Abstract
Inositol phospholipids have been implicated in almost all aspects of cellular physiology including spatiotemporal regulation of cellular signaling, acquisition of cellular polarity, specification of membrane identity, cytoskeletal dynamics, and regulation of cellular adhesion, motility, and cytokinesis. In this review, we examine the critical role phosphoinositides play in these processes to execute the establishment and maintenance of cellular architecture. Epithelial tissues perform essential barrier and transport functions in almost all major organs. Key to their development and function is the establishment of epithelial cell polarity. We place a special emphasis on highlighting recent studies demonstrating phosphoinositide regulation of epithelial cell polarity and how individual cells use phosphoinositides to further organize into epithelial tissues.
PtdIns(3,4,5)P3 specifies the basolateral surface of polarized epithelial cells, whereas PtdIns(4,5)P2 specifies the apical domain. Individual cells also use phosphoinositides to further organize into epithelial tissues.
Phosphoinositides (PIs) are essential components of cellular membranes in eukaryotes. Though these specialized lipids comprise less than 1% of the cellular lipid cohort, they play key roles in many fundamental biological processes (Di Paolo and De Camilli 2006; Saarikangas et al. 2010). PIs possess such far ranging roles by serving as specialized membrane docking sites for effectors of numerous cellular signal transduction cascades. PIs also serve as precursors of lipid second messengers. They are concentrated on the cytosolic face of cellular membranes (Fig. 1A) and rapidly diffuse within the plane of the membrane. Reversible phosphorylation of the myo-inositol head group of phosphatidylinositol (PtdIns) at positions 3, 4, and 5 (Fig. 1B) gives rise to the seven PI isoforms identified in eukaryotic cells. PtdIns(4)P and PtdIns(4,5)P2 are constitutively present in membranes and comprise the largest pool of cellular PIs, whereas PtdIns(3,4,5)P3 is essentially undetectable in most types of unstimulated cells (Lemmon and Ferguson 2000; Saarikangas et al. 2010).
Figure 1.
Phosphoinositide subcellular distribution, metabolism, and protein effectors. (A) Subcellular distribution of PI species. PIs concentrate in cytosolic membranes, serving as discrete determinants of membrane identity. The predominant localization of particular PI species in subcellular compartments is depicted. There is some overlap of PI signature between membrane compartments, and heterogeneity of PI distribution on membrane compartments also occurs. PtdIns(4,5)P2 and PtdIns(3,4,5)P3 are enriched at the plasma membrane, possibly in raft-like domains. PtdIns(3,4)P2 dominates in early endocytic membranes and at the plasma membrane. PtdIns(3)P is concentrated on early endosomal (EE) membranes, and the multivesicular body (MVB) compartment. PtdIns(4)P is enriched at the Golgi complex and in Golgi-derived carriers. PtdIns(3,5)P2 concentrates on late compartments of the endocytic pathway, the MVB, and lysosome. PtdIns(5)P is localized in the nucleus, and generation of nuclear PtdIns(4,5)P2 is key to regulating some aspects of gene expression (reviewed in Barlow et al. 2009). Parallels can be drawn between the generation of front–rear axis in migrating cells and apical–basal polarity in polarized cell types (for recent review, see Nelson 2009). Not all cellular compartments are illustrated and arrows are not intended to represent the entire cohort of known endocytic trafficking routes. Illustration based in part on data from Kutateladze (2010), with additional elements added. (B) Representation of the metabolic interconversions that generate the seven phosphoinositide species from PtdIns. Kinases and phosphatases involved in generating the PIs involved in apical and basolateral membrane identity are indicated. (C) PI binding modules present in cytosolic effectors and their reported PI binding preferences. The family of PI “code-breaking” modules includes PH, ANTH (AP180 amino-terminal homology), C2 (conserved region 2 of protein kinase C), ENTH (epsin amino-terminal homology), FERM (band 4.1, Ezrin, Radixin, Moesin), FYVE (Fab1, YOTB, Vac1, and EEA1), GOLPH3 (Golgi phosphoprotein 3), PROPPINS (B-propellors that bind PIs), PTB (phosphotyrosine binding), PX (Phox homology), and Tubby modules. Examples of protein interactions with different PIs are provided. (Panel based in part on data from DiPaolo and Di Camilli 2006; McCrea and De Camilli 2009; and Kutateladze 2010.)
The spatiotemporally regulated production and turnover of phosphoinositides is crucial for localized PI signaling and function. Numerous phosphatidylinositol kinases and phosphatases are involved in regulating the metabolism of the various PI isoforms (Fig. 1). The concerted action of PI kinases and phosphatases, that attach or remove phosphate groups respectively, and phospholipases, that cleave lipids, results in the generation of unique PI enrichment in distinct intracellular membranes (Di Paolo and De Camilli 2006; Kutateladze 2010). Individual PIs can then serve as a unique lipid signature or code for cellular organelle identity. Regulated PI interconversion from one isoform to another is also the underlying basis of appropriate vectorial vesicular delivery and fusion with destination membranes.
PIs FINE-TUNE CELLULAR SIGNALING
PIs integrate cellular signaling by acting as membrane designations for selective protein recruitment and assembly, thereby triggering downstream signaling cascades. PI-mediated signal specification occurs by a coincidence detection mechanism, whereby initial low affinity interaction of cytosolic proteins with PIs is amplified by cooperative association with one or more additional proteins and/or lipids within the membrane, the net result being enhanced membrane protein affinity (Di Paolo and De Camilli 2006; Saarikangas et al. 2010). These PI-protein interaction domains serve as signaling centers and frequently result in changes in actomyosin dynamics at the membrane–cytoskeleton interface.
PI effectors, though diverse, share some basic design features that enable them to interpret the unique membrane signature, the “PI code” (Kutateladze 2010). The initial discovery of selective PI binding by the pleckstrin homology (PH) domain of protein effectors was a turning point in our understanding of PI control of cellular signaling (Harlan et al. 1994). To date 11 PI-binding modules are known that selectively interact with unique PIs with variable affinity (Fig. 1C) (reviewed in Kutateladze 2010). The selective interaction of the differentially phosphorylated head groups of the PI with proteins containing one or more of these PI binding modules is the foundation for PI involvement in numerous cellular processes (Lemmon 2008). Multiple interactions between PIs and effector proteins, in combination with their unique subcellular distribution, generates a robust and powerful mechanism to spatiotemporally fine-tune the composition of the membrane–cytosol interface, providing intricate control of cellular signaling potential.
SMALL GTPases AND PHOSPHOINOSITIDES
One well established group of PI effector proteins is the large Ras superfamily of small GTPase proteins. These proteins function as molecular switches that cycle between active, GTP bound, and inactive, GDP bound forms to regulate cellular functions as diverse as trafficking, polarity, cell cycle regulation, and transcription. Ras superfamily proteins are frequently associated with membranes in which, upon activation, they recruit a variety of effector proteins to execute their diverse tasks (ten Klooster and Hordijk 2007). The activity status of small GTPases is primarily controlled by two classes of proteins, GTPase-activating proteins (GAPs) and guanine nucleotide-exchange factors (GEFs). A common feature of many of these regulatory proteins is the presence of PI binding domains, such as PH, FYVE, and BAR domains. Consequently, the spatiotemporal activity of many small GTPases is intimately linked with PI signaling. Furthermore, a number of small GTPase effectors are also involved in PI metabolism (Di Paolo and De Camilli 2006). This endows these small GTPases with the capacity to participate in feedback loops and cross talk with other PI signaling pathways.
