Abstract
Objective
We measured cortical gamma-oscillations in response to visual-language tasks consisting of picture naming and word reading in an effort to better understand human visual-language pathways.
Methods
We studied six patients with focal epilepsy who underwent extraoperative electrocorticography (ECoG) recording. Patients were asked to overtly name images presented sequentially in the picture naming task and to overtly read written words in the reading task.
Results
Both tasks commonly elicited gamma-augmentation (maximally at 80–100 Hz) on ECoG in the occipital, inferior-occipital-temporal and inferior-Rolandic areas, bilaterally. Picture naming, compared to reading task, elicited greater gamma-augmentation in portions of pre-motor areas as well as occipital and inferior-occipital-temporal areas, bilaterally. In contrast, word reading elicited greater gamma-augmentation in portions of bilateral occipital, left occipital-temporal and left superior-posterior-parietal areas. Gamma-attenuation was elicited by both tasks in portions of posterior cingulate and ventral premotor-prefrontal areas bilaterally. The number of letters in a presented word was positively correlated to the degree of gamma-augmentation in the medial occipital areas.
Conclusions
Gamma-augmentation measured on ECoG identified cortical areas commonly and differentially involved in picture naming and reading tasks. Longer words may activate the primary visual cortex for the more peripheral field.
Significance
The present study increases our understanding of the visual-language pathways.
Keywords: pediatric epilepsy surgery, gamma-band amplitude, electroencephalography (EEG), event-related synchronization
INTRODUCTION
Overt picture naming and reading are two major visual-language tasks repeatedly conducted in everyday life. Both tasks involve cognitive processes, including perception and analysis of shapes of given visual stimuli, retrieval of words as well as movement execution to articulate given words (Price and Devlin, 2003; Sinai et al., 2005). From a clinical point of view, identification of the cortical sites involved in picture naming and reading is important in presurgical evaluation of epileptic patients. Removal of such sites might increase the risk of functional deficits and potentially impair the quality of life of such patients. In the present study, we delineated the temporal-spatial dynamics of neural activities driven by picture naming and word reading, using intracranial electrocorticography (ECoG) recording.
Analysis of event-related gamma-oscillations was applied to quantitatively measure cortical activation elicited by tasks with high spatial (1 cm) and temporal resolution (10 msec). In short, augmentation of gamma-oscillations (most commonly involving 80–100 Hz) is considered to represent activation in a given cortical site (Crone et al., 1998; Pfurtscheller and Lopes da Silva, 1999; Tallon-Baudry and Bertrand, 1999; Lachaux et al., 2000; Niessing et al., 2005; Szurhaj and Derambure, 2006; Nishida et al., 2008; Ray et al., 2008); cortical sites showing gamma-augmentation can identify the eloquent areas with statistically-significant accuracy (Crone et al., 2006; Miller et al., 2007a; Brunner et al., 2009; Nagasawa et al., 2010a; 2010b). The benefits of functional brain mapping using ECoG include: (i) a better signal-to-noise ratio compared to scalp electroencephalography (EEG) and magnetoencephalography (MEG), which record cortical signals from outside of the scalp (Gaetz et al., 2008; Dalal et al 2009), (ii) better temporal resolution compared to functional MRI (fMRI) (Menon and Kim, 1999), and (iii) a shorter duration of a patient’s participation compared to electrical stimulation mapping using subdural electrodes (Brown et al., 2008; Lesser et al., 2010).
We specifically addressed the following three questions. (Question #1): We tested the hypothesis that picture naming, compared to word reading, elicits greater cortical activation in the left dorsolateral premotor-prefrontal areas. A previous lesion study showed that the damage to this area impaired naming more than word reading (Stuss et al., 2001). Previous fMRI studies showed that naming elicited greater cortical activation in this area compared to word reading (MacDonald et al., 2000; Polk et al., 2008). Intracranial neurophysiology studies using depth electrodes showed that reading tasks elicited cortical deactivation in the left anterior inferior frontal gyrus and activation in the left posterior inferior frontal gyrus (Lachaux et al., 2008; Mainy et al., 2008; Vidal et al., 2010). Partly because of spatial sampling limitations, the dynamic changes of neural activities in the caudal occipital or middle-superior frontal areas have not been well described in previous studies using subdural ECoG recording. Since ECoG sampling involved such areas in some of our patients, we expected that this study would enhance the literature.
(Question #2): We addressed the hypothesis that word reading, compared to picture naming, elicits greater cortical activation in the left ventral occipital-temporal area following presentation of visual stimuli. Evidence from lesion (Gaillard et al., 2006; Pflugshaupt et al., 2009), functional neuroimaging (Cohen et al., 2000; Dehaene and Cohen, 2007) and intracranial neurophysiology studies (Nobre et al., 1994; Tanji et al., 2005; Gaillard et al., 2006) suggested the presence of distinct visual-language function specific to reading in the left ventral occipital-temporal area. Yet, other studies using fMRI and ECoG failed to prove the presence of such reading-specific function in the left ventral occipital-temporal area (Price and Devlin, 2003; Mainy et al., 2008).
(Question #3): We addressed the hypothesis that longer words during the reading task elicit greater cortical activation in the medial occipital area following presentation of visual stimuli. Previous fMRI studies showed that longer words elicited greater activation in the caudal occipital lobe including the medial occipital area (Mechelli et al., 2000; Graves et al., 2010). A study using MEG showed that longer words elicited larger evoked potentials in the bi-occipital area (Wydell et al., 2003). A study of intracranial recording showed that an evoked potential was elicited in the medial occipital area by peripheral visual stimuli and in the lateral-polar occipital area by central stimuli (Yoshor et al., 2007).
MATERIALS and METHODS
Patients
The inclusion criteria of the present study consisted of: (i) patients with a history of focal epilepsy who underwent extraoperative subdural ECoG recording as a part of presurgical evaluation in Children’s Hospital of Michigan or Harper University Hospital, Detroit, between February 2008 and July 2010, (ii) age of 8 years or older, and (iii) measurement of amplitude modulations driven by picture naming and word reading tasks described below. The exclusion criteria consisted of: (i) presence of massive brain malformations (such as large perisylvian polymicrogyria or hemimegalencephaly) which confound the anatomical landmarks for the central and calcarine sulci, (ii) visual field deficits detected by confrontation, (iii) ECoG sampling limited to the temporal lobe, and (iv) history of previous epilepsy surgery. We retrospectively studied a consecutive series of six patients satisfying both inclusion and exclusion criteria (age range: 9 – 37 years; five females; Table 1). The study has been approved by the Institutional Review Board at Wayne State University, and written informed consent was obtained from the parents or guardians of all patients.
Table 1.
Clinical Profiles.
| Patient/ Gender |
Age (years) |
Handedness | Verbal Comprehension Index |
Language Dominant Side on Wada Test |
Medications prior to ECoG |
Subdural Electrode Placement |
Seizure onset zones on ECoG |
Histology |
|---|---|---|---|---|---|---|---|---|
| 1 / F | 9 | Left | 75 | NA | OXC, TPM | Lt FPOT | Lt T | Arachnoid cyst |
| 2 / M | 10 | Right | 71 | NA | OXC | Rt FPOT | Rt T & Rt F | Tumor |
| 3 / F | 16 | Right | 121 | Left | OXC, LEV | Lt FPOT & Rt T | Lt P & LtO | Gliosis |
| 4 / M | 17 | Right | 87 | Left | OXC, LEV | Rt FPOT | Rt T & Rt P | Gliosis |
| 5 / F | 17 | Right | 96 | NA | LTG | Lt FPOT & Rt FPO | Lt F | Gliosis |
| 6 / F | 37 | Right | NA* | NA | LEV | Lt FPO | NA* | Tumor |
F: female. M: male. Lt: left. Rt: right. F: frontal. P: parietal. O: occipital. T: temporal. NA: not applicable. ECoG: electrocorticography. LEV: levetiracetam. LTG: lamotrigine. OXC: oxcarbazepine. TPM: topiramate.
Patient #6 had a brain tumor in the left superior-medial parietal area and had mild weakness of the right leg. Otherwise, preoperative neurological examinations failed to find abnormalities in language function.
Subdural electrode placement
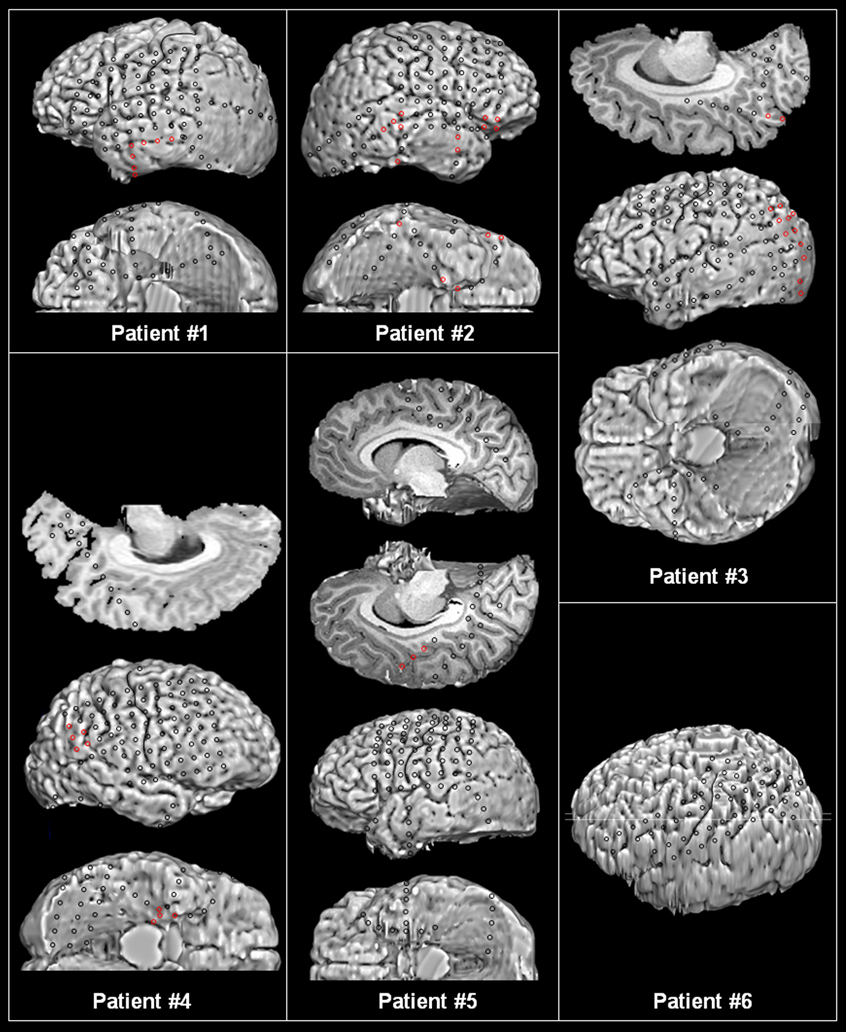
For extraoperative ECoG recording, platinum grid electrodes (10 mm intercontact distance, 4 mm diameter; Ad-tech, Racine, WI) were surgically implanted (Asano et al., 2009a; Figure 1). All electrode plates were stitched to adjacent plates and/or the edge of dura mater, to avoid movement of subdural electrodes after placement. In addition, intraoperative pictures were taken with a digital camera before dural closure, to confirm the spatial accuracy of electrode display on the three-dimensional brain surface reconstructed from MRI (Asano et al., 2005).
