Abstract
In the striatum, dopamine D1 receptors are preferentially expressed in striatonigral neurons, and increase the neuronal excitability, leading to the increase in GABAergic inhibitory output to substantia nigra pars reticulata. Such roles of D1 receptors are important for the control of motor functions. In addition, the roles of D1 receptors are implicated in reward, cognition, and drug addiction. Therefore, elucidation of mechanisms for the regulation of dopamine D1 receptor signaling is required to identify therapeutic targets for Parkinson’s disease and drug addiction. D1 receptors are coupled to Gs/olf/adenylyl cyclase/PKA signaling, leading to the phosphorylation of PKA substrates including DARPP-32. Phosphorylated form of DARPP-32 at Thr34 has been shown to inhibit protein phosphatase-1, and thereby controls the phosphorylation states and activity of many downstream physiological effectors. Roles of DARPP-32 and its phosphorylation at Thr34 and other sites in D1 receptor signaling are extensively studied. In addition, functional roles of the non-canonical D1 receptor signaling cascades that coupled to Gq/phospholipase C or Src family kinase become evident. We have recently shown that phosphodiesterases (PDEs), especially PDE10A, play a pivotal role in regulating the tone of D1 receptor signaling relatively to that of D2 receptor signaling. We review the current understanding of molecular mechanisms for the modulation of D1 receptor signaling in the striatum.
Keywords: dopamine, D1 receptor, signaling, DARPP-32, phosphodiesterase, striatum
Introduction
Dopamine plays critical roles in the regulation of psychomotor functions in the brain (Bromberg-Martin et al., 2010; Cools, 2011; Gerfen and Surmeier, 2011). The dopamine receptors are a superfamily of heptahelical G protein-coupled receptors, and are grouped into two categories, D1-like (D1, D5) and D2-like (D2, D3, D4) receptors, based on functional properties to stimulate adenylyl cyclase (AC) via Gs/olf and to inhibit AC via Gi/o, respectively (Kebabian and Calne, 1979; Jackson and Westlind-Danielsson, 1994; Missale et al., 1998). In the striatum, dopamine D1 and D2 receptor expressions are segregated in two types of medium spiny neurons, striatonigral/direct and striatopallidal/indirect pathway neurons, respectively (Hersch et al., 1995; Surmeier et al., 1996; Valjent et al., 2009; Bertran-Gonzalez et al., 2010). In the striatonigral/direct pathway neurons, D1 receptors are coupled to Gs/olf/AC/PKA signaling, and activation of PKA induces the phosphorylation of PKA substrates such as DARPP-32, a dopamine- and cAMP-regulated phosphoprotein of Mr 32 kDa, and a transcription factor, CREB, leading to alterations of neuronal functions (Greengard et al., 1999; Hyman and Malenka, 2001). In this review, the roles of DARPP-32 and its phosphorylation in D1 receptor signaling and the recent findings on the modulation of D1 receptor/Gs/olf/AC/PKA signaling by phosphodiesterase (PDE) inhibitors are discussed. In addition, the non-canonical D1 receptor signaling cascades that couple to Gq/phospholipase C (PLC) or Src family kinase (SFK) are overviewed.
Roles of DARPP-32 and its Phosphorylation in D1 Receptor/Gs/olf/AC/cAMP/PKA Signaling
Role of the PKA phosphorylation-site at Thr34 of DARPP-32
Dopamine, acting on D1 receptors, stimulates cAMP/PKA signaling via Gs/olf-mediated activation of AC (Herve et al., 2001). In postsynaptic striatal neurons, DARPP-32 is a major target for the cAMP/PKA signaling cascade (Greengard et al., 1999; Svenningsson et al., 2004). DARPP-32 is expressed in D1 receptor-enriched striatonigral neurons as well as D2 receptor-enriched striatopallidal neurons (Bateup et al., 2008). Phosphorylation at Thr34 by PKA converts DARPP-32 into a potent inhibitor of protein phosphatase-1 (PP-1; Figure 1). The inhibition of PP-1 thereby controls the phosphorylation state and activity of many downstream physiological effectors, including various neurotransmitter receptors (e.g., AMPA receptor GluR1 subunit, NMDA receptor NR1 subunit), ion channels and pumps (e.g., N/P-type Ca2+ channels, Na+ channel, Na+, K+-ATPase), and transcription factors (e.g., CREB, c-Fos, ΔFosB). Especially in cases of dual substrates for PKA and PP-1 such as GluR1 at Ser845 and NR1 at Ser897, activation of PKA signaling can efficiently increase the phosphorylation states of such substrates. Mice lacking DARPP-32 are deficient in their molecular, electrophysiological, and behavioral responses to dopamine, drugs of abuse, and antipsychotic medication, indicating an essential role for DARPP-32 in dopaminergic signaling (Fienberg et al., 1998; Fienberg and Greengard, 2000). Recently, a study using DARPP-32 conditional knockout mice, in which DARPP-32 is selectively deleted in D1 receptor-enriched striatonigral or D2 receptor-enriched striatopallidal neurons, revealed that DARPP-32 expressed in two types of medium spiny neurons differentially regulates striatal motor behaviors (Bateup et al., 2010). The loss of DARPP-32 in striatonigral neurons decreases basal and cocaine-induced locomotor activities and attenuates L-DOPA-induced dyskinesia in a 6-hydorxydopamine hemi-lesioned model of Parkinson’s disease, whereas the loss of DARPP-32 in striatopallidal neurons increases basal and cocaine-induced locomotor activities and abolishes haloperidol-induced catalepsy. These findings support the idea that DARPP-32 enhances D1 receptor functions in striatonigral neurons, but opposes D2 receptor functions in striatopallidal neurons.
Figure 1.
The D1 receptor signaling cascades in striatonigral/direct pathway neurons. D1 receptors couple to at least three distinct signaling cascades: (1) Gs/olf/adenylyl cyclase (AC)/cAMP/PKA/DARPP-32/protein phosphatase-1 (PP-1) signaling (blue; Svenningsson et al., 2004; Stipanovich et al., 2008), (2) Gq/phospholipase C (PLC)/inositol 1,4,5-trisphosphate (IP3)/IP3 receptor/Ca2+ signaling (orange; Rashid et al., 2007a; Kuroiwa et al., 2008; Hasbi et al., 2009), (3) Gβγ/Src family kinase (SFK)/NMDA receptor NR2B subunit/Ca2+/Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1)/ mitogen-activated protein kinase/ERK kinase (MEK)/ERK signaling (green; Girault et al., 2007; Pascoli et al., 2011). The phosphorylation levels of DARPP-32 are low at Thr34 and high at Thr75, Ser97, and Ser130 under basal conditions. Activation of PKA induces the phosphorylation of DARPP-32 at Thr34 and the dephosphorylation of DARPP-32 at Thr75 and Ser97 by PP-2A/B56δ complex, and phospho-Thr34/dephospho-Ser97 DARPP-32 accumulates in nucleus and inhibits PP-1, leading to the increase in histone H3 phosphorylation (Stipanovich et al., 2008). ERK, activated by two D1 receptor pathways, induces mitogen- and stress-activated kinase 1 (MSK1) activation and histone H3 and cAMP-response element binding protein (CREB) phosphorylation in the nucleus (Girault et al., 2007; Pascoli et al., 2011). Thus, D1 receptor-mediated activation of PKA, intracellular Ca2+, and ERK signaling induces the changes in downstream signaling cascades and the transcriptional activation of many genes. CaMK, Ca2+/calmodulin-dependent protein kinase; DAG, diacylglycerol; PDE, phosphodiesterase; STEP, striatal-enriched tyrosine phosphatase.
The phosphorylation state of DARPP-32 at Thr34 is regulated by the balance of phosphorylation by PKA and dephosphorylation by protein phosphatase 2B (calcineurin) and 2A (PP-2A). PKA signaling in direct pathway neurons is activated by β1-adrenceptors (Hara et al., 2010) and 5-HT4/6 receptors (Svenningsson et al., 2002, 2007) as well as D1 receptors (Bateup et al., 2008; Kuroiwa et al., 2008), and is inhibited by adenosine A1 receptors (Yabuuchi et al., 2006), α2-adrenceptors (Hara et al., 2010), and μ-opioid receptors (Lindskog et al., 1999). Dephosphorylation of phospho-Thr34 DARPP-32 is mainly regulated by calcineurin (Nishi et al., 1999). Activation of NMDA or AMPA receptors increases intracellular Ca2+ and activates calcineurin, leading to the dephosphorylation of DARPP-32 at Thr34 (Halpain et al., 1990; Nishi et al., 1999). D2 receptors are known to activate calcineurin via PLC/intracellular Ca2+ signaling (Hernandez-Lopez et al., 2000), leading to the dephosphorylation of DARPP-32 at Thr34 in striatal neurons where D1 and D2 receptors are co-expressed (Nishi et al., 1997). PP-2A also contributes to control the dephosphorylation process of DARPP-32 at Thr34 in a coordinated manner with calcineurin, as inhibition of PP-2A and calcineurin induces the synergistic and robust increase in DARPP-32 Thr34 phosphorylation in striatal slices (Nishi et al., 1999). However, the role of PP-2A in the dephosphorylation of DARPP-32 at Thr34 is not fully understood under physiological conditions.