The Ras superfamily can be further subdivided into five families: Ras, Rab, Ran, Rho, and Arf. Despite the considerable amount of functional diversity within each of these subfamilies, they are broadly characterized as participating in different cellular processes. For example, the Ras family of GTPases generally participate in proliferation, differentiation, and migration (Goldfinger 2008) the Arf and Rab families are commonly associated with vesicle trafficking and organelle structure (Behnia and Munro 2005; D’Souza-Schorey and Chavrier 2006); and Rho-family members are thought to regulate the cytoskeleton (Heasman and Ridley 2008). The many studies documenting the intersection of different Ras superfamily small GTPases and PI signaling are beyond the scope of this review. In the following sections, we will attempt to highlight specific small GTPases when they are relevant to PI signaling and the establishment of cellular architecture, with an emphasis on the well-studied members of the Rho family: RhoA, Rac1, and Cdc42.
PI CONTROL OF MEMBRANE IDENTITY
PI involvement in specifying the subcellular identity of membranes has been studied extensively (Di Paolo and De Camilli 2006). Polarized cell types use PI asymmetry at their plasma membrane as a way to generate and maintain polarity. PtdIns(3,4,5)P3 is restricted to the tip of the growing axon (Shi et al. 2003) and leading edge in neurons and migrating cells respectively. Conversely, PtdIns(4,5)P2 is concentrated at the trailing membrane of migrating cells. Epithelial cells constitute another type of polarized cyto-architecture with an apical and basolateral PM separated by tight junction protein complexes. It is now clear that PIs also play essential roles in the establishment and maintenance of epithelial polarity. Because of the importance of epithelial polarity to overall tissue architecture and organ development, and because PI involvement in neurons and migrating cells has been reviewed elsewhere (Cain and Ridley 2009; Tahirovic and Bradke 2009), we focus on PI specification of epithelial membrane identity.
A general link between PI signaling and epithelial polarity has been known for some time (Khwaja et al. 1998; Liu et al. 2004; Rosario and Birchmeier 2004). However, not until later work, on polarized Madin–Darby canine kidney (MDCK) cells, did a detailed picture of PI specification of epithelial cell membrane identity emerge (Gassama-Diagne et al. 2006). The investigators showed that PtdIns(3,4,5)P3 was normally restricted to the basolateral surface of polarized epithelial cells. Furthermore, the exogenous addition of PtdIns(3,4,5)P3 to the apical surface of cells transformed the identity of this membrane to basolateral, as assayed by the recruitment of phosphatidyl inositol-3 kinase (PI(3)-kinase) and other basolateral proteins, concomitant with the exclusion of apical proteins. PI(3)-kinase is an enzyme responsible for the conversion of PtdIns(4,5)P2 to PtdIns(3,4,5)P3. Treatment of polarized MDCK cells with PI(3)-kinase inhibitors reduced basolateral surface area and the height of cells, further demonstrating the requirement for PtdIns(3,4,5)P3 in basolateral membrane identity. Another study confirmed the importance of PI(3)-kinase signaling in regulating epithelial cell basolateral surface production and concluded that this regulation is independent of effects on polarity and adhesion, but could be mediated through Rac1 signaling (Jeanes et al. 2009).
If the leading edge of migrating cells is similar to the basolateral surface of epithelia in PtdIns(3,4,5)P3 composition, are there additional PIs that help specify the identity of the apical surface, similar to the role PtdIns(4,5)P2 plays in defining the trailing edge of polarized migrating cells? The answer to this question has emerged from the study of MDCK cells grown in three-dimensional (3D) culture. These cells form polarized cysts with a centrally located apical surface and fluid filled lumen (O’Brien et al. 2002). Analysis of lumen formation in this model system provides an excellent assay for the ability of cells to form the apical domain. During the early stages of cyst formation, PtdIns(4,5)P2 became concentrated at the apical surface of cells (Martin-Belmonte et al. 2007). PTEN, a lipid phosphatase that removes phosphate from the 3 position of PtdIns(3,4,5)P3, also strongly localized to the apical surface of cells during cell polarization and lumen formation. PTEN is a tumor suppressor that functionally antagonizes PI(3)-kinase signaling. Importantly, reduction of PTEN activity caused defects in the segregation of PtdIns(3,4,5)P3 and PtdIns(4,5)P2 and disruption of lumen formation. Analogous to the shift in membrane identity following addition of exogenous PtdIns(3,4,5)P3 to the apical surface, delivery of exogenous PtdIns(4,5)P2 to the basolateral surface of cysts caused lumen shrinkage and ectopic recruitment of apical PM proteins, indicative of a change in membrane identity.
It is unclear how widespread the use of PtdIns(3,4,5)P3 to specify the basolateral domain and PtdIns(4,5)P2 to specify the apical domain is across the many different types of epithelial cells found in different organisms; however, a high degree of conservation is suggested by the localization of PTEN and PtdIns(4,5)P2 to the apical membrane of Drosophila embryonic epithelia (von Stein et al. 2005; Pilot et al. 2006). However, it is important to note that PtdIns(3,4,5)P3 may not always be strictly localized to the basolateral domain. There are likely to be PI microdomains found within the greater apical and basolateral surfaces to carry out specific functions. In a specialized Drosophila photoreceptor cell, PTEN is localized to cell–cell junctions where it functions to restrict PtdIns(3,4,5)P3 to the apical membrane domain (Pinal et al. 2006). This photoreceptor domain is a modified cilia, which is a specialized organelle distinct from the bulk of the apical membrane (Reiter and Mostov 2006). Therefore, it is likely that fine tuning of both the levels and localization of PIs allows cells a degree of freedom to create specialized membrane domains to serve a wide variety of purposes.
Taken together, the above studies point to a critical function of PIs in epithelial cell membrane identity specification (Fig. 2). Less understood is how these PI asymmetries are initially established and coordinated with early polarization events. Work by many labs has led to a general model for the acquisition of epithelial polarity via signaling generated through cell interactions with the surrounding extracellular matrix (ECM) (O’Brien et al. 2002). β1-integrin is a critical component of this signaling pathway in MDCK cells and is a known modulator of PI(3)-kinase and PtdIns(3,4,5)P3 levels (Parise et al. 2000; Yu et al. 2005). Consequently, it is plausible to think that specification of basolateral membrane identity through the generation of integrin-mediated PtdIns(3,4,5)P3 production constitutes one of the earliest steps in epithelial polarization. E-cadherin-mediated PI(3)-kinase activation following cell–cell contact formation could also provide a significant source of PtdIns(3,4,5)P3 during initial polarization (Kovacs et al. 2002). Whatever the source, PtdIns(3,4,5)P3 can then function to recruit effectors, such as Rac1, that establish the axis of polarity and assign basolateral identity to the membrane.