Figure 1. Location of subdural electrodes.
Seizure onset zones are represented by red electrodes. Patient 2 had a tumor involving the right temporal area and the ictal discharges were originated from multiple areas simultaneously. Patient 4 had two distinct seizure onset zones; his habitual clinical seizures were associated with ictal discharges arising from the medial temporal area, whereas a subclinical seizure occurred from the parietal area.
Coregistration of subdural electrodes to the individual three-dimensional MRI
MRI including a T1-weighted spoiled gradient echo image as well as fluid-attenuated inversion recovery image was preoperatively obtained (Alkonyi et al., 2009; Fukuda et al., 2010b). Planar x-ray images (lateral and anteroposterior) were acquired with the subdural electrodes in place for electrode localization on the brain surface (von Stockhausen et al., 1997; Miller et al., 2007b; Muzik et al., 2007; Dalal et al., 2008); three metallic fiducial markers were placed at anatomically well-defined locations on the patient’s head for co-registration of the x-ray image with the MRI. A three-dimensional surface image was created with the location of electrodes directly defined on the brain surface (von Stockhausen et al., 1997; Muzik et al., 2007; Alkonyi et al., 2009). The accuracy of this procedure was reported previously as 1.2 ± 0.7 mm with a maximal misregistration of 2.7 mm (von Stockhausen et al., 1997), and was confirmed by intraoperative digital photographs showing in situ locations of the subdural electrodes (Rutka et al., 1999; Wellmer et al., 2002; Asano et al., 2005; Dalal et al., 2008).
Extraoperative video-ECoG recording
Extraoperative video-ECoG recordings were obtained for 3 to 5 days, using a 192-channel Nihon Kohden Neurofax 1100A Digital System (Nihon Kohden America Inc, Foothill Ranch, CA, USA), which has an input impedance of 200 Megaohm, a common mode rejection ratio greater than 110 dB, and an A/D conversion of 16 bits. This clinical recording system has adequate specifications for recording low-voltage gamma-oscillations (Crone et al., 2006; Fukuda et al., 2008; Kobayashi et al., 2010). The sampling frequency was set at 1,000 Hz with the amplifier band pass at 0.08 – 300 Hz. The averaged voltage of ECoG signals derived from the fifth and sixth intracranial electrodes of the ECoG amplifier (system reference potential) was used as the original reference (Fukuda et al., 2010b; Nagasawa et al., 2010a). ECoG signals were then re-montaged to a common average reference (Sinai et al., 2005; Canolty et al., 2007; Miller et al., 2007a; Nishida et al., 2008; Towle et al., 2008). Advantage and limitation of usage of a common average reference were previously discussed (Crone et al., 2001; Asano et al., 2009b). Channels contaminated with large interictal epileptiform discharges or artifacts were excluded from the common average reference (Fukuda et al., 2008). No notch filter was used for further analysis in any patients.
As a part of routine clinical procedures, surface electromyography electrodes were placed on the left and right deltoid muscles (Asano et al., 2005), and electrooculography electrodes were placed 2.5 cm below and 2.5 cm lateral to the left and right outer canthi (Asano et al., 2007). All antiepileptic medications were discontinued on the day of subdural electrode placement (i.e.: off antiepileptic medications during the picture naming and word reading tasks described below). Seizure onset zones were visually determined as previously described (Asano et al., 2009a). ECoG traces were visually inspected with a low-frequency filter at 53 Hz and a sensitivity of 20 µV/mm; thereby, broadband signals synchronized with facial and ocular muscle activities seen on electrooculography electrodes were treated as artifacts and excluded from further analysis (Otsubo et al., 2008; Ball et al., 2009; Jerbi et al., 2009; Kovach et al., 2011; Nagasawa et al., 2010a).
Visual-language tasks
None of the patients had a seizure within two hours prior to the visual-language tasks. Each patient was awake, unsedated, and comfortably seated on the bed in a dimly lit room. Patients #1, #3, and #5 completed picture naming prior to word reading task, whereas the remaining three patients completed word reading prior to picture naming task. Patients were instructed to overtly name objects presented sequentially in the picture naming task and to overtly read written words in the reading task. Stimuli were presented sequentially on a 19-inch Acer V193 LCD monitor (Acer America, San Jose, CA, USA) with a refresh rate of 75 Hz. The monitor was placed 60 cm in front of patients; the monitor cables and power cables were placed at least 60 cm away from the patient as well as the ECoG Recording System (Asano et al., 2009b). Picture stimuli consisted of 60 common line-drawn objects (such as ‘dog’ and ‘banana’; Rossion and Pourtois, 2004), of which size ranged from 11 to 16 cm in height and width. Word stimuli consisted of 60 nouns matched with those presented in the picture naming task, of which size was 3 cm in height and ranged from 7 to 18 cm in width. Picture and word stimuli were binocularly presented at the center of the monitor, in grayscale, on the black background, for 5,000 msec with an interstimulus interval randomly ranging 2,000 – 2,500 msec. TTL trigger signals synchronized with the onset and offset of each stimulus presentation were delivered to the ECoG recording system (Reale et al., 2007).
These audible visual-language sessions were recorded using a Digital Voice Recorder (WS-300M, Olympus America Inc, Hauppauge, NY, USA) concurrently with ECoG recording, and the amplified audio waveform was integrated into the Digital ECoG Recording System (Brown et al., 2008). The response time was defined as the period between the onset of stimulus presentation and the onset of overt responses. We determined whether the response time differed between picture naming and word reading tasks (paired t-test). We also determined whether the response time was correlated with the age of patients (Spearman’s rank test).
Measurement of ECoG amplitude modulations elicited by visual-language tasks
Each ECoG trial was transformed into the time-frequency domain, and we determined ‘when’ and ‘where’ gamma-oscillations were modulated. The time-frequency analysis used in the present study was previously validated (Hoechstetter et al., 2004; Brown et al., 2008; Asano et al., 2009b; Fukuda et al., 2010b; Koga et al., 2010; Nagasawa et al., 2010a; Thampratankul et al., 2010). In short, the measures of interest in the present study included a percent change of the amplitude of gamma-oscillations relative to that during the reference period (i.e.: the resting baseline) as well as statistical significance of task-related augmentation of gamma-oscillations. The details of analytic methods are described below.
Analysis of ECoG amplitude modulations relative to the onset of stimulus presentation
This analytic method was designed to evaluate sequential brain activation associated with perception and analysis of shapes of given visual stimuli. Since the response times were not uniform across trials, this analytic approach is not ideal to evaluate brain activation associated with movement execution to articulate answers. The inclusion criteria defining ECoG epochs suitable for this time-frequency analysis included: (i) a period of silence serving as a reference period lasting 400 msec was available between 600 to 200 msec prior to the onset of stimulus presentation. The exclusion criteria included: (i) ECoG trace was affected by movement artifacts; (ii) ECoG trace was affected by electrographic seizures; and (iii) ECoG trace from the occipital lobe was affected by runs of interictal epileptiform discharges lasting longer than 3 seconds (Nagasawa et al., 2010a). All 3,100-msec ECoG epochs (starting 600-msec prior to and ending 2,500-msec after the onset of stimulus presentation) which satisfied all of the inclusion and the exclusion criteria were utilized for the time-frequency ECoG analysis.
Time-frequency analysis was performed using BESA® EEG V.5.1.8 software (MEGIS Software GmbH, Gräfelfing, Germany); each suitable ECoG trial was transformed into the time-frequency domain using a complex demodulation technique (Papp and Ktonas, 1977; Hoechstetter et al., 2004). In this technique, the time-frequency transform was obtained by multiplication of the time-domain signal with a complex exponential, followed by a low-pass filter. The low-pass filter used here was a finite impulse response filter of Gaussian shape, making the complex demodulation effectively equivalent to a Gabor transform. The details of the complex demodulation technique for time-frequency transformation are described elsewhere (Papp and Ktonas, 1977; Hoechstetter et al., 2004). As the result of this transformation, a given ECoG signal was assigned a specific amplitude and phase as a function of frequency and time (relative to the onset of stimulus presentation). In this study, only the amplitude (also known as ‘square root of power’), averaged across all trials, was used for further analysis. Time-frequency transformation was performed for frequencies between 10 and 200 Hz and latencies between −600 msec and +2,500 msec relative to the onset of stimulus presentation, in steps of 5 Hz and 10 msec (Brown et al., 2008). This corresponded to a time-frequency resolution of ±7.1 Hz and ±15.8 msec (defined as the 50% power drop of the finite impulse response filter).
At each time-frequency bin, we analyzed the percent change in amplitude (averaged across trials) relative to the mean amplitude in a reference period. This parameter is commonly termed “event-related synchronization and desynchronization” (Pfurtscheller and Lopes da Silva, 1999) or “temporal spectral evolution” (TSE) (Salmelin and Hari, 1994). In the present study, ‘event-related’ modulations were defined as oscillatory responses consisting of both phase-locked (i.e.: a component present after averaging; also often known as ‘evoked’ oscillations) and non-phase-locked (i.e: a component absent after averaging; also often known as ‘induced’ oscillations) components (Tallon-Baudry and Bertrand, 1999; Crone et al., 2006; Nagasawa et al., 2010a).
To test for statistical significance for each obtained TSE value, two-step statistics was performed using the BESA software (Brown et al., 2008; Nagasawa et al., 2010a). First, a studentized bootstrap statistics (Davidson and Hinkley, 1999) was applied to obtain an uncorrected p-value independently for each time-frequency bin. This test compared the amplitude in each time-frequency bin with the averaged amplitude in the reference time period of the corresponding frequency. In a second step, correction for multiple testing was performed on these uncorrected p-values, accounting for the fact that TSE values at neighboring time bins are partially dependent. For that purpose, a modification of the correction developed by Simes (1986) was used as suggested by Auranen (2002): p-values of one frequency bin and channel were sorted in ascending order (pi, i = 1, …, N, where N is the number of trials). The maximum index m in the sorted array for which pi < α*i/N was determined. All TSE values with i<m were considered statistically significant. The corrected significance level α was set to 0.05. This approach is less conservative than the classic Bonferroni correction and is specifically suited for partially dependent multiple testing (Simes, 1986). In all figures, red color indicates augmentation of amplitude, and blue color attenuation of amplitude in the corresponding time-frequency bin relative to the reference period.
As described in our previous studies (Brown et al., 2008; Asano et al., 2009b; Fukuda et al., 2010a; Nagasawa et al., 2010a), an additional correction was employed as described below. TSE values in a given electrode were declared to be significant only if a minimum of eight time-frequency bins in the gamma-band range were arranged in a continuous array spanning (i) at least 20-Hz in width and (ii) at least 20-msec in duration. Event-related gamma-modulations in intracranial ECoG studies commonly involved wide-range frequency bands ranging at least 20-Hz in width (Lachaux et al., 2005; Tallon-Baudry et al., 2005; Brown et al., 2008; Asano et al., 2009b; Fukuda et al., 2010b; Nagasawa et al., 2010a), whereas some previous studies using scalp EEG recording showed augmentation of a narrow-range gamma-band oscillations around 40 Hz (Tallon-Baudry and Bertrand, 1999). Several human ECoG studies analyzed event-related gamma-modulations of 20-Hz width (Crone et al., 1998; Flinker et al., 2010). An epoch with duration of 20 msec can contain only a single cycle of gamma oscillation at 50 Hz. We recognize that our definition of significance may potentially underestimate gamma-modulations with a restricted frequency band (less than 20-Hz in width) or those with a short duration (less than 20-msec). Yet, discovery of very short-lasting gamma-augmentation confined to 15-Hz or less in width was not the purpose of our clinical study. We believe that our approach is warranted in statistical and clinical points of view. As the results of this additional correction, a very small probability of a Type-I error in determination of significant activation remained.
