1. Introduction
Abnormal development of the fetal brain in utero is now thought to contribute to the etiology of many functional and behavioral disorders that manifest throughout life. While differences in genetic makeup contribute to this, an ‘adverse’ intrauterine environment is a strong modulator of abnormal development. Maternal bacterial and viral infections during pregnancy represent a significant risk factor in several neuropsychiatric disorders with a presumed neurodevelopmental origin, including schizophrenia, autism, and cognitive delay.(Abecasis et al 2002; Brown et al 2004; Brown et al 2009; Ellman et al 2009; Fatemi et al 2002; Rantakallio et al 1997) However, specific infectious agents in this pathogenesis have not been demonstrated. It appears that prenatal inflammation is the greatest determinant of subsequent adverse outcomes for the offspring. While systemic infections during pregnancy are of concern, perhaps of greater concern, is the risk of in utero exposure to localized inflammation. A fetus is exposed to intrauterine inflammation in a woman with preterm labor and/or preterm birth or at any point in gestation when there evidence of chorioamnionitis (infection/inflammation of the fetal membranes). Inflammation is believed to be a leading cause of preterm birth which is defined as delivery at less than 37 weeks of gestation.(Andrews et al 2008; Romero 2007) Intrauterine inflammation, documented by histological examination of the placenta, occurs in approximately 20% of all pregnancies. However, the prevalence of histological chorioamnionitis is dramatically increased in preterm births with approximately 85% of very preterm births demonstrating this finding. (Yoon et al 2000b)
There are now several studies supporting an association between inflammation in preterm birth and cerebral palsy. (Bashiri et al 2006; Bracci and Buonocore 2003; Dammann et al 2003; Grether et al 2003; Hagberg et al 2005; Jacobsson and Hagberg 2004; Neufeld et al 2005; Wu 2002). These studies, along with animal studies,(Bell and Hallenbeck 2002; Borrell et al 2002; Cai et al 2000; Debillon et al 2000; Duncan et al 2002; Elovitz and Mrinalini 2004; Fatemi et al 2002; Hagberg et al 2002; Ito et al ; Kannan et al 2007; Meyer et al 2006; Ozawa et al 2006; Rousset et al 2006; Saadani-Makki et al 2008) suggests that inflammation is causal in adverse neurological outcomes for ex-preterm children. Further demonstrating a causal relation for prenatal inflammation in brain injury, ex-preterm children are at significant risk for other adverse neurobehavioral outcomes, aside from cerebral palsy.(Davis et al 2007; Johnson et al ; Johnson 2007; Kaukola et al 2006; Wood et al 2005) Chorioamnionitis has been reported to be an independent risk factor (with an odds ratio of 16) for a positive screening for early autistic features.(Pinelli and Zwaigenbaum 2008) To that end, emerging data has pointed to an astonishing prevalence of higher order neurodevelopmental impairment by the time these children reach school age.(Adams-Chapman and Stoll 2006; Hansen-Pupp et al 2008; Wood et al 2000; Wood et al 2005) In some studies, up to 50% of ex-preterm infants experience difficulties in executive functioning, as well as in the areas of attention and socioemotional behaviors, often requiring special academic support.(Bayless and Stevenson 2007; Davis et al 2007; Marlow et al 2007; Msall and Park 2008) Noting this growing body of evidence, it has been difficult to tease out the contribution of prematurity versus inflammation to long-term adverse outcomes for ex-preterm children.
In an effort to elucidate the mechanisms by which prenatal inflammation adversely affects the developing fetal brain, several types of animal models have been created.(Bell and Hallenbeck 2002; Elovitz and Mrinalini 2004; Gilles et al 1976; Gravett et al 1994; Hagberg et al 2002) While all of these models are useful in unraveling pathogenesis, it is crucial to understand the clinical and biological significance of each model.(Elovitz and Mrinalini 2004) The systemic models mimic the clinical scenario of a pregnant woman who has a bacteria (e.g. pyelonephritis, sepsis) or viral (e.g. influenza or CMV) infection. In contrast, the in utero or local models most aptly mirror inflammation in preterm birth or with chorioamnionitis at term; hence, these models may be more relevant in regards to the more common clinical exposure of a fetus to an inflammatory process.
Prior work from our laboratory has demonstrated that local intrauterine inflammation, sufficient to induce preterm birth, also causes significant fetal brain injury including loss of prooligodendrocytes, a reactive astrogliosis and a significant disruption in neuronal development.(Burd et al 2010a; Burd et al 2010b; Elovitz et al 2006; Ernst et al 2009) We have further demonstrated that neurons injured in utero by inflammation can continue to induce injury in other neurons in a cytokine-independent fashion.(Burd et al 2009) The question remains whether intrauterine inflammation, insufficient to induce preterm birth, can still evoke fetal brain injury. The fetus is most likely to be exposed to bacteria or bacterial byproducts through colonization of the uterine cavity; consequently, it is crucial to understand how infection and/or inflammation of the uterine cavity can evoke fetal brain injury and contribute to long term adverse neurological outcomes. Therefore, the objective of this study was to determine if a low-dose intrauterine inflammation, that was insufficient to induce preterm parturition, results in fetal and neonatal brain injury. We also sought to assess if the maternal and fetal response to intrauterine inflammation was dependent on gestational age of exposure. Using an established mouse model of intrauterine inflammation, we explored the effect of exposure to intrauterine inflammation in the preterm and term period on fetal brain development.
2. Methods
2.1 Animal model
A mouse model of intrauterine inflammation was used as previously reported. (Burd et al 2010a; Burd et al 2010b; Elovitz et al 2003; Elovitz et al 2006; Gonzalez et al 2009a; Gonzalez et al 2009b) CD-1 out-bred mice were used for these studies; this strain of mice has a gestational period of 19 days. We defined the preterm period as −70% of gestation which would be embryonic day 15 (E15) in this strain of mice. We defined the term period as the 12 hours prior to delivery which occurs on E18.5. We created the model of intrauterine inflammation in both preterm (E15) and term (E18.5) timed-pregnant CD-1 mice. For all experiments outlined, different sets of pregnant mice were utilized at each gestational time point. To induce intrauterine inflammation, at either E15 or E18.5, timed-pregnant CD-1 dams were placed under isoflurane anesthesia. A mini-laparotomy was performed and the lower right uterine horn was identified. Lipopolysaccharide (LPS) (Sigma, St Louis, MO), 50 μg in a hundred μL volume, or sterile saline (100 μL) was infused between two gestational sacs, with care not to inject into the amniotic cavity. Different experimental groups of mice, at each gestational time period, were used to assess our outcomes of interest 1) preterm birth and maternal morbidity and mortality; 2) immune response in the mother, placenta and fetus; 3) neuronal injury during fetal life, and 3) effect of exposure of prenatal inflammation on gene expression in the postnatal brain (P7).
2.2. Tissue collection (prenatal)
To assess the short term effect of intrauterine inflammation on the mother and fetus in both the preterm and term period, the following tissues were collected 6 hours after intrauterine infusion of LPS or saline: maternal serum, amniotic fluid, placentas, and fetal brains. For each gestational age and each treatment group, 4-6 dams per treatment group were used. Six hours after infusion, dams were euthanized with CO2 and a laparotomy was immediately performed. Maternal serum was obtained using a 22 gauge needle from the aorta with 0.8 to 1.0 mL collected per dam. Maternal blood was allowed to separate on ice and then serum was removed and centrifuged to remove debris. Serum was then placed in liquid nitrogen and stored at −80 degrees Celsius. To collect amniotic fluid, the lower 2-3 gestational sacs in both the left and right horn were identified. An 18 gauge needle on a 1 mL syringe was used to extract amniotic fluid from each sac and pooled as one sample. The sample was placed on ice, centrifuged to remove debris, and then placed in liquid nitrogen and stored at −80 degrees Celsius. The same gestational sacs used to collect amniotic fluid were used to collect placentas and fetal brains. The placentas were identified and fetal membranes were dissected off. Placentas were rinsed in sterile saline. The brain was removed from the cranium and placed in microfuge tubes. Both placenta and fetal brains placed in liquid nitrogen and stored at −80 degrees Celsius.
2.3 Tissue Collection (postnatal)
To determine the effect of prenatal (intrauterine) inflammation in the postnatal period, a separate group of timed-pregnant CD-1 mice at each gestational time period were used. Intrauterine LPS (LPS, 50 micrograms/dam) or saline was administered as described above on either E15 or E18.5 (N=8/group). Dams were observed in their cages every 12 hours for any sign of maternal morbidity or preterm birth. Preterm birth was defined as delivery of at least one pup. Morbidity was classified in a 1-4 scale with 1 being no signs of morbidity, 2 being minimal lethargy or piloerection, 3 was significant lethargy and/or piloerection and 4 being when a dam appear moribund. Dams were observed until after delivery of the litter at term. After delivery, pups were observed in their cages on a daily basis until collection on postnatal day (P7). Each dam and her litter were housed in separate cages so that there was no interaction between litters or mothers. The dam and litter were left undisturbed until P7.
