in these activities by corresponding PASE item weights and summing across all activities. Validity evidence for the PASE has been reported for a number of exercise trials (e.g., Dinger, Oman, Taylor, Vesely, & Able, 2004; Washburn, McAuley, Katula, Mihalko, & Boileau, 1999).
Cardiorespiratory Fitness
Participants completed a physician-supervised graded exercise test (GXT) using a modified Balke protocol (Balke & Ware, 1959; Froelicher, Thompson, Davis, Stewart, & Triebwasser, 1975). Participants walked at a brisk, self-selected pace on a motor-driven treadmill. The grade of the treadmill was set at 0% for a 3-minute warm-up and the first 2-minute stage of the test. For the second stage (minutes 3 and 4 of the test) the incline was increased by 2% with the incline increasing every 2 minutes by 2% or 3%, depending upon the subject’s respiratory exchange ratio (RER). If the participant’s RER was ≥ 1.0, the grade increased by 2% otherwise the grade was increased by 3%. The test was complete when the participant terminated the test volitionally or the physician stopped the test due to medical concerns. At this point the grade of the treadmill was returned to 0% for the cool down. A ParvoMedics® metabolic system and software were used to measure expired oxygen, carbon dioxide, ventilation, and respiratory exchange ratio. Blood pressure was monitored continuously and heart rate was measured via direct 12-lead electrocardiographic monitoring. Peak oxygen consumption was assessed via continuous sampling of expired gases and VO2peak was indicated as the highest value achieved when meeting two of three standard criteria (i.e., plateau of VO2; reaching age-predicted maximum heart rate; and RER greater than 1.10).
Hippocampal Volume
High-resolution (1.3 × 1.3 × 1.3 mm) T1-weighted brain images were acquired using a 3D MPRAGE (Magnetization Prepared Rapid Gradient Echo Imaging) protocol. All images were collected on a 3T Siemens Allegra scanner with an echo time (TE) = 3.87 ms, repetition time (TR) = 1,800 ms, field of view (FOV) = 256 mm, an acquisition matrix of 192 × 192 mm, and a flip angle of 8 degrees. FMRIB’s Integrated Registration and Segmentation Tool (FIRST) in FMRIB’s Software Library (FSL) version 4.0 was used for segmentation and volumetric analyses. As noted by Erickson et al. (2009), FIRST is a model-based subcortical segmentation tool which uses shape and appearance models obtained from manually segmented images from the Center for Morphometric Analysis, Massachusetts General Hospital, Boston. These images are modeled as a point distribution model in which the geometry and variation of the shape of the structure are submitted as priors. Volumetric labels are parameterized by a 3D deformation of a surface model based on multivariate Gaussian assumptions. Regional volumes are typically adjusted for height and sex by calculating intracranial volume (ICV; Raz et al., 2005). To determine ICV, we aggregated the sum of gray, white, and cerebrospinal fluid and subsequently used this to adjust the hippocampal regions using FMRIB’s automated segmentation tool in FSL version 4.0. (Smith et al., 2004; Zhang, Brady, & Smith, 2001). Finally, we used an analysis of covariance method for adjusting regions in these analyses in which the adjusted volume = raw volume − b × (ICV− mean ICV), where b is the slope of a regression of an ROI volume on IVC (Head, Rodrigue, Kennedy, & Raz, 2008; Kennedy et al., 2009; Raz, 2004; Raz et al., 2004). Adjusted volume was used in all statistical analyses reported herein.
Spatial Working Memory
To assess spatial working memory, we used a task which has been associated with age and also a genetic predisposition for AD (Greenwood, Lambert, Sunderland, & Parasuraman, 2005). Briefly, participants fixate on a crosshair for 1 seconds (s) whereupon one, two, or three black dots (set sizes) appeared randomly on the screen locations for 500 ms followed by the re-appearance of the crosshair for a period of 3 s. Following this a red dot appeared on the screen in either one of the same locations as the target dots (match condition) or at a different location (non-match condition). Participants had 2 s to determine whether the stimulus was matched or unmatched by pressing one of two keys on a standard keyboard. Each condition had 20 trials (Erickson et al., 2009) and participants were given several practice trials to familiarize themselves with the task and associated instructions. As noted above, three different set sizes were assessed in terms of reaction time and accuracy. Initial tests of the hypothesized model for each of the three set sizes produced identical fit indices (see preliminary model testing section). As we were interested in overall spatial working memory, we therefore used the average reaction time and average accuracy of response score by participants across three set sizes for all analyses.
