Abstract
Background/Aims
The aim of this study was to investigate the primary management experience for giant liver hemangiomas greater than 20 cm in size.
Methods
Records of patients referred for evaluation of radiologically and/or histopathologically proven giant liver hemangiomas between January 2007 and March 2010 were retrospectively analyzed. The reasons for referral, results of imaging studies, preoperative and surgical treatments, and outcome were reviewed.
Results
A retrospective analysis was performed for 14 patients diagnosed with a giant hemangioma on the basis of an imaging study and/or a histopathological examination. All cases were diagnosed as giant liver hemangioma with at least one lesion greater than 20 cm in size. Abdominal discomfort was the main presenting complaint for the referral in 9 patients (64.2%). Abdominal ultrasound established the diagnosis in 12 patients (85.7%). Twelve patients underwent liver resection, 2 of whom underwent staged resection. Enucleation was performed in 2 patients. Selective transcatheter arterial embolization was implemented in 9 patients. Postoperative morbidity occurred in 3 patients (21.4%). No complications related to the hemangiomas occurred during follow up.
Conclusions
Liver resection is indicated for giant liver hemangiomas with abdominal discomfort, especially for lesions greater than 20 cm in size. Staged operations are performed for patients with multiple lesions. Preoperative selective transcatheter arterial embolization alleviates progressive abdominal pain.
Keywords: Liver hemangioma, Liver resection, Transcatheter arterial embolization
INTRODUCTION
Hemangiomas are the most common benign tumor of the liver with an estimated prevalence of 0.4% to 20%.1 The majority of these patients are asymptomatic and seldom presented with specific clinical feature. Therefore, these lesions are usually detected incidentally through the increased use of imaging method for abdominal complaints. These tumors are soft and seldom cause compression of bile ducts, portal vein or inferior vena cava (IVC). Most hemangiomas are small and need no treatment or further follow-up as they have no hazardous effect and damage to adjacent organs. Only giant liver hemangiomas may give rise to mechanical complaints requiring surgical intervention. And giant liver hemangioma was defined as the one with diameter greater than 5 cm.2 In recent years, many reports regarding surgical treatment demonstrated good long-term outcome.3 However, there remains a very difficult challenge in surgical procedure for hemangioma greater than 20 cm because intraoperative bleeding is higher and it may difficult to control. Discussion about the management of giant liver hemangiomas with diameter greater than 20 cm is scarce.
More than 1,000 patients accepted liver resection in our center every year from January 2007, among which about 80 to 100 cases were for giant hemangioma, and 3 to 8 cases with at least one lesion greater than 20 cm underwent surgical treatment. Indications for surgery in our center are based on hemangiomas greater than 10 cm, as well as hemangiomas less than 10 cm with progressive abdominal pain, increase in size or with difficulty in excluding malignancy.
The aim of this study is to introduce our experiences of management of the patients with giant liver hemangioma greater than 20 cm.
MATERIALS AND METHODS
1. Patients
A search of the departmental database was carried out for the patients who underwent treatment of giant liver hemangioma. The 3-year period from January 2007 to March 2010 inclusive was reviewed. Patients were included in the study if they met the criteria of having one lesion greater than 20 cm, or multiple lesions with at least one greater than 20 cm. Patients were excluded if they were not amenable to hepatectomy, as well as those who complicated primary liver cancer confirmed preoperatively.
The medical records were reviewed for documentation of presenting symptoms or indications for further analysis, transcatheter arterial embolization, surgical procedure, blood transfusion and/or autologous transfusion, postoperative stay, and follow-up, including postoperative complications. Results of imaging studies including ultrasound (US) and computed tomography (CT) were reviewed to record total number, size and location of the liver hemangiomas.
2. Preoperative treatment
In each patient, the indication for surgery was discussed and written informed consent taken. Indocyanine green clearance test was performed in all patients to determine the hepatic function reserve. The choice of surgical procedure was at the discretion of the individual surgeon. The type of liver resection performed was based on the size and location of the hemangioma. For patients with multiple giant hemangiomas, we tried to resect all the lesions at one time. However, considering the hepatic function of liver remnant, staged resection was implemented in selected patients.
