Abstract
Voltage-gated calcium (Ca2+) channels are key transducers of membrane potential changes into intracellular Ca2+ transients that initiate many physiological events. There are ten members of the voltage-gated Ca2+ channel family in mammals, and they serve distinct roles in cellular signal transduction. The CaV1 subfamily initiates contraction, secretion, regulation of gene expression, integration of synaptic input in neurons, and synaptic transmission at ribbon synapses in specialized sensory cells. The CaV2 subfamily is primarily responsible for initiation of synaptic transmission at fast synapses. The CaV3 subfamily is important for repetitive firing of action potentials in rhythmically firing cells such as cardiac myocytes and thalamic neurons. This article presents the molecular relationships and physiological functions of these Ca2+ channel proteins and provides information on their molecular, genetic, physiological, and pharmacological properties.
The activity of a voltage-gated calcium channel is enhanced by the binding of an effector ready to respond to a calcium signal. This effector checkpoint mechanism may be a unifying theme in voltage-gated calcium channel regulation.
PHYSIOLOGICAL ROLES OF VOLTAGE-GATED Ca2+ CHANNELS
Ca2+ channels in many different cell types activate on membrane depolarization and mediate Ca2+ influx in response to action potentials and subthreshold depolarizing signals. Ca2+ entering the cell through voltage-gated Ca2+ channels serves as the second messenger of electrical signaling, initiating many different cellular events (Fig. 1). In cardiac and smooth muscle cells, activation of Ca2+ channels initiates contraction directly by increasing cytosolic Ca2+ concentration and indirectly by activating calcium-dependent calcium release by ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum (Reuter 1979; Tsien 1983; Bers 2002). In skeletal muscle cells, voltage-gated Ca2+ channels in the transverse tubule membranes interact directly with ryanodine-sensitive Ca2+ release channels in the sarcoplasmic reticulum and activate them to initiate rapid contraction (Catterall 1991; Tanabe et al. 1993). The same Ca2+ channels in the transverse tubules also mediate a slow Ca2+ conductance that increases cytosolic concentration and thereby regulates the force of contraction in response to high-frequency trains of nerve impulses (Catterall 1991). In endocrine cells, voltage-gated Ca2+ channels mediate Ca2+ entry that initiates secretion of hormones (Yang and Berggren 2006). In neurons, voltage-gated Ca2+ channels initiate synaptic transmission (Tsien et al. 1988; Dunlap et al. 1995; Catterall and Few 2008). In many different cell types, Ca2+ entering the cytosol via voltage-gated Ca2+ channels regulates enzyme activity, gene expression, and other biochemical processes (Flavell and Greenberg 2008). Thus, voltage-gated Ca2+ channels are the key signal transducers of electrical excitability, converting the electrical signal of the action potential in the cell surface membrane to an intracellular Ca2+ transient. Signal transduction in different cell types involves different molecular subtypes of voltage-gated Ca2+ channels, which mediate voltage-gated Ca2+ currents with different physiological, pharmacological, and regulatory properties.
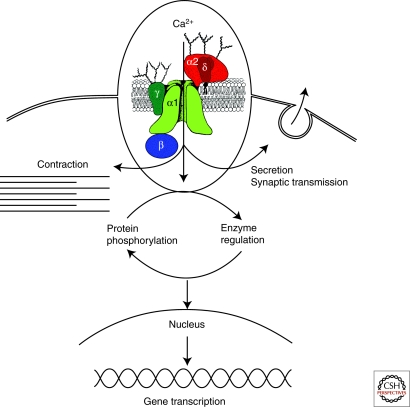
Figure 1.
Signal transduction by voltage-gated Ca2+ channels. Ca2+ entering cells initiates numerous intracellular events, including contraction, secretion, synaptic transmission, enzyme regulation, protein phosphorylation/dephosphorylation, and gene transcription. (Inset) Subunit structure of voltage-gated Ca2+ channels. The five-subunit complex that forms high-voltage-activated Ca2+ channels is illustrated with a central pore-forming α1 subunit, a disulfide-linked glycoprotein dimer of α2 and δ subunits, an intracellular β subunit, and a transmembrane glycoprotein γ subunit (in some Ca2+ channel subtypes). As described in the text, this model is updated from the original description of the subunit structure of skeletal muscle Ca2+ channels. (Adapted from Takahashi et al. 1987).
Ca2+ CURRENT TYPES DEFINED BY PHYSIOLOGICAL AND PHARMACOLOGICAL PROPERTIES
Since the first recordings of Ca2+ currents in cardiac myocytes (reviewed in Reuter 1979), it has become apparent that there are multiple types of Ca2+ currents as defined by physiological and pharmacological criteria (Tsien et al. 1988; Bean 1989a; Llinás et al. 1992). In cardiac, smooth, and skeletal muscle, the major Ca2+ currents are distinguished by high voltage of activation, large single channel conductance, slow voltage-dependent inactivation, marked up-regulation by cAMP-dependent protein phosphorylation pathways, and specific inhibition by Ca2+ antagonist drugs including dihydropyridines, phenylalkylamines, and benzothiazepines (Table 1) (Reuter 1979; Tsien et al. 1988). These Ca2+ currents have been designated L-type, as they have slow voltage-dependent inactivation and therefore are long lasting when Ba2+ is the current carrier and there is no Ca2+-dependent inactivation (Tsien et al. 1988). L-type Ca2+ currents are also recorded in endocrine cells where they initiate release of hormones (Yang and Berggren 2006) and in neurons where they are important in regulation of gene expression, integration of synaptic input, and initiation of neurotransmitter release at specialized ribbon synapses in sensory cells (Tsien et al. 1988; Bean 1989a; Flavell and Greenberg 2008). L-type Ca2+ currents are subject to regulation by second messenger–activated protein phosphorylation in several cell types as discussed below.
Table 1.
Subunit composition and function of Ca2+ channel types
| Ca2+ current type | α1 Subunits | Specific blocker | Principal physiological functions | Inherited diseases |
|---|---|---|---|---|
| L | Cav1.1 | DHPs | Excitation-contraction coupling in skeletal muscle, regulation of transcription | Hypokalemic periodic paralysis |
| Cav1.2 | DHPs | Excitation-contraction coupling in cardiac and smooth muscle, endocrine secretion, neuronal Ca2+ transients in cell bodies and dendrites, regulation of enzyme activity, regulation of transcription | Timothy syndrome: cardiac arrhythmia with developmental abnormalites and autism spectrum disorders | |
| Cav1.3 | DHPs | Endocrine secretion, cardiac pacemaking, neuronal Ca2+ transients in cell bodies and dendrites, auditory transduction | ||
| Cav1.4 | DHPs | Visual transduction | Stationary night blindness | |
| N | Cav2.1 | ω-CTx-GVIA | Neurotransmitter release, Dendritic Ca2+ transients |
|
| P/Q | Cav2.2 | ω-Agatoxin | Neurotransmitter release, Dendritic Ca2+ transients |
Familial hemiplegic migraine, cerebellar ataxia |
| R | Cav2.3 | SNX-482 | Neurotransmitter release, Dendritic Ca2+ transients |
|
| T | Cav3.1 | None | Pacemaking and repetitive firing | |
| Cav3.2 | Pacemaking and repetitive firing | Absence seizures | ||
| Cav3.3 |
Abbreviations: DHP, dihydropyridine; ω-CTx-GVIA, ω-conotoxin GVIA from the cone snail Conus geographus; SNX-482, a synthetic version of a peptide toxin from the tarantula Hysterocrates gigas.
Electrophysiological studies of Ca2+ currents in starfish eggs (Hagiwara et al. 1975) first revealed Ca2+ currents with different properties from L-type, and these were subsequently characterized in detail in voltage-clamped dorsal root ganglion neurons (Carbone and Lux 1984; Fedulova et al. 1985; Nowycky et al. 1985). In comparison to L-type, these novel Ca2+ currents activated at much more negative membrane potentials, inactivated rapidly, deactivated slowly, had small single channel conductance, and were insensitive to conventional Ca2+ antagonist drugs available at that time (Table 1). They were designated low-voltage-activated Ca2+ currents for their negative voltage dependence (Carbone and Lux 1984) or T-type Ca2+ currents for their transient openings (Nowycky et al. 1985).
Whole-cell voltage clamp and single-channel recording from dissociated dorsal root ganglion neurons revealed an additional Ca2+ current, N-type (Table 1) (Nowycky et al. 1985). N-type Ca2+ currents were initially distinguished by their intermediate voltage dependence and rate of inactivation—more negative and faster than L-type but more positive and slower than T-type (Nowycky et al. 1985). They are insensitive to organic L-type Ca2+ channel blockers but blocked by the cone snail peptide ω-conotoxin GVIA and related peptide toxins (Tsien et al. 1988; Olivera et al. 1994). This pharmacological profile has become the primary method to distinguish N-type Ca2+ currents, because the voltage dependence and kinetics of N-type Ca2+ currents in different neurons vary considerably.
Analysis of the effects of other peptide toxins revealed three additional Ca2+ current types (Table 1). P-type Ca2+ currents, first recorded in Purkinje neurons (Llinás and Yarom 1981; Llinás et al. 1989), are distinguished by high sensitivity to the spider toxin ω-agatoxin IVA (Mintz et al. 1992). Q-type Ca2+ currents, first recorded in cerebellar granule neurons (Randall and Tsien 1995), are blocked by ω-agatoxin IVA with lower affinity. R-type Ca2+ currents in cerebellar granule neurons are resistant to most subtype-specific organic and peptide Ca2+ channel blockers (Randall and Tsien 1995) and may include multiple channel subtypes (Tottene et al. 1996). They can be blocked selectively in some cell types by the peptide SNX-482 derived from the tarantula Hysterocrates gigas (Newcomb et al. 1998). Although L-type and T-type Ca2+ currents are recorded in a wide range of cell types, N-, P-, Q-, and R-type Ca2+ currents are most prominent in neurons.
MOLECULAR PROPERTIES OF Ca2+ CHANNELS
Subunit Structure
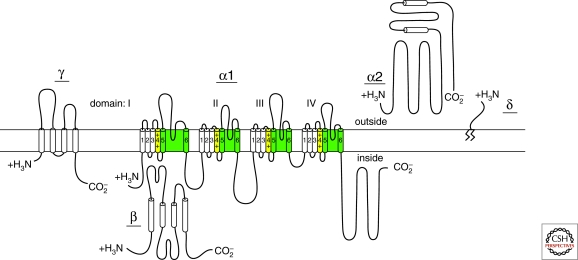
Ca2+ channels purified from skeletal muscle transverse tubules are complexes of α1, α2, β, γ, and δ subunits (Fig. 1) (Curtis and Catterall 1984, 1986; Flockerzi et al. 1986; Hosey et al. 1987; Leung et al. 1987; Striessnig et al. 1987; Takahashi et al. 1987). Analysis of the biochemical properties, glycosylation, and hydrophobicity of these five subunits led to a model comprising a principal transmembrane α1 subunit of 190 kDa in association with a disulfide-linked α2δ dimer of 170 kDa, an intracellular phosphorylated β subunit of 55 kDa, and a transmembrane γ subunit of 33 kDa (Fig. 1) (Takahashi et al. 1987).
The α1 subunit is a protein of about 2000 amino acid residues in length with an amino acid sequence and predicted transmembrane structure like the previously characterized, pore-forming α subunit of voltage-gated sodium channels (Fig. 2) (Tanabe et al. 1987). The amino acid sequence is organized in four repeated domains (I–IV), which each contains six transmembrane segments (S1–S6) and a membrane-associated loop between transmembrane segments S5 and S6. As expected from biochemical analysis (Takahashi et al. 1987a), the intracellular β subunit has predicted α helices but no transmembrane segments (Fig. 2) (Ruth et al. 1989), whereas the γ subunit is a glycoprotein with four transmembrane segments (Fig. 2) (Jay et al. 1990). The cloned α2 subunit has many glycosylation sites and several hydrophobic sequences (Ellis et al. 1988), but biosynthesis studies indicate that it is an extracellular, extrinsic membrane glycoprotein, attached to the membrane through disulfide linkage to the δ subunit (Fig. 2) (Gurnett et al. 1996). The δ subunit is encoded by the 3′ end of the coding sequence of the same gene as the α2 subunit, and the mature forms of these two subunits are produced by posttranslational proteolytic processing and disulfide linkage (Fig. 2) (De Jongh et al. 1990). Although it was initially assumed that the δ subunit was anchored to the membrane via a single membrane segment, recent work argues persuasively that further posttranslational processing actually cleaves the predicted transmembrane segment and replaces it with a glycophosphatidylinositol membrane anchor (Fig. 2) (Davies et al. 2010).
