Abstract
Background/Aims
The aim of this study was to evaluate the seroconversion rate of a hepatitis A virus (HAV) vaccination in patients with hepatitis B virus (HBV)-related chronic liver disease (CLD).
Methods
Analyses were conducted using clinical records from 94 patients with chronic HBV infection who were seronegative for IgG anti-HAV antibodies between September 2008 and June 2009. Two doses of an HAV vaccine were administered 24 weeks apart. A third vaccine dose was administered only for patients seronegative for anti-HAV antibodies at week 48.
Results
The seroconversion rate of anti-HAV following the two-dose vaccination was 86.17%. The seroconversion rate of anti-HAV was not significantly different according to age or status of liver disease. The rate was higher in female than in male patients. A third HAV vaccine dose was administered to 13 patients seronegative for anti-HAV after the two-dose regimen, and 84.62% of these patients showed seroconversion at week 72.
Conclusions
HAV vaccination is effective in most Korean patients with HBV-related CLD, and it might be necessary to evaluate three-dose vaccination approach for non-responders to the conventional regimen to maximize the success of an HAV vaccination program.
Keywords: Chronic hepatitis B, Hepatitis B virus related liver cirrhosis, Hepatitis A virus vaccination
INTRODUCTION
Hepatitis A virus (HAV) infection is an epidemiologically important disease and it causes acute hepatitis in humans with a worldwide distribution. The number of cases of adult hepatitis A has progressively been increasing during the last several decades in Korea since Korea has undergone a change from hyperendemicity to intermediate endemicity for hepatitis A virus infection due to the improvements of the socioeconomic and sanitation conditions.1-3
Lifelong protective antibodies are present after hepatitis A virus infection.4 The clinical manifestations of hepatitis A depend on the age of the host: less than 30% of infected young children showed symptomatic hepatitis, while about 80% of infected adults manifested with severe acute hepatitis with remarkably elevated levels of serum aminotransferases.5
Although the overall case-fatality rate of acute HAV among persons of all ages is only 0.01% to 0.3%,6-8 it is higher (1.8%) among adults who are 50 years of age or older.7 Several studies have also shown that super-infection with HAV in patients with chronic hepatitis B virus (HBV) infection results in significant increases in severe liver disease, acute liver failure and mortality.9-11 Korea is still highly endemic for hepatitis B infection, and this infection exhibits a strong tendency to turn towards chronic liver disease (CLD) when it is acquired during infancy or early childhood, and therefore vaccination for HAV is recommended for the patients with CLD. However, there are only limited studies that have evaluated the efficacy of HAV vaccine in the patients with CLD.
The aim of this study was to evaluate the seroconversion rate of IgG HAV antibody (anti-HAV) after having received the current HAV vaccination in the patients with HBV-related CLD in Korea.
MATERIALS AND METHODS
1. Study design, population and collection of data
We identified a total of 918 patients with CLD and who had undergone IgG anti-HAV testing between September 2008 and June 2009 at Samsung Medical Center, Seoul, Korea. Among them, 86.7% (796/918) of the patients already had seropositive anti-HAV. Ninety-four patients with HBV-related CLD were selected from 122 patients who showed seronegativity for IgG anti-HAV. The eligibility criteria included HBV surface antigen (HBsAg) positivity for at least 6 months. The patients with human immunodeficiency virus or hepatitis C virus infection and a past medical history of acute viral hepatitis A infection or HAV vaccination were excluded from this study.
The statuses of the underlying liver diseases were classified into chronic hepatitis and liver cirrhosis (LC). The diagnosis of LC was made if any one of the following findings was met: 1) compatible intraoperative gross findings or histologically compatible findings, 2) evidence of portal hypertension in the patients with liver disease, 3) compatible radiologic findings and platelet counts less than 100×109/L.
Blood samples for IgG anti-HAV testing were collected at weeks 0, 24, 48, and 72 after the first injection of vaccine.
The Institutional Review Board of Samsung Medical Center approved this retrospective study and informed consent was waived.
2. Materials
The vaccine used in this study was Havrix (GlaxoSmithKline, Philadelphia, PA, USA), which contains 1,440 enzyme-linked immunosorbent assay units of inactivated HM175 HAV per milliliter. Vaccination was implemented according to the recommended 2-dose schedule, and this consists of a primary dose of 1 mL followed by a booster dose of 1 mL 6 months later. At week 48 after the first injection, only the patients with seronegative IgG anti-HAV received a third dose of 1 mL of vaccine. The vaccine was administered in the deltoid muscle to all patients.
3. Laboratory procedures
Commercially available immunoassays (Anti-HAV IgG IRMA kit, North Institute of Biological Technology, Beijing, China; ARCHITECT HBsAg assay, Abbott Laboratories, Sligo, Ireland) were used to detect IgG anti-HAV and HBsAg.