Role of the Cdk5 phosphorylation-site at Thr75 of DARPP-32
In addition to Thr34, DARPP-32 is phosphorylated at multiple sites by several protein kinases. The major phosphorylation sites are at Thr75 for cyclin-dependent kinase 5 (Cdk5; Bibb et al., 1999), at Ser97 for CK2 (Girault et al., 1989), and at Ser130 for CK1 (Desdouits et al., 1995b), and these sites are highly phosphorylated in intact cells. DARPP-32 phosphorylated at Thr75 inhibits PKA activity and thereby reduces the efficacy of dopamine D1 receptor signaling (Bibb et al., 1999). Dopamine, via dopamine D1 receptors, activates PKA, which directly stimulates DARPP-32 Thr34 phosphorylation, and indirectly stimulates DARPP-32 Thr75 dephosphorylation by PP-2A (Nishi et al., 2000). PP-2A associated with B56δ regulatory subunit is involved in the dephosphorylation of Thr75. B56δ at Ser566 is phosphorylated by PKA, and the phosphorylation increases the activity of PP-2A/B56δ complex to dephosphorylate DARPP-32 at Thr75 (Ahn et al., 2007a). The ability of activated PKA to reduce the phosphorylation state of DARPP-32 at Thr75 and thereby de-inhibit PKA is important as a positive feedback mechanism for enhancing PKA signaling (Nishi et al., 2000). Further activation of PKA and inhibition of PP-1 via DARPP-32-dependent mechanisms synergistically increase the phosphorylation of various substrates.
Glutamate acting on NMDA or AMPA receptors also stimulates the dephosphorylation of DARPP-32 at Thr75 by PP-2A (Nishi et al., 2002). PP-2A associated with B′′/PR72 regulatory subunit mediates Ca2+-dependent dephosphorylation of Thr75 (Ahn et al., 2007b). PR72 contains two Ca2+-binding EF hands, EF1 and EF2 (Janssens et al., 2003). EF2 likely promotes the assembly of PP-2A/PR72 complex, and Ca2+ binding to EF1 activates PP-2A/PR72 activity for DARPP-32 at Thr75 in a substrate-specific manner (Janssens et al., 2003; Ahn et al., 2007b). By utilizing the Ca2+-dependent PP-2A/PR72 pathway, glutamate increases PKA activity by dephosphorylating Thr75 of DARPP-32, similarly to the D1 receptor-activated PP-2A/B56δ pathway.
The importance of DARPP-32 Thr75 phosphorylation is implicated in drug abuse. Chronic administration of psychostimulants such as cocaine induces the accumulation of a transcription factor, ΔFosB, resulting in the induction of a downstream target gene, Cdk5 (Bibb et al., 2001). The induced Cdk5 increases DARPP-32 Thr75 phosphorylation and therefore decreases D1 receptor/PKA signaling. The attenuation of D1 receptor/PKA signaling is considered as adaptive changes to cocaine addiction. The role of DARPP-32 at Thr75 is also demonstrated in stimulatory action of caffeine (Lindskog et al., 2002). Caffeine, by antagonizing adenosine A2A receptors in striatopallidal neurons, attenuates A2A receptor/PKA signaling and PP-2A activity and subsequently increases DARPP-32 Thr75 phosphorylation, which likely contribute to the stimulatory action of caffeine.
Role of the CK2 phosphorylation-site at Ser97 of DARPP-32 and the CK1 phosphorylation-site at Ser130 of DARPP-32
Phosphorylation of DARPP-32 at Ser97 (in mouse sequence; Ser102 in rat sequence) by CK2 is reported to increase the efficacy of DARPP-32 Thr34 phosphorylation by PKA (Girault et al., 1989). In parallel, phosphorylation of DARPP-32 at Ser130 (in mouse sequence; Ser137 in rat sequence) by CK1 decreases the rate of dephosphorylation of Thr34 by calcineurin (Desdouits et al., 1995a). Thus, DARPP-32 phosphorylation by CK2 or CK1 results in the increase in the phosphorylation states of DARPP-32 at Thr34, suggesting the role of CK2 and CK1 to enhance D1 receptor/PKA/DARPP-32/PP-1 signaling cascade. However, an opposing action of CK2 to inhibit D1 receptor/PKA signaling is demonstrated (Rebholz et al., 2009). CK2 directly interacts with Gs/olf, and negatively controls the functions of D1 receptors as well as A2A receptors by enabling faster internalization.
Recently, the phosphorylation state of DARPP-32 at Ser97 is found to be a key regulator of nuclear export of DARPP-32 (Stipanovich et al., 2008). DARPP-32 has been thought as cytoplasmic protein because majority of DARPP-32 was fractionated in the soluble fraction (Walaas and Greengard, 1984), although the immunoreactivity of DARPP-32 was noticed in some nuclei of medium spiny neurons (Ouimet and Greengard, 1990). Importantly, activation of D1 receptor/PKA signaling induces the nuclear accumulation of DARPP-32 (Stipanovich et al., 2008). The phosphorylation of DARPP-32 at Ser97 by CK2 functions as nuclear export signal of DARPP-32. Ser97 is highly phosphorylated under basal conditions (Girault et al., 1989), and only small fraction of DARPP-32 is located in the nucleus. When PKA is activated, Ser97 is dephosphorylated by PKA-activated PP-2A/B56δ complex, resulting in the accumulation of DARPP-32 (phospho-Thr34/dephospho-Ser97 form of DARPP-32) in the nucleus. The inhibition of PP-1 by phospho-Thr34 DARPP-32 promotes histone H3 phosphorylation and regulates the nuclear function via mechanisms of chromatin remodeling. These findings provide mechanisms for D1 receptor/PKA/DARPP-32 signaling to regulate gene expression, especially in conditions of drug addiction.
Regulation of D1 receptor/PKA/DARPP-32 signaling by neurotransmitters
The D1 receptor/PKA/DARPP-32 signaling cascade is modulated by various neurotransmitters including glutamate, GABA, acetylcholine, adenosine, serotonin, norepinephrine, nitric oxide, and neuropeptides (opioids, cholecystokinin, and neurotensin), in addition to dopamine (for the review, see Svenningsson et al., 2004). The release of dopamine is also regulated by neurotransmitters, therapeutic drugs, and drugs of abuse (Schmitz et al., 2003; Sulzer, 2011). We recently reported the role of glutamate (Nishi et al., 2005), PGE2 (Kitaoka et al., 2007), nitric oxide (Tanda et al., 2009), and norepinephrine (Hara et al., 2010) in the regulation of D1 receptor/PKA/DARPP-32 signaling. The role of DARPP-32 in integrating dopamine and other neurotransmitter signaling in D1-type striatonigral neurons as well as D2-type striatopallidal neurons has been proposed (Svenningsson et al., 2004; Fernandez et al., 2006). Furthermore, alterations of the D1 receptor/PKA/DARPP-32 signaling cascade in drug addiction and L-DOPA-induced dyskinesia are characterized (for the review, see Svenningsson et al., 2005; Borgkvist and Fisone, 2007; Fisone et al., 2007; Shuto and Nishi, 2011).
Dopamine D1 receptors are known to interact and form hetero-oligomers with other neurotransmitter receptors such as dopamine D2 receptors (Lee et al., 2004; Rashid et al., 2007b), dopamine D3 receptors (Fiorentini et al., 2008; Marcellino et al., 2008), adenosine A1 receptors (Gines et al., 2000; Toda et al., 2003), and NMDA receptors (Lee et al., 2002), resulting in alteration of D1 receptor functions (Figure 2). The role of D1 receptor hetero-oligomerization will be discussed in other reviews of this special topic.
Figure 2.
D1 receptors form hetero-oligomers with other receptors. Formation of hetero-oligomers with dopamine D1 receptors and other receptors such as D2, D3, A1, and NMDA receptors is shown. Biding of these receptors induces the changes in D1 receptor function and/or localization.
D1 Receptor/Gq/PLC/IP3 Signaling
In addition to the dopamine D1 (D1A) receptor that couples to Gs/olf/AC, the presence of a dopamine D1-like receptor that couples to Gq/PLC has been proposed (Felder et al., 1989; Undie and Friedman, 1990; Wang et al., 1995; Beaulieu and Gainetdinov, 2011; Figure 1). D1-like receptors are shown to couple to both Gs/AC and Gq/PLC signaling in the striatum and frontal cortex, but solely to Gq/PLC signaling in the hippocampus and amygdala (Undie and Friedman, 1990; Wang et al., 1995; Jin et al., 2001). It has been reported that Gq/PLC-coupled D1-like receptors are coded in mRNA with different size from that of Gs/olf/AC-coupled D1 receptors (Mahan et al., 1990), and are functional in dopamine receptor D1A knockout mice (Friedman et al., 1997; Tomiyama et al., 2002), but not in dopamine D5 receptor knockout mice (Sahu et al., 2009). However, there is a report showing the lack of Gq activation in the striatal membrane from D1A receptor knockout mice (Rashid et al., 2007b). It still needs to be determined whether the cloned D1A receptor (Drd1a), different types of D1-like receptors such as the D5 receptor, or both couple(s) to Gq/PLC signaling in the striatum.
It has been demonstrated that D1 receptors form the hetero-oligomer with D2 receptors, and that the D1–D2 receptor hetero-oligomer preferentially couples to Gq/PLC signaling (Rashid et al., 2007a,b). The expression of dopamine D1 and D2 receptors are largely segregated in direct and indirect pathway neurons in the dorsal striatum, respectively (Gerfen et al., 1990; Hersch et al., 1995; Heiman et al., 2008). However, some proportion of medium spiny neurons are known to expresses both D1 and D2 receptors (Hersch et al., 1995). Gene expression analysis using single cell RT-PCR technique estimated that 40% of medium spiny neurons express both D1 and D2 receptor mRNA (Surmeier et al., 1996). Recently, analysis using drd1a-EGFP and drd2-EGFP bacterial artificial chromosome (BAC) mice revealed that D1 and D2 receptors are co-expressed in medium spiny neurons at 5% in the dorsal striatum, 6% in the NAc core, and 17% in the NAc shell (Bertran-Gonzalez et al., 2008). Therefore, D1 receptors likely interact with D2 receptors in some populations of medium spiny neurons, where both D1 and D2 receptors are co-expressed.
