Abstract
Background/Aims
Gastric dysplasia is generally accepted to be the precursor lesion of gastric carcinoma. Approximately 25% to 35% of histological diagnoses based on endoscopic forcep biopsies for gastric dysplastic lesions change following endoscopic resection (ER). The aim of this study was to determine the predictive endoscopic features of high-grade gastric dysplasia (HGD) or early gastric cancer (EGC) following ER for lesions initially diagnosed as low-grade dysplasia (LGD) by a forceps biopsy.
Methods
To determine predictive variables for upgraded histology (LGD to HGD or EGC). The lesion size, gross endoscopic appearance, location, and surface nodularity or redness as well as the presence of a depressed portion, Helicobacter pylori infection, and intestinal metaplasia were retrospectively investigated.
Results
Among 251 LGDs diagnosed by an initial forceps biopsy, the diagnoses of 100 lesions (39.8%) changed following the ER; 56 of 251 LGDs (22.3%) were diagnosed as HGD, 39 (15.5%) as adenocarcinoma, and 5 (2.0%) as chronic gastritis. In a univariate analysis, large lesions (>15 mm), those with a depressed portion, and those with surface nodularity were significantly correlated with a upgraded histology classification following ER. In a multivariate analysis, a large size (>15 mm; odds ratio [OR], 2.8; 95% confidence interval [CI], 1.46 to 5.43) and a depressed portion in the lesion (OR, 2.7; 95% CI, 1.44 to 5.03) were predictive factors for upgraded histology following ER.
Conclusions
Our study shows that a substantial proportion of diagnoses of low-grade gastric dysplasias based on forceps biopsies were not representative of the entire lesion. We recommend ER for lesions with a depressed portion and for those larger than 15 mm.
Keywords: Gastric dysplasia, Endoscopic forcep biopsy, Endoscopic resection, Early gastric cancer
INTRODUCTION
Dysplasia refers to an unequivocal neoplastic transformation in the epithelium without penetration into the lamina propria (intramucosal carcinoma).1,2 Gastric dysplasia is generally accepted to be a precursor lesion for gastric carcinoma.3 The chance of carcinoma developing from gastric dysplasia may depend on the histological type and grade, size, and surface appearance of the gastric dysplasia.4,5 Therefore, a lesion diagnosed as high-grade dysplasia (HGD; category 4 in the Vienna classification) based on pathological examination of endoscopic forceps biopsy specimens should be considered for endoscopic resection (ER).6,7
In contrast to HGD, however, there is controversy as to what the best treatment option is for low-grade dysplasia (LGD). Some authors have asserted that endoscopic surveillance with re-biopsy should be scheduled periodically because of the low risk of malignant transformation,8,9 whereas others have suggested that the guiding principle for the management of LGD should be ER of the lesion.5,10 If the endoscopic forceps biopsy is representative of the entire lesion, endoscopic follow-up should be sufficient to manage LGD. However, a finding of low-grade dysplasia (category 3 in the Vienna classification) based on forceps biopsy material does not completely exclude the presence of HGD or carcinoma in other parts of the lesion.4,11,12 In particular, even though accurate diagnostic information can be obtained via endoscopic forceps biopsy, the histological discrepancy between endoscopic forceps biopsy specimens and ER has been reported to range from 25% to 40%.13-16 In this study, we investigated the predictive endoscopic features of HGD or early gastric cancer (EGC) of lesions originally diagnosed as LGD based on a forceps biopsy.
MATERIALS AND METHODS
1. Study population
From July 2005 to May 2009, 241 patients diagnosed with LGD lesions by initial endoscopic forceps biopsy were retrospectively enrolled and underwent ER at Yonsei University Wonju Christian Hospital, Korea. This study was approved by the local Institutional Review Board, Yonsei University Wonju College of Medicine, Wonju, Korea and all patients were provided written informed consent to participate in the study. After ER, all lesions were assigned to one of two groups based on the final histological findings: an up-graded histology group (UH; LGD to HGD or EGC) or a concordant histology group (CDH; LGD to gastritis or LGD).
