Abstract
Among Epstein-Barr virus (EBV)-associated neoplasms, EBV-associated gastric carcinoma (EBVaGC) is the most common tumor worldwide. In contrast to the predominant site of occurrence of EBV-negative gastric carcinoma in the antrum, EBVaGC occurs most frequently in the proximal stomach, including the cardia, fundus and body. Microscopically, EBVaGC can be subclassified into three histological subtypes according to the host cellular immune responses: lymphoepithelioma-like carcinoma, carcinoma with Crohn's disease-like lymphoid reaction, and conventional-type adenocarcinoma. Recent studies have shown that patients with the lymphoepithelioma-like carcinoma subtype of EBVaGC have the best overall and disease-free survival, followed by Crohn's disease-like reactions, which in turn have better survival than patients with conventional-type adenocarcinoma. Histologic subclassifications of EBVaGCs are based on the differing degree and pattern of infl ammatory response and the extent of desmoplasia. Because these subclassifications appear to be a powerful prognostic parameter, further research into the underlying mechanisms of the cellular immune reaction in these pathologic subtypes of EBVaGCs may play a key role in understanding the innate immune response of patients with this highly aggressive carcinoma.
Keywords: Epstein-Barr Virus, Stomach, Carcinoma, Pathology, Prognosis
INTRODUCTION
Epstein-Barr virus (EBV) is a ubiquitous γ-1 herpes virus usually acquired during childhood via salivary transmission, which establishes a life-long persistent infection of B cells in over 90% of adults.1 EBV infection is implicated in the etiology of B and T lymphomas, NK cell malignancies, nasopharyngeal undifferentiated carcinomas and a subset of smooth muscle tumors.2 EBV-associated gastric carcinoma (EBVaGC) is defined by the presence of EBV in gastric tumor cells by EBV-encoded RNA (EBER) in situ hybridization and constitutes about 8.7% of all GC,3 ranging between 1.3% to 20.1% (or 2% to 18%).4-6 Among EBV-associated neoplasms, EBVaGC is most common and distributed worldwide, while Burkitt's lymphoma and nasopharyngeal carcinoma are endemic to equatorial Africa and southeast China, respectively.
Lymphoepithelioma-like carcinoma (LELC) is a relatively rare type of GC, which constitutes approximately 1% to 4% of all GCs and is highly associated with EBV infection.7-9 The prevalence of EBVaGC among LELC of the stomach is over 90%,3 and more than 80% of LELCs have been found to be EBV-associated.10
It is now widely accepted that LELC of the stomach is associated with a significantly better prognosis. However, some studies have shown that EBVaGCs do not differ in the prognosis compared to their EBV-negative counterparts.11-13 This discrepancy in prognosis may be associated with diverse pathologic features of EBVaGC. Conventional histologic types of GC other than LELC also are associated with EBV in about 10% to 20% of all GCs.3,14 In the current article, we reviewed the relationship between pathologic features, especially host cellular immune responses and prognosis in EBVaGC.
CLINICAL FEATURES OF EBV-ASSOCIATED GASTRIC CARCINOMA
The clinicopathologic features of EBVaGC observed in metaanalyses are described in Table 1. Although some have demonstrated that EBVaGC occur more frequently in male and in younger age group, most studies showed no evident age dependence of EBVaGC.3,6 In our Korean cohorts, although male affected more frequently than female patients, we failed to demonstrate any age preference.11,14 Moreover, although lymph node metastasis was significantly frequent in EBVaGCs compared to EBV-negative GCs, there was no significant difference in the depth of invasion or clinical stage between EBVaGC and EBV-negative GC.6 Although the numbers of patients with pT3-4 stages were relatively smaller than control group, Song et al.14 showed that EBVaGCs are associated with lower pT stages, pN stages and International Union Against Cancer/American Joint Committee on Cancer (UICC/AJCC) stages than control patients.
Table 1.
The Clinicopathological Features of Epstein-Barr Virus (EBV)-Associated Gastric Carcinoma as Determined from a Meta-Analysis3,6
LELC, lymphoepithelioma-like carcinoma; EBVaGC, EBV-associated gastric carcinoma.
Most studies did not show any relationship between EBV presence and GC prognosis.15 Koriyama et al.16 first demonstrated that EBV was related to poor prognosis in intestinaltype carcinoma, whereas the diffuse-type EBVaGC had better prognosis even when LELC were excluded. This study is the first demonstrating the relationship between prognosis and histologic type of EBVaGCs. In our analyses with large number of EBVaGCs,14 although patients with EBVaGC showed longer overall and disease-free survival than patients with EBV-negative GC by univariate analysis, multivariate analysis with Cox proportional hazards failed to show any significant difference in spite of their lower UICC/AJCC stages. However, stratification of EBV-associated GCs by host cellular immune responses showed that patients with LELC and LELC+CLR have significantly longer overall survival time and disease-free survival (Table 2).14
Table 2.