Number of pups at postnatal day 7 (P7) was recorded. On P7, from each litter, one pup was selected at random to represent that litter. Pups were euthanized and the brain removed. The brain was dissected out from the cranium and overlying meninges. Three brain regions were collected: prefrontal cortex (PFC), cerebellum (CBL) and hippocampus (HC). Tissues were rinsed in sterile saline and placed in liquid nitrogen and stored at −80 degrees.
2.4 Measuring a Cytokine Response in Maternal Serum and Amniotic Fluid
To assess a cytokine response in the maternal serum and amniotic fluid, ELISAs were performed per manufacturer's instructions ((R&D Systems, Minneapolis, MN)). Each sample was run in duplicate and averages obtained. Five –six samples per treatment group (representing 5-6 dams as detailed in 2.2 above) at each gestational age were used. Mean and standard deviations were generated from the data and statistically analyzed using Sigma Stat software. T-test or Mann Whitney Rank Sum was utilized depending if the data was normally or non-normally distributed respectively.
2.5 Assessing Gene Expression in the Fetal and Neonatal Brain
To assess message expression in the placenta and brain (fetal and neonatal), quantitative PCR (QPCR) was performed as previously reported.(Burd et al 2010a; Burd et al 2010b; Elovitz et al 2006; Elovitz and Gonzalez 2008; Gonzalez et al 2009b; Xu et al 2008) Briefly, total RNA was extracted from placenta or brain tissues with trizol (Invitrogen) and complementary DNA (cDNA) was generated using high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). TaqMan Gene Expression Assays consisting of a 20X mix of unlabeled PCR primers and TaqMan minor groove binder probe, were purchased from Applied Biosystems (Foster City, CA). All TaqMan Gene Expression Assays were performed using TaqMan Universal PCR Master Mix from Applied Biosystems (Foster City, CA). QPCR reactions were carried out using equivalent dilutions of each cDNA sample on the Applied Biosystems Model 7900 sequence detector PCR machine. Target mRNA was normalized to the amount of 18S ribosomal RNA in each sample. The relative abundance of the target of interest was divided by the relative abundance of 18S in each sample to generate a standardized abundance for the target transcript of interest. All samples were analyzed in duplicates. Mean target mRNA expression was compared by Student T-test (for parametric data) or Mann Whiteny rank sum (for non-parametric data).
For placental tissues and fetal brains, 6 specimens from (representing 6 different dams) from each treatment group at each gestational time point were used. In placental and fetal brains, cytokine mRNA expression was assessed using QPCR as described above. For neonatal brains, 3-6 specimens (each specimen originating from a different dam) from each treatment group at each gestational age were used. For each brain region, gene expression in the following pathways was investigated: 1) white matter damage 2) neurobehavioral targets 3) glutamate-glutamine (GLU-GLN) cycle and regulation. (Table I)
Table I.
Fold Change in Gene Expression in Brain-Specific Regions on Postnatal Day 7 After Exposure to Low-Dose Intrauterine Inflammation
| Pathway | Gene | Exposure in Preterm Period (E15) | Exposure at Term (E18.5) | ||||
|---|---|---|---|---|---|---|---|
| PFC | CBL | HC | PFC | CBL | HC | ||
| White Matter Targets |
Gfap | NS | NS | NS | NS | NS | NS |
| Plp1 |
3.1 (P=0.006) |
2.2 (P=0.028) |
NS |
1.6 (P=0.05) |
1.1 (P=0.047) |
NS | |
| Mbp |
2.1 (P=0.021) |
NS | NS | NS | NS | NS | |
| Neuro- behavioral Targets |
MeCP2 | NS | NS | NS |
1.3 (p=0.005) |
1.2 (P=0.029) |
NS |
| Auts2 |
1.5 (P=0.004) |
NS | NS |
1.3 (P=0.002) |
NS |
1.2 (P=0.026) |
|
| Auts2 |
1.5 (P=0.004) |
NS | NS |
1.3 (P=0.002) |
NS |
1.2 (P=0.026) |
|
| TH | NS | NS | NS | NS | NS | NS | |
| Oxtr |
1.3 (P=0.019) |
NS | NS |
1.4 (P=0.02) |
NS | NS | |
| Nrxn1 | NS | NS | NS | NS | NS |
1.1 (P=0.03) |
|
| Rein | NS |
1..6 (P=0.028) |
NS | NS | NS |
1.2 ([=0.021) |
|
| Glutamate- Glutamine cycle |
Vglut1 |
1.4 (P=0.02) |
2.2 (P=0.004) |
NS |
1.3 (P=0.05) |
NS | NS |
| Vglut2 |
1.3 (p=0.03) |
NS | NS | NS |
1.1 (P=0.012) |
1.2 (P=0.007) |
|
| Vglut3 | NS | NS | NS | NS | NS | NS | |
| Eaat1 |
1.5 (P=0.02) |
1.8 (P=0.05) |
NS |
1.4 (P=0.049) |
NS |
1.2 (P=0.003) |
|
| Eaat2 | NS | NS | NS | NS | NS | NS | |
| Eaat3 | NS | NS | NS | NS | NS |
1.2 (P<0.001) |
|
| Eaat4 | NS |
1.2 (P=0.03) |
NS |
0.73 (P=0.012) |
NS |
1.2 (p=0.009) |
|
PFC-prefrontal cortex; CBL-cerebellum; HC-hippocampus; GFAP=glial fibrillary acid protein; PLP-proteolipid protein; MBP-myelin basic protein; TH-tyrosine hydroxylase; OXTR-oxytocin receptor, NRXN1-neurexin 1; RELN-reelin; VGLUT 1-3-glutamine transporter P value was calculated by T-test; NS=non-significant with P value >0.05
2.6 Assessing fetal neuronal injury: primary cortical neuronal cultures and quantitative assessment of dendritic processes
To assess neuronal injury (acutely) from exposure to intrauterine inflammation, primary cortical cultures were established as previously reported.(Burd et al 2010a; Burd et al 2010b; Burd et al 2009) For both gestational time points investigated, ix hours after intrauterine infusion of LPS or saline, fetal brains were collected. From each dam, 3-4 fetal brains were collected and treated as one sample (n=1). Using sterile technique, E15 and E18.5 fetal brains were harvested and placed into Petri dishes containing cold Ca++/Mg++-free Hanks Balanced Salt Solution (HBSS; Invitrogen, Carlsbad, CA), pH 7.4. The fetal cortex, was separated from meninges, olfactory bulbs, brain stem and cerebellum. Each cortex was minced, placed in 4 ml neurobasal medium (NBM; Invitrogen, Carlsbad, CA) containing 0.03% Trypsin (Invitrogen, Carlsbad, CA) and incubated for 15 minutes at 37°C and 5% CO2. Brain tissue was removed and placed in 4.5 ml NBM containing 10% fetal bovine serum (FBS) and allowed to settle to inactivate the trypsin. The medium was decanted and replaced with NBM supplemented with B-27 vitamin (Invitrogen, Carlsbad, CA) and 0.5mM L-glutamine and cells were dissociated by trituration. This media combination, NBM in the absence of fetal bovine serum, allows for the selective growth of neurons and not glia (astrocytes or microglia).(Burd et al 2009),(Monnerie et al 2003; Monnerie and Le Roux 2006; Monnerie and Le Roux 2007) Cells were plated at 4×104 cells/ml density on poly-L-lysine (1 mg/ml; Sigma-Aldrich, St. Louis, MO) coated glass coverslips, using 6- and 12-well culture plates. Groups were plated to equal density for each experiment. For each experiment, 4 fetal brains from one dam constituted one culture. Four dams from each treatment group at each gestational age were utilized for the analysis and the comparison of the dendritic counts. All experiments were performed in triplicate to assure the consistency of the results. To determine if intrauterine inflammation objectively altered neuronal morphology, quantitative analysis of dendritic processes was performed as previously reported.(Burd et al 2010a; Burd et al 2010b ; Burd et al 2009) Dendrite processes were analyzed at DIV 3, using previously described techniques. (Monnerie et al 2003; Monnerie and Le Roux 2006; Monnerie and Le Roux 2007) Briefly, cells were selected at random using at least 3 coverslips for each condition. One coverslip represented 4 fetal brains from 1 dam and three different dams were used for each condition in order to quantify processes emanating from each cell body. Neurons from each treatment group were evaluated at a final image magnification of 400X. Individual neurons were selected at random if they were clearly defined and not overlapping with other neurons. Approximately 10 neurons were evaluated from each coverslip. Each coverslip represented 1 dam (with pooled cultures from 4 fetal brains from that same dam). This experiment was repeated 3 times. Fluorescent images were recorded and analyzed using a Dell Latitude D620, using an image processing program (Image J 1.37v).
2.7 Statistical Analysis
Statistical analysis was performed using SigmaStat 3.5 (Aspire Software International, Asburn, VA). Data are expressed as the mean standard deviation of the mean (SD) or as the median where the data is not normally distributed. Statistical significance was defined as a two-sided p < 0.05. For each outcome measure, statistical analyses were performed comparing saline-exposed to LPS-exposed for each gestational time period as delineated in each section above.