2. Methods
ER was performed by endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD). Gastric lesions were first identified and demarcated using white-light endoscopy and chromoendoscopy with an indigo-carmine solution (GIF-Q 240, 260; Olympus Optical Co., Ltd., Tokyo, Japan). Marking around the lesions was achieved with spotty cautery using argon plasma coagulation. Isotonic saline mixed with epinephrine (1:10,000) was injected into the submucosal layer to produce a mucosal bleb. A circumferential mucosal incision was made around the lesion and then resection with a snare (EMR or polypectomy) or a hook knife and/or insulated tipped knife (Olympus Optical Co., Ltd.) ESD was performed. All patients were sedated by intravenous injection of 3 to 4 mg of midazolam and/or 20 mg of propofol. Ten to 20 mg of propofol was additionally given for conscious sedation as needed throughout the procedure.
To determine predictive variables for UH, the size, number of forceps biopsy specimens, location of the dysplastic lesion, surface nodularity or redness, presence of a depressed portion, Helicobacter pylori infection, and intestinal metaplasia were retrospectively investigated as potential factors. The location of the dysplastic lesion, surface nodularity and redness, and the presence of a depressed portion were investigated by white-light endoscopy and chromoendoscopy using indigo-carmine. A mucosal depressed portion was defined as any lesions with mucosal defect, erosion, or scar. Surface nodularity of the membrane to have the curvature of 2 mm or more was defined as positive. The size of the dysplastic lesion, presence of H. pylori infection, and coexistence of intestinal metaplasia were extracted from the pathology report of the resected specimen.
One expert gastrointestinal pathologist reviewed the histopathological findings of both endoscopic forceps biopsy materials and the endoscopically resected specimen. Biopsy specimens from the gastric lesion were fixed in formalin and bisected for hematoxylin-eosin (H&E) staining. The resected specimens were also fixed on a flat board and observed macroscopically; they were then fixed in formalin and examined in step sections. The resected specimens were sectioned perpendicularly at 2 mm intervals. All of the lesions were classified according to the standardized Vienna classification guidelines for gastrointestinal neoplasia.12
3. Statistical analysis
All statistical tests performed were two-sided tests and a p value of less than 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS PC software program (SPSS Inc., Chicago, IL, USA). Associations between the categorical parameters and sub-groups of UH and CDH were assessed by the chi-square test. Multiple logistic regression analyses to determine predictive factors for an upgraded histology after ER were performed to examine the effects of independent variables, and adjustments were made for the effects of each of the variables on the other variables. Medical statistician supported the study design and analysis of data.
RESULTS
1. Clinicopathological characteristics of the patients and their gastric lesions
A total of 241 patients (mean age, 62.6±10 years; M:F=175:66) were enrolled in this study, for a total of 251 lesions. Among 241 cases, 23 (9.5%) have multiple lesions; 21 cases had double lesions and the rest 2 had triple lesions. All of multiple lesions, the initial biopsies were performed in 10 cases. The mean size of the lesions was 12.8±7.9 mm and the number of forceps biopsies performed per lesion was 2.5±1.3. When we divided the gastric area into three sections (fundus, angle, and antrum), 160 cases were located on the antrum. The frequencies of a depressed portion, surface nodularity, and redness were 46%, 55%, and 39%, respectively. The frequencies of H. pylori infection and intestinal metaplasia were 48% and 85%, respectively.
2. Histological comparison between forcep biopsy specimens and resected specimens
Among 241 patients diagnosed with LGD on forceps biopsy, 151 cases (60%) showed a concordant histology after ER whereas 100 cases (40%) had a different histology: 39 cases of adenocarcinoma, 56 cases of HGD, and 5 cases of chronic gastritis. Among 39 cases of adenocarcinoma, all except one moderately differentiated cancer were well differentiated tumor. Therefore, 38% (95/251) of lesions initially diagnosed as LGD on forceps biopsy were upgraded after ER (Table 1).