LELC, lymphoepithelioma-like carcinoma; CLR, carcinoma with Crohn's disease-like lymphoid reaction; CA, conventional adenocarcinoma.
From a clinical perspective, endoscopy is the most useful modality for the diagnosis of EBVaGC. Although distinguishing endoscopic findings are not described, some have reported submucosal nodules of carcinoma with lymphoid stroma.17,18 In these circumstances, characteristic submucosal tumor-like protrusions without clear tumor margins are described. The submucosal mass lesion, one of the various appearances of LELC, can be misdiagnosed as submucosal tumors.18 In these cases, endoscopic ultrasonography demonstrating hypoechoic submucosal mass (corresponding to lymphoid stroma composed of carcinoma cells and infiltrating lymphocytes) located in the hyperechoic submucosal layer of the gastric wall is useful distinguishing LELC from other submucosal tumors.17,19 The authors have experienced small numbers of LELC cases mimicking submucosal tumor on endoscopic and radiologic findings. In these cases, multiple deep biopsies usually confirmed the presence of carcinoma. Hence, during the endoscopic follow up of submucosal tumors, when the endoscopists experience rapid increase of size or ulcer in spite of small sizes, multiple deep biopsies are requested to rule out the possibility of EBVaGCs.
PATHOLOGIC FEATURES OF EBV-ASSOCIATED GASTRIC CARCINOMAS
Table 2 describes macroscopic and microscopic features of EBVaGC.
1. Macroscopic findings
EBVaGC develops most often in the proximal stomach, including cardia, fundus and body, in contrast to the predominance of antrum in EBV-negative GC in the GC prevalent areas such as Korea. EBVaGC was detected in 11.8% of the cases in the cardia, in 13.0% in the body, and in 5.9% in the antrum, indicating that there is a significant relationship between the tumor location in the stomach and EBVaGC.6
Some have reported that macroscopic findings of advanced EBVaGC show markedly increased ratio of thickness to width compared to EBV-negative GC.18-20 However, these observations were based on adopting only LELC and excluding other histologic types of EBVaGC. If a certain EBVaGC is conventional-type adenocarcinoma rather than LELC, it can show conventional gross findings similar to EBV-negative counterparts.
There are scattered reports indicating that the incidence of multiple synchronous carcinomas appears to be higher in EBVaGC than in EBV-negative GC.21,22
2. Microscopic findings
EBVaGC can be divided into three histologic subtypes according to microscopic findings based on host cellular immune responses.14 A recent study on 123 EBVaGCs showed that 53 (43.1%) was lymphoepithelioma-like carcinoma (LELC), 52 (42.3%) was Crohn's disease-like lymphocytic reaction (CLR), and 18 (14.6%) was conventional adenocarcinoma (CA).14
1) Lymphoepithelioma-like carcinoma
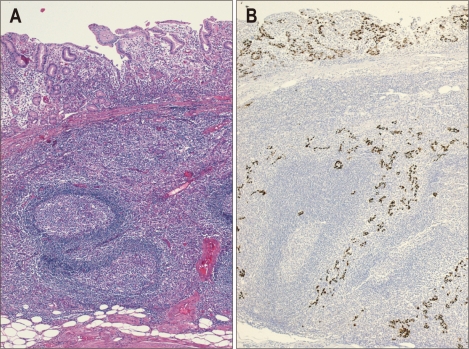
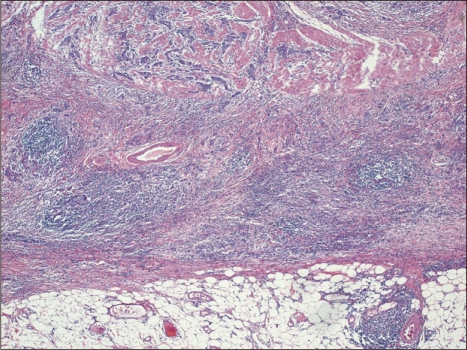
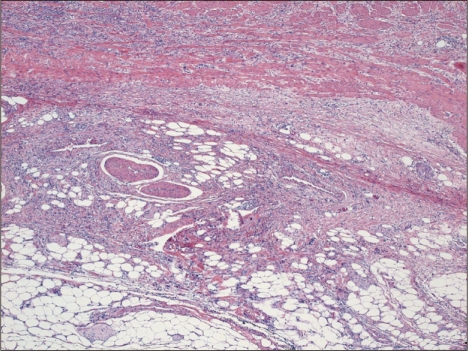
Typical microscopic findings of LELC are defined by 1) well-defined tumor margin, 2) dense lymphocytic infiltration of a degree whereby the number of tumor infiltrating lymphocytes was greater than the tumor cells throughout the tumor, 3) indistinct cytoplasmic borders and a syncytial growth pattern with poorly formed glandular structures, and 4) no desmoplasia (Fig. 1).14 Particularly, in the intramucosal stage, LELC shows a 'lace pattern' which consists of the connection and fusion of neoplastic glands (Fig. 2).23
Fig. 1.
