Abstract
Background/Aims
The aim of this study was to evaluate the usefulness of health screening for early detection and improved prognosis in pancreatic cancer.
Methods
Between 1995 and 2008, 176,361 examinees visited the Health Promotion Center (HPC). Twenty patients diagnosed with pancreatic cancer were enrolled. During the same period, 40 patients were randomly selected from 2,202 patients diagnosed with pancreatic cancer at the Out Patient Clinic (OPC) for comparison.
Results
Within the HPC group, 10 patients were initially suspected of having pancreatic cancer following abnormal ultrasonographic findings, and 9 patients had suspected cases following the detection of elevated serum CA 19-9. The curative resection rate was higher in the HPC group than in the OPC group (p=0.011). The median survival was longer in the HPC group than in the OPC group (p=0.000). However, there was no significant difference in the 3-year survival rate between the two groups. Asymptomatic patients (n=6/20) in the HPC group showed better curative resection and survival rates than symptomatic patients. However, the difference was not statistically significant.
Conclusions
Health screening is somewhat helpful for improving the curative resection rate and median survival of patients with pancreatic cancer detected by screening tests. However, the benefit of this method in improving long-term survival is limited by how early the cancer is detected.
Keywords: Pancreatic cancer, Screening, Early detection, Prognosis
INTRODUCTION
Pancreatic cancer is the fourth leading cause of cancer death in most Western countries. Actually, an estimated 42,470 new cases of pancreatic cancer are expected to occur and an estimated 35,240 deaths are expected to occur in the United States during 2009.1
The prognosis of pancreatic cancer is very poor. For all stages combined, the 5-year survival rate is 5% and even for those people diagnosed with resectable disease, the 5-year survival rate is only 20%. Approximately only 20% of patients have disease amendable to surgical resection at the time of presentation.2 In addition, a subset of patients undergoing curative resection (up to 30%) will have positive resection margins, reflecting an incomplete resection. Eventually less than 10% of patients with pancreatic cancer undergo a margin-negative (R0) resection.3 The dismal prognosis of this disease is due to the silent nature of pancreatic cancer until late in the disease process.4 Patients present for a medical evaluation only when the cancer is advanced; this is when they experience the signs and symptoms of obstructive jaundice, abdominal pain, and weight loss.
The only hope for long-term survival in pancreatic cancer is a curative resection. While some progress has been made in chemotherapy of pancreatic cancer and newer biologic agents promise better results, overall adjuvant therapy only prolongs life and rarely cures pancreatic cancer.5 Therefore, early detection and curative resection are important strategies to improve outcomes. Unfortunately, there is no rationale to investigate early detection strategies in the general population owing to the relatively low incidence of pancreatic cancer and the lack of cost-effective screening tool.6
Recently, as the public awareness about health has increased, general health screening is becoming increasingly popular. These health screenings are being periodically conducted by individuals, welfare program in workplace, or health insurance companies. Since a health screening program is not only for the detection of pancreatic cancer but also for general health screening including all kinds of cancer and metabolic disorders, the cost-effectiveness related to the early detection of pancreatic cancer is not an important issue under this screening program. So, in this study, we evaluated whether the health screening was useful for the early detection and improving the prognosis of pancreatic cancer.
MATERIALS AND METHODS
Twenty patients were diagnosed with pancreatic cancer among 176,361 healthy examinees visiting the Health Promotion Center (HPC) at Samsung Medical Center, Seoul, Korea, from 1995 to 2008. There were seven missed cases thought to be benign conditions of the pancreas at the health promotion center, but diagnosed with pancreatic cancer within one year thereafter. During the same period, among 2,202 patients that were diagnosed with pancreatic cancer at the Out Patient Clinic (OPC) of Samsung Medical Center, 40 patients (two times more than HPC group) were randomly selected for comparison after matching for age and gender to the HPC. This study was approved by the Institutional Review Board of Samsung Medical Center.