Transcatheter arterial embolization was implemented in selected patients for alleviating progressive abdominal discomfort if the operation was not performed within 3 to 5 days.
Before considering surgical resection, other possible causes of abdominal complaints such as gallstones, gastroesophageal reflux disease or pancreatitis were excluded.
What's more, the cell-saver apparatus was prepared for autologous transfusion in all cases before surgical resection.
3. Surgical technique
A right shape incision was made below the bilateral costal margin and a variable upper abdominal self-retaining retractor was used to expose the operating field. Each hepatic ligament was liberated and cut off. The infra- and suprahepatic IVC was liberated and then taped to control blood flow, and the same was done for the hepatic pedicle. Hepatic artery and portal vein of lesion side was cut down in patients who need hemihepatectomy to decrease bleeding.
Before separation, the plane of demarcation was delineated by intraoperative ultrasound. When it was considered that a large amount of blood would be lost and/or the operating area was near a vital vessel, the infra- and suprahepatic IVC were excluded in turn to block the total hepatic blood supply so that the vital vessels could be operated on safely and the blood leakage could be corrected. The opening of the vascular exclusion was in the reverse order.
The resection together with absorption was done from the boundary between the hemangioma and the normal hepatic tissue with CUSA, which could decrease the bleeding at the incisional edge. Small diameter nutrient vessels were electrocoagulated and titanium clips had been implemented to small vessels, while large ones were ligated. Only the tumor was removed by this method with little hepatic tissue damaged. The resection along the boundary between the hemangioma and the normal hepatic tissue led to little blood loss.
4. Postoperative treatment
The postoperative treatment included the return to oral feeding after 48 hours via nasogastric aspiration, inhibitors of gastric acid secretion and broad-spectrum antibiotics, daily biochemical monitoring of blood tests, and of hepatic function for the first 5 postoperative days.
5. Follow-up
After discharge, the clinical conditions as well as the hepatic function of all patients were monitored with a median follow-up of 16.3±1.6 months (range, 3 to 43 months). Morphological findings of liver were obtained by US and/or abdominal CT.
RESULTS
A total of 14 patients were treated with liver resection over the 3-year period of the study, which were radiologically and histologically proven liver hemangioma. The characteristics of 14 patients with giant liver hemangiomas are shown in Table 1. The size of liver hemangioma in our subjects ranged from 20.1 to 28.4 cm. All cases were diagnosed as giant liver hemangioma with at least one lesion greater than 20 cm. There were 12 women (85.8%) and 2 men (14.2%) with a mean age of 48.5 (range, 29 to 68) years.
Table 1.
Characteristics of 14 Patients with Giant Liver Hemangiomas
No., number.
Abdominal discomfort was the main presenting feature for referral in 9 patients (64.2%), in which 6 patients complained of abdominal pain and 3 of them presented with abdominal distention. Two patients (14.2%) were diagnosed because of abnormal liver function tests. An incidental finding on abdominal imaging for other reasons occurred in 3 patients (21.4%).
In all the patients, the imaging findings were obtained from the case records. The extent and severity of the disease were evaluated by instrumental examination including hepatic US and abdominal spiral CT scan or magnetic resonance imaging (MRI). Abdominal US established the diagnosis in 12 patients (85.7%). Subsequent CT or MRI established the diagnosis in the other 2 patients. On US, liver hemangioma was usually shown as homogenous, hyperechoic, well-defined lesions (Fig. 1), and was characterized as a well-defined, homogenous, hypodense lesion with peripheral nodular enhancement followed by progressive centripetal enhancement on CT scan image (Fig. 2).
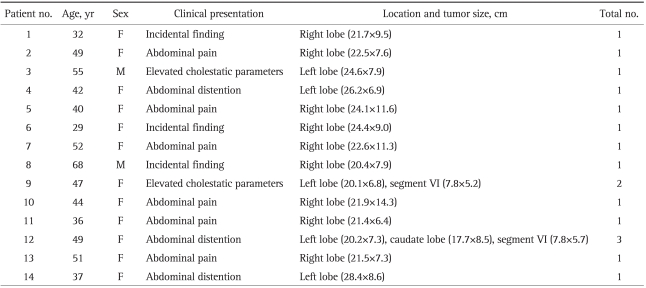
Fig. 1.