Figure 2.
Subunit structure of Ca2+ channels. The structures of Ca2+ channel subunits are illustrated as transmembrane folding models; predicted α helices are depicted as cylinders; the lengths of lines correlate approximately to the lengths of the polypeptide segments represented; and the zigzag line on the δ subunit illustrates its glycophosphatidylinositol anchor.
Purification of cardiac Ca2+ channels labeled by dihydropyridine Ca2+ antagonists identified subunits of the sizes of the α1, α2δ, β, and γ subunits of skeletal muscle Ca2+ channels (Chang and Hosey 1988; Schneider and Hofmann 1988; Kuniyasu et al. 1992), whereas immunoprecipitation of Ca2+ channels from neurons labeled by dihydropyridine Ca2+ antagonists revealed α1, α2δ, and β subunits but no γ subunit (Ahlijanian et al. 1990). Purification and immunoprecipitation of N-type and P/Q-type Ca2+ channels labeled by ω-conotoxin GVIA and ω-agatoxin IVA, respectively, from brain membrane preparations also revealed α1, α2δ, and β subunits but not γ subunits (McEnery et al. 1991; Martin-Moutot et al. 1995; Witcher et al. 1995a; Liu et al. 1996). More recent experiments have unexpectedly revealed a novel γ subunit (stargazin), which is the target of the stargazer mutation in mice (Letts et al. 1998), and a related series of seven γ subunits is expressed in brain and other tissues (Klugbauer et al. 2000). These γ-subunit-like proteins can modulate the voltage dependence of CaV2.1 channels expressed in nonneuronal cells, so they may be associated with these Ca2+ channels in vivo. However, the stargazin-like γ subunits (also called transmembrane AMPA receptor modulators [TARPs]) are the primary modulators of glutamate receptors in the postsynaptic membranes of brain neurons (Nicoll et al. 2006), and it remains to be determined whether they are also associated with voltage-gated Ca2+ channels in brain neurons in vivo.
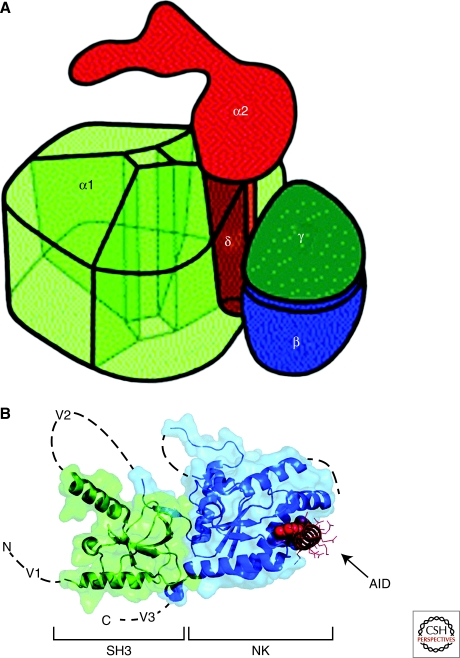
Three-Dimensional Structure of Ca2+ Channels
The three-dimensional structure of Ca2+ channels is not known at high resolution. Low-resolution structural models have been developed from image reconstruction analysis of CaV1.1 channels purified from skeletal muscle membranes (Serysheva et al. 2002; Wang et al. 2002; Wolf et al. 2003), and some of the structural features have been associated with the α1, β, and α2δ subunits (Fig. 3A). Further high-resolution structural analysis will be required to confirm these initial structural models. The three-dimensional structure of the CaVβ subunits has been determined at high resolution by X-ray crystallography (Fig. 3B) (Chen et al. 2004; Van Petegem et al. 2004). These subunits contain conserved SH3 and guanylate kinase domains like the MAGUK family of scaffolding proteins. These two domains are arrayed side-by-side in the CaVβ subunit (Fig. 3B). The CaVβ subunits bind to a single site in the α1 subunits (the α interaction domain, AID) (Pragnell et al. 1994), which is located in the first half of the intracellular loop connecting domains I and II. The AID forms an α helix that is bound tightly to a groove in the guanylate kinase domain of the CaVβ subunit. This tight, multipoint binding interaction likely sustains the association between Ca2+ channel α1 and β subunits throughout the lifetime of the Ca2+ channel complex at the cell surface membrane. MAGUK proteins often bind more than one protein partner, so CaVβ subunits may also interact with other intracellular proteins, and several potential binding partners are under active investigation.
Figure 3.
Three-dimensional architecture of Ca2+ channels. (A) Illustration of the skeletal muscle CaV1.1 channel based on cryo-electronmicroscopy. This drawing assumes pseudo-fourfold symmetry of the α1 subunit. The view shows the extracellular side with the α2 subunit. The α1, γ, and δ subunits are embedded into the lipid membrane (not shown), which separates the extracellular α2 subunit from the cytosol. α2 is anchored via the disulfide-linked δ subunit within the α1 subunit. The proposed model allows for a tight interaction between α1 and δ as well as α1 and γ. (B) Structure of the CaVβ subunit with the α interaction domain (AID). Coordinates are for the CaVβ2a–CaV1.2 AID complex with SH3 (green) and NK (blue) domains are indicated. V1, V2, and V3 show the locations of the three variable domains that are absent from the structure. The AID (red) binds to a deep groove in the NK domain. AID residues tyrosine, tryptophan, and isoleucine are shown as CPK. The remaining residues are shown as lines.
Functions of Ca2+ Channel Subunits
Expression of the α1 subunit is sufficient to produce functional skeletal muscle Ca2+ channels, but with low expression level and abnormal kinetics and voltage dependence of the Ca2+ current (Perez-Reyes et al. 1989). Coexpression of the α2δ subunit and especially the β subunit enhanced the level of expression and conferred more normal gating properties (Lacerda et al. 1991; Singer et al. 1991). As for skeletal muscle Ca2+ channels, coexpression of β subunits has a large effect on the level of expression and the voltage dependence and kinetics of gating of cardiac and neuronal Ca2+ channels (reviewed in Hofmann et al. 1994; Dolphin 2003). In general, the level of expression is increased and the voltage dependence of activation and inactivation is shifted to more negative membrane potentials, and the rate of inactivation is increased. However, these effects are different for the individual β subunit isoforms. For example, the β2a subunit slows channel inactivation in most subunit combinations. Coexpression of α2δ subunits also increases expression and enhances function of Ca2+ channels, but to a lesser extent and in a more channel-specific way than do β subunits (Arikkath and Campbell 2003; Davies et al. 2007). In general, γ subunits have smaller effects.
Ca2+ Channel Diversity
The different types of Ca2+ currents are primarily defined by different α1 subunits, and ten different ones have been characterized by cDNA cloning and functional expression in mammalian cells or Xenopus oocytes (Table 1). These subunits can be divided into three structurally and functionally related families (CaV1, CaV2, and CaV3) (Snutch and Reiner 1992; Ertel et al. 2000). L-type Ca2+ currents are mediated by the CaV1 type of α1 subunits, which have about 75% amino acid sequence identity among them. The CaV2 type Ca2+ channels form a distinct subfamily with <40% amino acid sequence identity with CaV1 α1 subunits but >70% amino acid sequence identity among themselves. Cloned CaV2.1 subunits (Mori et al. 1991; Starr et al. 1991) conduct P- or Q-type Ca2+ currents, which are inhibited by ω-agatoxin IVA. CaV2.2 subunits conduct N-type Ca2+ currents blocked with high affinity by ω-conotoxin GVIA (Dubel et al. 1992; Williams et al. 1992). Cloned CaV2.3 subunits form R-type Ca2+ channels, which are resistant to both organic Ca2+ antagonists specific for L-type Ca2+ currents and the peptide toxins specific for N-type or P/Q-type Ca2+ currents (Soong et al. 1994). T-type Ca2+ currents are mediated by the CaV3 Ca2+ channels (Perez-Reyes et al. 1998). These α1 subunits are only distantly related to the other known homologs, with <25% amino acid sequence identity. These results reveal a surprising structural dichotomy between the T-type, low-voltage-activated Ca2+ channels and the high-voltage-activated Ca2+ channels. Evidently, these two lineages of Ca2+ channels diverged very early in evolution of multicellular organisms. Single representatives of the CaV1, CaV2, and CaV3 subfamilies are present in invertebrate genomes, including the worm Caenorhabditis elegans and the fruit fly Drosophila.
The diversity of Ca2+ channel structure and function is substantially enhanced by multiple β subunits. Four β subunit genes have been identified, and each is subject to alternative splicing to yield additional isoforms (reviewed in Hofmann et al. 1994; Dolphin 2003). In Ca2+ channel preparations isolated from brain, individual Ca2+ channel α1 subunit types are associated with multiple types of β subunits, although there is a different rank order in each case (Pichler et al. 1997; Witcher et al. 1995b). The different β subunit isoforms cause different shifts in the kinetics and voltage dependence of gating, so association with different β subunits can substantially alter the physiological function of an α1 subunit. Genes encoding four α2δ subunits have been described (Klugbauer et al. 1999), and the α2δ isoforms produced by these different genes have selective effects on the level of functional expression and the voltage dependence of different α1 subunits (Davies et al. 2007).
Molecular Basis for Ca2+ Channel Function
Intensive studies of the structure and function of the related pore-forming subunits of Na+, Ca2+, and K+ channels have led to identification of their principal functional domains (reviewed in Catterall 2000a,b; Yi and Jan 2000; Bichet et al. 2003; Yu et al. 2005). Each domain of the principal subunits consists of six transmembrane α helices (S1–S6) and a membrane-associated loop between S5 and S6 (Fig. 2). The S4 segments of each homologous domain serve as the voltage sensors for activation, moving outward and rotating under the influence of the electric field and initiating a conformational change that opens the pore. The S5 and S6 segments and the membrane-associated pore loop between them form the pore lining of the voltage-gated ion channels. The narrow external end of the pore is lined by the pore loop, which contains a pair of glutamate residues in each domain that are required for Ca2+ selectivity, a structural feature that is unique to Ca2+ channels (Heinemann et al. 1992). Remarkably, substitutions that add only three glutamate residues in the pore loops between the S5 and S6 segments in domains II, III, and IV of sodium channels are sufficient to confer Ca2+ selectivity (Heinemann et al. 1992; Sather and McCleskey 2003). The inner pore is lined by the S6 segments, which form the receptor sites for the pore-blocking Ca2+ antagonist drugs specific for L-type Ca2+ channels (Hockerman et al. 1997a,b). All Ca2+ channels share these general structural features, but the amino acid residues that confer high affinity for the organic Ca2+ antagonists used in therapy of cardiovascular diseases are present only in the CaV1 family of Ca2+ channels, which conduct L-type Ca2+ currents.
CaV1 CHANNELS AND EXCITATION-RESPONSE COUPLING
CaV1 channels serve to couple depolarization of the plasma membrane to a wide range of cellular responses (Fig. 1). Three widely studied examples are excitation-contraction coupling in muscle, excitation-transcription coupling in nerve and muscle, and excitation-secretion coupling in endocrine cells and at specialized ribbon synapses.
CaV1 CHANNELS AND EXCITATION-CONTRACTION COUPLING
Mechanisms of Excitation-Contraction Coupling
CaV1 channels initiate excitation-contraction coupling in skeletal, cardiac, and smooth muscle. There are striking mechanistic differences between excitation-contraction coupling in skeletal muscle and cardiac muscle. In skeletal muscle, entry of external Ca2+ is not required for initiation of contraction (Armstrong et al. 1972). CaV1.1 channels in the transverse tubules are thought to interact directly with the ryanodine-sensitive Ca2+ release channels (RyR1) of the sarcoplasmic reticulum (Numa et al. 1990), as observed in high-resolution electron microscopy (Block et al. 1988), and the voltage-driven conformational changes in their voltage-sensing domains are thought to directly induce activation of RyR1 (Numa et al. 1990). Reconstitution of excitation-contraction coupling in myocytes from mutant mice requires both CaV1.1 and RyR1 proteins and their relevant sites of protein–protein interaction (Tanabe et al. 1990; Nakai et al. 1998), and functional expression of the CaV1.1 channel in skeletal muscle requires its RyR1 binding partner (Nakai et al. 1996).
