Abstract
Human immunodeficiency virus (HIV) infection is associated with the loss of the two principal types of dendritic cell (DC), myeloid DC (mDC) and plasmacytoid DC (pDC), but the mechanism of this loss and its relationship to AIDS pathogenesis remain ill-defined. The nonhuman primate is a powerful model to dissect this response for several reasons. Both DC subsets have been well characterized in nonhuman primates and shown to have strikingly similar phenotypic and functional characteristics to their counterparts in the human. Moreover, decline of mDC and pDC occurs in rhesus macaques with end-stage simian immunodeficiency virus (SIV) infection, the model of HIV infection in humans. In this brief review, we discuss what is known about DC subsets in pathogenic and nonpathogenic nonhuman primate models of HIV infection and highlight the advances and controversies that currently exist in the field.
Keywords: Innate immunity, Nonhuman primate, Lymphoid tissue, In vivo, Pathogenesis
Nonhuman primate models of human immunodeficiency virus infection
Nonhuman primate models are invaluable for advancing our understanding of virus–host interactions and disease pathogenesis, and none has been more informative in this regard than the simian immunodeficiency virus (SIV) model of human immunodeficiency virus (HIV) infection. Experimental SIV infection of rhesus macaques and other Asian nonhuman primate species results in progressive disease similar to HIV infection in humans, although with an accelerated course of decline; SIV infection of rhesus macaques is therefore a key model to study AIDS pathogenesis [1–5]. In contrast, SIV infection in “natural” African nonhuman primate hosts of SIV, such as the African green monkey and sooty mangabey, is nonpathogenic, and disease does not generally develop despite productive virus replication. This model has therefore been useful to dissect the mechanisms of disease control [6–9]. Acute SIV infection in both pathogenic and nonpathogenic models is defined by a rapid and strong type I interferon (IFN) response, similar to that observed in HIV-infected humans [10–12]. However, SIV-infected African green monkeys quickly and efficiently resolve this response, whereas SIV-infected rhesus macaques do not [8, 13–16]. Thus, development of AIDS in progressive disease models has been linked to continuous activation of the innate immune system during chronic infection [17, 18]. Indeed, chronic activation of the immune system is a reliable predictor of disease progression in both HIV-infected humans and SIV-infected macaques [19–22]. Because of the central role that dendritic cells (DC) play in the innate response to pathogens, these cells have recently come under the spotlight as potentially mediating chronic immune activation, but the picture is far from clear.
Identification and characteristics of DC in the primate
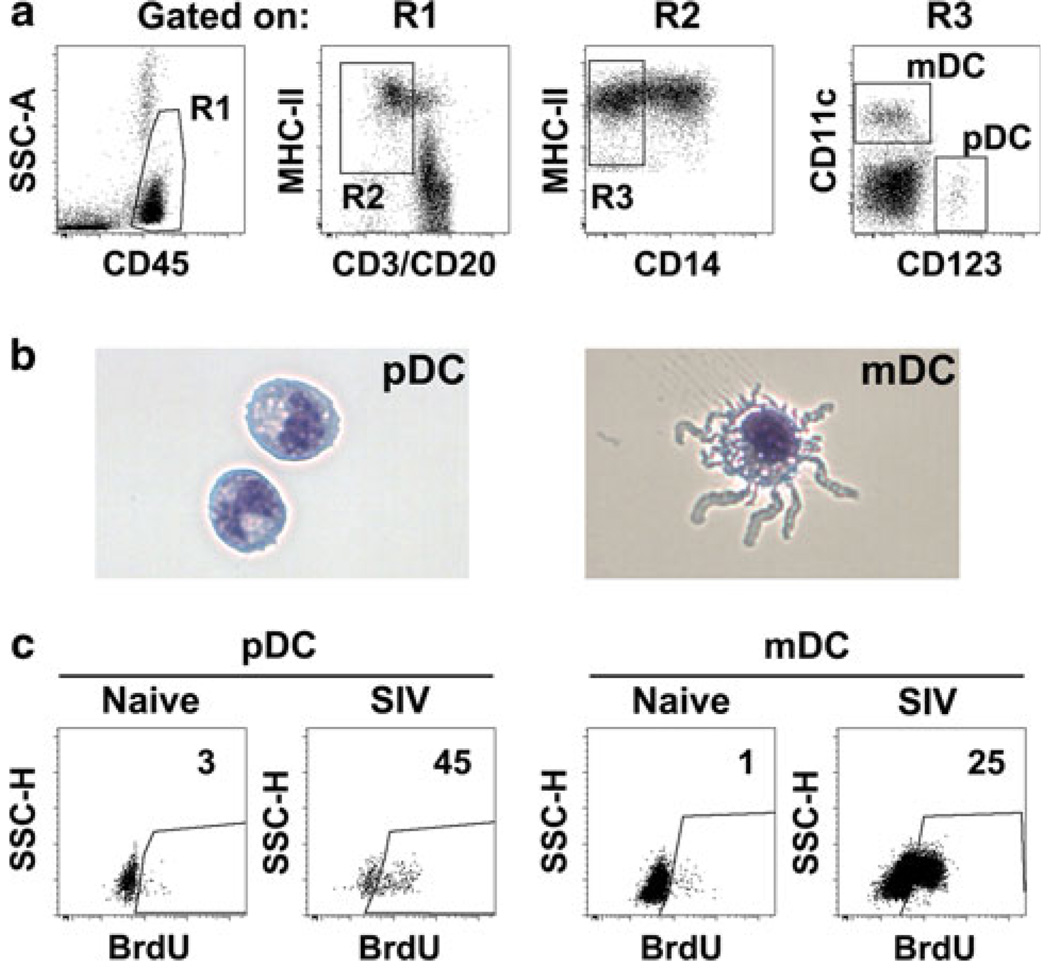
DC are antigen-presenting cells (APC) that form an indispensible bridge between the innate and adaptive immune responses. Human DC are grouped into two major subsets: myeloid DC (mDC) and plasmacytoid DC (pDC) [23]. Similarly, in rhesus macaques and other nonhuman primate species, we and others have identified mDC and pDC in blood, lymphoid tissues, and mucosal tissues [11, 24–29]. mDC are classically defined in monkey blood as MHC-class II + CD11c + CD123− cells lacking expression of the lineage markers CD3, CD14, and CD20, whereas pDC are identified as Lin–MHC-class II + CD123 + CD11c−, similar to the phenotype in humans [24, 26, 30]. Rhesus macaque mDC and pDC also have distinct morphologies, as is clear on cytospins of sorted cells (Fig. 1) [26]. Both cell types are resident in lymphoid tissues in the steady state, but during an inflammatory response, pDC and mDC are actively recruited to these tissues [31–33]. Recruitment of mDC is mediated by CCR7-CCL19/CCL21 interactions, whereas recruitment of pDC to inflamed lymph nodes is CXCL9- and E-selectin-dependent [32, 34]. An identifying feature of the pDC response to virus exposure is the production of the antiviral cytokine IFN-α, and this characteristic is seen in both human and monkey pDC [26, 27, 35–38].