3. Results
3.1 Preterm birth, maternal morbidity and pup survival
As previously reported, intrauterine infusion of a higher dose of LPS (250 micrograms per dam) consistently results in greater than 90% preterm birth rate.(Elovitz and Mrinalini 2006; Elovitz et al 2003) In contrast, on E15, low dose LPS resulted in a 30% preterm birth rate. Exposure to low dose LPS on E18.5 resulted in no preterm birth; all dams exposed delivered at term on E19. There was no evidence of maternal morbidity (piloerection, decreased movement) or mortality at either gestational time point. All dams (saline and LPS-exposed) had a score of 1. After exposure on E15, mean litter sizes on P7 were not significantly different in saline-exposed dams (9.8+/−4.8) and LPS-exposed dams (9.8+/−2.5). Similarly, after E18.5 exposure, mean litter size on P7 was not significantly different in dams who were exposed to saline (10.8 +/− 2.7) or LPS (9.7 +/−3.9).
3.2 Cytokine response in mother, placenta and amniotic fluid
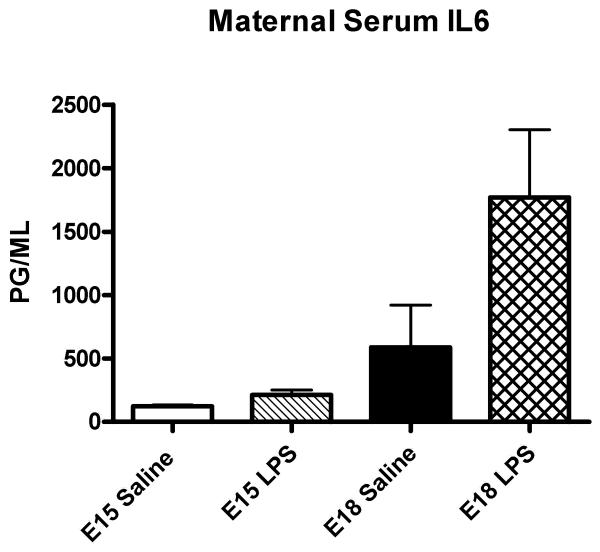
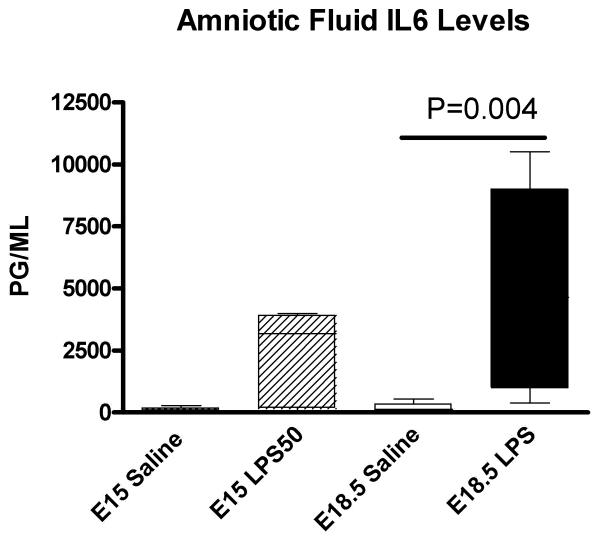
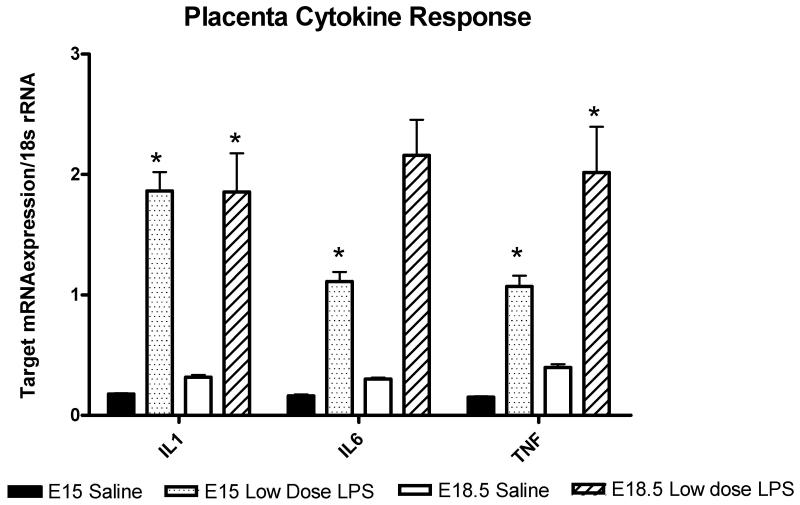
A cytokine response was assessed in the dam, placenta and amniotic fluid six hours after exposure to intrauterine inflammation on either E15 or E18.5. In maternal serum, intrauterine infusion of low-dose LPS resulted in a mild elevation of IL6 levels on E18 but did not alter levels after exposure to inflammation on E15 compared to saline-exposed (Figure 1). In the amniotic fluid, low-dose LPS induced a non-significant increase in IL6 levels on E15 and a 27-fold increase in IL6 on E18 (Figure 2). Exposure to low dose LPS increased cytokine mRNA expression in the placenta in both the preterm (E15) and term (E18.5) period. (Figure 3)
Figure 1.
IL6 levels in maternal serum. Bar graph depicting means and standard deviations of IL6 levels in maternal serum from preterm (E15) and term (E18.5) exposure to low dose intrauterine LPS. Low dose intrauterine LPS did not significantly alter maternal IL6 levels in the preterm period. At term (E18.5), IL6 levels increased 3-fold but this was not statistically significant (P=0.09, T-test).
Figure 2.
IL6 levels in amniotic fluid. Bar graph depicting means and standard deviations of IL6 levels in amniotic fluid from preterm (E15) and term (E18.5) exposure to low dose intrauterine LPS. There was wide variation in IL6 levels between the dams; the data were not normally distributed. IL6 levels were increased in the amniotic fluid in the preterm period but this did not reach statistical significance (Mann Whitney Rank Sum). In the term period, intrauterine inflammation significantly increased IL6 levels 27-fold compared to saline-exposed (P=0.004, Mann Whitney Rank Sum)
Figure 3.
Cytokine expression in the placenta. Bar graph representing mean mRNA expression normalized to the amount of 18s rRNA in placentas exposed to saline or low dose LPS at both gestational time points (E15 and E18.5). Exposure to low dose LPS increased placental IL1β 12-fold (*P=0.008) on E15 and 5.8-fold on E18.5 (*P=0.009). Low dose LPS increased IL6 mRNA expression 8.6-fold (P=0.008) on E15 and 87-fold (P=0.06) on E18.5. Low dose LPS increased TNF 7-fold (P=0.002) on E15 and 5-fold (P=0.04) on E18.5. MRNA expression was compared using T-test when data was normally distributed and Mann Whitney Rank Sum test when the data was non-parametric.
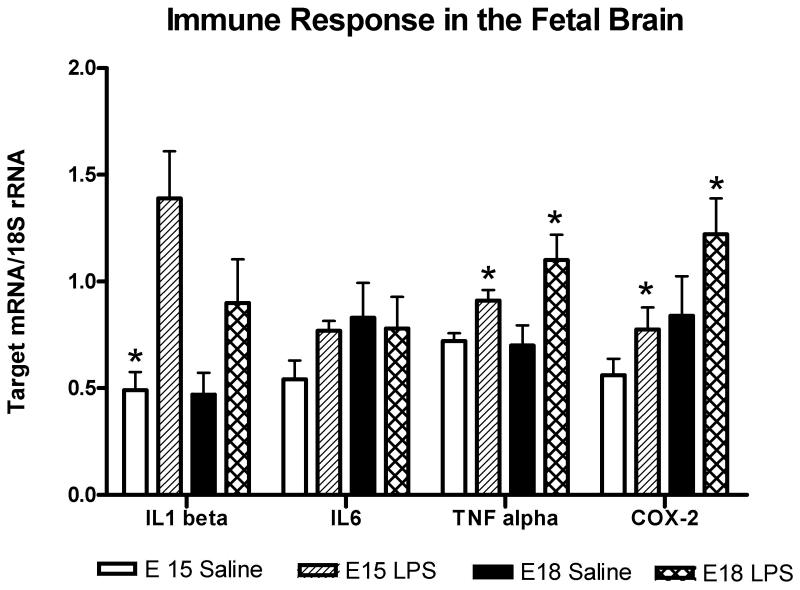
3.3 Immune response in the fetal brain
In the preterm (E15) period, compared to saline, exposure to intrauterine low dose LPS resulted in a significant increase in mRNA expression of IL1β, TNFα and cyclooxygenase-2 (COX-2) (Figure 4). On E18.5, compared to saline, exposure to intrauterine low dose LPS significantly increased TNFα and COX2 (1.6-fold, P=0.04). IL6 mRNA expression was not significantly different in brains exposed to saline compared to LPS. (Figure 4)
Figure 4.