4. Statistical analysis
The categorical and continuous variables were compared using Fisher's exact test and independent two-sample t-tests, respectively, for comparing the clinical characteristics between the seropositive anti-HAV and seronegative anti-HAV groups after HAV vaccination.
A p value of less than 0.05 was considered statistically significant. All the statistical analyses were run on SPSS version 15.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
1. Patient demographics
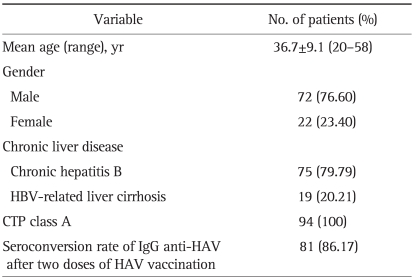
The patient characteristics are detailed in Table 1. The mean age of the patients was 36.7 years (range, 20 to 58 years). A male preponderance (76.6%) was observed and the vast majority of patients had chronic viral hepatitis B (79.79%). The overall seroconversion rate of IgG anti-HAV in the patients with CLD after the recommended two doses of HAV vaccine was 86.17% (81/94). None of the patients showed any serious adverse events associated with the vaccine.
Table 1.
Patient Characteristics
HBV, hepatitis B virus; CTP, Child-Turcotte-Pugh; HAV, hepatitis A virus.
2. Seroconversion rate of IgG anti-HAV according to age, gender, and status of liver disease after two doses of HAV vaccine
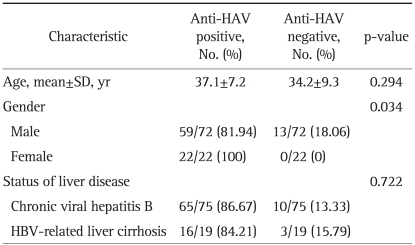
The seroconversion rate of IgG anti-HAV according to age, gender, and status of liver disease in the patients with HBV-related CLD after two doses of HAV vaccine is shown in Table 2. There was no significant difference in age between the seropositive and seronegative anti-HAV groups (mean age, 37.1 vs 34.2 years, respectively; p=0.294). The seroconversion rate was higher for the female patients than that for the male patients (100% vs 81.94%, respectively; p=0.034). As for the status of the liver disease, the chronic viral hepatitis B and HBV related LC groups showed no significant difference of the seroconversion rate after vaccination (86.67% vs 84.21%, respectively; p=0.722).
Table 2.
Seroconversion Rate of IgG Anti-HAV with Respect to Age, Gender, and Status of Liver Disease in Patients with HBV-Related CLD Following Two HAV Vaccine Doses
HAV, hepatitis A virus; HBV, hepatitis B virus; CLD, chronic liver disease.
3. Seroconversion rate of IgG anti-HAV after three doses of HAV vaccination
At weeks 48 after the first injection, an additional third dose of HAV vaccination was administered to only the 13 patients who showed seronegativity for IgG anti-HAV even after two doses of vaccination. The mean age of these 13 patients was 34±7.2 years (range, 21 to 45 years) and all of them were male. They consisted of ten patients with chronic hepatitis and three patients with LC. One patient with chronic hepatitis and one with LC did not attend the follow-up visit at week 72. These two patients were considered to have failed seroconversion at the specified time points. On an intention-to-treat basis, the seroconversion rate for these patients was 84.62% (11/13).
DISCUSSION
Based on the potential risk for fulminant hepatic failure and death from acute HAV superinfection, the 1996 Advisory Committee on Immunization Practices recommended HAV vaccination for all patients with chronic liver disease.12 These recommendations have also been endorsed by the World Health Organization,13 the National Institute of Health14 and others.
There are few published studies that have evaluated the safety and efficacy of HAV vaccine in patients with chronic liver diseases.15-20 For the patients with well-compensated disease, the vaccine shows the same safety and immunogenicity profile as that in the general population, and the seroconversion rate reaches over 80% or 90% after the complete vaccination course. Lee et al.15 reported that the seroconversion rate was 100% in chronic liver disease patients after two doses of vaccine. In that study, 93.3% of the patients (56/60) had HBV infection, and the severity or chronicity of the HBV-related liver disease was not mentioned. In another study by Keeffe et al.16, 94% of all those patients with liver disease and who were vaccinated had seroconversion after two doses of vaccine that were given 6 months apart. However, only a minority of patients in that study had chronic HBV infection (10%). Tsang and Sung19 reported that the seroconversion rate was 80% in 65 patients with chronic HBV infection after two doses of vaccine. Although that study included patients with LC, the Child-Pugh classes of them were not provided.
