Abstract
Background
Rhabdomyosarcoma (RMS), the most common soft-tissue sarcoma in children, occurs less commonly in infants. Historically, poorer outcomes have been seen for infants diagnosed with RMS than for older children.
Methods
We analyzed the characteristics, treatment administered, outcomes, and patterns of failure for infants < 1 year of age with non-metastatic RMS who were treated with multimodal therapy on Intergroup Rhabdomyosarcoma Study (IRS) protocols IRS-IV, D9602, and D9803.
Results
Seventy-six infants with non-metastatic RMS were treated on the 3 protocols from 1991 to 2005. Median age was 7.4 months (range 0.1–12 months). Tumor histology included embryonal (57%), alveolar (21%), and undifferentiated sarcoma/other (22%). Parameningeal primary site was less common in this infant cohort (3%) than for all patients treated on IRS-IV (25%). Estimated 5-year failure-free survival and overall survival (95% confidence intervals) are 57% (44%, 67%) and 76% (65%, 85%) for infants compared to 81% (79%, 83%) and 87% (85%, 89%) for ages 1–9 years. Twenty-three of 32 infants with treatment failure had local recurrence/progression with (n=3) or without (n=20) distant failure. The overall local failure rate was 30%. Median time to treatment failure was 13 months. FFS was worse for infants with Group III tumors and for those who received less than protocol recommended radiation therapy.
Conclusions
Infants with RMS appear to have worse outcomes than older patients, in part due to high rates of local failure. Concerns regarding morbidity in infants and reluctance to perform aggressive local control measures may lead to higher rates of local failure.
Keywords: pediatric, sarcoma, infant, radiotherapy, rhabdomyosarcoma
Introduction
Rhabdomyosarcoma (RMS) is a malignancy of mesenchymal cell origin that primarily occurs in children and young adults. While the majority of cases of RMS are diagnosed in children less than 6 years of age,1 RMS is uncommon in infants. Previous studies have demonstrated that age is a prognostic factor in RMS with poorer outcomes seen in infants than in older children.2–4 Since there is no clear evidence that the biology of RMS is different in infants, the reason for worse outcome is unclear. A potential explanation for inferior outcome for infants could be less aggressive local therapy. Concerns regarding the morbidity and long-term adverse effects of surgery or radiation therapy (RT) may lead treating physicians and parents to omit or reduce local therapy for infants. Alternatively, infants may tolerate intensive systemic therapy less well. Higher therapy-related mortality rates have been seen in infants with Wilms tumor, and the risk of hepatopathy is higher in younger children with RMS.5, 6 Therefore, reduced doses of chemotherapy are often administered.
The Intergroup Rhabdomyosarcoma Study Group (IRSG) has conducted consecutive multi-institutional clinical trials to improve outcome for children with RMS.7–12 We describe the characteristics, outcome, prognostic factors, and patterns of failure for infants with non-metastatic RMS treated on the 4th and 5th Intergroup Rhabdomyosarcoma Studies (IRS). We specifically evaluated adherence to protocol recommended RT as a prognostic factor for local failure and failure-free survival (FFS).
Patients and Methods
Study Population
The study population consists of infants with non-metastatic RMS who were treated on intergroup RMS studies, IRS-IV11 and IRS-V7, 12. IRS-V was conducted through the Children’s Oncology Group (COG) as studies D9803 for “intermediate risk” RMS and D9602 for “low risk” RMS. Informed consent was obtained from the parents/guardians of all infants included in this analysis at the original time of enrollment on one of the IRS studies.
All patients in this analysis were < 1 year of age at the time of diagnosis. The IRS studies assigned a presurgical Stage based on tumor site, size, clinical lymph node involvement, and presence or absence of metastases.13 A post-surgical “Group” was also assigned to all tumors based on IRS classification.9 Group I includes localized tumors that are completely excised with no microscopic residual disease. Group II includes grossly resected tumors with microscopic residual disease and/or resected regional disease with involved lymph nodes. Group III consists of those patients with gross residual disease after resection or biopsy only. Group IV consists of patients with metastatic disease.
Treatment
Patients on all 3 protocols received multimodal therapy including chemotherapy with or without surgery and/or radiation therapy as assigned by protocol according to IRS Stage and Group. Outcome for patients treated on IRS-IV was similar to those treated on IRS-V,7, 11 allowing for pooled analysis of data from these studies. Treatment by study is summarized below.
IRS-IV enrolled patients from 1991 to 1997.11 Patients without metastatic disease were randomized to receive vincristine and dactinomycin with cyclophosphamide (VAC), ifosfamide (VAI), or etoposide (VIE). Patients with Group III tumors were randomized to receive either conventional RT (50.4 Gy in 28 1.8 Gy fractions) or hyperfractionated RT (59.4 Gy in 54 1.1 Gy fractions twice daily). The COG study D9803 enrolled patients with “intermediate risk” RMS from 1999 to 2005.7 Eligible patients included those with Stage 1–3/Group I-III alveolar RMS and undifferentiated sarcoma, those with Stage 2–3/Group III embryonal RMS, and patients < 10 years of age with Stage 4/Group IV embryonal RMS. Three infants with Stage 4/Group IV embryonal RMS treated on D9803 are excluded from this analysis. Patients were randomized to receive either VAC chemotherapy or VAC alternating with vincristine, topotecan, and cyclophosphamide (VTC). The D9602 study enrolled patients with “low risk” RMS (Stage 1–3/Groups I–II with embryonal or botryoid histology) from 1997 to 2004.12 Patients were treated with either VAC or VA chemotherapy with or without RT based on pre-treatment Stage and Group.