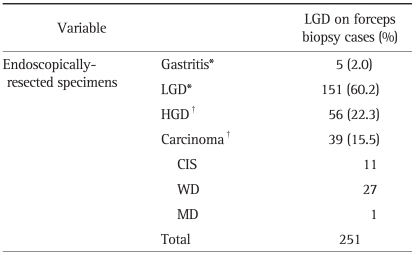
Table 1.
Histological Comparison of Forceps Biopsy Specimens and Resected Specimens
LGD, low-grade dysplasia; HGD, high-grade dysplasia; CIS, carcinoma in situ; WD, well differentiated; MD, moderately differentiated. *Concordant or down-graded histology (CDH) group; †Up-graded histology (UH) group.
Examples of histological discrepancies are provided in Figs. 1 and 2. Fig. 1 shows a lesion that was upgraded to HGD, whereas Fig. 2 shows a lesion that was upgraded to an adenocarcinoma.
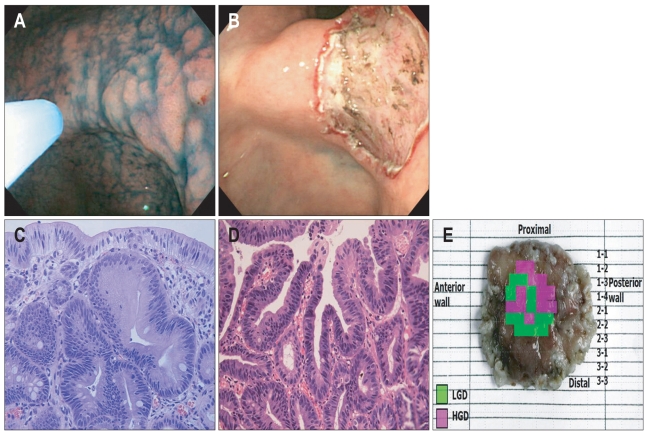
Fig. 1.
A lesion with a histologic upgrade from low-grade dysplasia (LGD) to high-grade dysplasia (HGD) following endoscopic resection. (A) Endoscopic findings of the lesion based on indigo-carmine spray. Endoscopy reveales a 15 mm elevated mucosal lesion with surface nodularity and redness on the posterior wall of the angle. (B) Following endoscopic resection, a 2 cm mucosal defect is observed. (C) Microscopic features of the forceps biopsy. The biopsy specimen shows mild glandular disarray and increased cellularity with basally located, enlarged hyperchromatic nuclei. These findings are consistent with LGD (H&E stain, ×400). (D) Microscopic features of the resected specimen. This portion of the lesion shows marked glandular disarray with vesicular, round nuclei and a marked increase in mitosis. These findings are consistent with HGD (H&E stain, ×400). (E) Map of the resected specimen. The tumor is 15 mm in diameter, and LGD is mixed with HGD.
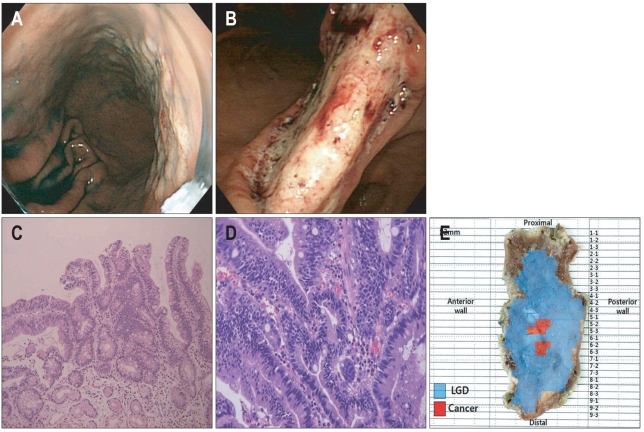
Fig. 2.