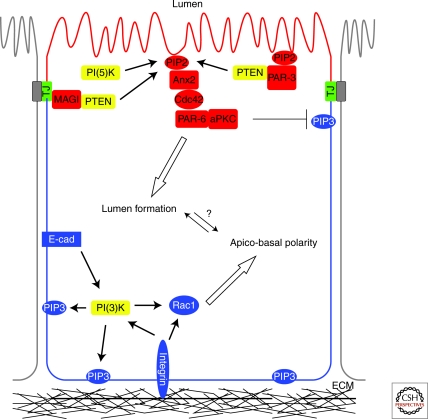
Figure 2.
PIs specify membrane identity in epithelial cells. PI(3)-kinase generated PIP3 (PtdIns(3,4,5)P3) at the basolateral surface contributes to apico-basal polarity specification. This is coordinated with PTEN and PI(5)-kinase enrichment of PIP2 (PtdIns(4,5)P2) at the apical surface. A PTEN/PIP2/Anx2/Cdc42 pathway links the production of PIP2 to actin reorganization during apical membrane biogenesis and lumen formation.
PI INVOLVEMENT IN APICAL DOMAIN BIOGENESIS
The temporal and molecular relationship between apical surface specification and the initial establishment of the apico-basolateral polarity axis, initiated by cell–ECM interactions, remains unclear; however, it appears that these two events are separable because the loss of components affecting apical surface formation, such as PTEN, does not affect axis of polarity. Despite this lack of clarity, a number of studies have begun to address the mechanisms by which PtdIns(4,5)P2 is generated at the nascent apical surface and the downstream signaling events required for apical surface expansion and lumen formation.
As discussed above, PTEN is an important regulator of apical membrane identity. How then is PTEN localization controlled in polarizing cells? The evolutionarily conserved PAR/aPKC complex is a tripartite complex that regulates polarity in embryos, neurons, epithelia, and migrating cells (Suzuki and Ohno 2006; Lee and Vasioukhin 2008). Bazooka (Baz or PAR-3 in mammals) is the first protein of the PAR/aPKC complex to show asymmetric localization to the apical cortex during epithelial polarity establishment (Harris and Peifer 2005). An initial clue that the Baz/PAR-3 scaffolding protein could play an important role in the regulation of PI signaling was the observation that it directly binds to PTEN through its PDZ domains (von Stein et al. 2005). Additional work shows that the interaction between PAR-3 and PTEN was conserved in mammals and necessary for the establishment of epithelial polarity (Feng et al. 2008). Interestingly, two recent studies have reported that Baz/PAR-3 can bind directly to PIs in flies and mammals (Wu et al. 2007; Krahn et al. 2010). There is a lack of agreement on which region of Baz/PAR-3 is required for binding, but this could be because of intrinsic differences in Drosophila and mammalian Baz/PAR-3. Both groups showed an ability for Baz/PAR-3 binding to PtdIns(3,4,5)P3 and PtdIns(4,5)P2. The Baz/PAR-3 PI binding region was also necessary and sufficient for cortical localization and is required for proper mammalian PAR-3 function in epithelia. These findings point to a key role for Baz/PAR-3 in the integration of phosphoinositide signaling during polarization. In this context, Baz/PAR-3 could recruit PTEN to the membrane by binding PIs. This would then lead to removal of the basolateral identifying PtdIns(3,4,5)P3 and localized production of PtdIns(4,5)P2 resulting in the establishment of apical membrane identity (Fig. 2). In this scenario, other factors would still be needed to refine Baz/PAR-3 localization to a specific region of the cortex in concert with apico-basal axis specification and junction formation. Without these additional factors, Baz/PAR-3 would be expected to localize indiscriminately at PIs over the entire plasma membrane.
A point worth considering is that PTEN is unlikely to be the only regulator of PtdIns(4,5)P2 during apical membrane specification. It might therefore be more accurate to think of PTEN function as simply preventing PtdIns(3,4,5)P3 accumulation at the apical surface than as a major source of PtdIns(4,5)P2. Consistent with this idea is the observation that phosphatidylinositol 5-kinase (PI(5)-kinase), a kinase known to stimulate PtdIns(4,5)P2 production, localized to the apical region of MDCK cells and increased biosynthetic delivery of apical proteins (Guerriero et al. 2006). There are likely to be additional mechanisms to generate and amplify apical PtdIns(4,5)P2 that await discovery.
Following establishment of PtdIns(4,5)P2 asymmetry, biogenesis of the apical surface and lumen must be coupled with reorganization of the cytoskeleton. The actin cytoskeleton in particular becomes highly enriched in the subapical region of epithelial cells during lumen formation and is required for numerous cellular processes such as vesicle trafficking and junction formation/stability. Annexins could provide a critical link between PtdIns(4,5)P2 and cytoskeletal reorganization. Anx2 binds PtdIns(4,5)P2 and is recruited to sites of actin assembly (Rescher et al. 2004). During MDCK cyst formation, disruption of Anx2 function compromised lumen formation in a similar manner to loss of PTEN (Martin-Belmonte et al. 2007). Anx2 was localized to the apical surface of cells in developing cysts and delivery of exogenous PtdIns(4,5)P2 to the basal surface of cysts resulted in ectopic recruitment of Anx2 to this surface. Although there are likely to be other effectors of PtdIns(4,5)P2 at the apical membrane, these results point to a potential role of Annexins in PtdIns(4,5)P2-dependent apical actin remodeling. An additional connection between Anx2 and the actin cytoskeleton came from the observation that Anx2 binds the Rho-family small GTPase Cdc42, a known modulator of actin. Support for a Cdc42 role in this process was further shown by RNAi of Cdc42, which disrupted the apical actin cytoskeleton and caused similar lumen formation defects to reduction of Anx2 and PTEN. Collectively, these results define a molecular pathway, involving PTEN/Anx2/Cdc42, that link the production of PtdIns(4,5)P2 to actin reorganization during apical membrane biogenesis and lumen formation (Fig. 2).