Each protocol recommended that infants receive 50% prescribed chemotherapy doses in the initial phase of chemotherapy with escalation to 75% and then 100% doses in subsequent phases if no significant toxicity occurred. Chemotherapy doses were calculated by weight (kilogram) or by body surface area (meter squared) depending on the protocol and chemotherapeutic agent.
RT was assigned for all patients with Group II or Group III tumors and for patients with Group I alveolar histology. Patients with Group III tumors, Group II tumors, and Group I alveolar/undifferentiated histology received RT at doses of 50.4 or 59.4 Gy 41.4 Gy, and 36 Gy, respectively.
Study Design
We analyzed data regarding clinical features for infants < 1 year of age in comparison to older cohorts of patients treated on the same studies. We also assessed chemotherapy and radiation therapy delivered and operative procedures performed. Initial and second look operations as well as outcome in regards to loss of cosmesis, loss of organ function, and resection of vital structures were evaluated. “Loss of cosmesis” was defined as any resection that resulted in a noticeable change in appearance, including resection of face/head/neck. “Loss of function” was defined as any resection resulting in a permanent loss of a functional organ (e.g. bladder). “Resection of vital structures” was defined as removal of at least some portion of a critical structure (e.g. ureter, kidney, colon) that was associated with morbidity with or without “loss of function”. Adherence to protocol-specified RT was analyzed and the proportion of patients who received less than prescribed radiation was determined. RT was considered to not be “as specified by protocol” if (1) RT was withheld despite protocol guidelines specifying that the patient should receive RT based on IRS Stage and Group, (2) there was a major deviation from protocol specified dose and/or target volumes. Lack of adherence to assigned fractionation (conventional or hyperfractionated) was not considered a major deviation. In regards to chemotherapy, mean percentage of delivered to calculated dose was determined for each agent in each “phase” of treatment. FFS and overall survival (OS) were determined for infants and compared to older age groups treated on the same studies. FFS was assessed and compared by Group and by whether or not protocol specified RT was received. Sites of failure and time to failure were determined.
Statistical Analysis
Patient characteristics were compared across cohorts using Fisher’s Exact test. FFS and OS were estimated using Kaplan-Meier life table analysis14 and were compared between age cohorts, by Group, and by radiation received using the log-rank test.15
Results
Patient and Disease Characteristics
Among the 1843 patients with non-metastatic RMS enrolled on IRS-IV, D9602, and D9803, 76 (4%) were < 1 year of age at the time of diagnosis (IRS-IV:n=41; D9602:n=20; D9803:n=15). Characteristics of the study population compared to patients 1 to 9 years and ≥ 10 years with non-metastatic RMS are shown in Table 1. Thirty-one (41%) infants were female. The median age at diagnosis was 7.4 months (range 0.1 to 12 months). Thirteen patients (17%) were < 3 months of age and 4 patients (5%) were < 1 month at the time of diagnosis.
Table 1. Patient Characteristics.
Comparison of characteristics by age for patients with non-metastatic RMS treated on IRS-IV, D9602, and D9803
| Characteristic | < 1 year (N = 76) | 1 – 9 years (N = 1230) | ≥10 years (N = 537) | p-value | |||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Sex | |||||||
| Male | 45 | 59 | 770 | 63 | 341 | 64 | 0.76 |
| Female | 31 | 41 | 460 | 37 | 196 | 36 | |
| Histology | |||||||
| Embryonal | 43 | 57 | 882 | 72 | 271 | 50 | <0.0001 |
| Alveolar | 16 | 21 | 275 | 22 | 202 | 38 | |
| UDS/Other | 17 | 22 | 73 | 6 | 64 | 12 | |
| Primary Tumor Site | |||||||
| Parameningeal | 3 | 4 | 291 | 24 | 141 | 26 | <0.0001 |
| Orbit | 4 | 5 | 157 | 13 | 48 | 9 | |
| Head & Neck | 13 | 17 | 98 | 8 | 38 | 7 | |
| Extremity | 12 | 16 | 127 | 10 | 73 | 14 | |
| Trunk/Abdomen | 16 | 21 | 132 | 11 | 40 | 7 | |
| GU B/P | 12 | 16 | 141 | 11 | 21 | 4 | |
| GU non-B/P | 12 | 16 | 242 | 20 | 153 | 28 | |
| Other, unspecified | 4 | 5 | 24 | 2 | 10 | 2 | |
| IRS Stage | |||||||
| 1 | 29 | 38 | 502 | 41 | 238 | 44 | 0.015 |
| 2 | 19 | 25 | 259 | 21 | 78 | 15 | |
| 3 | 28 | 37 | 469 | 38 | 220 | 41 | |
| IRS Group | |||||||
| I | 18 | 24 | 255 | 21 | 123 | 23 | 0.44 |
| II | 15 | 20 | 204 | 17 | 100 | 19 | |
| III | 43 | 57 | 771 | 63 | 314 | 58 | |
| Tumor Size | |||||||
| ≤ 5cm | 45 | 59 | 698 | 57 | 232 | 44 | <0.0001 |
| > 5cm | 31 | 41 | 523 | 43 | 300 | 56 | |
| T-Stage | |||||||
| T-1 | 45 | 59 | 747 | 61 | 280 | 53 | 0.0037 |
| T-2 | 31 | 41 | 474 | 39 | 252 | 47 | |
| Nodes | |||||||
| N0 | 63 | 84 | 1068 | 88 | 412 | 78 | <0.0001 |
| N1 | 9 | 12 | 126 | 10 | 107 | 20 | |
| NX | 3 | 4 | 24 | 2 | 11 | 2 | |
UDS, undifferentiated sarcoma; B/P, Bladder/Prostate
Includes GI/liver, retroperitoneum, pelvis, chest wall
Includes paratesticular
Forty-three infants (57%) had tumors with embryonal histology and 16 (21%) had alveolar histology. The remaining 17 patients (22%) had “other” histology, which includes undifferentiated sarcoma and ectomesenchymoma. Infants had a higher proportion of undifferentiated sarcoma than older patients. The distribution of primary tumor sites differed for infants compared to older patients (p<0.0001). Infants had a lower incidence of parameningeal tumors (4%) and a higher proportion of tumors in the trunk/abdomen (includes chest wall, paraspinous region, and retroperitoneum).
