Abstract
Background
Tamoxifen is standard adjuvant treatment for postmenopausal women with hormone receptor-positive breast cancer. The benefit of adding chemotherapy and optimal timing of tamoxifen with chemotherapy are unknown.
Methods
We conducted a parallel randomized phase III trial in postmenopausal women with hormone receptor-positive, node-positive breast cancer to test whether disease-free survival (DFS) with cyclophosphamide, doxorubicin (AdriamycinR), and 5-fluorouracil (CAF) plus 5 years of tamoxifen was longer than with tamoxifen alone; and whether DFS with CAF followed by tamoxifen (CAF-T) was better than CAF plus concurrent tamoxifen (CAFT). Overall survival and toxicity were predefined, important secondary outcomes. Randomization was a 2:3:3 (T:CAF-T:CAFT) unblinded allocation and analysis was by intention to treat. This trial is registered with ClinicalTrials.gov, number NCT00929591.
Findings
Of 1558 women randomized, 1477 (95%) were eligible (tamoxifen, 361; CAF-T, 566; CAFT, 550). The combined CAF groups were superior to tamoxifen for the primary outcome of DFS (P=0.002), with adjusted Cox regression hazard ratio (HR) =0.76 (95% CI 0.64,0.91). CAF-T was marginally better than CAFT for DFS (P=0.055) with adjusted HR 0.84 (0.70,1.01). Ten-year DFS for CAF-T, CAFT, and T were 60%, 53%, and 48%, respectively. The planned secondary outcome, overall survival, showed a similar pattern of results, with combined CAF groups seem superior to tamoxifen [p=0.043, adjusted HR 0.83 (0.68,1.01)]. Neutropenia, stomatitis, thromboembolism, congestive heart failure and leukemia were more frequent with CAF than tamoxifen alone.
Interpretation
Chemotherapy with CAF plus tamoxifen resulted in longer survival over tamoxifen in endocrine-responsive, node-positive breast cancer, with greater benefit when tamoxifen followed CAF.
Funding
National Cancer Institute (NIH-USA).
The most common presentation of breast cancer is an estrogen receptor-positive (ER+) tumor in postmenopausal women, for whom tamoxifen is the gold standard against which other systemic adjuvant treatments are compared.(1–4) Whether to add chemotherapy to endocrine therapy is attractive in theory(5), but there is no consensus regarding such treatment in postmenopausal women with tamoxifen-responsive disease.(3,4) Individual phase III trials that compared chemotherapy plus tamoxifen versus tamoxifen alone did not show a significant survival benefit in older women.(6–9) A recent meta-analysis of all existing trials based on individual patient data found that the addition of chemotherapy to tamoxifen is only marginally beneficial in older women, in contrast to major survival improvements in premenopausal populations (see Figure 4, reference 10 for the EBCTCG metaanalysis)
Figure 4.
Southwest Oncology Group trial SWOG-8814 (North American Breast Intergroup 0100) forest plots of hazard ratios and 95% confidence intervals for major subsets for disease-free survival. Panel A describes the disease-free survival advantage for chemotherapy by possible subsets unadjusted for other covariates. Panel B shows the benefit of CAF-T over CAFT in each subset. The dashed vertical line represents the overall unadjusted hazard ratio in each plot.
Most individual trials in postmenopausal women tested the addition of regimens based on cyclophosphamide, methotrexate and 5-fluorouracil (CMF) to tamoxifen(3,4, 6–8, 10), but in certain breast cancer study populations, CMF may be inferior to anthracycline-based regimens(11–16). No clinical trials have shown, however, that anthracycline-based therapy adds to the benefit of tamoxifen specifically in postmenopausal patients with ER+ disease. Moreover, interference with drug-induced cytotoxicity has been found in vitro when tamoxifen is added to cancer cell lines concurrently with chemotherapy(17–20), yet concurrent tamoxifen and CMF has been a common practice in clinical trials.
Our two objectives were to determine if chemotherapy, consisting of 6 months of cyclophosphamide, doxorubicin (AdriamycinR), and 5-fluorouracil (CAF) plus 5 years of tamoxifen, was superior to tamoxifen alone; and to assess if CAF followed by tamoxifen was better than CAF plus concurrent tamoxifen. The CAF program we used was the most dose-intense combination among the commonly used regimens when this trial was designed.(11) This report presents 10-year outcomes for both objectives.
METHODS
The trial was approved by the National Cancer Institute’s Central Institutional Review Board managing all cooperative group trials and the local review board at each institution. All patients gave written informed consent in the presence of an independent witness after the trial was explained by the treating oncologist. Progress of the trial and adverse event rates were reviewed by an independent data and safety monitoring committee every six months.
Eligibility Criteria
Postmenopausal women (defined in the protocol using standard NCI criteria employed across all intergroup trials) with pathologic stage T1-3N1-2 (1988 criteria, 21; excluding clinical N2) infiltrating adenocarcinoma of the breast were eligible. Tumors were either ER+ and/or progesterone receptor positive (PgR+) by biochemical assay (≥10 fmol/mg) or classified as positive by immunohistochemistry according to institutional standards, with all tests performed locally. Liver enzymes, chest radiograph, contralateral mammogram and bone scan had to show no evidence of cancer. Definitive local therapy was modified radical mastectomy or lumpectomy with microscopically-negative margins and axillary dissection. Radiotherapy was mandatory if a lumpectomy was performed and optional after mastectomy if the stage was T3,≥4 positive nodes, or if there was extranodal extension. Left ventricular ejection fraction had to be normal, if performed. Adequate renal, hepatic and bone marrow function were required.