D1 and D2 receptors, co-expressed in transfected cells and striatal neurons, form the hetero-oligomer (Lee et al., 2004). The D1 and D2 receptor hetero-oligomer couples to Gq and activates PLC and intracellular Ca2+ signaling (Lee et al., 2004; Rashid et al., 2007b). For activation of Gq/PLC signaling, both D1 and D2 receptors are required, because either D1 or D2 antagonist and genetic deletion of either D1 or D2 receptors abolish the effect (Rashid et al., 2007b). It is likely that SKF83959, which selectively activate Gq/PLC signaling but not Gs/olf/PKA signaling, acts as a full agonist for D1 receptors and a partial agonist for D2 receptors in the form of hetero-oligomer (Rashid et al., 2007a,b). The functional role of the D1 and D2 receptor hetero-oligomer to activate Ca2+/calmodulin-dependent kinase IIα, which may lead to the induction of brain-derived neurotrophic factor (BDNF) expression, has been suggested (Hasbi et al., 2009), and therefore the D1 and D2 receptor hetero-oligomer is implicated as a potential therapeutic target for schizophrenia and drug addiction (Hasbi et al., 2010).
The Gs/olf/AC-coupled and Gq/PLC-coupled D1-like receptor signaling cascades likely play differential roles in dopaminergic signaling. Gq/PLC-coupled D1-like receptors show different sensitivity to benzazepine dopamine D1 receptor agonists from Gs/olf/AC-coupled D1 receptors (Undie et al., 1994). Many D1 receptor agonists such as SKF38393 and SKF81297 activate both Gs/olf/AC and Gq/PLC signaling (Undie et al., 1994). In contrast, SKF83959 preferentially stimulates Gq/PLC-coupled D1-like receptors (Panchalingam and Undie, 2001; Jin et al., 2003), and SKF83822 selectively stimulates Gs/olf/AC-coupled D1 receptors (Undie et al., 1994). By utilizing SKF83959 and SKF83822, we investigated the D1-like receptor signaling cascades, which regulate DARPP-32 phosphorylation at Thr34 (the PKA-site) in mouse striatal slices (Kuroiwa et al., 2008). Dopamine D1 receptor agonists activate at least three D1-like receptor signaling cascades in striatonigral/direct pathway neurons: (i) SCH23390-sensitive Gs/olf/AC/PKA signaling, (ii) SCH23390-insensitive Gs/olf/AC/PKA signaling, and (iii) Gq/PLC signaling. Activation of SCH23390-sensitive D1 receptors stimulates AC/cAMP/PKA signaling, leading to the phosphorylation of DARPP-32 at Thr34 in striatonigral neurons. Activation of Gq-coupled D1 receptors stimulates PLC signaling, resulting in the suppression of DARPP-32 Thr34 phosphorylation.
Behavioral studies revealed that activation of D1 receptor/ Gs/olf/AC signaling by SKF83822 mediates sniffing, locomotion, rearing, stereotypy, and seizure (O’Sullivan et al., 2004), whereas activation of D1-like receptor/Gq/PLC signaling by SKF83959 as well as SKF38393 mediates vacuous jaw movements and intense grooming (Deveney and Waddington, 1995; Undie et al., 2000). Molecular mechanisms by which Gq/PLC-coupled D1-like receptors regulate the function of neostriatal neurons, and their interaction with the D1 receptor/Gs/olf/AC/PKA signaling cascades need to be clarified.
D1 Receptors Coupled to Src Family Kinase Signaling
Activation of D1 receptors with simultaneous activation of NMDA receptors by drugs of abuse such as cocaine and d-amphetamine induces extracellular-signal regulated kinase (ERK) activation selectively in striatonigral/direct pathway neurons (Valjent et al., 2005; Bertran-Gonzalez et al., 2008), leading to activation of transcription for genes critical for drug-induced plasticity (Girault et al., 2007; Bertran-Gonzalez et al., 2008; Figure 1). The role of D1 receptor/PKA/DARPP-32 signaling is also implicated for ERK activation, since the inhibition of PP-1 by phospho-Thr34 DARPP-32 induces activation of mitogen-activated protein kinase/ERK kinase (MEK), which phosphorylates ERK, and inhibition of striatal-enriched tyrosine phosphatase (STEP), which dephosphorylates ERK (Valjent et al., 2005; Girault et al., 2007). In addition, the Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1), a neuronal specific activator of Ras/ERK signaling, is identified as an upstream of MEK/ERK signaling (Fasano et al., 2009). However, mechanisms for activation of NMDA receptors by drugs of abuse were not clearly understood, because the changes in extracellular glutamate levels induced by drugs of abuse were variable (Reid et al., 1997; Zhang et al., 2001). Recently, Pascoli et al. (2011) reported that D1 receptors potentiate NMDA receptor function via phosphorylation of NR2B subunit (at Tyr1472) of NMDA receptors by SFK, which is responsible for activation of the Ras-GRF1/MEK/ERK signaling cascade by cocaine (Fasano et al., 2009; Pascoli et al., 2011). The activation of SFK by D1 receptors is mediated through Gβγ subunit. The findings are in agreement with previous reports showing that D1 receptor-mediated increase in NMDA receptor currents requires NR2B subunit and SFK activity (Wittmann et al., 2005), and that activation of D1 receptors induces trafficking of NMDA receptors to the postsynaptic membrane mediated through NR2B phosphorylation by SFK, especially Fyn (Dunah and Standaert, 2001; Hallett et al., 2006). The novel D1 receptor/Gβγ/SFK/NR2B signaling cascade, which results in activation of NMDA receptors/Ca2+/Ras-GRF1/MEK/ERK signaling, likely plays a critical role in pathological conditions such as drug addiction and L-DOPA-induced dyskinesia in the animal model of Parkinson’s disease, in which NR2B tyrosine phosphorylation (Menegoz et al., 1995; Pascoli et al., 2011) and activation of ERK signaling by D1 receptors (Gerfen et al., 2002; Girault et al., 2007; Santini et al., 2007) are enhanced.
Modulation of cAMP/PKA Signaling in D1-Type Direct Pathway Neurons as Wells as D2-Type Indirect Pathway Neurons by Phosphodiesterase (PDE) Inhibition
Activation of Gs/olf-coupled dopamine D1 receptors causes an increase in cAMP synthesis, while the subsequent hydrolysis of cAMP is mediated by PDEs. PDEs are subdivided into 11 families, encoded by 21 genes (Conti and Beavo, 2007). Multiple PDEs with different substrate specificities and subcellular localization are expressed in the striatum. PDE10A, PDE1B, and PDE7B are enriched in the striatum, and PDE4 (A, B, and D isoforms), PDE2A and PDE9A, which are widely distributed in the brain, are also expressed in the striatum (Menniti et al., 2006).
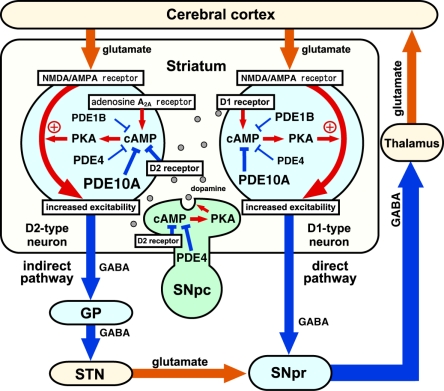
Several types of PDEs such as PDE10A, PDE4, and PDE1B are expressed in direct and indirect pathway neurons. The inhibition of PDEs can result in activation of cAMP/PKA signaling both in direct and indirect pathway neurons. If the function of the PDE (e.g., PDE1B) is predominant in direct pathway neurons, the inhibition of the PDE and activation of cAMP/PKA signaling results in activation of direct pathway neurons, leading to inhibition of GPi/SNpr inhibitory output neurons and activation of thalamocortical motor circuits. Conversely, if the function of the PDE (e.g., PDE10A and PDE4) is predominant in indirect pathway neurons, the inhibition of the PDE and activation of cAMP/PKA signaling results in activation of indirect pathway neurons, leading to activation of GPi/SNpr inhibitory output neurons and inhibition of thalamocortical motor circuits. Thus, PDE inhibitors that predominantly act in direct pathway neurons work like dopamine D1 receptor agonists and activate motor function, whereas PDE inhibitors that predominantly act in indirect pathway neurons work like dopamine D2 receptor antagonists and inhibit motor function (Nishi and Snyder, 2010). The balance of action of each PDE inhibitor in indirect and direct pathway neurons determines the behavioral effects (Figure 3).
Figure 3.