Distribution of IRS Stage, tumor size, nodal status, and T-stage were statistically different across the 3 age categories. Similar to the older age groups, more than half of infants had Group III tumors after initial operative procedure.
Treatment Outcome
Thirty-two of 76 infants experienced treatment failure and 20 died. Eighteen patients died from progressive disease and 2 died from treatment related toxicity. Outcome for patients with non-metastatic RMS treated on IRS-IV, D9602, and D9803 differed by age at diagnosis (Figure 1). The estimated 5-year FFS (95% confidence intervals (CI)) for infants is 57% (44%,67%) compared to 81% (79%,93%) for age 1–9 years, and 68% (63%,72%) for age ≥ 10 years (p-value < 0.001). Five-year OS for age < 1 year, age 1–9 years, and age ≥ 10 years are 76% (65%,85%), 87% (85%, 89%), and 75% (71%,79%), respectively (p-value < 0.001).
Figure 1.
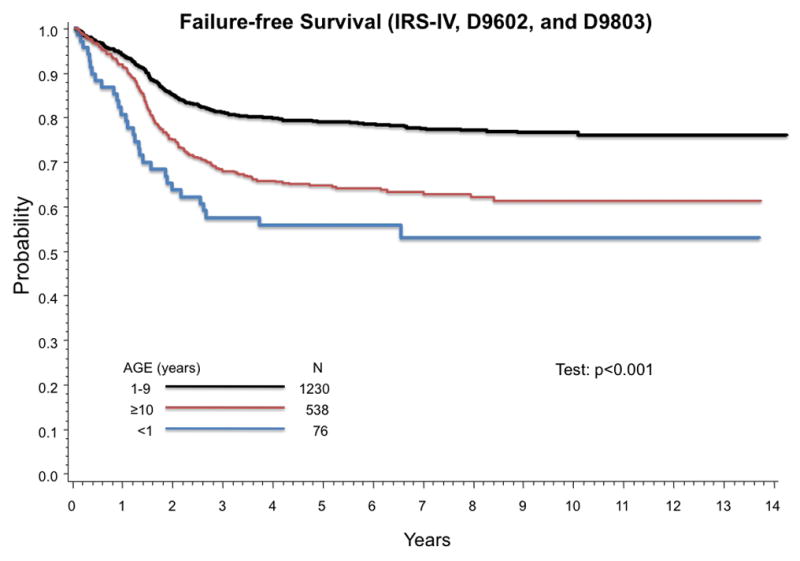
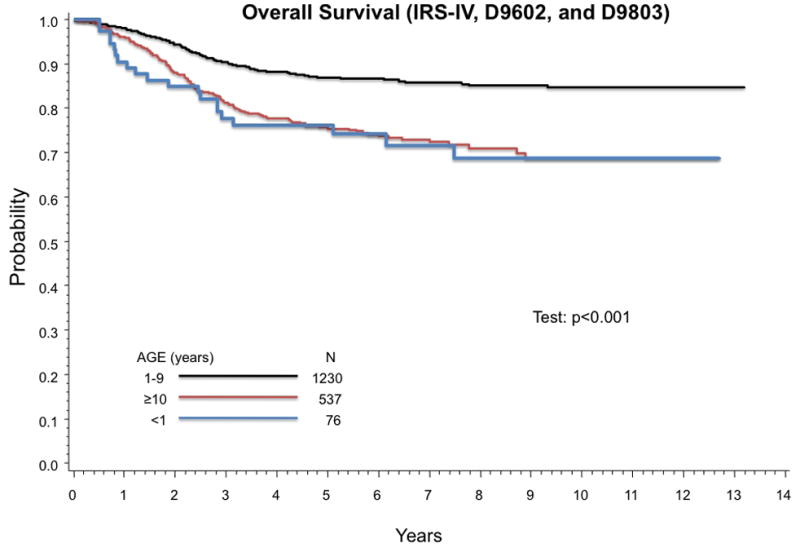
Age at diagnosis is prognostic of outcome in RMS. Infants have poorer FFS than older patients with RMS. (A) Estimated 5-year FFS (95% CI) by age group are 57% (44%,67%) for age < 1 year, 81% (79%,93%) for age 1–9 years, and 68% (63%,72%) for age ≥ 10 years (p-value < 0.001). (B) Estimated 5-year OS (95% CI) are 76% (65%,85%) for age < 1 year, 87% (85%, 89%) for age 1–9 years, and 75% (71%–79%) for age ≥ 10 years (p-value < 0.001).