Analysis of ECoG amplitude modulations relative to the onset of patient’s overt responses
This analytic method was designed to evaluate sequential brain activation associated with movement execution to overtly articulate and hearing answers. The inclusion criteria defining ECoG epochs suitable for this time-frequency analysis included: (i) patient provided a correct response within 2,000 msec from the onset of stimulus presentation, (ii) duration of overt response lasted less than 1,500 msec, (iii) a period of silence serving as a reference period lasting 400 msec was available between 2,000 to 2,400 msec following the onset of overt response. Since the response times were not uniform across trials and the reference period was set while stimuli were presented, this analytic approach is not ideal to evaluate occipital activation associated with initial perception of shapes of given visual stimuli. The exclusion criteria were same as those applied in the analysis relative to the onset of stimulus presentation. All 4,500-msec ECoG epochs (starting 2,000-msec prior to and ending 2,500-msec after the onset of overt responses) which satisfied all of the inclusion and the exclusion criteria were utilized for the time-frequency ECoG analysis. ECoG amplitude modulations were determined using the statistical approach described above.
Localization of differential gamma-augmentation
This analytic method was designed to determine whether the degree of gamma-augmentation in a cortical site was greater on a task compared to the other task, when significant gamma-augmentation was elicited in a given site by both picture naming and word reading tasks. Comparison of amplitude between picture naming and word reading tasks was performed on ECoG traces relative to the onset of stimulus presentation, in order to determine whether locations of cortical sites involved in perception and analysis of shapes of given visual stimuli differed between the two tasks. Similarly, ECoG traces were analyzed relative to the onset of overt responses to determine whether locations of cortical sites involved in overt articulation differed between the two tasks. As described above, a given electrode site was declared to have significant gamma-oscillations differentially augmented by a task, only if TSE values differed between two tasks in a minimum of eight bins in the gamma-band range in a continuous array spanning at least 20-Hz in width and at least 20-msec in duration (Fukuda et al., 2010b).
Correlation between the length of words and the degree of gamma-augmentation
This analysis was employed in cortical sites showing significant gamma-augmentation elicited by presentation of word stimuli at the time period 0 to 500 msec following stimulus presentation. The length of word (i.e.: number of characters; range: 3 to 10) was correlated with ‘the maximum gamma-amplitude80–100Hz’ at each cortical site, using Spearman’s Rank Test. Thereby, ‘the maximum gamma-amplitude80–100Hz’ was defined as the maximum of gamma-amplitudes at 80–100 Hz at the time period 0 to 500 msec following word presentation in the present study. Previous ECoG studies showed that language-related spectral modulations commonly involved this range of gamma-oscillations (Sinai et al., 2005; Tanji et al., 2005; Canolty et al., 2007; Towle et al., 2008; Brown et al., 2008; Dalal et al., 2009; Fukuda et al., 2010b; Jacobs and Kahana, 2009; Edwards et al., 2010; Wu et al., 2010); thus, ‘the maximum gamma-amplitude80–100Hz’ is an appropriate summary measure to delineate cortical activation elicited by presentation of word stimuli (Crone et al., 2006). We recognize that epileptogenic lesions may alter the results of event-related gamma-oscillations (Bragin et al., 1995); we, therefore, conducted group analyses with including and excluding cortical sites classified as seizure onset zones as well as those involved by a structural lesion.
RESULTS
Behavioral data
The response time was longer in picture naming (mean: 1.24 sec) compared to word reading task (mean: 1.02 sec) (p=0.006; paired t-test; Table 2). No difference in the response time was found between the first- and second-assigned tasks (p=0.7; paired t-test). No correlation was found between the age and response time in either picture naming (p=0.2) or word reading task (p=0.3; Spearman’s Rank Test).
Table 2.
Behavioral results.
| Patient / Gender | Age (yrs) |
Average Response Time (sec) | |
|---|---|---|---|
| Naming | Reading | ||
| 1 / F | 9 | 1.64 | 1.39 |
| 2 / M | 10 | 1.50 | 1.19 |
| 3 / F | 16 | 0.89 | 0.61 |
| 4 / M | 17 | 1.36 | 1.11 |
| 5 / F | 17 | 1.06 | 0.80 |
| 6 / F | 37 | 0.98 | 1.00 |
| Average | 1.24 | 1.02 | |
Results of time-frequency analysis relative to the onset of stimulus presentation
Gamma-augmentation in the occipital area (Brodmann Areas 17/18/19)
The results of time-frequency analysis relative to the onset of stimulus presentation are provided in Figures 2–8. In short, both naming and reading tasks elicited significant gamma-augmentation at 40–200 Hz (maximally at 80–100 Hz) initially in the occipital sites (Brodmann Areas 17, 18, and 19), bilaterally, in all five patients whose ECoG sampling involved these areas. The total number of occipital electrode sites showing significant gamma-augmentation elicited by either task was 65. Seventeen occipital sites showed gamma-augmentation equally elicited by both naming and reading tasks. Forty-one occipital sites showed gamma-augmentation elicited by naming alone or by naming greater than reading, whereas six occipital sites showed gamma-augmentation elicited by reading alone or by reading greater than naming. A single occipital site showed gamma-augmentation initially greater in the reading task and subsequently in the naming task (Channel 3 in Figure 4). Regardless of adding or excluding five occipital sites classified as seizure onset zone, the McNemar’s test suggested that the picture naming task, compared to the word reading task, more frequently elicited greater gamma-augmentation in occipital sites (p<0.0001). The median onset latency of occipital gamma-augmentation was 85 msec on average (mean: 116 msec; SD: 86 msec) in naming and 110 msec in reading task (mean: 199 msec; SD: 187 msec). After excluding five occipital sites classified as seizure onset zone, the median onset latency of such gamma-augmentation was 90 msec (mean: 122 msec; SD: 87 msec) in naming and 150 msec in reading task (mean: 212 msec; SD: 192 msec).
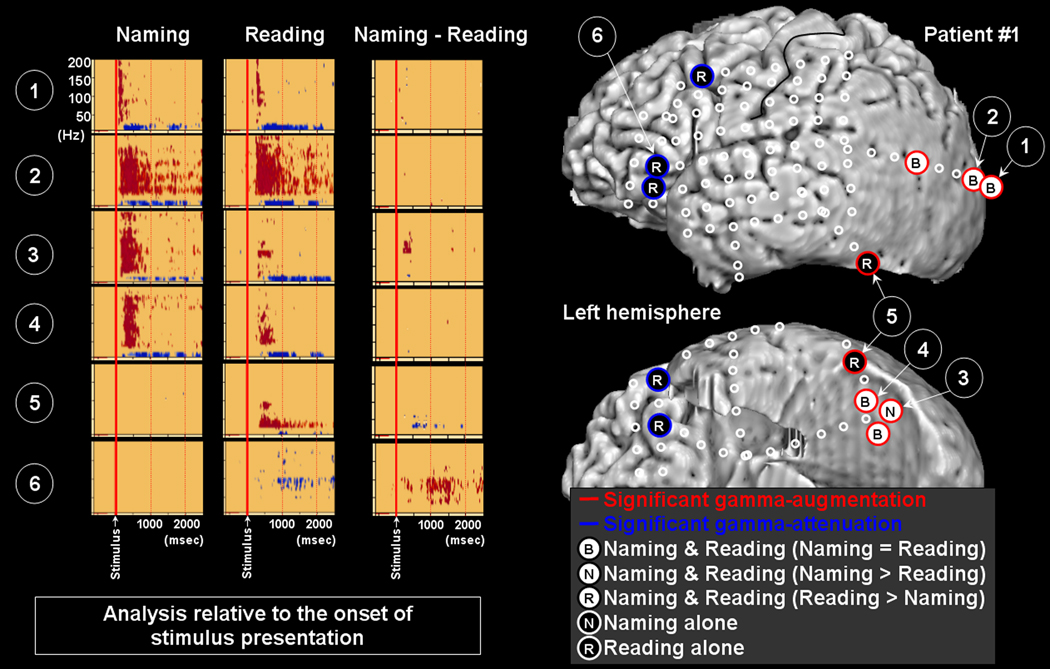
Figure 2. Gamma-oscillations modulated by naming and reading in patient #1.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Both naming and reading tasks elicited gamma-augmentation involving the left lateral occipital sites (Channels 1 and 2). Subsequently, gamma-augmentation involved the inferior occipital sites (Channels 3 and 4). The task-related degree of gamma-augmentation in Channel 3 was significantly larger in naming compared to reading. Gamma-augmentation involved Channel 5 in the occipital-temporal junction only during the reading task. Gamma-attenuation involved the left ventral prefrontal areas (Channel 6) as well as the left premotor area only during the reading task.
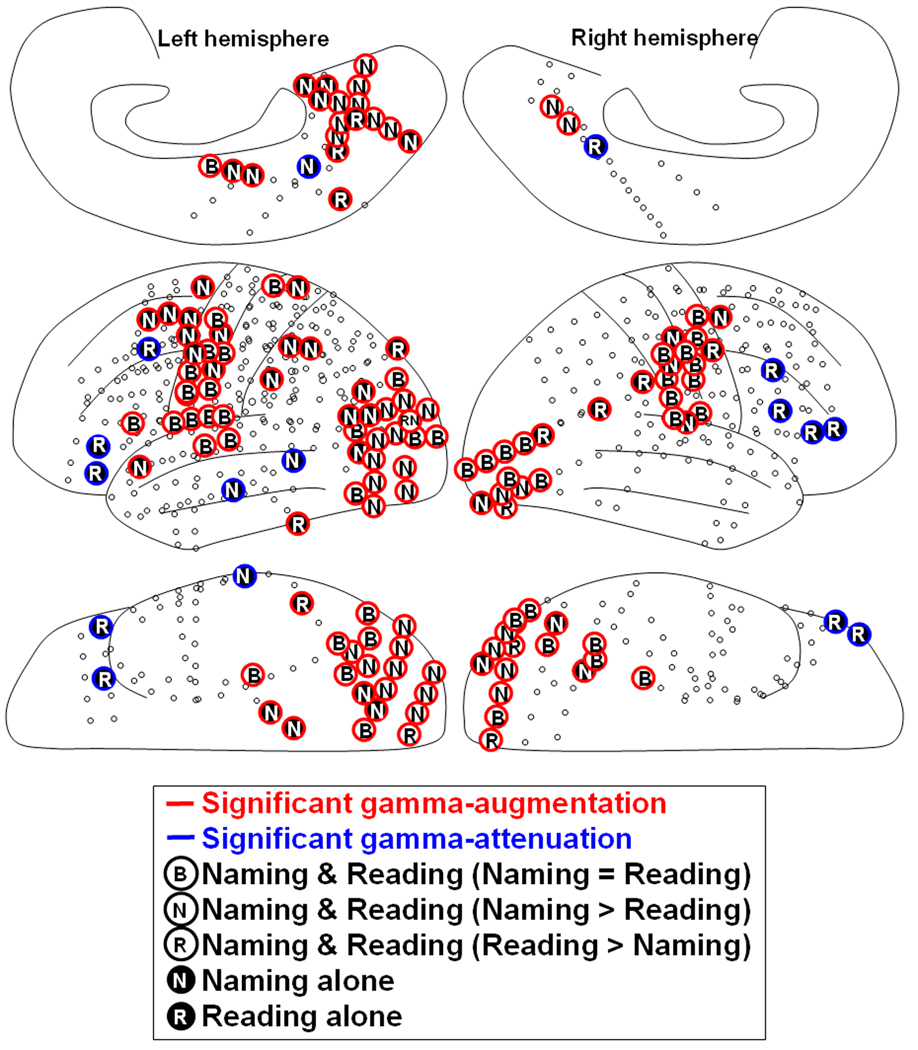
Figure 8. Summary illustration.
