Abstract
Background/Aims
There are limited data regarding the clinical outcomes of self-expandable metal stents in the treatment of proximal colon obstruction. We compared the clinical outcomes of stent placement in patients with malignant proximal to distal colon obstructions.
Methods
We reviewed medical records from 37 consecutive patients from three institutions (19 men; mean age, 72 years) who underwent endoscopic stent placement at a malignant obstruction of the proximal colon. We also examined the records from 99 patients (50 men; mean age, 65 years) who underwent endoscopic stent placement for a distal colon obstruction. Technical success, clinical improvements, complications and stent patency were compared between treatments.
Results
The technical success rate tended to be lower in stents inserted to treat proximal colon obstructions than in those used to treat distal colon obstructions (86% vs 97%, p=0.06). Clinical improvement was achieved in 78% of patients (29/37) with proximal colonic stenting and in 91% of patients (90/99) with distal colonic stenting (p=0.08). Complications (24% vs 27%), stent migration (8% vs 8%) and stent reocclusion rates (11% vs 17%) did not differ significantly between groups. Two cases of bowel perforation related to stenting (5%) occurred in patients with proximal colonic stenting.
Conclusions
The technical success and clinical improvement associated with self-expandable metal stents used to treat proximal colon obstruction tend to be lower than cases of distal colon obstruction. Technical failure is an important cause of poor clinical improvement in patients with proximal colon stenting. Complication rates and stent patency appear to be similar in both groups.
Keywords: Self-expandable metal stent, Colon cancer, Proximal colon, Distal colon
INTRODUCTION
Placement of a self-expandable metal stent (SEMS) is a safe and effective treatment for left-sided malignant colorectal obstruction, for palliation, or for allowing a single-stage resection.1 Recent reviews have reported technical and clinical success rates of greater than 90% and low rates of complications including stent migration (10% to 12%), stent blockage (7% to 10%) and perforation (3% to 4%).2,3 Most of these data were from procedures to insert an SEMS for a distal colon obstruction. However, there are limited data regarding stent placement for proximal colon obstruction because fewer than 5% of reported cases have involved this region.4,5 The main reason for the limited data on stent placement in the right colon is that this type of acute obstruction is usually handled by resection and primary anastomosis without the need for formal bowel preparation.6
Since the first report on stent placement in the transverse colon in 1997,7 a few studies have discussed the clinical outcomes of the use of stents in the management of proximal colon obstruction.6,8-11 These studies reported that stent placement for a proximal colon obstruction is a safe, feasible and effective treatment for palliation or as a bridge to surgery. However, long-term follow-up data on complications and stent patency are limited.
We evaluated the feasibility and efficacy of endoscopic stent insertion to treat proximal colon obstructions caused by primary colon cancer. We compared the technical feasibility and clinical outcomes of SEMS insertion for treating proximal colon obstruction with those for distal colon obstruction.
MATERIALS AND METHODS
1. Subjects
The data for 37 consecutive patients who had documented primary colon adenocarcinoma and underwent endoscopic stent insertion for colon obstruction proximal to the splenic flexure at three institutions (Seoul St. Mary's Hospital, St. Vincent's Hospital, and Incheon St. Mary's Hospital, Korea) were compared with those of 99 consecutive patients who underwent endoscopic stent insertion for malignant distal colon obstruction at Seoul St. Mary's Hospital from January 2004 to March 2009. SEMSs were inserted for palliative treatment or as a bridge to curative surgery. Patients with colon obstruction caused by other than colorectal malignancies were excluded from this study.
This study was performed by retrospective review. Data were gathered on patient age and sex, use of SEMS for palliation or bridge to surgery, type of stent, stent diameter and length, location of any obstruction, use of chemotherapy or radiotherapy after stent placement, type of surgery, surgical complications and outcomes and survival rate. Patients were considered to have subtotal obstruction if they manifested symptoms such as bowel distension, difficulty in passing solid stool or presence of narrowed stool caliber, or the ability to only pass small amounts of liquid stool or gas. Patients were considered to have complete obstruction if they presented with nausea, vomiting, abdominal distension, decreased or absent bowel sounds, or the inability to pass any stool or gas per anus.