The following data from a self administered questionnaire and/or physical examination by physicians were collected: age, gender, history of smoking and alcohol, a medical history such as diabetes and chronic pancreatitis, family medical history, body mass index (BMI), and whether a specific symptom was present or absent. BMI was calculated by dividing the weight (kg) by height (m2) on the basis of the usual weight before losing weight. In addition, the medical records on pancreatic cancer such as modality of diagnosis, imaging finding, tumor stage, resection status, and survival rate were reviewed. The CA 19-9 level, as a tumor marker was collected. The upper limit of the normal range of CA 19-9 was defined as 37 U/mL, as suggested by Del Villano et al.7 The modality of diagnosis was an initial test used for a suspected diagnosis of pancreatic cancer. If surgery was impossible, the tumor stage was determined based on the imaging finding. If surgery was performed, the tumor stage was determined by the clinical staging system of the American Joint Committee on Cancer (AJCC) TNM classification. A curative resection was defined as a margin-negative (R0) resection by microscopy.
Statistical analysis was performed using the χ2 test for comparison of discrete variables and the t-test was used for comparison of continuous variables. The BMI measured in this study was expressed as the median. Statistical analysis was performed using PASW Statistics 17.0 (SPSS Inc., Chicago, IL, USA). All p-values were two-tailed and a value of p<0.05 was considered significant.
RESULTS
1. Clinical characteristics
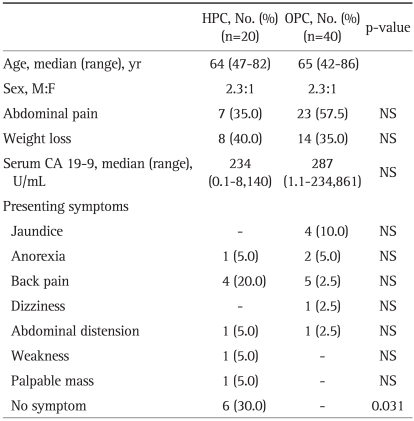
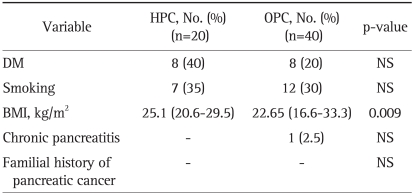
The median age of HPC and OPC groups was 64 years (range, 47 to 82 years) and 65 years (range, 42 to 86 years), respectively. The ratio of men to women was 2.3:1 in both groups. While all patients in the OPC group had presenting symptoms, 6 patients in the HPC group were asymptomatic at the time of diagnosis (p=0.031). The common symptoms were abdominal pain (35% vs 57.5%), weight loss (40.0% vs 35%), back pain (20% vs 12.5%), and jaundice (0% vs 10%) in each group (HPC group vs OPC group) (Table 1). Table 2 shows the risk factors associated with pancreatic cancer. Eight patients in the HPC group (40%) and 8 in the OPC group (20%) had diabetes mellitus at the time of diagnosis. Seven patients in the HPC group (35%) and 12 in the OPC group (30%) were smokers. Only 1 patient in the OPC group had a past medical history of chronic pancreatitis. None of the patients in either group had a family history of pancreatic cancer. There was no significant difference in the risk factors between the two groups except for the BMI. In the HPC group, the BMI was statistically higher than in the OPC group (25.1 kg/m2 vs 22.65 kg/m2, p=0.009).
Table 1.
Baseline Characteristics and Presenting Symptoms of the HPC and OPC Groups
HPC, Health Promotion Center; OPC, Outpatient Clinic; NS, not significant.
Table 2.
Comparison of Risk Factors between the HPC and OPC Groups
HPC, Health Promotion Center; OPC, Outpatient Clinic; DM, diabetes mellitus, BMI, body mass index; NS, not significant.