Roles of phosphodiesterases (PDEs) in the control of basal ganglia–thalamocortical circuitry. Output neurons in the striatum are medium spiny neurons (MSNs), which consist of striatonigral/direct pathway and striatopallidal/indirect pathway neurons. Direct pathway neurons are GABAergic, and inhibit tonically active neurons in globus pallidus interna (GPi)/substantia nigra pars reticulata (SNpr). Indirect pathway neurons are also GABAergic, and activate neurons in GPi/SNpr via inhibition of globus pallidus externa (GPe) GABAergic neurons and activation of subthalamic nucleus (STN) glutamatergic neurons. Direct and indirect pathway neurons induce opposing effects on the output neurons in GPi/SNpr, resulting in dis-inhibition and pro-inhibition of output, respectively, to motor areas of the thalamus and cortex. The inhibition of PDEs increases cAMP/PKA signaling in both direct and indirect pathway neurons. PDE inhibitors that predominantly act in direct pathway neurons work like dopamine D1 receptor agonists and activate motor function, whereas PDE inhibitors that predominantly act in indirect pathway neurons work like dopamine D2 receptor antagonists and inhibit motor function. SNpc, substantia nigra pars compacta. Reproduced with permission from reference Nishi and Snyder (2010).
Role of PDE10A in dopamine signaling
PDE10A is a dual substrate PDE that hydrolyzes both cAMP and cGMP, and has a higher affinity for cAMP than for cGMP by ∼20-fold (Fujishige et al., 1999; Bender and Beavo, 2006). In the striatum, PDE10A is expressed in two types of medium spiny neurons (direct and indirect pathway neurons), but not in interneurons (Xie et al., 2006; Nishi et al., 2008; Sano et al., 2008). Papaverine, an opium alkaloid primarily used for the treatment of visceral spasm and vasospasm, was found to selectively inhibit PDE10A with an IC50 of 36 nM (Siuciak et al., 2006). Papaverine was used to explore the physiological role of PDE10A in the regulation of striatal function. Recently, the potent PDE10A inhibitors, TP-10 (IC50 0.3 nM) and MP-10 (IC50 0.18 nM), were developed (Schmidt et al., 2008). Using these PDE10A inhibitors, PDE10A was shown to hydrolyze both cAMP and cGMP in the striatum in vivo (Siuciak et al., 2006; Schmidt et al., 2008; Grauer et al., 2009). We examined the effect of papaverine on the phosphorylation of PKA substrates including DARPP-32 using neostriatal slices. Papaverine robustly increased the phosphorylation of DARPP-32 at Thr34 and GluR1 at Ser845 in striatal medium spiny neurons in slices as well as in vivo (Nishi et al., 2008). The effect of papaverine was mediated through the potentiation of cAMP/PKA signaling, but not cGMP/PKG signaling. Similarly to papaverine, inhibition of PDE10A by TP-10 and/or MP-10 in the striatum in vivo was demonstrated to induce the phosphorylation of DARPP-32, GluR1, and CREB at PKA-sites (Schmidt et al., 2008; Grauer et al., 2009).
PDE10A is abundantly expressed in direct and indirect pathway neurons, and the expression levels are similar in the two types of neurons (Xie et al., 2006; Nishi et al., 2008; Sano et al., 2008). In agreement, PDE10A regulates cAMP/PKA signaling (Nishi et al., 2008) as well as gene expression (Strick et al., 2010) in both direct and indirect pathway neurons. In direct pathway neurons, PDE10A inhibition by papaverine activates cAMP/PKA signaling, leading to the potentiation of dopamine D1 receptor signaling. In indirect pathway neurons, PDE10A inhibition by papaverine also activates cAMP/PKA signaling by simultaneously potentiating adenosine A2A receptor signaling and inhibiting dopamine D2 receptor signaling. Since the balance of cAMP/PKA signaling between the direct and indirect pathways determines the output from the basal ganglia, neuronal type-specific regulation of DARPP-32 Thr34 phosphorylation was studied using neostriatal slices from D1R-DARPP-32-Flag/D2R-DARPP-32-Myc mice (Nishi et al., 2008), in which Flag-tagged DARPP-32 and Myc-tagged DARPP-32 are expressed selectively in direct and indirect pathway neurons under the control of D1 and D2 receptor promoters, respectively (Bateup et al., 2008). PDE10A inhibition by papaverine increases Myc-tagged DARPP-32 phosphorylation sixfold in indirect pathways, whereas Flag-tagged DARPP-32 phosphorylation only twofold in direct pathway neurons. Thus, PDE10A inhibitors activate cAMP/PKA signaling in indirect and direct pathway neurons, but the action of PDE10A inhibitors predominates in indirect pathway neurons. A recent electrophysiological study showing that PDE10A inhibition has greater facilitatory effect on corticostriatal synaptic activity in indirect pathway neurons supports the interpretation (Threlfell et al., 2009). The biochemical features of PDE10A inhibitors resemble those of antipsychotic drugs, which act primarily as D2 receptor antagonists and increase DARPP-32 phosphorylation in indirect pathway neurons (Bateup et al., 2008). In agreement, PDE10A inhibition by papaverine, TP-10 and MP-10 and genetic deletion of PDE10A display behavioral phenotypes of antipsychotics with therapeutic potency for negative symptoms and cognitive deficits as well as positive symptoms in schizophrenics (Sano et al., 2008; Grauer et al., 2009; Nishi and Snyder, 2010).
Role of PDE4 in dopamine signaling
PDE4 is a cAMP-specific PDE with high affinity for cAMP (Km 1–10 μM; Beavo, 1995; Bender and Beavo, 2006). The PDE4 family is encoded by four genes (PDE4A–PDE4D), and each isoform has multiple variants (Houslay and Adams, 2003; McCahill et al., 2008). In the CNS, PDE4A, PDE4B, and PDE4D are widely distributed, but the expression of PDE4C is restricted to the olfactory bulb in rodent brain (Cherry and Davis, 1999; Perez-Torres et al., 2000). Each PDE4 variant has a modular structure consisting of a variant-specific N-terminal domain, regulatory domains termed upstream conserved region 1 (UCR1) and UCR2, and a catalytic domain. The phosphorylation of UCR1 by PKA disrupts the inhibitory interaction of UCR2 with the catalytic domain and activates PDE4 activity (McCahill et al., 2008), leading to the downregulation of cAMP/PKA signaling. The N-terminal domain and UCR1/2 interact with PDE4 variant-specific binding proteins including A-kinase anchor proteins (AKAP) and disrupted in schizophrenia 1 (DISC1), leading to the compartmentalization of cAMP signaling in cells (McCahill et al., 2008; Houslay, 2010). The involvement of PDE4B in the molecular mechanisms of schizophrenia is supported by its interaction with DISC1 (Millar et al., 2005; Clapcote et al., 2007; Murdoch et al., 2007), which is a genetic susceptibility factor for schizophrenia (Chubb et al., 2008).
Function of PDE4 has been analyzed using a selective PDE4 inhibitor, rolipram (IC50 1 μM; Bender and Beavo, 2006). Inhibition of PDE4 by rolipram robustly increases tyrosine hydroxylase (TH) phosphorylation at Ser40 (PKA-site) at dopaminergic terminals in neostriatal slices and in vivo, leading to the enhancement of dopamine synthesis and metabolism (Nishi et al., 2008). In addition, the inhibition of PDE4 by rolipram weakly enhances cAMP/PKA signaling in striatal neurons, leading to the phosphorylation of DARPP-32 at Thr34 in neostriatal slices (Nishi et al., 2008). Rolipram treatment augments adenosine A2A receptor-mediated phosphorylation of DARPP-32 at Thr34, but has no effect on dopamine D1 receptor-mediated phosphorylation. However, in neostriatal slices from D1R-Flag/D2R-Myc DARPP-32 mice, rolipram induces the phosphorylation of both Flag- and Myc-tagged DARPP-32 in direct and indirect pathway neurons, respectively. Immunohistochemical analysis in D1R-Flag/D2R-Myc DARPP-32 mice revealed that PDE4B expression is higher in indirect pathway neurons than that in direct pathway neurons. These data suggest that PDE4 preferentially regulates cAMP/PKA signaling coupled to adenosine A2A receptors in indirect pathway neurons compared to that coupled to dopamine D1 receptors in direct pathway neurons. Activation of cAMP/PKA signaling in indirect pathway neurons elicited by the PDE4 inhibitor, rolipram, is expected to oppose dopamine D2 receptor signaling. At the same time, rolipram stimulates dopamine synthesis, indicating that PDE4 inhibition raises dopaminergic tone in the striatum. Therefore, rolipram mimics the biochemical effects of dopamine D2 antagonists and to some extent D1 agonists.
The pharmacological profile of the PDE4 inhibitor, including positive effects on mood and cognition, supports its possible efficacy for the treatment of negative symptoms and cognitive deficits in addition to positive symptoms in schizophrenics (Siuciak, 2008). However, the studies in PDE4B knockout mice generally fail to recapitulate the antipsychotic effects of rolipram, and the discrepancy might be explained by the lack of PDE4B selectivity of rolipram and chronic compensatory mechanisms for PDE4B gene deletion (Siuciak, 2008; Nishi and Snyder, 2010).
