Abstract
Invasive gastric Candida infection in patient with co-morbidity can cause stenotic change if it is developed at anatomically narrowing portion, such as distal antrum, pylorus, or duodenal bulb. However, proper management of benign stenosis by diffuse gastric Candidasis is still under controversy and palliative bypass surgery has several shortcomings because high risk operative group may be included in this case. Palliative placement of self-expandable metallic stent has been settled as a standard management of malignant gastric pyloric obstruction and it is expected to be applied in benign stenotic lesions due to its gradual dilation effect. We described a case of stenosis by diffuse gastric Candidasis at anastomosis of subtotal gastrectomy, which was managed by temporary placement of self-expandable metallic stent.
Keywords: Candidiasis, Stenosis, Self-expandable metallic stent
INTRODUCTION
Normally colonized Candida species in digestive tract may grow abnormally and cause plaques or ulcers at mucosa under various conditions (i.e., diabetes, administration of steroids, antibiotics or anti-cancer drugs, malignancy, gastric resection).1,2 Most of Candida infection in the stomach is presented as ulcerations or plaques, or both,3-6 and may cause stenotic change if diffuse Candida infection is developed at anatomically narrowing portion, such as distal antrum, pylorus, or duodenal bulb. We describe a case of pyloric stenosis due to diffuse gastric Candida infection, which was successfully managed by temporary insertion of self-expandable metallic stent (SEMS).
CASE REPORT
A 69-year-old man with type 2 diabetes mellitus was referred to Division of Gastroenterology, Korea University Guro Hospital with anorexia, nausea, vomiting, and epigastric pain. He was diagnosed as diabetes 15 years ago and his blood sugar was controlled by oral hypoglycemic agents. He had undergone subtotal gastrectomy with Billroth-I anastomosis due to advanced gastric cancer (Borrman type 3, T2N0M0) before one month from referral. He had suffered from drowsiness and purulent sputum, and was treated with intravenous antibiotics due to aspiration pneumonia during post-operative care. He did not receive any anti-acid agents, anti-H-2 receptors or oral proton pump inhibitors (PPIs) at that time. His absolute neutrophil count was within normal limit and anti-human immunodeficiency virus (HIV) antibody was negative. An esophagogastroduodenoscopy showed a diffuse mucosal defect at remnant stomach body, which was covered with greenish to yellowish plaque and exudates (Fig. 1). Biopsies were performed at ulcerative lesions and its histologic findings demonstrated that there were many yeast forms of fungal organism with chronic active ulcer, which was compatible with gastric Candidiasis (Fig. 2). Oral fluconazole was administered for more than two weeks, however follow-up esophagogastroduodenoscopy could not show any improvement of above mentioned lesion. Therefore, amphoterecin B was given intravenously for 10 days. His symptoms and endoscopic findings were improved and he was discharged after completion of intravenous amphoterecin B treatment.
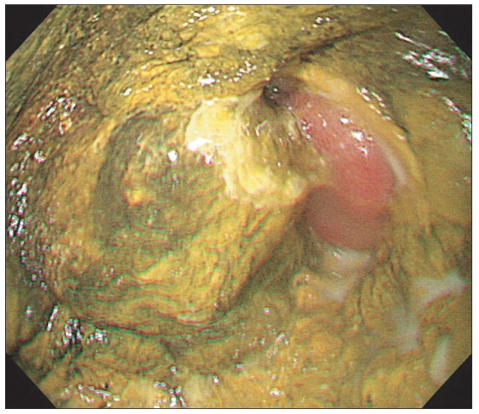
Fig. 1.
Esophagogastroduodenoscopic findings at the time of diagnosis of invasive gastric Candidiasis. A diffuse mucosal defect covered with a greenish to yellow plaque is noted at the anastomosis site.
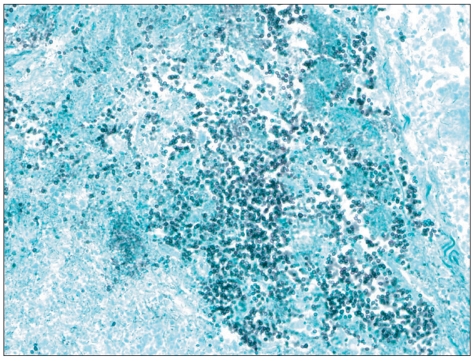
Fig. 2.
Histopathologic findings of a chronic active ulcer using Grocott's Methenamine Silver stain (×400). Yeast forms of fungal organisms were compatible with invasive gastric Candidiasis.