There are several possible models for how Cdc42 and the actin cytoskeleton might regulate lumen formation (Martin-Belmonte and Mostov 2007). The presence of large intracellular vacuoles following disruption of the PTEN/Anx2/Cdc42 pathway indicates that there could be defects in vesicle trafficking and exocytosis at the apical surface. This model fits with Cdc42’s regulation of exocytosis (Kroschewski et al. 1999; Musch et al. 2001; Rojas et al. 2001; Wu et al. 2008). Because Cdc42 is known to bind PAR-6 and regulate PAR/aPKC localization, it is also possible that compromised aPKC activity leads to defects in lumen formation (Munro 2006). Interestingly, normal aPKC activity is required to restrict PtdIns(3,4,5)P3 to the basal surface (Takahama et al. 2008). This highlights a potential mechanism for reinforcement or amplification of initial PTEN generated PI asymmetry. Last, a role for Cdc42 activity in mitotic spindle positioning and lumen formation has recently been described (Jaffe et al. 2008; Schluter et al. 2009; Rodriguez-Fraticelli et al. 2010). Intersectin 2, a Cdc42-specific GEF, was proposed to regulate Cdc42-dependent spindle positioning and lumen formation; however, there are probably other regulators of Cdc42, because RNAi of Intersectin 2 does not recapitulate the strong apical membrane biogenesis defects and accumulation of vacuoles seen when total Cdc42 levels are reduced. An RNAi screen of 70 Rho-family GEFs identified the Cdc42 GEF, Tuba, as well as a number of additional potential regulators of Cdc42, that also function in lumen formation (Qin et al. 2010).
DYNAMIC STABILITY: PI CONTROL OF ACTIN ASSEMBLY AND ORGANIZATION
As discussed above, Rho-family GTPases are a major class of PI effector and the net outcome of many PI signaling events is to reorganize the cytoskeleton via spatiotemporal regulation of actin machinery. Key to regulated cell shape changes during morphogenesis is the actin cytoskeleton. The actin cytoskeleton is a dynamic scaffold that underpins a diverse array of cellular processes in eukaryotes. Actin-dependent processes typically involve dynamic membrane reorganization that is achieved by spatiotemporally controlled interplay between the plasma membrane and the underlying cortical actin cytoskeleton (Vicente-Manzanares et al. 2009; Martin 2010). Such control of the cortical actomyosin network is achieved via tightly controlled actin filament turnover and organization and regulation of myosin-II activity. Polymerization and myosin-II-mediated reorganization of actin filaments provides cellular force to drive assembly, maintenance and remodeling of cell–cell contacts (Schwartz and DeSimone 2008). We will not provide a detailed review of actin machinery here, as this has been reviewed recently (Kurisu and Takenawa 2009; Padrick and Rosen 2010; Saarikangas et al. 2010). Instead, we highlight how PI control of actin machinery contributes to adherens junction (AJ) stability (Fig. 3).
Figure 3.
PI control of actin assembly and organization supports junctional integrity and tissue cohesion. (A) Coordination of RhoGTPase directed Arp2/3-dependent actin assembly supports AJ stability. PI control of RhoGTPase localization and activity directs differential Arp2/3-mediated actin assembly that contributes to AJ stability by regulating endocytosis and stability of E-cadherin at the cell surface. A gradient of Rac activation along the apical–basal polarity axis corresponds to different modes of membrane protrusive activity (filopodial vs. lamellipodial), contributing to adhesion dynamics. Phosphorylation of PAR-3 by ROCK modulates Rac activity at the apical pole. (B) Integration of mechanical signals by adhesion proteins controls cellular morphology. Potential molecular feedback loop whereby actomyosin-mediated tension contributes to AJ stabilization and control of PTEN stability and activity. ROCK activity is a potential point of convergence between the pathways controlling orientation of polarity and establishment of the apical surface. ROCK activity modulates PAR-3 and PTEN complex formation and stability, thereby contributing to lipid modification and subsequent control of actin assembly and organization.
Branched Actin Networks
A large number of proteins come together to generate new actin filaments and many of these proteins interact directly with PIs. Arp2/3 (a nucleation promoting factor) activation is controlled by small GTPases, Wiskott–Aldrich syndrome protein (WASP)-family proteins and phosphoinositides (Saarikangas et al. 2010). WASP exists in an autoinhibited conformation (Miki et al. 1998). The mechanism of WASP-family protein regulation involves overcoming an autoinhibitory intramolecular interaction and subsequent oligomerization to drive Arp2/3-dependent actin assembly (Padrick et al. 2008). N-WASP is allosterically activated and oligomerized by binding to PtdIns(4,5)P2 and GTP-Cdc42 (Prehoda et al. 2000; Rohatgi et al. 2000). Interestingly other WASP-family proteins, WAVE/Scar proteins, act somewhat similarly mechanistically to WASP proteins except they are not autoinhibited (Machesky et al. 1999) and are activated by Rac and PtdIns(3,4,5)P3 (Padrick and Rosen 2010). So, PI asymmetries can differentially direct actin nucleation, but how do these complexes contribute to stability of AJs?
The ability of cells to turn over their junctions and thus respond to external stimuli during development is in part achieved by endocytosis and recycling of cadherin to the cell surface (Cavey and Lecuit 2009; Nelson 2009). PAR polarity proteins are essential for appropriate control of cytoskeletal, and thus cellular, polarity in a wide variety of polarized cell types (Mertens et al. 2005; Nishimura et al. 2005; Zhang and Macara 2006, 2008; Pegtel et al. 2007; Nakayama et al. 2008; St Johnston and Ahringer 2010). Baz/Par interaction with the Rac-GEF, TIAM, regulates actin dynamics in a number of different systems (Nishimura et al. 2005; Zhang and Macara 2006; Heasman and Ridley 2008), and contributes to TJ formation in epithelia (Chen and Macara 2005; Mertens et al. 2005; Gopalakrishnan et al. 2007). Recent studies in Drosophila show the critical role of Cdc42, WASP, Arp2/3, and actin in AJ dynamics. Cdc42, PAR-6, and aPKC regulate junctional maintenance and integrity by controlling Arp2/3-dependent endocytosis and recycling of the AJ protein, E-cadherin (Georgiou et al. 2008; Harris and Tepass 2008; Leibfried et al. 2008). These mechanisms are likely conserved in mammalian epithelia (Shen and Turner 2008). Importantly, mutation of Rac and SCAR did not produce the same effects on cadherin distribution as observed on loss of Cdc42 (Georgiou et al. 2008). Further, Rac-WAVE-dependent actin polymerization is required for organization and maintenance of cell–cell contacts (Yamazaki et al. 2007) and high-affinity WAVE activity is restricted to sites of PtdIns(3,4,5)P3 enrichment—namely, the basolateral surface (Suetsugu et al. 2006). More recently the apical polarity proteins Cdc42, PAR-6, aPKC, and Baz/PAR-3 were shown to act together with the Rac-GEF, Sif/TIAM, to define the type of membrane protrusion generated by Rac/WAVE/SCAR-Arp2/3 machinery along the apical–basal axis (Georgiou and Baum 2010). The investigators suggest a gradient of Rac activity, under polarity protein control, that spatially controls the dynamic form of the membrane–actin interface. Considered collectively, the unique subcellular distribution of PIs controls the differential recruitment of subsets of actin assembly machinery to tune the membrane–actin interface to dynamically regulate AJ stability.