Role of PDE1B in dopamine signaling
PDE1B is a dual substrate PDE with a higher affinity for cGMP (Km 2.4 μM) than for cAMP (Km 24 μM; Bender and Beavo, 2006). PDE1B is activated by Ca2+ and calmodulin, providing a mechanism for crosstalk between Ca2+ and cyclic nucleotide signaling. PDE1B is abundantly expressed in the striatum (Polli and Kincaid, 1994), and striatal PDE1B is localized to all DARPP-32-positive medium spiny neurons, indicating the PDE1B expression in both direct and indirect pathway neurons (A. Nishi and M. Kuroiwa, unpublished observations). Biochemical studies in PDE1B knockout mice revealed that the function of dopamine D1 receptors to stimulate the phosphorylation of DARPP-32 and GluR1 at PKA-sites is potentiated in striatal slices from PDE1B knockout mice (Reed et al., 2002). In behavioral analysis, PDE1B knockout mice exhibited the increase in spontaneous locomotor activity and psychostimulant- and NMDA receptor antagonist-stimulated locomotor activity and the cognitive deficit in Morris water maze test (Reed et al., 2002; Ehrman et al., 2006; Siuciak et al., 2007). In spite of similar expression pattern of PDE1B with PDE10A in the striatum, the behavioral profiles of PDE1B knockout mice are pro-psychotic and completely opposite to those of PDE10A knockout mice. We hypothesize that PDE1B predominantly regulates cyclic nucleotide signaling in direct pathway neurons, whereas a predominant role of PDE10A and PDE4 in indirect pathway neurons.
Conclusion
D1 receptor signaling in striatonigral/direct pathway neurons plays an essential role in motor functions (Gerfen and Surmeier, 2011) as well as reward and cognition (Bromberg-Martin et al., 2010; Cools, 2011). The alterations of D1 receptor signaling are implicated in drug addiction (Shuto and Nishi, 2011) and L-DOPA-induced dyskinesia (Fisone et al., 2007). Besides the importance of D1 receptor signaling under pathophysiological conditions, dopaminergic drugs are mainly acting on D2 receptors, and therapeutic agents that selectively modulate D1 receptor signaling are not currently utilized. Functional roles of the canonical and non-canonical D1 receptor signaling cascades are implicated in the same category of diseases. Further understanding of each D1 receptor signaling cascade and its regulation is required for the development of therapeutic agents targeting D1 receptor signaling.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Ahn J. H., McAvoy T., Rakhilin S. V., Nishi A., Greengard P., Nairn A. C. (2007a). Protein kinase A activates protein phosphatase 2A by phosphorylation of the B56delta subunit. Proc. Natl. Acad. Sci. U.S.A. 104, 2979–2984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn J. H., Sung J. Y., McAvoy T., Nishi A., Janssens V., Goris J., Greengard P., Nairn A. C. (2007b). The B”/PR72 subunit mediates Ca2+-dependent dephosphorylation of DARPP-32 by protein phosphatase 2A. Proc. Natl. Acad. Sci. U.S.A. 104, 9876–9881 10.1073/pnas.0611532104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H. S., Santini E., Shen W., Birnbaum S., Valjent E., Surmeier D. J., Fisone G., Nestler E. J., Greengard P. (2010). Distinct subclasses of medium spiny neurons differentially regulate striatal motor behaviors. Proc. Natl. Acad. Sci. U.S.A. 107, 14845–14850 10.1073/pnas.1009874107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateup H. S., Svenningsson P., Kuroiwa M., Gong S., Nishi A., Heintz N., Greengard P. (2008). Cell type-specific regulation of DARPP-32 phosphorylation by psychostimulant and antipsychotic drugs. Nat. Neurosci. 11, 932–939 10.1038/nn.2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J. M., Gainetdinov R. R. (2011). The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 63, 182–217 10.1124/pr.110.002642 [DOI] [PubMed] [Google Scholar]
- Beavo J. A. (1995). Cyclic nucleotide phosphodiesterases: functional implications of multiple isoforms. Physiol. Rev. 75, 725–748 [DOI] [PubMed] [Google Scholar]
- Bender A. T., Beavo J. A. (2006). Cyclic nucleotide phosphodiesterases: molecular regulation to clinical use. Pharmacol. Rev. 58, 488–520 10.1124/pr.58.3.5 [DOI] [PubMed] [Google Scholar]
- Bertran-Gonzalez J., Bosch C., Maroteaux M., Matamales M., Herve D., Valjent E., Girault J. A. (2008). Opposing patterns of signaling activation in dopamine D1 and D2 receptor-expressing striatal neurons in response to cocaine and haloperidol. J. Neurosci. 28, 5671–5685 10.1523/JNEUROSCI.1039-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertran-Gonzalez J., Herve D., Girault J. A., Valjent E. (2010). What is the degree of segregation between striatonigral and striatopallidal projections? Front. Neuroanat. 4:136. 10.3389/fnana.2010.00136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibb J. A., Chen J., Taylor J. R., Svenningsson P., Nishi A., Snyder G. L., Yan Z., Sagawa Z. K., Ouimet C. C., Nairn A. C., Nestler E. J., Greengard P. (2001). Effects of chronic exposure to cocaine are regulated by the neuronal protein Cdk5. Nature 410, 376–380 10.1038/35066591 [DOI] [PubMed] [Google Scholar]
- Bibb J. A., Snyder G. L., Nishi A., Yan Z., Meijer L., Fienberg A. A., Tsai L. H., Kwon Y. T., Girault J. A., Czernik A. J., Huganir R. L., Hemmings H. C., Jr., Nairn A. C., Greengard P. (1999). Phosphorylation of DARPP-32 by Cdk5 modulates dopamine signalling in neurons. Nature 402, 669–671 10.1038/45251 [DOI] [PubMed] [Google Scholar]
- Borgkvist A., Fisone G. (2007). Psychoactive drugs and regulation of the cAMP/PKA/DARPP-32 cascade in striatal medium spiny neurons. Neurosci. Biobehav. Rev. 31, 79–88 10.1016/j.neubiorev.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Bromberg-Martin E. S., Matsumoto M., Hikosaka O. (2010). Dopamine in motivational control: rewarding, aversive, and alerting. Neuron 68, 815–834 10.1016/j.neuron.2010.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry J. A., Davis R. L. (1999). Cyclic AMP phosphodiesterases are localized in regions of the mouse brain associated with reinforcement, movement, and affect. J. Comp. Neurol. 407, 287–301 [DOI] [PubMed] [Google Scholar]
- Chubb J. E., Bradshaw N. J., Soares D. C., Porteous D. J., Millar J. K. (2008). The DISC locus in psychiatric illness. Mol. Psychiatry 13, 36–64 10.1038/sj.mp.4002106 [DOI] [PubMed] [Google Scholar]
- Clapcote S. J., Lipina T. V., Millar J. K., Mackie S., Christie S., Ogawa F., Lerch J. P., Trimble K., Uchiyama M., Sakuraba Y., Kaneda H., Shiroishi T., Houslay M. D., Henkelman R. M., Sled J. G., Gondo Y., Porteous D. J., Roder J. C. (2007). Behavioral phenotypes of Disc1 missense mutations in mice. Neuron 54, 387–402 10.1016/j.neuron.2007.04.015 [DOI] [PubMed] [Google Scholar]
- Conti M., Beavo J. (2007). Biochemistry and physiology of cyclic nucleotide phosphodiesterases: essential components in cyclic nucleotide signaling. Annu. Rev. Biochem. 76, 481–511 10.1146/annurev.biochem.76.060305.150444 [DOI] [PubMed] [Google Scholar]
- Cools R. (2011). Dopaminergic control of the striatum for high-level cognition. Curr. Opin. Neurobiol. 21, 402–407 [DOI] [PubMed] [Google Scholar]
- Desdouits F., Siciliano J. C., Greengard P., Girault J.-A. (1995a). Dopamine- and cAMP-regulated phosphoprotein DARPP-32: phosphorylation of Ser-137 by casein kinase I inhibits dephosphorylation of Thr-34 by calcineurin. Proc. Natl. Acad. Sci. U.S.A. 92, 2682–2685 10.1073/pnas.92.7.2682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desdouits F., Cohen D., Nairn A. C., Greengard P., Girault J.-A. (1995b). Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase I in vitro and in vivo. J. Biol. Chem. 270, 8772–8778 10.1074/jbc.270.15.8772 [DOI] [PubMed] [Google Scholar]
- Deveney A. M., Waddington J. L. (1995). Pharmacological characterization of behavioural responses to SK&F 83959 in relation to “D1-like” dopamine receptors not linked to adenylyl cyclase. Br. J. Pharmacol. 116, 2120–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunah A. W., Standaert D. G. (2001). Dopamine D1 receptor-dependent trafficking of striatal NMDA glutamate receptors to the postsynaptic membrane. J. Neurosci. 21, 5546–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrman L. A., Williams M. T., Schaefer T. L., Gudelsky G. A., Reed T. M., Fienberg A. A., Greengard P., Vorhees C. V. (2006). Phosphodiesterase 1B differentially modulates the effects of methamphetamine on locomotor activity and spatial learning through DARPP32-dependent pathways: evidence from PDE1B-DARPP32 double-knockout mice. Genes Brain Behav. 5, 540–551 10.1111/j.1601-183X.2006.00209.x [DOI] [PubMed] [Google Scholar]