Study Design
A phase III, randomized parallel three-group unblinded controlled trial was conducted. Randomization was to tamoxifen alone (T), CAF followed by tamoxifen (CAF-T), or CAF with concurrent tamoxifen (CAFT) in a 2:3:3 allocation. Eligible patients were stratified by number of involved nodes (1–3 versus ≥4), PgR (positive versus negative), and interval from surgery (≤6 weeks versus >6 weeks). Patients were randomized by a central software program that randomized within the cross-classification of the three stratifying variables with allocation probability to each treatment being determined by the sample size goals. After a center entered the patient’s study ID and stratification variables, the computer generated, reported, and recorded the randomized assignment. It was not possible to know the next assignment since it was to be generated at the next registration. Blocking was not used. Both patient and treating physician were unmasked to randomization assignment.in this open label study.
Treatment
The CAF was given every 4 weeks for 6 cycles: cyclophosphamide, 100 mg/m2 orally days 1–14; doxorubicin (Adriamycin®), 30 mg/m2 and 5-fluorouracil, 500 mg/m2, both intravenously on days 1 and 8. The tamoxifen dose was 20 mg orally daily for 5 years. Dose reduction and toxicity reporting criteria were specified in the protocol. Standardized radiation treatment prescriptions were stipulated in the protocol. The radiation had to be completed prior to CAF or initiated after completion of CAF at the discretion of the physician. It was begun on day 1 in the tamoxifen group. Patients were followed every 4 months for 5 years, every 6 months for 3 years, then yearly thereafter, even if patients withdrew early from treatment. Follow-up for late recurrence was terminated due to financial constraints, but mortality information is still collected where possible.
Statistical Considerations
Target sample size for each CAF group (530/group) gave 89% power to detect a 33% increase in the hazard rate for disease-free survival for CAFT versus CAF-T. Target sample size for the tamoxifen group (350) gave 90% power to detect a 25% reduction in the hazard rate for disease-free survival for the combined CAF groups compared to tamoxifen alone. While the protocol specified 1-sided tests (with overall α=0.05), only 2-sided P-values are reported here. The first interim analysis was planned after 75% of the accrual (α=0.010) and the second (α=0.013), 18 months after accrual was complete, with the final analysis at α=0.04 level so that the combined α=0.05 over all analyses.(22) Disease-free survival (DFS) was defined as the time from registration (randomization) to breast cancer relapse (local or distant), new primary breast cancer, or death due to any cause, whichever came first. Patients who did not have an event were censored at the last follow-up visit.
The intent-to-treat analyses were performed on all eligible patients. Documentation of eligibility was assessed within a few months of randomization and centrally reviewed before outcome information was available. Patients who did not meet eligibility criteria after final review were excluded from the analysis (Figure 1). Analyses included all eligible patients regardless of the actual treatment subsequently received. The primary endpoint was disease-free survival, with overall survival a secondary endpoint. Two planned analyses compared tamoxifen with the combined CAF groups (CAF-T plus CAFT), and CAF-T with CAFT. Secondary analyses comparing all three groups were added when interim results disclosed effect of tamoxifen timing on benefit from chemotherapy. The method of Kaplan and Meier was used for estimation of survival times and stratified log-rank tests were employed to test treatment effects using the three stratifying variables from randomization.(23) Cox models estimated hazard ratios of treatment benefit adjusted for significant prognostic factors. Adverse events were graded according to the Southwest Oncology Group Toxicity Criteria included in the protocol. This trial is registered with ClinicalTrials.gov, number NCT00929591.
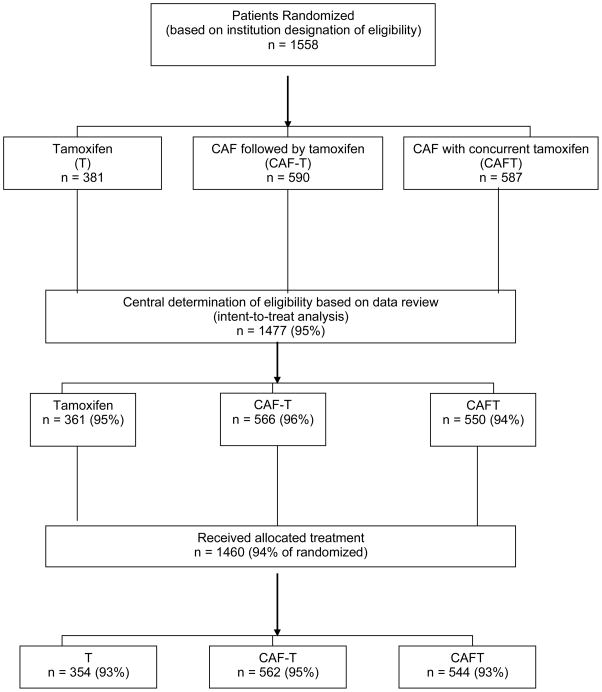
Figure 1.
Consort diagram of study design, accrual and eligibility for Southwest Oncology Group trial SWOG-8814 (The Breast Cancer Intergroup of North America 0100). The figure shows the number randomized, the number who were fully eligible (and analyzed), and the number who actually received their assigned treatment.
Role of the funding source
This trial was solely supported by the National Cancer Institute (NCI) as a High Priority Trial and was administered by the Southwest Oncology Group (SWOG) for The Breast Cancer Intergroup of North America (TBCI). The sponsor had no role in the data analysis, writing the report, or the decision to submit for publication. The Southwest Oncology Group was responsible for data gathering and analysis. The corresponding author with the Southwest Oncology Group had responsibility for the decision to submit for publication.