Immune response in the fetal brain. Bar graph representing mean mRNA expression normalized to the amount of 18s rRNA in fetal brains exposed to saline or low dose LPS at both gestational time points (E15 and E18.5). In the preterm (E15) period, compared to saline, exposure to intrauterine low dose LPS resulted in an 2.8-fold increase in IL1 beta (P=0.007), a 1.4-fold increase in TNF (P=0.018) and 1.6-fold increase in COX-2 (P=0.02) mRNA expression. On E18.5, compared to saline, exposure to intrauterine low dose LPS increased IL1 beta 1.9-fold (P=0.13), TNF 1.8-fold (P=0.04) and COX2 (1.6-fold, P=0.04). IL6 mRNA expression was not significantly different in brains exposed to saline compared to LPS. Mean mRNA expression was compared using T-test for each gestational time period.
3.4 Acute neuronal injury
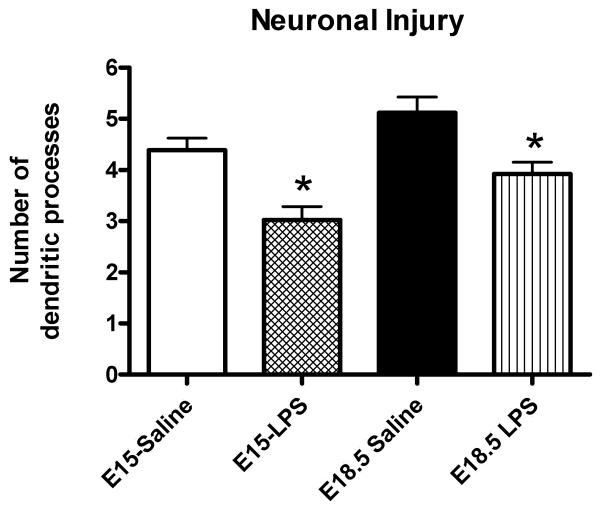
Neurons exposed to intrauterine saline demonstrated abnormal cell bodies, decreased aggregation, and abnormal processes, similar to our prior reports with a higher dose of LPS.(Burd et al 2010a ; Burd et al 2010b; Burd et al 2009) To provide objective measures of neuronal injury, we assessed the number of dendritic processes in each treatment group at each gestational age. Exposure to intrauterine inflammation on either E15 or E18.5 resulted in a decreased number of dendritic processes (Figure 5) The number of dendritic processes in the saline-exposed cortical cultures is similar to what we have previously reported for saline-exposed as well as for non-infectious induced preterm birth.(Burd et al 2010a)
Figure 5.
Fetal neuronal injury. Bar graph representing means and standard deviations of dendritic counts in the preterm and term period after exposure to low dose intrauterine inflammation or saline. Exposure to low dose LPS significantly decreased dendritic counts on E15 and E18.5 compared to saline-exposed (P<0.001 for both gestational time periods).
3.5 Gene expression changes in the neonatal brain
Exposure to low dose intrauterine inflammation significantly altered gene expression in all three pathways at both gestational age periods. (Table 1) 1) White matter damage: Proteolipid protein (PLP) and myelin basic protein (MBP) mRNA were differentially expressed in a region-specific manner after exposure to LPS at both gestational time periods. Glial fibrillary acidic protein (GFAP) was not increased in any brain region after E15 or E18 exposure. 2) Neurobehavioral targets: In a region-specific manner and dependent on gestational age of exposure, AUTS2, MECP2, Oxytocin receptor (OXTR), reelin (RLN) and neurexin (NRXN) mRNA expression were differentially expressed after after exposure to intrauterine inflammation. 3) Glutamate-glutamine cycle: Exposure to intrauterine inflammation on E15 and E18.5 increased mRNA expression of VGLUT1, VGLUT2 and VGLUT3 in a region specific in P7 neonatal brains (Table I). Similarly, EAAT1-4 were differently expressed in P7 neonatal brains exposed to intrauterine inflammation during the prenatal period (both preterm and term).
4. Conclusions
Exposure to prenatal inflammation is believed to be an important causal factor in adverse neurological outcomes for children born preterm or at term. While there are conceptual paradigms of how prenatal inflammation may affect the developing fetal brain, the precise mechanisms by which prenatal inflammation results in fetal brain injury and how the ‘injured’ fetal brain contributes to long-term adverse neurobehavioral outcomes remains to be fully elucidated. The studies presented herein advances our understanding of this pathogenesis by revealing the following: 1) intrauterine inflammation, that is insufficient to cause parturition, is sufficient to induce fetal brain injury in both the preterm and term period; 2) a maternal immune response (IL6) does not appear necessary for fetal or neonatal brain injury to occur; 3) exposure to prenatal inflammation alters gene expression in the brain of the exposed offspring; 4) the gestational age of the fetus at the time of exposure to prenatal (intrauterine) inflammation dictates gene expression patterns in the brain; 5) exposure to intrauterine inflammation has a differential effect on brain regions and this is also dependent on gestational age of exposure; 6) exposure to prenatal inflammation in either the preterm or term period has significant effects on several gene pathways in the brain that are likely involved in the diverse neurobehavioral, motor and psychosocial deficits observed in children exposed to inflammation during fetal life.
Clinical studies support the association between prenatal inflammation and adverse neurobehavioral outcomes. Animal models have been utilized to investigate if there is a causal relationship between prenatal inflammation, brain injury and adverse outcomes. Animal models that utilized the systemic administration of inflammatory agents (Poly I:C, LPS) or bacteria demonstrate that exposure to such agents alters neurobehavioral function in the offspring.(Bakos et al 2004; Golan et al 2005; Meyer et al 2006) While these models clearly demonstrate a link between exposure to adverse neurobehavioral outcomes and exposure to prenatal inflammation, they do not address the most common clinical scenario in which a fetus is exposed to inflammation. Infection and/or inflammation of the uterine cavity and/or amniotic sac, referred to clinically as chorioamnionitis, is a much more prevalent event that women presenting with systemic illness. Intrauterine inflammation, defined clinically or histologically as chorioamnionitis affects greater than 85% of pregnancies ending in very preterm births and is present in approximately 20% of all pregnancies. (Edwards 2005; Yoon et al 2000b) Thus, exposure to intrauterine inflammation is a common event in human pregnancy. Consequently, understanding the effect of intrauterine inflammation to fetal brain development and subsequent adverse outcomes is of great clinical significance. Our mouse model serves to aptly mimic the human condition of inflammation/infection the uterine cavity. Hence, the studies described herein can provide insight into adverse outcomes from exposure to intrauterine inflammation.
Regardless of the route of inflammation, the mechanisms linking prenatal inflammation to fetal injury must be understood if therapeutic strategies are our goal. Prior work suggests that maternal IL6 is an essential mediator linking prenatal inflammation to adverse outcomes for the offspring.(Smith et al 2007) Our work demonstrates that maternal IL6 levels are, in fact, not altered in the setting of intrauterine inflammation in the preterm period. Yet, despite no change in maternal IL6 levels, we observed a significant cytokine response in the placenta, fetus (amniotic fluid) and fetal brain suggesting that maternal IL6 is not the critical link to fetal injury in the setting of intrauterine inflammation. Differences between our study and that of Smith et al include 1) different inflammatory agent (LPS vs PolyI:C and 2) a local versus a systemic model of inflammation. It is possible that maternal IL6 dictates fetal injury in the settting of systemic but not local (intrauterine) inflammation.
There has been little research exploring the neonatal outcomes after exposure to intrauterine inflammation in animal models. Recent work in rabbits suggest that intrauterine inflammation leads to changes in white matter development, alters cortical serotonin and disrupts serotonin-regulated thalamocortical development in the neonatal brain (Kannan et al ; Saadani-Makki et al 2009); this same model demonstrated that exposed offspring manifest motor deficits, similar to what is observed in cerebral palsy.(Saadani-Makki et al 2008). These studies begin to demonstrate a definitive causal relationship between exposure to intrauterine inflammation and adverse neurological outcomes.
Similarly, our work suggests proposed mechanisms for how intrauterine inflammation may lead to a spectrum of neurobehavioral disorders. Most importantly, we have found, similar to what we report with a higher dose of intrauterine LPS, that low dose intrauterine LPS results in a specific neuronal insult.(Burd et al 2010a; Burd et al 2009) A method to objectively measure neuronal injury is to assess the number of dendritic processes; a decrease in the number of processes suggest aberrant arboritization and can lead to disrupted synaptic communication. (Esquenazi et al 2002; Labelle and Leclerc 2000) Abnormalities in cytoskeletal structure and neuronal aboritization have been demonstrated to alter synaptic connectively and implicated in diverse neurobehavioral disorders.(Moretti et al 2006; Snow et al 2008) These studies demonstrate that low dose inflammation elicits neuronal injury and that this injury is independent of gestational-age. These findings along with our prior work suggest that neuronal dysfunction may underlie several neurobehavioral disorders observed after exposure to prenatal inflammation.(Burd et al 2010a; Burd et al 2009) Our findings in the P7 neonatal brains further support the concept that neuronal dysfunction may be a common mechanism in adverse neurobehavioral outcomes after exposure to prenatal inflammation. Our data demonstrates that expression of several genes in the GLU-GLN cycle is altered in the P7 brain. Exploration of regulators and transporters (VGLUT and EAAT) is a novel part of our work; these mediators are essential for glutamate homeostasis in the brain and, specifically, are involved in glial-neuronal and neuronal-neuronal communication.(Bak et al 2005; Broer and Brookes 2001) We also found that reelin, neurexin and MECP2 were differently expressed after exposure to inflammation; these three genes have been implicated in 1) neuronal development and synaptic communication and 2) specific neurobehavioral disorders that have been reported to be associated with prenatal inflammation. Reelin is a candidate gene for autism spectrum disorder and is involved in neuronal migration.(Fatemi et al 2005; Gong et al 2007; Qiu et al 2006) Neurexin plays a role in dendrite formation and synapse formation(Chen et al ; Dean and Dresbach 2006) has also been implicated in autism spectrum disorder (Arking et al 2008). MECP2 is associated with neuropsychiatric phentoypes, specifically Rett's syndrome.(Adegbola et al 2009; Amano et al 2000; Bienvenu et al 2000). On a molecular level, MECP2 is involved in neuronal architecture, synaptogenesis and regulates glial-neuronal crosstalk.(Ballas et al 2009; Fukuda et al 2005) Whether the observed gene expression changes in the postnatal brain from exposure to prenatal inflammation contribute to specific neurobehavioral phenotype requires further investigation.