2. Modalities with initial suspicion of pancreatic cancer
Among the 20 patients in the HPC group that underwent abdominal ultrasonography, 10 patients (n=10/20, 50%) were initially suspected of pancreatic cancer with abnormal ultrasonographic (USG) findings. Among the 17 patients in the HPC group that had serum CA 19-9 measured, 9 patients (n=9/17, 53%) were initially suspected of having pancreatic cancer with elevated serum CA 19-9. Fourteen out of 17 patients had elevated serum CA 19-9 (median value, 361.5 U/mL; range, 43.3 to 8,140 U/mL); 5 patients had abnormal findings on the USG at the same time. One patient (n=1/1, 100%) was initially suspected to have pancreatic cancer based on an abnormal computed tomography (CT). The patient reported severe epigastric pain and weight loss at the time of health screening, and abdominal CT was performed. The serum CA 19-9 levels and USG findings were normal. On the other hand, more than half of the OPC patients were evaluated initially with CT because they were suspected of having pancreatic cancer at their primary medical institutions. Also, among the 37 patients in OPC group that had serum CA 19-9 measured initially, 27 patients had elevated serum CA 19-9 (median value, 1,170 U/mL; range, 44.9 to 234,867 U/mL).
For the HPC group, the abnormal findings on the USG were focal lesions of the pancreas (7/20, 35%) and pancreatic duct dilatation (2/20, 10%). In addition, liver metastasis was detected by USG in one patient.
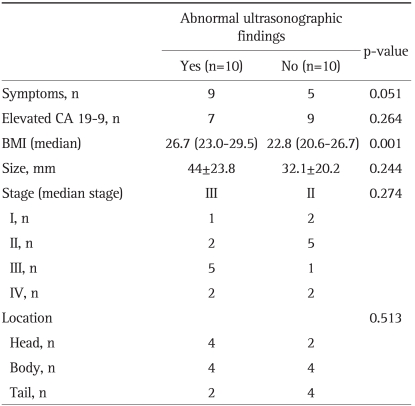
The HPC group was divided into two groups: patients with normal USG findings (n=10) and patients with abnormal USG findings (n=10) (Table 3). There was no significant difference between the two groups with regard to tumor size, stage and location. However, the BMI of patients with abnormal USG findings was higher than in patients with normal abdominal USG findings (p=0.001).
Table 3.
Characteristics of Patients with Abnormal Ultrasonographic Findings in the HPC Group
HPC, Health Promotion Center; BMI, body mass index.
3. Prognosis
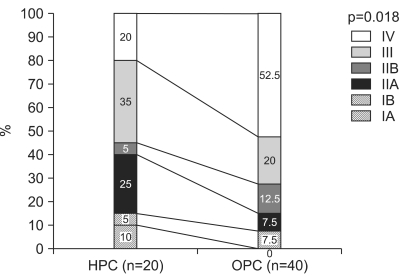
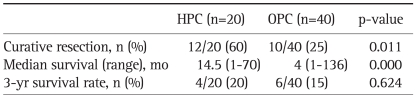
For the HPC group, 15% had stage I, 30% had stage II, 35% had stage III, and 20% had stage IV disease by the AJCC TNM classification. For the OPC group, 7.5% had stage I, 20% had stage II, 20% had stage III, and 52.5% had stage IV disease (Fig. 1). The curative resection rate was higher in the HPC group (n=12/20, 60%) than in OPC group (n=10/40, 25%, p=0.011). The median survival was longer in HPC group (14.5 months) than in the OPC group (4 months, p=0.000). However, there was no significant difference in the 3-year survival rate between the two groups (HPC group vs OPC, 20% vs 15.0%, p=0.624) (Table 4).
Fig. 1.
Comparison of pancreatic cancer stage between the Health Promotion Center (HPC) and Outpatient Clinic (OPC) groups based on American Joint Committee on Cancer (AJCC) TNM classification.
Table 4.
Comparison of Prognoses between the HPC and OPC Groups
HPC, Health Promotion Center; OPC, Outpatient Clinic.
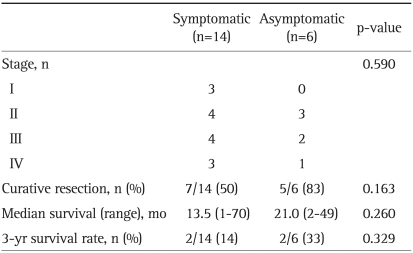
When the HPC group was divided into symptomatic and asymptomatic groups at the time of diagnosis, tumor stage, curative resection rate, median survival, and 3-year survival rate did not significantly differ (Table 5).
Table 5.
Differences between Symptomatic and Asymptomatic Patients in the HPC Groups
HPC, Health Promotion Center.