FINDINGS
Study Conduct and Reporting
Accrual occurred from June, 1989 to July, 1995. Criteria for early stopping were not met at the first planned interim analysis. At the second interim analysis (after reaching the accrual goal), the comparison of tamoxifen alone with the combined CAF groups met criteria for reporting of the primary outcome, DFS.(24) Additional follow-up was required for overall survival(25) and the first comparison of the 2 CAF groups(26). Definitive 10-year estimates of disease-free survival and overall survival for both primary questions are now available. The early analyses reported hazard ratios as tamoxifen versus CAF, while in this analysis they are reported as CAF versus tamoxifen to be consistent with current clinical trial publications.
Study Population and Treatment Delivered
Of 1,558 randomized women, 1477 (95%) were eligible, with 361 assigned to tamoxifen; 566 to CAF-T; and 550 to CAFT (Figure 1). The reasons for ineligibility were wrong stage or incomplete staging. The ineligibility rate did not differ by treatment assignment (P=0.22). Patient and tumor characteristics were well balanced across the treatment groups (Table 1).
Table 1.
North American Breast Intergroup Trial 0100 (SWOG-8814): Patient and Tumor Characteristics
| Characteristic* | Tamoxifen | CAF-T | CAFT | All patients |
|---|---|---|---|---|
| Number randomized (6/89-7/95) | 381 | 590 | 587 | 1558 |
| Number ineligible | 20 (5%) | 24 (4%) | 37 (6%) | 81 (5%) |
| Number included | 361 (95%) | 566 (96%) | 550 (94%) | 1477 (95%) |
| Age | ||||
| Median (years) | 60.0 | 60.7 | 61.8 | 61.3 |
| Range (years) | 37 – 79 | 42 – 81 | 33 – 89 | 33–89 |
| ≥age 65 | 117 (32%) | 162 (29%) | 191 (35%) | 470 (32%) |
| ≥age 70 | 46 (13%) | 62 (11%) | 82 (15%) | 190 (13%) |
| Race/Ethnicity | ||||
| White | 307 (85%) | 492 (87%) | 453 (82%) | 1252 (84.8%) |
| Black | 38 (11%) | 44 (8%) | 57 (10%) | 139 (9.4%) |
| Hispanic | 11 (3%) | 16 (3%) | 23 (4%) | 50 (3.4%) |
| Other | 5 (1%) | 14 (3%) | 17 (3%) | 36 (2.4%) |
| Number involved axillary nodes | ||||
| 1–3(+) | 207 (57%) | 334 (59%) | 311 (57%) | 852 (58%) |
| ≥ 4(+) | 154 (43%) | 232 (41%) | 239 (43%) | 625 (42%) |
| Receptor status of tumor | ||||
| PgR(+)/ER(+) | 261 (73%) | 416 (74%) | 414 (75%) | 1091 (74%) |
| PgR(−)/ER(+) | 84 (23%) | 125 (22%) | 111 (20%) | 320 (22%) |
| PgR(+)/ER(−) | 16 (4%) | 25 (4%) | 25 (5%) | 66 (4%) |
| Tumor size T3 | 27 (7%) | 40 (7%) | 38 (7%) | 105 (7%) |
| Type of primary therapy | ||||
| Breast conservation | 69 (19%) | 101 (18%) | 109 (20%) | 279 (19%) |
| Mastectomy | 292 (81%) | 465 (82%) | 441 (80%) | 1198 (81%) |
| Interval from definitive surgery | ||||
| ≥6 weeks | 257 (71%) | 409 (72%) | 378 (69%) | 1044 (71%) |
| >6 weeks | 104 (29%) | 157 (28%) | 172 (31%) | 433 (29%) |
| Prior postmenopausal estrogens | ||||
| Yes | 76 (21%) | 136 (24%) | 121 (22%) | 333 (23%) |
| No | 285 (79%) | 430 (76%) | 429 (78%) | 1144 (77%) |
All patient and tumor characteristics were very well balanced among the 3 treatment groups;
CAF-T, cyclophosphamide, doxorubicin, 5-fluorouracil followed by tamoxifen; CAFT: T initiated concurrent with CAF
Fifteen percent of patients did not complete 6 cycles of CAF due to toxicity (10%), disease progression or death (1%), or other reasons (4%). There was no significant difference between the two CAF groups. Mean doses delivered (as percent of planned doses) for cycles 1–3 were cyclophosphamide, 86.6%; doxorubicin, 86.1%; and 5-fluorouracil 85.8%; for cycles 4–6 these figures were 72.4%, 74.4%, and 71.8%, respectively. Six percent of patients stopped tamoxifen due to toxicity, 1% did not receive tamoxifen, and 8% discontinued tamoxifen early for miscellaneous reasons. Less than 1% of patients continued to receive tamoxifen beyond 5 years.
Primary Disease-Free and Overall Survival Comparisons: Tamoxifen versus Combined CAF Groups
With tamoxifen alone, there were 179/361 (50%) events (149 relapses, 9 new primary breast cancers, 21 deaths without breast cancer) and in the combined CAF groups there were 458/1116 (41%) events (318 relapses, 27 new primary breast cancers, 113 deaths without breast cancer). Disease-free survival (Figure 2A) was significantly longer in the combined CAF groups than in the tamoxifen alone group, stratified log-rank test P=0.002. The 10-year disease-free survival estimates were 57% (95% CI 53%,60%) for the combined CAF groups and 48% (95% CI 42%,53%) for tamoxifen alone. Table 2 shows that, compared with the tamoxifen group, the hazard ratio for disease-free survival in the combined CAF groups was 0.76 (95% CI 0.64,0.91; P=0.002) by Cox regression, adjusted for the other significant independent prognostic factors of nodal status, receptor status, tumor size, and African American race/ethnicity.
Figure 2.