Outcome was similar for infants < 3 months of age compared to older infants. Five failures were observed among 13 infants younger than 3 months. Outcome varied by tumor histology, but not other clinical parameters such as tumor site, tumor size, T-stage, and nodal status (Table 2). The subset of infants with alveolar histology had a high risk of failure with an estimated 5-year FFS (95% CI) of 22% (5%, 45%) and 5-year OS of 65% (35%, 85%). Infants in this study with undifferentiated sarcoma had outcomes similar to those with embryonal RMS.
Table 2.
Failure-free Survival for Infants by Patient Characteristics
| Characteristic | Total | % | 5-yr FFS (%) | p-value |
|---|---|---|---|---|
| Age | ||||
| < 3 months | 13 | 17 | 59 | 0.86 |
| ≥ 3 months | 63 | 83 | 56 | |
| Site | ||||
| Favorable | 29 | 38 | 52 | 0.43 |
| Unfavorable | 43 | 57 | 61 | |
| Histology | ||||
| Embryonal | 43 | 57 | 64 | 0.04 |
| Alveolar | 16 | 21 | 22 | |
| UDS/Other | 17 | 22 | 69 | |
| Tumor Size | ||||
| ≤ 5cm | 45 | 59 | 59 | 0.86 |
| > 5cm | 31 | 41 | 54 | |
| T-Stage | ||||
| T-1 | 45 | 59 | 59 | 0.48 |
| T-2 | 31 | 41 | 53 | |
| Nodes | ||||
| N0 | 63 | 84 | 56 | 0.97 |
| N1 | 9 | 12 | 65 | |
Favorable Site: non-parameningeal Head & Neck, Orbit, GU non-Bladder/Prostate
Unfavorable Site: Parameningeal, Extremity, Trunk/Abdomen, Bladder/Prostate
UDS, undifferentiated sarcoma
Outcome by IRS Group
Infants with Group III tumors had a significantly higher probability of poor outcome than those with Groups I or II tumors (Figure 2). Estimated 5-year FFS (95% CI) for infants with Group III tumors is 39% (24%, 53%) compared to 69% (37%, 87%) for Group II and 89% (62%, 97%) for Group I (p-value = 0.0022). Estimated 5-year OS (95% CI) are 60% (44%, 74%), 92% (57%, 99%), and 100% for patients with Group III, Group II, and Group I tumors, respectively (p-value = 0012).
Figure 2.
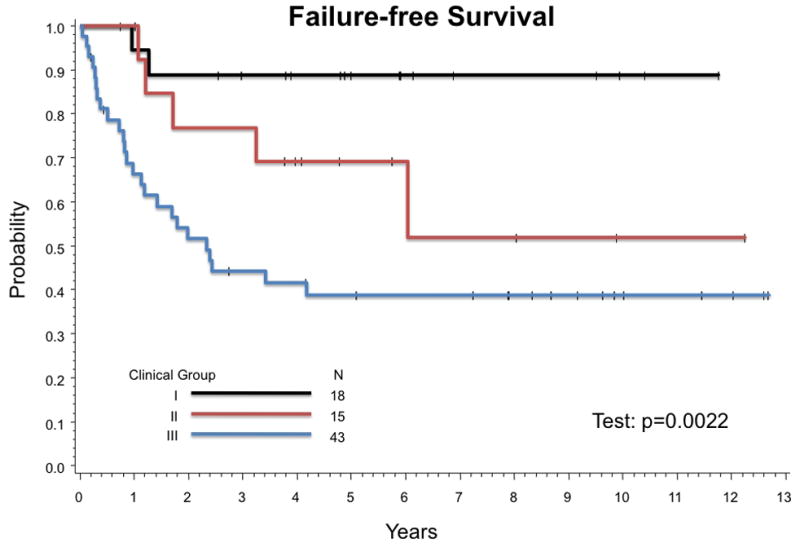
Failure-Free Survival by IRS Group. Estimated 5-year FFS (95% CI) by IRS Group are 89% (62%,97%) for Group I, 69% (37%, 87%) for Group II, and 39% (24%,53%) for Group III (p-value = 0.0022).
Treatment Received
Surgery
Thirty-four of 58 infants (59%) with Group II or Group III tumors underwent second look operations (SLO) after the initiation of protocol therapy: re-biopsy in 11 patients, incomplete excision in 9 patients, and complete excision (defined as resection with negative margins) in 14 patients. Of the 14 infants who had second complete excisions, 6 patients also received RT. Five of 14 patients with second complete excisions, including 2 who also received RT, experienced treatment failure (3 local, 1 distant, and 1 death from toxicity).