Contractile Actomyosin Networks
Nonmuscle myosin II (NM-II) plays a crucial role in processes that involve cell shape changes and movement, including cell adhesion, migration, cytokinesis, and differentiation, to name but a few. Although the contribution of mechanical force to developmental and normal physiology is well established, the exact molecular mechanisms used by cells to sense and respond to changing environmental stimuli is comparatively less well understood (Lopez et al. 2008; Martin 2010). The role for actomyosin generated mechanical force and contractility in regulating individual cellular behaviors and cooperative cellular behavior in tissue morphogenesis and function are areas of ongoing intense investigation (Bershadsky et al. 2006; Schwartz and DeSimone 2008; Vicente-Manzanares et al. 2009). Here we consider how NM-II contributes to cellular force transmission under PI control.
NM-II is an actin-binding protein family that cross links actin filaments, and is regulated by heavy and light chain phosphorylation (Vicente-Manzanares et al. 2009). The actin cross-linking and contractile capacity of NM-II filaments enables NM-II to control the assembly and concomitantly the tensile capacity of the actin cytoskeleton that is intimately involved in tissue organization (Fernandez-Gonzalez and Zallen 2009; Harris and Tepass 2010). There are key kinetic differences and upstream regulatory pathways that in part explain the unique cellular functionality of the different NM-II motors (Kovacs et al. 2003; Wang et al. 2003, 2010; Bao et al. 2007; Smutny et al. 2010). Dozens of kinases have been reported that reversibly phosphorylate the regulatory light chains (RLCs) of NM-II. Among this group are the Rho-associated, coiled coil kinase (ROCK) proteins that are major effectors of the Rho signaling pathway. The two ROCK isoforms are highly homologous, apart from their PH domains, and act on the same cellular substrates (Yoneda et al. 2005, 2007). ROCKII recruitment to the membrane is GTP-RhoA dependent (Sin et al. 1998) and the PH domain of ROCKII bound PtdIns(3,4,5)P3 (Yoneda et al. 2005). Therefore, membrane recruitment of ROCKII downstream from Rho could depend on localized PtdIns(3,4,5)P3 enrichment. In knockout mice studies, ROCKI was unable to compensate for loss of ROCKII (Thumkeo et al. 2003), arguing that ROCKs regulate discrete cellular NM-II pools. Collectively, these studies indicate that subcellular targeting by differential PI binding restricts ROCK isoform activity, affording spatial control of actin organization.
The orientation of cellular polarity, particularly in epithelial tissues, depends on the interactions of the cell with the ECM (Lopez et al. 2008). Important insights into how cells identify and interpret the polarizing cues coming from the ECM have been obtained from studies of epithelial cells grown in 3D (Yu et al. 2005; O’Brien et al. 2006; Liu et al. 2007). These studies support a role for Rac1, PI(3)-kinase, and β1-integrin/ECM engagement in establishing pole of polarity. Appropriate ECM organization is dependent on the actin cytoskeleton and Rho activity. In the β1-integrin blocked 3D model, selective depletion of the ROCK isoforms revealed a differential requirement for ROCKI in establishing pole of polarity downstream of cell-ECM engagement. Because inversion of polarity has been observed in vivo during tumor progression (Adams et al. 2004), these studies provide important mechanistic insight into the pathway underlying this clinically relevant process.
The pathway that establishes pole of polarity could be coupled to the pathway that generates the apical surface, the PTEN/Annexin/Cdc42 axis mentioned above. ROCK activity is a likely candidate because it contributes to both PAR-3 and PTEN function (Fig. 3). ROCK phosphorylation of PAR-3 disrupted PAR-3/aPKC/PAR-6 complex formation in migrating cells, spatially controlling TIAM-mediated Rac activity (Nakayama et al. 2008). Zallen and colleagues reported a mechanism whereby ROCK regulates the Baz/PAR-3 PI interaction, thus controlling Baz/PAR-3 association with the cortex (Simoes Sde et al. 2010). PAR-3 recruits PTEN to establish epithelial polarity (Feng et al. 2008). The RhoA/ROCK pathway is known to enhance PTEN phosphatase activity (Li et al. 2005). PTEN stability and activity is regulated by phosphorylation of the carboxy-terminal tail and by interactions with specific binding partners (Leslie et al. 2008). Of note PTEN protein levels are controlled by AJ association and PTEN in turn stabilizes AJs (Kotelevets et al. 2005; Subauste et al. 2005; Li et al. 2007; Fournier et al. 2009). Interestingly, a direct link between PTEN and ROCKI was shown in primary bone marrow macrophages, in which ROCKI was required for PTEN stabilization, phosphorylation, and activity (Vemula et al. 2010). Collectively, these studies suggest an integral role, and perhaps a point of convergence, for ROCK in regulation of acquisition of polarity and membrane specification. Recruitment of PAR-3 and PTEN to the cortex via binding of PIs contributes to cellular polarization, if we couple this with ROCK-mediated regulation of Rho-GTPase/PAR complex formation and PTEN stability, we add another layer of regulation to the polarization machinery. Future research will determine the exact molecular mechanisms involved.
PI CONTROL OF JUNCTIONAL STABILITY
Key to polarization are the development of adherens junctions (AJs) and tight junctions (TJs) that regulate cell–cell adhesion (Wang and Margolis 2007). PAR-3 is a critical determinant of cellular polarity, acting to control actin assembly and the development of tight and adherens junctions. In addition to interacting with PTEN, Baz/PAR-3 also interacts with components of the nectin- and cadherin-based adhesion machinery to promote cadherin clustering, a well-recognized determinant of cadherin adhesive strengthening (Leckband and Prakasam 2006; Harris and Tepass 2010). During the early stages of intestinal epithelial cell polarization in C. elegans, cortical foci containing PAR-3 recruit E-cadherin, PAR-6, and aPKC (Achilleos et al. 2010). Furthermore, appropriate positioning of Baz is critical to defining the apical/lateral border (Morais-de-Sa et al. 2010). PAR-3 also interacts directly with PIs (Wu et al. 2007). Collectively, these observations place differential PI driven polarity protein complex regulation at the core of cellular membrane asymmetry and domain amplification. How cell–cell adhesions are established and coordinated with polarity has been the focus of a number of recent reviews (Suzuki and Ohno 2006; Goldstein and Macara 2007; Martin-Belmonte and Rodriquez-Fraticelli 2009; St Johnston and Ahringer 2010), here we consider PI regulation of junctional stability and the emerging role of AJs as mechanosensors.
AJs AS MECHANOSENSORS
How does the actin cytoskeleton contribute to AJ stability and cellular mechanosensing? Many labs have reported that NM-II activity is required for adherens junction organization and stability (Shewan et al. 2005; Ivanov et al. 2007; Yamada and Nelson 2007; Vicente-Manzanares et al. 2009; Smutny et al. 2010). Yet the molecular mechanisms that underlie integration of contractile force at cell–cell adhesions are less well defined, although recent research provides some clues (Fig. 3). NM-II-dependent recruitment of vinculin to AJs revealed a mechanosensing role for E-cadherin (le Duc et al. 2010). Yonemura and colleagues showed an NM-II-dependent mechanism for stabilization of AJs that involves a force-dependent interaction between vinculin and α-catenin (Miyake et al. 2006; Yonemura et al. 2010). Further, direct application of an exogenous tugging force was sufficient, in addition to myosin activity, to trigger AJ formation and stabilization (Liu et al. 2010). Interestingly, vinculin controls PTEN protein levels by modulating the interaction of the E-cadherin binding protein, β-catenin, with membrane-associated guanylate-kinase 2 (MAGI-2) (Subauste et al. 2005). Vinculin is recruited to the membrane by association with PtdIns(4,5)P2 (Palmer et al. 2009). Whether stretch activation of α-catenin and associated vinculin binding is required to stabilise the β-catenin/MAGI interaction, thus controlling PTEN protein levels and PtdIns(4,5)P2, is an open question. These studies highlight the complex interplay between force and adhesion and how translation of multiple signals into actomyosin-generated force at cell–cell and cell–ECM contacts collaboratively support global tissue architecture.