Fig. 1.
Dendritic cell identification in rhesus macaques. a Representative flow cytometry dot plots revealing the gating strategy used to define pDC and mDC within peripheral blood mononuclear cells of an SIV-naïve macaque. b Representative images of pDC and mDC sorted from lymph node cell suspensions of a rhesus macaque. Cells have been stained with modified Wright–Giemsa. c Representative dot plots demonstrating increased blood pDC and mDC incorporation of 5-bromo-2′-deoxyuridine in vivo in acute SIV infection of rhesus macaques relative to pre-infection. Numbers represent percentage of cells in the indicated gate
Chronic HIV and SIV infection and the DC response
Relatively early in the AIDS epidemic, it was appreciated that HIV infection disrupts DC homeostasis. During chronic HIV infection, pDC and mDC are lost from the blood, and this depletion correlates with high plasma viral load and low CD4 + T-cell counts [39–52]. We confirmed that SIV-infected rhesus macaques with end-stage disease also have DC loss from both blood and lymphoid tissues [25]. Depletion of blood pDC is not thought to occur in nonpathogenic models of SIV infection [38, 53], and elevated blood pDC counts have been noted in HIV-infected individuals that have controlled infection, so-called long-term nonprogressors [52]. However, the relevance of pDC loss to disease progression is unclear, as it was recently reported that HIV-2 infection of humans, which is significantly attenuated relative to HIV-1 infection, is nevertheless associated with a substantial depletion of pDC from blood [54]. Whether mDC accumulate in or are lost from lymph nodes in chronic HIV infection prior to AIDS is also a matter of debate, as both increases and decreases have been reported [55–57].
We have recently addressed this issue in the pathogenic SIV model. We found that animals with long-term stable infection of more than 1 year had average increases in blood mDC of 200% over pre-infection levels at virus set point (8–12 weeks post-infection), whereas animals that progressed rapidly to AIDS within 32 weeks had significant loss of mDC at set point. Progressive infection was associated with increased expression of CCR7 on blood mDC and an eightfold increase in CCL19 expression in lymph node tissues, consistent with increased mDC recruitment that was nevertheless offset by increased apoptosis [58]. These data suggest that the inflamed lymph node serves as a sink for mDC in progressive SIV infection beginning relatively early in infection before disease manifests, consistent with a role in disease pathogenesis [59].
The DC response in acute SIV infection
Studies of DC kinetics in acute HIV infection have been slow to emerge, perhaps because at these early stages, the majority of patients are unaware of their infection status. We have taken advantage of the rhesus macaque/SIV model to begin to initially define the pDC response in acute SIV infection. We have combined absolute blood cell counts with 5-bromo-2′-deoxyuridine delivery in vivo to label recently divided cells that have been mobilized from bone marrow into blood and tissues (Fig. 1) [24, 60]. We found that as early as 3 days after intravenous virus inoculation, pDC experience a significant mobilization resulting in a three- to sevenfold increase in pDC in blood [60]. This mobilization may be related to a systemic surge in proinflammatory cytokines such as TNF-α [61], which in mouse models causes rapid pDC mobilization [32]. The increase in pDC in blood tapers rapidly, and by 14 days post-infection, blood and lymph node pDC are depleted to levels that inversely correlate with plasma viral loads. Interestingly, while the absolute number of pDC in lymph nodes decreased in acute infection, the proportion of lymph node pDC that had recently divided based on 5-bromo-2′-deoxyuridine incorporation increased 10- to 20-fold above that seen in uninfected monkeys [60]. These data indicate that pDC death exceeds recruitment within these tissues, a condition that is not consistent with a pathologic role for pDC in disease [62].