(A) Abdominal ultrasound US showing a hyperechoic mass (21.9×14.3 cm) in the right lobe with radiological characteristics of a giant liver hemangioma. (B) Arrow (a) shows the compressed right portal vein; (b) shows the compressed inferior vena cava with slight shifting to the left and anterior to the aorta (arrow c).
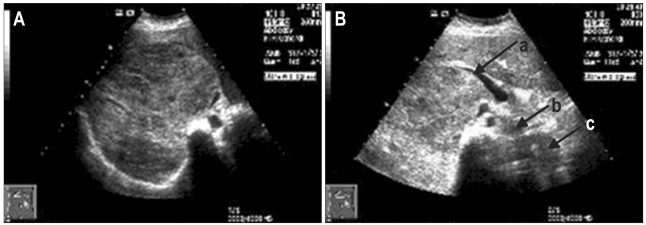
Fig. 2.
(A) Abdominal computed tomography showing a homogenous hypodense lesion with characteristics of a hemangioma (21.9×14.3 cm) in the right lobe. Arrows show the characteristic nodular enhancement. (B) Arrows show three giant hemangiomas. (a) A lesion of 7.8×5.7 cm in S6; (b) A giant hemangioma of 20.2×7.3 cm in left lobe; (c) Another giant hemangioma of 17.7×8.5 cm in the caudate lobe.
Transcatheter arterial embolization was implemented in 9 patients with abdominal discomfort. Abdominal pain was alleviated to some extent in 6 patients with progressive abdominal pain. However, 3 patients got no alleviation of their abdominal distension. The mean size of the tumor did not show any significant change on follow-up imaging studies.
Autologous transfusion was performed by the cell-saver apparatus in all cases. Three patients had blood transfusion of 400 to 1,200 mL, and the other 11 patients had no blood transfusion. All patients had hepatic function restored after 3 to 12 days of symptomatic treatment.
The type of liver resection had been performed on the basis of the size, location and typical morphology of the hemangioma (Table 2). Hemihepatectomy was done in 8 patients, trisectionectomy was done in 4 patients, and enucleation was done in 2 cases.
Table 2.
Characteristics of 14 Patients for Surgical Procedures
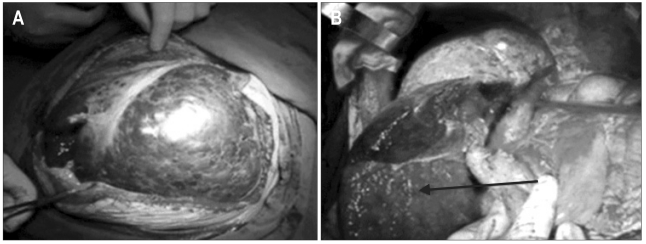
One patient with two giant hemangiomas underwent left hemihatectomy initially and S6 resection one year later. Another patient with 3 hemangiomas located in left and caudate lobe and S6 underwent left hemi-hepatectomy and caudate lobectomy (Fig. 3), and the lesion of S6 had been preserved for next resection to prevent hepatic failure.
Fig. 3.
(A) Giant liver hemangiomas on the operating table. (B) The remnant of the liver before abdominal wall closure. The arrow shows the lesion of S6, which was preserved to prevent hepatic failure.
All the patients had good outcomes without post operative complications except abdominal serous collection in the raw surface of liver remnant in 2 cases and lung infection in 1 case. During the follow-up of surgical procedures, all the patients had no abdominal discomfort with normal liver function, except one case who had hepatitis B infection with markedly elevated ALT and AST level.
DISCUSSION
Liver hemangioma occurs more frequently in women than in men, and is believed to be related to levels of female hormones.4 Majority of the patients in present study has solitary lesion and multiple lesions found in only 14.2% of the patients, comparable to other reports.5,6 The main reason for referral was abdominal discomfort in our series (64.2%), and abdominal US established the diagnosis in most cases. Helical CT or MRI was also important in precise location of the hemangioma diagnosis and selection of operation methods.