Frequency of Forgetting (F of F)
Zelinski and Gilewski (2004) have recently developed a 10-item version of the 33-item Frequency of Forgetting (F of F) scale from the Memory Functioning Questionnaire (MFQ; Zelinski, Gilewski, & Anthony-Bergstone, 1990). The 10-item F of F scale assesses the frequency with which participants have forgotten such things as names, where they have put things, faces, and directions. Such items are relational in nature and therefore one would expect them to be associated with hippocampal volume and also with spatial memory tasks which involve a spatial instantiation of relational memory.
Items were rated on a seven point Likert scale with lower ratings indicating more negative self-report, or a greater frequency of forgetting. Mean rating was calculated by summing all items and dividing by 10. The 10-item measure was developed by using the 10 best discriminating items from the longer 33-item measure using Rasch scaling procedures. Zelinski & Gilewski (2004) report excellent reliability across items and persons for the shorter version of the scale. Construct validity was demonstrated by theorized relationships with depression, conscientiousness and actual memory performance (Zelinski & Gilewski, 2004).
Data Analysis
Initial analyses examined the distribution of the data for skewness and kurtosis. All model variables followed a normal distribution, and there were no missing data for any of the study variables. Bivariate correlation analyses examined the initial associations among all primary variables. This was followed by a series of path analyses within a covariance modeling framework using the MLR estimator in Mplus (version 6.0, Muthén & Muthén, 1998–2010) which is highly robust with non-normal, categorical, and missing data. The analyses examined the direct effects of sex, BMI, age, education, and physical activity levels on fitness; the direct effects of fitness on hippocampal volume and indirect effects of fitness on, spatial memory (accuracy and reaction time) and F of F; the direct effects of hippocampal volume on spatial memory; and the direct effect of spatial memory on F of F. All paths between fitness, hippocampal volume, spatial working memory, and F of F were saturated for sex, age, and education and mMMSE score (i.e., treated as covariates on each path).
Model fit
Model-data fit was assessed using the chi-square statistic, standardized root mean square residual (SRMR), and comparative fit index (CFI). The chi-square statistic assessed absolute fit of the model to the data (Jöreskog & Sörbom, 1996). The SRMR is the average standardized residual value derived between the variance/covariance matrix for the hypothesized model and the variance/covariance matrix of the sample data (Bollen, 1989). The value of the SRMR should be less than .08 for a good fitting model (Hu & Bentler, 1999). The CFI tests the proportionate improvement in fit by comparing the hypothesized model with the independence model (Bentler, 1990). The value of the CFI should approximate 0.95 or greater for a good fitting model (Hu & Bentler, 1999).
Results
Descriptive Characteristics
The sample had a mean age of 66.49 yr (± 5.59), was overweight (BMI = 29.91 (± 4.39)), low fit (VO2max = 20.94 ml/min/kg (± 4.74)), well-educated (80% with 1–3 years of college or more), and primarily female (65.4%). One participant was 59 yr at study entry and turned 60 yr shortly after baseline. Table 1 presents further descriptive data on all variables for males, females, and the total sample. Table 2 shows the frequency of self-reported health conditions within the sample.
Table 2.
Frequency of participant self-reported health conditions
| Health Condition | % for Men | % for Women | % for Sample |
|---|---|---|---|
| Cardiovascular disease, surgery, or interventions | 9.4% | 1.9% | 4.4% |
| Significant disorders of heart rhythm | 11.3% | 7.6% | 8.9% |
| Peripheral vascular disease | 1.9% | 1.0% | 1.3% |
| Pulmonary disease (asthma) | 5.7% | 7.6% | 7.0% |
| Central nervous system disorders or residuals | 0.0% | 0.0% | 0.0% |
| Osteoporosis | 1.9% | 29.5% | 20.3% |
| Severe back problems | 11.3% | 9.5% | 10.1% |
| Severe arthritis | 5.7% | 14.3% | 11.4% |
| Hypertension | 52.8% | 44.8% | 47.5% |
| Hyperlipidemia | 35.8% | 51.4% | 46.2% |
| Diabetes | 7.5% | 12.4% | 10.8% |
| Anemia or bleeding disorder | 1.9% | 3.8% | 3.2% |
| Phlebitis or emboli | 0.0% | 1.0% | 0.6% |
| Cancer | 28.3% | 14.4% | 19.1% |
| Emotional disorders | 11.3% | 13.3% | 12.7% |
| Ulcers | 1.9% | 1.0% | 1.3% |
| Edema | 11.3% | 15.2% | 9.5% |
| Infectious disease | 0.0% | 1.0% | 0.6% |
| Hearing loss | 30.2% | 21.9% | 24.7% |
Correlational Analyses
As can be seen in Table 3, all hypothesized correlations are significant at the bivariate level. That is, being male, younger, better educated, more active, and having lower BMI, were significantly associated with having higher cardiorespiratory fitness (all ps < .05). BMI and physical activity were not significantly correlated with hippocampal volume, spatial memory, or F of F. However, being fitter was associated with greater hippocampal volume, better spatial working memory performance, and less frequent forgetting episodes (p < .05). Cardiovascular conditions and hypertension were associated with lower fitness levels and depression was correlated with memory complaints. Additionally, being older was associated with lower hippocampal volume, poorer working memory, and more memory complaints.