CYTOSKELETAL CONTROL OF TISSUE ARCHITECTURE
How does the interaction of PIs with actin regulatory proteins translate to cell shape changes and subsequent control of tissue architecture? Important insights have emerged from work in model systems. Actomyosin networks play a central role in the morphogenetic movements that underpin model organism development (Quintin et al. 2008; Martin 2010). Though the contributions of actin and myosin in developmental processes are established, the exact mechanisms by which actomyosin networks drive cell shape changes remain to be fully characterized.
Actomyosin networks underlying the cell surface are capable of generating planar tensile forces that contribute to cortical tension. Because PIs are intimately involved in regulating polarity and actin assembly machinery, PIs are likely central players in force integration. Cells employ tensile cortical actin meshworks to balance and respond to forces experienced within the tissue, originating from other cells and the ECM (Lopez et al. 2008). In response to complex patterns of tension input, the cells respond by locally changing shape to accommodate changing requirements of the tissue. A key developmental cell shape change is driven by apical constriction. Epithelial cells undergo spatially regulated and timed constriction of the apical actomyosin belt to convert columnar cells to a wedge-shaped morphology, which drives folding of the epithelial sheet (Sawyer et al. 2010). This elegant cellular mechanism underlies the formation of many epithelial tissue topologies, including: folds, pits, and tubules. Biochemical signals are necessary and sufficient to recruit NM-II to the cortex, though mechanical signals play an important role too. Recent studies have provided evidence that mechanical tension is sufficient to recruit NM-II to the apical surface to control actomyosin dynamics (Blankenship et al. 2006; Fernandez-Gonzalez et al. 2009; Pouille et al. 2009). Interestingly cortical actomyosin acts as a ratchet, whereby pulsed contractions of the cortical actomyosin network drive and stabilize incremental apical constriction (Martin et al. 2009; Solon et al. 2009). Subsequent work showed that AJs balance the forces generated by the tensile actomyosin network to transmit tension across the tissue (Martin et al. 2010). Of particular note, PTEN recruits NM-II to the cortex in response to application of force in Dictyostelium (Pramanik et al. 2009). Additionally, Baz and PAR-6/aPKC regulate distinct phases of the myosin assembly disassembly cycle during amnioserosa apical constriction during Drosophila dorsal closure (David et al. 2010). Although, it is possible that apical constriction alone may not be sufficient to induce sheet folding because integration of both circumapical and lateral contraction of the endoderm was required to drive early ascidian gastrulation (Sherrard et al. 2010). Because NM-II can be recruited to the cortex by tension and by protein interactions the involvement of polarity proteins in apical constriction is particularly germane (Choi and Sokol 2009; Hava et al. 2009; David et al. 2010). Polarity protein involvement in apical constriction suggests that mechanical tension is integrated by modifying the properties of the plasma membrane and, by extension, the PI composition of the cell surface. An in-depth understanding of cellular mechanisms for controlling and responding to force is crucial if we are to understand the cellular basis of tissue and organismal development.
CONCLUDING REMARKS
In this review, we have examined how a group of membrane-associated phospholipids contribute to a wide variety of signaling pathways to control cellular and tissue architecture. Regulation of the interconversion of various PIs is able to assign unique membrane identity to epithelial cell domains in conjunction with pole of polarity specification. Once PI-mediated membrane identity is initially established, PIs act by participating in cross-talk and feedback amplification loops to reinforce membrane identity. PIs are further able to integrate membrane identity with aspects of cellular physiology by recruiting and enriching membranes with downstream effectors that posses PI-binding modules such as PH domains. Frequently, these effectors act to reorganize the cytoskeleton proximal to membrane domains in a manner that helps further define their unique identity. These cytoskeletal changes play an important part in cell–cell adhesion and the establishment of actomyosin contractility across epithelial sheets that are required for coordinating overall tissue architecture.
Epithelial cells play important roles in development and disease. Significantly, loss of epithelial cell polarity and tissue architecture is one of the defining hallmarks of cancer and it is estimated that the majority of all cancers are epithelial in origin. Given the essential nature of PI regulation of epithelial cell architecture, it is not surprising that PTEN and PI(3)-kinase are two of the most frequently mutated genes in cancer (Bunney and Katan 2010). Consequently, further study of PI regulation of epithelial cell polarity will yield important insight into both the development of this important cell type as well as potential mechanisms of oncogenic signaling.
ACKNOWLEDGMENTS
We thank Ross Metzger for thoughtful comments on the manuscript. We extend our apologies to the authors of the studies we were unable to cite because of space limitations. This work was supported by a National Health and Medical Research Council C.J. Martin Fellowship to A.S., a Ruth L. Kirschstein NRSA Postdoctoral Fellowship to D.J.E., and National Institutes of Health R01s DK074398, DK067153, and AI25144 to K.M.