The overall results of time-frequency analysis relative to the onset of stimulus presentation are shown. The locations of subdural electrodes in six patients were superimposed on a brain template.
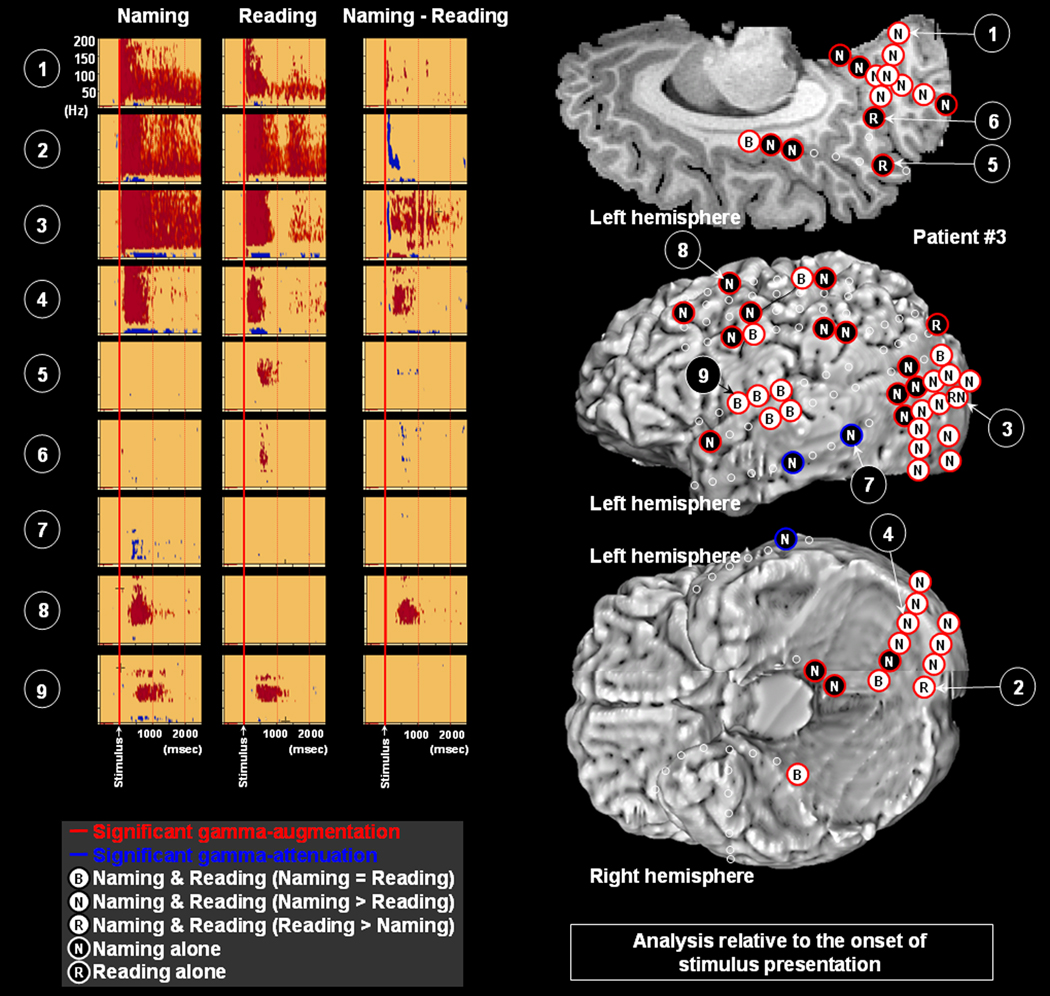
Figure 4. Gamma-oscillations modulated by naming and reading in patient #3.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Both naming and reading tasks elicited gamma-augmentation involving the left lateral, medial and inferior occipital sites (Channels 1–4). The task-related degree of gamma-augmentation in Channels 1 and 4 was significantly larger in naming; that in Channel 2 was larger in reading; that in Channel 3 was initially larger during reading but subsequently larger during naming. Gamma-augmentation involved Channels 5 and 6 in the left superior-posterior-medial parietal area (Brodmann Area 7) only during the reading task. Gamma-attenuation involved Channel 7 in the left posterior-lateral temporal area only during the naming task. Gamma-augmentation involved Channel 8 in the premotor area only during the naming task and Channel 9 in the inferior Rolandic area during both naming and reading tasks.
Gamma-augmentation in the temporal-occipital junction (Brodmann Area 37)
Five sites at Brodmann Area 37 (temporal-occipital junction) showed gamma-augmentation elicited by either task in three patients (patient #1, #2, and #4); two sites on the right side showed gamma-augmentation equally elicited by both naming and reading tasks; one on the right side showed gamma-augmentation elicited by naming alone; one on each side showed gamma-augmentation elicited by reading alone (Figure 2). The median onset latency of such gamma-augmentation at Brodmann Area 37 was 210 msec in naming (mean: 210 msec; SD: 20 msec) and 235 msec in reading task (mean: 248 msec; SD: 109 msec).
Gamma-augmentation in the superior-posterior parietal area (Brodmann Area 7)
Three sites at Brodmann Area 7 on the left side (superior posterior parietal area) in patient #3 showed gamma-augmentation elicited by reading task alone (Figure 4). The median onset latency of such gamma-augmentation was 470 msec (mean: 453 msec; SD: 47 msec). Two of these three sites were classified as seizure onset zone.
Gamma-attenuation in the posterior cingulate area (Brodmann Area 31)
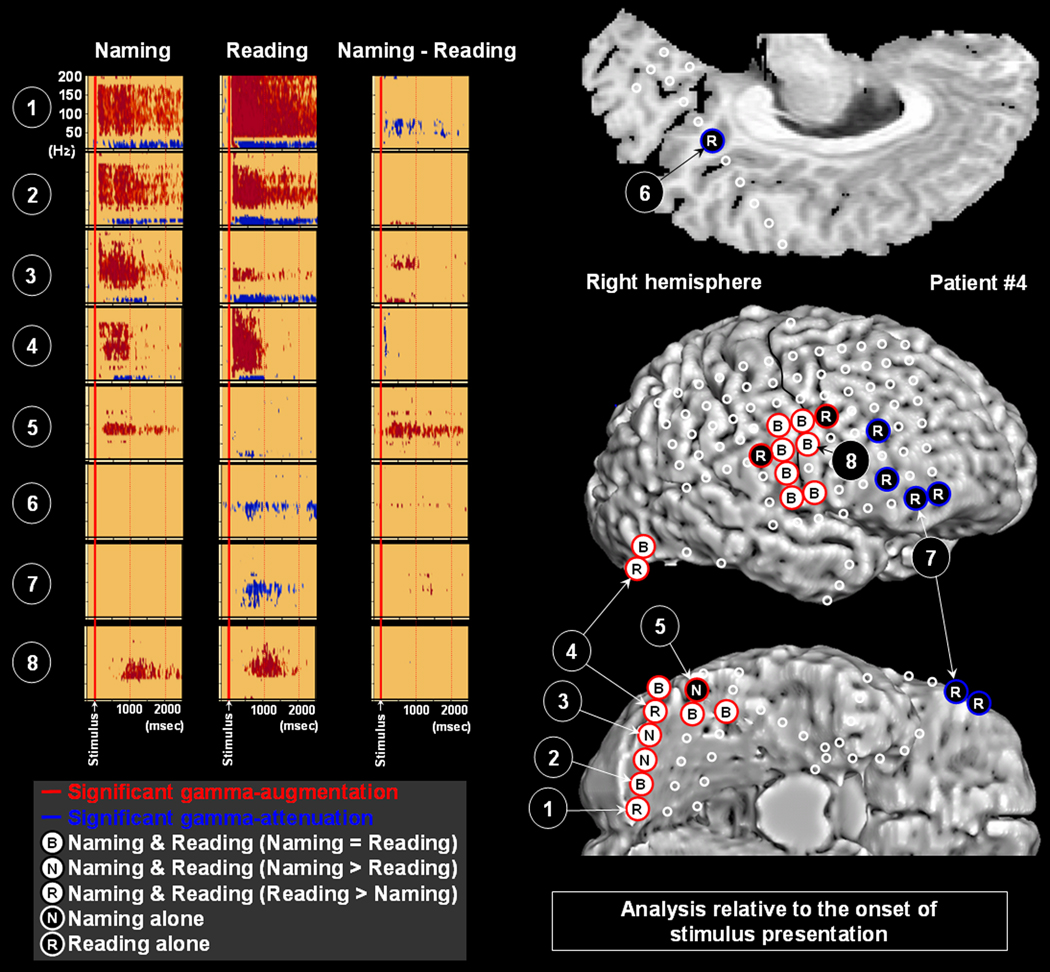
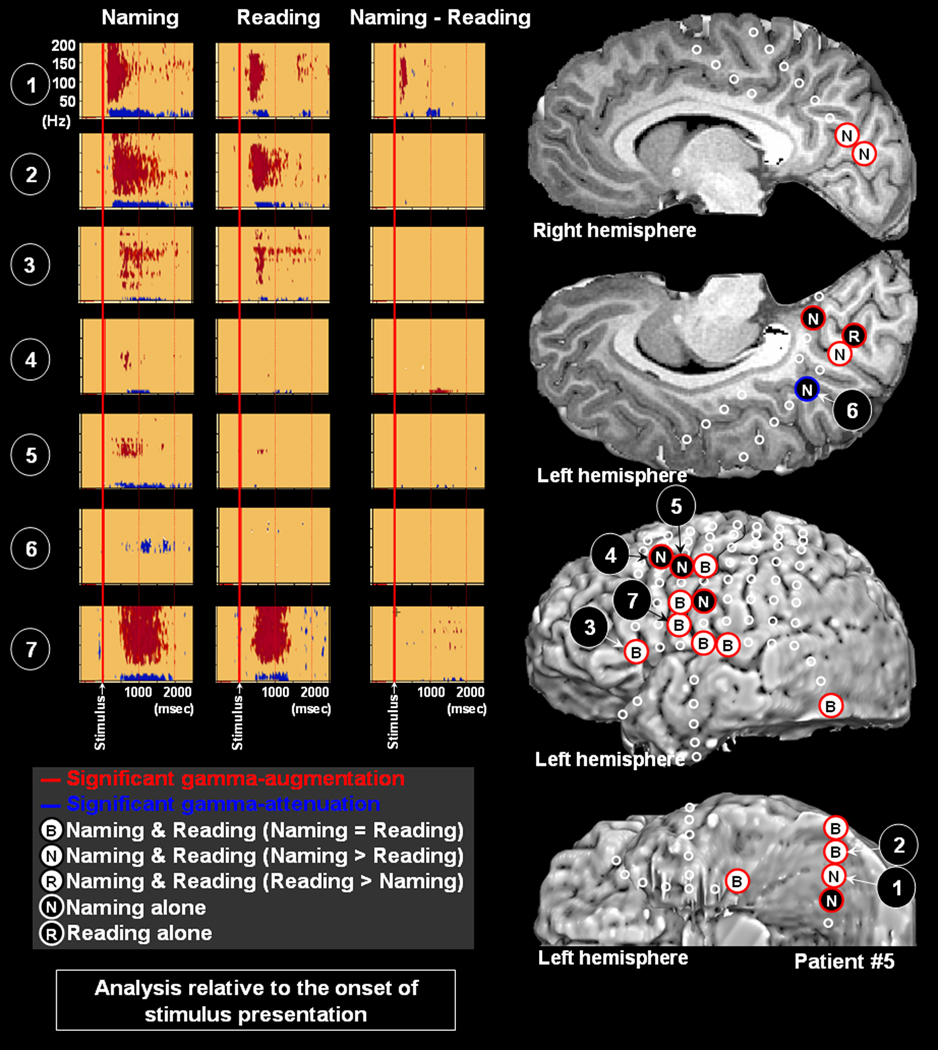
Two sites at Brodmann Area 31 (posterior cingulate area) showed gamma-attenuation. Reading task elicited gamma-attenuation at Brodmann Area 31 on the right side in patient #4 (Figure 5); the onset latency of such gamma-attenuation was 460 msec. Naming task likewise elicited gamma-augmentation on the left side in patient #5 (Figure 6); the onset latency of such gamma-attenuation was 1080 msec.