In patients with advanced cirrhosis and in transplant patients, the vaccination appeared less effective, and seroconversion is achieved in only about 50% of the cases.17,18,20 Arguedas et al.20 reported that for patients with compensated liver disease, including the patients with chronic hepatitis or the Child-Pugh class A patients, a 98% response rate was achieved after a two-dose course of vaccination, as compared with a 65.7% response rate for patients with decompensated liver disease. Among this latter group, the Child-Pugh class B patients had a response rate of 71.4%, whereas a response of 57% was achieved in the Child-Pugh class C patients. In that study, the majority of the patients had chronic viral liver disease, yet the specific viral etiologies were not provided.
In our study, the seroconversion rate of IgG anti-HAV in 94 Korean patients with HBV-related CLD after the recommended two-dose vaccination was 86.17%, which was quite comparable to that of other studies.15,16,19,20
In this current study, the seroconversion rate of anti-HAV after two-dose vaccination was not significantly different according to age and this result is consistent with the results of other studies. Reuman et al.21 reported that adults older than 40 years appear to respond less well than do younger adults to a single dose of hepatitis A vaccine, but both groups of adults responded equally well after two doses of vaccine.
The seroconversion rate of anti-HAV after two-dose vaccination was not significantly different according to the status of liver disease. This is probably attributable to the fact that all the patients had Child-Pugh class A, compensated liver disease.
As has also been reported in another clinical trial,16 the female patients in this study exhibited a higher seroconversion rate than did the male patients. Although the exact reason could not be found, the female patients usually have lower body surface area and conventional dose of vaccination might be relatively higher for them.
Inactivated hepatitis A vaccines have been commercially available in Korea since 1997. Studies have shown that they are safe and effective in healthy subjects and they also seem equally safe in patients with chronic liver diseases, as mentioned earlier. Initially, it was recommended that individuals should be vaccinated with three doses at weeks 0, 4, and 24 and thereafter a single double dose regimen at weeks 0 and 24 has been found to be just as immunogenic.22 Nearly all the patients receiving a single double dose regimen achieved seroconversion, with protective levels of antibody persisting for up to 12 months23 and a single double dose regimen has become the commonly recommended regimen of vaccination.
In the current study, 13.83% of the patients (13/94) were still seronegative for IgG anti-HAV even after the recommended two doses of vaccination. They received an additional third dose of vaccination and seroconversion rate was 84.62% (11/13) on an intension-to-treat basis. To the best of our knowledge, this three-dose regimen of vaccination at weeks 0, 24, and 48 has never been tried in other studies and the current study suggests the possible benefit of this regimen for the patients with CLD and who are non-responsive to the conventional two-dose regimen of HAV vaccination.
The current study has a couple of limitations. First, since less than 50% of the patients completed blood sample tests at week 24, the seroconversion rate and the factors affecting seroconversion after the first dose of vaccination could not be evaluated. Second, only Child-Pugh class A cirrhotic patients were included in this study, and they might not properly represent the features of the patients with HBV-related liver cirrhosis. Third, the current study included a relatively small number of HBV-related CLD patients and the study was performed at a single medical center. So, the patients may not be representative of the whole CLD population of Korea. A large population based prospective controlled study to confirm our results should be considered.
In conclusion, the recommended two-dose HAV vaccination is effective in most Korean patients with HBV-related CLD and it might be necessary to evaluate three-dose vaccination approach for patients who were non-responsive to the conventional regimen to maximize the success of a vaccination program for HAV.
ACKNOWLEDGEMENTS
The authors who have taken part in this study state they do not have anything to declare regarding conflicts of interest or funding from industry with respect to this manuscript.