A lesion with a histologic upgraded from low-grade dysplasia (LGD) to adenocarcinoma following endoscopic resection. (A) Endoscopic findings of the lesion based on indigo-carmine spray. Endoscopy reveales a 40 mm flat mucosal lesion with surface nodularity in the lesser curvature side of the angle to the mid body. (B) A large mucosal defect following endoscopic submucosal dissection is noted over the gastric angle. (C) Microscopic features of the forceps biopsy specimen. The biopsy specimen shows increased cellularity, and the surface epithelium had a villous appearance with elongated cigar-shaped nuclei confined to the basal half of the epithelial cells. These findings are consistent with LGD (H&E stain, ×200). (D) Microscopic features of the resected specimen. The glandular architecture is severely distorted by marked proliferation of disarrayed glands with invasion. This finding is consistent with well differentiated adenocarcinoma confined to the lamina propria (H&E stain, ×400). (E) Mapping of the resected specimen. The tumor is 45 mm in size, and focal cancerous lesions mixed with LGD are evident. The lateral and vertical margins are free from tumor.
3. Factors related to the histological discrepancy between forceps biopsy specimens and ER specimens
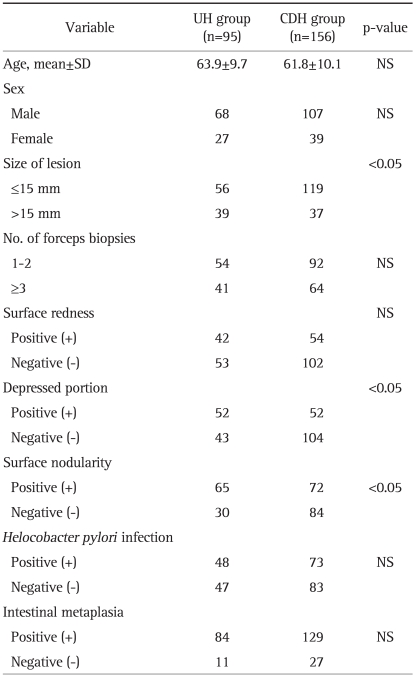
A comparison of the lesion and patient characteristics according to UH or CDH group is provided in Table 2. In 39 of 95 cases (41%) with UH, the lesion size was larger than 15 mm. Of 156 cases with CDH, only 37 cases (23.7%) were larger than 15 mm. Fifty-two of 95 cases (54.7%) with UH had a depressed portion present in the lesion; however, 52 of 156 cases (33.3%) with CDH had a depressed portion in the lesion. Sixty-five of 95 cases (68.4%) with UH had surface nodularity, while 72 of 156 cases (46.2%) with CDH had surface nodularity.
Table 2.
Comparison between UH and CDH Groups Following Endoscopic Resection
UH, upgraded histology; CDH, concordant or down-graded histology; NS, not significant.
On univariate analysis, a large size, presence of a depressed portion, and surface nodularity were significantly related to UH (Table 2). On multivariate analysis of risk factors of UH, a large size and a depressed lesion were significant risk factors for UH after ER of lesions diagnosed as LGD by forceps biopsy (odds ratios of 2.8 and 2.7, respectively) (Table 3). There were no significant associations between UH and age, sex, surface redness, number of forceps biopsy specimens, H. pylori infection, or intestinal metaplasia (Tables 2 and 3).
Table 3.
Multivariate Analysis of Risk Factors for UH Following Endoscopic Resection
UH, upgraded histology; OR, odds ratio; CI, confidence interval.
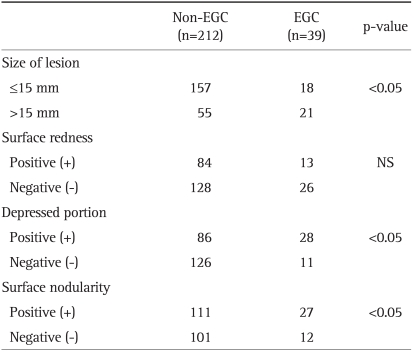
4. Factors related to the endoscopic finding between non-EGC and EGC
A comparison of the lesion according to non-EGC (early gastric cancer) or EGC group is provided in Table 4. In 21 of 39 cases (53.8%) with EGC, the lesion size was larger than 15 mm. Of 212 cases with non-EGC, only 55 cases (25.9%) were larger than 15 mm. Twenty-eight of 39 cases (71.8%) with EGC had a depressed portion present in the lesion; however, 86 of 212 cases (40.6%) with non-EGC had a depressed portion in the lesion. Twenty-seven of 39 cases (71.8%) with EGC had surface nodularity, while 111 of 212 cases (52.4%) with non-EGC had surface nodularity.