Prior work using systemic models of inflammation have suggested that the gestational age of exposure dictates the presence and type of neurobehavioral phenotype in the exposed offspring. (Aguilar-Valles and Luheshi ; Meyer et al 2006) The studies herein confirm that are gestational age dependent and independent events in terms of the ‘type’ of brain injury that occurs after exposure to intrauterine inflammation. Indeed, our findings of neuronal injury—with exposure at either gestational time point—and our prior work demonstrating this finding at higher doses of inflammation,(Burd et al 2010a; Burd et al 2009) suggest that neuronal injury and/or dysfunction is a central mechanism leading to adverse outcomes. Our work also suggests that the immune response in the fetal brain is more potent in the preterm period; whether this heightened immune response dictates specific changes in gene expression observed on P7 or would correlated to different behavioral phenotypes is not yet known. The different patterns of gene expression in the P7 brain do appear to be a gestational age dependent event suggesting a differential response of the fetal brain to an immune challenge. Yet, the selected pathways appear to be disrupted from both gestational time points with the expression changes differing by gene and by brain region. It is likely that gene expression changes in one region (i.e cerebellum versus prefrontal cortex) will manifest in a different behavioral phenotype.
Our study has limitations. We are using a rodent model and hence brain development does not parallel human fetal development specifically in regards to myelination. Yet, these types of studies are not feasible in humans and quite costly in non-human primates. While it is accepted to correlate E15 with the ‘preterm period’ and E18.5 with ‘term’, these designations may be most appropriate in regards to parturition pathways. Glial development, specifically the oligodendrocyte lineage, is a postnatal event in the mouse as compared to the human. These limitations of the mouse model are acknowledged and must be understood when interpreting results. We did not look at a panel of cytokines in the maternal serum or amniotic fluid but instead choose to focus on IL6. Systemic models of inflammation have suggested that IL6 is essential for adverse outcomes while clinical studies suggest IL6 levels in the amniotic fluid predict development of cerebral palsy.(Smith et al 2007; Yoon et al 1996; Yoon et al 1997; Yoon et al 2000a) Despite these reports, we did not find IL6 to be altered in maternal serum or in the fetal brain. While IL6 was increased in maternal serum on E18.5, this did not reach significance. In addition, the level of IL6 remained much lower than demonstrated in our high dose model of LPS.(Gonzalez et al 2009a) In comparing prior studies with our findings, we propose that IL6 may play a role in causing brain injury from a systemic or high dose intrauterine inflammatory exposure but that it is not necessary for brain injury to occur. It is possible that other cytokines may be altered in the mother after low dose intrauterine inflammation and that these cytokines can contribute to adverse outcomes. Another limitation of this work is that we choose to investigate only certain genes in each pathway in the postnatal brain and thus can only provide limited information regarding gene expression patterns. However, genes were picked based on 1) knock-out studies that suggest certain proteins are critical for neuronal development or lead to a specific neurobehavioral phenotype;(Bakker et al 1994; Bouwknecht et al 2001; Chang et al 2006; Cheh et al 2006; Comery et al 1997; Degano et al 2009) 2) genetic association studies have demonstrated that a gene is associated with a neurobehavioral disorder(Adegbola et al 2009; Alarcon et al 2002; Han et al 2004); or 3) clinical studies demonstrate changes in these pathways (i.e white matter development) in children born preterm or with specific neurobehavioral disorders common to preterm infants or those exposed to chorioamnionitis.(Dammann et al 2009; Jacobsson and Hagberg 2004; O'Shea et al 2008; O'Shea et al 2009; Polam et al 2005; Wu et al 2003; Yoon et al 2003) While our method of gene selection provides is based on biological plausibility, other unstudied gene pathways may also play a role in adverse neurobehavioral phenotypes. Numerous reports demonstrate an association between aberrations in gene expression (of pathways studied herein) and neurobehavioral phenotypes in both animal models and humans.(Abdolmaleky et al 2005; Adachi et al 2009; Adegbola et al 2009; Dean and Dresbach 2006; Fatemi et al 2005; Hung et al 2008; Jugloff et al 2008; Kaufmann et al 2000; Medrihan et al 2008; Samaco et al 2008) Yet, while these studies confirm the probably importance of these gene expression changes in the P7 brain, further work demonstrating a link between these gene expression patterns on P7, in this model, and behavioral phenotypes will be necessary.
Understanding this limitation, this study and our prior work begin to ascribe specific mechanisms by which exposure to prenatal inflammation (intrauterine) results in adverse neurological outcomes. Specifically, activation of the immune system in the placenta and fetus (even in the absence of significant maternal response) is sufficient to ‘compromise’ the fetal brain. The pathways activated in the fetal brain result in permanent gene expression changes in the offspring. Whether there are epigenetic modifications, resulting from an inflammatory exposure, requires further investigation. There are emerging data to suggest that inflammatory pathways can result in epigenetic modifications leading to altered gene expression.(Angrisano et al 2010; Lyn-Kew et al 2010). A recent opinion paper hypothesize that epigenetic modifications contribute to adverse outcomes in neonatal brain injury.(Kumral et al 2009) While our data support some epigenetic modification occurring in the fetal period, future work will need to explore this possible mechanism. Important for extrapolation to the clinical realm, our results do suggest that ‘surviving’ an inflammatory insult is not protective again future brain injury and perhaps, more importantly, these studies demonstrate that the insult occurred in the fetal period continues to propagate after birth.
Our prior work used a higher dose of LPS and demonstrated significant disturbances in glial and neuronal development with this dose.(Burd et al 2010a; Burd et al 2009; Elovitz et al 2006) This higher dose of LPS in the preterm period resulted in nearly 100% of preterm birth.(Elovitz et al 2003) As preterm birth in mice results in neonatal death, the long term effect of intrauterine inflammation on the fetus could not be studied. What we now demonstrate is that intrauterine inflammation—that is insufficient to cause parturition—still evokes injury to the developing fetal brain. This is a critical finding. The conceptual paradigm for brain injury in preterm birth has assumed that if inflammatory pathways were insufficient to induce parturition than they were insufficient to cause fetal harm (or were not present). Our study suggests that this paradigm is incorrect. Our findings argue that small doses of inflammation in the uterus—that do not cause a maternal systemic immune response as measured here by IL6—are still quite capable of inducing a potent immune response in the placenta, fetus (amniotic fluid) and fetal brain. If we begin to extrapolate these findings to the clinical realm, these findings might suggest that women with preterm labor—who do not have a preterm birth—may be at significant risk for adverse neurological outcomes for that infant despite the absence of prematurity. Thus, clinically, our challenge will be, if a maternal immune response is not required for this pathogenesis to occur, than identifying fetuses at risk antenatally will be a daunting task. Exploring mechanisms how acute events lead to chronic changes in the neonatal brain may be more realistic targets in preventing adverse outcomes.
In conclusion, these studies demonstrate that intrauterine inflammation, which evokes a limited or no maternal response and does not induce preterm birth, can induce acute injury in the fetal brain and result in permanent changes in gene expression in the offspring. Collectively, our data support that inflammatory pathways activated in the uterus and placenta are sufficient to induce fetal neuronal injury in utero and may be a causative factor in adverse neurobehavioral outcomes in children exposed to prenatal inflammation.