(A) Lymphoepithelioma-like carcinoma with dense lymphocytic infiltration and a pushing margin. Lymphocytes outnumber tumor cells (H&E stain, ×4). (B) Photomicrograph of Epstein-Barr virus-encoded RNA as determined by in situ hybridization (×4). Only tumor cell nuclei are positive for staining, whereas lymphocytes are negative.
Fig. 2.
(A) Tumor cells show a 'lace pattern,' the connection and fusion of neoplastic glands (H&E stain, ×10). (B) In situ hybridization for Epstein-Barr virus-encoded RNA in early gastric carcinoma (×10).
Although many EBVaGCs show the pathologic findings similar to LELC, diagnosis of EBVaGC need for caution because many lymphocytes can infiltrate around tumor cells in other subtypes of EBVaGC and confirmation of EBV is prerequisite. So, strict criteria to diagnose LELC should be applied in order to subclassify EBVaGC and further validate their clinical significances.
2) Carcinoma with Crohn's disease-like lymphoid reaction
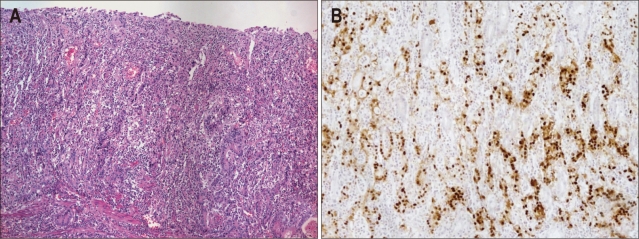
Carcinoma with Crohn's disease-like lymphoid reaction (CLR) was defined by 1) patchy lymphocytic infiltration with 3 or more lymphoid follicles with active germinal centers per tissue section at the advancing edge of the tumor, 2) smaller numbers of lymphocytes than tumor cells, 3) frequent tubule or gland formation, and 4) minimal or no desmoplasia (Fig. 3). In this pathologic subtype, it is characteristic that desmoplastic reaction is less prominent than conventional-type adenocarcinoma, especially of diffuse-type.
Fig. 3.
Carcinoma with Crohn's disease-like lymphoid reaction (H&E stain, ×4). Well-formed lymphoid follicles are easily found, but the tumor margin is somewhat infiltrative and tumor cells outnumber lymphocytes.
3) Conventional-type adenocarcinoma
Carcinomas other than LELC or CLR can be classified as conventional-type adenocarcinoma (CA). In contrast to extensive lymphocytic infiltrations found in LELC or CLR, CAs show scattered lymphocytes infiltration with prominent desmoplasia and rarely forms lymphoid follicles with prominent germinal centers (Fig. 4). Sometimes, in the periphery of CAs, only 1 or 2 lymphoid aggregates per tissue section are observed. This subtype is least common among EBVaGCs and cannot be delineated histologically from EBV-negative counterparts without confirmation of EBV within tumor cells.
Fig. 4.
Conventional-type adenocarcinoma (H&E stain, ×4). Lymphocytic infiltration is rarely identified.
PROGNOSIS OF EBV-ASSOCIATED GASTRIC CARCINOMA IN RELATION TO PATHOLOGY
Among the three histologic subtypes described earlier, patients with LELC subtype of EBVaGC show the best overall and disease-free survival followed by CLR, which showed better survival than CA patients.14 So this histologic subclassification of EBVaGC into 3 distinct subsets seems to be a powerful prognostic parameter.
In patients with GC, tumor infiltrating lymphocytes or dendritic cells have been associated with favorable prognosis.24-26 Several studies have reported that the infiltrating cells in EBVaGC are CD8+ T cells and mature dendritic cells.27-29 Lymphocytic infiltration around tumor can be considered as a host immune reaction against tumor cells, although the exact cellular characteristics or their roles are not certain at the present time. More dense lymphocytic infiltration could be considered as a stronger host immune reaction, and this assumption is well correlated with prognostic differences.