Operative therapy resulted in resection of vital structures in 18 (24%) of the 76 infants with resulting loss of organ function in 17 (22%). An additional 5 patients (8%) had loss of cosmesis. Resection of vital structures and loss of organ function occurred more frequently in patients with complete resection. However, 6 of 36 patients who never had complete excisions had operations that resulted in resection of vital structures and loss of function.
Radiation Therapy
Thirty-three infants (43%) received RT (3 with Group I, 10 with Group II, and 20 with Group III tumors). RT was often withheld by local investigators or because of parent refusal. As such, deviations from protocol specified RT were also analyzed for 72 infants for whom complete data was available. Data regarding target volume and dose were not available for 4 patients. Thirty (42%) of 72 infants had major deviations from protocol-specified radiation dose or volume. This group of 30 infants includes 20 patients with Group II, Group III, or Group I alveolar tumors who had radiation withheld as well as 10 patients (2 Group II and 8 Group III) who received lower than specified doses or target volumes. The percentages of patients within each Group who received RT as assigned by protocol are shown in Table 3. Estimated 5-year FFS (95% CI) and 5-year OS for infants who did not receive protocol-assigned RT are 46% (27%, 63%) and 64% (43%, 79%) compared to 70% (53%, 82%) and 85% (70%, 93%) for those whose RT plan followed protocol guidelines (p-values = 0.074 for FFS and 0.032 for OS).
Table 3. Radiation Therapy Delivered.
Radiation received and radiation received as assigned by protocol for each IRS Group
| Group I (N = 18) | Group II (N = 15) | Group III (N = 43) | Total | 5-yr FFS (95% CI) | p-value |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||
| RT | ||||||||||
| Yes | 3 | 17 | 10 | 68 | 20 | 46 | 33 | 43 | 58% (39%,73%) | |
| No | 15 | 83 | 5 | 33 | 23 | 54 | 43 | 57 | 56% (40%,70%) | 0.77 |
| * RT as assigned | ||||||||||
| Yes | 16 | 89 | 10 | 71 | 16 | 40 | 42 | 58 | 70% (53%,82%) | |
| No | 2 | 11 | 4 | 29 | 24 | 60 | 30 | 42 | 46% (27%,63%) | 0.074 |
Excludes 4 patients (Group II = 1, Group III = 3) for whom details of XRT were not available or were not sufficient to make a determination.
Twenty-three infants (54%) with Group III tumors did not receive any RT. Twenty-four of 40 infants (60%) with Group III tumors for whom sufficient information was available did not receive RT as specified by protocol. Estimated 5-year FFS (95% CI) with or without RT for infants with Group III tumors are 47% (24%, 67%) and 32% (14%, 51%), respectively (p-value = 0.17) (Figure 3A). Estimated 5-year OS (95% CI) for Group III patients is 63% (37%, 80%) with RT and 58% (35%, 76%) without (p-value = 0.49) (Figure 3B).
Figure 3.
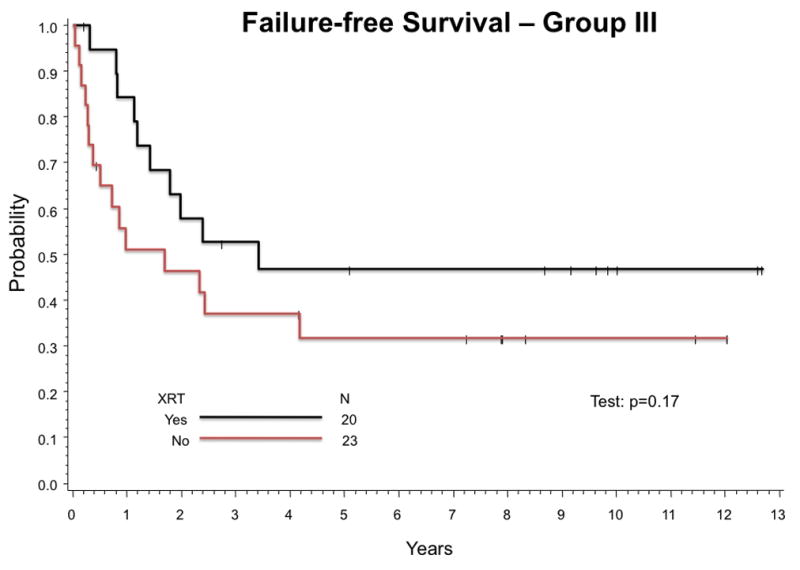
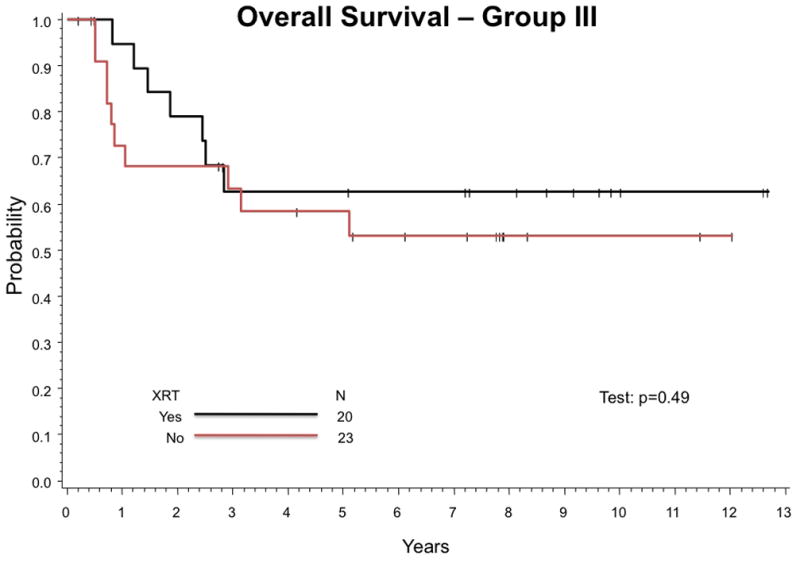
Outcome for infants Group III tumors was compared between those who received any RT and those who received no RT. (A) Estimated 5-year FFS are 47% (24%,67%) with RT and 32% (14%,51%) without (p-value = 0.17). (B) Estimated 5-year OS are 63% (37%,80%) with RT and 58% (35%,76%) without (p-value = 0.49).