In contrast to skeletal muscle, entry of Ca2+ is required for excitation-contraction coupling in cardiac myocytes, and Ca2+ entry via CaV1.2 channels triggers activation of the RyR2 and initiates Ca2+-induced Ca2+-release, activation of actomyosin, and contraction (Fabiato 1983; Bers 2002). Release of Ca2+ from the sarcoplasmic reticulum via RyR2 greatly amplifies the cellular Ca2+ transient and is required for effective initiation of contraction. All three steps in the cascade of Ca2+ transport processes—Ca2+ entry via CaV1.2 channels, Ca2+ release via RyR, and Ca2+ uptake into the sarcoplasmic reticulum by SERCA Ca2+ pumps—are tightly regulated by second messenger signaling networks (Bers 2002). The section below considers the regulation of CaV1 channels in excitation-contraction coupling.
Regulation of Excitation-Contraction Coupling via CaV1 Channels
As part of the flight-or-flight response, the rate and force of contraction of both skeletal and cardiac muscle are increased through the activity of the sympathetic nervous system. Release of catecholamines stimulates β-adrenergic receptors (β-ARs), which increases the force of skeletal and cardiac muscle contraction and the heart rate (Reuter 1983; Tsien et al. 1986). In cardiac muscle, Ca2+ influx through Cav1.2 channels is responsible for initiating excitation-contraction coupling, and increased Ca2+ channel activity via the PKA pathway is primarily responsible for the increase in contractility. Cav1.2 channels are modulated by the β-adrenergic receptor/cAMP signaling. Activation of β-adrenergic receptors increases L-type Ca2+ currents through PKA-mediated phosphorylation of the Cav1.2 channel protein and/or associated proteins (Tsien 1973; Reuter and Scholz 1977; Osterrieder et al. 1982; McDonald et al. 1994).
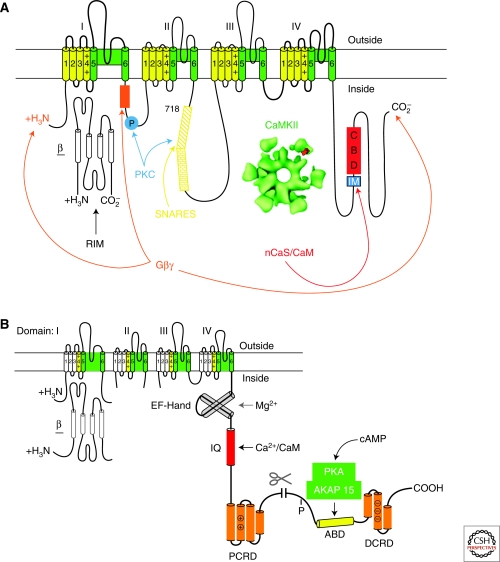
The pore-forming α1 subunit and the auxiliary β subunits of skeletal muscle CaV1.1 channels (Curtis and Catterall 1985; Flockerzi et al. 1986; Takahashi et al. 1987) and cardiac CaV1.2 channels (Hell et al. 1993b; De Jongh et al. 1996; Haase et al. 1996; Puri et al. 1997) are phosphorylated by PKA. These α1 subunits are also truncated by proteolytic processing of the carboxy-terminal domain (Fig. 4) (De Jongh et al. 1989, 1991, 1996; Hulme et al. 2005). Voltage-dependent potentiation of Cav1.1 channels on the 50-msec time scale requires PKA phosphorylation (Sculptoreanu et al. 1993) as well as PKA anchoring via an A kinase anchoring protein (AKAP) (Johnson et al. 1994, 1997), suggesting close association of PKA and Ca2+ channels. A novel, plasma membrane–targeted AKAP (AKAP15) is associated with both Cav1.1 channels (Gray et al. 1997, 1998) and CaV1.2 channels (Hulme et al. 2003), and may mediate their regulation by PKA. This AKAP (also known as AKAP18 [Fraser et al. 1998]) binds to the carboxy-terminal domain of Cav1.1 channels (Hulme et al. 2002) and CaV1.2 channels (Hulme et al. 2003) via a novel modified leucine zipper interaction near the primary sites of PKA phosphorylation. Block of this interaction by competing peptides prevents PKA regulation of Ca2+ currents in intact skeletal and cardiac myocytes (Hulme et al. 2002, 2003, 2006b). These physiological results suggest that a Ca2+ channel signaling complex containing AKAP15 and PKA is formed in both skeletal and cardiac muscle, and this conclusion is supported by specific colocalization of these proteins in both skeletal and cardiac myocytes and specific coimmunoprecipitation of this complex from both tissues (Hulme et al. 2002, 2003, 2006a). Remarkably, block of kinase anchoring is as effective as block of kinase activity in preventing Cav1.1 and Cav1.2 channel regulation, consistent with the conclusion that PKA targeting via leucine zipper interactions is absolutely required for regulation of Cav1 channels in intact skeletal and cardiac myocytes.
Figure 4.
Ca2+ channel signaling complexes. (A) The presynaptic Ca2+ channel signaling complex. A presynaptic Ca2+ channel α1 subunit is illustrated as a transmembrane folding diagram as in Figure 2. Sites of interaction of SNARE proteins (the synprint site), Gβγ subunits, protein kinase C (PKC), CaMKII, and CaM and CaS proteins are illustrated. IM, IQ-like motif; CBD, CaM binding domain. (B) The cardiac Ca2+ channel signaling complex. The carboxy-terminal domain of the cardiac Ca2+ channels is shown in expanded presentation to illustrate the regulatory interactions clearly. ABD, AKAP15 binding domain; DCRD, distal carboxy-terminal regulatory domain; PCRD, proximal carboxy-terminal regulatory domain; scissors, site of proteolytic processing. The DCRD binds to the PCRD through a modified leucine zipper interaction.
Proteolytic Processing and Regulation via the Carboxy-Terminal Domain
The distal carboxy-terminal domains of skeletal muscle and cardiac Ca2+ channels are proteolytically processed in vivo (Fig. 4B) (De Jongh et al. 1991, 1996). Nevertheless, the most prominent in vitro PKA phosphorylation sites of both proteins are located beyond the site of proteolytic truncation (Rotman et al. 1992, 1995; De Jongh et al. 1996; Mitterdorfer et al. 1996), and interaction of AKAP15 and PKA with the distal carboxy-terminal domain through a leucine zipper motif is required for regulation of cardiac Ca2+ channels in intact myocytes (Hulme et al. 2003). These results imply that the distal carboxy-terminal domain remains associated with the proteolytically processed cardiac CaV1.2 channel, and this is supported by evidence that the distal carboxyl-terminus can bind to the truncated CaV1.1 and CaV1.2 channels in vitro (Gerhardstein et al. 2000; Gao et al. 2001; Hulme et al. 2005) and in transfected cells (Hulme et al. 2002; Hulme et al. 2006b). Moreover, formation of this complex dramatically inhibits cardiac Ca2+ channel function in intact mammalian cells (Hulme et al. 2006b). Deletion of the distal carboxy-terminal near the site of proteolytic processing increases Ca2+ channel activity (Wei et al. 1994; Hulme et al. 2006b). However, noncovalent association of the cleaved distal carboxy-terminal reduces channel activity more than 10-fold, to a level much below that of channels with an intact carboxyl-terminus (Hulme et al. 2006b). Thus, proteolytic processing produces an autoinhibited Ca2+ channel complex containing noncovalently bound distal carboxyl-terminus with AKAP15 and PKA associated through a modified leucine zipper interaction (Fig. 4B). This autoinhibited complex appears to be the primary substrate for regulation of cardiac Ca2+ channels by the β-adrenergic receptor/PKA pathway in vivo, and PKA up-regulation results from phosphorylation of a single site near the end of the proximal carboxy-terminal domain at the interface with the distal carboxy-terminal domain (Fig. 4B) (Hulme et al. 2006b; Emrick et al. 2010; Fuller et al. 2010).
Ca2+ Binding Proteins
In addition to their regulation by the PKA/AKAP15 signaling complex, cardiac Ca2+ channels have calmodulin bound to their carboxy-terminal domain through an IQ motif (Fig. 4B), and Ca2+ binding to calmodulin causes Ca2+-dependent inactivation (Peterson et al. 1999; Qin et al. 1999; Zühlke et al. 1999). Activation of CaV1.2 channels in the presence of Ba2+ as the permeant ion results in inward Ba2+ currents that activate rapidly and inactivate slowly via a voltage-dependent inactivation process. In contrast, in the presence of Ca2+ as the permeant ion, Ca2+ currents are rapidly inactivated via Ca2+/calmodulin-dependent inactivation. The Ca2+-dependent inactivation process is crucial for limiting Ca2+ entry during long cardiac action potentials. In light of these results, it is evident that both the cAMP and Ca2+ second messenger pathways regulate CaV1.2 channels locally, dependent on associated regulatory proteins in Ca2+ channel signaling complexes.
CaV1 CHANNELS IN EXCITATION-TRANSCRIPTION COUPLING
Ca2+ entering neurons through L-type Ca2+ currents conducted by CaV1 channels has a privileged role in regulation of gene transcription, compared to similar amounts of Ca2+ entering via other voltage-gated or ligand-gated ion channels (Flavell and Greenberg 2008). This unique access of CaV1 channels to regulation of transcription might arise from preferential localization, which could provide Ca2+ in the vicinity of transcriptional regulators, preferential interaction with binding partners, which could be activated by local Ca2+ entry and carry the regulatory signal to the nucleus, or nuclear targeting of a subunit or domain of the CaV1 channel itself, which would serve to regulate transcription directly. It is likely that all three of these mechanisms are involved based on recent experiments.
CaV1 channels are localized in higher density in the cell bodies and proximal dendrites of neurons compared to CaV2 and CaV3 channels, which are more prevalent in nerve terminals and dendrites, respectively (Westenbroek et al. 1990; Hell et al. 1993a). This preferential localization would favor Ca2+ entry through these channels in control of transcription in the nucleus. However, this effect seems insufficient to fully account for the dominance of this Ca2+ entry pathway.
Studies with selective Ca2+ buffers indicate that only a local increase in Ca2+ is required for up-regulation of transcription in neurons (Wheeler et al. 2008). These findings suggest that specifically bound Ca2+-dependent regulatory proteins may respond to local Ca2+ entering via CaV1 channels and regulate transcription. Calmodulin is a resident Ca2+-dependent regulator of CaV1 channels (Pitt et al. 2001), and calmodulin binding to the proximal carboxy-terminal domain of CaV1.2 channels is required for regulation of transcription in neurons (Bito et al. 1996; Dolmetsch et al. 2001). Thus, calmodulin itself might serve as a regulator by binding local Ca2+, changing conformation to the active form, and moving to the nucleus (Bito et al. 1996; Deisseroth et al. 1998). However, there are large pools of free and Ca2+-bound calmodulin throughout the cell, so additional mechanisms must be engaged to specifically move Ca2+/calmodulin complexes from the CaV1 channels to the nucleus in the context of this mode of regulation. Calcineurin bound to the distal carboxy-terminal domain of CaV1 channels also is a potential transcriptional regulator through dephosphorylation of regulatory proteins (Oliveria et al. 2007). In cultured hippocampal neurons, dephosphorylation of the nuclear factor of activated T cells (NFAT) by calcineurin bound to CaV1.2 channels induces its dissociation, movement to the nucleus, and regulation of transcription (Oliveria et al. 2007). This pathway appears to have all of the necessary elements for selective regulation of gene transcription by Ca2+ entering neurons via CaV1.2 channels and has the precedent that it is a crucial element in gene regulation in lymphocytes by a similar mechanism.
The distal carboxy-terminal domain of the CaV1 channel itself has also been proposed as a transcriptional regulator (Gomez-Ospina et al. 2006). The large carboxy-terminal domain of CaV1.1 and CaV1.2 channels is proteolytically processed in vivo near its center (De Jongh et al. 1991, 1996), leaving a noncovalently associated distal carboxy-terminal domain of more than 300 residues intact to regulate channel activity (Fig. 4B) (Hulme et al. 2006b). In neurons, this proteolytic cleavage process is regulated by Ca2+ and blocked by calpain inhibitors (Hell et al. 1996). The distal carboxy-terminal domain can be detected in the nuclei of a subset of neurons in the developing brain and in neurons in cell culture (Gomez-Ospina et al. 2006), opening the possibility of direct effects on transcription in the nucleus. Indeed, the distal carboxy-terminal domain can regulate the transcription of a substantial set of other genes in neurons (Gomez-Ospina et al. 2006), as well as the transcription of the gene encoding the CaV1.2 channel itself in cardiac myocytes (Schroder et al. 2009). This regulatory mechanism also has all of the necessary elements to give selective regulation of gene expression by CaV1.2 channels, but it remains unknown how the parallel effects of the distal carboxyl-terminus on regulation of channel activity versus migration to the nucleus and regulation of transcription are controlled. At least in neurons, it seems that only a small fraction of the distal carboxy-terminal is located in the nucleus (Gomez-Ospina et al. 2006), so it may be that most of the proteolytically processed distal carboxy-terminal domain remains associated with CaV1.2 channels as an autoinhibitory regulator of channel activity while a small fraction dissociates and moves to the nucleus to regulate transcription.