However, his nausea and vomiting recurred after discharge, thus he underwent follow-up esophagogastroduodenoscopy. Ulcerative lesion was much improved comparing with previous findings, however stenotic change at pre-anastomosis site was developed and tip of the scope could not be passed through the narrowing portion (Fig. 3A). Gastroduodenography also indicated partial narrowing near anastomosis site (Fig. 3B). Therefore we inserted SEMS (Bonastent®, covered; Standard Sci Tech, Seoul, Korea) through the anastomosis site at three months after referral (Fig. 4A). After the procedure, his symptom was nearly resolved and did not recur thereafter. On follow-up esophagogastroduodenoscopy which was performed at two months after procedure, the stent was migrated from the anastomosis site and expelled outside the gastrointestinal tract spontaneously. However, anastomosis site remained dilated and the tip of endoscope could be passed through well (Fig. 4B).
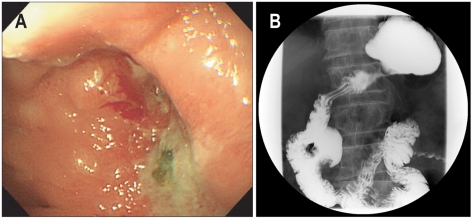
Fig. 3.
(A) An esophagogastroduodenoscopic finding following treatment of gastric Candidiasis. The tip of the scope can not be passed through the stenotic portion at the anastomosis site. (B) A gastroduodenographic finding. Partial narrowing near the anastomosis site is detected.
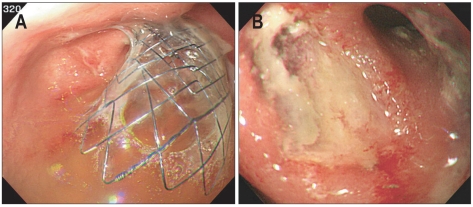
Fig. 4.
Esophagogastroduodenoscopic findings. (A) A self-expandable metallic stent is inserted through the stenotic lesion of the anastomosis site. (B) A follow-up esophagogastroduodenoscopy is performed two months following the procedure, at which point a widened lumen at the anastomosis site was observed.
DISCUSSION
There are many conditions which are known to contribute to the colonization of Candida in gastrointestinal tract, especially in the stomach.7 Hypoacidity after treatment with H-2 receptor blockers or PPIs is associated with colonization of fungus at stomach, and impaired gastric emptying and stasis due to gastric resection or gastroneuropathy may also cause colonization by Candida.7 Other systemic disease, such as immune-deficiency status, especially HIV-infected patients, chronic alcoholism, malignancy or diabetes may predispose to invasive Candida infection in extraesophageal gastrointestinal tract.4,8 In our case, the patient had diabetes and underwent subtotal gastrectomy and these factors would contribute to the development of invasive gastric Candidiasis.
Endoscopically, gastric Candida infection may aggravate and present as diffuse superficial erosions to large ulcers.9 However, there are limited reports about the endoscopic features of Candida-infected ulcers. A previous study reported that unclear border, dirty aspect, bezoar-like morphology (i.e., foreign body), larger diameter (>2.0 cm) and proximal lesion were the characteristic endoscopic findings of Candida infected ulcers.4 Another previous study suggested that majority of benign gastric ulcers associated with Candida infection were found more frequently in fundic area and were rare in antrum or pre-pylorus.3 Radiologic features of Candida-associated gastric ulcers are also little known. Few cases showed radiologic findings resembled those of metastatic gastric cancers or pyloric stenosis.10 It is obvious that endoscopists should be careful to distinguish Candida-infected ulcers from gastric malignancy, especially advanced gastric cancer or recurrent lesions.3
As we mentioned above, stenosis can be developed if diffuse Candida infection occurs at pre-pylorus or anastomosis site of subtotal gastrectomy. If such lesions failed to resolve spontaneously, optimal management is controversial and clinicians may consider palliative surgery (i.e., gastroenterostomy). However, this approach is not available in case of high risk operative group, such as old age or immune-deficiency status. Pyloric stenosis due to benign causes has been treated with endoscopic balloon dilatation as an alternative to surgery, however, although its short-term clinical outcome is favorable, the long-term results are often disappointing, with 51% of patients requiring subsequent surgery, 33% experiencing recurrent obstructions, and there is an associated risk of bleeding or perforation.11-13 In these cases, endoscopic procedure could be an alternative option. Temporary placement of SEMS may be effective for symptomatic improvement in benign pyloric stenosis as well as malignant stricture, although inserted stent could be migrated.14-16 In our previous pilot study,17 we observed 70% of sustained symptomatic improvement in patients with benign pyloric stricture due to recurrent gastric or duodenal ulcers in spite of high rate of stent migration (57%). Stent application in benign stenosis is expected to be beneficial due to more gradual and sustained dilatation in stenotic portion. In our case, symptomatic improvement was sustained and dilated lumen remained for more than 2 months in spite of stent migration.
As far we know, there has been no reported case of stenotic change after fungal infection and its management with temporary stent insertion. We report a case of successful placement of SEMS in patient who had subtotal gastrectomy and stenotic change at anastomosis site after diffuse invasive gastric Candida infection and whose symptom was improved by the procedure.