Table 3.
Bivariate relationship among all study variables.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Age | --- | −.033 | −.121 | −.165* | −.095 | .019 | −.111 | .148 | −.430** | −.414** | .210** | −.307** | −.224** |
| 2. Sex | --- | .053 | .173* | .076 | .113 | .090 | .497** | .290** | −.312** | .135 | .023 | ||
| 3. Education | --- | −.016 | −.027 | −.027 | −.065 | −.214** | .229** | .128 | −.061 | .038 | .241** | ||
| 4. BMI | --- | .080 | .242** | .012 | −.049 | −.215** | .009 | −.029 | −.004 | .072 | |||
| 5. CVD | --- | .103 | −.103 | −.087 | −.168* | .012 | .049 | −.039 | −.036 | ||||
| 6. Hypertension | --- | −.126 | −.056 | −.218** | .018 | −.090 | −.126 | −.094 | |||||
| 7. PASE | --- | −.075 | .157* | .059 | −.117 | .030 | −.025 | ||||||
| 8. GDS | --- | -.001 | −.052 | −.023 | −.122 | −.170 | |||||||
| 9. VO2 | --- | .534** | -.333** | .309** | .168* | ||||||||
| 10. Hip. Vol. | --- | −.329** | .311** | .231** | |||||||||
| 11. SpWM RT | --- | −.395** | −.203* | ||||||||||
| 12. SpWM Acc | --- | .176* | |||||||||||
| 13. F of F | --- |
Correlation is significant at p<.05
Correlation is significant at p<.01
Notes: Sex = Women: 1, Men: 2; BMI= Body Mass Index; CVD= Cardiovascular Disease; PASE= Physical Activity Scale for the Elderly; GDS= Geriatric Depression Scale; VO2= Cardiorespiratory fitness; Hip. Vol.= Hippocampus Volume; SpWM RT= Spatial working memory reaction time; SpWM Acc= Spatial working memory accuracy; F of F= Frequency of forgetting.
Path Models
Preliminary Model Testing
As the spatial working memory task was composed of three set size tasks, we conducted six preliminary path analyses (2 (behavioral outcomes; RT and accuracy) × 3 (set sizes)) and compared the model fit for each size against the model using average set size scores. In all cases, the model fit indices mirrored those of the average set size model with identical CFI (1.00) and SRMR values (.02). The paths between hippocampal volume, reaction time and accuracy for each set size, and memory complaints were then statistically compared. None of these paths were significantly different from each other within each behavioral outcome, supporting our decision to use the average scores to examine overall spatial working memory in subsequent model testing.
Because hypertension and cardiovascular risk factors have been associated with hippocampal atrophy (Raz & Rodrigue, 2006) and depression is correlated with more frequent memory complaints (Zelinski & Gilewski, 2004), we next conducted analyses using these variables as covariates. Participants were classified as self-reporting hypertension or presence of cardiovascular disease based on their health history. Depression scores were derived from screening assessments using the 5-item Geriatric Depression Scale (Hoyl et al., 1999). Neither hypertension nor cardiovascular disease status were associated with hippocampal volume, spatial working memory, or F of F and were subsequently dropped in final models. Similarly, the bivariate association between depression and memory complaints was no longer significant when all variables were included in the path models. Interestingly, modification indices suggested that a direct path from hippocampal volume to F of F would improve the model fit. Consequently we added this path in final model testing.