CaV1 CHANNELS IN EXCITATION-SECRETION COUPLING
Ca2+ entry via CaV1 channels initiates secretion of hormones from endocrine cells (Artalejo et al. 1994; Yang and Berggren 2006) and release of neurotransmitters at specialized ribbon synapses in sensory-transduction neurons (Table 1) (Kollmar et al. 1997; Barnes and Kelly 2002). The relative role of individual CaV1 channel subtypes in secretion, as well as the contribution of CaV2 channels, differs among cell types and species. In the pancreas, the requirement for L-type Ca2+ currents for insulin secretion is greater in mouse than in human β cells (Eliasson et al. 2008; Braun et al. 2009). In adrenal chromaffin cells, L-type Ca2+ currents conducted by CaV1.2 and CaV1.3 channels trigger secretion of catecholamines, and their activity is strongly regulated by second messenger signaling pathways, including cAMP (Marcantoni et al. 2007).
Neurotransmitter release at specialized ribbon synapses is continuous, similar to hormone secretion in some physiological circumstances, and CaV1 channels are specifically required for this mode of synaptic transmission. In photoreceptors, CaV1.4 channels are primarily responsible for Ca2+ entry that triggers exocytosis of neurotransmitters (Table 1) (Barnes and Kelly 2002). Mutations in the CaV1.4 channel in humans lead to stationary night blindness (Bech-Hansen et al. 1998; Striessnig et al. 2010). In auditory hair cells, CaV1.3 channels conduct the L-type Ca2+ currents that trigger neurotransmitter release (Kollmar et al. 1997). Deletion of the gene encoding CaV1.3 channels causes deafness in mice (Platzer et al. 2000). The distal carboxy-terminal domain plays an autoregulatory role in both CaV1.3 and CaV1.4 channels (Singh et al. 2006, 2008), but it is not known whether it is subject to proteolytic processing in vivo. CaV1.3 channels are regulated by multiple interacting proteins (Cui et al. 2007; Jenkins et al. 2010), which may be important in tuning their activity to fit the specific requirements of hair cells transmitting auditory information at different frequencies.
CaV2 CHANNELS IN SYNAPTIC TRANSMISSION
Presynaptic Ca2+ channels conduct P/Q-, N-, and R-type Ca2+ currents, which initiate synaptic transmission (Table 1). The efficiency of neurotransmitter release depends on the third or fourth power of the entering Ca2+. This steep dependence of neurotransmission on Ca2+ entry makes the presynaptic Ca2+ channel an exceptionally sensitive and important target of regulation. In the nervous system, CaV2.1 channels conducting P/Q-type Ca2+ currents and CaV2.2 channels conducting N-type Ca2+ currents are the predominant pathways for Ca2+ entry initiating fast release of classical neurotransmitters like glutamate, acetylcholine, and GABA. Extensive studies indicate that they are controlled by many different protein interactions with their intracellular domains, which serve as a platform for Ca2+-dependent signal transduction (Fig. 4A).
SNARE Proteins
Ca2+ entry through voltage-gated Ca2+ channels initiates exocytosis by triggering the fusion of secretory vesicle membranes with the plasma membrane through actions on the SNARE protein complex of syntaxin, SNAP-25, and VAMP/synaptobrevin (reviewed in Bajjalieh and Scheller 1995; Sudhof 1995, 2004). The function of the SNARE protein complex is regulated by interactions with numerous proteins, including the synaptic vesicle Ca2+-binding protein synaptotagmin. Presynaptic CaV2.1 and CaV2.2 channels interact directly with the SNARE proteins through a specific synaptic protein interaction (synprint) site in the large intracellular loop connecting domains II and III (Fig. 4A) (Sheng et al. 1994; Rettig et al. 1996). This interaction is regulated by Ca2+ and protein phosphorylation (Sheng et al. 1996; Yokoyama et al. 1997, 2005). Synaptotagmin also binds to the synprint site of CaV2 channels (Charvin et al. 1997; Sheng et al. 1997; Wiser et al. 1997). Injection into presynaptic neurons of peptides that block SNARE protein interactions with CaV2 channels inhibits synaptic transmission, consistent with the conclusion that interaction with SNARE proteins is required to position docked synaptic vesicles near Ca2+ channels for fast exocytosis (Mochida et al. 1996; Rettig et al. 1997). These results define a second functional activity of the presynaptic Ca2+ channel–targeting docked synaptic vesicles to a source of Ca2+ for effective transmitter release.
In addition to this functional role of interaction between Ca2+ channels and SNARE proteins in the anterograde process of synaptic transmission, these interactions also have retrograde regulatory effects on Ca2+ channel function. Coexpression of the plasma membrane SNARE proteins syntaxin or SNAP-25 with CaV2.1 or CaV2.2 channels reduces the level of channel expression and inhibits Ca2+ channel activity by shifting the voltage dependence of steady-state inactivation during long depolarizing prepulses toward more negative membrane potentials (Bezprozvanny et al. 1995; Wiser et al. 1996; Zhong et al. 1999). The inhibitory effects of syntaxin are relieved by coexpression of SNAP-25 and synaptotagmin to form a complete SNARE complex (Wiser et al. 1997; Tobi et al. 1999; Zhong et al. 1999), which has the effect of enhancing activation of CaV2 channels with nearby docked synaptic vesicles that have formed complete SNARE complexes and are ready for release. These processes fine-tune the efficiency of neurotransmitter release at frog neuromuscular junctions, where peptide and cDNA reagents can be used to modify synaptic function in vivo (Keith et al. 2007).
G Protein Modulation
N-type and P/Q-type Ca2+ currents are regulated through multiple G protein coupled pathways (Hille 1994; Jones et al. 1997; Ikeda and Dunlap 1999). Although there are several G protein signaling pathways that regulate these channels, one common pathway that has been best studied at both cellular and molecular levels is voltage dependent and membrane delimited (i.e., a pathway without soluble intracellular messengers whose effects can be reversed by strong depolarization). Inhibition of Ca2+ channel activity is typically caused by a positive shift in the voltage dependence and a slowing of channel activation (Bean 1989b). These effects are relieved by strong depolarization resulting in facilitation of Ca2+ currents (Marchetti et al. 1986; Bean 1989b). Synaptic transmission is inhibited by neurotransmitters through this mechanism. G-protein α subunits are thought to confer specificity in receptor coupling, but Gβγ subunits are responsible for modulation of Ca2+ channels. Cotransfection of cells with the Ca2+ channel α1 and β subunits plus Gβγ causes a shift in the voltage dependence of Ca2+ channel activation to more positive membrane potentials and reduces the steepness of voltage-dependent activation, effects that closely mimic the actions of neurotransmitters and guanyl nucleotides on N-type and P/Q-type Ca2+ channels in neurons and neuroendocrine cells (Herlitze et al. 1996). In contrast, transfection with a range of Gα subunits does not have this effect. This voltage shift can be reversed by strong positive prepulses resulting in voltage-dependent facilitation of the Ca2+ current in the presence of Gβγ, again closely mimicking the effects of neurotransmitters and guanyl nucleotides on Ca2+ channels. Similarly, injection or expression of Gβγ subunits in sympathetic ganglion neurons induces facilitation and occludes modulation of N-type channels by norepinephrine, but Gα subunits do not (Herlitze et al. 1996; Ikeda 1996). These results point to the Gβγ subunits as the primary regulators of presynaptic Ca2+ channels via this voltage-dependent pathway through direct protein–protein interactions (Fig. 4A).
Possible sites of G protein βγ subunit interaction with Ca2+ channels have been extensively investigated by construction and analysis of channel chimeras, by G protein binding experiments, and by site-directed mutagenesis and expression (Fig. 4A). Evidence from G protein binding and site-directed mutagenesis experiments points to the intracellular loop between domains I and II (LI-II) as a crucial site of G protein regulation, and peptides from this region of CaV2.2 prevent inhibition of channel activity by Gβγ, presumably by binding to Gβγ and competitively inhibiting its access to Ca2+ channels (De Waard et al. 1997; Herlitze et al. 1997; Zamponi et al. 1997). This region of the channel binds Gβγ in vitro as well as in vivo in the yeast two-hybrid assay (De Waard et al. 1997; Zamponi et al. 1997; Garcia et al. 1998). Increasing evidence also points to segments in the amino- and carboxy-terminal domains of Ca2+ channels that are also required for G protein regulation (Zhang et al. 1996; Page et al. 1997, 1998; Qin et al. 1997; Canti et al. 1999; Li et al. 2004). As the amino- and carboxy-terminal domains are likely to interact with each other in the folded channel protein, a second site of interaction for G proteins may be formed at their intersection.
Ca2+ Binding Proteins
Ca2+-dependent facilitation and inactivation of presynaptic Ca2+ channels was observed in patch clamp recordings of presynaptic nerve terminals in the rat neurohypophysis (Branchaw et al. 1997) and the calyx of Held synapse in the rat brainstem (Forsythe et al. 1998b). During tetanic stimulation at this synapse, Cav2.1 channel currents show both Ca2+-dependent facilitation and inactivation (Borst and Sakmann 1998; Cuttle et al. 1998; Forsythe et al. 1998a), which results in facilitation and depression of excitatory postsynaptic responses (Borst and Sakmann 1998; Cuttle et al. 1998; Forsythe et al. 1998b). Ca2+-dependent facilitation and inactivation are also observed for cloned and expressed Cav2.1 channels expressed in mammalian cells (Lee et al. 1999, 2000). A novel CaM-binding site was identified by yeast two-hybrid screening in the carboxy-terminal domain of the pore-forming α12.1 subunit of Cav2.1 channels (Lee et al. 1999). This CaM-binding domain (CBD) (Fig. 4A) is located on the carboxy-terminal side of the sequence in α12.1 that corresponds to the IQ-domain that is required for CaM modulation of cardiac Cav1.2 channels (Peterson et al. 1999; Qin et al. 1999; Zühlke et al. 1999). The modified IQ domain of α12.1 begins with the amino acid sequence IM instead of IQ and has other changes that would be predicted to substantially reduce its affinity for CaM. CaM binding to the CBD is Ca2+-dependent. Both Ca2+-dependent facilitation and inactivation are blocked by coexpression of a CaM inhibitor peptide (Lee et al. 1999), suggesting that Ca2+-dependent modulation of Cav2.1 channels in neurons is caused by two sequential interactions with CaM or a related Ca2+-binding protein.
The mechanism for Ca2+-dependent facilitation and inactivation of Cav2.1 channels involves CaM binding to two adjacent subsites—the CBD and the upstream IQ-like motif (Lee et al. 2003). The IQ-like motif is required for facilitation, whereas the CBD is required for inactivation. In addition, the two lobes of CaM are also differentially involved in these two processes. Mutation of the two EF hands in the carboxy-terminal lobe primarily prevents facilitation, whereas mutation of the EF hands in the amino-terminal lobe primarily prevents inactivation (DeMaria et al. 2001; Erickson et al. 2001; Lee et al. 2003). FRET studies indicate that apo-calmodulin can bind to Cav2.1 channels in intact cells and binding is enhanced by Ca2+ binding to calmodulin (Erickson et al. 2001). Altogether, these results support a model in which the two lobes of CaM interact differentially with the modified IQ domain and the CBD to effect bi-directional regulation, with the high-affinity carboxy-terminal lobe primarily controlling facilitation through interactions with the IQ-like domain and the lower-affinity amino-terminal lobe primarily controlling inactivation through interactions with the CBD. This biphasic regulation of CaV2.1 channels causes synaptic facilitation and depression in transfected sympathetic ganglion neuron synapses in which neurotransmission is initiated by transfected CaV2.1 channels (Mochida et al. 2008).