Figure 5. Gamma-oscillations modulated by naming and reading in patient #4.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Both naming and reading tasks elicited gamma-augmentation involving the right lateral and inferior occipital sites (Channels 1–4). The task-related degree of gamma-augmentation in Channels 1 and 4 was significantly larger during reading, and that in Channel 3 was larger during naming. Gamma-augmentation involved Channel 5 in the occipital-temporal junction only during the naming task. Reading-related gamma-attenuation involved Channel 6 over posterior cingulate cortex as well Channel 7 in the prefrontal area. Gamma-augmentation involved Channel 8 in the inferior Rolandic area during both naming and reading tasks.
Figure 6. Gamma-oscillations modulated by naming and reading in patient #5.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Both naming and reading tasks elicited gamma-augmentation involving the inferior and medial occipital sites (Channels 1 and 2). The task-related degree of gamma-augmentation in Channel 1 was larger during naming compared to reading. Subsequently, both naming and reading tasks elicited gamma-augmentation in the left prefrontal area (Channel 3). Gamma-augmentation involved Channels 4 and 5 in the premotor areas only during the naming task. Gamma-attenuation was noted in the left posterior cingulate region (Channel 6) during naming task alone. Gamma-augmentation involved Channel 7 in the inferior Rolandic area during naming and reading tasks.
Gamma-augmentation and attenuation in the prefrontal areas (Brodmann Areas 44/45/47)
Only a single site in the left prefrontal areas (Brodmann Areas 44/45) showed gamma-augmentation, which was noted in patient #5 and elicited by both naming and reading tasks equally (Figure 6); the onset latency of such gamma-attenuation was 510 msec in naming and 450 msec in reading. Conversely, seven sites in the prefrontal areas (3 sites at left Broadmann Area 47; 4 sites at right Brodmann Areas 44/45) showed gamma-attenuation elicited by reading alone (Figures 2 and 5); the median onset latency of such gamma-attenuation was 390 msec (mean: 509 msec; SD: 217 msec).
Gamma-augmentation and attenuation in the premotor area (Brodmann Area 6)
Six premotor sites at Broadmann Area 6 (4 sites in the left middle frontal gyrus; 1 site in the left superior frontal gyrus; 1 site in the right middle frontal gyrus) showed gamma-augmentation elicited by naming alone (Figures 3, 4, 6 and 7). The median onset latency of such gamma-augmentation was 385 msec (mean: 420 msec; SD: 157 msec). A single premotor site at Broadmann Area 6 (right middle frontal gyrus) showed gamma-augmentation elicited by reading alone (Figure 5); the onset latency of such gamma-augmentation was 250 msec. A single premotor site at Broadmann Area 6 in the left middle frontal gyrus showed gamma-attenuation elicited by reading alone; the onset latency of such gamma-attenuation was 860 msec (Figure 2).
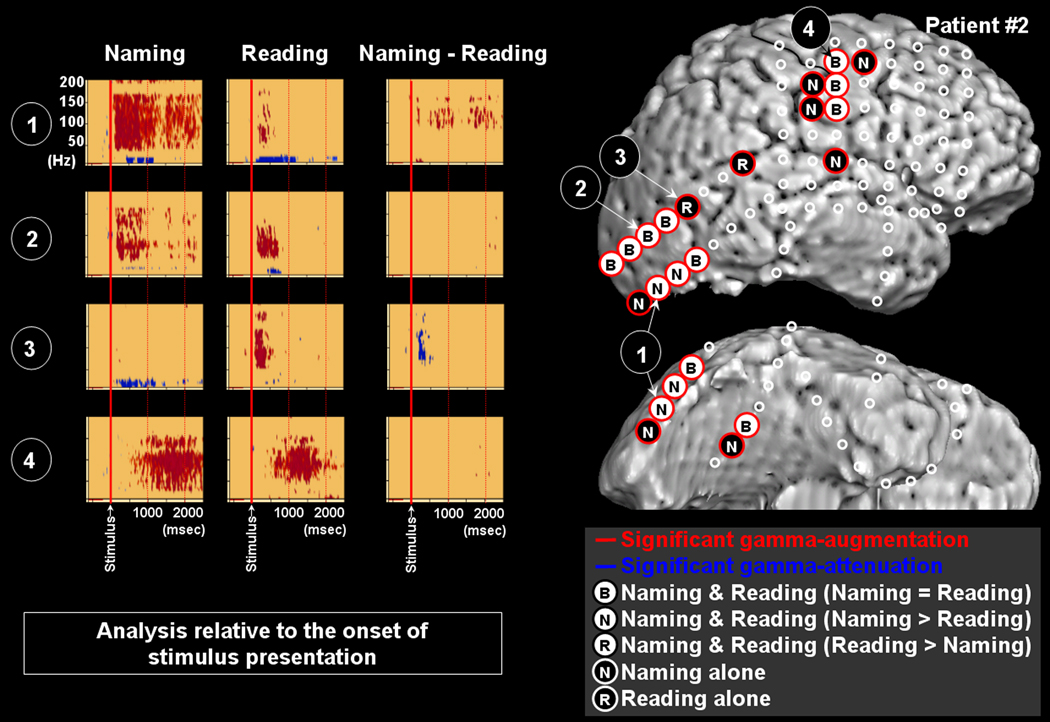
Figure 3. Gamma-oscillations modulated by naming and reading in patient #2.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Both naming and reading tasks elicited gamma-augmentation involving the right lateral occipital sites (Channels 1 and 2). The task-related degree of gamma-augmentation in Channel 1 was significantly larger in naming compared to reading. Gamma-augmentation involved Channel 3 in the lateral occipital area only during the reading task. Gamma-augmentation involved Channel 4 in the Rolandic area during naming and reading tasks.
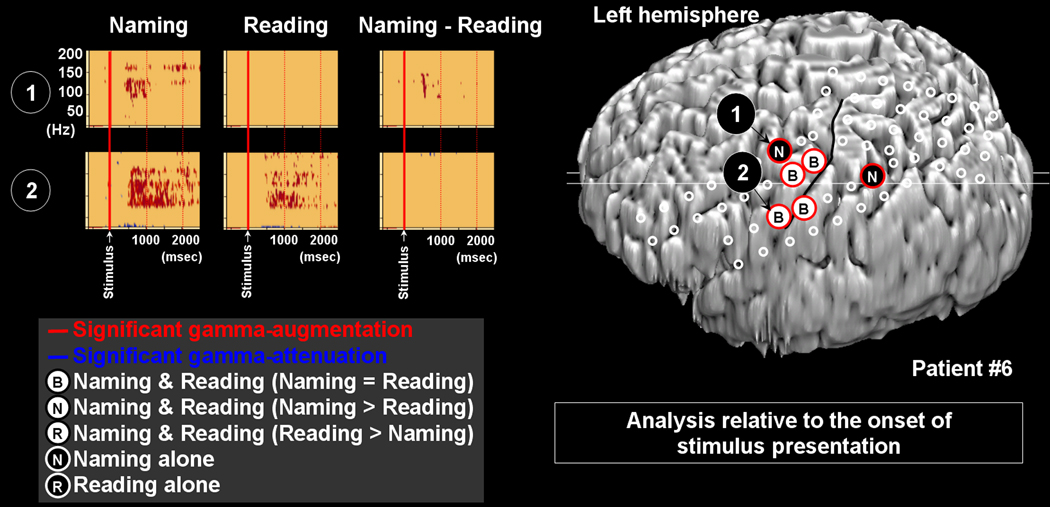
Figure 7. Gamma-oscillations modulated by naming and reading in patient #6.
The results of time-frequency analysis relative to the onset of stimulus presentation are shown. Gamma-augmentation involved Channel 1 in the left premotor area only during naming task. Gamma-augmentation involved Channel 2 in the inferior Rolandic area during both naming and reading tasks.
Results of time-frequency analysis relative to the onset of vocalization
Gamma-augmentation in the inferior Rolandic area (Brodmann Areas 4/1/2/3)
In Supplementary Figures S1–S6, the results of time-frequency analysis relative to the onset of stimulus presentation are provided; this analytic method was designed to evaluate brain activation associated with overt articulation and hearing answers. Twenty-eight inferior Rolandic sites (Brodmann Areas 4, 1, 2 and 3, bilaterally) showed gamma-augmentation equally elicited by both naming and reading responses, whereas five inferior Rolandic sites showed gamma-augmentation elicited by naming responses alone. The median onset latency of gamma-augmentation in the inferior Rolandic sites was −330 msec (i.e.: 330 msec prior to the onset of responses; mean: −322 msec; SD: 191 msec) in naming and −380 msec (mean: −344 msec; SD: 162 msec) in reading task.
Gamma-augmentation in the superior temporal gyrus (Brodmann Areas 41/42/22)
Significant gamma-augmentation was elicited in seven sites of the posterior superior temporal gyrus or superior temporal sulcus (Brodmann Areas 41/42/22) following overt responses (four sites during both naming and reading tasks; one site during naming task alone; two sites during reading task alone). The median onset latency of such gamma-augmentation was 180 msec (i.e.: 180 msec following the onset of responses; mean: 194 msec; SD: 85 msec) in naming and 185 msec (mean: 223 msec; SD: 143 msec) in reading task.
Correlation between the length of words and the degree of gamma-augmentation
A total of 67 sites showed significant reading-related gamma-augmentation within 500 msec following stimulus presentation and these electrodes were included into further analysis (50 occipital, 4 occipital-temporal, 3 superior-posterior parietal, 1 prefrontal, 1 premotor, 1 para-hippocampal and 7 Rolandic sites). Analysis of individual electrode sites demonstrated that the length of words was positively correlated to ‘the maximum gamma-amplitude80–100Hz’ in 11 occipital sites and one premotor site (p<0.05 on the Spearman’s Rank Test) and that the mean rho-value among these 12 sites was 0.39 (median: 0.36; range: 0.29–0.61; Figure 9).