All patients displayed clinical features and symptoms of colorectal obstruction. All patients had endoscopic features of colonic obstruction and a colonoscope of 12 to 14 mm diameter could not pass through the stricture.
2. Endoscopic stent placement
Eighty-four Hanaro® stents (M. I. Tech Co. Ltd., Seoul, Korea) and 52 Bona® stents (Standard Sci-Tech Inc., Seoul, Korea) were used. These were 22 to 24 mm in diameter and 6 to 16 cm long. Bowel preparation was performed using repeated sodium phosphate enemas for patients with total bowel obstruction or polyethylene glycol for some patients with subtotal bowel obstruction. Under endoscopic and fluoroscopic guidance, an endoscopic retrograde cholangiopancreatography (ERCP) catheter was passed over the guidewire, through the stricture, and advanced to the proximal region. The guidewire was then removed and a contrast dye was injected through the catheter to define the degree, length and site of the stricture. After that, a guidewire was reintroduced through the stricture. The SEMS, preloaded into a delivery system, was advanced over the guidewire and positioned through the stricture under endoscopic and fluoroscopic guidance. The stent was then released and its position and location were assessed.
Patients started an oral diet after they had passed flatus and the symptoms of obstruction had improved. Endoscopic or radiological follow-up evaluation was performed only in those patients with recurrent obstructive symptoms.
3. Definitions
Outcomes of SEMS placement were evaluated according to the following parameters: 1) technical success, 2) clinical improvement, 3) complications, and 4) stent patency. Technical success was defined as accurate placement of the stent through the entire length of stricture. Clinical improvement was defined as the ability of the patient to defecate and the relief of obstructive symptoms without procedure-related complications. Stent patency was defined as the period between the initial stent placement and the recurrence of obstructive symptoms caused by a stent occlusion.
4. Statistical analysis
Continuous variables for patient characteristics are expressed as the mean±SD. The percentage of patients for whom stenting was performed as a bridge to surgery, the technical success rate and the clinical outcomes of proximal colonic stenting and distal colonic stenting were compared using chi-squared tests. The overall survival and stent patency were estimated using Kaplan-Meier life table analysis.
RESULTS
1. Patient characteristics
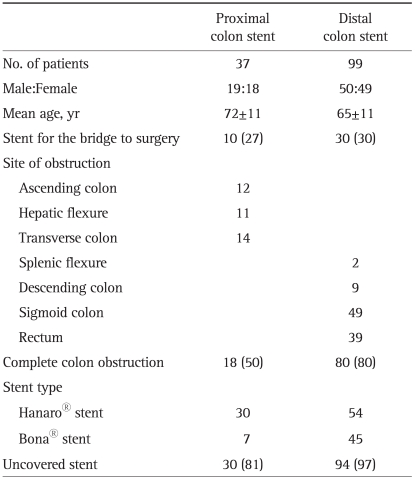
The patients' characteristics are summarized in Table 1. During the study period, stenting was attempted in 37 patients with colon obstruction proximal to the splenic flexure and in 99 patients with distal colon obstruction. Left-sided colon obstruction including the splenic flexure was regarded as the distal colon. The site of obstruction was the ascending colon (n=12), hepatic flexure (n=11), transverse colon (n=14), splenic flexure (n=2), descending colon (n=9), and rectosigmoid colon (n=88). Colon obstruction was complete in 18 patients (49%) and subtotal in 19 patients. Stenting was attempted as a bridge to surgery in 10 patients (27%) treated with proximal colonic stenting and in 30 patients (30%) treated with distal colonic stenting. These distributions were not significantly different (p=0.90).
Table 1.
Clinical Characteristics of Patients Who Underwent Stent Placement for Proximal or Distal Colon Obstruction
Data are presented as mean±SD or number (%).