Acknowledgement
This research was funded by the National Institutes of Health, RO1-HD046544-0 (MAE) and a Transdisciplinary Award in Translational Medicine and Therapeutics from the University of Pennsylvania
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdolmaleky HM, Cheng KH, Russo A, Smith CL, Faraone SV, Wilcox M, Shafa R, Glatt SJ, Nguyen G, Ponte JF, Thiagalingam S, Tsuang MT. Hypermethylation of the reelin (RELN) promoter in the brain of schizophrenic patients: a preliminary report. Am J Med Genet B Neuropsychiatr Genet. 2005;134B:60–6. doi: 10.1002/ajmg.b.30140. [DOI] [PubMed] [Google Scholar]
- Abecasis GR, Cherny SS, Cookson WO, Cardon LR. Merlin--rapid analysis of dense genetic maps using sparse gene flow trees. Nature Genetics. 2002;30:97–101. doi: 10.1038/ng786. [DOI] [PubMed] [Google Scholar]
- Adachi M, Autry AE, Covington HE, 3rd, Monteggia LM. MeCP2-mediated transcription repression in the basolateral amygdala may underlie heightened anxiety in a mouse model of Rett syndrome. J Neurosci. 2009;29:4218–27. doi: 10.1523/JNEUROSCI.4225-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams-Chapman I, Stoll BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis. 2006;19:290–7. doi: 10.1097/01.qco.0000224825.57976.87. [DOI] [PubMed] [Google Scholar]
- Adegbola AA, Gonzales ML, Chess A, LaSalle JM, Cox GF. A novel hypomorphic MECP2 point mutation is associated with a neuropsychiatric phenotype. Hum Genet. 2009;124:615–23. doi: 10.1007/s00439-008-0585-6. [DOI] [PubMed] [Google Scholar]
- Aguilar-Valles A, Luheshi GN. Alterations in cognitive function and behavioral response to amphetamine induced by prenatal inflammation are dependent on the stage of pregnancy. Psychoneuroendocrinology. 2010 doi: 10.1016/j.psyneuen.2010.09.006. epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Alarcon M, Cantor RM, Liu J, Gilliam TC, Geschwind DH. Evidence for a language quantitative trait locus on chromosome 7q in multiplex autism families. Am J Hum Genet. 2002;70:60–71. doi: 10.1086/338241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amano K, Nomura Y, Segawa M, Yamakawa K. Mutational analysis of the MECP2 gene in Japanese patients with Rett syndrome. J Hum Genet. 2000;45:231–6. doi: 10.1007/s100380070032. [DOI] [PubMed] [Google Scholar]
- Andrews WW, Cliver SP, Biasini F, Peralta-Carcelen AM, Rector R, Alriksson-Schmidt AI, Faye-Petersen O, Carlo W, Goldenberg R, Hauth JC. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. Am J Obstet Gynecol. 2008;198:466 e1–e11. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angrisano T, Pero R, Peluso SK, sacchetti SB, CHiariotti L, Lembo F. LPS-induced IL-8 activation in human intestinal epithelial cells is accompanied by specific histone H3 acetylation and methylation changes. BMC Microbiol. 2010;10:172. doi: 10.1186/1471-2180-10-172. S. CB. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arking DE, Cutler DJ, Brune CW, Teslovich TM, West K, Ikeda m, Rea A, Guy M, Lin S, Cook EH, Jr., Chakravarti A. A common genetic variant in the neurexin superfamily member CNTNAP2 increases familial risk of autism. American Journal of Human Genetics. 2008;82:160–4. doi: 10.1016/j.ajhg.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak LK, Sickmann HM, Schousboe A, Waagepetersen HS. Activity of the lactate-alanine shuttle is independent of glutamate-glutamine cycle activity in cerebellar neuronal-astrocytic cultures. J Neurosci Res. 2005;79:88–96. doi: 10.1002/jnr.20319. [DOI] [PubMed] [Google Scholar]
- Bakker C, Verheij C, Willemsen R, van der Helm R, Oerlemans F, Vermey M, Bygrave A, Hoogeveen A, Oostra B, Reyniers E, De Boulle K, D'Hooge R, Cras P, van Velzen D, Nagels G, Martin J, De Deyn R, Darby J, Willems P. Fmr1 knockout mice: a model to study fragile X mental retardation. Cell. 1994;78:23–33. [PubMed] [Google Scholar]
- Bakos J, Duncko R, Makatsori A, Pirnik Z, Kiss A, Jezova D. Prenatal immune challenge affects growth, behavior, and brain dopamine in offspring. Ann N Y Acad Sci. 2004;1018:281–7. doi: 10.1196/annals.1296.033. [DOI] [PubMed] [Google Scholar]
- Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–7. doi: 10.1038/nn.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashiri A, Burstein E, Mazor M. Cerebral palsy and fetal inflammatory response syndrome: a review. J Perinat Med. 2006;34:5–12. doi: 10.1515/JPM.2006.001. [DOI] [PubMed] [Google Scholar]
- Bayless S, Stevenson J. Executive functions in school-age children born very prematurely. Early Hum Dev. 2007;83:247–54. doi: 10.1016/j.earlhumdev.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Bell MJ, Hallenbeck JM. Effects of intrauterine inflammation on developing rat brain. J Neurosci Res. 2002;70:570–9. doi: 10.1002/jnr.10423. [DOI] [PubMed] [Google Scholar]
- Bienvenu T, Carrie A, de Roux N, Vinet MC, Jonveaux P, Couvert P, Villard L, Arzimanoglou A, Beldjord C, Fontes M, Tardieu M, Chelly J. MECP2 mutations account for most cases of typical forms of Rett syndrome. Hum Mol Genet. 2000;9:1377–84. doi: 10.1093/hmg/9.9.1377. [DOI] [PubMed] [Google Scholar]
- Borrell J, Vela JM, Arevalo-Martin A, Molina-Holgado E, Guaza C. Prenatal immune challenge disrupts sensorimotor gating in adult rats. Implications for the etiopathogenesis of schizophrenia. Neuropsychopharmacology. 2002;26:204–15. doi: 10.1016/S0893-133X(01)00360-8. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RAA, Hen R, Olivier B. Absence of 5-HT1B receptors is associated with impaired impulse control in male 5-HT1B knockout mice. Biological Psychiatry. 2001;49:557–68. doi: 10.1016/s0006-3223(00)01018-0. [DOI] [PubMed] [Google Scholar]
- Bracci R, Buonocore G. Chorioamnionitis: a risk factor for fetal and neonatal morbidity. Biol Neonate. 2003;83:85–96. doi: 10.1159/000067956. [DOI] [PubMed] [Google Scholar]
- Broer S, Brookes N. Transfer of glutamine between astrocytes and neurons. J Neurochem. 2001;77:705–19. doi: 10.1046/j.1471-4159.2001.00322.x. [DOI] [PubMed] [Google Scholar]
- Brown AG, Leite RS, Strauss JF., 3rd Mechanisms underlying “functional” progesterone withdrawal at parturition. Ann N Y Acad Sci. 2004;1034:36–49. doi: 10.1196/annals.1335.004. [DOI] [PubMed] [Google Scholar]
- Brown AS, Vinogradov S, Kremen WS, Poole JH, Deicken RF, Penner JD, McKeague IW, Kochetkova A, Kern D, Schaefer CA. Prenatal exposure to maternal infection and executive dysfunction in adult schizophrenia. Am J Psychiatry. 2009;166:683–90. doi: 10.1176/appi.ajp.2008.08010089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Bentz AI, Chai J, Gonzalez J, Monnerie H, Le Roux PD, Cohen AS, Yudkoff M, Elovitz MA. Inflammation-induced preterm birth alters neuronal morphology in the mouse fetal brain. J Neurosci Res. 2010a;88:1872–81. doi: 10.1002/jnr.22368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Breen K, Friedman A, Chai J, Elovitz MA. Magnesium sulfate reduces inflammation-associated brain injury in fetal mice. Am J Obstet Gynecol. 2010b;202:292 e1–9. doi: 10.1016/j.ajog.2010.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burd I, Chai J, Gonzalez J, Ofori E, Monnerie H, Le Roux PD, Elovitz MA. Beyond white matter damage: fetal neuronal injury in a mouse model of preterm birth. Am J Obstet Gynecol. 2009;201:279 e1–8. doi: 10.1016/j.ajog.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Z, Pan ZL, Pang Y, Evans OB, Rhodes PG. Cytokine induction in fetal rat brains and brain injury in neonatal rats after maternal lipopolysaccharide administration. Pediatr Res. 2000;47:64–72. doi: 10.1203/00006450-200001000-00013. [DOI] [PubMed] [Google Scholar]
- Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron. 2006;49:341–8. doi: 10.1016/j.neuron.2005.12.027. [DOI] [PubMed] [Google Scholar]
- Cheh MA, Millonig JH, Roselli LM, Ming X, Jacobsen E, Kamdar S, Wagner GC. En2 knockout mice display neurobehavioral and neurochemical alterations relevant to autism spectrum disorder. Brain Research. 2006;1116:166–76. doi: 10.1016/j.brainres.2006.07.086. [DOI] [PubMed] [Google Scholar]
- Chen SX, Tari PK, She K, Haas K. Neurexin-neuroligin cell adhesion complexes contribute to synaptotropic dendritogenesis via growth stabilization mechanisms in vivo. Neuron. 2010;67:967–83. doi: 10.1016/j.neuron.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Comery TA, Harris JB, Willems PJ, Oostra BA, Irwin SA, Weiler IJ, Greenough WT. Abnormal dendritic spines in fragile X knockout mice: maturation and pruning deficits. Proceedings of the National Academy of Sciences USA. 1997;94:5401–4. doi: 10.1073/pnas.94.10.5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Drescher J, Veelken N. Maternal fever at birth and non-verbal intelligence at age 9 years in preterm infants. Dev Med Child Neurol. 2003;45:148–51. doi: 10.1017/s001216220300029x. [DOI] [PubMed] [Google Scholar]