Southwest Oncology Group trial SWOG-8814 (The Breast Cancer Intergroup of North America 0100) Kaplan-Meier disease-free survival and overall survival distributions for 1116 eligible patients at risk on the combined CAF plus tamoxifen groups (566, CAF-T; 550, CAFT) and 361 on tamoxifen alone. Panel A shows the disease-free survival for the combined CAF plus tamoxifen groups versus tamoxifen alone, log-rank P=0.002, stratified by number of positive nodes, hormone receptor status, and time from definitive surgery.. Panel B depicts the overall survival for the CAF plus tamoxifen versus tamoxifen comparison, stratified log-rank P=0.043. Panel C represents the disease-free survival for the CAFT versus CAF-T comparison, stratified log-rank P=0.055. Panel D shows the overall survival with 10-year estimates for the CAFT versus CAF-T comparison, stratified log-rank P=0.27. Ten-year survival estimates with 95% confidence intervals are also given.
Table 2.
North American Breast Intergroup Trial 0100 (SWOG-8814) Survival Outcomes by Treatment Adjusted for Factors Significant in the Cox Multivariate Model*
| Treatment Comparison | Disease-Free Survival | Overall Survival | ||
|---|---|---|---|---|
| HR (95% CI) | 2-sided P-value | HR (95% CI) | 2-sided P-value | |
| Primary comparisons | ||||
| Combined CAF groups versus Tamoxifen alone | 0.76 (0.64,0.91) | 0.002 | 0.83 (0.68,1.01) | 0.057 |
| CAF-T vs CAFT | 0.84 (0.70,1.01) | 0.061 | 0.90 (0.73,1.10) | 0.30 |
| Secondary comparisons | ||||
| CAF-T vs Tamoxifen | 0.70 (0.57,0.85) | <0.001 | 0.79 (0.63,0.98) | 0.032 |
| CAFT vs Tamoxifen | 0.83 (0.69,1.01) | 0.062 | 0.87 (0.70,1.08) | 0.22 |
| Relevant subset comparisons for combined CAF versus Tamoxifen alone | ||||
| Positive Nodes | ||||
| 1–3 nodes | 0.98 (0.75,1.29) | 0.91 | 0.95 (0.70,1.29) | 0.76 |
| 4+ nodes | 0.63 (0.50, 0.79) | <0.001 | 0.75 (0.59, 0.97) | 0.026 |
| Interaction p= 0.015 | Interaction p = 0.26 | |||
| Age | ||||
| < 65 years | 0.70 (0.57,0.86) | 0.001 | 0.79 (0.62,1.00) | 0.049 |
| >= 65 years | 0.94 (0.69, 1.29) | 0.72 | 0.95 (0.68, 1.33) | 0.76 |
| Interaction p= 0.13 | Interaction p = 0.44 | |||
C, cyclophosphamide; A, Adriamycin®; F, 5-fluorouracil; T, tamoxifen
African American race/ethnicity, nodal status, receptor status, tumor size
Overall survival (Figure 2B) was significantly longer in the combined CAF groups than in the tamoxifen group, stratified log-rank test P=0.043. The Kaplan-Meier survival curves began to diverge after the fourth year and remained separated for the remainder of the study. The 10-year overall survival estimate for the combined CAF groups was 65% (95% CI 62%,68%) and for tamoxifen alone, 60% (95% CI 54%,65%). After adjustment for nodal status, receptor status, tumor size, and African American race/ethnicity, the hazard ratio for death (Table 3) for the combined CAF groups compared to tamoxifen was 0.83 (95% CI 0.68,1.01; P=0.057).
Table 3.
North American Breast Intergroup Trial 0100 (SWOG-8814): Mortality and Morbidity during the First and Subsequent Years of Treatment.
| Early Events (first year of treatment) | Tamoxifen (n=354) | CAF Arms* (n=1106) | |||||
|---|---|---|---|---|---|---|---|
| Numbers of deaths (%) | 0 | 4 (0.36%) | |||||
| Grade 4 neutropenia | 0 | 491 (44%) | |||||
| ≥ Grade 2 emesis | 1 patient | 255 (23%) | |||||
| ≥ Grade 2 stomatitis/mucositis | 2 patients | 294 (27%) | |||||
| Thromboembolic episodes | 0 | 40 (3.6%) | |||||
| Cardiac | |||||||
| Grade 1–2: EF decline | 0 | 4 (0.36%) | |||||
| Grade 3–4: CHF | 0 | 10 (0.9%) | |||||
| Late Events (after year 1, no relapse) | Tamoxifen/Rate** (n=346) | CAF Arms*/Rate** (n=1084) | |||||
| Congestive heart failure*** | 1 | .048 | 25 | .36 | |||
| Any grade thromboembolic event | 7 | .33 | 24∞ | .34 | |||
| Uterine neoplasm | 4† | .16 | 15‡ | .19 | |||
| Endometrial | 3 | .12 | 14 | .17 | |||
| Sarcoma | 1 | .041 | 1 | .012 | |||
| AML/MDS | 0 | 0 | 9 | .14 | |||
T, tamoxifen; C, cyclophosphamide; A, Adriamycin®; F, 5-fluorouracil; EF, ejection fraction; CHF, congestive heart failure; AML, acute myeloid leukemia; MDS, myelodysplastic syndrome
No significant difference in any event between the CAF groups (CAFT, CAF-T)
Rate per 100 person-years of follow-up to relapse or last contact
Excludes coronary artery disease causation
Includes 1 death from pulmonary embolism at year 1.8
Includes 1 death from uterine sarcoma
Includes 2 deaths (1, uterine sarcoma; 1, endometrial carcinoma)
Exploratory, unplanned analyses showed that benefit from the addition of CAF for disease-free survival and overall survival was observed in most major subsets in Table 1. Figure 4A shows unadjusted DFS hazard ratios for subsets based on Table 1 variables, suggesting that there may be variation in the efficacy of chemotherapy particularly with number of positive nodes and age. For disease-free survival patients with ≥4 positive nodes derived more benefit than those with 1–3 positive nodes (test for interaction p=0.015) and patients <65 years may have had a greater degree of benefit than older patients (test for interaction p=0.13) adjusting for prognostic factors (Table 2). Additional variation of the unadjusted hazard ratios in Figure 4A may be attributable to small numbers or especially association with number of positive nodes, such as the type of surgical procedure used.