The mechanisms responsible for pDC and mDC loss are still under investigation. Research suggests that depletion of pDC in the blood could be caused by direct infection [63–65] or by apoptosis as a result of pDC fusing with HIV-infected cells [66]. We have studied DC infection during the acute phase of SIV infection of macaques when virus load in blood and lymph nodes is at its peak. We found that lymph node mDC had a negligible level of infection, whereas around 4% of pDC were productively infected, based on a quantitative PCR assay for proviral DNA in sorted cells [60]. This frequency of pDC infection in vivo is not likely to account for the substantial cell loss seen at the same time. Interestingly, lymph node pDC uniformly upregulated expression of CD95 in lymph nodes at day 14 post-infection, and death of cells could potentially result from engagement of this receptor by ligands expressed in inflamed lymph nodes [60].
Effect of infection on DC maturation and its consequences
In addition to quantitative changes that have been observed in the blood and lymph nodes, disturbances in the quality of DC have also been reported. HIV infection causes pDC to have a diminished ability to migrate toward the CXCR4 ligand, CXCL12, while mDC retain their migratory ability [56]. HIV and SIV infection is associated with increased expression of immunostimulatory molecules CD40, CD80, and CD86 on mDC [39, 58] as well as the regulatory molecule B7-H1, the ligand for PD-1 that is concomitantly upregulated on T cells [29].Expression of PD-1 on virus-specific T cells is associated with disease progression in HIV infection of humans [67]. Interestingly, we have observed that mDC from lymph nodes of macaques with stable long-term SIV infection have a less-activated, semi-mature phenotype relative to progressive infection or naïve individuals [58]. Semi-mature DC isolated from HIV-infected lymph nodes have been shown to induce CD4 + FoxP3 + T regulatory cells (Treg) in vitro [57]. Similarly, HIV-activated pDC stimulate Treg in vitro [68]. These effects have been interpreted as an immunosuppressive function that could dampen HIV immunity. Consistent with this possibility, increased proportions of FoxP3 + CD4 + T cells have been noted in progressive HIV infection [69]. However, in pathogenic SIV infection, we have noted a loss of Treg, which correlated with immune activation in vivo [70], and others have defined only a transient and delayed Treg response in pathogenic SIV infection as opposed to nonpathogenic infection of African green monkeys, associated with an imbalance with Th17 cells [71]. The capacity for DC to induce Treg or alternatively to polarize CD4 + T cells into Th1, Th2, or even Th17 cells and its relationship to early pathogenesis are a significant issue that needs to be addressed.
Friend or foe: the innate immune response to HIV and SIV infection
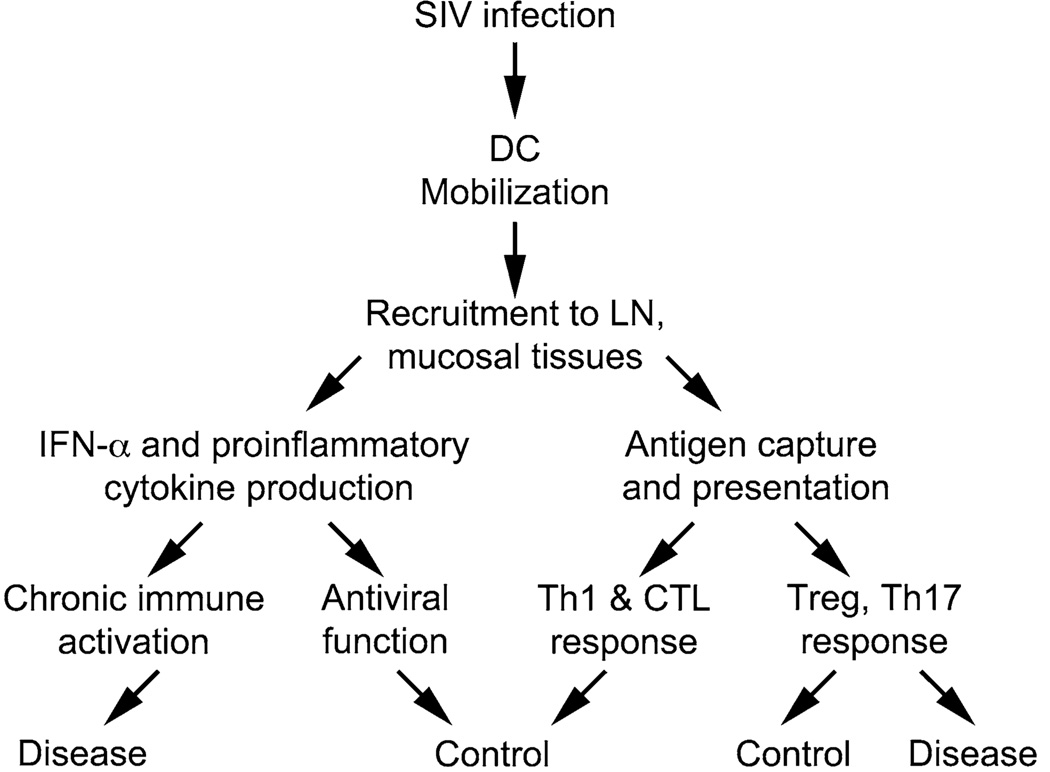
pDC and mDC express distinct arrays of the Toll-like receptor (TLR) family of pattern recognition receptors, with pDC expressing TLR7 and TLR9 and mDC expressing TLR2, TLR3, TLR4, TLR5, TLR6, and TLR8 [72, 73]. Endocytosed HIV has been shown to directly activate pDC through TLR7 recognition of the viral RNA genome, leading to the production of IFN-α [74]. HIV particles do not appear to directly stimulate TLR8 in mDC; however, HIV-encoded TLR8 agonists are effective inducers of proinflammatory cytokines in these cells, suggesting mDC are susceptible to HIV-mediated activation [75, 76]. Whether DC activation is beneficial or deleterious in HIV infection is still a matter of debate [77, 78], and there are many downstream consequences of activation and recruitment that could conceivably lead to widely different clinical outcomes (Fig. 2).