ACKNOWLEDGEMENTS
The authors report that there are no disclosures relevant to this publication.
References
- 1.Zwolinska-Wcisło M, Budak A, Bogdał J, Trojanowska D, Stachura J. Fungal colonization of gastric mucosa and its clinical relevance. Med Sci Monit. 2001;7:982–988. [PubMed] [Google Scholar]
- 2.Scott BB, Jenkins D. Gastro-oesophageal candidiasis. Gut. 1982;23:137–139. doi: 10.1136/gut.23.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirasaki S, Koide N, Ogawa H, Tsuji T. Benign gastric ulcer associated with Canidida infection in a healthy adult. J Gastroenterol. 1999;34:688–693. doi: 10.1007/s005350050320. [DOI] [PubMed] [Google Scholar]
- 4.Morishita T, Kamiya T, Munakata Y, Tsuchiya M. Radiologic and endoscopic studies of gastric ulcers associated with Candida infection. Acta Gastroenterol Latinoam. 1993;23:223–229. [PubMed] [Google Scholar]
- 5.Rajablou M, Ganz RA, Batts KP. Candida infection presenting as multiple ulcerated masses. Gastrointest Endosc. 2007;65:164–166. doi: 10.1016/j.gie.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Sari R, Altunbas H, Mahsereci E, Meric M, Gelen T, Karayalcin U. Multiple gastric ulcers caused by gastric candidiasis in a diabetic patient: a rare cause of upper GI bleeding. Gastrointest Endosc. 2003;58:309–311. doi: 10.1067/mge.2003.330. [DOI] [PubMed] [Google Scholar]
- 7.Goenka MK, Kochhar R, Chakrabarti A, et al. Candida overgrowth after treatment of duodenal ulcer. A comparison of cimetidine, famotidine, and omeprazole. J Clin Gastroenterol. 1996;23:7–10. doi: 10.1097/00004836-199607000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Höchter W, Wagner N, Hemmer E, Kunert H, Ottenjann R. Fungal infestation of gastroduodenal ulcers: incidence and significance (author's transl) Dtsch Med Wochenschr. 1982;107:845–848. doi: 10.1055/s-2008-1070031. [DOI] [PubMed] [Google Scholar]
- 9.Lee EL, Feldman M. Gastritis and gastropathy. In: Sleisenger MH, Feldman M, Friedman LS, Brandt LJ, editors. Sleisenger & Fordtran's gastrointestinal and liver disease: pathophysiology, diagnosis, management. 8th ed. Philadelphia: Saunders; 2006. pp. 1074–1075. [Google Scholar]
- 10.Nakada K, Watanabe T, Nakazawa K, Takahashi S, Umezono A, Mukai M. Two cases of multiple gastric ulcers with Candida infection disguised themselves as submucosal tumor. Progr Dig Endosc. 1982;21:205–208. [Google Scholar]
- 11.Lau JY, Chung SC, Sung JJ, et al. Through-the-scope balloon dilation for pyloric stenosis: long-term results. Gastrointest Endosc. 1996;43(2 Pt 1):98–101. doi: 10.1016/s0016-5107(06)80107-0. [DOI] [PubMed] [Google Scholar]
- 12.Misra SP, Dwivedi M. Long-term follow-up of patients undergoing ballon dilation for benign pyloric stenoses. Endoscopy. 1996;28:552–554. doi: 10.1055/s-2007-1005553. [DOI] [PubMed] [Google Scholar]
- 13.Solt J, Bajor J, Szabó M, Horváth OP. Long-term results of balloon catheter dilation for benign gastric outlet stenosis. Endoscopy. 2003;35:490–495. doi: 10.1055/s-2003-39664. [DOI] [PubMed] [Google Scholar]
- 14.Fiori E, Lamazza A, Volpino P, et al. Palliative management of malignant antro-pyloric strictures. Gastroenterostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:269–271. [PubMed] [Google Scholar]
- 15.Mittal A, Windsor J, Woodfield J, Casey P, Lane M. Matched study of three methods for palliation of malignant pyloroduodenal obstruction. Br J Surg. 2004;91:205–209. doi: 10.1002/bjs.4396. [DOI] [PubMed] [Google Scholar]
- 16.Profili S, Meloni GB, Bifulco V, Conti M, Feo CF, Canalis GC. Self-expandable metal stents in the treatment of antro-pyloric and/or duodenal strictures. Acta Radiol. 2001;42:176–180. doi: 10.1080/028418501127346477. [DOI] [PubMed] [Google Scholar]
- 17.Kim HJ, Park JJ, Kang CD, et al. Effect of the temporary placement of stent in benign pyloric stenosis. Gastrointest Endosc. 2004;59:P153. [Google Scholar]