CaM is the most well-characterized member of a superfamily of Ca2+ sensor (CaS) proteins, many of which differ from CaM in having neuron-specific localization, amino-terminal myristoylation, and amino acid substitutions that prevent Ca2+ binding to one or two of the EF hands (Haeseleer and Palczewski 2002). The CaS protein CaBP1 binds to the CBD, but not the IQ-like domain, of α12.1 and its binding is Ca2+-independent (Lee et al. 2002). CaBP1 causes a strong enhancement of the rate of inactivation, a positive shift in the voltage-dependence of activation, and a loss of Ca2+-dependent facilitation of Cav2.1 channels, which would combine to reduce the activity of these channels. Because it coimmunoprecipitates and colocalizes with Cav2.1 channels in the brain (Lee et al. 2002), CaBP1 may be an important determinant of Cav2.1 channel function in neurons and may contribute to the diversity of function of these channels in the nervous system. Visinin-like protein 2 (VILIP-2) is a neuronal Ca2+-binding protein that is distantly related to CaBP-1 (Haeseleer and Palczewski 2002). Consistent with these structural differences, VILIP-2 has opposite effects on CaV2.1 channels than CaBP-1 (Lautermilch et al. 2005). Coexpression of VILIP-2 causes slowed inactivation and enhanced facilitation, but its binding and effects are Ca2+-independent like CaBP-1. VILIP-2 may serve as a positive modulator of synaptic transmission, prolonging Ca2+ channel opening, and enhancing facilitation. Differential expression of CaBP1 and VILIP-2 at synapses would lead to opposite modulation of synaptic transmission in response to trains of action potentials and opposing input–output functions at the synapse.
CaV3 CHANNELS AND FREQUENCY MODULATION
Molecular Properties of CaV3 Channels
Ca2+ channels of the CaV3 subfamily conduct T-type Ca2+ currents (Catterall et al. 2005). These Ca2+ currents are activated at comparatively negative membrane potentials, in the same range as Na+ currents in most cells, and they have fast voltage-dependent inactivation compared to other Ca2+ currents (Nowycky et al. 1985). These Ca2+ currents are therefore well-suited for rhythmic firing of action potentials. They are also well-suited for generation of large Ca2+ transients because they are activated at negative membrane potentials where the driving force for Ca2+ entry is large. A family of three CaV3 channel α1 subunits have been characterized by cDNA cloning and sequencing (Catterall et al. 2005). Remarkably, these Ca2+ channel subunits have the same molecular organization as CaV1 and CaV2 channels but are only ∼25% identical in amino acid sequence (Catterall et al. 2005). This is a similar level of amino acid sequence identity as Ca2+ channels have with Na+ channels, indicating that these subfamilies of Ca2+ channels separated from each other at the same point of evolution as Na+ channels separated from Ca2+ channels. Although CaV3 channels are similar in structure to CaV1 and CaV2 channels, there is no clear evidence at present that they interact with the same set of auxiliary subunits. In fact, the prevailing view is that the α1 subunits function independently of other subunits. This would be unique among the families of Na+ and Ca2+ channels.
Functional Roles of CaV3 Channels
As expected from their functional properties, CaV3 channels are important in repetitively firing tissues. In the sino-atrial node of the heart, they conduct an important component of the pacemaker current that generates the heartbeat (Mangoni et al. 2006). In the relay neurons of the thalamus, they are crucial for generation of the rhythmic bursts of action potentials that drive sleep spindles and control sleep (Lee et al. 2004). Moreover, mutations in CaV3 channels cause absence epilepsy, in which the affected individuals transiently enter a sleep-like state that interrupts their normal activities (Kim et al. 2001; Song et al. 2004). In the adrenal cortex, they are important in regulation of synthesis and secretion of aldosterone (Welsby et al. 2003).
Regulation of CaV3 Channels
In neurons, dopamine and other neurotransmitters inhibit T-type Ca2+ currents via a pathway that is specific for the Gβ2 subunit (Wolfe et al. 2003). As for CaV2 channels, G protein βγ subunits bind directly to CaV3 channels and regulate them (DePuy et al. 2006). The site of interaction is in the intracellular loop connecting domains II and III (DePuy et al. 2006). In addition, in adrenal glomerulosa cells, angiotensin II regulates aldosterone secretion via enhanced activation of CaV3.2 channels (Welsby et al. 2003). This regulation is mediated by a signaling complex of CaMKII bound to the intracellular loop connecting domains II and III (Yao et al. 2006). Phosphorylation of a single serine residue in this intracellular loop negatively shifts the voltage dependence of activation and thereby substantially increases Ca2+ current at negative membrane potentials (Yao et al. 2006). It is unknown at this stage whether binding of CaMKII is required for physiological regulation or whether binding of the kinase per se has any regulatory effect.
THE EFFECTOR CHECKPOINT MODEL OF Ca2+ CHANNEL REGULATION
In closing this article on Ca2+ signaling via voltage-gated Ca2+ channels, it is interesting to introduce an emerging theme that unites several aspects of the localized regulation of these proteins. Ca2+ channel signaling complexes are formed when the effectors and regulators of the Ca2+ signal bind to the intracellular domains of Ca2+ channels to effectively receive and respond to the local Ca2+ signal. In four cases, binding of the effectors of the Ca2+ signal has been shown to enhance the activity of the CaV1 and CaV2 channels. First, in skeletal muscle, interactions of the plasma membrane CaV1.1 channel with the ryanodine-sensitive Ca2+ release channel in the sarcoplasmic reticulum, which serves as the effector of excitation-contraction coupling, greatly increase the functional activity of the CaV1.1 channels (Nakai et al. 1996a). Second, as described above, interaction with individual plasma membrane SNARE proteins inhibits the activity of CaV2 channels, but formation of complete SNARE complex containing synaptotagmin, the effector of exocytosis, relieves this inhibition and enhances Ca2+ channel activity (Bezprozvanny et al. 1995; Wiser et al. 1996, 1997; Zhong et al. 1999). Third, binding of Ca2+/CaM-dependent protein kinase II, an effector of Ca2+-dependent regulatory events, to a site in the carboxy-terminal domain of CaV2.1 channels substantially increases their activity (Jiang et al. 2008). Finally, binding of RIM, a regulator of SNARE protein function, to the CaVβ subunits substantially increases CaV2 channel activity (Kiyonaka et al. 2007). The common thread in all of these diverse examples of Ca2+ channel regulation by interacting proteins is that binding of an effector ready to respond to the Ca2+ signal enhances the activity of the Ca2+ channel. Thus, this mechanism provides a functional checkpoint of the fitness of a Ca2+ channel to carry out its physiological role, and enhances its activity if it passes this checkpoint criterion. This “effector checkpoint” mechanism would serve to focus Ca2+ entry on the CaV channels that are ready to use the resulting Ca2+ signal to initiate a physiological intracellular signaling process. It seems likely that further studies will reveal more examples of this form of regulation and that it may be a unifying theme in the regulation of Ca2+ signaling by CaV channels.
Footnotes
Editors: Martin Bootman, Michael J. Berridge, James W. Putney, and H. Llewelyn Roderick
Additional Perspectives on Calcium Signaling available at www.cshperspectives.org
REFERENCES
- Ahlijanian MK, Westenbroek RE, Catterall WA 1990. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron 4: 819–832 [DOI] [PubMed] [Google Scholar]
- Arikkath J, Campbell KP 2003. Auxiliary subunits: Essential components of the voltage-gated calcium channel complex. Curr Opin Neurobiol 13: 298–307 [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Bezanilla FM, Horowicz P 1972. Twitches in the presence of ethylene glycol bis (-aminoethyl ether)-N,N′-tetracetic acid. Biochim Biophys Acta 267: 605–608 [DOI] [PubMed] [Google Scholar]
- Artalejo CR, Adams ME, Fox AP 1994. Three types of calcium channel trigger secretion with different efficacies in chromaffin cells. Nature 367: 72–76 [DOI] [PubMed] [Google Scholar]
- Bajjalieh SM, Scheller RH 1995. The biochemistry of neurotransmitter secretion. J Biol Chem 270: 1971–1974 [DOI] [PubMed] [Google Scholar]
- Barnes S, Kelly ME 2002. Calcium channels at the photoreceptor synapse. Adv Exp Med Biol 514: 465–476 [DOI] [PubMed] [Google Scholar]
- Bean BP 1989a. Classes of calcium channels in vertebrate cells. Annu Rev Physiol 51: 367–384 [DOI] [PubMed] [Google Scholar]
- Bean BP 1989b. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature 340: 153–156 [DOI] [PubMed] [Google Scholar]
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM 1998. Loss-of-function mutations in a calcium-channel α1 subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet 19: 264–267 [DOI] [PubMed] [Google Scholar]
- Bers DM 2002. Cardiac excitation-contraction coupling. Nature 415: 198–205 [DOI] [PubMed] [Google Scholar]
- Bezprozvanny I, Scheller RH, Tsien RW 1995. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature 378: 623–626 [DOI] [PubMed] [Google Scholar]
- Bichet D, Haass FA, Jan LY 2003. Merging functional studies with structures of inward-rectifier potassium channels. Nat Rev Neurosci 4: 957–967 [DOI] [PubMed] [Google Scholar]
- Bito H, Deisseroth K, Tsien RW 1996. CREB phosphorylation and dephosphorylation: A Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell 87: 1203–1214 [DOI] [PubMed] [Google Scholar]
- Block BA, Imagawa T, Campbell KP, Franzini-Armstrong C 1988. Structural evidence for direct interaction between the molecular components of the transverse tubule/sarcoplasmic reticulum junction in skeletal muscle. J Cell Biol 107: 2587–2600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst JG, Sakmann B 1998. Facilitation of presynaptic calcium currents in the rat brainstem. J Physiol 513: 149–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branchaw JL, Banks MI, Jackson MB 1997. Ca2+- and voltage-dependent inactivation of Ca2+ channels in nerve terminals of the neurohypophysis. J Neurosci 17: 5772–5781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun M, Ramracheya R, Johnson PR, Rorsman P 2009. Exocytotic properties of human pancreatic β-cells. Ann NY Acad Sci 1152: 187–193 [DOI] [PubMed] [Google Scholar]
- Canti C, Page KM, Stephens GJ, Dolphin AC 1999. Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J Neurosci 19: 6855–6864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbone E, Lux HD 1984. A low voltage-activated, fully inactivating Ca channel in vertebrate sensory neurones. Nature 310: 501–502 [DOI] [PubMed] [Google Scholar]
- Catterall WA 1991. Excitation-contraction coupling in vertebrate skeletal muscle: A tale of two calcium channels. Cell 64: 871–874 [DOI] [PubMed] [Google Scholar]
- Catterall WA 2000a. From ionic currents to molecular mechanisms: The structure and function of voltage-gated sodium channels. Neuron 26: 13–25 [DOI] [PubMed] [Google Scholar]
- Catterall WA 2000b. Structure and regulation of voltage-gated calcium channels. Annu Rev Cell Dev Biol 16: 521–555 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Few AP 2008. Calcium channel regulation and presynaptic plasticity. Neuron 59: 882–901 [DOI] [PubMed] [Google Scholar]
- Catterall WA, Perez-Reyes E, Snutch TP, Striessnig J 2005. International Union of Pharmacology. XLVIII. Nomenclature and structure-function relationships of voltage-gated calcium channels. Pharmacol Rev 57: 411–425 [DOI] [PubMed] [Google Scholar]
- Chang FC, Hosey MM 1988. Dihydropyridine and phenylalkylamine receptors associated with cardiac and skeletal muscle calcium channels are structurally different. J Biol Chem 263: 18929–18937 [PubMed] [Google Scholar]
- Charvin N, Lévêque C, Walker D, Berton F, Raymond C, Kataoka M, Shoji-Kasai Y, Takahashi M, De Waard M, Seagar MJ 1997. Direct interaction of the calcium sensor protein synaptotagmin I with a cytoplasmic domain of the α1A subunit of the P/Q-type calcium channel. EMBO J 16: 4591–4596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YH, Li MH, Zhang Y, He LL, Yamada Y, Fitzmaurice A, Shen Y, Zhang H, Tong L, Yang J 2004. Structural basis of the α1β subunit interaction of voltage-gated Ca2+ channels. Nature 429: 675–680 [DOI] [PubMed] [Google Scholar]