- Dammann O, Naples M, Bednarek F, Shah B, Kuban KC, O'Shea TM, Paneth N, Allred EN, Leviton A. SNAP-II and SNAPPE-II and the Risk of Structural and Functional Brain Disorders in Extremely Low Gestational Age Newborns: The ELGAN Study. Neonatology. 2009;97:71–82. doi: 10.1159/000232588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis NM, Ford GW, Anderson PJ, Doyle LW. Developmental coordination disorder at 8 years of age in a regional cohort of extremely-low-birthweight or very preterm infants. Dev Med Child Neurol. 2007;49:325–30. doi: 10.1111/j.1469-8749.2007.00325.x. [DOI] [PubMed] [Google Scholar]
- Dean C, Dresbach T. Neuroligins and neurexins: linking cell adhesion, synapse formation and cognitive function. Trends Neurosci. 2006;29:21–9. doi: 10.1016/j.tins.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Debillon T, Gras-Leguen C, Verielle V, Winer N, Caillon J, Roze JC, Gressens P. Intrauterine infection induces programmed cell death in rabbit periventricular white matter. Pediatr Res. 2000;47:736–42. doi: 10.1203/00006450-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Degano AL, Pasterkamp RJ, Ronnett GV. MeCP2 deficiency disrupts axonal guidance, fasciculation, and targeting by altering Semaphorin 3F function. Mol Cell Neurosci. 2009;42:243–54. doi: 10.1016/j.mcn.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan JR, Cock ML, Scheerlinck JP, Westcott KT, McLean C, Harding R, Rees SM. White matter injury after repeated endotoxin exposure in the preterm ovine fetus. Pediatr Res. 2002;52:941–9. doi: 10.1203/00006450-200212000-00021. [DOI] [PubMed] [Google Scholar]
- Edwards RK. Chorioamnionitis and labor. Obstet Gynecol Clin North Am. 2005;32:287–96. doi: 10.1016/j.ogc.2004.12.002. x. [DOI] [PubMed] [Google Scholar]
- Ellman LM, Yolken RH, Buka SL, Torrey EF, Cannon TD. Cognitive functioning prior to the onset of psychosis: the role of fetal exposure to serologically determined influenza infection. Biol Psychiatry. 2009;65:1040–7. doi: 10.1016/j.biopsych.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz M, Mrinalini C. Preterm labor and 17-P: lessons from a mouse model. American Journal of Obstetrics and Gynecology. 2006;193:S49. doi: 10.1016/j.ajog.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol. 2003;163:2103–11. doi: 10.1016/S0002-9440(10)63567-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab. 2004;15:479–87. doi: 10.1016/j.tem.2004.10.009. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Mrinalini C, Sammel MD. Elucidating the early signal transduction pathways leading to fetal brain injury in preterm birth. Pediatr Res. 2006;59:50–5. doi: 10.1203/01.pdr.0000191141.21932.b6. [DOI] [PubMed] [Google Scholar]
- Elovitz MA, Gonzalez J. Medroxyprogesterone acetate modulates the immune response in the uterus, cervix and placenta in a mouse model of preterm birth. J Matern Fetal Neonatal Med. 2008;21:223–30. doi: 10.1080/14767050801923680. [DOI] [PubMed] [Google Scholar]
- Ernst LM, Gonzalez J, Ofori E, Elovitz M. Inflammation-Induced Preterm Birth in a Murine Model is Associated with Increases in Fetal Macrophages and Circulating Erythroid Precursors. Pediatr Dev Pathol. 2009 doi: 10.2350/09-05-0649-OA.1. [DOI] [PubMed] [Google Scholar]
- Esquenazi S, Monnerie H, Kaplan P, Le Roux P. BMP-7 and excess glutamate: opposing effects on dendrite growth from cerebral cortical neurons in vitro. Exp Neurol. 2002;176:41–54. doi: 10.1006/exnr.2002.7906. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Snow AV, Stary JM, Araghi-Niknam M, Reutiman TJ, Lee S, Brooks AI, Pearce DA. Reelin signaling is impaired in autism. Biological Psychiatry. 2005;57:777–87. doi: 10.1016/j.biopsych.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Fukuda T, Itoh M, Ichikawa T, Washiyama K, Goto Y. Delayed maturation of neuronal architecture and synaptogenesis in cerebral cortex of Mecp2-deficient mice. J Neuropathol Exp Neurol. 2005;64:537–44. doi: 10.1093/jnen/64.6.537. [DOI] [PubMed] [Google Scholar]
- Gilles FH, Leviton A, Kerr CS. Endotoxin leucoencephalopathy in the telencephalon of the newborn kitten. J Neurol Sci. 1976;27:183–91. doi: 10.1016/0022-510x(76)90060-5. [DOI] [PubMed] [Google Scholar]
- Golan HM, Lev V, Hallak M, Sorokin Y, Huleihel M. Specific neurodevelopmental damage in mice offspring following maternal inflammation during pregnancy. Neuropharmacology. 2005;48:903–17. doi: 10.1016/j.neuropharm.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Gong C, Wang T-W, Huang HS, Parent JM. Reelin regulates neuronal progenitor migration in intact and epileptic hippocampus. The Journal of Neuroscience. 2007;27:1803–11. doi: 10.1523/JNEUROSCI.3111-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Ofori E, Burd I, Chai J, Scholler N, Elovitz MA. Maternal mortality from systemic illness: unraveling the contribution of the immune response. Am J Obstet Gynecol. 2009a;200:430 e1–8. doi: 10.1016/j.ajog.2009.01.049. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Xu H, Chai J, Ofori E, Elovitz MA. Preterm and term cervical ripening in CD1 Mice (Mus musculus): similar or divergent molecular mechanisms? Biol Reprod. 2009b;81:1226–32. doi: 10.1095/biolreprod.108.075309. [DOI] [PubMed] [Google Scholar]
- Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol. 1994;171:1660–7. doi: 10.1016/0002-9378(94)90418-9. [DOI] [PubMed] [Google Scholar]
- Grether JK, Nelson KB, Walsh E, Willoughby RE, Redline RW. Intrauterine exposure to infection and risk of cerebral palsy in very preterm infants. Arch Pediatr Adolesc Med. 2003;157:26–32. doi: 10.1001/archpedi.157.1.26. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Peebles D, Mallard C. Models of white matter injury: comparison of infectious, hypoxic-ischemic, and excitotoxic insults. Ment Retard Dev Disabil Res Rev. 2002;8:30–8. doi: 10.1002/mrdd.10007. [DOI] [PubMed] [Google Scholar]
- Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. Bjog. 2005;112(Suppl 1):16–8. doi: 10.1111/j.1471-0528.2005.00578.x. [DOI] [PubMed] [Google Scholar]
- Han DH, Park DB, Na C, Kee BS, Lee YS. Association of aggressive behavior in Korean male schizophrenic patients with polymorphisms in the serotonin transporter promoter and catecholamine-O-methyltransferase genes. Psychiatry Research. 2004;129:29–37. doi: 10.1016/j.psychres.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Hansen-Pupp I, Hallin AL, Hellstrom-Westas L, Cilio C, Berg AC, Stjernqvist K, Fellman V, Ley D. Inflammation at Birth is associated with Subnormal Development in Very Preterm Infants. Pediatr Res. 2008 doi: 10.1203/PDR.0b013e318176144d. [DOI] [PubMed] [Google Scholar]
- Hung AY, Futai K, Sala C, Valtschanoff JG, Ryu J, Woodworth MA, Kidd FL, Sung CC, Miyakawa T, Bear MF, Weinberg RJ, Sheng M. Smaller dendritic spines, weaker synaptic transmission, but enhanced spatial learning in mice lacking Shank1. J Neurosci. 2008;28:1697–708. doi: 10.1523/JNEUROSCI.3032-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Smith SE, Hsiao E, Patterson PH. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav Immun. 2010;24:930–41. doi: 10.1016/j.bbi.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsson B, Hagberg G. Antenatal risk factors for cerebral palsy. Best Pract Res Clin Obstet Gynaecol. 2004;18:425–36. doi: 10.1016/j.bpobgyn.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Johnson S, Hollis C, Kochhar P, Hennessy E, Wolke D, Marlow N. Autism Spectrum Disorders in Extremely Preterm Children. J Pediatr. 2010;156:525–31. doi: 10.1016/j.jpeds.2009.10.041. [DOI] [PubMed] [Google Scholar]
- Johnson S. Cognitive and behavioural outcomes following very preterm birth. Semin Fetal Neonatal Med. 2007;12:363–73. doi: 10.1016/j.siny.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Jugloff DG, Vandamme K, Logan R, Visanji NP, Brotchie JM, Eubanks JH. Targeted delivery of an Mecp2 transgene to forebrain neurons improves the behavior of female Mecp2-deficient mice. Hum Mol Genet. 2008;17:1386–96. doi: 10.1093/hmg/ddn026. [DOI] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Balakrishnan B, Dai H, Chakraborty PK, Janisse J, Muzik O, Romero R, Chugani DC. Decreased cortical serotonin in neonatal rabbits exposed to endotoxin in utero. J Cereb Blood Flow Metab. 2011;31:738–49. doi: 10.1038/jcbfm.2010.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan S, Saadani-Makki F, Muzik O, Chakraborty P, Mangner TJ, Janisse J, Romero R, Chugani DC. Microglial activation in perinatal rabbit brain induced by intrauterine inflammation: detection with 11C-(R)-PK11195 and small-animal PET. J Nucl Med. 2007;48:946–54. doi: 10.2967/jnumed.106.038539. [DOI] [PubMed] [Google Scholar]