Primary Disease-Free and Overall Survival Comparisons: CAFT versus CAF-T
Event rates were lower than predicted and there was late separation of the CAFT and CAF-T survival curves for disease-free survival (Figure 2C) and overall survival (Figure 2D). The disease-free survival for CAF-T was marginally superior to CAFT (stratified log-rank test, P=0.055), with 10-year estimates of 60% and 53%, respectively. The hazard ratio for disease-free survival (Table 2) adjusted for nodal status, receptor status, tumor size, and African American race/ethnicity in the Cox model was 0.84 (95% CI 0.70,1.01; P=0.061) for CAF-T compared with CAFT. Figure 4B shows unadjusted DFS hazard ratios comparing CAF-T to CAFT for subsets based on Table 1 variables. The CAF-T benefit is seen in most subsets but confidence intervals are very wide in others. The difference in overall survival was not significant (stratified log-rank test, P=0.27), with 10-year estimates of 68% and 62%, and adjusted hazard ratio (Table 2) of 0.90 (95% CI 0.73,1.10; P=0.30) for CAF-T compared to CAFT.
Secondary Disease-Free Survival and Overall Survival Comparisons
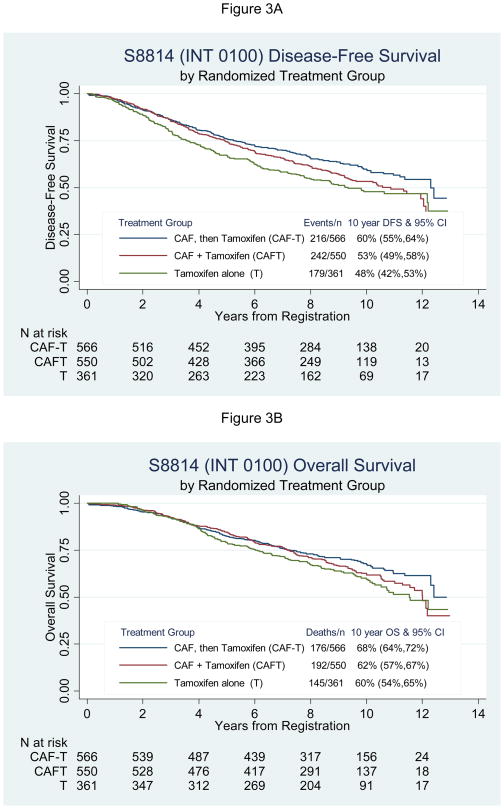
Because the timing of tamoxifen treatment affected the degree of benefit from CAF for both disease-free survival and overall survival, secondary three-way comparisons were undertaken (Figure 3). The three-sample stratified log-rank tests were significant for DFS (P=0.002), but did not quite meet statistical significance for overall survival (P=0.074). Table 2 shows the adjusted hazard ratios for disease-free survival and overall survival for each CAF group compared to tamoxifen alone. The CAF-T hazard ratio for DFS was 0.70 (95% CI 0.57,0.85; P<0.001) and the CAFT hazard ratio was 0.83 (95% CI 0.69,1.01; P=0.062) compared to T alone. For overall survival the adjusted hazard ratios were 0.79 (95% CI 0.63,0.98; P=0.032) and 0.87 (95% CI 0.70,1.08; P=0.22) for CAF-T and CAFT, respectively, compared to tamoxifen alone. The absolute 10-year DFS benefit for CAF-T and CAFT over tamoxifen was 12% and 5%, respectively (Figure 3A).
Figure 3.
Southwest Oncology Group trial SWOG-8814 (North American Breast Intergroup 0100) Kaplan-Meier disease-free survival and overall survival distributions for 566 patients on CAF-T, 550 on CAFT, and 361 on tamoxifen alone. Panel A describes the disease-free survival advantage for the sequential approach, log-rank P=0.002 stratified by number of positive nodes, hormone receptor status, and time of surgery, and Panel B shows superior overall survival the CAF-T group, stratified log-rank P=0.074. Ten-year survival estimates and hazard ratios adjusted for prognostic covariates with 95% confidence intervals are also given.
Deaths from causes other than acute treatment toxicity or breast cancer occurred in 8.8% of patients (25.3% of all deaths). Twenty-two percent of deaths in women <65 years at study entry versus 42% of deaths for women ≥65 years at enrollment was not due to breast cancer or acute toxicity. Specific ascertainment of the reason for these deaths (competing or unrelated cause versus late toxicity) was not possible.
Adverse Events and Toxicity
Mortality and morbidity during year 1 of treatment for tamoxifen and the CAF groups are summarized in Table 3. Four deaths occurred during CAF treatment. The incidence of grade 4 neutropenia was 44% in the CAF groups, but neutropenic fever was uncommon. Grade ≥2 emesis or stomatitis was observed in 23% and 27% of the CAF groups, respectively. There were 40 (3.6%) thrombotic events, mainly deep venous thromboses, in the combined CAF groups and none in the tamoxifen group. There were 10 (0.9%) reports of grade 3–4 congestive heart failure and 4 other patients in the CAF groups had grade 1–2 decline of ejection fraction. No differences in toxicity were noted between CAFT and CAF-T.