2. Technical and clinical outcomes
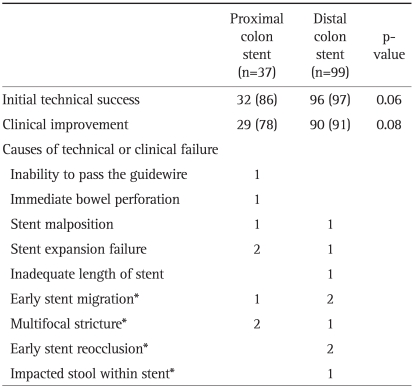
The technical and clinical outcomes are summarized in Table 2. Technical success was achieved in 86% (32/37) of patients with proximal colon obstruction and in 97% (96/99) of patients with distal colon obstruction (p=0.06). The causes of technical failure of proximal colonic stenting were inability to pass the guidewire through the completely obstructed site (n=1), a bowel perforation related to stenting (n=1), stent expansion failure (n=2), and stent malposition (n=1). The causes of technical failure in patients receiving left colonic stenting were stent malposition (n=1), inadequate length of the stent for traversing the lesion (n=1) and stent expansion failure (n=1). There was no procedure-related mortality.
Table 2.
Initial Technical Success and Clinical Improvement Following Stent Placement
Data are presented as number (%).
*These occurred in patients whose stents were inserted successfully.
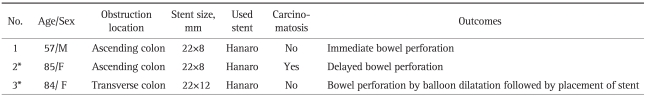
One case of immediate bowel perforation was caused by a stent passing through the wall of the ascending colon during insertion. There were two cases of stent expansion failure in patients with proximal colon obstructions. The first was a patient with ascending colon obstruction. Her obstructive symptoms did not improve and ileus persisted despite stent placement. Delayed bowel perforation was recognized six days after stent placement. She was treated with an emergency palliative operation. The second case was a patient with a transverse colon obstruction. Immediately after stenting, dilatation using a CRE™ Balloon (Boston Scientific Co., Boston, MA, USA) was performed because the initial expansion of the stent was unsatisfactory. Bowel perforation was recognized during the balloon dilatation. She was treated with surgery.
In summary, there were three cases of bowel perforation in patients who had proximal colon obstructions. One case was immediate and one was a delayed bowel perforation related to stenting. One case was not related to stenting. The demographic and procedural details for these patients are listed in Table 3. None of the patients with distal colon obstruction had bowel perforation.
Table 3.
Demographics, Procedure Details, and Outcomes in 3 Patients with Bowel Perforations
*Initial stent expansion failed in those patients.
Stent malposition occurred in a patient with excessive angulated obstruction at the hepatic flexure. A case of failure to pass the guidewire though the obstructive lesion also occurred in another patient who had obstruction at the hepatic flexure.
Clinical improvement was achieved in 78% (29/37) of patients with proximal colonic stenting. Three patients whose stent had been successfully inserted did not improve because of early stent migration (n=1) or combined obstruction of the terminal ileum and ascending colon (n=2). Compared with proximal colonic stenting, a clinical improvement was achieved in 91% (90/99) of patients with distal colonic stenting (p=0.08).
3. Complications and subsequent interventions
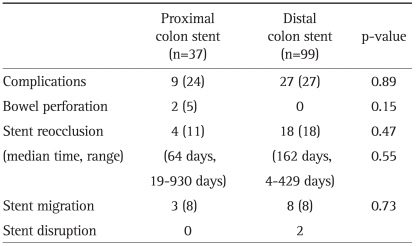
Stent-related complications occurred in 9 instances for 8 patients (24%) for SEMSs inserted in the proximal colon obstruction and 27 cases for insertions in the distal colon obstruction (27%, p=0.89). The complications are summarized in Table 4. The complications in patients with proximal colonic stenting were immediate or delayed bowel perforation related to stenting (n=2), stent migration (n=3), and stent reocclusion (n=4).
Table 4.
Stent Related Complications in Proximal and Distal Colon Stent Placement
Data are presented as number (%).
The stent migration rate did not differ between the two patients with proximal colon obstruction. In one patient with an obstruction in the hepatic flexure, the first stent migrated within one day and the reinserted stent also migrated. Nine cases of stent migration occurred during chemotherapy. Six patients experienced no reobstructive symptoms.
Stent reocclusion caused by tumor ingrowth or overgrowth occurred in four patients in the proximal colonic stent group (11%) at a median of 64 days (range, 19 to 930) and in 18 patients in the distal colonic stent group (18%) at median of 161 days (range, 4 to 429). The reocclusion rate and time to stent reocclusion did not differ significantly between groups (p=0.47).