- Fasano S., D’Antoni A., Orban P. C., Valjent E., Putignano E., Vara H., Pizzorusso T., Giustetto M., Yoon B., Soloway P., Maldonado R., Caboche J., Brambilla R. (2009). Ras-guanine nucleotide-releasing factor 1 (Ras-GRF1) controls activation of extracellular signal-regulated kinase (ERK) signaling in the striatum and long-term behavioral responses to cocaine. Biol. Psychiatry 66, 758–768 10.1016/j.biopsych.2009.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felder C. C., Jose P. A., Axelrod J. (1989). The dopamine-1 agonist, SKF 82526, stimulates phospholipase-C activity independent of adenylate cyclase. J. Pharmacol. Exp. Ther. 248, 171–175 [PubMed] [Google Scholar]
- Fernandez E., Schiappa R., Girault J. A., Le Novere N. (2006). DARPP-32 is a robust integrator of dopamine and glutamate signals. PLoS Comput. Biol. 2, e176. 10.1371/journal.pcbi.0020176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fienberg A. A., Greengard P. (2000). The DARPP-32 knockout mouse. Brain Res. Brain Res. Rev. 31, 313–319 10.1016/S0165-0173(99)00047-8 [DOI] [PubMed] [Google Scholar]
- Fienberg A. A., Hiroi N., Mermelstein P., Song W.-J., Snyder G. L., Nishi A., Cheramy A., O’Callaghan J. P., Miller D., Cole D., Corbett R., Haile C., Cooper D., Onn S., Grace A. A., Ouimet C., White F. J., Hyman S. E., Surmeier D. J., Girault J. A., Nestler E., Greengard P. (1998). DARPP-32, regulator of the efficacy of dopaminergic neurotransmission. Science 281, 838–842 10.1126/science.281.5378.838 [DOI] [PubMed] [Google Scholar]
- Fiorentini C., Busi C., Gorruso E., Gotti C., Spano P., Missale C. (2008). Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 74, 59–69 10.1124/mol.107.043885 [DOI] [PubMed] [Google Scholar]
- Fisone G., Hakansson K., Borgkvist A., Santini E. (2007). Signaling in the basal ganglia: postsynaptic and presynaptic mechanisms. Physiol. Behav. 92, 8–14 10.1016/j.physbeh.2007.05.028 [DOI] [PubMed] [Google Scholar]
- Friedman E., Jin L. Q., Cai G. P., Hollon T. R., Drago J., Sibley D. R., Wang H. Y. (1997). D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: evidence from D1A knockout mice. Mol. Pharmacol. 51, 6–11 [DOI] [PubMed] [Google Scholar]
- Fujishige K., Kotera J., Michibata H., Yuasa K., Takebayashi S., Okumura K., Omori K. (1999). Cloning and characterization of a novel human phosphodiesterase that hydrolyzes both cAMP and cGMP (PDE10A). J. Biol. Chem. 274, 18438–18445 [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Engber T. M., Mahan L. C., Susel Z., Chase T. M., Monsma F. J., Jr., Sibley D. R. (1990). D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 250, 1429–1432 10.1126/science.2147780 [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Miyachi S., Paletzki R., Brown P. (2002). D1 dopamine receptor supersensitivity in the dopamine-depleted striatum results from a switch in the regulation of ERK1/2/MAP kinase. J. Neurosci. 22, 5042–5054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R., Surmeier D. J. (2011). Modulation of striatal projection systems by dopamine. Annu. Rev. Neurosci. 34, 441–466 10.1146/annurev-neuro-061010-113641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gines S., Hillion J., Torvinen M., Le Crom S., Casado V., Canela E. I., Rondin S., Lew J. Y., Watson S., Zoli M., Agnati L. F., Verniera P., Lluis C., Ferre S., Fuxe K., Franco R. (2000). Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. U.S.A. 97, 8606–8611 10.1073/pnas.150241097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault J. A., Valjent E., Caboche J., Herve D. (2007). ERK2: a logical AND gate critical for drug-induced plasticity? Curr. Opin. Pharmacol. 7, 77–85 10.1016/j.coph.2006.08.012 [DOI] [PubMed] [Google Scholar]
- Girault J.-A., Hemmings H. C., Jr., Williams K. R., Nairn A. C., Greengard P. (1989). Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphorprotein, by casein kinase II. J. Biol. Chem. 264, 21748–21759 [PubMed] [Google Scholar]
- Grauer S. M., Pulito V. L., Navarra R. L., Kelly M., Kelley C., Graf R., Langen B., Logue S., Brennan J., Jiang L., Charych E., Egerland U., Liu F., Marquis K. L., Malamas M., Hage T., Comery T. A., Brandon N. J. (2009). PDE10A inhibitor activity in preclinical models of the positive, cognitive and negative symptoms of schizophrenia. J. Pharmacol. Exp. Ther. 331, 574–590 10.1124/jpet.109.155994 [DOI] [PubMed] [Google Scholar]
- Greengard P., Allen P. B., Nairn A. C. (1999). Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron 23, 435–447 10.1016/S0896-6273(00)80798-9 [DOI] [PubMed] [Google Scholar]
- Hallett P. J., Spoelgen R., Hyman B. T., Standaert D. G., Dunah A. W. (2006). Dopamine D1 activation potentiates striatal NMDA receptors by tyrosine phosphorylation-dependent subunit trafficking. J. Neurosci. 26, 4690–4700 10.1523/JNEUROSCI.0792-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpain S., Girault J.-A., Greengard P. (1990). Activation of NMDA receptors induced dephosphorylation of DARPP-32 in rat striatal slices. Nature 343, 369–372 10.1038/343369a0 [DOI] [PubMed] [Google Scholar]
- Hara M., Fukui R., Hieda E., Kuroiwa M., Bateup H. S., Kano T., Greengard P., Nishi A. (2010). Role of adrenoceptors in the regulation of dopamine/DARPP-32 signaling in neostriatal neurons. J. Neurochem. 113, 1046–1059 10.1111/j.1471-4159.2010.06668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M. L., O’Dowd B. F., George S. R. (2009). Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. U.S.A. 106, 21377–21382 10.1073/pnas.0903676106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasbi A., O’Dowd B. F., George S. R. (2010). Heteromerization of dopamine D2 receptors with dopamine D1 or D5 receptors generates intracellular calcium signaling by different mechanisms. Curr. Opin. Pharmacol. 10, 93–99 10.1016/j.coph.2009.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M., Schaefer A., Gong S., Peterson J. D., Day M., Ramsey K. E., Suarez-Farinas M., Schwarz C., Stephan D. A., Surmeier D. J., Greengard P., Heintz N. (2008). A translational profiling approach for the molecular characterization of CNS cell types. Cell 135, 738–748 10.1016/j.cell.2008.10.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Lopez S., Tkatch T., Perez-Garci E., Galarraga E., Bargas J., Hamm H., Surmeier D. J. (2000). D2 dopamine receptors in striatal medium spiny neurons reduce L-type Ca2+ currents and excitability via a novel PLC[beta]1-IP3-calcineurin-signaling cascade. J. Neurosci. 20, 8987–8995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hersch S. M., Ciliax B. J., Gutekunst C. A., Rees H. D., Heilman C. J., Yung K. K., Bolam J. P., Ince E., Yi H., Levey A. I. (1995). Electron microscopic analysis of D1 and D2 dopamine receptor proteins in the dorsal striatum and their synaptic relationships with motor corticostriatal afferents. J. Neurosci. 15, 5222–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve D., Le Moine C., Corvol J. C., Belluscio L., Ledent C., Fienberg A. A., Jaber M., Studler J. M., Girault J. A. (2001). Galpha(olf) levels are regulated by receptor usage and control dopamine and adenosine action in the striatum. J. Neurosci. 21, 4390–4399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay M. D. (2010). Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci. 35, 91–100 10.1016/j.tibs.2009.09.007 [DOI] [PubMed] [Google Scholar]
- Houslay M. D., Adams D. R. (2003). PDE4 cAMP phosphodiesterases: modular enzymes that orchestrate signalling cross-talk, desensitization and compartmentalization. Biochem. J. 370, 1–18 10.1042/BJ20021698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman S. E., Malenka R. C. (2001). Addiction and the brain: the neurobiology of compulsion and its persistence. Nat. Rev. Neurosci. 2, 695–703 10.1038/35094560 [DOI] [PubMed] [Google Scholar]
- Jackson D. M., Westlind-Danielsson A. (1994). Dopamine receptors: molecular biology, biochemistry and behavioural aspects. Pharmacol. Ther. 64, 291–370 10.1016/0163-7258(94)90041-8 [DOI] [PubMed] [Google Scholar]
- Janssens V., Jordens J., Stevens I., Van Hoof C., Martens E., De Smedt H., Engelborghs Y., Waelkens E., Goris J. (2003). Identification and functional analysis of two Ca2+-binding EF-hand motifs in the B”/PR72 subunit of protein phosphatase 2A. J. Biol. Chem. 278, 10697–10706 10.1074/jbc.M208857200 [DOI] [PubMed] [Google Scholar]
- Jin L. Q., Goswami S., Cai G., Zhen X., Friedman E. (2003). SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J. Neurochem. 85, 378–386 10.1046/j.1471-4159.2003.01698.x [DOI] [PubMed] [Google Scholar]
- Jin L. Q., Wang H. Y., Friedman E. (2001). Stimulated D(1) dopamine receptors couple to multiple Galpha proteins in different brain regions. J. Neurochem. 78, 981–990 10.1046/j.1471-4159.2001.00470.x [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. (1979). Multiple receptors for dopamine. Nature 277, 93–96 10.1038/277093a0 [DOI] [PubMed] [Google Scholar]