Late treatment-related adverse events among 1,430 patients who completed 1 year without early relapse are summarized in Table 3 (rates per 100 person-years of follow-up). Congestive heart failure was reported in 25 patients (0.36) in the combined CAF groups and 1 patient (0.048) in the tamoxifen group. Pulmonary embolism, deep venous thrombosis or stroke occurred in 24 patients (0.34) who received CAF plus tamoxifen and in 7 (0.33) on tamoxifen alone. The distributions of non-breast second primary malignancies between tamoxifen alone and the CAF groups were similar except for secondary acute myelogenous leukemia or myelodysplastic syndrome (9 events or 0.14, CAF groups; 0, tamoxifen group). There were 19 uterine malignancies, rates of 0.16 for tamoxifen alone and 0.19 for the CAF groups. Rates for each of these events were similar for CAFT and CAF-T.
DISCUSSION
We found that adjuvant treatment with a combination of CAF plus tamoxifen significantly improved disease-free survival, as compared with tamoxifen alone in postmenopausal women with node-positive, hormone receptor-positive breast cancer. This advantage was greater in women with 4 or more positive nodes and younger postmenopausal women, though benefit cannot be ruled out for women with 1–3 positive nodes or for older women. A separate paper describes subsets of patients who do not appear to benefit (based on multi-gene analyses of the tumors), despite the overall trial finding that CAF is beneficial compared to tamoxifen alone.
The other primary objective of this study was to investigate whether potential antagonism between tamoxifen and chemotherapy suggested by preclinical data(17–20) was manifested by a worse clinical outcome for concomitant therapy compared with sequential treatment. The magnitude of benefit for disease-free survival when CAF was added to tamoxifen seemed greater when tamoxifen followed chemotherapy than when given concurrently (Figure 2C; Figure 3A, Table 2). Two small trials prospectively addressed timing of tamoxifen administration, but neither found a significant difference.(27,28) There are a number of reasons why concurrent tamoxifen could interfere with chemotherapy (17–20), but none of them has been conclusively proven.
Overall survival was an important secondary outcome in this postmenopausal population. While OS showed the same general trends as DFS, the hazard ratios were attenuated and statistical significance was not achieved in several analyses. This study supports using DFS as a primary outcome due to increased timeliness, but also demonstrates that overall survival significance may require longer follow-up in endocrine-responsive breast cancer.
Our data support sequential anthracycline-based chemotherapy followed by tamoxifen in clinical practice. The recent St. Gallen consensus recommended that beginning tamoxifen after chemotherapy should be the new standard(4) based on our trial results, and this approach is current policy for major cooperative group adjuvant studies. However, the concerns regarding concomitant tamoxifen with chemotherapy raised by this study should not be extrapolated to aromatase inhibitors, the other major form of endocrine adjuvant therapy, that work by a different mechanism in the cell. The optimal timing of these agents with respect to chemotherapy has not been studied.
Common practice is to omit chemotherapy from the systemic therapy adjuvant prescription in postmenopausal women. This practice standard, recommended for certain nodal and ER-level subsets in the St. Gallen consensus, is based on the small benefit of chemotherapy reported in the Oxford meta-analysis, and in individual trials that found no added benefit of chemotherapy.(7–10) Most of these studies were CMF-based, prescribed tamoxifen concurrently with chemotherapy, used intravenous cyclophosphamide in the CMF regimen, or prospectively lowered doses of chemotherapy based on older age.
Instead, our results suggest that anthracycline-based approaches for approximately 6 months may help achieve maximum benefit from chemoendocrine therapy in postmenopausal women with hormone-receptor positive breast cancer. The NSABP reported a disease-free survival advantage from 4 cycles of doxorubicin plus cyclophosphamide added to tamoxifen over tamoxifen alone, but overall survival in the two groups was not significantly different (P=0.08), although premenopausal patients and those with receptor-negative disease were also included in the study.(29) Another group described a disease-free survival but not overall survival advantage to 6 cycles of FEC (E, epirubicin) in postmenopausal women with ER+ breast cancer and 1–3 positive nodes.(30)
However, it is uncertain which women would benefit from chemotherapy compared to endocrine therapy alone. A subset of women with ER+ breast cancer have endocrine-responsive tumors (defined by very high levels of receptor expression) that exhibit different biology and may not be as responsive to chemotherapy.(4, 9) It is probable that the benefit of CAF observed in our study may not occur in all patient subsets. A separate paper will address this important question.
The survival advantage from CAF-based treatment was not without considerable toxicity in some women, such that the common as well as more infrequent toxicities and late events observed must be fully presented at time of the treatment decision-making process. There was an increased risk of thrombotic episodes during the first year of chemoendocrine therapy, as observed by others (8), and no difference in the risk between CAFT and CAF-T. Congestive heart failure was uncommon, but occurred at greater frequency in the CAF groups than in the tamoxifen groups; the anthracycline was likely responsible for this adverse event. The risk of late acute myelogenous leukemia or myelodysplastic syndrome was less than 1%, but it occurred only in the combined CAF groups (no difference between CAFT and CAF-T) and not in the tamoxifen group.
We believe that for postmenopausal women with minimal comorbidities who have a substantial risk of recurrence or death based on the prognostic profile of their tumor, the risk-benefit balance favors anthracycline-based chemotherapy followed by tamoxifen. However, characteristics of the tumor must be factored into the risk-benefit ratio as well. This study illustrates the necessity of long-term follow-up of adjuvant therapies to determine the outcomes of treatment. Many events appeared very late in this study. Without prolonged follow-up, the overall survival advantage to CAF would have been missed and the benefit from delaying tamoxifen until after CAF may have remained hidden.