Fig. 2.
Potential beneficial and detrimental outcomes of the DC response to SIV infection. The mobilization and recruitment of DC to lymph nodes and mucosal tissues could be related to beneficial immune responses, which could control virus replication and mitigate disease progression. In contrast, chronic stimulation of DC and subsequent cytokine production could promote disease development through establishment or maintenance of deleterious chronic immune activation
pDC and pDC-derived IFN-α have a well-established role in the control of virus infection including HIV-1 (the latter demonstrated in vitro) [66, 79–82]. Our preliminary data indicate that pDC are recruited and accumulated within gut and lung mucosal tissues in acute infection (M. Kader and Z. Swan, unpublished data) where they could serve an antiviral function, as with other viral infections [80, 81]. In contrast, it has been suggested that continual IFN-α production by persistently stimulated pDC could cause chronic immune activation and subsequent dysfunction [18, 38, 83, 84]. Furthermore, products of the IFN-signaling pathway remain elevated throughout chronic infection, associated with increased CD4 + T-cell activation and apoptosis [85]. HIV appears to induce persistent pDC activation in vitro [86], and pDC derived from women (who progress more rapidly to AIDS than do men at a given virus load [87–89]) produce more IFN-α in response to HIV-1-derived TLR7 ligands [90]. Again, this is a controversial issue, as therapy with IFN-α has been shown to have a beneficial effect in chronic HIV infection, significantly reducing virus loads [91]. pDC have also been shown to induce immunosuppressive effects by expressing the tryptophan-catabolizing enzyme, indoleamine-pyrrole 2,3-dioxygenase (IDO) [17]. IDO has been shown to be immunosuppressive by inhibiting expansion of activated T cells [92] and furthermore by influencing naïve CD4 + T cells to become regulatory T cells [93]. In HIV-infected patients, high plasma levels of IDO correlate with increased plasma viremia and inhibit maturation of bystander mDC [94], suggesting that pDC activation is causing adaptive immune system suppression and inhibiting further antigen presentation.
Differential effects of infection of pDC and mDC responsiveness to stimulation
In vitro, DC that are activated for the first time with TLR agonists have a robust ability to induce Th1 polarization (production of IFN-γ), whereas cells that are repeatedly stimulated exhibit “exhaustion” which is reflected in Th2 polarization (production of IL-4) or nonpolarization [95–97]. Likewise, pDC from chronically HIV-infected individuals have been shown to be refractory to ex vivo stimulation with either ssRNA or HSV DNA [41, 48, 98]. These data suggest that pDC are bombarded with activating agonists leading to a chronic state of stimulation [99, 100], suggesting an exhausted, refractory phenotype, which could lead to impaired stimulation of Th1 responses and a preferential induction of Th2 responses. In contrast, mDC have been shown to remain relatively functional at producing proinflammatory cytokines in HIV infection [101, 102] and inducing a repertoire of NK-cell functions [103]. Our preliminary studies in SIV-infected macaques are consistent with this finding. We find that mDC remain responsive to virus-encoded TLR agonists in acute infection, while pDC and monocytes become rapidly refractory. Moreover, continued hyper-responsiveness of mDC appears to be linked with long-term stable infection, whereas mDC isolated from monkeys that progress rapidly to AIDS have reduced responsiveness at an earlier stage (E. Wonderlich, unpublished data). The relationship between mDC activation and disease control is an active area of investigation.
Summary
While considerable advances have been made to define the role of DC in HIV pathogenesis, the fundamental question of whether the DC response is good or bad remains unanswered. Clarity on this topic is essential if we are to effectively intervene in the innate response to the betterment of HIV-infected individuals. Studies in the nonhuman primate models of SIV infection, both pathogenic and nonpathogenic, have provided key insight into the DC response and its relationship to AIDS pathogenesis. We believe the ensuing years will see continued contributions of this model in defining the contribution of the different DC subsets to virus control and disease progression.
Acknowledgments
We wish to thank current and former members of the Barratt-Boyes laboratory who contributed to work discussed in this review. Studies in the Barratt-Boyes laboratory cited in the review were supported by National Institutes of Health grants R01 AI071777 and the ARRA supplement to this grant R01 AI071777-03S1.
Biography

Simon M. Barratt-Boyes
Contributor Information
Elizabeth R. Wonderlich, Center for Vaccine Research, University of Pittsburgh, Pittsburgh, PA, USA Department of Infectious Diseases and Microbiology, University of Pittsburgh, Pittsburgh, PA, USA.
Muhamuda Kader, Center for Vaccine Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Infectious Diseases and Microbiology, University of Pittsburgh, Pittsburgh, PA, USA.
Viskam Wijewardana, Center for Vaccine Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Microbiology and Molecular Genetics, University of Pittsburgh, Pittsburgh, PA, USA.
Simon M. Barratt-Boyes, Email: smbb@pitt.edu, Center for Vaccine Research, University of Pittsburgh, Pittsburgh, PA, USA; Department of Infectious Diseases and Microbiology, University of Pittsburgh, Pittsburgh, PA, USA; Department of Immunology, University of Pittsburgh, 9014 Biomedical Science Tower 3, 3501 Fifth Avenue, Pittsburgh, PA 15261, USA.