Final Model Testing
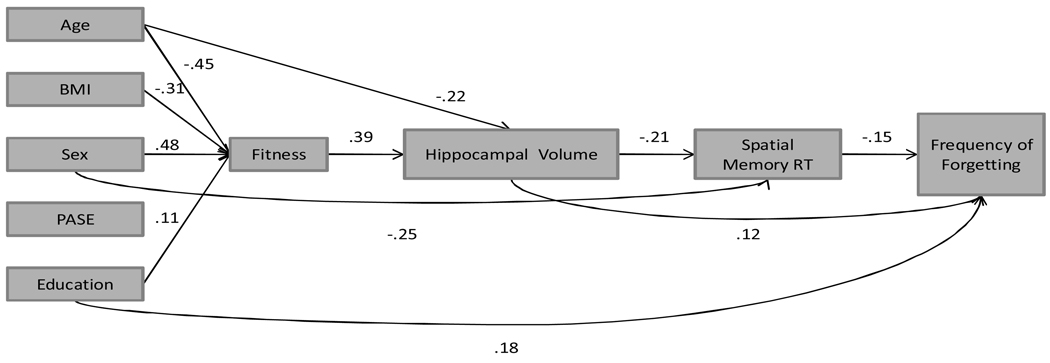
Figures 2a (RT) and 2b (accuracy) show the final models. All paths shown are significant and values reflect standardized path coefficients. Model 1 (see Figure 2a) provided an excellent fit to the data (χ2 = 3.85, df = 11, p = ns; SRMR = 0.01, CFI = 1.0). In this model, there were significant (p <.05) associations of sex (β = .48), age (β = −.45), BMI (β = −.31), and education (β = .11) but not physical activity (β = .07) with fitness. These findings are consistent for both models and will not be subsequently repeated. As hypothesized, being male, younger, better educated, and having lower BMI were associated with higher fitness levels. Being younger (β = −.22) and fitter (β = .39) were significantly associated with having greater hippocampal volume. In turn, there was a significant direct effect of hippocampal volume on mean spatial memory reaction time (β = −.21). Additionally, males had faster reaction (β = −.25). Both hippocampal volume (β = .12) and spatial working memory reaction time (β = −.15) were associated with lower F of F. Finally, participants with higher levels of education reported fewer memory complaints (β = .18). Overall, the model accounted for 53% of the variance in fitness, 34% of the variance in hippocampal volume, 17.1% in reaction time, and 15% in F of F.
Figure 2.
a. Path model showing direct and indirect effects of fitness, hippocampal volume, spatial working memory (RT) on frequency of forgetting.
Notes: BMI = Body Mass Index; PASE = Physical Activity Scale for the Elderly
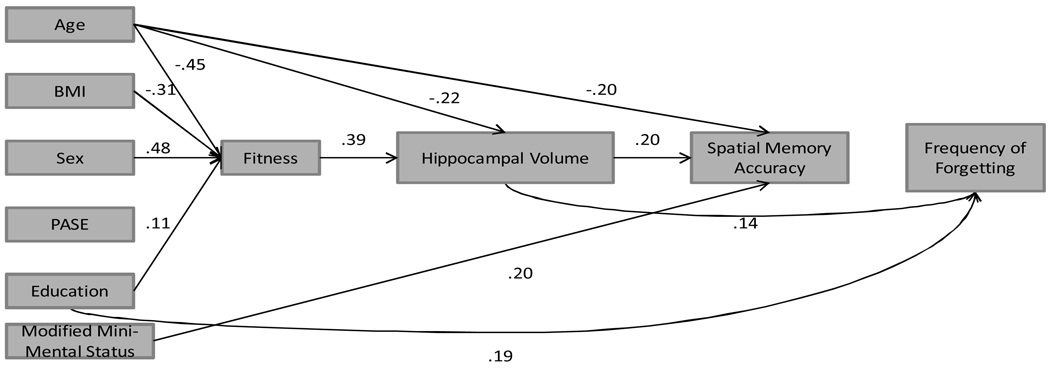
b. Path model showing direct and indirect effects of fitness, hippocampal volume, spatial working memory (Accuracy) on frequency of forgetting.