DISCUSSION
Pancreatic cancer remains a lethal malignancy. Due to the rapid progression of pancreatic cancers, early detection through screening will be required to improve long-term outcomes.8 In this respect, pancreatic neoplasias may meet some of the World Health Organization criteria for principles of screening. Sohn et al.9 showed that survival was markedly better in patients that had small tumors, negative resection margins, and no lymph node involvement. Ariyama et al. reported a postoperative 5-year cumulative survival rate of 100% for patients with tumors less than 1 cm in size.10,11 These data provide some evidence that early detection through screening may improve the long-term outcome for adenocarcinoma of the pancreas. However, since the prevalence of pancreatic cancer remains too low (8 to 12 per 100,000), it would not be cost effective and the yield of screening would be extremely low in the general population.4,12 At present, as it is known that 3% to 16% of pancreatic cancers are either syndromic or familial, patients with known genetic syndromes predispose them to the disease or with a strong familial history may be offered screening and surveillance in an attempt to detect pancreatic neoplasia at a curable stage.8 Whereas routine check-up for detecting of early pancreatic cancer in general population was not recommended to date. This study focused on a general health screening program. Abdominal USG which was usually included in the health screening program, screened several diseases related with hepatobiliary system, kidney and other organ of the abdominal cavity, not only for pancreatic cancers. Therefore, the cost-effectiveness for the detection of pancreatic cancer is not an important issue under this screening program. Moreover, there is a chance that the increasing volume of health screening improves the detection rate of early pancreatic cancer.
A remarkable thing about this study was that the curative resection rate and median survival were greater for the pancreatic cancer patients diagnosed at the HPC compared to patients diagnosed at the OPC. In addition, pancreatic cancer was detected at an earlier stage in the HPC group than in the OPC group. However there was a limitation to detect pancreatic cancer as early as the long-term survival was possible. In order to improve long-term survival, pancreatic cancer must be detected when patients are asymptomatic and the tumor is small (≤2 cm) and classified as T1 stage. However, in this study, only 30% of the HPC group was asymptomatic and 15% were classified as T1 stage. So, the long-term survival was not significantly improved in the HPC group compared to the OPC group.
A common problem in patients with pancreatic cancer is delayed diagnosis. This delay in diagnosis is largely due to the fact that abdominal pain and jaundice, which are the main symptoms of pancreatic cancer, appear relatively late during the course of the disease when the tumor is already at an advanced stage.13,14 In this study, 30% of patients with pancreatic cancer diagnosed at the HPC had no symptoms, and all patients diagnosed at the OPC had symptoms at the time of diagnosis. Most of the patients with symptoms presented with typical symptoms such as abdominal pain, back pain, weight loss, and jaundice. For the HPC group, asymptomatic patients were shown better curative resection rate and survival rate than symptomatic patients, but unfortunately, this difference was not statistically significant (Table 5). A large series of asymptomatic patients with pancreatic cancer are needed for further study.
In the present study, there were 6 missed cases judged as benign condition of pancreas at the health promotion center, however diagnosed with pancreatic cancer within 1 year. The median period for a delayed diagnosis was 7.5 months. On the initial USG findings at the HPC, 5 of 6 cases were normal and the remaining one had pancreatic duct dilatation. These cases suggest that patients with abnormal USG findings such as pancreatic duct dilatation, require further workup particularly if the patient is of advanced ages. In the 5 patients with serum CA 19-9 measured at the time of screening, all had normal level of CA19-9. The remaining one measured serum CA 19-9 at the time of diagnosis of pancreatic cancer and serum CA 19-9 was elevated. Although the positive predictive value CA 19-9 for detecting pancreatic cancer was very low, patients with an elevated CA 19-9 require careful follow-up. However, there still remains a possibility that abdominal USG and serum CA 19-9 may miss a significant number of cases of pancreatic cancer.