Acknowledgments
This investigation was supported in part by the following PHS Cooperative Agreement grant numbers awarded by the National Cancer Institute, DHHS: CA32102, CA38926 CA21115, CA02599, CA60138, CA25224, CA77202-06, CA04920, CA58658, CA13612, CA37981, CA76447, CA22433, CA58416, CA20319, CA58686, CA04919, CA46441, CA58861, CA27057, CA32734, CA35281, CA12644, CA16385, CA45560, CA58882, CA14028, CA35176, CA46282, CA46113, CA52650, CA03096, CA28862, CA35090, CA58723, CA35283, CA45807, CA35200, CA35119, CA45450, CA46136, CA42777, CA35261, CA45466, CA35117, CA46368, CA58348, CA12213, CA52654, CA35128, CA58415, CA52623, CA35192, CA45377, CA35996, CA52757, CA76132, CA35431, CA76462, CA45461, CA35084, CA76429, CA35178, CA67663, CA63844, CA52772.
The authors thank the North American Breast Intergroup discipline leaders for their advice or assistance during the design or conduct of this study. Also, we thank the membership of the Breast Committees of SWOG, ECOG, NCCTG, CALGB and NCIC-CTG for their support of this study over the long accrual period and especially the breast cancer survivors who were treated and followed on this protocol.
Footnotes
AUTHOR CONTRIBUTIONS AND CONFLICT OF INTEREST
The corresponding author (KSA), as the principal investigator, participated in all phases of this study, including design and writing of the protocol, study and data/toxicity monitoring, analysis, interpretation and manuscript preparation. The co-investigators from each of the cooperative groups comprising The Breast Cancer Intergroup of North America reviewed the final protocol and participated in ongoing data interpretation. The study biostatisticians (initially SJG, then subsequently WEB, with DL) conducted all the analyses. Coauthors reviewed the manuscript contents and approved the submission version except Dr. Martin D. Abeloff, who is deceased. We are retaining Dr. Abeloff as an author in recognition of his critical leadership within the Intergroup when this study was designed, his role in review of the prior meeting presentations and in his memory. There are no relevant conflicts of interest for any author.
References
- 1.Early Breast Cancer Trialists’ Collaborative Group. Effects of adjuvant tamoxifen and of cytotoxic and of cytotoxic therapy on mortality in early breast cancer: an overview of 61 randomised trials among 28,896 women. N Engl J Med. 1988;319:1681–92. doi: 10.1056/NEJM198812293192601. [DOI] [PubMed] [Google Scholar]
- 2.Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351:1451–67. [PubMed] [Google Scholar]
- 3.National Institutes of Health Consensus Development Panel. NIH consensus development conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. J National Cancer Inst. 2001;93:979–89. doi: 10.1093/jnci/93.13.979. [DOI] [PubMed] [Google Scholar]
- 4.Goldhirsch A, Glick JH, Gelber RD, Coates AS, Thurlimann B, Senn H-J International Consensus Panel members. Meeting Highlights: International expert consensus on the primary therapy of early breast cancer. Ann Onc. 2005;16:1569–1583. doi: 10.1093/annonc/mdi326. [DOI] [PubMed] [Google Scholar]
- 5.Osborne CK. Combined chemohormonal therapy in breast cancer: a hypothesis. Breast Cancer Res Treat. 1981;1:121–23. doi: 10.1007/BF01805864. [DOI] [PubMed] [Google Scholar]
- 6.Crivellari D, Bonetti M, Castiglione-Gertsch M, et al. Burdens and benefits of adjuvant cyclophosphamide, methotrexate, and fluorouracil and tamoxifen for elderly patients with breast cancer: the International Breast Cancer Study Group Trial VII. J Clin Oncol. 2000;18:1412–22. doi: 10.1200/JCO.2000.18.7.1412. [DOI] [PubMed] [Google Scholar]
- 7.Rivkin SE, Green S, Metch B, et al. Adjuvant CMFVP versus tamoxifen versus concurrent CMFVP and tamoxifen for postmenopausal, node-positive, and estrogen receptor-positive breast cancer patients: A Southwest Oncology Group study. J Clin Oncol. 1994;12:2078–85. doi: 10.1200/JCO.1994.12.10.2078. [DOI] [PubMed] [Google Scholar]
- 8.Pritchard KI, Paterson AH, Fine S, et al. Randomized trial of cyclophosphamide, methotrexate and fluorouracil chemotherapy added to tamoxifen as adjuvant therapy in postmenopausal women with node-positive estrogen and/or progesterone receptor-positive breast cancer: a report of the National Cancer Institute of Canada Clinical Trials Group. Breast Cancer Site Group. J Clin Oncol. 1997;15:2302–11. doi: 10.1200/JCO.1997.15.6.2302. [DOI] [PubMed] [Google Scholar]
- 9.Castiglione-Gertsch M, Price KN, Goldhirsch A, et al. Endocrine responsiveness and tailoring adjuvant therapy for postmenopausal lymph node-negative breast cancer: A randomized trial of the International Breast Cancer Study Group (Trial IX) J Natl Cancer Inst. 2002;994:1054–65. doi: 10.1093/jnci/94.14.1054. [DOI] [PubMed] [Google Scholar]