References
- 1.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–477. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 2.Smith SM, Holland B, Russo C, et al. Retrospective analysis of viral load and SIV antibody responses in rhesus macaques infected with pathogenic SIV: predictive value for disease progression. AIDS Res Hum Retroviruses. 1999;15:1691–1701. doi: 10.1089/088922299309739. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch VM, Johnson PR. Pathogenic diversity of simian immunodeficiency viruses. Virus Res. 1994;32:183–203. doi: 10.1016/0168-1702(94)90041-8. [DOI] [PubMed] [Google Scholar]
- 4.Letvin NL, King NW. Immunologic and pathologic manifestations of the infection of rhesus monkeys with simian immunodeficiency virus of macaques. J Acquir Immune Defic Syndr. 1990;3:1023–1040. [PubMed] [Google Scholar]
- 5.Watson A, Ranchalis J, Travis B, et al. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liovat AS, Jacquelin B, Ploquin MJ, Barre-Sinoussi F, Muller-Trutwin MC. African non human primates infected by SIV—why don’t sick? Lessons from studies on the early phase of non-pathogenic SIV infection. Curr HIV Res. 2009;7:39–50. doi: 10.2174/157016209787048546. [DOI] [PubMed] [Google Scholar]
- 7.Paiardini M, Pandrea I, Apetrei C, Silvestri G. Lessons learned from the natural hosts of HIV-related viruses. Annu Rev Med. 2009;60:485–495. doi: 10.1146/annurev.med.60.041807.123753. [DOI] [PubMed] [Google Scholar]
- 8.Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol. 2008;29:419–428. doi: 10.1016/j.it.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sodora DL, Allan JS, Apetrei C, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med. 2009;15:861–865. doi: 10.1038/nm.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abel K, Alegria-Hartman MJ, Rothaeusler K, Marthas M, Miller CJ. The relationship between simian immunodeficiency virus RNA levels and the mRNA levels of alpha/beta interferons (IFN-alpha/beta) and IFN-alpha/beta-inducible Mx in lymphoid tissues of rhesus macaques during acute and chronic infection. J Virol. 2002;76:8433–8445. doi: 10.1128/JVI.76.16.8433-8445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Malleret B, Maneglier B, Karlsson I, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood. 2008;112:4598–4608. doi: 10.1182/blood-2008-06-162651. [DOI] [PubMed] [Google Scholar]
- 12.Stacey AR, Norris PJ, Qin L, et al. Induction of a striking systemic cytokine cascade prior to peak viremia in acute human immunodeficiency virus type 1 infection, in contrast to more modest and delayed responses in acute hepatitis B and C virus infections. J Virol. 2009;83:3719–3733. doi: 10.1128/JVI.01844-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sodora DL, Silvestri G. Immune activation and AIDS pathogenesis. Aids. 2008;22:439–446. doi: 10.1097/QAD.0b013e3282f2dbe7. [DOI] [PubMed] [Google Scholar]
- 14.Jacquelin B, Mayau V, Targat B, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest. 2009;119:3544–3555. doi: 10.1172/JCI40093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bosinger SE, Li Q, Gordon SN, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest. 2009;119:3556–3572. doi: 10.1172/JCI40115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lederer S, Favre D, Walters KA, et al. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000296. e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boasso A, Herbeuval JP, Hardy AW, et al. HIV inhibits CD4 + T cell proliferation by inducing indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells. Blood. 2007;109:3351–3359. doi: 10.1182/blood-2006-07-034785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manches O, Bhardwaj N. Resolution of immune activation defines nonpathogenic SIV infection. J Clin Invest. 2009;119:3512–3515. doi: 10.1172/JCI41509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4 + T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 20.Fahey JL, Taylor JM, Manna B, et al. Prognostic significance of plasma markers of immune activation, HIV viral load and CD4 T-cell measurements. Aids. 1998;12:1581–1590. doi: 10.1097/00002030-199813000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Giorgi JV, Hultin LE, McKeating JA, et al. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J Infect Dis. 1999;179:859–870. doi: 10.1086/314660. [DOI] [PubMed] [Google Scholar]
- 22.Hazenberg MD, Otto SA, van Benthem BH, et al. Persistent immune activation in HIV-1 infection is associated with progression to AIDS. AIDS. 2003;17:1881–1888. doi: 10.1097/00002030-200309050-00006. [DOI] [PubMed] [Google Scholar]
- 23.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151–161. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 24.Brown KN, Barratt-Boyes SM. Surface phenotype and rapid quantification of blood dendritic cell subsets in the rhesus macaque. J Med Primatol. 2009;38:272–278. doi: 10.1111/j.1600-0684.2009.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown KN, Trichel A, Barratt-Boyes SM. Parallel loss of myeloid and plasmacytoid dendritic cells from blood and lymphoid tissue in simian AIDS. J Immunol. 2007;178:6958–6967. doi: 10.4049/jimmunol.178.11.6958. [DOI] [PubMed] [Google Scholar]
- 26.Coates PT, Barratt-Boyes SM, Zhang L, et al. Dendritic cell subsets in blood and lymphoid tissue of rhesus monkeys and their mobilization with Flt3 ligand. Blood. 2003;102:2513–2521. doi: 10.1182/blood-2002-09-2929. [DOI] [PubMed] [Google Scholar]