Most liver hemangiomas remain stable in size. It has been proposed that asymptomatic patients with hemangiomas less than 5 cm require no intervention therapy. Also, the patients with giant hemangiomas with no symptoms can be observed safely without the chance of developing complications.7 Schnelldorfer et al.8 reported that clinical observation is prefered in most patients with giant hemangioma in a recent retrospective cohort study. The occurrence of spontaneous rupture is rare, even in giant hemangiomas.9 However, in our experience, most patients with hemangioma greater than 20 cm have abdominal discomfort, among which some patients have progressive abdominal pain or distension. For those patients, selective transcatheter arterial embolization was performed for alleviating symptom during the period of waiting for surgical procedure. As a result in our center, abdominal pains were alleviated to some extent in 6 (6/9) patients and no rupture occurred. However, the mean size of the tumor did not show any significant change on follow-up imaging studies, and no alleviation for distention in all 3 patients.
Different treatment modalities of liver hemangiomas apart from resection have been described, such as ligation of hepatic artery10 or liver transplantation for giant unresectable lesions.11 Selective transcatheter arterial embolization12 and radiation therapy13 have also been reported. However, these methods are unsuccessful in the long term except for liver transplantation. Not all the patients with giant hemangioma are fit for liver transplantation for their good hepatic function. The lack of donors, high expenses for transplantation and postoperative immune rejections are all the hazards which we took concern about. Therefore, the treatment choice remains liver resection.
Some reports have mentioned that enucleation is a better option than resection for giant hemangioma with less intraoperative blood loss and less postoperative hospital stay.14,15 However, the mean size of lesions reported was almost not greater than 10 cm, and the majority of subjects taken into account were solitary hemangioma. Hamaloglu et al.16 reported enucleation should be considered in the case of anterior and superficial hemangiomas. In our experience, enucleation for giant lesions greater than 20 cm is difficult and would result in more intro-operative blood loss due to attachment of many vascular structures adjacent to it. However, for liver resection, the infra- and suprahepatic IVC were liberated and then taped to control blood flow, and the same was done for hepatic pedicle. The hepatic artery and portal vein of lesion side were ligated and cut down, and the diminution in size of the hemangioma when squeezed was visualized, which could decrease the bleeding at the incisional edge.
For multiple giant liver hemangiomas, it is difficult to deal with all the lesions at one time for the reason of preservation of enough liver parenchyma, which is critical for the success of the operation. In our center, we tried to resect all the lesions at one time. However, considering the small liver remnant, long operating time, large intraoperative bleeding and poor coagulation for massive blood transfusion, we strongly recommended staged resection for multiple giant liver hemangiomas. For example, one patient with two giant hemangiomas underwent left hemihetactomy initially and S6 resection one year later. She had recovered successfully and had a good follow-up. Another patient with abdominal pain had three giant hemangiomas, for which we recommended liver transplantation. However, she strongly asked for resection because of donor shortage and high expenses for transplantation. Considering her undamaged hepatic function, we implemented left hemihepatectomy and caudate resection at first (Fig. 3). The patient recovered 14 days after operation and would undergo S6 resection one year later. She was asked for follow up every 3 months consecutively, by which her hepatic function and liver imaging can be assessed.
Patient with multiple giant hemangioma who underwent first operation, was needed strict follow-up to assess liver function and regeneration. If the lesion did not affect the major vessels or not get bigger in size or not cause any clinical manifestation, we did not go for second resection, but we asked them regular follow-up for clinical observation.
In conclusion, liver resection is indicated for giant liver hemangiomas with abdominal discomfort, especially for lesions greater than 20 cm, and staged operation is adopted for the patients with multiple lesions. Preoperative selective transcatheter arterial embolization is good to alleviate progessive abdominal pain, but no use to change the size of lesions.
Our study can be criticized for its patient and treatment selection, the lack of report of nonoperative management when introducing surgical management, as well as the retrospective nature of assessment of outcome. However, it highlights the possibility of surgical management for hemangiomas greater than 20 cm.