Chemotherapy Doses Administered
Table 4 shows the mean percentage of prescribed doses administered (with 100% defined as the prescribed dose for children over 1 year of age) by phase of treatment for alkyating agents (cyclophosphamide or ifosfamide), vincristine and dactinomycin. Doses were escalated successfully for the majority of infants. In the final phase of therapy, infants on average received 83% of prescribed doses of alkylating agents and vincristine, and 89% of prescribed dose of dactinomycin. Fifteen of 43 patients (35%) received at least 90% of prescribed alkylator dose in the final phase.
Table 4. Chemotherapy Doses.
Mean percent doses of chemotherapeutic agents administered per treatment phase
| Phase | Alykylators (Cyclophosphamide or Ifosfamide) | Vincristine | Dactinomycin | Etoposide | ||||
|---|---|---|---|---|---|---|---|---|
| N | Mean % | N | Mean % | N | Mean % | N | Mean% | |
| 1 | 60 | 58 | 74 | 60 | 58 | 67 | 16 | 58 |
| 2 | 53 | 78 | 64 | 75 | 46 | 87 | 15 | 93 |
| 3 | 47 | 78 | 59 | 78 | 48 | 87 | 13 | 92 |
| 4 | 43 | 83 | 53 | 83 | 52 | 89 | - | - |
Patterns of Failure
Among the 32 patients with initial treatment failure, 20 had local/regional recurrence or progression, 6 developed distant metastases, 3 had local and metastatic progression, and 2 died from toxicity (Table 5). Site of recurrence/progression was not reported for 1 patient. The mean and median times to failure are 17 months and 13 months, respectively (range: 1 to 72 months). Fifteen patients experienced treatment failure within one year of diagnosis, and 8 treatment failures occurred within 6 months.
Table 5.
Site of Failure and Time to Failure by Site
| Time to First Failure (months) | |||||
|---|---|---|---|---|---|
| N | % | Mean | Median | Range | |
| All | 32 | 17 | 13 | 1 – 72 | |
| Local/Regional | 20 | 62 | 16 | 14 | 1 – 50 |
| Metastatic | 6 | 19 | 25 | 18 | 1 – 72 |
| Local + Mets | 3 | 9 | 19 | 24 | 5 – 28 |
| Toxic Death | 2 | 6 | 8 | 8 | 6 – 10 |
| Unknown | 1 | 3 | |||
The overall incidence of local failure (defined as any local failure with or without failure at other sites) in this infant cohort was 30% compared to 13% for all patients treated on IRS-IV, D9602, and D9803. Eighteen of 23 patients with local failure had Group III tumors. The rate of local failure for infants with Group III tumors was 42% compared to 17% for all Group III patients on IRS-IV, D9602, and D9803. Among the 18 infants with Group III tumors and local failure, 6 had received RT, 1 patient had a delayed complete resection without RT, and 11 patients had received no local therapy.
Discussion
Management of infants with RMS remains a challenge, particularly regarding local therapy. Previous studies have demonstrated that, after adjusting for other features, age < 1 year at diagnosis remains a significant adverse prognostic factor.3, 11 Outcome for our cohort of infants is similar to what was demonstrated by Joshi et al for infants treated on IRS-IV (5-year FFS 53%) and by Ferrari et al for infants treated in the Italian Cooperative Group (5-year FFS 42%).2, 3 While there has been a significant improvement in FFS for children with RMS over sequential IRS studies, the same improvement in outcome has not been seen for infants.7–12
The reason for poorer outcomes for infants remains unclear. In adolescents, an increased risk of failure can be explained by a higher frequency of alveolar histology and tumors in unfavorable sites. Conversely, our cohort of infants did not have a higher incidence of these known adverse prognostic factors. Previous analysis of IRS-III and IRS-IV demonstrated that outcomes were worse for infants compared to older patients within each histologic subtype.16 Other factors currently used in risk stratification, including IRS Stage and Group, were similar in infants compared to older patients in our study.