- 27.Diop OM, Ploquin MJ, Mortara L, et al. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol. 2008;82:5145–5152. doi: 10.1128/JVI.02433-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teleshova N, Kenney J, Jones J, et al. CpG-C immunostimulatory oligodeoxyribonucleotide activation of plasmacytoid dendritic cells in rhesus macaques to augment the activation of IFN-gamma-secreting simian immunodeficiency virus-specific T cells. J Immunol. 2004;173:1647–1657. doi: 10.4049/jimmunol.173.3.1647. [DOI] [PubMed] [Google Scholar]
- 29.Xu H, Wang X, Pahar B, et al. Increased B7-H1 expression on dendritic cells correlates with programmed death 1 expression on T cells in simian immunodeficiency virus-infected macaques and may contribute to T cell dysfunction and disease progression. J Immunol. 2010;185:7340–7348. doi: 10.4049/jimmunol.1001642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teleshova N, Jones J, Kenney J, et al. Short-term Flt3L treatment effectively mobilizes functional macaque dendritic cells. J Leukoc Biol. 2004;75:1102–1110. doi: 10.1189/jlb.1103588. [DOI] [PubMed] [Google Scholar]
- 31.Cella M, Jarrossay D, Facchetti F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919–923. doi: 10.1038/11360. [DOI] [PubMed] [Google Scholar]
- 32.Yoneyama H, Matsuno K, Zhang Y, et al. Evidence for recruitment of plasmacytoid dendritic cell precursors to inflamed lymph nodes through high endothelial venules. Int Immunol. 2004;16:915–928. doi: 10.1093/intimm/dxh093. [DOI] [PubMed] [Google Scholar]
- 33.Yoneyama H, Matsuno K, Toda E, et al. Plasmacytoid DCs help lymph node DCs to induce anti-HSV CTLs. J Exp Med. 2005;202:425–435. doi: 10.1084/jem.20041961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 35.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 36.Jaehn PS, Zaenker KS, Schmitz J, Dzionek A. Functional dichotomy of plasmacytoid dendritic cells: antigen-specific activation of T cells versus production of type I interferon. Eur J Immunol. 2008;38:1822–1832. doi: 10.1002/eji.200737552. [DOI] [PubMed] [Google Scholar]
- 37.McKenna K, Beignon AS, Bhardwaj N. Plasmacytoid dendritic cells: linking innate and adaptive immunity. J Virol. 2005;79:17–27. doi: 10.1128/JVI.79.1.17-27.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mandl JN, Barry AP, Vanderford TH, et al. Divergent TLR7 and TLR9 signaling and type I interferon production distinguish pathogenic and nonpathogenic AIDS virus infections. Nat Med. 2008;14:1077–1087. doi: 10.1038/nm.1871. [DOI] [PubMed] [Google Scholar]
- 39.Barron MA, Blyveis N, Palmer BE, MaWhinney S, Wilson CC. Influence of plasma viremia on defects in number and immunophenotype of blood dendritic cell subsets in human immunodeficiency virus 1-infected individuals. J Infect Dis. 2003;187:26–37. doi: 10.1086/345957. [DOI] [PubMed] [Google Scholar]
- 40.Donaghy H, Pozniak A, Gazzard B, et al. Loss of blood CD11c(+) myeloid and CD11c(−) plasmacytoid dendritic cells in patients with HIV-1 infection correlates with HIV-1 RNA virus load. Blood. 2001;98:2574–2576. doi: 10.1182/blood.v98.8.2574. [DOI] [PubMed] [Google Scholar]
- 41.Feldman S, Stein D, Amrute S, et al. Decreased interferon-alpha production in HIV-infected patients correlates with numerical and functional deficiencies in circulating type 2 dendritic cell precursors. Clin Immunol. 2001;101:201–210. doi: 10.1006/clim.2001.5111. [DOI] [PubMed] [Google Scholar]
- 42.Grassi F, Hosmalin A, McIlroy D, et al. Depletion in blood CD11c-positive dendritic cells from HIV-infected patients. AIDS. 1999;13:759–766. doi: 10.1097/00002030-199905070-00004. [DOI] [PubMed] [Google Scholar]
- 43.Killian MS, Fujimura SH, Hecht FM, Levy JA. Similar changes in plasmacytoid dendritic cell and CD4 T-cell counts during primary HIV-1 infection and treatment. AIDS. 2006;20:1247–1252. doi: 10.1097/01.aids.0000232231.34253.bd. [DOI] [PubMed] [Google Scholar]
- 44.Pacanowski J, Kahi S, Baillet M, et al. Reduced blood CD123 + (lymphoid) and CD11c + (myeloid) dendritic cell numbers in primary HIV-1 infection. Blood. 2001;98:3016–3021. doi: 10.1182/blood.v98.10.3016. [DOI] [PubMed] [Google Scholar]
- 45.Soumelis V, Scott I, Gheyas F, et al. Depletion of circulating natural type 1 interferon-producing cells in HIV-infected AIDS patients. Blood. 2001;98:906–912. doi: 10.1182/blood.v98.4.906. [DOI] [PubMed] [Google Scholar]
- 46.Knight SC, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 47.Macatonia SE, Lau R, Patterson S, Pinching AJ, Knight SC. Dendritic cell infection, depletion and dysfunction in HIV-infected individuals. Immunology. 1990;71:38–45. [PMC free article] [PubMed] [Google Scholar]
- 48.Chehimi J, Campbell DE, Azzoni L, et al. Persistent decreases in blood plasmacytoid dendritic cell number and function despite effective highly active antiretroviral therapy and increased blood myeloid dendritic cells in HIV-infected individuals. J Immunol. 2002;168:4796–4801. doi: 10.4049/jimmunol.168.9.4796. [DOI] [PubMed] [Google Scholar]
- 49.Finke JS, Shodell M, Shah K, Siegal FP, Steinman RM. Dendritic cell numbers in the blood of HIV-1 infected patients before and after changes in antiretroviral therapy. J Clin Immunol. 2004;24:647–652. doi: 10.1007/s10875-004-6250-5. [DOI] [PubMed] [Google Scholar]
- 50.Schmidt B, Fujimura SH, Martin JN, Levy JA. Variations in plasmacytoid dendritic cell (PDC) and myeloid dendritic cell (MDC) levels in HIV-infected subjects on and off antiretroviral therapy. J Clin Immunol. 2006;26:55–64. doi: 10.1007/s10875-006-8401-3. [DOI] [PubMed] [Google Scholar]