Recent meta-analyses revealed that there was no significant association between EBVaGC and EBV-negative GC in the same tumor invasion depth (T stage) or lymph node metastasis (N stage).6 Considering the prognostic significances of host cellular immune reaction, further studies are needed. Application of this type of histologic subclassification of EBVaGC, based mainly on host cellular immune response, may be helpful in predicting the prognosis of not only the patients with EBVaGC but also in the patients with other histological types of gastric cancer with negative EBV. Further elucidation of the underlying mechanisms of host cellular immune responses may guide the future development of immunotherapy as a therapeutic strategy for patients with GC.
CONCLUSION AND FUTURE PERSPECTIVES
EBV in situ hybridization is not routinely performed in daily pathology practice in many hospitals worldwide. Therefore, it is important to identify the characteristic pathological features of EBVaGC. Furthermore, pathologic subclassification of EBVaGCs according to histology reflecting host immune responses against tumor is an important prognostic factor. It will also be helpful to develop simple and easily applicable immunohistochemical methods to detect the EBV in the fixed tissue sections. Further research on underlying mechanisms of cellular immune reaction in EBVaGCs may play a key role in stimulating the innate immune response in patients with this highly aggressive carcinoma.
References
- 1.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 2.Delecluse HJ, Feederle R, O'Sullivan B, Taniere P. Epstein Barr virus-associated tumours: an update for the attention of the working pathologist. J Clin Pathol. 2007;60:1358–1364. doi: 10.1136/jcp.2006.044586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murphy G, Pfeiffer R, Camargo MC, Rabkin CS. Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location. Gastroenterology. 2009;137:824–833. doi: 10.1053/j.gastro.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morewaya J, Koriyama C, Akiba S, Shan D, Itoh T, Eizuru Y. Epstein-Barr virus-associated gastric carcinoma in Papua New Guinea. Oncol Rep. 2004;12:1093–1098. [PubMed] [Google Scholar]
- 5.Ojima H, Fukuda T, Nakajima T, Takenoshita S, Nagamachi Y. Discrepancy between clinical and pathological lymph node evaluation in Epstein-Barr virus-associated gastric cancers. Anticancer Res. 1996;16(5B):3081–3084. [PubMed] [Google Scholar]
- 6.Lee JH, Kim SH, Han SH, An JS, Lee ES, Kim YS. Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis. J Gastroenterol Hepatol. 2009;24:354–365. doi: 10.1111/j.1440-1746.2009.05775.x. [DOI] [PubMed] [Google Scholar]
- 7.Corvalan A, Ding S, Koriyama C, et al. Association of a distinctive strain of Epstein-Barr virus with gastric cancer. Int J Cancer. 2006;118:1736–1742. doi: 10.1002/ijc.21530. [DOI] [PubMed] [Google Scholar]
- 8.Horiuchi K, Mishima K, Ohsawa M, Aozasa K. Carcinoma of stomach and breast with lymphoid stroma: localisation of Epstein-Barr virus. J Clin Pathol. 1994;47:538–540. doi: 10.1136/jcp.47.6.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burke AP, Yen TS, Shekitka KM, Sobin LH. Lymphoepithelial carcinoma of the stomach with Epstein-Barr virus demonstrated by polymerase chain reaction. Mod Pathol. 1990;3:377–380. [PubMed] [Google Scholar]
- 10.Takada K. Epstein-Barr virus and gastric carcinoma. Mol Pathol. 2000;53:255–261. doi: 10.1136/mp.53.5.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park ES, Do IG, Park CK, et al. Cyclooxygenase-2 is an independent prognostic factor in gastric carcinoma patients receiving adjuvant chemotherapy and is not associated with EBV infection. Clin Cancer Res. 2009;15:291–298. doi: 10.1158/1078-0432.CCR-08-0848. [DOI] [PubMed] [Google Scholar]
- 12.Chang MS, Lee HS, Kim CW, Kim YI, Kim WH. Clinicopathologic characteristics of Epstein-Barr virus-incorporated gastric cancers in Korea. Pathol Res Pract. 2001;197:395–400. doi: 10.1078/0344-0338-00052. [DOI] [PubMed] [Google Scholar]