As there is no clear evidence of different biology of RMS in infants, one must consider less aggressive treatment as a possible reason for poorer outcome. While the incidence of Group III tumors was similar to older children, the outcome for these infants was significantly worse, with a 5-year FFS of 39%. In comparison, 3-year FFS for all patients with Group III tumors treated on IRS-IV was 73%.11 Ragab et al also found inferior survival for infants with Group III tumors (3-year OS 41%) treated on IRS-I and IRS-II, but no difference in survival for other Groups compared to older children.17 As in our study, Ragab et al found a higher local recurrence rate for infants with Group III RMS (29%) than for older patients treated on the same studies (11%). The high incidence of local failure in our infants as well as in the Ragab cohort suggests the possibility of less aggressive local control. In our cohort, more than half of infants with Group III tumors had major deviations from protocol-specified RT, and the majority of infants with Group III tumors and local failure had received no radiation at all. This finding is consistent with the study by Ferarri et al, where RT was withheld in 54% of infants with RMS who met protocol guidelines to receive radiation.2 Accordingly, a high rate of local failure was seen in their infant cohort, with 85% of treatment failures being local.2
There is clearly a dilemma in administering local therapy to infants. There are valid concerns regarding the long-term morbidity of RT in infants and young children.18 Depending on the location and size of tumor, local control with an operative procedure can be equally problematic. However, our data suggest that without adequate local control, the risk of local failure is very high. In an analysis of patients with Group II RMS, Million et al found a similar high rate of local recurrence associated with deviations from protocol guidelines.19 However, reasonable local control rates can be achieved with appropriate radiation therapy, even in very young children.20 Finding the balance between long-term effects, quality of life, and the potential for cure is difficult. Therefore, treatment decisions often need to be individualized for each particular case. The fact that we did not see worse OS for infants with Group III RMS for whom RT was withheld suggests that many of these patients may be salvaged after local recurrence. Current COG guidelines for local therapy in children < 24 months allow for individualization of treatment to permit careful balancing of long-term morbidity against the increased risk of local failure and death.
There is a paucity of pharmacokinetic data in infants to help guide dosing of chemotherapeutic agents.21 Due to higher mortality in infants on IRS-I with full dose chemotherapy, initial chemotherapy in subsequent studies has started at 50% of the calculated doses for older children with dose-escalation as tolerated.9 In our infant cohort, chemotherapy was relatively well tolerated and doses were successfully escalated for most patients. The rate of death from toxicity was only 3%. It is unclear if the lower intensity of initial chemotherapy contributed to the inferior outcome for infants in our study. However, with improvements in supportive care and monitoring, this degree of chemotherapy dose reduction for infants may no longer be necessary. Increasing chemotherapy intensity could potentially contribute to successful prevention of local failure.22 Data from a current COG study evaluating the pharmacokinetics and pharmacogenetics of vincristine and dactinomycin in children (including infants) will help guide dosing of these agents in future trials.23
In summary, our results corroborate previous studies in infants with RMS and suggest that local control is critical to successful treatment. While achieving local control in infants with solid tumors remains difficult, advancement of surgical techniques and improved methods for delivering highly conformal RT are necessary to overcome this challenge. In addition, the development of novel systemic agents and incorporation of these agents into treatment plans should help to improve outcome for infants with RMS.
Acknowledgments
Financial Support: Supported by grants No. U10 CA 98543 and U10 CA 98413 from the National Cancer Institute, National Institutes of Health
Footnotes
Presented in abstract form at the 23rd annual meeting of the American Society of Pediatric Hematology/Oncology in April, 2010.
Disclosures: None of the authors have financial relationships or interests to disclose
References
- 1.Ries LAGSM, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR. NIH Pub No 99–4649. Bethesda, MD: 1999. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975–1995. National Cancer Institute, SEER Program. [Google Scholar]
- 2.Ferrari A, Casanova M, Bisogno G, et al. Rhabdomyosarcoma in infants younger than one year old: a report from the Italian Cooperative Group. Cancer. 2003;97(10):2597–604. doi: 10.1002/cncr.11357. [DOI] [PubMed] [Google Scholar]
- 3.Joshi D, Anderson JR, Paidas C, Breneman J, Parham DM, Crist W. Age is an independent prognostic factor in rhabdomyosarcoma: a report from the Soft Tissue Sarcoma Committee of the Children’s Oncology Group. Pediatr Blood Cancer. 2004;42(1):64–73. doi: 10.1002/pbc.10441. [DOI] [PubMed] [Google Scholar]