- 10.Early Breast Cancer Trialists’ Collaborative Group. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;365:1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 11.Bull JM, Tormey DC, Li SH, et al. A randomized comparative trial of Adriamycin versus methotrexate in combination drug therapy. Cancer. 1978;41:1649–57. doi: 10.1002/1097-0142(197805)41:5<1649::aid-cncr2820410501>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 12.Buzdar A, Hortobagyi G, Marcus C, Smith T, Martin R, Gehan E. Results of adjuvant chemotherapy trials in breast cancer at M. D. Anderson Hospital and Tumor Institute. NCI Monogr. 1986;1:81–85. [PubMed] [Google Scholar]
- 13.Cummings F, Gelman R, Horton J. Comparison of CAF versus CMFP in metastatic breast cancer: analysis of prognostic factors. J Clin Oncol. 1985;3:932–40. doi: 10.1200/JCO.1985.3.7.932. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Redmond C, Wickerham DL, et al. Doxorubicin-containing regimens for the treatment of stage II breast cancer: the National Surgical Breast and Bowel Project experience (B11, B12) J Clin Oncol. 1989;7:572–82. doi: 10.1200/JCO.1989.7.5.572. [DOI] [PubMed] [Google Scholar]
- 15.Levine M, Bramwell V, Pritchard K, et al. Randomized trial of intensive cyclophosphamide, methotrexate and fluorouracil in premenopausal women with node-positive breast cancer. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1998;16:2651–58. doi: 10.1200/JCO.1998.16.8.2651. [DOI] [PubMed] [Google Scholar]
- 16.Perloff M, Norton L, Korzun AH, et al. Postsurgical adjuvant chemotherapy of stage II breast carcinoma with or without crossover to a non-cross-resistant regimen: a Cancer and Leukemia Group B study. J Clin Oncol. 1996;14:1589–98. doi: 10.1200/JCO.1996.14.5.1589. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg GJ, Froese EK. Antagonism of the cytocidal activity and uptake of melphalan in human breast cancer cells in vitro. Biochem Pharmacol. 1985;34:763–70. doi: 10.1016/0006-2952(85)90755-5. [DOI] [PubMed] [Google Scholar]
- 18.Osborne CK, Kitten L, Arteaga CL. Antagonism of chemotherapy-induced cytotoxicity for human breast cancer cells by antiestrogens. J Clin Oncol. 1989;7:710–717. doi: 10.1200/JCO.1989.7.6.710. [DOI] [PubMed] [Google Scholar]
- 19.Hug V, Hortobagyi G, Drewinko B, Finders F. Tamoxifen citrate counteracts antitumor effects of cytotoxic drugs in vitro. J Clin Oncol. 1985;3:1672–77. doi: 10.1200/JCO.1985.3.12.1672. [DOI] [PubMed] [Google Scholar]
- 20.Sutherland RL, Green MD, Hall RE, et al. Tamoxifen induces accumulation of MCF7 cells in the G0/G1 phase of the cell cycle. Eur J Cancer Clin Oncol. 1983;19:615–21. doi: 10.1016/0277-5379(83)90177-3. [DOI] [PubMed] [Google Scholar]
- 21.American Joint Commission on Cancer (AJCC) Staging Manual. 5. Lippincott Raven; Philadelphia, PA: 1988. [Google Scholar]
- 22.Fleming T, O’Brien P, Harrington D. Designs for group sequential tests. Controlled Clinical Trials. 1984:348–61. doi: 10.1016/s0197-2456(84)80014-8. [DOI] [PubMed] [Google Scholar]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemotherapy Reports. 1966;50:163–70. [PubMed] [Google Scholar]
- 24.Albain K, Green S, Osborne K, et al. Tamoxifen (T) vs. cyclophosphamide, Adriamycin, and 5-FU (CAF) plus either concurrent or sequential T in postmenopausal, receptor(+), node(+) breast cancer: a Southwest Oncology Group phase III Intergroup trial (SWOG-8814, INT-0100) Proc Amer Soc Clin Oncol. 1997;16:128a. [Google Scholar]
- 25.Albain K, Green S, Ravdin P, et al. Overall survival after cyclophosphamide, Adriamycin, 5-FU and tamoxifen is superior to tamoxifen alone in postmenopausal, receptor(+), node(+) breast cancer: new findings from phase III Southwest Oncology Group Intergroup Trial S8814 (INT-0100) Proc Amer Soc Clin Oncol. 2001;20:24a. [Google Scholar]
- 26.Albain KS, Green SJ, Ravdin PM, et al. Adjuvant chemohormonal therapy for primary breast cancer should be sequential instead of concurrent: Initial results from Intergroup trial 0100 (SWOG-8814) Proc Amer Soc Clin Oncol. 2002;21:37a. [Google Scholar]
- 27.Pico C, Martin M, Jara C, et al. Epirubicin-cyclophosphamide chemotherapy plus tamoxifen administered concurrent versus sequential: randomized phase III trial in postmenopausal node-positive breast cancer patients. GEICAM 9401 study. Proc Amer Soc Clin Oncol. 2002;21:37a. doi: 10.1093/annonc/mdh016. [DOI] [PubMed] [Google Scholar]
- 28.Sertoli MR, Pronzato P, Ventorini M, et al. A randomized study of concurrent versus sequential adjuvant chemotherapy and tamoxifen in stage II breast cancer. Proc Amer Soc Clin Oncol. 2002;21:46a. [Google Scholar]
- 29.Fisher B, Redmond C, Legault-Poisson S, et al. Postoperative chemotherapy and tamoxifen compared with tamoxifen alone in the treatment of positive-node breast cancer patients aged 50 years and older with tumors responsive to tamoxifen: results from the national Surgical Adjuvant Breast and Bowel Project B-16. J Clin Oncol. 1990;8:1005–18. doi: 10.1200/JCO.1990.8.6.1005. [DOI] [PubMed] [Google Scholar]
- 30.Namer M, Fargeot P, Roche H, et al. Improved disease-free survival with epirubicin-based chemoendocrine adjuvant therapy compared with tamoxifen alone in non-positive, estrogen-receptor-positive breast cancer patients: 9pyear follow-up analysis from pooled FASG 02 and 07 trials. Breast Cancer Res Treat. 2004;88(suppl 1):S54. [Google Scholar]