Notes: BMI = Body Mass Index; PASE = Physical Activity Scale for the Elderly
Model 2 (see Figure 2b) was also an excellent fit to the data (χ2 = 6.45, df = 11, p = ns; SRMR = 0.02, CFI = 1.00). Age had a significant direct effect on hippocampal volume (β = −.23) and mean spatial memory for accuracy (β = −.21). There was a direct effect of fitness on hippocampal volume (β = .39), which in turn was significantly related to mean spatial memory for accuracy (β = .18). Additionally, there was a direct effect of mMMSE status on spatial memory for accuracy (β = .20). Greater accuracy was not significantly associated with lower F of F (β = .06); however, hippocampal volume had a significant direct effect on F of F (β = .14). Again, greater levels of education were associated with fewer memory complaints (β = .21). Overall, the model accounted for 53% of the variance in fitness, 33% of the variance in hippocampal volume, 14% of spatial working memory accuracy, and 12.6% in F of F.
Discussion
In the present study we tested the hypothesis that cardiorespiratory fitness is associated with frequency of forgetting indirectly through its influence on hippocampal volume and, in turn, spatial working memory. This hypothesized model used sex, age, physical activity, education and BMI as determinants of cardiorespiratory fitness and saturated all other hypothesized paths for demographic characteristics. For the most part, our hypotheses were supported. These data may have important implications for understanding the everyday memory complaints that are associated with the aging process and, to our knowledge, represent the first data showing associations between fitness, brain structure, cognition, and frequency of forgetting.
Several issues are of interest herein. First, much of the literature examining relationships between cognitive function and factors such as age, sex, physical activity level, and BMI has typically considered these factors as independent determinants or, alternatively, confounding variables for cognitive function. However, as others have noted, these variables are determinants of cardiovascular fitness (Steinhaus et al., 1988) and may have indirect as well as direct effects on aspects of cognitive function. Our findings suggest this to be the case with one exception; physical activity, although associated with fitness at the bivariate level, was a non-significant contributor when age, sex, and BMI were included as predictors. This may perhaps be a function of the manner in which physical activity was measured, i.e., via the PASE, and further examination of this is warranted. Indeed, it would be of interest to determine whether objective measures of physical activity, such as accelerometers, operate in a similar fashion. Alternatively, our sample was predominantly female, older, and overweight and it may be that these demographic and body compositional factors are simply suppressing the effect of physical activity.
Interestingly, as might be expected, several demographic factors influenced other components of the hypothesized models, as well as being a primary determinant of cardiorespiratory fitness. For example, being older was associated with lower hippocampal volume and less accurate spatial working memory accuracy. Importantly, the bivariate correlations between age, hippocampal volume and spatial memory RT and accuracy were all significant. However, these associations were attenuated or rendered nonsignificant when cardiorespiratory fitness is included in the model. These findings suggest two things. First, the relationship between age and spatial working memory operates through multiple pathways. Second, they support the position that higher levels of fitness may partially offset age-related declines in brain structure and function in older adults (Colcombe, Kramer, McAuley, Erickson, & Scalf, 2004). Additionally, being better educated appears to have a direct effect on the frequency of forgetting even when controlling for all other variables in the model. Possibly, education allows individuals to develop strategies to offset memory lapses as we age. Finally, men had faster reaction time than women but there were no differences in their accuracy of responses. Other reports of reaction time in older adults have also suggested that males have faster responses than females (Lord & Fitzpatrick, 2001).
Although our earlier study (Erickson et al., 2009) supports other reports in the literature suggesting that fitness offers a protective effect on brain health (Cotman, Berchtold, & Christie, 2007; Hertzog, Kramer, Wilson, & Lindenberger, 2009), the major contribution of this report is the subsequent link to memory self-appraisals. Older adults’ assessments of their memory are typically voiced as difficulties in their inability to remember names, places, directions, etc. Our data suggest an indirect effect of fitness on frequency of forgetting, in part, through the hippocampus-spatial memory pathway. This latter link appears to be the case with speed of spatial working memory responses but not in terms of accuracy. Additionally, there was also a direct association of hippocampal volume with frequency of forgetting, indicating that the retention of hippocampus volume, in part as a function of higher levels of fitness, contributes to fewer memory complaints.
Although we attempted to recruit participants who were cognitively intact, and our conclusions are drawn on this assumption, we cannot rule out the possibility that some of our participants, who reported frequently forgetting names, places, locations, etc., might meet the criteria for being diagnosed as having mild cognitive impairment. Indeed, a meta-analysis by Heyn, Abreu, and Ottenbacher (2004) reported an overall medium effect size for fitness training effects on cardiorespiratory fitness and cognitive function outcomes in older adults with cognitive impairment. Additionally, positive effects of exercise training have recently been reported in women with mild cognitive impairment (Baker et al., 2010). Nevertheless, whether the relationships described in this paper hold in cognitively impaired samples remains to be determined.