- 4.Orbach D, Rey A, Oberlin O, et al. Soft tissue sarcoma or malignant mesenchymal tumors in the first year of life: experience of the International Society of Pediatric Oncology (SIOP) Malignant Mesenchymal Tumor Committee. J Clin Oncol. 2005;23(19):4363–71. doi: 10.1200/JCO.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 5.Arndt C, Hawkins D, Anderson JR, Breitfeld P, Womer R, Meyer W. Age is a risk factor for chemotherapy-induced hepatopathy with vincristine, dactinomycin, and cyclophosphamide. J Clin Oncol. 2004;22(10):1894–901. doi: 10.1200/JCO.2004.08.075. [DOI] [PubMed] [Google Scholar]
- 6.Morgan E, Baum E, Breslow N, Takashima J, D’Angio G. Chemotherapy-related toxicity in infants treated according to the Second National Wilms’ Tumor Study. J Clin Oncol. 1988;6(1):51–5. doi: 10.1200/JCO.1988.6.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Arndt CA, Stoner JA, Hawkins DS, et al. Vincristine, actinomycin, and cyclophosphamide compared with vincristine, actinomycin, and cyclophosphamide alternating with vincristine, topotecan, and cyclophosphamide for intermediate-risk rhabdomyosarcoma: children’s oncology group study D9803. J Clin Oncol. 2009;27(31):5182–8. doi: 10.1200/JCO.2009.22.3768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crist W, Gehan EA, Ragab AH, et al. The Third Intergroup Rhabdomyosarcoma Study. J Clin Oncol. 1995;13(3):610–30. doi: 10.1200/JCO.1995.13.3.610. [DOI] [PubMed] [Google Scholar]
- 9.Maurer HM, Beltangady M, Gehan EA, et al. The Intergroup Rhabdomyosarcoma Study-I. A final report. Cancer. 1988;61(2):209–20. doi: 10.1002/1097-0142(19880115)61:2<209::aid-cncr2820610202>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Maurer HM, Gehan EA, Beltangady M, et al. The Intergroup Rhabdomyosarcoma Study-II. Cancer. 1993;71(5):1904–22. doi: 10.1002/1097-0142(19930301)71:5<1904::aid-cncr2820710530>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Crist WM, Anderson JR, Meza JL, et al. Intergroup rhabdomyosarcoma study-IV: results for patients with nonmetastatic disease. J Clin Oncol. 2001;19(12):3091–102. doi: 10.1200/JCO.2001.19.12.3091. [DOI] [PubMed] [Google Scholar]
- 12.Walterhouse DO, Meza JL, Raney RB, et al. Dactinomycin (A) and vincristine (V) with or without cyclophosphamide (C) and radiation therapy (RT) for newly diagnosed patients with low risk embryonal/botryoid rhabdomyosarcoma (RMS). An IRS-V report from the Soft Tissue Sarcoma Committe of the Children’s Oncology Group. J Clin Oncol. 2006;24:18s. doi: 10.1200/JCO.2010.30.4469. (abstr 9001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawrence W, Jr, Gehan EA, Hays DM, Beltangady M, Maurer HM. Prognostic significance of staging factors of the UICC staging system in childhood rhabdomyosarcoma: a report from the Intergroup Rhabdomyosarcoma Study (IRS-II) J Clin Oncol. 1987;5(1):46–54. doi: 10.1200/JCO.1987.5.1.46. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 15.Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meza JL, Anderson J, Pappo AS, Meyer WH. Analysis of prognostic factors in patients with nonmetastatic rhabdomyosarcoma treated on intergroup rhabdomyosarcoma studies III and IV: the Children’s Oncology Group. J Clin Oncol. 2006;24(24):3844–51. doi: 10.1200/JCO.2005.05.3801. [DOI] [PubMed] [Google Scholar]
- 17.Ragab AH, Heyn R, Tefft M, Hays DN, Newton WA, Jr, Beltangady M. Infants younger than 1 year of age with rhabdomyosarcoma. Cancer. 1986;58(12):2606–10. doi: 10.1002/1097-0142(19861215)58:12<2606::aid-cncr2820581209>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Paulino AC, Wen BC, Brown CK, et al. Late effects in children treated with radiation therapy for Wilms’ tumor. Int J Radiat Oncol Biol Phys. 2000;46(5):1239–46. doi: 10.1016/s0360-3016(99)00534-9. [DOI] [PubMed] [Google Scholar]
- 19.Million L, Anderson J, Breneman J, et al. Influence of Noncompliance with Radiation Therapy Protocol Guidelines and Operative Bed Recurrences for Children with Rhabdomyosarcoma and Microscopic Residual Disease: A Report from the Children’s Oncology Group. Int J Radiat Oncol Biol Phys. 2010 doi: 10.1016/j.ijrobp.2010.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Puri DR, Wexler LH, Meyers PA, La Quaglia MP, Healey JH, Wolden SL. The challenging role of radiation therapy for very young children with rhabdomyosarcoma. Int J Radiat Oncol Biol Phys. 2006;65(4):1177–84. doi: 10.1016/j.ijrobp.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 21.Adamson PC. It’s not easy being small. Pediatr Blood Cancer. 54(3):341–3. doi: 10.1002/pbc.22343. [DOI] [PubMed] [Google Scholar]
- 22.Stevens MC, Rey A, Bouvet N, et al. Treatment of nonmetastatic rhabdomyosarcoma in childhood and adolescence: third study of the International Society of Paediatric Oncology--SIOP Malignant Mesenchymal Tumor 89. J Clin Oncol. 2005;23(12):2618–28. doi: 10.1200/JCO.2005.08.130. [DOI] [PubMed] [Google Scholar]
- 23.Study of the Pharmacokinetics, Pharmacodynamics, and Pharmacogenetics of Dactinomycin and Vincristine in Pediatric Patients With Cancer. (Children’s Oncology Group Study Number ADVL06B1), NCT00674193. http://www.cancer.gov/search/ViewClinicalTrials.aspx?cdrid=559243&version=HealthProfessional&protocolsearchid=7793878.