- Cui G, Meyer AC, Calin-Jageman I, Neef J, Haeseleer F, Moser T, Lee A 2007. Ca2+-binding proteins tune Ca2+-feedback to Cav1.3 channels in mouse auditory hair cells. J Physiol 585: 791–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BM, Catterall WA 1984. Purification of the calcium antagonist receptor of the voltage-sensitive calcium channel from skeletal muscle transverse tubules. Biochem 23: 2113–2118 [DOI] [PubMed] [Google Scholar]
- Curtis BM, Catterall WA 1985. Phosphorylation of the calcium antagonist receptor of the voltage-sensitive calcium channel by cAMP-dependent protein kinase. Proc Natl Acad Sci 82: 2528–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis BM, Catterall WA 1986. Reconstitution of the voltage-sensitive calcium channel purified from skeletal muscle transverse tubules. Biochemistry 25: 3077–3083 [DOI] [PubMed] [Google Scholar]
- Cuttle MF, Tsujimoto T, Forsythe ID, Takahashi T 1998. Facilitation of the presynaptic calcium current at an auditory synapse in rat brainstem. J Physiol 512: 723–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies A, Hendrich J, Van Minh AT, Wratten J, Douglas L, Dolphin AC 2007. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharmacol Sci 28: 220–228 [DOI] [PubMed] [Google Scholar]
- Davies A, Kadurin I, Alvarez-Laviada A, Douglas L, Nieto-Rostro M, Bauer CS, Pratt WS, Dolphin AC 2010. The α2δ subunits of voltage-gated calcium channels form GPI-anchored proteins, a posttranslational modification essential for function. Proc Natl Acad Sci 107: 1654–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh KS, Merrick DK, Catterall WA 1989. Subunits of purified calcium channels: A 212-kDa form of α1 and partial amino acid sequence of a phosphorylation site of an independent β subunit. Proc Natl Acad Sci 86: 8585–8589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Catterall WA 1990. Subunits of purified calcium channels. α2 and δ are encoded by the same gene. J Biol Chem 265: 14738–14741 [PubMed] [Google Scholar]
- De Jongh KS, Warner C, Colvin AA, Catterall WA 1991. Characterization of the two size forms of the α1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci 88: 10778–10782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh KS, Murphy BJ, Colvin AA, Hell JW, Takahashi M, Catterall WA 1996. Specific phosphorylation of a site in the full-length form of the α1 subunit of the cardiac L-type calcium channel by cAMP-dependent protein kinase. Biochemistry 35: 10392–10402 [DOI] [PubMed] [Google Scholar]
- De Waard M, Liu HY, Walker D, Scott VES, Gurnett CA, Campbell KP 1997. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature 385: 446–450 [DOI] [PubMed] [Google Scholar]
- Deisseroth K, Heist EK, Tsien RW 1998. Translocation of calmodulin to the nucleus supports CREB phosphorylation in hippocampal neurons. Nature 392: 198–202 [DOI] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT 2001. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature 411: 484–489 [DOI] [PubMed] [Google Scholar]
- DePuy SD, Yao J, Hu C, McIntire W, Bidaud I, Lory P, Rastinejad F, Gonzalez C, Garrison JC, Barrett PQ 2006. The molecular basis for T-type Ca2+ channel inhibition by G protein β2γ2 subunits. Proc Natl Acad Sci 103: 14590–14595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME 2001. Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294: 333–339 [DOI] [PubMed] [Google Scholar]
- Dolphin AC 2003. β subunits of voltage-gated calcium channels. J Bioenerg Biomembr 35: 599–620 [DOI] [PubMed] [Google Scholar]
- Dubel SJ, Starr TVB, Hell J, Ahlijanian MK, Enyeart JJ, Catterall WA, Snutch TP 1992. Molecular cloning of the α1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci 89: 5058–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K, Luebke JI, Turner TJ 1995. Exocytotic calcium channels in mammalian central neurons. Trends Neurosci 18: 89–98 [PubMed] [Google Scholar]
- Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P 2008. Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol 586: 3313–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis SB, Williams ME, Ways NR, Brenner R, Sharp AH, Leung AT, Campbell KP, McKenna E, Koch WJ, Hui A, et al. 1988. Sequence and expression of mRNAs encoding the α1 and α2 subunits of a DHP-sensitive calcium channel. Science 241: 1661–1664 [DOI] [PubMed] [Google Scholar]
- Emrick MA, Sadilek M, Konoki K, Catterall WA 2010. β-adrenergic-regulated phosphorylation of the skeletal muscle CaV1.1 channel in the fight-or-flight response. Proc Natl Acad Sci 107: 18712–18717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT 2001. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron 31: 973–985 [DOI] [PubMed] [Google Scholar]
- Ertel EA, Campbell KP, Harpold MM, Hofmann F, Mori Y, Perez-Reyes E Schwartz A, Snutch TP, Tanabe T, Birnbaumer L, et al. 2000. Nomenclature of voltage-gated calcium channels. Neuron 25: 533–535 [DOI] [PubMed] [Google Scholar]
- Fabiato A 1983. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol 245: C1–C14 [DOI] [PubMed] [Google Scholar]
- Fedulova SA, Kostyuk PG, Veselovsky NS 1985. Two types of calcium channels in the somatic membrane of new-born rat dorsal root ganglion neurones. J Physiol 359: 431–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Greenberg ME 2008. Signaling mechanisms linking neuronal activity to gene expression and plasticity of the nervous system. Annu Rev Neurosci 31: 563–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flockerzi V, Oeken HJ, Hofmann F, Pelzer D, Cavalie A, Trautwein W 1986. Purified dihydropyridine-binding site from skeletal muscle t-tubules is a functional calcium channel. Nature 323: 66–68 [DOI] [PubMed] [Google Scholar]
- Forsythe ID, Tsujimoto T, Barnes-Davies M, Cuttle MF, Takahashi T 1998. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron 20: 797–807 [DOI] [PubMed] [Google Scholar]
- Fraser IDC, Tavalin SJ, Lester LB, Langeberg LK, Westphal AM, Dean RA, Marrion NV, Scott JD 1998. A novel lipid-anchored A-kinase anchoring protein facilitates cAMP-responsive membrane events. EMBO J 17: 2261–2272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller MD, Emrick MA, Sadilek M, Scheuer T, Catterall WA 2010. Molecular mechanism of calcium channel regulation in the fight-or-flight response. Sci Signal 3: ra70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao T, Cuadra AE, Ma H, Bunemann M, Gerhardstein BL, Cheng T, Eick RT, Hosey MM 2001. C-terminal fragments of the α1C (Cav1.2) subunit associate with and regulate L-type calcium channels containing C-terminal-truncated α1C subunits. J Biol Chem 276: 21089–21097 [DOI] [PubMed] [Google Scholar]
- Garcia DE, Li B, Garcia-Ferreiro RE, Hernández-Ochoa EO, Yan K, Gautam N, Catterall WA, Mackie K, Hille B 1998. G-protein β subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels. J Neurosci 18: 9163–9170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhardstein BL, Gao T, Bunemann M, Puri TS, Adair A, Ma H, Hosey MM 2000. Proteolytic processing of the C terminus of the α1C subunit of L-type calcium channels and the role of a proline-rich domain in membrane tethering of proteolytic fragments. J Biol Chem 275: 8556–8563 [DOI] [PubMed] [Google Scholar]
- Gomez-Ospina N, Tsuruta F, Barreto-Chang O, Hu L, Dolmetsch R 2006. The C terminus of the L-type voltage-gated calcium channel CaV1.2 encodes a transcription factor. Cell 127: 591–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PC, Tibbs VC, Catterall WA, Murphy BJ 1997. Identification of a 15-kDa cAMP-dependent protein kinase-anchoring protein associated with skeletal muscle L-type calcium channels. J Biol Chem 272: 6297–6302 [DOI] [PubMed] [Google Scholar]
- Gray PC, Johnson BD, Westenbroek RE, Hays LG, Yates JR 3rd, Scheuer T, Catterall WA, Murphy BJ 1998. Primary structure and function of an A kinase anchoring protein associated with calcium channels. Neuron 20: 1017–1026 [DOI] [PubMed] [Google Scholar]
- Gurnett CA, De Waard M, Campbell KP 1996. Dual function of the voltage-dependent Ca2+ channel α2δ subunit in current stimulation and subunit interaction. Neuron 16: 431–440 [DOI] [PubMed] [Google Scholar]
- Haase H, Bartel S, Karczewski P, Morano I, Krause EG 1996. In-vivo phosphorylation of the cardiac L-type calcium channel β subunit in response to catecholamines. Mol Cell Biochem 163–164: 99–106 [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Palczewski K 2002. Calmodulin and calcium-binding proteins: Variations on a theme. Adv Exp Med Biol 514: 303–317 [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Ozawa S, Sand O 1975. Voltage clamp analysis of two inward current mechanisms in the egg cell membrane of a starfish. J Gen Physiol 65: 617–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann SH, Terlau H, Stühmer W, Imoto K, Numa S 1992. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature 356: 441–443 [DOI] [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Warner C, Ahlijanian MK, Prystay W, Gilbert MM, Snutch TP, Catterall WA 1993a. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel α1 subunits. J Cell Biol 123: 949–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell JW, Yokoyama CT, Wong ST, Warner C, Snutch TP, Catterall WA 1993b. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel α1 subunit. J Biol Chem 268: 19451–19457 [PubMed] [Google Scholar]
- Hell JW, Westenbroek RE, Breeze LJ, Wang KKW, Chavkin C, Catterall WA 1996. N-methyl-D-aspartate receptor-induced proteolytic conversion of postsynaptic class C L-type calcium channels in hippocampal neurons. Proc Natl Acad Sci 93: 3362–3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA 1996. Modulation of calcium channels by G protein βγ subunits. Nature 380: 258–262 [DOI] [PubMed] [Google Scholar]
- Herlitze S, Hockerman GH, Scheuer T, Catterall WA 1997. Molecular determinants of inactivation and G protein modulation in the intracelular loop connecting domains I and II of the calcium channel α1A subunit. Proc Natl Acad Sci 94: 1512–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B 1994. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci 17: 531–536 [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Peterson BZ, Johnson BD, Catterall WA 1997a. Molecular determinants of drug binding and action on L-type calcium channels. Annu Rev Pharmacol Toxicol 37: 361–396 [DOI] [PubMed] [Google Scholar]
- Hockerman GH, Peterson BZ, Sharp E, Tanada TN, Scheuer T, Catterall WA 1997b. Construction of a high-affinity receptor site for dihydropyridine agonists and antagonists by single amino acid substitutions in a non-L-type calcium channel. Proc Natl Acad Sci 94: 14906–14911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann F, Biel M, Flockerzi V 1994. Molecular basis for calcium channel diversity. Annu Rev Neurosci 17: 399–418 [DOI] [PubMed] [Google Scholar]
- Hosey MM, Barhanin J, Schmid A, Vandaele S, Ptasienski J, O’Callahan C, Cooper C, Lazdunski M 1987. Photoaffinity labelling and phosphorylation of a 165 kilodalton peptide associated with dihydropyridine and phenylalkylamine-sensitive calcium channels. Biochem Biophys Res Commun 147: 1137–1145 [DOI] [PubMed] [Google Scholar]
- Hulme JT, Ahn M, Hauschka SD, Scheuer T, Catterall WA 2002. A novel leucine zipper targets AKAP15 and cyclic AMP-dependent protein kinase to the C terminus of the skeletal muscle calcium channel and modulates its function. J Biol Chem 277: 4079–4087 [DOI] [PubMed] [Google Scholar]
- Hulme JT, Lin TW, Westenbroek RE, Scheuer T, Catterall WA 2003. β-adrenergic regulation requires direct anchoring of PKA to cardiac CaV1.2 channels via a leucine zipper interaction with A kinase-anchoring protein 15. Proc Natl Acad Sci 100: 13093–13098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Konoki K, Lin TW, Gritsenko MA, Camp DG 2nd, Bigelow DJ, Catterall WA 2005. Sites of proteolytic processing and noncovalent association of the distal C-terminal domain of CaV1.1 channels in skeletal muscle. Proc Natl Acad Sci 102: 5274–5279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Westenbroek RE, Scheuer T, Catterall WA 2006a. Phosphorylation of serine 1928 in the distal C-terminal of cardiac Cav1.2 channels during β-adrenergic regulation. Proc Natl Acad Sci 103: 16574–16579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme JT, Yarov-Yarovoy V, Lin TW-C, Scheuer T, Catterall WA 2006b. Autoinhibitory control of the Cav1.2 channel by its proteolytically cleaved distal C-terminal domain. J Physiol (Lond) 576: 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR 1996. Voltage-dependent modulation of N-type calcium channels by G-protein β/γ subunits. Nature 380: 255–258 [DOI] [PubMed] [Google Scholar]