- 13.Gulley ML, Pulitzer DR, Eagan PA, Schneider BG. Epstein-Barr virus infection is an early event in gastric carcinogenesis and is independent of bcl-2 expression and p53 accumulation. Hum Pathol. 1996;27:20–27. doi: 10.1016/s0046-8177(96)90133-1. [DOI] [PubMed] [Google Scholar]
- 14.Song HJ, Srivastava A, Lee J, et al. Host inflammatory response predicts survival of patients with Epstein-Barr virus-associated gastric carcinoma. Gastroenterology. 2010;139:84–92.e2. doi: 10.1053/j.gastro.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Akiba S, Koriyama C, Herrera-Goepfert R, Eizuru Y. Epstein-Barr virus associated gastric carcinoma: epidemiological and clinicopathological features. Cancer Sci. 2008;99:195–201. doi: 10.1111/j.1349-7006.2007.00674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koriyama C, Akiba S, Itoh T, et al. Prognostic significance of Epstein-Barr virus involvement in gastric carcinoma in Japan. Int J Mol Med. 2002;10:635–639. [PubMed] [Google Scholar]
- 17.Yanai H, Nishikawa J, Mizugaki Y, et al. Endoscopic and pathologic features of Epstein-Barr virus-associated gastric carcinoma. Gastrointest Endosc. 1997;45:236–242. doi: 10.1016/s0016-5107(97)70265-7. [DOI] [PubMed] [Google Scholar]
- 18.Kim SW, Shin HC, Kim IY, et al. Epstein-Barr virus-associated lymphoepithelioma-like gastric carcinoma presenting as a submucosal mass: CT findings with pathologic correlation. Korean J Radiol. 2010;11:697–700. doi: 10.3348/kjr.2010.11.6.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nishikawa J, Yanai H, Mizugaki Y, Takada K, Tada M, Okita K. Case report: hypoechoic submucosal nodules. A sign of Epstein-Barr virus-associated early gastric cancer. J Gastroenterol Hepatol. 1998;13:585–590. doi: 10.1111/j.1440-1746.1998.tb00694.x. [DOI] [PubMed] [Google Scholar]
- 20.Fukayama M. Epstein-Barr virus and gastric carcinoma. Pathol Int. 2010;60:337–350. doi: 10.1111/j.1440-1827.2010.02533.x. [DOI] [PubMed] [Google Scholar]
- 21.Matsunou H, Konishi F, Hori H, et al. Characteristics of Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma in Japan. Cancer. 1996;77:1998–2004. doi: 10.1002/(SICI)1097-0142(19960515)77:10<1998::AID-CNCR6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 22.Arikawa J, Tokunaga M, Tashiro Y, et al. Epstein-Barr virus-positive multiple early gastric cancers and dysplastic lesions: a case report. Pathol Int. 1997;47:730–734. doi: 10.1111/j.1440-1827.1997.tb04450.x. [DOI] [PubMed] [Google Scholar]
- 23.Arikawa J, Tokunaga M, Satoh E, Tanaka S, Land CE. Morphological characteristics of Epstein-Barr virus-related early gastric carcinoma: a case-control study. Pathol Int. 1997;47:360–367. doi: 10.1111/j.1440-1827.1997.tb04509.x. [DOI] [PubMed] [Google Scholar]
- 24.Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99:1704–1711. doi: 10.1038/sj.bjc.6604738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grogg KL, Lohse CM, Pankratz VS, Halling KC, Smyrk TC. Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival. Mod Pathol. 2003;16:641–651. doi: 10.1097/01.MP.0000076980.73826.C0. [DOI] [PubMed] [Google Scholar]
- 26.Ishigami S, Natsugoe S, Tokuda K, et al. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- 27.van Beek J, zur Hausen A, Snel SN, et al. Morphological evidence of an activated cytotoxic T-cell infiltrate in EBV-positive gastric carcinoma preventing lymph node metastases. Am J Surg Pathol. 2006;30:59–65. doi: 10.1097/01.pas.0000176428.06629.1e. [DOI] [PubMed] [Google Scholar]
- 28.Kuzushima K, Nakamura S, Nakamura T, et al. Increased frequency of antigen-specific CD8(+) cytotoxic T lymphocytes infiltrating an Epstein-Barr virus-associated gastric carcinoma. J Clin Invest. 1999;104:163–171. doi: 10.1172/JCI6062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chapel F, Fabiani B, Davi F, et al. Epstein-Barr virus and gastric carcinoma in Western patients: comparison of pathological parameters and p53 expression in EBV-positive and negative tumours. Histopathology. 2000;36:252–261. doi: 10.1046/j.1365-2559.2000.00843.x. [DOI] [PubMed] [Google Scholar]
- 30.Chang MS, Lee JH, Kim JP, et al. Microsatellite instability and Epstein-Barr virus infection in gastric remnant cancers. Pathol Int. 2000;50:486–492. doi: 10.1046/j.1440-1827.2000.01072.x. [DOI] [PubMed] [Google Scholar]