All cases of stenting as a bridge to an operation were successful clinically. The median time from stenting to curative operation was 7 days (range, 4 to 18) in patients who underwent proximal colonic stenting and 6 days (range, 3 to 27) in patients who underwent distal colonic stenting. Bowel preparation was possible in all patients after stent placement. No major surgical complications occurred in these patients and none required temporary stoma formation.
4. Survival and stent patency
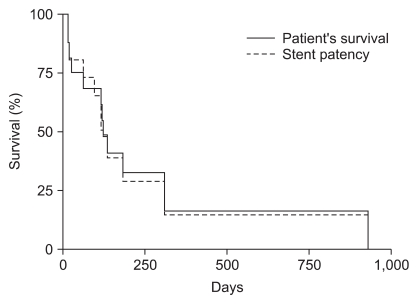
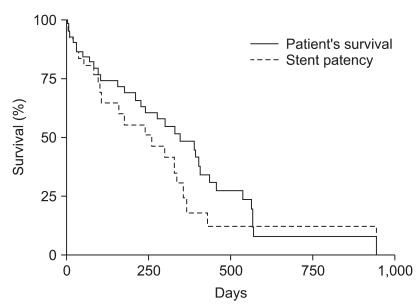
Among the patients who underwent colonic stenting for palliative care, three (11%) with proximal colon obstruction and nine (13%) with distal colon obstruction were lost to follow-up within one month after stent insertion. In patients who underwent proximal colonic stenting, the median stent patency was 120 days (range, 12 to 930 days) and the median survival was 124 days (range, 12 to 930 days; Fig. 1). In patients who underwent distal colonic stenting, the median stent patency was 186 days (range, 3 to 943 days) and the median survival was 348 days (range, 3 to 943 days; Fig. 2).
Fig. 1.
Kaplan-Meier curve of patient survival and stent patency in proximal colonic stent placement.
Fig. 2.
Kaplan-Meier curve of patient survival and stent patency in distal colonic stent placement.
DISCUSSION
SEMS placement for palliation is associated with significant reductions in hospital stay, mortality and medical complications compared with surgery for left-sided malignant colon obstruction.12 However, there has been a reluctance to use stents in the management of patients with proximal colon lesions primarily because such cancers are managed mainly by one-stage surgery without the need for bowel preparation or stoma formation. We aimed to evaluate the technical feasibility and clinical outcomes of SEMS placement to treat patients with proximal colon obstructions and compared these in patients with distal colon obstruction caused by primary colon cancer.
In our study, the clinical improvement in the proximal colon obstruction group was not significantly different from the distal colon obstruction group (78% vs 91%, p=0.08); however, these data seem to be lower than those reported for stenting of the distal colon. In a systemic review of colorectal stenting-largely on distal colonic stents-the median technical success rate was 96% (range, 67% to 100%) and the median clinical success rate was 92% (range, 46% to 100%).5,13-15 The low rate of clinical improvement in the proximal colon obstruction group was mostly caused by technical failure of stent insertion. In our study, the technical success rate tends to be lower in stents inserted to treat a proximal colon obstruction than a distal colon obstruction (86% vs 97%, p=0.06). Until now, two studies on proximal colonic stenting reported a 95% technical success rate,6,16 similar to those reported for stenting of distal colon lesions.5 The 86% technical success rate in this study was lower than these two previous studies. The main reasons for technical failure and higher complication rates were bowel perforation and stent expansion failure. Technical failure of SEMSs is caused most commonly by an inability to pass the guidewire or stent delivery system through an excessively angulated site, such as the hepatic flexure, and through a fixated stricture.16 The long distance from the anus and tortuosity of the bowel can prevent advancement and positioning of the stent,17,18 and the poor endoscopic view resulting from incomplete bowel preparation can disturb access to the obstructive lesion. In this study, most of the proximal colon obstructions were complete, and this feature might lead to technical failure.
In addition, one important variable determining technical or clinical success may be the angulation of the colon. In this study, there was one case of stent malposition and one case of failure to pass the guidewire, respectively. These patients had obstruction at angulated hepatic flexures. The characteristics of the lesion is also important. Tumor extension over ascending colon to cecum, a cul-de-sac, also can disturb the initial expansion of the stents.