Table 4.
Comparison of Non-EGCs and EGCs Following Endoscopic Resection
EGC, early gastric cancer; NS, not significant.
On univariate analysis, a large size, presence of a depressed portion, and surface nodularity were significantly related to EGC (Table 4).
DISCUSSION
Gastric dysplasia is known to be a precursor lesion of gastric carcinoma and is classified as "noninvasive neoplasia" by the Padova International Classification system.2 Gastric dysplasia can be categorized as low-grade or high-grade based on the severity of histological abnormalities using a two-tier system.12 The characteristics of LGD are multiple small, round, glandular structures similar to adenomatous polyps in the colon. However, it is often difficult to discriminate gastric dysplasia in practice for several reasons. The first reason is interobserver variability. Fertitta et al.8 reported that 51% of cases initially diagnosed as moderate dysplasia by general pathologists were confirmed as hyperplastic or metaplastic lesions. The second reason is specimen size. Because the specimens obtained using forceps biopsy are tiny and often break into splinters, the whole lesion is not represented and the disease severity may be under-diagnosed. A third reason is the pathophysiology of gastric carcinogenesis. A series of changes have been identified as precursors to the intestinal type of gastric carcinoma, representing apparently sequential steps in the precancerous process, namely superficial gastritis, chronic atrophic gastritis, intestinal metaplasia, dysplasia, and cancer.13 Similarly, the genetic evolution of cancer involves the accumulation of multiple mutations. In gastric cancer, altered loci include p53, APC, K-ras, and there is also microsatellite instability in cancers with a replication error (RER) or ubiquitous somatic mutation (USM) phenotype.14 Thus, various degrees of dysplasia can coexist in the same lesion, making the lesion heterogeneous. Despite these limitations, however, endoscopic biopsy results are essential for clinicians to formulate their treatment plan. In this study, we investigated which endoscopic features were risk factors for upgrading a lesion initially diagnosed as LGD based on forceps biopsy. We focused on LGD histology for several important reasons. First, LGD has a histologically vague position. Until very recently, there was a substantial lack of agreement on the issue of dysplasia and its grading among pathologists, especially between those from Japan and Western countries. As a result of two consensus conferences held in Padova and Vienna, we now have the Padova International Classification2 and the Vienna Classification.12 When the pathologists involved in creating the Padova Classification participated in a test of variability after the conference, there was general agreement between them 77.7% to 86.5% of the time.17 The κ coefficients were a little over 0.6, indicating moderately good agreement. However, interobserver variability in diagnosing dysplasia is inevitable whenever a continuous spectrum is subjectively divided. The difficulty in differentiating reactive from dysplastic changes may account for reports of the reversibility of LGD.18 For this reason, interobserver variability could occur more often in LGD cases. In our study, a single expert gastrointestinal pathologist reviewed forceps biopsy materials and resected specimens, thereby ensuring no interobserver variability.
Second, the best treatment option for LGD is unclear. Using the two-tier system of classification, low-grade dysplasia was shown to regress in between 38% and 49% of cases, to persist in 19% to 28%, and to progress to high-grade dysplasia in between 0% and 15% of cases. High-grade dysplasia regressed in about 5% of cases, persisted in 14%, and progressed in 81% to 85%.17,19 For these reasons, a lesion diagnosed as high-grade dysplasia by endoscopic forceps biopsy material should be considered for ER.6,7 Several long-term follow-up studies have demonstrated that LGD lesions do not progress rapidly to HGD or carcinoma, thus some authors have advocated a management approach of scheduled endoscopic surveillance and re-biopsy.9,20 However, other groups have suggested removal of the LGD lesions because of the histological discrepancy between forceps biopsy specimens and resected specimens.21-23 In our study, substantial lesions initially characterized as LGD based on forceps biopsy were up-graded after ER (39%), whereas only 2% of lesions were down-graded. Furthermore, scarring changes in the lesion due to multiple biopsies could interfere with ER. Hull et al.24 reported that EMR is superior to biopsy for the diagnostic evaluation of large lesions. Therefore, endoscopic forceps biopsy findings as well as endoscopic findings known to be related to upgrading of lesions should be considered when planning the management of LGD lesions.