- Ikeda SR, Dunlap K 1999. Voltage-dependent modulation of N-type calcium channels: Role of G protein subunits. Adv Second Messenger Phosphoprotein Res 33: 131–151 [DOI] [PubMed] [Google Scholar]
- Jay SD, Ellis SB, McCue AF, Williams ME, Vedvick TS, Harpold MM, Campbell KP 1990. Primary structure of the γ subunit of the DHP-sensitive calcium channel from skeletal muscle. Science 248: 490–492 [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Christel CJ, Jiao Y, Abiria S, Kim KY, Usachev YM, Obermair GJ, Colbran RJ, Lee A 2010. Ca2+-dependent facilitation of Cav1.3 Ca2+ channels by densin and Ca2+/calmodulin-dependent protein kinase II. J Neurosci 30: 5125–5135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Lautermilch NJ, Watari H, Westenbroek RE, Scheuer T, Catterall WA 2008. Modulation of Cav2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc Natl Acad Sci 105: 341–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Scheuer T, Catterall WA 1994. Voltage-dependent potentiation of L-type Ca2+ channels in skeletal muscle cells requires anchored cAMP-dependent protein kinase. Proc Natl Acad Sci 91: 11492–11496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Brousal JP, Peterson BZ, Gallombardo PA, Hockerman GH, Lai Y, Scheuer T, Catterall WA 1997. Modulation of the cloned skeletal muscle L-type Ca2+ channel by anchored cAMP-dependent protein kinase. J Neurosci 17: 1243–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LP, Patil PG, Snutch TP, Yue DT 1997. G-protein modulation of N-type calcium channel gating current in human embryonic kidney cells (HEK 293). J Physiol (Lond) 498: 601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith RK, Poage RE, Yokoyama CT, Catterall WA, Meriney SD 2007. Bidirectional modulation of transmitter release by calcium channel/syntaxin interactions in vivo. J Neurosci 27: 265–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS 2001. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking α1G T-type Ca2+ channels. Neuron 31: 35–45 [DOI] [PubMed] [Google Scholar]
- Kiyonaka S, Wakamori M, Miki T, Uriu Y, Nonaka M, Bito H, Beedle AM, Mori E, Hara Y, De Waard M, et al. 2007. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic calcium channels. Nat Neurosci 10: 691–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Lacinová L, Marais E, Hobom M, Hofmann F 1999. Molecular diversity of the calcium channel α2δ subunit. J Neurosci 19: 684–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klugbauer N, Dai S, Specht V, Lacinova L, Marais E, Bohn G, Hofmann F 2000. A family of γ-like calcium channel subunits. FEBS Lett 470: 189–197 [DOI] [PubMed] [Google Scholar]
- Kollmar R, Montgomery LG, Fak J, Henry LJ, Hudspeth AJ 1997. Predominance of the α1D subunit in L-type voltage-gated Ca2+ channels of hair cells in the chicken’s cochlea. Proc Natl Acad Sci 94: 14883–14888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuniyasu A, Oka K, Ide-Yamada T, Hatanaka Y, Abe T, Nakayama H, Kanaoka Y 1992. Structural characterization of the dihydropyridine receptor-linked calcium channel from porcine heart. J Biochem (Tokyo) 112: 235–242 [DOI] [PubMed] [Google Scholar]
- Lacerda AE, Kim HS, Ruth P, Perez-Reyes E, Flockerzi V, Hofmann F, Birnbaumer L, Brown AM 1991. Normalization of current kinetics by interaction between the α1 and β subunits of the skeletal muscle dihydropyridine-sensitive calcium channel. Nature 352: 527–530 [DOI] [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA 2005. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci 25: 7062–7070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Wong ST, Gallagher D, Li B, Storm DR, Scheuer T, Catterall WA 1999. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature 399: 155–159 [DOI] [PubMed] [Google Scholar]
- Lee A, Scheuer T, Catterall WA 2000. Calcium/calmodulin dependent inactivation and facilitation of P/Q-type calcium channels. J Neurosci 20: 6830–6838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Westenbroek RE, Haeseleer F, Palczewski K, Scheuer T, Catterall WA 2002. Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat Neurosci 5: 210–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A, Zhou H, Scheuer T, Catterall WA 2003. Molecular determinants of Ca2+/calmodulin-dependent regulation of Cav2.1 channels. Proc Natl Acad Sci 100: 16059–16064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kim D, Shin HS 2004. Lack of δ waves and sleep disturbances during non-rapid eye movement sleep in mice lacking α1G subunit of T-type calcium channels. Proc Natl Acad Sci 101: 18195–18199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letts VA, Felix R, Biddlecome GH, Arikkath J, Mahaffey CL, Valenzuela A, Bartlett IFS, Mori Y, Campbell KP, Frankel WN 1998. The mouse stargazer gene encodes a neuronal calcium-channel γ subunit. Nature Genet 19: 340–347 [DOI] [PubMed] [Google Scholar]
- Leung AT, Imagawa T, Campbell KP 1987. Structural characterization of the 1,4-dihydropyridine receptor of the voltage-dependent calcium channel from rabbit skeletal muscle. Evidence for two distinct high molecular weight subunits. J Biol Chem 262: 7943–7946 [PubMed] [Google Scholar]
- Li B, Zhong H, Scheuer T, Catterall WA 2004. Functional role of a C-terminal Gβγ-binding domain of Cav2.2 channels. Mol Pharmacol 66: 761–769 [DOI] [PubMed] [Google Scholar]
- Liu H, De Waard M, Scott VES, Gurnett CA, Lennon VA, Campbell KP 1996. Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive calcium channel. J Biol Chem 271: 13804–13810 [PubMed] [Google Scholar]
- Llinás R, Yarom Y 1981. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol (Lond) 315: 569–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás RR, Sugimori M, Cherksey B 1989. Voltage-dependent calcium conductances in mammalian neurons. The P channel . Ann NY Acad Sci 560: 103–111 [DOI] [PubMed] [Google Scholar]
- Llinás R, Sugimori M, Hillman DE, Cherksey B 1992. Distribution and functional significance of the P-type, voltage-dependent calcium channels in the mammalian central nervous system. Trends Neurosci 15: 351–355 [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Traboulsie A, Leoni AL, Couette B, Marger L, Le Quang K, Kupfer E, Cohen-Solal A, Vilar J, Shin HS, et al. 2006. Bradycardia and slowing of the atrioventricular conduction in mice lacking CaV3.1/α1G T-type calcium channels. Circ Res 98: 1422–1430 [DOI] [PubMed] [Google Scholar]
- Marcantoni A, Baldelli P, Hernandez-Guijo JM, Comunanza V, Carabelli V, Carbone E 2007. L-type calcium channels in adrenal chromaffin cells: Role in pace-making and secretion. Cell Calcium 42: 397–408 [DOI] [PubMed] [Google Scholar]
- Marchetti C, Carbone E, Lux HD 1986. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflugers Arch 406: 104–111 [DOI] [PubMed] [Google Scholar]
- Martin-Moutot N, Leveque C, Sato K, Kato R, Takahashi M, Seagar M 1995. Properties of omega conotoxin MVIIC receptors associated with α1A calcium channel subunits in rat brain. FEBS Lett 366: 21–25 [DOI] [PubMed] [Google Scholar]
- McDonald TF, Pelzer S, Trautwein W, Pelzer DJ 1994. Regulation and modulation of calcium channels in cardiac, skeletal, and smooth muscle cells. Physiol Rev 74: 365–507 [DOI] [PubMed] [Google Scholar]
- McEnery MW, Snowman AM, Sharp AH, Adams ME, Snyder SH 1991. Purified omega-conotoxin GVIA receptor of rat brain resembles a dihydropyridine-sensitive L-type calcium channel. Proc Natl Acad Sci 88: 11095–11099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz IM, Adams ME, Bean BP 1992. P-type calcium channels in rat central and peripheral neurons. Neuron 9: 85–95 [DOI] [PubMed] [Google Scholar]
- Mitterdorfer J, Froschmayr M, Grabner M, Moebius FF, Glossmann H, Striessnig J 1996. Identification of PKA phosphorylation sites in the carboxyl terminus of L-type calcium channel α-1 subunits. Biochemistry 35: 9400–9406 [DOI] [PubMed] [Google Scholar]
- Mochida S, Sheng ZH, Baker C, Kobayashi H, Catterall WA 1996. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron 17: 781–788 [DOI] [PubMed] [Google Scholar]
- Mochida S, Few AP, Scheuer T, Catterall WA 2008. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron 57: 210–216 [DOI] [PubMed] [Google Scholar]
- Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, et al. 1991. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature 350: 398–402 [DOI] [PubMed] [Google Scholar]
- Nakai J, Dirksen RT, Nguyen HT, Pessah IN, Beam KG, Allen PD 1996. Enhanced dihydropyridine receptor channel activity in the presence of ryanodine receptor. Nature 380: 72–75 [DOI] [PubMed] [Google Scholar]
- Nakai J, Sekiguchi N, Rando TA, Allen PD, Beam KG 1998. Two regions of the ryanodine receptor involved in coupling with L-type Ca2+ channels. J Biol Chem 273: 13403–13406 [DOI] [PubMed] [Google Scholar]
- Newcomb R, Szoke B, Palma A, Wang G, Chen XH, Hopkins W, Cong R, Miller J, Urge L, Tarczy-Hornoch K, et al. 1998. Selective peptide antagonist of the class E calcium channel from the venom of the tarantula Hysterocrates gigas. Biochemistry 37: 15353–15362 [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS 2006. Auxiliary subunits assist AMPA-type glutamate receptors. Science 311: 1253–1256 [DOI] [PubMed] [Google Scholar]
- Nowycky MC, Fox AP, Tsien RW 1985. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature 316: 440–443 [DOI] [PubMed] [Google Scholar]
- Numa S, Tanabe T, Takeshima H, Mikami A, Niidome T, Nishimura S, Adams BA, Beam KG 1990. Molecular insights into excitation-contraction coupling. Cold Spring Harb Symp Quant Biol 55: 1–7 [DOI] [PubMed] [Google Scholar]
- Olivera BM, Miljanich GP, Ramachandran J, Adams ME 1994. Calcium channel diversity and neurotransmitter release: The omega-conotoxins and omega-agatoxins. Annu Rev Biochem 63: 823–867 [DOI] [PubMed] [Google Scholar]
- Oliveria SF, Dell’Acqua ML, Sather WA 2007. AKAP79/150 anchoring of calcineurin controls neuronal L-type Ca2+ channel activity and nuclear signaling. Neuron 55: 261–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterrieder W, Brum G, Hescheler J, Trautwein W, Flockerzi V, Hofmann F 1982. Injection of subunits of cyclic AMP-dependent protein kinase into cardiac myocytes modulates Ca2+ current. Nature 298: 576–578 [DOI] [PubMed] [Google Scholar]
- Page KM, Stephens GJ, Berrow NS, Dolphin AC 1997. The intracellular loop between domains I and II of the B-type calcium channel confers aspects of G-protein sensitivity to the E-type calcium channel. J Neurosci 17: 1330–1338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page KM, Cantí C, Stephens GJ, Berrow NS, Dolphin AC 1998. Identification of the amino terminus of neuronal Ca2+ channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J Neurosci 18: 4815–4824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Reyes E, Kim HS, Lacerda AE, Horne W, Wei XY, Rampe D, Campbell KP, Brown AM, Birnbaumer L 1989. Induction of calcium currents by the expression of the α1 subunit of the dihydropyridine receptor from skeletal muscle. Nature 340: 233–236 [DOI] [PubMed] [Google Scholar]
- Perez-Reyes E, Cribbs LL, Daud A, Lacerda AE, Barclay J, Williamson MP, Fox M, Rees M, Lee JH 1998. Molecular characterization of a neuronal low-voltage-activated T-type calcium channel. Nature 391: 896–900 [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Yue DT 1999. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron 22: 549–558 [DOI] [PubMed] [Google Scholar]
- Pichler M, Cassidy TN, Reimer D, Haase H, Krause R, Ostler D, Striessnig J 1997. β subunit heterogeneity in neuronal L-type calcium channels. J Biol Chem 272: 13877–13882 [DOI] [PubMed] [Google Scholar]
- Pitt GS, Zuhlke RD, Hudmon A, Schulman H, Reuter H, Tsien RW 2001. Molecular basis of calmodulin tethering and calcium-dependent inactivation of L-type calcium channels. J Biol Chem 276: 30794–30802 [DOI] [PubMed] [Google Scholar]
- Platzer J, Engel J, Schrott-Fischer A, Stephan K, Bova S, Chen H, Zheng H, Striessnig J 2000. Congenital deafness and sinoatrial node dysfunction in mice lacking class D L-type Ca2+ channels. Cell 102: 89–97 [DOI] [PubMed] [Google Scholar]