Bowel perforation is the most serious complication because subsequent fecal peritonitis may be fatal. Delayed colonic perforation that is not related to the stent insertion procedure itself is rare and is usually caused by erosion of the colonic wall by the wire ends of the stent at the tumor site.19-21 In our study, the three cases of bowel perforation occurred exclusively in patients with proximal colon obstruction. Two cases were related to the stent and one was not. The first case was caused by erosion from the stent over the guidewire deployed through the obstruction site in the ascending colon. The second case was of a delayed bowel perforation at an ascending colon obstruction. The wall of the ascending colon is thinner than that of the rectum, so the stent might have eroded the wall. This might explain the predominance of bowel perforation at a right colon obstruction. The third case occurred during balloon dilatation followed by stent placement in the transverse colon obstruction. In this study, no other patients underwent hydrostatic balloon dilatation. Because initial stent expansion was insufficient in the second and the third patient, unequal radial force from the stent and balloon dilatation might have predisposed to bowel perforation.
The tendency of lower technical success and clinical improvement of SEMS in proximal colonic obstruction than in distal colonic obstruction, although not statistically significant, suggests the clinically effectiveness of SEMS in the proximal colonic obstruction is lower than in distal colonic obstruction.
Colonic stenting as a bridge to surgery allows preoperative bowel cleaning and full preoperative staging. It is important for reducing the risk of a two-staged operation and perioperative complications. In our study, effective colon preparation was possible after stent placement in all patients, and none had any postsurgical complications.
In our study, the migration rate was 8% in patients receiving proximal colonic stenting, the same as for distal colonic stenting. In most of the patients, the dilating effect of the stent on the stricture and the response to chemotherapy persisted for some time after migration. The rate of stent reocclusion caused by tumor ingrowth or overgrowth and the time until stent reocclusion did not differ significantly between the two groups.
This multicenter study had extensive long-term follow-up data because most of the SEMSs were placed for palliation. The median patency of stents used for palliation and the median survival in patients receiving proximal colonic stenting did not differ from those for the distal colon and are similar to the median duration of stent patency in a previous study for mainly distal colonic stenting.5
We included the patients with primary colon cancer exclusively. Our study had some limitations in that it included retrospective data and only two types of stents were used. However, the features of Hanaro® stents and Bona® stents are similar in that both stents have a woven structure and high flexibility.
In conclusion, the technical success and clinical improvement of SEMS in proximal colonic obstruction tend to be lower in distal colonic obstruction, although not statistically significant. Technical failure was an important cause of poor clinical improvement in patients receiving proximal colonic stenting. The complication rate and stent patency seem to be similar in stents inserted to treat both a proximal and a distal colonic obstruction.
References
- 1.Ptok H, Meyer F, Marusch F, et al. Palliative stent implantation in the treatment of malignant colorectal obstruction. Surg Endosc. 2006;20:909–914. doi: 10.1007/s00464-005-0594-7. [DOI] [PubMed] [Google Scholar]
- 2.Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051–2057. doi: 10.1111/j.1572-0241.2004.40017.x. [DOI] [PubMed] [Google Scholar]
- 3.Khot UP, Lang AW, Murali K, Parker MC. Systematic review of the efficacy and safety of colorectal stents. Br J Surg. 2002;89:1096–1102. doi: 10.1046/j.1365-2168.2002.02148.x. [DOI] [PubMed] [Google Scholar]
- 4.Mergener K, Kozarek RA. Stenting of the gastrointestinal tract. Dig Dis. 2002;20:173–181. doi: 10.1159/000067488. [DOI] [PubMed] [Google Scholar]