References
- 1.Kang JH, Lee KY, Kim CH, Sim D. Changing hepatitis A epidemiology and the need for vaccination in Korea. Asian Pac J Allergy Immunol. 2004;22:237–242. [PubMed] [Google Scholar]
- 2.Song HJ, Kim TH, Song JH, et al. Emerging need for vaccination against hepatitis A virus in patients with chronic liver disease in Korea. J Korean Med Sci. 2007;22:218–222. doi: 10.3346/jkms.2007.22.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moon HW, Cho JH, Hur M, et al. Laboratory characteristics of recent hepatitis A in Korea: ongoing epidemiological shift. World J Gastroenterol. 2010;16:1115–1118. doi: 10.3748/wjg.v16.i9.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacobsen KH, Koopman JS. Declining hepatitis A seroprevalence: a global review and analysis. Epidemiol Infect. 2004;132:1005–1022. doi: 10.1017/s0950268804002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tong MJ, el-Farra NS, Grew MI. Clinical manifestations of hepatitis A: recent experience in a community teaching hospital. J Infect Dis. 1995;171(Suppl 1):S15–S18. doi: 10.1093/infdis/171.supplement_1.s15. [DOI] [PubMed] [Google Scholar]
- 6.Bell BP, Shapiro CN, Alter MJ, et al. The diverse patterns of hepatitis A epidemiology in the United States: implications for vaccination strategies. J Infect Dis. 1998;178:1579–1584. doi: 10.1086/314518. [DOI] [PubMed] [Google Scholar]
- 7.Advisory Committee on Immunization Practices (ACIP) Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1999;48(RR-12):1–37. [PubMed] [Google Scholar]
- 8.Bianco E, Stroffolini T, Spada E, et al. Case fatality rate of acute viral hepatitis in Italy: 1995-2000. An update. Dig Liver Dis. 2003;35:404–408. doi: 10.1016/s1590-8658(03)00157-9. [DOI] [PubMed] [Google Scholar]
- 9.Yao GB. Clinical spectrum and natural history of viral hepatitis A in 1988 Shanghai epidemic. In: Hollinger FB, Lemon SM, Margolis H, editors. Viral hepatitis and liver disease: proceedings of the 1990 International Symposium on Viral Hepatitis and Liver Disease--contemporary issues and future prospects. Baltimore: Willams and Wilkins; 1991. pp. 76–78. [Google Scholar]
- 10.Hadler SC. Global impact of hepatitis A virus infection-changing patterns. In: Hollinger FB, Lemon SM, Margolis H, editors. Viral hepatitis and liver disease: proceedings of the 1990 International Symposium on Viral Hepatitis and Liver Disease--contemporary issues and future prospects. Baltimore: Willams and Wilkins; 1991. pp. 14–20. [Google Scholar]
- 11.Keeffe EB. Is hepatitis A more severe in patients with chronic hepatitis B and other chronic liver diseases? Am J Gastroenterol. 1995;90:201–205. [PubMed] [Google Scholar]
- 12.Advisory Committee on Immunization Practices (ACIP) Prevention of hepatitis A through active or passive immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 1996;45(RR-15):1–30. [PubMed] [Google Scholar]
- 13.Public health control of hepatitis A: memorandum from a WHO meeting. Bull World Health Organ. 1995;73:15–20. [PMC free article] [PubMed] [Google Scholar]
- 14.National Institutes of Health. National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: 2002--June 10-12, 2002. Hepatology. 2002;36:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 15.Lee SD, Chan CY, Yu MI, et al. Safety and immunogenicity of inactivated hepatitis A vaccine in patients with chronic liver disease. J Med Virol. 1997;52:215–218. doi: 10.1002/(sici)1096-9071(199706)52:2<215::aid-jmv16>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 16.Keeffe EB, Iwarson S, McMahon BJ, et al. Safety and immunogenicity of hepatitis A vaccine in patients with chronic liver disease. Hepatology. 1998;27:881–886. doi: 10.1002/hep.510270336. [DOI] [PubMed] [Google Scholar]
- 17.Dumot JA, Barnes DS, Younossi Z, et al. Immunogenicity of hepatitis A vaccine in decompensated liver disease. Am J Gastroenterol. 1999;94:1601–1604. doi: 10.1111/j.1572-0241.1999.01150.x. [DOI] [PubMed] [Google Scholar]
- 18.Stark K, Günther M, Neuhaus R, et al. Immunogenicity and safety of hepatitis A vaccine in liver and renal transplant recipients. J Infect Dis. 1999;180:2014–2017. doi: 10.1086/315125. [DOI] [PubMed] [Google Scholar]
- 19.Tsang SW, Sung JJ. Inactivated hepatitis A vaccine in Chinese patients with chronic hepatitis B infection. Aliment Pharmacol Ther. 1999;13:1445–1449. doi: 10.1046/j.1365-2036.1999.00628.x. [DOI] [PubMed] [Google Scholar]
- 20.Arguedas MR, Johnson A, Eloubeidi MA, Fallon MB. Immunogenicity of hepatitis A vaccination in decompensated cirrhotic patients. Hepatology. 2001;34:28–31. doi: 10.1053/jhep.2001.25883. [DOI] [PubMed] [Google Scholar]
- 21.Reuman PD, Kubilis P, Hurni W, Brown L, Nalin D. The effect of age and weight on the response to formalin inactivated, alum-adjuvanted hepatitis A vaccine in healthy adults. Vaccine. 1997;15:1157–1161. doi: 10.1016/s0264-410x(96)00310-6. [DOI] [PubMed] [Google Scholar]
- 22.Van Damme P, Matheï C, Thoelen S, Meheus A, Safary A, André FE. Single dose inactivated hepatitis A vaccine: rationale and clinical assessment of the safety and immunogenicity. J Med Virol. 1994;44:435–441. doi: 10.1002/jmv.1890440422. [DOI] [PubMed] [Google Scholar]
- 23.Van Damme P, Thoelen S, Cramm M, Meheus A. Safety and immunogenicity of a high-potency inactivated hepatitis A vaccine. J Travel Med. 1996;3:83–90. doi: 10.1111/j.1708-8305.1996.tb00711.x. [DOI] [PubMed] [Google Scholar]