Footnotes
Editor: Kai Simons
Additional Perspectives on The Biology of Lipids available at www.cshperspectives.org
REFERENCES
- Achilleos A, Wehman AM, Nance J 2010. PAR-3 mediates the initial clustering and apical localization of junction and polarity proteins during C. elegans intestinal epithelial cell polarization. Development 137: 1833–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SA, Smith ME, Cowley GP, Carr LA 2004. Reversal of glandular polarity in the lymphovascular compartment of breast cancer. J Clin Pathol 57: 1114–1117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao J, Ma X, Liu C, Adelstein RS 2007. Replacement of nonmuscle myosin II-B with II-A rescues brain but not cardiac defects in mice. J Biol Chem 282: 22102–22111 [DOI] [PubMed] [Google Scholar]
- Barlow CA, Laishram RS, Anderson RA 2009. Nuclear phosphoinositides: A signaling enigma wrapped in a compartmental conundrum. Trends Cell Biol 20: 25–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behnia R, Munro S 2005. Organelle identity and the signposts for membrane traffic. Nature 438: 597–604 [DOI] [PubMed] [Google Scholar]
- Bershadsky A, Kozlov M, Geiger B 2006. Adhesion-mediated mechanosensitivity: a time to experiment, and a time to theorize. Curr Opin Cell Biol 18: 472–481 [DOI] [PubMed] [Google Scholar]
- Blankenship JT, Backovic ST, Sanny JS, Weitz O, Zallen JA 2006. Multicellular rosette formation links planar cell polarity to tissue morphogenesis. Dev Cell 11: 459–470 [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M 2010. Phosphoinositide signalling in cancer: Beyond PI3K and PTEN. Nat Rev Cancer 10: 342–352 [DOI] [PubMed] [Google Scholar]
- Cain RJ, Ridley AJ 2009. Phosphoinositide 3-kinases in cell migration. Biol Cell 101: 13–29 [DOI] [PubMed] [Google Scholar]
- Cavey M, Lecuit T 2009. Molecular bases of cell–cell junctions stability and dynamics. Cold Spring Harb Perspect Biol 1: a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macara IG 2005. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol 7: 262–269 [DOI] [PubMed] [Google Scholar]
- Choi SC, Sokol SY 2009. The involvement of lethal giant larvae and Wnt signaling in bottle cell formation in Xenopus embryos. Dev Biol 336: 68–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Souza-Schorey C, Chavrier P 2006. ARF proteins: Roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7: 347–358 [DOI] [PubMed] [Google Scholar]
- David DJ, Tishkina A, Harris TJ 2010. The PAR complex regulates pulsed actomyosin contractions during amnioserosa apical constriction in Drosophila. Development 137: 1645–1655 [DOI] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Feng W, Wu H, Chan LN, Zhang M 2008. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem 283: 23440–23449 [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Zallen JA 2009. Cell mechanics and feedback regulation of actomyosin networks. Sci Signal 2: e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Simoes Sde M, Roper JC, Eaton S, Zallen JA 2009. Myosin II dynamics are regulated by tension in intercalating cells. Dev Cell 17: 736–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier MV, Fata JE, Martin KJ, Yaswen P, Bissell MJ 2009. Interaction of E-cadherin and PTEN regulates morphogenesis and growth arrest in human mammary epithelial cells. Cancer Res 69: 4545–4552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gassama-Diagne A, Yu W, ter Beest M, Martin-Belmonte F, Kierbel A, Engel J, Mostov K 2006. Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat Cell Biol 8: 963–970 [DOI] [PubMed] [Google Scholar]
- Georgiou M, Baum B 2010. Polarity proteins and Rho GTPases cooperate to spatially organise epithelial actin-based protrusions. J Cell Sci 123: 1089–1098 [DOI] [PubMed] [Google Scholar]
- Georgiou M, Marinari E, Burden J, Baum B 2008. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol 18: 1631–1638 [DOI] [PubMed] [Google Scholar]
- Goldfinger LE 2008. Choose your own path: Specificity in Ras GTPase signaling. Mol Biosyst 4: 293–299 [DOI] [PubMed] [Google Scholar]
- Goldstein B, Macara IG 2007. The PAR proteins: Fundamental players in animal cell polarization. Dev Cell 13: 609–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Hallett MA, Atkinson SJ, Marrs JA 2007. aPKC-PAR complex dysfunction and tight junction disassembly in renal epithelial cells during ATP depletion. Am J Physiol Cell Physiol 292: C1094–1102 [DOI] [PubMed] [Google Scholar]
- Guerriero CJ, Weixel KM, Bruns JR, Weisz OA 2006. Phosphatidylinositol 5-kinase stimulates apical biosynthetic delivery via an Arp2/3-dependent mechanism. J Biol Chem 281: 15376–15384 [DOI] [PubMed] [Google Scholar]
- Harlan JE, Hajduk PJ, Yoon HS, Fesik SW 1994. Pleckstrin homology domains bind to phosphatidylinositol-4,5-bisphosphate. Nature 371: 168–170 [DOI] [PubMed] [Google Scholar]
- Harris TJ, Peifer M 2005. The positioning and segregation of apical cues during epithelial polarity establishment in Drosophila. J Cell Biol 170: 813–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KP, Tepass U 2008. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J Cell Biol 183: 1129–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris TJ, Tepass U 2010. Adherens junctions: From molecules to morphogenesis. Nat Rev Mol Cell Biol 11: 502–514 [DOI] [PubMed] [Google Scholar]
- Hava D, Forster U, Matsuda M, Cui S, Link BA, Eichhorst J, Wiesner B, Chitnis A, Abdelilah-Seyfried S 2009. Apical membrane maturation and cellular rosette formation during morphogenesis of the zebrafish lateral line. J Cell Sci 122: 687–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ 2008. Mammalian Rho GTPases: New insights into their functions from in vivo studies. Nat Rev Mol Cell Biol 9: 690–701 [DOI] [PubMed] [Google Scholar]
- Ivanov AI, Bachar M, Babbin BA, Adelstein RS, Nusrat A, Parkos CA 2007. A unique role for nonmuscle myosin heavy chain IIA in regulation of epithelial apical junctions. PLoS One 2: e658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe AB, Kaji N, Durgan J, Hall A 2008. Cdc42 controls spindle orientation to position the apical surface during epithelial morphogenesis. J Cell Biol 183: 625–633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanes A, Smutny M, Leerberg JM, Yap AS 2009. Phosphatidylinositol 3′-kinase signalling supports cell height in established epithelial monolayers. J Mol Histol 40: 395–405 [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS 1988. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell 52: 311–320 [DOI] [PubMed] [Google Scholar]
- Khwaja A, Lehmann K, Marte BM, Downward J 1998. Phosphoinositide 3-kinase induces scattering and tubulogenesis in epithelial cells through a novel pathway. J Biol Chem 273: 18793–18801 [DOI] [PubMed] [Google Scholar]
- Kotelevets L, van Hengel J, Bruyneel E, Mareel M, van Roy F, Chastre E 2005. Implication of the MAGI-1b/PTEN signalosome in stabilization of adherens junctions and suppression of invasiveness. FASEB J 19: 115–117 [DOI] [PubMed] [Google Scholar]
- Kovacs EM, Ali RG, McCormack AJ, Yap AS 2002. E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J Biol Chem 277: 6708–6718 [DOI] [PubMed] [Google Scholar]
- Kovacs M, Wang F, Hu A, Zhang Y, Sellers JR 2003. Functional divergence of human cytoplasmic myosin II: Kinetic characterization of the non-muscle IIA isoform. J Biol Chem 278: 38132–38140 [DOI] [PubMed] [Google Scholar]
- Krahn MP, Klopfenstein DR, Fischer N, Wodarz A 2010. Membrane targeting of Bazooka/PAR-3 is mediated by direct binding to phosphoinositide lipids. Curr Biol 20: 636–642 [DOI] [PubMed] [Google Scholar]