The histological discrepancy between endoscopic biopsy specimens and surgical specimens has been reported to range from 25% to 40%.13-16,25-27 In our study, a histological discrepancy between the forceps biopsy specimens and the ER specimens was confirmed in 100/251 (39.8%) of total cases. We therefore hypothesized that important information could be extracted from the endoscopic findings. Recent studies reported that large lesions (>15 mm), redness, nodularity of surface lesions, and the presence of a depressed portion were markers for malignancy risk.4,5 Furthermore, adenomatous polyps with a diameter greater than 2 cm size have been regarded as having malignant potential.28 In our study, lesions larger than 15 mm, those with a depressed portion, and those with surface nodularity were more often presented in the UH group than the CDH group. In addition, these parameters were more often presented in the EGC group than the non-EGC group based on final pathologic reports (Table 3). In the multivariate analysis, the risk of UH was increased significantly by 2.8-fold in lesions larger than 15 mm and 2.7-fold in lesions with a depressed portion. These results suggest that the size of lesions and the existence of a depressed portion may be risk factors related with UH. In our study 39/251 (15.5%) cases were diagnosed as EGC after ER. According to several studies in Korea, the proportion of cancer patients who were initially diagnosed as LGD but finally diagnosed by resection pathology ranged from 7.8% to 34%,29-33 which was consistent with our data. One explanation for high percentage of EGCs in almost study was that every study included only endoscopically resected cases which were likely to be high risk of cancer. Interestingly, 5 cases (2.1%) were reported as gastritis after ER. With regard to this point, Kim et al.34 reported that 3.2% was found to be negative after ER and this was similar to our result. They suggested several possible reasons for this; small tumors removed by the previous forceps biopsy, sampling error and a different location.
However, our study had some limitations. First, there is the possibility of selection bias because this study was performed retrospectively. Unfortunately, we could not present the exact number of LGD patients who might not be treated due to old age or comorbidity, or treated in another hospital because we searched the cases with key words of LGD and ER in a retrospective manner. Also, we had a difficulty to find the number of LGD diagnosed in our institution during the study period, because our medical records was determined by the final diagnosis of ER, the gold standard of this study. Endoscopic ablation for LGD was performed in only 2 cases. Because we included all cases of endoscopically resection during the study period, the number of LGD cases who may not be included in the study should be small. Therefore, it's very unlikely these cases affect the results of this study. Second, the concordance rate of endoscopic findings between observers was not investigated. Third, the number of forceps biopsies performed per patient differed. Further prospective studies are required to address these limitations.
In conclusion, we suggest that additional ER should be preferentially considered for lesions with a depressed portion or those larger than 15 mm, even if endoscopic biopsy shows LGD.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2011-0006074).