As shown in these missed cases, another problem in patients with pancreatic cancer is limited accuracy of available screening tests for detection of small pancreatic cancer. The tools for diagnosis of pancreatic cancer can be categorized as serologic biomarkers such as CA 19-9 and imaging techniques. The results of this study showed that nearly half of the patients in the HPC group were diagnosed with pancreatic cancer by only increased serum CA 19-9; there were no definite abnormal lesion in pancreas on their abdominal USG findings. Five out of 9 patients that were initially suspected of having pancreatic cancer with elevated serum CA 19-9, had no symptoms. These results looked as if serum CA 19-9 was effective tests to detect small pancreatic cancer in an asymptomatic population. However, caution is necessary for the interpretation of increased serum CA 19-9 as a marker for pancreatic cancer. Recent reports in asymptomatic subjects demonstrated that the incidence of carcinoma in asymptomatic subjects with elevated CA 19-9 was very low (2.8%, 10/353) and among them, subjects with pancreatic cancer were only 1.1% (4/353).15 Other studies have reported that the positive predictive value CA 19-9 for detecting pancreatic cancer was only 0.9% in the asymptomatic population.16-18
Also, in this study, half of the patients in the HPC group were initially suspected of having pancreatic cancer with abnormal USG findings. However, as mentioned earlier, the other half in the HPC group had normal USG findings. Between patients with normal and abnormal USG findings in the HPC group, there was no significant difference except for the BMI. Contrary to expectations, the BMI was higher in patients with abnormal USG findings than in patients with normal USG findings. But, the BMI was not always correlated with abdominal obesity and the sonographic view could be affected by bowel gas too. Of all the imaging studies, the endoscopic ultrasound (EUS) and helical CT scans are the most sensitive imaging modalities for detecting pancreatic tumor.4 Some studies reported that the EUS was useful for surveillance in high risk group patients.4,6 But, using the EUS directly to screen the general population may be problematic due to invasiveness of methods and limited availabiliaty.3,19 Multi-detector CT is the current preferred abdominal imaging test for pancreatic disease, including the diagnosis and staging of pancreatic cancer.13,20 But, because of the concern for repeated exposure to radiation, the role of the CT in screening for small pancreatic cancers remains to be established.3
The limitations of the present study include the following. First, the HPC group did not precisely represent the general population because they were interested in health enough to have private health screening and some of them had presenting symptoms at the time of their checkups. Second, since this hospital is a tertiary referral center, the patients that registered in the OPC group likely reflects a selection bias. Finally, the target number of pancreatic cancer patients diagnosed at the health promotion center was small. But considering the incidence of pancreatic cancer in the general population,21,22 the number of cases identified in the HPC group was not small.
In conclusion, general health screening is somewhat helpful for improving the curative resection rate and median survival of patients with pancreatic cancer detected as part of a general screening visit. However, there is a limitation to detect early enough to improve long-term survival. Also general health screening misses a significant portion of pancreatic cancer. Therefore, more sensitive and cost-effective screening tool is needed to improve the detection, early treatment and outcome of patients with pancreatic cancer.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Chang DK, Merrett ND, Biankin AV NSW Pancreatic Cancer Network. Improving outcomes for operable pancreatic cancer: is access to safer surgery the problem? J Gastroenterol Hepatol. 2008;23(7 Pt 1):1036–1045. doi: 10.1111/j.1440-1746.2008.05471.x. [DOI] [PubMed] [Google Scholar]