There are several features of this work that bear further discussion. For example, one might question why we have focused upon the hippocampus rather than other areas of the brain. There are several reasons for this. First, the spatial working memory task used in the present study assesses a type of relational memory, which is highly dependent on the hippocampus (see Konkel & Cohen, 2009, for a review). Second, it is important to underscore that we do not view the hippocampus as the only, or perhaps even the most important, structure for spatial working memory performance. Indeed, our findings and those of Erikson et al. (2009) suggest that the volume of the hippocampus only partially mediates task performance. Thus, the hippocampus can be thought of as a part of a network of regions involved with spatial working memory performance.
Although our findings suggest that lower cardiorespiratory fitness is associated with decreased hippocampal volume, it is clear that other factors are involved in atrophy. As noted by Raz & Rodrigue (2006), healthy older adults show volume shrinkage rates which are twice that of their younger counterparts. Our data show an independent direct effect of age on hippocampal volume with our older participants having greater atrophy. Additionally, physical health conditions such as hypertension and cardiovascular conditions have been reported to detrimentally influence brain structure (Raz & Rodrigue, 2006). However, our analyses were unable to detect any effect of these conditions on hippocampal volume, although we acknowledge that we employed relatively healthy participants in our study and fairly crude assessments of medical conditions. Moreover, we did not have data to determine who had controlled versus uncontrolled hypertension. Depressive mood has previously been associated with frequency of forgetting (Zelinski & Gilewski, 2004) and in the present study there was a significant association at the bivariate level. However, this relationship was no longer significant when tested in the path analyses.
We are conscious of the limitations of our cross-sectional design and the obvious inability to assign cause and effect relationships in the models tested. Although the hypothesized sequencing of variable relationships make intuitive sense it is also quite possible that a reciprocal pattern of associations exist. In addition, the present design did not allow us to examine potential cross-lagged effects of some model constructs on other components of the model. Longitudinal panel models would be required to test these latter effects. Of course, the definitive test of such relationships will be in the context of a randomized controlled trial whereby an exercise condition designed to improve cardiorespiratory fitness is compared with a non-aerobic activity condition. In this way, the extent to which the proposed models hold when reflecting changes in the key constructs due to the intervention can be tested. Only under such conditions can we determine whether improvements in cardiorespiratory fitness result in a preservation of hippocampal volume and subsequent spatial memory performance and frequency of forgetting. Additionally, there are ample data to suggest that nutrition and other lifestyle activities (i.e., engagement in activities associated with cognitive demand) are implicated in brain health and processing. The extent to which these are independent or interact with fitness has yet to be definitively determined. In addition, it will be of interest to determine whether the hypothesized relationships hold true when analyzed separately by sex. Unfortunately, our sample was not large enough to conduct such analyses. Finally, personality characteristics such as conscientiousness have been associated with a number of health conditions (Bogg & Roberts, 2004). Inclusion of such measures in subsequent studies may lend further insight into the veracity of the model tested. To our knowledge, this is the first study to examine the proposed behavioral, physiological, brain structure, and cognitive influences on subjective memory complaints, a condition that reliably affects a considerable proportion of older adults (De Jager et al., 2005; Jonker et al., 2000). Our findings will require replication and extension possibly by testing the effects of a systematic intervention to enhance cardiorespiratory fitness and thereby change in hippocampal volume, spatial working memory, and memory complaints.
ACKNOWLEDGEMENT
This work was supported by the National Institute on Aging at the National Institutes of Health (grant number 05 R37 AG025667). The authors express their appreciation to Ms Susan Herrel for coordination of this project.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/neu
REFERENCES
- Baker LD, Frank LL, Foster-Schubert K, Green PS, Wilkinson CW, McTiernan A, Plymate SR, Craft S. Effects of aerobic exercise on mild cognitive impairment: a controlled trial. Archives of Neurology. 2010;67(1):71–79. doi: 10.1001/archneurol.2009.307. 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balke B, Ware RW. An experimental study of physical fitness of Air Force personnel. United States Armed Forces Medical Journal. 1959;10(6):675. [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107(2):238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kampert JB, Kohl HW, Barlow CE, Macera CA, Paffenbarger RS, Gibbons LW. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. JAMA. 1996;276(3):205–210. [PubMed] [Google Scholar]
- Bogg BW, Roberts T. Conscientiousness and health-related behaviors: A meta-analysis of the leading behavioral contributors to mortality. Psychol Bull. 2004;130(6):887–949. doi: 10.1037/0033-2909.130.6.887. [DOI] [PubMed] [Google Scholar]
- Bollen KA. A new incremental fit index for general structural equation models. Sociol Method Res. 1989;17(3):303–316. [Google Scholar]
- Carnethon MR, Gulati M, Greenland P. Prevalence and cardiovascular disease correlates of low cardiorespiratory fitness in adolescents and adults. JAMA. 2005;294(23):2981–2988. doi: 10.1001/jama.294.23.2981. [DOI] [PubMed] [Google Scholar]
- CDC. Difficulty in physical functioning, ages 18+ U.S., 1997–2008. [Retrieved March 8, 2010];2008 from http://cdc.gov.
- Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58(2):176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, McAuley E, Erickson KI, Scalf P. Neurocognitive aging and cardiovascular fitness - Recent findings and future directions. J Mol Neurosci. 2004;24(1):9–14. doi: 10.1385/JMN:24:1:009. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends Neurosci. 2007;30:489–489. doi: 10.1016/j.tins.2007.06.011. (vol 9, pg 464, 2007) [DOI] [PubMed] [Google Scholar]
- De Jager C, Blackwell AD, Budge MM, Sahakian BJ. Predicting cognitive decline in healthy older adults. Am J Geriat Psychiat. 2005;13(8):735–740. doi: 10.1176/appi.ajgp.13.8.735. [DOI] [PubMed] [Google Scholar]
- Dinger MK, Oman F, Taylor EL, Vesely SK, Able J. Stability and convergent validity of the Physical Activity Scale for the Elderly (PASE) J Sport Med Phys Fit. 2004;44(2):186–192. [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, Chaddock L, Hu L, Morris KS, Kramer AF. Aerobic Fitness is Associated With Hippocampal Volume in Elderly Humans. Hippocampus. 2009;19(10):1030–1039. doi: 10.1002/hipo.20547. [Article] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froelicher VF, Thompson AJ, Davis G, Stewart AJ, Triebwasser JH. Prediction of maximal oxygen consumption: Comparison of the Bruce and Balke treadmill protocols. Chest. 1975;68(3):331–336. doi: 10.1378/chest.68.3.331. [DOI] [PubMed] [Google Scholar]
- Greenwood PM, Lambert C, Sunderland T, Parasuraman R. Effects of apolipoprotein E genotype on spatial attention, working memory, and their interaction in healthy, middle-aged adults: Results from the National Institute of Mental Health's BIOCARD study. Neuropsychology. 2005;19(2):199–211. doi: 10.1037/0894-4105.19.2.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J, Paul RH, Cohen RA, Tate DF, Spitznagel MB, Gordon E. Elevated body mass index is associated with executive dysfunction in otherwise healthy adults. Compr Psychiat. 2007;48(1):57–61. doi: 10.1016/j.comppsych.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22(4):491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyn P, Abreu BC, Ottenbacher KJ. The effects of exercise training on elderly persons with cognitive impairment and dementia: a meta-analysis. Archives of Physical Medicine and Rehabilitation. 2004;85:1694–1704. doi: 10.1016/j.apmr.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Hertzog C, Kramer AF, Wilson RS, Lindenberger U. Enrichment effects on cognitive developement: Can the functional capacity of older adults be preserved and enhanced? Psycological Sciences in Public Interest. 2009;9(1):1–65. doi: 10.1111/j.1539-6053.2009.01034.x. [DOI] [PubMed] [Google Scholar]
- Hoyl MT, Alessi CA, Harker JO, Josephson KR, Pietruszka FM, Koelfgen M, Rubenstein LZ. Development and testing of a five-item version of the Geriatric Depression Scale. J Am Geriatr Soc. 1999;47(7):873–878. doi: 10.1111/j.1532-5415.1999.tb03848.x. [DOI] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6(1):1–55. [Google Scholar]
- Jonker C, Geerlings MI, Schmaud B. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int J Geriatr Psych. 2000;15(11):983–991. doi: 10.1002/1099-1166(200011)15:11<983::aid-gps238>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Jöreskog KG, Sörbom D. LISREL8:User's reference guide. Chicago: Scientific Software International; 1996. [Google Scholar]
- Kelley WM, Miezin FM, McDermott KB, Buckner RL, Raichle ME, Cohen NJ, Petersen SE. Hemispheric asymmetry for verbal and nonverbal memory encoding in human dorsal frontal cortex. J Cognitive Neurosci. 1998:46–46. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kennedy KM, Erickson KI, Rodrigue KM, Voss MW, Colcombe SJ, Kramer AF, Raz N. Age-related differences in regional brain volumes: A comparison of optimized voxel-based morphometry to manual volumetry. Neurobio Aging. 2009;30(10):1657–1676. doi: 10.1016/j.neurobiolaging.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konkel A, Cohen NJ. Relational memory and the hippocampus: representations and methods. Front Neurosci. 2009;3(2):166–174. doi: 10.3389/neuro.01.023.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Colcombe A. Ageing, fitness and neurocognitive function. Nature. 