- Kitaoka S., Furuyashiki T., Nishi A., Shuto T., Koyasu S., Matsuoka T., Miyasaka M., Greengard P., Narumiya S. (2007). Prostaglandin E2 acts on EP1 receptor and amplifies both dopamine D1 and D2 receptor signaling in the striatum. J. Neurosci. 27, 12900–12907 10.1523/JNEUROSCI.3257-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa M., Bateup H. S., Shuto T., Higashi H., Tanaka M., Nishi A. (2008). Regulation of DARPP-32 phosphorylation by three distinct dopamine D1-like receptor signaling pathways in the neostriatum. J. Neurochem. 107, 1014–1026 [DOI] [PubMed] [Google Scholar]
- Lee F. J., Xue S., Pei L., Vukusic B., Chery N., Wang Y., Wang Y. T., Niznik H. B., Yu X. M., Liu F. (2002). Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell 111, 219–230 10.1016/S0092-8674(02)00962-5 [DOI] [PubMed] [Google Scholar]
- Lee S. P., So C. H., Rashid A. J., Varghese G., Cheng R., Lanca A. J., O’Dowd B. F., George S. R. (2004). Dopamine D1 and D2 receptor co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 279, 35671–35678 10.1074/jbc.M406611200 [DOI] [PubMed] [Google Scholar]
- Lindskog M., Svenningsson P., Fredholm B., Greengard P., Fisone G. (1999). Mu- and delta-opioid receptor agonists inhibit DARPP-32 phosphorylation in distinct populations of striatal projection neurons. Eur. J. Neurosci. 11, 2182–2186 10.1046/j.1460-9568.1999.00597.x [DOI] [PubMed] [Google Scholar]
- Lindskog M., Svenningsson P., Pozzi L., Kim Y., Fienberg A. A., Bibb J. A., Fredholm B. B., Nairn A. C., Greengard P., Fisone G. (2002). Involvement of DARPP-32 phosphorylation in the stimulant action of caffeine. Nature 418, 774–778 10.1038/nature00817 [DOI] [PubMed] [Google Scholar]
- Mahan L. C., Burch R. M., Monsma F. J., Jr., Sibley D. R. (1990). Expression of striatal D1 dopamine receptors coupled to inositol phosphate production and Ca2+ mobilization in Xenopus oocytes. Proc. Natl. Acad. Sci. U.S.A. 87, 2196–2200 10.1073/pnas.87.6.2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcellino D., Ferre S., Casado V., Cortes A., Le Foll B., Mazzola C., Drago F., Saur O., Stark H., Soriano A., Barnes C., Goldberg S. R., Lluis C., Fuxe K., Franco R. (2008). Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 283, 26016–26025 10.1074/jbc.M710349200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCahill A. C., Huston E., Li X., Houslay M. D. (2008). PDE4 associates with different scaffolding proteins: modulating interactions as treatment for certain diseases. Handb. Exp. Pharmacol. 186, 125–166 [DOI] [PubMed] [Google Scholar]
- Menegoz M., Lau L. F., Herve D., Huganir R. L., Girault J. A. (1995). Tyrosine phosphorylation of NMDA receptor in rat striatum: effects of 6-OH-dopamine lesions. Neuroreport 7, 125–128 10.1097/00001756-199512000-00030 [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Faraci W. S., Schmidt C. J. (2006). Phosphodiesterases in the CNS: targets for drug development. Nat. Rev. Drug Discov. 5, 660–670 10.1038/nrd2058 [DOI] [PubMed] [Google Scholar]
- Millar J. K., Pickard B. S., Mackie S., James R., Christie S., Buchanan S. R., Malloy M. P., Chubb J. E., Huston E., Baillie G. S., Thomson P. A., Hill E. V., Brandon N. J., Rain J. C., Camargo L. M., Whiting P. J., Houslay M. D., Blackwood D. H., Muir W. J., Porteous D. J. (2005). DISC1 and PDE4B are interacting genetic factors in schizophrenia that regulate cAMP signaling. Science 310, 1187–1191 10.1126/science.1112915 [DOI] [PubMed] [Google Scholar]
- Missale C., Nash S. R., Robinson S. W., Jaber M., Caron M. G. (1998). Dopamine receptors: from structure to function. Physiol. Rev. 78, 189–225 [DOI] [PubMed] [Google Scholar]
- Murdoch H., Mackie S., Collins D. M., Hill E. V., Bolger G. B., Klussmann E., Porteous D. J., Millar J. K., Houslay M. D. (2007). Isoform-selective susceptibility of DISC1/phosphodiesterase-4 complexes to dissociation by elevated intracellular cAMP levels. J. Neurosci. 27, 9513–9524 10.1523/JNEUROSCI.1493-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Bibb J. A., Matsuyama S., Hamada M., Higashi H., Nairn A. C., Greengard P. (2002). Regulation of DARPP-32 dephosphorylation at PKA- and Cdk5-sites by NMDA and AMPA receptors: distinct roles of calcineurin and protein phosphatase-2A. J. Neurochem. 81, 832–841 10.1046/j.1471-4159.2002.00876.x [DOI] [PubMed] [Google Scholar]
- Nishi A., Bibb J. A., Snyder G. L., Higashi H., Nairn A. C., Greengard P. (2000). Amplification of dopaminergic signaling by a positive feedback loop. Proc. Natl. Acad. Sci. U.S.A. 97, 12840–12845 10.1073/pnas.220410397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Kuroiwa M., Miller D. B., O’Callaghan J. P., Bateup H. S., Shuto T., Sotogaku N., Fukuda T., Heintz N., Greengard P., Snyder G. L. (2008). Distinct roles of PDE4 and PDE10A in the regulation of cAMP/PKA signaling in the striatum. J. Neurosci. 28, 10460–10471 10.1523/JNEUROSCI.2518-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Snyder G. L. (2010). Advanced research on dopamine signaling to develop drugs for the treatment of mental disorders: biochemical and behavioral profiles of phosphodiesterase inhibition in dopaminergic neurotransmission. J. Pharmacol. Sci. 114, 6–16 10.1254/jphs.10R01FM [DOI] [PubMed] [Google Scholar]
- Nishi A., Snyder G. L., Greengard P. (1997). Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J. Neurosci. 17, 8147–8155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi A., Snyder G. L., Nairn A. C., Greengard P. (1999). Role of calcineurin and protein phosphatase-2A in the regulation of DARPP-32 dephosphorylation in neostriatal neurons. J. Neurochem. 72, 2015–2021 10.1046/j.1471-4159.1999.0722015.x [DOI] [PubMed] [Google Scholar]
- Nishi A., Watanabe Y., Higashi H., Tanaka M., Nairn A. C., Greengard P. (2005). Glutamate regulation of DARPP-32 phosphorylation in neostriatal neurons involves activation of multiple signaling cascades. Proc. Natl. Acad. Sci. U.S.A. 102, 1199–1204 10.1073/pnas.0409138102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan G. J., Roth B. L., Kinsella A., Waddington J. L. (2004). SK&F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioural topography. Eur. J. Pharmacol. 486, 273–280 10.1016/j.ejphar.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Ouimet C. C., Greengard P. (1990). Distribution of DARPP-32 in the basal ganglia: an electron microscopic study. J. Neurocytol. 19, 39–52 10.1007/BF01188438 [DOI] [PubMed] [Google Scholar]
- Panchalingam S., Undie A. S. (2001). SKF83959 exhibits biochemical agonism by stimulating [(35)S]GTP gamma S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacology 40, 826–837 10.1016/S0028-3908(01)00011-9 [DOI] [PubMed] [Google Scholar]
- Pascoli V., Besnard A., Herve D., Pages C., Heck N., Girault J. A., Caboche J., Vanhoutte P. (2011). Cyclic adenosine monophosphate- independent tyrosine phosphorylation of NR2B mediates cocaine-induced extracellular signal-regulated kinase activation. Biol. Psychiatry 69, 218–227 10.1016/j.biopsych.2010.08.031 [DOI] [PubMed] [Google Scholar]
- Perez-Torres S., Miro X., Palacios J. M., Cortes R., Puigdomenech P., Mengod G. (2000). Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and[3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J. Chem. Neuroanat. 20, 349–374 10.1016/S0891-0618(00)00097-1 [DOI] [PubMed] [Google Scholar]
- Polli J. W., Kincaid R. L. (1994). Expression of calmodulin-dependent phosphodiesterase isoform (PDE1B1) correlates with brain regions having extensive dopaminergic innervation. J. Neurosci. 14, 1251–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashid A. J., O’Dowd B. F., Verma V., George S. R. (2007a). Neuronal Gq/11-coupled dopamine receptors: an uncharted role for dopamine. Trends Pharmacol. Sci. 28, 551–555 10.1016/j.tips.2007.10.001 [DOI] [PubMed] [Google Scholar]
- Rashid A. J., So C. H., Kong M. M., Furtak T., El-Ghundi M., Cheng R., O’Dowd B. F., George S. R. (2007b). D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. U.S.A. 104, 654–659 10.1073/pnas.0604049104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebholz H., Nishi A., Liebscher S., Nairn A. C., Flajolet M., Greengard P. (2009). CK2 negatively regulates Galphas signaling. Proc. Natl. Acad. Sci. U.S.A. 106, 14096–14101 10.1073/pnas.0906857106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed T. M., Repaske D. R., Snyder G. L., Greengard P., Vorhees C. V. (2002). Phosphodiesterase 1B knock-out mice exhibit exaggerated locomotor hyperactivity and DARPP-32 phosphorylation in response to dopamine agonists and display impaired spatial learning. J. Neurosci. 22, 5188–5197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid M. S., Hsu K., Jr., Berger S. P. (1997). Cocaine and amphetamine preferentially stimulate glutamate release in the limbic system: studies on the involvement of dopamine. Synapse 27, 95–105 [DOI] [PubMed] [Google Scholar]