References
- 1.Coumbaras M, Wendum D, Monnier-Cholley L, Dahan H, Tubiana JM, Arrivé L. CT and MR imaging features of pathologically proven atypical giant hemangiomas of the liver. AJR Am J Roentgenol. 2002;179:1457–1463. doi: 10.2214/ajr.179.6.1791457. [DOI] [PubMed] [Google Scholar]
- 2.Adam YG, Huvos AG, Fortner JG. Giant hemangiomas of the liver. Ann Surg. 1970;172:239–245. doi: 10.1097/00000658-197008000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herman P, Costa ML, Machado MA, et al. Management of hepatic hemangiomas: a 14-year experience. J Gastrointest Surg. 2005;9:853–859. doi: 10.1016/j.gassur.2005.01.292. [DOI] [PubMed] [Google Scholar]
- 4.Tuncer I, Arslan H, Harman M. Two giant cavernous hemangioma caused cavernous transformation of the portal vein in a pregnant woman. Turk J Gastroenterol. 2002;13:229–231. [PubMed] [Google Scholar]
- 5.Gandolfi L, Leo P, Solmi L, Vitelli E, Verros G, Colecchia A. Natural history of hepatic haemangiomas: clinical and ultrasound study. Gut. 1991;32:677–680. doi: 10.1136/gut.32.6.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takayasu K, Makuuchi M, Takayama T. Computed tomography of a rapidly growing hepatic hemangioma. J Comput Assist Tomogr. 1990;14:143–145. doi: 10.1097/00004728-199001000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Farges O, Daradkeh S, Bismuth H. Cavernous hemangiomas of the liver: are there any indications for resection? World J Surg. 1995;19:19–24. doi: 10.1007/BF00316974. [DOI] [PubMed] [Google Scholar]
- 8.Schnelldorfer T, Ware AL, Smoot R, Schleck CD, Harmsen WS, Nagorney DM. Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg. 2010;211:724–730. doi: 10.1016/j.jamcollsurg.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 9.Corigliano N, Mercantini P, Amodio PM, et al. Hemoperitoneum from a spontaneous rupture of a giant hemangioma of the liver: report of a case. Surg Today. 2003;33:459–463. doi: 10.1007/s10595-002-2514-z. [DOI] [PubMed] [Google Scholar]
- 10.Nishida O, Satoh N, Alam AS, Uchino J. The effect of hepatic artery ligation for irresectable cavernous hemangioma of the liver. Am Surg. 1988;54:483–486. [PubMed] [Google Scholar]
- 11.Russo MW, Johnson MW, Fair JH, Brown RS., Jr Orthotopic liver transplantation for giant hepatic hemangioma. Am J Gastroenterol. 1997;92:1940–1941. [PubMed] [Google Scholar]
- 12.Giavroglou C, Economou H, Ioannidis I. Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol. 2003;26:92–96. doi: 10.1007/s00270-002-2648-8. [DOI] [PubMed] [Google Scholar]
- 13.Gaspar L, Mascarenhas F, da Costa MS, Dias JS, Afonso JG, Silvestre ME. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29:45–50. doi: 10.1016/0167-8140(93)90172-5. [DOI] [PubMed] [Google Scholar]
- 14.Singh RK, Kapoor S, Sahni P, Chattopadhyay TK. Giant haemangioma of the liver: is enucleation better than resection? Ann R Coll Surg Engl. 2007;89:490–493. doi: 10.1308/003588407X202038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lerner SM, Hiatt JR, Salamandra J, et al. Giant cavernous liver hemangiomas: effect of operative approach on outcome. Arch Surg. 2004;139:818–821. doi: 10.1001/archsurg.139.8.818. [DOI] [PubMed] [Google Scholar]
- 16.Hamaloglu E, Altun H, Ozdemir A, Ozenc A. Giant liver hemangioma: therapy by enucleation or liver resection. World J Surg. 2005;29:890–893. doi: 10.1007/s00268-005-7661-z. [DOI] [PubMed] [Google Scholar]