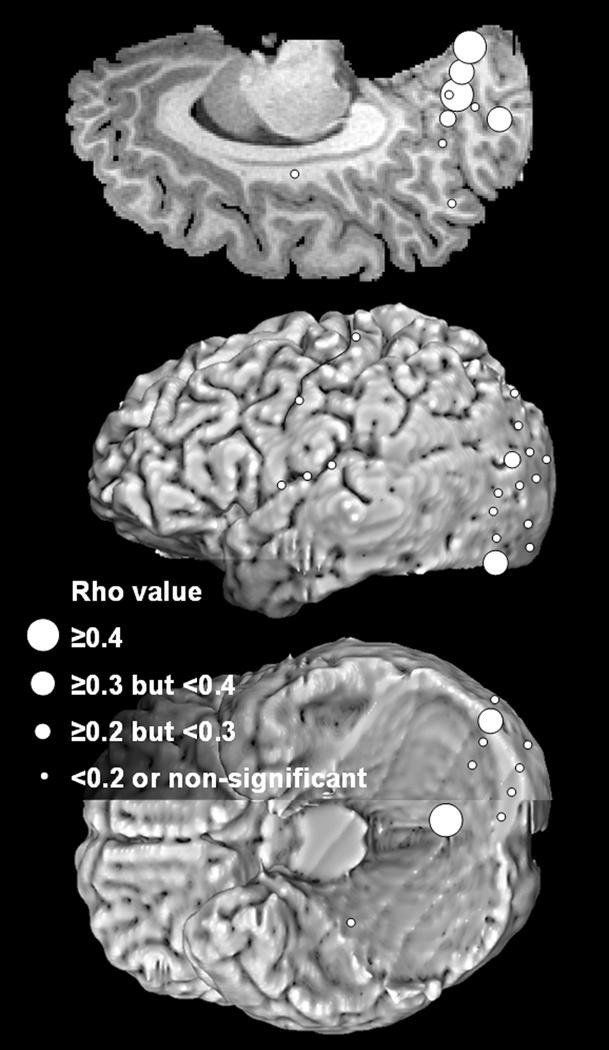
Figure 9. Correlation between the length of words and the degree of gamma-augmentation in patient #3.
The Spearman’s rho-value representing the strength of correlation between the length of words and ‘the maximum gamma-amplitude80–100Hz’ is shown in a given cortical site.
Group analysis of 50 occipital sites suggested that the mean rho-value was 0.26 in the medial surface of the occipital lobe (95%CI: 0.15 to 0.38; N=9 sites), 0.17 in the inferior surface (95%CI: 0.054 to 0.29; N=15 sites) and 0.063 in the lateral surface (95%CI: 0.013 to 0.11; N=26 sites). After excluding the five lateral occipital sites classified as seizure onset zone, the mean rho-value in the lateral surface was still as small as 0.054 (95%CI: −0.003 to 0.11; N=21 sites). These findings suggest that longer words in reading task elicited larger gamma-augmentation in the occipital lobe, especially in the medial surface of the occipital lobe and modestly in the lateral surface (Figure 9). Group analysis of four sites in the occipital-temporal junction (Brodmann Area 37) suggested that the mean rho-value was −0.040 (range: −0.064 to −0.027; 95%CI: −0.070 to −0.009). Group analysis of three sites in the superior-posterior parietal area (Brodmann Area 7) suggested that the mean rho-value was 0.13 (95%CI: 0.059 to 0.20). Group analysis of seven inferior Rolandic sites suggested that the mean rho-value was 0.14 (95%CI: 0.043 to 0.23).
Concordance between sites showing gamma augmentation and alpha-beta attenuation
Visual assessment of time-frequency bins indicated that cortical sites showing significant event-related gamma-augmentation often showed alpha-beta attenuation at 10–30 Hz. As an ancillary analysis, therefore, we explored the agreement in localization between sites showing gamma-augmentation and those showing alpha-beta attenuation, using Cohen’s kappa measures. Thereby, TSE values at 10–30 Hz in a given electrode were declared to be statistically significant only if a minimum of eight time-frequency bins were arranged in a continuous array spanning (i) at least 10-Hz in width and (ii) at least 40-msec in duration. An epoch with a duration of 40 msec can contain a single cycle of beta oscillation at 25 Hz.
In the time-frequency analysis relative to the onset of stimulus presentation, naming task elicited both gamma-augmentation and alpha-beta attenuation in 41 occipital sites, gamma-augmentation alone in 20 occipital sites, and alpha-beta attenuation alone in 17 occipital sites and no significant amplitude changes in 6 occipital sites; thereby, Cohen’s kappa was −0.064 (−0.32 to 0.19). Similarly, reading task elicited both gamma-augmentation and alpha-beta attenuation in 38 occipital sites, gamma-augmentation alone in 14 occipital sites, and alpha-beta attenuation alone in 21 occipital sites and no significant amplitude changes in 11 occipital sites; Cohen’s kappa was 0.078 (95%CI: −0.16 to 0.31). These results suggest poor agreement in localization between the occipital sites showing gamma-augmentation and those showing alpha-beta attenuation.
In the time-frequency analysis relative to the onset of responses, naming task elicited gamma-augmentation and alpha-beta attenuation in 10 Rolandic sites, gamma-augmentation alone in 19 Rolandic sites, and alpha-beta attenuation alone in 9 Rolandic sites and no significant amplitude changes in 67 Rolandic sites; Cohen’s kappa was 0.253 (95%CI: 0.017 to 0.49). Similarly, reading task elicited gamma-augmentation and alpha-beta attenuation in 7 Rolandic sites, gamma-augmentation alone in 16 Rolandic sites, and alpha-beta attenuation alone in 4 Rolandic sites and no significant amplitude changes in 78 Rolandic sites; Cohen’s kappa was 0.315 (95%CI: 0.044 to 0.59). These results suggest fair agreement in localization between the Rolandic sites showing gamma-augmentation and those showing alpha-beta attenuation. Measurement of Cohen’s kappa was not feasible in areas other than occipital and Rolandic areas, since only a small number of sites showed significant gamma-augmentation or alpha-beta attenuation.
Surgical outcome
Patient #1, #2 and #4 underwent temporal lobectomy and have been free from seizures (mean follow-up period: 11 months). Neither occipital nor occipital-temporal sites showing task-related gamma-augmentation were removed. Postoperative gross confrontation revealed upper quadrant hemianopia contralateral to the side of surgery in patients #1 and #2 but not in patient #4. None of these three patients developed apparent naming or reading difficulties.
The seizure onset zone in patient #3 involved two superior-posterior parietal sites (Brodmann Area 7) as well as five lateral occipital sites (Brodmann Areas 19/18/17) showing gamma-augmentation elicited by either reading and/or naming task. The patient underwent resection of the seizure onset zone involving Brodmann Area 7 and Brodmann Area 19 as well as multiple subpial transactions involving Brodmann Areas 17 and 18. Immediately following surgery, right-sided hemianopia was noted. Four months after surgery, the patient significantly recovered from such a visual field deficit but she still stated that her right peripheral vision was slightly darker compared to the left; no apparent reading or naming deficits were noted. Postoperatively, she experienced visual auras but no complex partial or secondarily generalized seizures (follow-up period: 5 months).
Surgical resection in patient #5 involved the left superior frontal and cingulate gyrus; a single premotor site showing naming-related gamma-augmentation was affected by collateral damage. The patient developed temporary difficulty in naming immediately following surgery and underwent an inpatient speech therapy for two weeks. Two months after surgery, no apparent speech deficit was noted and she denied any difficulty in naming. She has been free from seizures (follow-up period: 30 months).
Surgical resection in patient #6 involved the left superior parietal lobule and cingulate cortex in addition to the tumor. Postoperatively, the patient had seizure-like episodes, which turned out to be nonepileptic according to follow-up video-EEG monitoring; otherwise, she has been free from epileptic seizures (follow-up period: 4 months). Following surgery, naming was intact but she developed a mild numbness in the right lower extremity and perceived a reduced memory capacity (e.g.: difficulty in remembering a phone number).
DISCUSSION
In the present study, measurement of gamma-modulations identified cortical areas commonly and differentially involved in picture naming and reading tasks. Specifically, we found that picture naming, compared to word reading, elicited greater cortical activation in the left dorsolateral premotor areas. We also found that word reading, compared to picture naming, elicited greater cortical activation in portions of the left ventral occipital-temporal area. We found that longer words, compared to shorter ones, activated the primary visual cortex for the more peripheral field.
Gamma-augmentation in the occipital area
In the present study, both naming and reading tasks elicited gamma-augmentation bilaterally in the occipital areas following stimulus presentation. Yet, group analysis suggested that picture naming task, compared to word reading task, more frequently elicited greater gamma-augmentation in the occipital sites. This finding can be explained by difference in attentiveness to presented stimuli as well as difference in physical properties of stimuli between naming and reading tasks. A previous ECoG study demonstrated that attentive reading, compared to ignoring presented words, elicited larger gamma-augmentation in the left posterior temporal region as well as the inferior frontal gyrus (Jung et al., 2008). According to our behavioral data, it generally took a longer time for subjects to name objects compared to read words. This finding suggests that subjects may have paid a greater or longer visual attention to real life objects compared to written words. Visual stimuli were not controlled between naming and reading tasks in the present study; a real life object (such as a dog) contains greater complexities in contour or shading compared to a written word. The response time may also depend on the familiarity to a given presented word (Frishkoff et al., 2009).
A significant positive correlation between the length of words and the degree of gamma-augmentation in the occipital lobe was a novel observation in our ECoG study. This finding is consistent with the notion derived from other diagnostic modalities that the degree of gamma-augmentation in the visual cortex is dependent on the size of visual stimuli (Mechelli et al., 2000; Wydell et al., 2003; Graves et al., 2010). We found that the effect of word length on gamma-augmentation was largest in the medial occipital lobe. This finding suggests that longer words, compared to shorter ones, activate the primary visual cortex for the peripheral field (Wong and Sharpe, 1999; Levy et al., 2001; Rols et al., 2001; Stenbacka and Vanni, 2007; Yoshor et al., 2007).
Gamma-augmentation in the temporal-occipital junction
One site at Brodmann Area 37 on the left side showed gamma-augmentation elicited by reading alone. There was no correlation between the length of words and the degree of gamma-augmentation in this area. These observations support the notion that the left ventral occipital-temporal area plays a distinct role in word reading (Nobre et al., 1994; Cohen et al., 2000; Tanji et al., 2005; Dehaene and Cohen, 2007). Previous lesion studies reported that damage of this area resulted in a reading impairment not simply explained by visual field deficits (Gaillard et al., 2006; Pflugshaupt et al., 2009)
Gamma-augmentation in the superior-posterior parietal area (Brodmann Area 7)
Three sites at Brodmann Area 7 on the left side (superior posterior parietal area) in patient #3 showed gamma-augmentation elicited by reading task alone. Two of these three sites were classified as seizure onset zones and surgically removed. Subsequently, this patient developed a right-sided visual field deficit but no apparent deficit in reading or naming. These results suggest that the superior-posterior parietal area was involved in but not essential for word reading in this patient. Previous neuroimaging studies showed that this area was activated by sight music score reading in healthy subjects (Sergent et al., 1992), Braille reading in blind subjects (Sadato et al., 1996) and letter-by-letter reading in a patient who developed hemorrhagic stroke involving the left ventral occipital-temporal junction (Ino et al., 2008).