- 3.Chari ST. Detecting early pancreatic cancer: problems and prospects. Semin Oncol. 2007;34:284–294. doi: 10.1053/j.seminoncol.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klapman J, Malafa MP. Early detection of pancreatic cancer: why, who, and how to screen. Cancer Control. 2008;15:280–287. doi: 10.1177/107327480801500402. [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Stocken DD, Friess H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004;350:1200–1210. doi: 10.1056/NEJMoa032295. [DOI] [PubMed] [Google Scholar]
- 6.Brand RE, Lerch MM, Rubinstein WS, et al. Advances in counselling and surveillance of patients at risk for pancreatic cancer. Gut. 2007;56:1460–1469. doi: 10.1136/gut.2006.108456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Villano BC, Brennan S, Brock P, et al. Radioimmunometric assay for a monoclonal antibody-defined tumor marker, CA 19-9. Clin Chem. 1983;29:549–552. [PubMed] [Google Scholar]
- 8.Larghi A, Verna EC, Lecca PG, Costamagna G. Screening for pancreatic cancer in high-risk individuals: a call for endoscopic ultrasound. Clin Cancer Res. 2009;15:1907–1914. doi: 10.1158/1078-0432.CCR-08-1966. [DOI] [PubMed] [Google Scholar]
- 9.Sohn TA, Yeo CJ, Cameron JL, et al. Resected adenocarcinoma of the pancreas-616 patients: results, outcomes, and prognostic indicators. J Gastrointest Surg. 2000;4:567–579. doi: 10.1016/s1091-255x(00)80105-5. [DOI] [PubMed] [Google Scholar]
- 10.Ariyama J, Suyama M, Ogawa K, et al. The detection and prognosis of small pancreatic carcinoma. Int J Pancreatol. 1990;7:37–47. doi: 10.1007/BF02924218. [DOI] [PubMed] [Google Scholar]
- 11.Kern S, Hruban R, Hollingsworth MA, et al. A white paper: the product of a pancreas cancer think tank. Cancer Res. 2001;61:4923–4932. [PubMed] [Google Scholar]
- 12.Vitone LJ, Greenhalf W, McFaul CD, Ghaneh P, Neoptolemos JP. The inherited genetics of pancreatic cancer and prospects for secondary screening. Best Pract Res Clin Gastroenterol. 2006;20:253–283. doi: 10.1016/j.bpg.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 13.Canto MI. Strategies for screening for pancreatic adenocarcinoma in high-risk patients. Semin Oncol. 2007;34:295–302. doi: 10.1053/j.seminoncol.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Gullo L, Tomassetti P, Migliori M, Casadei R, Marrano D. Do early symptoms of pancreatic cancer exist that can allow an earlier diagnosis? Pancreas. 2001;22:210–213. doi: 10.1097/00006676-200103000-00017. [DOI] [PubMed] [Google Scholar]
- 15.Kim BJ, Lee KT, Moon TG, et al. How do we interpret an elevated carbohydrate antigen 19-9 level in asymptomatic subjects? Dig Liver Dis. 2009;41:364–369. doi: 10.1016/j.dld.2008.12.094. [DOI] [PubMed] [Google Scholar]
- 16.Kim JE, Lee KT, Lee JK, Paik SW, Rhee JC, Choi KW. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J Gastroenterol Hepatol. 2004;19:182–186. doi: 10.1111/j.1440-1746.2004.03219.x. [DOI] [PubMed] [Google Scholar]
- 17.Lee JK. Screening and diagnosis for pancreatic cancer. Korean J Gastroenterol. 2008;51:84–88. [PubMed] [Google Scholar]
- 18.Boeck S, Stieber P, Holdenrieder S, Wilkowski R, Heinemann V. Prognostic and therapeutic significance of carbohydrate antigen 19-9 as tumor marker in patients with pancreatic cancer. Oncology. 2006;70:255–264. doi: 10.1159/000094888. [DOI] [PubMed] [Google Scholar]
- 19.Helmstaedter L, Riemann JF. Pancreatic cancer: EUS and early diagnosis. Langenbecks Arch Surg. 2008;393:923–927. doi: 10.1007/s00423-007-0275-1. [DOI] [PubMed] [Google Scholar]
- 20.American Gastroenterological. American Gastroenterological Association medical position statement: epidemiology, diagnosis, and treatment of pancreatic ductal adenocarcinoma. Gastroenterology. 1999;117:1463–1484. doi: 10.1016/s0016-5085(99)70297-0. [DOI] [PubMed] [Google Scholar]
- 21.Holly EA, Chaliha I, Bracci PM, Gautam M. Signs and symptoms of pancreatic cancer: a population-based case-control study in the San Francisco Bay area. Clin Gastroenterol Hepatol. 2004;2:510–517. doi: 10.1016/s1542-3565(04)00171-5. [DOI] [PubMed] [Google Scholar]
- 22.Khorana AA, Fine RL. Pancreatic cancer and thromboembolic disease. Lancet Oncol. 2004;5:655–663. doi: 10.1016/S1470-2045(04)01606-7. [DOI] [PubMed] [Google Scholar]