- Sahu A., Tyeryar K. R., Vongtau H. O., Sibley D. R., Undieh A. S. (2009). D5 dopamine receptors are required for dopaminergic activation of phospholipase C. Mol. Pharmacol. 75, 447–453 10.1124/mol.108.053017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano H., Nagai Y., Miyakawa T., Shigemoto R., Yokoi M. (2008). Increased social interaction in mice deficient of the striatal medium spiny neuron-specific phosphodiesterase 10A2. J. Neurochem. 105, 546–556 10.1111/j.1471-4159.2007.05152.x [DOI] [PubMed] [Google Scholar]
- Santini E., Valjent E., Usiello A., Carta M., Borgkvist A., Girault J. A., Herve D., Greengard P., Fisone G. (2007). Critical involvement of cAMP/DARPP-32 and extracellular signal-regulated protein kinase signaling in L-DOPA-induced dyskinesia. J. Neurosci. 27, 6995–7005 10.1523/JNEUROSCI.0852-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C. J., Chapin D. S., Cianfrogna J., Corman M. L., Hajos M., Harms J. F., Hoffman W. E., Lebel L. A., McCarthy S. A., Nelson F. R., Proulx-LaFrance C., Majchrzak M. J., Ramirez A. D., Schmidt K., Seymour P. A., Siuciak J. A., Tingley F. D., III, Williams R. D., Verhoest P. R., Menniti F. S. (2008). Preclinical characterization of selective phosphodiesterase 10A inhibitors: a new therapeutic approach to the treatment of schizophrenia. J. Pharmacol. Exp. Ther. 325, 681–690 10.1124/jpet.107.132910 [DOI] [PubMed] [Google Scholar]
- Schmitz Y., Benoit-Marand M., Gonon F., Sulzer D. (2003). Presynaptic regulation of dopaminergic neurotransmission. J. Neurochem. 87, 273–289 10.1046/j.1471-4159.2003.02050.x [DOI] [PubMed] [Google Scholar]
- Shuto T., Nishi A. (2011). Treatment of the psychostimulant-sensitized animal model of schizophrenia. CNS Neurosci. Ther. 17, 133–139 10.1111/j.1755-5949.2010.00218.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siuciak J. A. (2008). The role of phosphodiesterases in schizophrenia: therapeutic implications. CNS Drugs 22, 983–993 10.2165/0023210-200822120-00002 [DOI] [PubMed] [Google Scholar]
- Siuciak J. A., Chapin D. S., Harms J. F., Lebel L. A., McCarthy S. A., Chambers L., Shrikhande A., Wong S., Menniti F. S., Schmidt C. J. (2006). Inhibition of the striatum-enriched phosphodiesterase PDE10A: a novel approach to the treatment of psychosis. Neuropharmacology 51, 386–396 10.1016/j.neuropharm.2006.01.012 [DOI] [PubMed] [Google Scholar]
- Siuciak J. A., McCarthy S. A., Chapin D. S., Reed T. M., Vorhees C. V., Repaske D. R. (2007). Behavioral and neurochemical characterization of mice deficient in the phosphodiesterase-1B (PDE1B) enzyme. Neuropharmacology 53, 113–124 10.1016/j.neuropharm.2007.04.009 [DOI] [PubMed] [Google Scholar]
- Stipanovich A., Valjent E., Matamales M., Nishi A., Ahn J. H., Maroteaux M., Bertran-Gonzalez J., Brami-Cherrier K., Enslen H., Corbille A. G., Filhol O., Nairn A. C., Greengard P., Herve D., Girault J. A. (2008). A phosphatase cascade by which rewarding stimuli control nucleosomal response. Nature 453, 879–884 10.1038/nature06994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick C. A., James L. C., Fox C. B., Seeger T. F., Menniti F. S., Schmidt C. J. (2010). Alterations in gene regulation following inhibition of the striatum-enriched phosphodiesterase, PDE10A. Neuropharmacology 58, 444–451 10.1016/j.neuropharm.2009.09.008 [DOI] [PubMed] [Google Scholar]
- Sulzer D. (2011). How addictive drugs disrupt presynaptic dopamine neurotransmission. Neuron 69, 628–649 10.1016/j.neuron.2011.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier D. J., Song W. J., Yan Z. (1996). Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 16, 6579–6591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Nairn A. C., Greengard P. (2005). DARPP-32 mediates the actions of multiple drugs of abuse. AAPS J. 7, E353–E360 10.1208/aapsj070235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Nishi A., Fisone G., Girault J. A., Nairn A. C., Greengard P. (2004). DARPP-32: an integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 44, 269–296 10.1146/annurev.pharmtox.44.101802.121415 [DOI] [PubMed] [Google Scholar]
- Svenningsson P., Tzavara E. T., Liu F., Fienberg A. A., Nomikos G. G., Greengard P. (2002). DARPP-32 mediates serotonergic neurotransmission in the forebrain. Proc. Natl. Acad. Sci. U.S.A. 99, 3188–3193 10.1073/pnas.052712699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenningsson P., Tzavara E. T., Qi H., Carruthers R., Witkin J. M., Nomikos G. G., Greengard P. (2007). Biochemical and behavioral evidence for antidepressant-like effects of 5-HT6 receptor stimulation. J. Neurosci. 27, 4201–4209 10.1523/JNEUROSCI.3110-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanda K., Nishi A., Matsuo N., Nakanishi K., Yamasaki N., Sugimoto T., Toyama K., Takao K., Miyakawa T. (2009). Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol. Brain 2, 19. 10.1186/1756-6606-2-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfell S., Sammut S., Menniti F. S., Schmidt C. J., West A. R. (2009). Inhibition of phosphodiesterase 10A increases the responsiveness of striatal projection neurons to cortical stimulation. J. Pharmacol. Exp. Ther. 328, 785–795 10.1124/jpet.108.146332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda S., Alguacil L. F., Kalivas P. W. (2003). Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J. Neurochem. 87, 1478–1484 10.1046/j.1471-4159.2003.02121.x [DOI] [PubMed] [Google Scholar]
- Tomiyama K., McNamara F. N., Clifford J. J., Kinsella A., Drago J., Tighe O., Croke D. T., Koshikawa N., Waddington J. L. (2002). Phenotypic resolution of spontaneous and D1-like agonist-induced orofacial movement topographies in congenic dopamine D1A receptor “knockout” mice. Neuropharmacology 42, 644–652 10.1016/S0028-3908(02)00023-0 [DOI] [PubMed] [Google Scholar]
- Undie A. S., Berki A. C., Beardsley K. (2000). Dopaminergic behaviors and signal transduction mediated through adenylate cyclase and phospholipase C pathways. Neuropharmacology 39, 75–87 10.1016/S0028-3908(99)00106-9 [DOI] [PubMed] [Google Scholar]
- Undie A. S., Friedman E. (1990). Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J. Pharmacol. Exp. Ther. 253, 987–992 [PubMed] [Google Scholar]
- Undie A. S., Weinstock J., Sarau H. M., Friedman E. (1994). Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in brain. J. Neurochem. 62, 2045–2048 10.1046/j.1471-4159.1994.62052045.x [DOI] [PubMed] [Google Scholar]
- Valjent E., Bertran-Gonzalez J., Herve D., Fisone G., Girault J. A. (2009). Looking BAC at striatal signaling: cell-specific analysis in new transgenic mice. Trends Neurosci. 32, 538–547 10.1016/j.tins.2009.06.005 [DOI] [PubMed] [Google Scholar]
- Valjent E., Pascoli V., Svenningsson P., Paul S., Enslen H., Corvol J. C., Stipanovich A., Caboche J., Lombroso P. J., Nairn A. C., Greengard P., Herve D., Girault J. A. (2005). Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc. Natl. Acad. Sci. U.S.A. 102, 491–496 10.1073/pnas.0408305102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. (1984). DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated phosphoprotein enriched in dopamine-innervated brain regions. I. Regional and cellular disrtribution in rat brain. J. Neurosci. 4, 84–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H. Y., Undie A. S., Friedman E. (1995). Evidence for the coupling of Gq protein to D1-like dopamine sites in rat striatum: possible role in dopamine-mediated inositol phosphate formation. Mol. Pharmacol. 48, 988–994 [PubMed] [Google Scholar]
- Wittmann M., Marino M. J., Henze D. A., Seabrook G. R., Conn P. J. (2005). Clozapine potentiation of N-methyl-D-aspartate receptor currents in the nucleus accumbens: role of NR2B and protein kinase A/Src kinases. J. Pharmacol. Exp. Ther. 313, 594–603 10.1124/jpet.104.080200 [DOI] [PubMed] [Google Scholar]
- Xie Z., Adamowicz W. O., Eldred W. D., Jakowski A. B., Kleiman R. J., Morton D. G., Stephenson D. T., Strick C. A., Williams R. D., Menniti F. S. (2006). Cellular and subcellular localization of PDE10A, a striatum-enriched phosphodiesterase. Neuroscience 139, 597–607 10.1016/j.neuroscience.2005.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabuuchi K., Kuroiwa M., Shuto T., Sotogaku N., Snyder G. L., Higashi H., Tanaka M., Greengard P., Nishi A. (2006). Role of adenosine A(1) receptors in the modulation of dopamine D(1) and adenosine A(2a) receptor signaling in the neostriatum. Neuroscience 141, 19–25 10.1016/j.neuroscience.2006.04.047 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Loonam T. M., Noailles P. A., Angulo J. A. (2001). Comparison of cocaine- and methamphetamine-evoked dopamine and glutamate overflow in somatodendritic and terminal field regions of the rat brain during acute, chronic, and early withdrawal conditions. Ann. N. Y. Acad. Sci. 937, 93–120 10.1111/j.1749-6632.2001.tb03560.x [DOI] [PubMed] [Google Scholar]