Gamma-augmentation in the premotor area
This ECoG study demonstrated that six premotor sites (five on the left) showed gamma-augmentation elicited by naming alone. This finding is consistent with the observation in a lesion study showing that the damage to this area impaired naming more than word reading (Stuss et al., 2001) and also with imaging studies showing that naming elicited greater cortical activation in this area compared to word reading (MacDonald et al., 2000; Polk et al., 2008). We have recently measured gamma-oscillations elicited by word reading and color naming while subjects were presented a word printed in an incongruent color; color naming specifically elicited gamma-augmentation in the left premotor area (Koga et al., 2010). Thus, we speculate that naming-specific premotor gamma-augmentation observed in the present study can be explained by difference in tasks rather than difference in physical properties of the stimuli. The behavioral data in the present study demonstrated that the response time was longer in picture naming compared to the word reading task. One patient developed a temporary naming deficit following resective surgery. Taken together, we speculate that the left premotor area plays an essential role in picture naming, at least in some human subjects.
Gamma-attenuation in the prefrontal and premotor areas
Eight prefrontal-premotor sites showed gamma-attenuation elicited by reading alone, while two posterior cingulate sites showed gamma-attenuation elicited by either reading or naming. These findings are consistent with previous ECoG studies demonstrating task-related gamma-attenuations with similar spatial characteristics (Lachaux et al., 2008; Miller et al., 2009; Jerbi et al., 2010). Such task-related gamma-attenuations may be associated with cortical activation during resting periods. Functional neuroimaging studies have shown that portions of brain are active while human subjects are apparently resting and not engaged in an externally given task. Such brain regions have been commonly called as the default-mode network, which includes the ventral prefrontal as well as posterior cingulate areas (Raichle et al, 2001; Greicius et al., 2003).
Concordance between sites showing gamma augmentation and alpha-beta attenuation
An ancillary analysis in the present study indicated that occipital sites showing event-related gamma-augmentation often showed alpha-beta attenuation but that agreement in localization between sites showing gamma-augmentation and alpha-beta attenuation was poor. This finding may suggest that visually-driven alpha-beta attenuation represents a visual process distinct from that represented by gamma-augmentation. The mechanism of such alpha-beta attenuation still remains hypothetical, but previous studies have shown association between greater alpha-beta attenuation and greater awareness or attention to stimuli (Sauseng et al., 2005; Dalal et al., 2009; Wyart and Tallon-Baudry, 2009; Snyder and Foxe, 2010).
Methodological limitations
Inevitable limitations of ECoG recording include: sampling limitation, antiepileptic drugs, and inability to study healthy volunteers. Many of our patients had subdural electrodes placed only on the cortical surface of the presumed epileptogenic hemisphere; we were not able to evaluate the other hemisphere or subcortical structures. Since large bridging veins were present, we did not place large grid subdural electrodes but strip electrodes in the occipital-temporal junction. Antiepileptic drugs might have affected the findings of time-frequency ECoG analysis. Phenytoin was reported to elevate motor thresholds to transcranial magnetic stimulation but had no effect on motor-evoked potential amplitudes (Chen et al., 1997). A human study using macro-electrodes showed that reduction of antiepileptic drugs was followed by a 3% increase in duration of epileptogenic high-frequency oscillations at 80 Hz and above spontaneously arising from the seizure onset zone (Zijlmans et al., 2009). It has been reported that short-lasting saccade-driven artifacts can confound EEG and ECoG signals (Yuval-Greenberg et al., 2008; Ball et al., 2009; Jerbi et al., 2009; Kovach et al., 2011); previous ECoG studies commonly reported that the anterior temporal pole was most susceptible for saccade-driven artifacts and that the effects of such artifacts became gradually smaller on the areas more distant from the anterior temporal pole. In the present study, we failed to find a gradual change in gamma amplitudes across locations in the present study. Time-frequency analysis using bipolar montage also showed significant gamma-augmentation which is very difficult to explain by the effects of saccade-driven artifacts (Supplementary Figure S7). Thus, we speculate that the effects of saccade-driven artifacts not time-locked to stimulus presentation were small in the present study.
Supplementary Material
Acknowledgement
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano) as well as by Wayne State University School of Medicine MD/PhD program (to H. Wu). We are grateful to Harry T. Chugani, M.D., Carol Pawlak, R.EEG./EP.T., Ruth Roeder, R.N., M.S., Sarah Minarik, R.N., B.S.N., Alanna Carlson, M.A. and the staff of the Division of Electroneurodiagnostics at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- Alkonyi B, Juhász C, Muzik O, Asano E, Saporta A, Shah A, Chugani HT. Quantitative brain surface mapping of an electrophysiologic/metabolic mismatch in human neocortical epilepsy. Epilepsy Res. 2009;87:77–87. doi: 10.1016/j.eplepsyres.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Muzik O, Chugani DC, Shah J, Sood S, Chugani HT. Origin and propagation of epileptic spasms delineated on electrocorticography. Epilepsia. 2005;46:1086–1097. doi: 10.1111/j.1528-1167.2005.05205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Mihaylova T, Juhász C, Sood S, Chugani HT. Effect of sleep on interictal spikes and distribution of sleep spindles on electrocorticography in children with focal epilepsy. Clin Neurophysiol. 2007;118:1360–1368. doi: 10.1016/j.clinph.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Juhász C, Shah A, Sood S, Chugani HT. Role of subdural electrocorticography in prediction of long-term seizure outcome in epilepsy surgery. Brain. 2009a;132:1038–1047. doi: 10.1093/brain/awp025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009b;45:477–489. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auranen T. Master's Thesis. Espoo, Finland: Helsinki University of Technology, Department of Electrical and Communications Engineering; 2002. Nonparametric statistical analysis of time-frequency representations of magnetoencephalographic data. [Google Scholar]
- Ball T, Kern M, Mutschler I, Aertsen A, Schulze-Bonhage A. Signal quality of simultaneously recorded invasive and non-invasive EEG. Neuroimage. 2009;46:708–716. doi: 10.1016/j.neuroimage.2009.02.028. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15:47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunner P, Ritaccio AL, Lynch TM, Emrich JF, Wilson JA, Williams JC, Aarnoutse EJ, Ramsey NF, Leuthardt EC, Bischof H, Schalk G. A practical procedure for real-time functional mapping of eloquent cortex using electrocorticographic signals in humans. Epilepsy Behav. 2009;15:278–286. doi: 10.1016/j.yebeh.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canolty RT, Soltani M, Dalal SS, Edwards E, Dronkers NF, Nagarajan SS, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal dynamics of word processing in the human brain. Front Neurosci. 2007;1:185–196. doi: 10.3389/neuro.01.1.1.014.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R, Samii A, Caños M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- Cohen L, Dehaene S, Naccache L, Lehéricy S, Dehaene-Lambertz G, Hénaff MA, Michel F. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000;123:291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998;121:2301–2315. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- Dalal SS, Edwards E, Kirsch HE, Barbaro NM, Knight RT, Nagarajan SS. Localization of neurosurgically implanted electrodes via photograph-MRI-radiograph coregistration. J Neurosci Methods. 2008;174:106–115. doi: 10.1016/j.jneumeth.2008.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalal SS, Baillet S, Adam C, Ducorps A, Schwartz D, Jerbi K, Bertrand O, Garnero L, Martinerie J, Lachaux JP. Simultaneous MEG and intracranial EEG recordings during attentive reading. Neuroimage. 2009;45:1289–1304. doi: 10.1016/j.neuroimage.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Davidson AC, Hinkley DV. Studentized bootstrap method. In: Davison AC, Hinkley DV, editors. Bootstrap Methods and their Application. Cambridge: Cambridge University Press; 1999. pp. 161–175. [Google Scholar]
- Dehaene S, Cohen L. Cultural recycling of cortical maps. Neuron. 2007;56:384–398. doi: 10.1016/j.neuron.2007.10.004. [DOI] [PubMed] [Google Scholar]
- Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal imaging of cortical activation during verb generation and picture naming. Neuroimage. 2010;50:291–301. doi: 10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flinker A, Chang EF, Kirsch HE, Barbaro NM, Crone NE, Knight RT. Single-trial speech suppression of auditory cortex activity in humans. J Neurosci. 2010;30:16643–16650. doi: 10.1523/JNEUROSCI.1809-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frishkoff GA, Perfetti CA, Westbury C. ERP measures of partial semantic knowledge: left temporal indices of skill differences and lexical quality. Biol Psychol. 2009;80:130–147. doi: 10.1016/j.biopsycho.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, Asano E. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–1805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E. Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin Neurophysiol. 2010a;121:366–375. doi: 10.1016/j.clinph.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010b;49:2735–2745. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetz W, Otsubo H, Pang EW. Magnetoencephalography for clinical pediatrics: the effect of head positioning on measurement of somatosensory-evoked fields. Clin Neurophysiol. 2008;119:1923–1933. doi: 10.1016/j.clinph.2008.04.291. [DOI] [PubMed] [Google Scholar]
- Gaillard R, Naccache L, Pinel P, Clémenceau S, Volle E, Hasboun D, Dupont S, Baulac M, Dehaene S, Adam C, Cohen L. Direct intracranial, FMRI, and lesion evidence for the causal role of left inferotemporal cortex in reading. Neuron. 2006;50:191–204. doi: 10.1016/j.neuron.2006.03.031. [DOI] [PubMed] [Google Scholar]
- Graves WW, Desai R, Humphries C, Seidenberg MS, Binder JR. Neural systems for reading aloud: a multiparametric approach. Cereb Cortex. 2010;20:1799–1815. doi: 10.1093/cercor/bhp245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100:253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoechstetter K, Bornfleth H, Weckesser D, Ille N, Berg P, Scherg M. BESA source coherence: A new method to study cortical oscillatory coupling. Brain Topography. 2004;16:233–238. doi: 10.1023/b:brat.0000032857.55223.5d. [DOI] [PubMed] [Google Scholar]
- Ino T, Tokumoto K, Usami K, Kimura T, Hashimoto Y, Fukuyama H. Longitudinal fMRI study of reading in a patient with letter-by-letter reading. Cortex. 2008;44:773–781. doi: 10.1016/j.cortex.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band electrocorticographic activity. J Neurosci. 2009;29:10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Dalal S, Kahane P, Bertrand O, Berthoz A, Lachaux JP. Saccade related gamma-band activity in intracerebral EEG: dissociating neural from ocular muscle activity. Brain Topogr. 2009;22:18–23. doi: 10.1007/s10548-009-0078-5. [DOI] [PubMed] [Google Scholar]