- 5.Watt AM, Faragher IG, Griffin TT, Rieger NA, Maddern GJ. Self-expanding metallic stents for relieving malignant colorectal obstruction: a systematic review. Ann Surg. 2007;246:24–30. doi: 10.1097/01.sla.0000261124.72687.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Repici A, Adler DG, Gibbs CM, Malesci A, Preatoni P, Baron TH. Stenting of the proximal colon in patients with malignant large bowel obstruction: techniques and outcomes. Gastrointest Endosc. 2007;66:940–944. doi: 10.1016/j.gie.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 7.Campbell KL, Hussey JK, Eremin O. Expandable metal stent application in obstructing carcinoma of the proximal colon: report of a case. Dis Colon Rectum. 1997;40:1391–1393. doi: 10.1007/BF02050829. [DOI] [PubMed] [Google Scholar]
- 8.García-Cano J, González-Huix F, Juzgado D, et al. Use of self-expanding metal stents to treat malignant colorectal obstruction in general endoscopic practice (with videos) Gastrointest Endosc. 2006;64:914–920. doi: 10.1016/j.gie.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 9.Elsberger B, Rourke K, Brush J, Glancy S, Collie M. Self-expanding metallic stent insertion in the proximal colon. Colorectal Dis. 2008;10:194–196. doi: 10.1111/j.1463-1318.2007.01336.x. [DOI] [PubMed] [Google Scholar]
- 10.Shim CS, Cho JY, Jung IS, et al. Through-the-scope double colonic stenting in the management of inoperable proximal malignant colonic obstruction: a pilot study. Endoscopy. 2004;36:426–431. doi: 10.1055/s-2004-814332. [DOI] [PubMed] [Google Scholar]
- 11.Dronamraju SS, Ramamurthy S, Kelly SB, Hayat M. Role of self-expanding metallic stents in the management of malignant obstruction of the proximal colon. Dis Colon Rectum. 2009;52:1657–1661. doi: 10.1007/DCR.0b013e3181a8f4af. [DOI] [PubMed] [Google Scholar]
- 12.Carne PW, Frye JN, Robertson GM, Frizelle FA. Stents or open operation for palliation of colorectal cancer: a retrospective, cohort study of perioperative outcome and long-term survival. Dis Colon Rectum. 2004;47:1455–1461. doi: 10.1007/s10350-004-0624-x. [DOI] [PubMed] [Google Scholar]
- 13.Wholey MH, Levine EA, Ferral H, Castaneda-Zuniga W. Initial clinical experience with colonic stent placement. Am J Surg. 1998;175:194–197. doi: 10.1016/s0002-9610(97)00285-7. [DOI] [PubMed] [Google Scholar]
- 14.Meisner S, Hensler M, Knop FK, West F, Wille-Jørgensen P. Self-expanding metal stents for colonic obstruction: experiences from 104 procedures in a single center. Dis Colon Rectum. 2004;47:444–450. doi: 10.1007/s10350-003-0081-y. [DOI] [PubMed] [Google Scholar]
- 15.Binkert CA, Ledermann H, Jost R, Saurenmann P, Decurtins M, Zollikofer CL. Acute colonic obstruction: clinical aspects and cost-effectiveness of preoperative and palliative treatment with self-expanding metallic stents--a preliminary report. Radiology. 1998;206:199–204. doi: 10.1148/radiology.206.1.9423673. [DOI] [PubMed] [Google Scholar]
- 16.Stimac D. Colonic stents for the palliation of malignant colonic obstruction. Dig Dis. 2008;26:336–341. doi: 10.1159/000177019. [DOI] [PubMed] [Google Scholar]
- 17.Keymling M. Colorectal stenting. Endoscopy. 2003;35:234–238. doi: 10.1055/s-2003-37265. [DOI] [PubMed] [Google Scholar]
- 18.Bhardwaj R, Parker MC. Palliative therapy of colorectal carcinoma: stent or surgery? Colorectal Dis. 2003;5:518–521. doi: 10.1046/j.1463-1318.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- 19.Camúñez F, Echenagusia A, Simó G, Turégano F, Vázquez J, Barreiro-Meiro I. Malignant colorectal obstruction treated by means of self-expanding metallic stents: effectiveness before surgery and in palliation. Radiology. 2000;216:492–497. doi: 10.1148/radiology.216.2.r00au12492. [DOI] [PubMed] [Google Scholar]
- 20.Han YM, Lee JM, Lee TH. Delayed colon perforation after palliative treatment for rectal carcinoma with bare rectal stent: a case report. Korean J Radiol. 2000;1:169–171. doi: 10.3348/kjr.2000.1.3.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trovato C, Fiori G, Ravizza D, et al. Delayed colonic perforation after metal stent placement for malignant colorectal obstruction. Endoscopy. 2006;38(Suppl 2):E96. doi: 10.1055/s-2006-944621. [DOI] [PubMed] [Google Scholar]