- 51.Nilsson J, Boasso A, Velilla PA, et al. HIV-1-driven regulatory T-cell accumulation in lymphoid tissues is associated with disease progression in HIV/AIDS. Blood. 2006;108:3808–3817. doi: 10.1182/blood-2006-05-021576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Almeida M, Cordero M, Almeida J, Orfao A. Different subsets of peripheral blood dendritic cells show distinct phenotypic and functional abnormalities in HIV-1 infection. AIDS. 2005;19:261–271. [PubMed] [Google Scholar]
- 53.Campillo-Gimenez L, Laforge M, Fay M, et al. Non pathogenesis of SIV infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol. 2009 doi: 10.1128/JVI.01496-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cavaleiro R, Baptista AP, Soares RS, et al. Major depletion of plasmacytoid dendritic cells in HIV-2 infection, an attenuated form of HIV disease. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000667. e1000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Biancotto A, Grivel JC, Iglehart SJ, et al. Abnormal activation and cytokine spectra in lymph nodes of people chronically infected with HIV-1. Blood. 2007;109:4272–4279. doi: 10.1182/blood-2006-11-055764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dillon SM, Robertson KB, Pan SC, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krathwohl MD, Schacker TW, Anderson JL. Abnormal presence of semimature dendritic cells that induce regulatory T cells in HIV-infected subjects. J Infect Dis. 2006;193:494–504. doi: 10.1086/499597. [DOI] [PubMed] [Google Scholar]
- 58.Wijewardana V, Soloff AC, Liu X, Brown KN, Barratt-Boyes SM. Early myeloid dendritic cell dysregulation is predictive of disease progression in simian immunodeficiency virus infection. PLoS Pathog. 2010;6 doi: 10.1371/journal.ppat.1001235. e1001235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barratt-Boyes SM, Wijewardana V. A divergent myeloid dendritic cell response at virus set-point predicts disease outcome in SIV-infected rhesus macaques. J Med Primatol. doi: 10.1111/j.1600-0684.2011.00484.x. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000413. e1000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Graziosi C, Gantt KR, Vaccarezza M, et al. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barratt-Boyes SM, Wijewardana V, Brown KN. In acute pathogenic SIV infection plasmacytoid dendritic cells are depleted from blood and lymph nodes despite mobilization. J Med Primatol. 2010;39:235–242. doi: 10.1111/j.1600-0684.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fong L, Mengozzi M, Abbey NW, Herndier BG, Engleman EG. Productive infection of plasmacytoid dendritic cells with human immunodeficiency virus type 1 is triggered by CD40 ligation. J Virol. 2002;76:11033–11041. doi: 10.1128/JVI.76.21.11033-11041.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lore K, Smed-Sorensen A, Vasudevan J, Mascola JR, Koup RA. Myeloid and plasmacytoid dendritic cells transfer HIV-1 preferentially to antigen-specific CD4 + T cells. J Exp Med. 2005;201:2023–2033. doi: 10.1084/jem.20042413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Patterson S, Rae A, Hockey N, Gilmour J, Gotch F. Plasmacytoid dendritic cells are highly susceptible to human immunodeficiency virus type 1 infection and release infectious virus. J Virol. 2001;75:6710–6713. doi: 10.1128/JVI.75.14.6710-6713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Meyers JH, Justement JS, Hallahan CW, et al. Impact of HIV on cell survival and antiviral activity of plasmacytoid dendritic cells. PLoS One. 2007;2:e458. doi: 10.1371/journal.pone.0000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 68.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Suchard MS, Mayne E, Green VA, et al. FOXP3 expression is upregulated in CD4T cells in progressive HIV-1 infection and is a marker of disease severity. PLoS One. 2010;5:e11762. doi: 10.1371/journal.pone.0011762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qin S, Sui Y, Soloff AC, et al. Chemokine and cytokine mediated loss of regulatory T cells in lymph nodes during pathogenic simian immunodeficiency virus infection. J Immunol. 2008;180:5530–5536. doi: 10.4049/jimmunol.180.8.5530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Favre D, Lederer S, Kanwar B, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog. 2009;5 doi: 10.1371/journal.ppat.1000295. e1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarrossay D, Napolitani G, Colonna M, Sallusto F, Lanzavecchia A. Specialization and complementarity in microbial molecule recognition by human myeloid and plasmacytoid dendritic cells. Eur J Immunol. 2001;31:3388–3393. doi: 10.1002/1521-4141(200111)31:11<3388::aid-immu3388>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 73.Kadowaki N, Ho S, Antonenko S, et al. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J Exp Med. 2001;194:863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–5232. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meier A, Alter G, Frahm N, et al. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–186. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fitzgerald-Bocarsly P, Jacobs ES. Plasmacytoid dendritic cells in HIV infection: striking a delicate balance. J Leukoc Biol. 2010;87:609–620. doi: 10.1189/jlb.0909635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cervantes-Barragan L, Zust R, Weber F, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lund JM, Linehan MM, Iijima N, Iwasaki A. Cutting edge: plasmacytoid dendritic cells provide innate immune protection against mucosal viral infection in situ. J Immunol. 2006;177:7510–7514. doi: 10.4049/jimmunol.177.11.7510. [DOI] [PubMed] [Google Scholar]