- Pragnell M, De Waard M, Mori Y, Tanabe T, Snutch TP, Campbell KP 1994. Calcium channel β subunit binds to a conserved motif in the I-II cytoplasmic linker of the α1 subunit. Nature 368: 67–70 [DOI] [PubMed] [Google Scholar]
- Puri TS, Gerhardstein BL, Zhao XL, Ladner MB, Hosey MM 1997. Differential effects of subunit interactions on protein kinase A- and C-mediated phosphorylation of L-type calcium channels. Biochemistry 36: 9605–9615 [DOI] [PubMed] [Google Scholar]
- Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L 1997. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca2+ channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci 94: 8866–8871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L 1999. Ca2+ induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc Natl Acad Sci 96: 2435–2438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A, Tsien RW 1995. Pharmacological dissection of multiple types of calcium channel currents in rat cerebellar granule neurons. J Neurosci 15: 2995–3012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Sheng Z-H, Kim DK, Hodson CD, Snutch TP, Catterall WA 1996. Isoform-specific interaction of the α1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc Natl Acad Sci 93: 7363–7368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettig J, Heinemann C, Ashery U, Sheng ZH, Yokoyama CT, Catterall WA, Neher E 1997. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J Neurosci 17: 6647–6656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H 1979. Properties of two inward membrane currents in the heart. Annu Rev Physiol 41: 413–424 [DOI] [PubMed] [Google Scholar]
- Reuter H 1983. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature 301: 569–574 [DOI] [PubMed] [Google Scholar]
- Reuter H, Scholz H 1977. The regulation of calcium conductance of cardiac muscle by adrenaline. J Physiol 264: 49–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman EI, De Jongh KS, Florio V, Lai Y, Catterall WA 1992. Specific phosphorylation of a COOH-terminal site on the full-length form of the α1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem 267: 16100–16105 [PubMed] [Google Scholar]
- Rotman EI, Murphy BJ, Catterall WA 1995. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel α1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem 270: 16371–16377 [DOI] [PubMed] [Google Scholar]
- Ruth P, Röhrkasten A, Biel M, Bosse E, Regulla S, Meyer HE, Flockerzi V, Hofmann F 1989. Primary structure of the β subunit of the DHP-sensitive calcium channel from skeletal muscle. Science 245: 1115–1118 [DOI] [PubMed] [Google Scholar]
- Sather WA, McCleskey EW 2003. Permeation and selectivity in calcium channels. Annu Rev Physiol 65: 133–159 [DOI] [PubMed] [Google Scholar]
- Schneider T, Hofmann F 1988. The bovine cardiac receptor for calcium channel blockers is a 195-kDa protein. Eur J Biochem 174: 369–375 [DOI] [PubMed] [Google Scholar]
- Schroder E, Byse M, Satin J 2009. L-type calcium channel C terminus autoregulates transcription. Circ Res 104: 1373–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sculptoreanu A, Scheuer T, Catterall WA 1993. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature 364: 240–243 [DOI] [PubMed] [Google Scholar]
- Serysheva II, Ludtke SJ, Baker MR, Chiu W, Hamilton SL 2002. Structure of the voltage-gated L-type Ca2+ channel by electron cryomicroscopy. Proc Natl Acad Sci 99: 10370–10375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Takahashi M, Catterall WA 1994. Identification of a syntaxin-binding site on N-type calcium channels. Neuron 13: 1303–1313 [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Rettig J, Cook T, Catterall WA 1996. Calcium-dependent interaction of N-type calcium channels with the synaptic core-complex. Nature 379: 451–454 [DOI] [PubMed] [Google Scholar]
- Sheng Z-H, Yokoyama C, Catterall WA 1997. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc Natl Acad Sci 94: 5405–5410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer D, Biel M, Lotan I, Flockerzi V, Hofmann F, Dascal N 1991. The roles of the subunits in the function of the calcium channel. Science 253: 1553–1557 [DOI] [PubMed] [Google Scholar]
- Singh A, Hamedinger D, Hoda JC, Gebhart M, Koschak A, Romanin C, Striessnig J 2006. C-terminal modulator controls Ca2+-dependent gating of Cav1.4 L-type Ca2+ channels. Nat Neurosci 9: 1108–1116 [DOI] [PubMed] [Google Scholar]
- Singh A, Gebhart M, Fritsch R, Sinnegger-Brauns MJ, Poggiani C, Hoda JC, Engel J, Romanin C, Striessnig J, Koschak A 2008. Modulation of voltage- and Ca2+-dependent gating of CaV1.3 L-type calcium channels by alternative splicing of a C-terminal regulatory domain. J Biol Chem 283: 20733–20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snutch TP, Reiner PB 1992. Calcium channels: Diversity of form and function. Curr Opin Neurobiol 2: 247–253 [DOI] [PubMed] [Google Scholar]
- Song I, Kim D, Choi S, Sun M, Kim Y, Shin HS 2004. Role of the α1G T-type calcium channel in spontaneous absence seizures in mutant mice. J Neurosci 24: 5249–5257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP 1994. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science 260: 1133–1136 [DOI] [PubMed] [Google Scholar]
- Starr TVB, Prystay W, Snutch TP 1991. Primary structure of a calcium channel that is highly expressed in the rat cerebellum. Proc Natl Acad Sci 88: 5621–5625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striessnig J, Knaus HG, Grabner M, Moosburger K, Seitz W, Lietz H, Glossmann H 1987. Photoaffinity labelling of the phenylalkylamine receptor of the skeletal muscle transverse-tubule calcium channel. FEBS Lett 212: 247–253 [DOI] [PubMed] [Google Scholar]
- Striessnig J, Bolz HJ, Koschak A 2010. Channelopathies in Cav1.1, Cav1.3, and Cav1.4 voltage-gated L-type Ca2+ channels. Pflugers Arch 460: 361–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudhof TC 1995. The synaptic vesicle cycle: A cascade of protein-protein interactions. Nature 375: 645–653 [DOI] [PubMed] [Google Scholar]
- Sudhof TC 2004. The synaptic vesicle cycle. Annu Rev Neurosci 27: 509–547 [DOI] [PubMed] [Google Scholar]
- Takahashi M, Seagar MJ, Jones JF, Reber BF, Catterall WA 1987. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc Natl Acad Sci 84: 5478–5482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe T, Takeshima H, Mikami A, Flockerzi V, Takahashi H, Kangawa K, Kojima M, Matsuo H, Hirose T, Numa S 1987. Primary structure of the receptor for calcium channel blockers from skeletal muscle. Nature 328: 313–318 [DOI] [PubMed] [Google Scholar]
- Tanabe T, Beam KG, Adams BA, Niidome T, Numa S 1990. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature 346: 567–569 [DOI] [PubMed] [Google Scholar]
- Tanabe T, Mikami A, Niidome T, Numa S, Adams BA, Beam KG 1993. Structure and function of voltage-dependent calcium channels from muscle. Ann NY Acad Sci 707: 81–86 [DOI] [PubMed] [Google Scholar]
- Tobi D, Wiser O, Trus M, Atlas D 1999. N-type voltage-sensitive calcium channel interacts with syntaxin, synaptotagmin and SNAP-25 in a multiprotein complex. Receptor Channel 6: 89–98 [PubMed] [Google Scholar]
- Tottene A, Moretti A, Pietrobon D 1996. Functional diversity of P-type and R-type calcium channels in rat cerebellar neurons. J Neurosci 16: 6353–6363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien RW 1973. Adrenaline-like effects of intracellular iontophoresis of cyclic AMP in cardiac Purkinje fibres. Nat New Biol 245: 120–122 [DOI] [PubMed] [Google Scholar]
- Tsien RW 1983. Calcium channels in excitable cell membranes. Annu Rev Physiol 45: 341–358 [DOI] [PubMed] [Google Scholar]
- Tsien RW, Bean BP, Hess P, Lansman JB, Nilius B, Nowycky MC 1986. Mechanisms of calcium channel modulation by β-adrenergic agents and dihydropyridine calcium agonists. J Mol Cell Cardiol 18: 691–710 [DOI] [PubMed] [Google Scholar]
- Tsien RW, Lipscombe D, Madison DV, Bley KR, Fox AP 1988. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci 11: 431–438 [DOI] [PubMed] [Google Scholar]
- Van Petegem F, Clark KA, Chatelain FC, Minor DL Jr 2004. Structure of a complex between a voltage-gated calcium channel β subunit and an α subunit domain. Nature 429: 671–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MC, Velarde G, Ford RC, Berrow NS, Dolphin AC, Kitmitto A 2002. 3D structure of the skeletal muscle dihydropyridine receptor. J Mol Biol 323: 85–98 [DOI] [PubMed] [Google Scholar]
- Wei XNA, Lacerda AE, Olcese R, Stefani E, Perez-Reyes E, Birnbaumer L 1994. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac α1 subunit. J Biol Chem 269: 1635–1640 [PubMed] [Google Scholar]
- Welsby PJ, Wang H, Wolfe JT, Colbran RJ, Johnson ML, Barrett PQ 2003. A mechanism for the direct regulation of T-type calcium channels by Ca2+/calmodulin-dependent kinase II. J Neurosci 23: 10116–10121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Ahlijanian MK, Catterall WA 1990. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature 347: 281–284 [DOI] [PubMed] [Google Scholar]
- Wheeler DG, Barrett CF, Groth RD, Safa P, Tsien RW 2008. CaMKII locally encodes L-type channel activity to signal to nuclear CREB in excitation-transcription coupling. J Cell Biol 183: 849–863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams ME, Brust PF, Feldman DH, Patthi S, Simerson S, Maroufi A, McCue AF, Velicelebi G, Ellis SB, Harpold MM 1992. Structure and functional expression of an omega-conotoxin-sensitive human N-type calcium channel. Science 257: 389–395 [DOI] [PubMed] [Google Scholar]
- Wiser O, Bennett MK, Atlas D 1996. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. EMBO J 15: 4100–4110 [PMC free article] [PubMed] [Google Scholar]
- Wiser O, Tobi D, Trus M, Atlas D 1997. Synaptotagmin restores kinetic properties of a syntaxin-associated N-type voltage sensitive calcium channel. FEBS Lett 404: 203–207 [DOI] [PubMed] [Google Scholar]
- Witcher DR, De Waard M, Kahl SD, Campbell KP 1995a. Purification and reconstitution of N-type calcium channel complex from rabbit brain. Method Enzymol 238: 335–348 [DOI] [PubMed] [Google Scholar]
- Witcher DR, De Waard M, Liu H, Pragnell M, Campbell KP 1995b. Association of native calcium channel β subunits with the α1 subunit interaction domain. J Biol Chem 270: 18088–18093 [DOI] [PubMed] [Google Scholar]
- Wolf M, Eberhart A, Glossmann H, Striessnig J, Grigorieff N 2003. Visualization of the domain structure of an L-type Ca2+ channel using electron cryo-microscopy. J Mol Biol 332: 171–182 [DOI] [PubMed] [Google Scholar]
- Wolfe JT, Wang H, Howard J, Garrison JC, Barrett PQ 2003. T-type calcium channel regulation by specific G-protein βγ subunits. Nature 424: 209–213 [DOI] [PubMed] [Google Scholar]
- Yang SN, Berggren PO 2006. The role of voltage-gated calcium channels in pancreatic β-cell physiology and pathophysiology. Endocr Rev 27: 621–676 [DOI] [PubMed] [Google Scholar]
- Yao J, Davies LA, Howard JD, Adney SK, Welsby PJ, Howell N, Carey RM, Colbran RJ, Barrett PQ 2006. Molecular basis for the modulation of native T-type Ca2+ channels in vivo by Ca2+/calmodulin-dependent protein kinase II. J Clin Invest 116: 2403–2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi BA, Jan LY 2000. Taking apart the gating of voltage-gated potassium channels. Neuron 27: 423–425 [DOI] [PubMed] [Google Scholar]
- Yokoyama CT, Sheng Z-H, Catterall WA 1997. Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J Neurosci 17: 6929–6938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama CT, Myers SJ, Fu J, Mockus SM, Scheuer T, Catterall WA 2005. Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site. Mol Cell Neurosci 28: 1–17 [DOI] [PubMed] [Google Scholar]
- Yu FH, Yarov-Yarovoy V, Gutman GA, Catterall WA 2005. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol Rev 57: 387–395 [DOI] [PubMed] [Google Scholar]
- Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP 1997. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature 385: 442–446 [DOI] [PubMed] [Google Scholar]
- Zhang JF, Ellinor PT, Aldrich RW, Tsien RW 1996. Multiple structural elements in voltage-dependent Ca2+ channels support their inhibition by G proteins. Neuron 17: 991–1003 [DOI] [PubMed] [Google Scholar]
- Zhong H, Yokoyama C, Scheuer T, Catterall WA 1999. Reciprocal regulation of P/Q-type calcium channels by SNAP-25, syntaxin and synaptotagmin. Nat Neurosci 2: 939–941 [DOI] [PubMed] [Google Scholar]
- Zühlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H 1999. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature 399: 159–162 [DOI] [PubMed] [Google Scholar]