References
- 1.Ming SC, Bajtai A, Correa P, et al. Gastric dysplasia: significance and pathologic criteria. Cancer. 1984;54:1794–1801. doi: 10.1002/1097-0142(19841101)54:9<1794::aid-cncr2820540907>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 2.Rugge M, Correa P, Dixon MF, et al. Gastric dysplasia: the Padova international classification. Am J Surg Pathol. 2000;24:167–176. doi: 10.1097/00000478-200002000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Serck-Hanssen A. Precancerous lesions of the stomach. Scand J Gastroenterol Suppl. 1979;54:104–105. [PubMed] [Google Scholar]
- 4.Park DI, Rhee PL, Kim JE, et al. Risk factors suggesting malignant transformation of gastric adenoma: univariate and multivariate analysis. Endoscopy. 2001;33:501–506. doi: 10.1055/s-2001-15089. [DOI] [PubMed] [Google Scholar]
- 5.Morson BC, Sobin LH, Grundmann E, Johansen A, Nagayo T, Serck-Hanssen A. Precancerous conditions and epithelial dysplasia in the stomach. J Clin Pathol. 1980;33:711–721. doi: 10.1136/jcp.33.8.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue H. Treatment of esophageal and gastric tumors. Endoscopy. 2001;33:119–125. doi: 10.1055/s-2001-11664. [DOI] [PubMed] [Google Scholar]
- 7.Ponchon T. Endoscopic mucosal resection. J Clin Gastroenterol. 2001;32:6–10. doi: 10.1097/00004836-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Fertitta AM, Comin U, Terruzzi V, et al. Gastrointestinal Endoscopic Pathology Study Group. Clinical significance of gastric dysplasia: a multicenter follow-up study. Endoscopy. 1993;25:265–268. doi: 10.1055/s-2007-1010311. [DOI] [PubMed] [Google Scholar]
- 9.Srivastava A, Lauwers GY. Gastric epithelial dysplasia: the Western perspective. Dig Liver Dis. 2008;40:641–649. doi: 10.1016/j.dld.2008.02.039. [DOI] [PubMed] [Google Scholar]
- 10.Park SY, Jeon SW, Jung MK, et al. Long-term follow-up study of gastric intraepithelial neoplasias: progression from low-grade dysplasia to invasive carcinoma. Eur J Gastroenterol Hepatol. 2008;20:966–970. doi: 10.1097/MEG.0b013e3283013d58. [DOI] [PubMed] [Google Scholar]
- 11.Weinstein WM, Goldstein NS. Gastric dysplasia and its management. Gastroenterology. 1994;107:1543–1545. doi: 10.1016/0016-5085(94)90561-4. [DOI] [PubMed] [Google Scholar]
- 12.Schlemper RJ, Riddell RH, Kato Y, et al. The Vienna classification of gastrointestinal epithelial neoplasia. Gut. 2000;47:251–255. doi: 10.1136/gut.47.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Correa P, Haenszel W, Cuello C, et al. Gastric precancerous process in a high risk population: cross-sectional studies. Cancer Res. 1990;50:4731–4736. [PubMed] [Google Scholar]
- 14.Mironov NM, Aguelon MA, Potapova GI, et al. Alterations of (CA)n DNA repeats and tumor suppressor genes in human gastric cancer. Cancer Res. 1994;54:41–44. [PubMed] [Google Scholar]
- 15.Sung HY, Cheung DY, Cho SH, et al. Polyps in the gastrointestinal tract: discrepancy between endoscopic forceps biopsies and resected specimens. Eur J Gastroenterol Hepatol. 2009;21:190–195. doi: 10.1097/MEG.0b013e3283140ebd. [DOI] [PubMed] [Google Scholar]
- 16.Kim YD, Cho JY, Jung IS, et al. Comparison of endoscopic forcep biopsy and the histopathologic diagnosis after endoscopic submucosal dissection. Korean J Gastrointest Endosc. 2009;38:188–192. [Google Scholar]
- 17.Bearzi I, Brancorsini D, Santinelli A, Rezai B, Mannello B, Ranaldi R. Gastric dysplasia: a ten-year follow-up study. Pathol Res Pract. 1994;190:61–68. doi: 10.1016/s0344-0338(11)80497-8. [DOI] [PubMed] [Google Scholar]
- 18.You WC, Blot WJ, Li JY, et al. Precancerous gastric lesions in a population at high risk of stomach cancer. Cancer Res. 1993;53:1317–1321. [PubMed] [Google Scholar]