- Kaufmann WE, MacDonald SM, Altamura CR. Dendritic cytoskeletal protein expression in mental retardation: an immunohistochemical study of the neocortex in Rett syndrome. Cereb Cortex. 2000;10:992–1004. doi: 10.1093/cercor/10.10.992. [DOI] [PubMed] [Google Scholar]
- Kaukola T, Herva R, Perhomaa M, Paakko E, Kingsmore S, Vainionpaa L, Hallman M. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr Res. 2006;59:478–83. doi: 10.1203/01.pdr.0000182596.66175.ee. [DOI] [PubMed] [Google Scholar]
- Kumral A, Tuzun F, Yesilirmak DC, Duman N, Oskan H. Role of epigenetic regulatory mechanisms in neonatal hypoxic-ischemic brain injury. Med Hypotheses. 2009;72:692. doi: 10.1016/j.mehy.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Labelle C, Leclerc N. Exogenous BDNF, NT-3 and NT-4 differentially regulate neurite outgrowth in cultured hippocampal neurons. Brain Res Dev Brain Res. 2000;123:1–11. doi: 10.1016/s0165-3806(00)00069-9. [DOI] [PubMed] [Google Scholar]
- Lyn-Kew K, Rich E, Zeng X, Wen H, Kunkel SL, Newstead M, BHan U, Standiford T. IRAK-M regulates chromatin remodeling in lung macrophages during experimental sepsis. PLoS ONE. 2010;5:11145. doi: 10.1371/journal.pone.0011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow N, Hennessy EM, Bracewell MA, Wolke D. Motor and executive function at 6 years of age after extremely preterm birth. Pediatrics. 2007;120:793–804. doi: 10.1542/peds.2007-0440. [DOI] [PubMed] [Google Scholar]
- Medrihan L, Tantalaki E, Aramuni G, Sargsyan V, Dudanova I, Missler M, Zhang W. Early defects of GABAergic synapses in the brain stem of a MeCP2 mouse model of Rett syndrome. J Neurophysiol. 2008;99:112–21. doi: 10.1152/jn.00826.2007. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–62. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnerie H, Shashidhara S, Le Roux PD. Effect of excess extracellular glutamate on dendrite growth from cerebral cortical neurons at 3 days in vitro: Involvement of NMDA receptors. J Neurosci Res. 2003;74:688–700. doi: 10.1002/jnr.10797. [DOI] [PubMed] [Google Scholar]
- Monnerie H, Le Roux PD. Glutamate receptor agonist kainate enhances primary dendrite number and length from immature mouse cortical neurons in vitro. J Neurosci Res. 2006;83:944–56. doi: 10.1002/jnr.20805. [DOI] [PubMed] [Google Scholar]
- Monnerie H, Le Roux PD. Reduced dendrite growth and altered glutamic acid decarboxylase (GAD) 65- and 67-kDa isoform protein expression from mouse cortical GABAergic neurons following excitotoxic injury in vitro. Exp Neurol. 2007;205:367–82. doi: 10.1016/j.expneurol.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, Armstrong D, Arancio O, Sweatt JD, Zoghbi HY. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci. 2006;26:319–27. doi: 10.1523/JNEUROSCI.2623-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Msall ME, Park JJ. The spectrum of behavioral outcomes after extreme prematurity: regulatory, attention, social, and adaptive dimensions. Semin Perinatol. 2008;32:42–50. doi: 10.1053/j.semperi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Neufeld MD, Frigon C, Graham AS, Mueller BA. Maternal infection and risk of cerebral palsy in term and preterm infants. J Perinatol. 2005;25:108–13. doi: 10.1038/sj.jp.7211219. [DOI] [PubMed] [Google Scholar]
- O'Shea TM, Kuban KC, Allred EN, Paneth N, Pagano M, Dammann O, Bostic L, Brooklier K, Butler S, Goldstein DJ, Hounshell G, Keller C, McQuiston S, Miller A, Pasternak S, Plesha-Troyke S, Price J, Romano E, Solomon KM, Jacobson A, Westra S, Leviton A. Neonatal cranial ultrasound lesions and developmental delays at 2 years of age among extremely low gestational age children. Pediatrics. 2008;122:e662–9. doi: 10.1542/peds.2008-0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, Leviton A. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–54. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Pinelli J, Zwaigenbaum L. Chorioamnionitis, gestational age, male sex, birth weight, and illness severity predicted positive autism screening scores in very-low-birth-weight preterm infants. Evid Based Nurs. 2008;11:122. doi: 10.1136/ebn.11.4.122. [DOI] [PubMed] [Google Scholar]
- Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Arch Pediatr Adolesc Med. 2005;159:1032–5. doi: 10.1001/archpedi.159.11.1032. [DOI] [PubMed] [Google Scholar]
- Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurobiol Learn Mem. 2006;85:228–42. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Rantakallio P, Jones P, Moring J, Von Wendt L. Association between central nervous system infections during childhood and adult onset schizophrenia and other psychoses: a 28-year follow-up. Int J Epidemiol. 1997;26:837–43. doi: 10.1093/ije/26.4.837. [DOI] [PubMed] [Google Scholar]
- Romero R. Prevention of spontaneous preterm birth: the role of sonographic cervical length in identifying patients who may benefit from progesterone treatment. Ultrasound Obstet Gynecol. 2007;30:675–86. doi: 10.1002/uog.5174. [DOI] [PubMed] [Google Scholar]
- Rousset CI, Chalon S, Cantagrel S, Bodard S, Andres C, Gressens P, Saliba E. Maternal exposure to LPS induces hypomyelination in the internal capsule and programmed cell death in the deep gray matter in newborn rats. Pediatr Res. 2006;59:428–33. doi: 10.1203/01.pdr.0000199905.08848.55. [DOI] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199:651 e1–7. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, Makki M, Muzik O, Janisse J, Romero R, Chugani D. Intrauterine endotoxin administration leads to white matter diffusivity changes in newborn rabbits. J Child Neurol. 2009;24:1179–89. doi: 10.1177/0883073809338213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaco RC, Fryer JD, Ren J, Fyffe S, Chao HT, Sun Y, Greer JJ, Zoghbi HY, Neul JL. A partial loss of function allele of methyl-CpG-binding protein 2 predicts a human neurodevelopmental syndrome. Hum Mol Genet. 2008;17:1718–27. doi: 10.1093/hmg/ddn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow WM, Hartle K, Ivanco TL. Altered morphology of motor cortex neurons in the VPA rat model of autism. Dev Psychobiol. 2008;50:633–9. doi: 10.1002/dev.20337. [DOI] [PubMed] [Google Scholar]
- Wood NS, Marlow N, Costeloe K, Gibson AT, Wilkinson AR. Neurologic and developmental disability after extremely preterm birth. EPICure Study Group. N Engl J Med. 2000;343:378–84. doi: 10.1056/NEJM200008103430601. [DOI] [PubMed] [Google Scholar]
- Wood NS, Costeloe K, Gibson AT, Hennessy EM, Marlow N, Wilkinson AR. The EPICure study: associations and antecedents of neurological and developmental disability at 30 months of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2005;90:F134–40. doi: 10.1136/adc.2004.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW. Systematic review of chorioamnionitis and cerebral palsy. Ment Retard Dev Disabil Res Rev. 2002;8:25–9. doi: 10.1002/mrdd.10003. [DOI] [PubMed] [Google Scholar]
- Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. Jama. 2003;290:2677–84. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- Xu H, Gonzalez JM, Ofori E, Elovitz MA. Preventing cervical ripening: the primary mechanism by which progestational agents prevent preterm birth? Am J Obstet Gynecol. 2008;198:314 e1–8. doi: 10.1016/j.ajog.2008.01.029. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Yang SH, Jun JK, Kim IO, Choi JH, Syn HC. Interleukin-6 concentrations in umbilical cord plasma are elevated in neonates with white matter lesions associated with periventricular leukomalacia. Am J Obstet Gynecol. 1996;174:1433–40. doi: 10.1016/s0002-9378(96)70585-9. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, Park KH, Gomez R, Choi JH, Kim IO. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am J Obstet Gynecol. 1997;177:19–26. doi: 10.1016/s0002-9378(97)70432-0. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH, Han TR. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol. 2000a;182:675–81. doi: 10.1067/mob.2000.104207. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ, Jun JK. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol. 2000b;183:1124–9. doi: 10.1067/mob.2000.109035. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Park CW, Chaiworapongsa T. Intrauterine infection and the development of cerebral palsy. Bjog. 2003;110:124–7. doi: 10.1016/s1470-0328(03)00063-6. [DOI] [PubMed] [Google Scholar]