- Jerbi K, Vidal JR, Ossandon T, Dalal SS, Jung J, Hoffmann D, Minotti L, Bertrand O, Kahane P, Lachaux JP. Exploring the electrophysiological correlates of the default-mode network with intracerebral EEG. Front Syst Neurosci. 2010;4:27. doi: 10.3389/fnsys.2010.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. The neural bases of attentive reading. Hum Brain Mapp. 2008;29:1193–1206. doi: 10.1002/hbm.20454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Agari T, Oka M, Yoshinaga H, Date I, Ohtsuka Y, Gotman J. Detection of seizure-associated high-frequency oscillations above 500Hz. Epilepsy Res. 2010;88:139–144. doi: 10.1016/j.eplepsyres.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koga S, Rothermel R, Juhász C, Nagasawa N, Sood S, Asano E. Electrocorticographic correlates of cognitive control in a Stroop task. -Intracranial recording in epileptic patients. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovach CK, Tsuchiya N, Kawasaki H, Oya H, Howard MA, 3rd, Adolphs R. Manifestation of ocular-muscle EMG contamination in human intracranial recordings. Neuroimage. 2011;54:213–233. doi: 10.1016/j.neuroimage.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Rodriguez E, Martinerie J, Adam C, Hasboun D, Varela FJ. A quantitative study of gamma-band activity in human intracranial recordings triggered by visual stimuli. Eur J Neurosci. 2000;12:2608–2622. doi: 10.1046/j.1460-9568.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, George N, Tallon-Baudry C, Martinerie J, Hugueville L, Minotti L, Kahane P, Renault B. The many faces of the gamma band response to complex visual stimuli. Neuroimage. 2005;25:491–501. doi: 10.1016/j.neuroimage.2004.11.052. [DOI] [PubMed] [Google Scholar]
- Lachaux JP, Jung J, Mainy N, Dreher JC, Bertrand O, Baciu M, Minotti L, Hoffmann D, Kahane P. Silence is golden: transient neural deactivation in the prefrontal cortex during attentive reading. Cereb Cortex. 2008;18:443–450. doi: 10.1093/cercor/bhm085. [DOI] [PubMed] [Google Scholar]
- Lesser RP, Crone NE, Webber WR. Subdural electrodes. Clin Neurophysiol. 2010;121:1376–1392. doi: 10.1016/j.clinph.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- Mainy N, Jung J, Baciu M, Kahane P, Schoendorff B, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Cortical dynamics of word recognition. Hum Brain Mapp. 2008;29:1215–1230. doi: 10.1002/hbm.20457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechelli A, Humphreys GW, Mayall K, Olson A, Price CJ. Differential effects of word length and visual contrast in the fusiform and lingual gyri during reading. Proc Biol Sci. 2000;267:1909–1913. doi: 10.1098/rspb.2000.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon RS, Kim SG. Spatial and temporal limits in cognitive neuroimaging with fMRI. Trends Cogn Sci. 1999;3:207–216. doi: 10.1016/s1364-6613(99)01329-7. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Leuthardt EC, Schalk G, Rao RP, Anderson NR, Moran DW, Miller JW, Ojemann JG. Spectral changes in cortical surface potentials during motor movement. J Neurosci. 2007a;27:2424–2432. doi: 10.1523/JNEUROSCI.3886-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller KJ, Makeig S, Hebb AO, Rao RP, denNijs M, Ojemann JG. Cortical electrode localization from X-rays and simple mapping for electrocorticographic research: The "Location on Cortex" (LOC) package for MATLAB. J Neurosci Methods. 2007b;162:303–308. doi: 10.1016/j.jneumeth.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Miller KJ, Weaver KE, Ojemann JG. Direct electrophysiological measurement of human default network areas. Proc Natl Acad Sci USA. 2009;106:12174–12177. doi: 10.1073/pnas.0902071106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzik O, Chugani DC, Zou G, Hua J, Lu Y, Lu S, Asano E, Chugani HT. Multimodality Data Integration in Epilepsy. Int J Biomed Imaging. 2007 doi: 10.1155/2007/13963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhasz C, Fukuda M, Nishida M, Akiyama T, Sood S, Asano E. Cortical gamma-oscillations modulated by auditory-motor tasks. -Intracranial recording in patients with epilepsy. Hum Brain Mapp. 2010a;31:1627–1642. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by visuomotor tasks: Intracranial recording in patients with epilepsy. Epilepsy Behav. 2010b;18:254–261. doi: 10.1016/j.yebeh.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Nishida M, Juhász C, Sood S, Chugani HT, Asano E. Cortical glucose metabolism positively correlates with gamma-oscillations in nonlesional focal epilepsy. Neuroimage. 2008;42:1275–1284. doi: 10.1016/j.neuroimage.2008.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobre AC, Allison T, McCarthy G. Word recognition in the human inferior temporal lobe. Nature. 1994;372:260–263. doi: 10.1038/372260a0. [DOI] [PubMed] [Google Scholar]
- Otsubo H, Ochi A, Imai K, Akiyama T, Fujimoto A, Go C, Dirks P, Donner EJ. High-frequency oscillations of ictal muscle activity and epileptogenic discharges on intracranial EEG in a temporal lobe epilepsy patient. Clin Neurophysiol. 2008;119:862–868. doi: 10.1016/j.clinph.2007.12.014. [DOI] [PubMed] [Google Scholar]
- Papp N, Ktonas P. Critical evaluation of complex demodulation techniques for the quantification of bioelectrical activity. Biomed Sci Instrum. 1977;13:135–145. [PubMed] [Google Scholar]
- Pflugshaupt T, Gutbrod K, Wurtz P, von Wartburg R, Nyffeler T, de Haan B, Karnath HO, Mueri RM. About the role of visual field defects in pure alexia. Brain. 2009;132:1907–1917. doi: 10.1093/brain/awp141. [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH. Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol. 1999;110:1842–1857. doi: 10.1016/s1388-2457(99)00141-8. [DOI] [PubMed] [Google Scholar]
- Polk TA, Drake RM, Jonides JJ, Smith MR, Smith EE. Attention enhances the neural processing of relevant features and suppresses the processing of irrelevant features in humans: a functional magnetic resonance imaging study of the Stroop task. J Neurosci. 2008;28:13786–13792. doi: 10.1523/JNEUROSCI.1026-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Devlin JT. The myth of the visual word form area. Neuroimage. 2003;19:473–481. doi: 10.1016/s1053-8119(03)00084-3. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Crone NE, Niebur E, Franaszczuk PJ, Hsiao SS. Neural correlates of high-gamma oscillations (60–200 Hz) in macaque local field potentials and their potential implications in electrocorticography. J Neurosci. 2008;28:11526–11536. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reale RA, Calvert GA, Thesen T, Jenison RL, Kawasaki H, Oya H, Howard MA, Brugge JF. Auditory-visual processing represented in the human superior temporal gyrus. Neuroscience. 2007;145:162–184. doi: 10.1016/j.neuroscience.2006.11.036. [DOI] [PubMed] [Google Scholar]
- Rols G, Tallon-Baudry C, Girard P, Bertrand O, Bullier J. Cortical mapping of gamma oscillations in areas V1 and V4 of the macaque monkey. Vis Neurosci. 2001;18:527–540. doi: 10.1017/s0952523801184038. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–236. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Rutka JT, Otsubo H, Kitano S, Sakamoto H, Shirasawa A, Ochi A, Snead OC., 3rd Utility of digital camera-derived intraoperative images in the planning of epilepsy surgery for children. Neurosurgery. 1999;45:1186–1191. doi: 10.1097/00006123-199911000-00033. [DOI] [PubMed] [Google Scholar]
- Sadato N, Pascual-Leone A, Grafman J, Ibañez V, Deiber MP, Dold G, Hallett M. Activation of the primary visual cortex by Braille reading in blind subjects. Nature. 1996;380:526–528. doi: 10.1038/380526a0. [DOI] [PubMed] [Google Scholar]
- Salmelin R, Hari R. Spatiotemporal characteristics of sensorimotor MEG rhythms related to thumb movement. Neuroscience. 1994;60:537–550. doi: 10.1016/0306-4522(94)90263-1. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N. A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci. 2005;22:2917–2926. doi: 10.1111/j.1460-9568.2005.04482.x. [DOI] [PubMed] [Google Scholar]
- Sergent J, Zuck E, Terriah S, MacDonald B. Distributed neural network underlying musical sight-reading and keyboard performance. Science. 1992;257:106–109. doi: 10.1126/science.1621084. [DOI] [PubMed] [Google Scholar]
- Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika. 1986;73:751–754. [Google Scholar]
- Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- Snyder AC, Foxe JJ. Anticipatory attentional suppression of visual features indexed by oscillatory alpha-band power increases: a high-density electrical mapping study. J Neurosci. 2010;30:4024–4032. doi: 10.1523/JNEUROSCI.5684-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbacka L, Vanni S. fMRI of peripheral visual field representation. Clin Neurophysiol. 2007;118:1303–1314. doi: 10.1016/j.clinph.2007.01.023. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Floden D, Alexander MP, Levine B, Katz D. Stroop performance in focal lesion patients: dissociation of processes and frontal lobe lesion location. Neuropsychologia. 2001;39:771–786. doi: 10.1016/s0028-3932(01)00013-6. [DOI] [PubMed] [Google Scholar]
- Szurhaj W, Derambure P. Intracerebral study of gamma oscillations in the human sensorimotor cortex. Prog Brain Res. 2006;159:297–310. doi: 10.1016/S0079-6123(06)59020-X. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O. Oscillatory gamma activity in humans and its role in object representation. Trends Cogn Sci. 1999;3:151–162. doi: 10.1016/s1364-6613(99)01299-1. [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C, Bertrand O, Hénaff MA, Isnard J, Fischer C. Attention modulates gamma-band oscillations differently in the human lateral occipital cortex and fusiform gyrus. Cereb Cortex. 2005;15:654–662. doi: 10.1093/cercor/bhh167. [DOI] [PubMed] [Google Scholar]
- Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thampratankul L, Nagasawa T, Rothermel R, Juhasz C, Sood S, Asano E. Cortical gamma oscillations modulated by word association tasks: intracranial recording. Epilepsy Behav. 2010;18:116–118. doi: 10.1016/j.yebeh.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JR, Ossandón T, Jerbi K, Dalal SS, Minotti L, Ryvlin P, Kahane P, Lachaux JP. Category-specific visual responses: an intracranial study comparing gamma, beta, alpha, and ERP response selectivity. Front Hum Neurosci. 2010;4:195. doi: 10.3389/fnhum.2010.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stockhausen HM, Thiel A, Herholz K, Pietrzyk U. A convenient method for topographical localization of intracranial electrodes with MRI and a conventional radiograph. Neuroimage. 1997;5:S514. [Google Scholar]
- Wellmer J, von Oertzen J, Schaller C, Urbach H, König R, Widman G, Van Roost D, Elger CE. Digital photography and 3D MRI-based multimodal imaging for individualized planning of resective neocortical epilepsy surgery. Epilepsia. 2002;43:1543–1550. doi: 10.1046/j.1528-1157.2002.30002.x. [DOI] [PubMed] [Google Scholar]
- Wong AM, Sharpe JA. Representation of the visual field in the human occipital cortex: a magnetic resonance imaging and perimetric correlation. Arch Ophthalmol. 1999;117:208–217. doi: 10.1001/archopht.117.2.208. [DOI] [PubMed] [Google Scholar]
- Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, Gaona C, Leuthardt EC. Electrocorticographic frequency alteration mapping for extraoperative localization of speech cortex. Neurosurgery. 2010;66:E407–E409. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. How ongoing fluctuations in human visual cortex predict perceptual awareness: baseline shift versus decision bias. J Neurosci. 2009;29:8715–8725. doi: 10.1523/JNEUROSCI.0962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wydell TN, Vuorinen T, Helenius P, Salmelin R. Neural correlates of letter-string length and lexicality during reading in a regular orthography. J Cogn Neurosci. 2003;15:1052–1062. doi: 10.1162/089892903770007434. [DOI] [PubMed] [Google Scholar]
- Yoshor D, Bosking WH, Ghose GM, Maunsell JH. Receptive fields in human visual cortex mapped with surface electrodes. Cereb Cortex. 2007;17:2293–2302. doi: 10.1093/cercor/bhl138. [DOI] [PubMed] [Google Scholar]
- Yuval-Greenberg S, Tomer O, Keren AS, Nelken I, Deouell LY. Transient induced gamma-band response in EEG as a manifestation of miniature saccades. Neuron. 2008;58:429–441. doi: 10.1016/j.neuron.2008.03.027. [DOI] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–986. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.