- 19.Lansdown M, Quirke P, Dixon MF, Axon AT, Johnston D. High grade dysplasia of the gastric mucosa: a marker for gastric carcinoma. Gut. 1990;31:977–983. doi: 10.1136/gut.31.9.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamada H, Ikegami M, Shimoda T, Takagi N, Maruyama M. Long-term follow-up study of gastric adenoma/dysplasia. Endoscopy. 2004;36:390–396. doi: 10.1055/s-2004-814330. [DOI] [PubMed] [Google Scholar]
- 21.Farinati F, Rugge M, Di Mario F, Valiante F, Baffa R. Early and advanced gastric cancer in the follow-up of moderate and severe gastric dysplasia patients: a prospective study. I.G.G.E.D.--Interdisciplinary Group on Gastric Epithelial Dysplasia. Endoscopy. 1993;25:261–264. doi: 10.1055/s-2007-1010310. [DOI] [PubMed] [Google Scholar]
- 22.Di Gregorio C, Morandi P, Fante R, De Gaetani C. Gastric dysplasia: a follow-up study. Am J Gastroenterol. 1993;88:1714–1719. [PubMed] [Google Scholar]
- 23.Saraga EP, Gardiol D, Costa J. Gastric dysplasia: a histological follow-up study. Am J Surg Pathol. 1987;11:788–796. [PubMed] [Google Scholar]
- 24.Hull MJ, Mino-Kenudson M, Nishioka NS, et al. Endoscopic mucosal resection: an improved diagnostic procedure for early gastroesophageal epithelial neoplasms. Am J Surg Pathol. 2006;30:114–118. doi: 10.1097/01.pas.0000180438.56528.a0. [DOI] [PubMed] [Google Scholar]
- 25.Palli D, Bianchi S, Cipriani F, et al. Reproducibility of histologic classification of gastric cancer. Br J Cancer. 1991;63:765–768. doi: 10.1038/bjc.1991.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakim NS, Sarr MG, van Heerden JA. Does endoscopy really help the surgeon evaluate gastric cancer? Can J Surg. 1989;32:175–177. [PubMed] [Google Scholar]
- 27.Namieno T, Koito K, Higashi T, et al. Assessing the suitability of gastric carcinoma for limited resection: histologic differentiation of endoscopic biopsy. World J Surg. 1998;22:865–868. doi: 10.1007/s002689900483. [DOI] [PubMed] [Google Scholar]
- 28.Tomasulo J. Gastric polyps. Histologic types and their relationship to gastric carcinoma. Cancer. 1971;27:1346–1355. doi: 10.1002/1097-0142(197106)27:6<1346::aid-cncr2820270612>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 29.Kim EY, Kim JJ, Kim B, Park SM. Treatment of gastric epithelial dysplasia that is diagnosed by endoscopic biopsy. J Korean Gastric Cancer Assoc. 2010;10:1–4. [Google Scholar]
- 30.Kim JY, Lee SW, Park LC, et al. Histological comparison of endoscopic forceps biopsy with endoscopic mucosal resection in the gastric lesions. Korean J Med. 2006;71:600–608. [Google Scholar]
- 31.Hwang JY, Park KS, Hwang JS, Ahn SH, Park SK. Histological comparison of endoscopic forceps biopsy with endoscopic resection in gastric mucosal elevated lesion. Korean J Gastrointest Endosc. 2003;26:68–72. [Google Scholar]
- 32.Park EH, Kang KT, Kim BH, et al. The Histologic discrepancy before and after endoscopic submucosal dissection of gastric adenoma and early gastric cancer. Korean J Gastrointest Endosc. 2007;34:125–131. [Google Scholar]
- 33.Yoon WJ, Lee DH, Jung YJ, et al. Histologic characteristics of gastric polyps in Korea: emphasis on discrepancy between endoscopic forceps biopsy and endoscopic mucosal resection specimen. World J Gastroenterol. 2006;12:4029–4032. doi: 10.3748/wjg.v12.i25.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim ES, Jeon SW, Park SY, et al. Where has the tumor gone? The characteristics of cases of negative pathologic diagnosis after endoscopic mucosal resection. Endoscopy. 2009;41:739–745. doi: 10.1055/s-0029-1215043. [DOI] [PubMed] [Google Scholar]