- 81.Smit JJ, Rudd BD, Lukacs NW. Plasmacytoid dendritic cells inhibit pulmonary immunopathology and promote clearance of respiratory syncytial virus. J Exp Med. 2006;203:1153–1159. doi: 10.1084/jem.20052359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Swiecki M, Gilfillan S, Vermi W, Wang Y, Colonna M. Plasmacytoid dendritic cell ablation impacts early interferon responses and antiviral NK and CD8(+) T cell accrual. Immunity. 2010;33:955–966. doi: 10.1016/j.immuni.2010.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Boasso A, Shearer GM. Chronic innate immune activation as a cause of HIV-1 immunopathogenesis. Clin Immunol. 2008;126:235–242. doi: 10.1016/j.clim.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Herbeuval JP, Shearer GM. HIV-1 immunopathogenesis: how good interferon turns bad. Clin Immunol. 2007;123:121–127. doi: 10.1016/j.clim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sedaghat AR, German J, Teslovich TM, et al. Chronic CD4 + T-cell activation and depletion in human immunodeficiency virus type 1 infection: type I interferon-mediated disruption of T-cell dynamics. J Virol. 2008;82:1870–1883. doi: 10.1128/JVI.02228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.O’Brien M, Manches O, Sabado RL, et al. Spatiotemporal trafficking of HIV in human plasmacytoid dendritic cells defines a persistently IFN-alpha-producing and partially matured phenotype. J Clin Invest. 2011;121:1088–1101. doi: 10.1172/JCI44960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Farzadegan H, Hoover DR, Astemborski J, et al. Sex differences in HIV-1 viral load and progression to AIDS. Lancet. 1998;352:1510–1514. doi: 10.1016/S0140-6736(98)02372-1. [DOI] [PubMed] [Google Scholar]
- 88.Gandhi M, Bacchetti P, Miotti P, et al. Does patient sex affect human immunodeficiency virus levels? Clin Infect Dis. 2002;35:313–322. doi: 10.1086/341249. [DOI] [PubMed] [Google Scholar]
- 89.Sterling TR, Lyles CM, Vlahov D, et al. Sex differences in longitudinal human immunodeficiency virus type 1 RNA levels among seroconverters. J Infect Dis. 1999;180:666–672. doi: 10.1086/314967. [DOI] [PubMed] [Google Scholar]
- 90.Meier A, Chang JJ, Chan ES, et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV-1. Nat Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tavel JA, Huang CY, Shen J, et al. Interferon-alpha produces significant decreases in HIV load. J Interferon Cytokine Res. 2010;30:461–464. doi: 10.1089/jir.2009.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Munn DH, Mellor AL. Indoleamine 2, 3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Manches O, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2, 3-dioxygenase-dependent mechanism. J Clin Invest. 2007;118:3431–3439. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Boasso A, Hardy AW, Anderson SA, Dolan MJ, Shearer GM. HIV-induced type I interferon and tryptophan catabolism drive T cell dysfunction despite phenotypic activation. PLoS One. 2008;3:e2961. doi: 10.1371/journal.pone.0002961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1:311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 96.Karp CL, Wysocka M, Ma X, et al. Potent suppression of IL-12 production from monocytes and dendritic cells during endotoxin tolerance. Eur J Immunol. 1998;28:3128–3136. doi: 10.1002/(SICI)1521-4141(199810)28:10<3128::AID-IMMU3128>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 97.Ma X, Sun J, Papasavvas E, et al. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722–1729. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 98.Lopez C, Fitzgerald PA, Siegal FP. Severe acquired immune deficiency syndrome in male homosexuals: diminished capacity to make interferon-alpha in vitro associated with severe opportunistic infections. J Infect Dis. 1983;148:962–966. doi: 10.1093/infdis/148.6.962. [DOI] [PubMed] [Google Scholar]
- 99.Derdeyn CA, Silvestri G. Viral and host factors in the pathogenesis of HIV infection. Curr Opin Immunol. 2005;17:366–373. doi: 10.1016/j.coi.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Moanna A, Dunham R, Paiardini M, Silvestri G. CD4 + T-cell depletion in HIV infection: killed by friendly fire? Curr HIV/AIDS Rep. 2005;2:16–23. doi: 10.1007/s11904-996-0004-3. [DOI] [PubMed] [Google Scholar]
- 101.Martinson JA, Roman-Gonzalez A, Tenorio AR, et al. Dendritic cells from HIV-1 infected individuals are less responsive to toll-like receptor (TLR) ligands. Cell Immunol. 2007;250:75–84. doi: 10.1016/j.cellimm.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.St John EP, Martinson J, Simoes JA, Landay AL, Spear GT. Dendritic cell activation and maturation induced by mucosal fluid from women with bacterial vaginosis. Clin Immunol. 2007;125:95–102. doi: 10.1016/j.clim.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Benlahrech A, Gotch F, Kelleher P, Patterson S. Loss of NK stimulatory capacity by plasmacytoid and monocyte-derived DC but not myeloid DC in HIV-1 infected patients. PLoS One. 2011;6:e17525. doi: 10.1371/journal.pone.0017525. [DOI] [PMC free article] [PubMed] [Google Scholar]