1999;400(6743):418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Lee I, Jerman TS, Kesner RP. Disruption of delayed memory for a sequence of spatial locations following CA1- or CA3-lesions of the dorsal hippocampus. Neurobiol of Learn Mem. 2005;84(2):138–147. doi: 10.1016/j.nlm.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Leuner B, Waddell J, Gould E, Shors TJ. Temporal discontiguity is neither necessary nor sufficient for learning-induced effects on adult neurogenesis. J Neurosci. 2006;26(52):13437–13442. doi: 10.1523/JNEUROSCI.2781-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frackowiak RSJ, Frith CD. Recalling routes around London: Activation of the right hippocampus in taxi drivers. J Neurosci. 1997;17(18):7103–7110. doi: 10.1523/JNEUROSCI.17-18-07103.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira AC, Huddleston DE, Brickman AM, Sosunov AA, Hen R, McKhann GM, Small SA. An in vivo correlate of exercise-induced neurogenesis in the adult dentate gyrus. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(13):5638–5643. doi: 10.1073/pnas.0611721104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N. The aging brain observed in vivo: differential changes and their modifiers. In: Cabeza R, Nyberg L, Park DC, editors. Cognitive Neuroscience of Aging: Linking Cognitive and Cerebral Aging. New York: Oxford University Press; 2004. pp. 18–55. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobio Aging. 2004;25(3):377–396. doi: 10.1016/S0197-4580(03)00118-0. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Acker JD. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15(11):1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: Patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30(6):730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield PW, Marder M, Dooneief G, Jacobs DM, Sano M, Stern Y. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Amer J Psychiat. 1997;154(5):609–615. doi: 10.1176/ajp.154.5.609. [DOI] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D-1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J Neurosci. 1998;18(4):1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Larue A, Komo S, Kaplan A, Mandelkern MA. Predictors of cognitive change in middle-aged and older adults with memory loss. Amer J Psychiat. 1995;152(12):1757–1764. doi: 10.1176/ajp.152.12.1757. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Steinhaus LA, Dustman RE, Ruhling RO, Emmerson RY, Johnson SC, Shearer DE, Bonekat WH. Cardio-respiratory fitness of young and older active and sedentary men. Br J Sports Med. 1988;22(4):163–166. doi: 10.1136/bjsm.22.4.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern Y, Sano M, Paulsen J, Mayeux R. Modified Mini-Mental State Examination: Validity and Reliability. Neurology. 1987;37:179. [Google Scholar]
- van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao CM, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. Journal of Neuroscience. 2005;25(38):8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn RA, McAuley E, Katula J, Mihalko SL, Boileau RA. The physical activity scale for the elderly (PASE): Evidence for validity. J Clin Epidemiol. 1999;52(7):643–651. doi: 10.1016/s0895-4356(99)00049-9. [DOI] [PubMed] [Google Scholar]
- Washburn RA, Smith KW, Jette AM, Janney CA. The physical-activity scale for the elderly (PASE)- Developement and evaluation. J Clin Epidemiol. 1993;46(2):153–162. doi: 10.1016/0895-4356(93)90053-4. [Article] [DOI] [PubMed] [Google Scholar]
- Weuve J, Kang JH, Manson JE, Breteler MMB, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- Zelinski EM, Gilewski MJ, Anthony-Bergstone CR. Memory functioning questionaire: Concurrent validity with memory performance and self-report memory failures. Psychol Aging. 1990;5(3):388–399. doi: 10.1037/0882-7974.5.3.388. [DOI] [PubMed] [Google Scholar]
- Zelinski M, Gilewski MJ. A 10-item rasch model memeory self-efficacy scale. Aging Ment Health. 2004;8(4):293–306. doi: 10.1080/13607860410001709665. [DOI] [PubMed] [Google Scholar]
- Zhang YY, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Trans Med Imaging. 2001;20(1):45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]