- Kroschewski R, Hall A, Mellman I 1999. Cdc42 controls secretory and endocytic transport to the basolateral plasma membrane of MDCK cells. Nat Cell Biol 1: 8–13 [DOI] [PubMed] [Google Scholar]
- Kurisu S, Takenawa T 2009. The WASP and WAVE family proteins. Genome Biol 10: 226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutateladze TG 2010. Translation of the phosphoinositide code by PI effectors. Nat Chem Biol 6: 507–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Duc Q, Shi Q, Blonk I, Sonnenberg A, Wang N, Leckband D, de Rooij J 2010. Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J Cell Biol 189: 1107–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leckband D, Prakasam A 2006. Mechanism and dynamics of cadherin adhesion. Annu Rev Biomed Eng 8: 259–287 [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V 2008. Cell polarity and cancer—Cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci 121: 1141–1150 [DOI] [PubMed] [Google Scholar]
- Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y 2008. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol 18: 1639–1648 [DOI] [PubMed] [Google Scholar]
- Lemmon MA 2008. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol 9: 99–111 [DOI] [PubMed] [Google Scholar]
- Lemmon MA, Ferguson KM 2000. Signal-dependent membrane targeting by pleckstrin homology (PH) domains. Biochem J 350: 1–18 [PMC free article] [PubMed] [Google Scholar]
- Leslie NR, Batty IH, Maccario H, Davidson L, Downes CP 2008. Understanding PTEN regulation: PIP2, polarity and protein stability. Oncogene 27: 5464–5476 [DOI] [PubMed] [Google Scholar]
- Li Z, Dong X, Wang Z, Liu W, Deng N, Ding Y, Tang L, Hla T, Zeng R, Li L, et al. 2005. Regulation of PTEN by Rho small GTPases. Nat Cell Biol 7: 399–404 [DOI] [PubMed] [Google Scholar]
- Li Z, Wang L, Zhang W, Fu Y, Zhao H, Hu Y, Prins BP, Zha X 2007. Restoring E-cadherin-mediated cell–cell adhesion increases PTEN protein level and stability in human breast carcinoma cells. Biochem Biophys Res Commun 363: 165–170 [DOI] [PubMed] [Google Scholar]
- Liu KD, Datta A, Yu W, Brakeman PR, Jou TS, Matthay MA, Mostov KE 2007. Rac1 is required for reorientation of polarity and lumen formation through a PI 3-kinase-dependent pathway. Am J Physiol Renal Physiol 293: F1633–1640 [DOI] [PubMed] [Google Scholar]
- Liu H, Radisky DC, Wang F, Bissell MJ 2004. Polarity and proliferation are controlled by distinct signaling pathways downstream of PI3-kinase in breast epithelial tumor cells. J Cell Biol 164: 603–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Tan JL, Cohen DM, Yang MT, Sniadecki NJ, Ruiz SA, Nelson CM, Chen CS 2010. Mechanical tugging force regulates the size of cell–cell junctions. Proc Natl Acad Sci 107: 9944–9949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez JI, Mouw JK, Weaver VM 2008. Biomechanical regulation of cell orientation and fate. Oncogene 27: 6981–6993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machesky LM, Mullins RD, Higgs HN, Kaiser DA, Blanchoin L, May RC, Hall ME, Pollard TD 1999. Scar, a WASp-related protein, activates nucleation of actin filaments by the Arp2/3 complex. Proc Natl Acad Sci 96: 3739–3744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC 2010. Pulsation and stabilization: contractile forces that underlie morphogenesis. Dev Biol 341: 114–125 [DOI] [PubMed] [Google Scholar]
- Martin AC, Kaschube M, Wieschaus EF 2009. Pulsed contractions of an actin-myosin network drive apical constriction. Nature 457: 495–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AC, Gelbart M, Fernandez-Gonzalez R, Kaschube M, Wieschaus EF 2010. Integration of contractile forces during tissue invagination. J Cell Biol 188: 735–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K 2007. Phosphoinositides control epithelial development. Cell Cycle 6: 1957–1961 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Rodriguez-Fraticelli AE 2009. Chapter 3: Acquisition of membrane polarity in epithelial tube formation patterns, signaling pathways, molecular mechanisms, and disease. Int Rev Cell Mol Biol 274: 129–182 [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, Mostov K 2007. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea HJ, De Camilli P 2009. Mutations in phosphoinositide metabolizing enzymes and human disease. Physiology (Bethesda) 24: 8–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG 2005. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol 170: 1029–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki H, Sasaki T, Takai Y, Takenawa T 1998. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 391: 93–96 [DOI] [PubMed] [Google Scholar]
- Miyake Y, Inoue N, Nishimura K, Kinoshita N, Hosoya H, Yonemura S 2006. Actomyosin tension is required for correct recruitment of adherens junction components and zonula occludens formation. Exp Cell Res 312: 1637–1650 [DOI] [PubMed] [Google Scholar]
- Morais-de-Sa E, Mirouse V, St Johnston D 2010. aPKC phosphorylation of Bazooka defines the apical/lateral border in Drosophila epithelial cells. Cell 141: 509–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro EM 2006. PAR proteins and the cytoskeleton: A marriage of equals. Curr Opin Cell Biol 18: 86–94 [DOI] [PubMed] [Google Scholar]
- Musch A, Cohen D, Kreitzer G, Rodriguez-Boulan E 2001. cdc42 regulates the exit of apical and basolateral proteins from the trans-Golgi network. EMBO J 20: 2171–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, Amano M, Kaibuchi K 2008. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell 14: 205–215 [DOI] [PubMed] [Google Scholar]
- Nelson WJ 2009. Remodeling epithelial cell organization: Transitions between front–rear and apical–basal polarity. Cold Spring Harb Perspect Biol 1: a000513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, Hoshino M, Kaibuchi K 2005. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol 7: 270–277 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Zegers MM, Mostov KE 2002. Opinion: Building epithelial architecture: Insights from three-dimensional culture models. Nat Rev Mol Cell Biol 3: 531–537 [DOI] [PubMed] [Google Scholar]
- O’Brien LE, Yu W, Tang K, Jou TS, Zegers MM, Mostov KE 2006. Morphological and biochemical analysis of Rac1 in three-dimensional epithelial cell cultures. Methods Enzymol 406: 676–691 [DOI] [PubMed] [Google Scholar]
- Padrick SB, Rosen MK 2010. Physical mechanisms of signal integration by WASP family proteins. Annu Rev Biochem 79: 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padrick SB, Cheng HC, Ismail AM, Panchal SC, Doolittle LK, Kim S, Skehan BM, Umetani J, Brautigam CA, Leong JM, et al. 2008. Hierarchical regulation of WASP/WAVE proteins. Mol Cell 32: 426–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer SM, Playford MP, Craig SW, Schaller MD, Campbell SL 2009. Lipid binding to the tail domain of vinculin: Specificity and the role of the N and C termini. J Biol Chem 284: 7223–7231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parise LV, Lee J, Juliano RL 2000. New aspects of integrin signaling in cancer. Semin Cancer Biol 10: 407–414 [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG 2007. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front–rear polarity. Curr Biol 17: 1623–1634 [DOI] [PubMed] [Google Scholar]
- Pilot F, Philippe JM, Lemmers C, Lecuit T 2006. Spatial control of actin organization at adherens junctions by a synaptotagmin-like protein Btsz. Nature 442: 580–584 [DOI] [PubMed] [Google Scholar]
- Pinal N, Goberdhan DC, Collinson L, Fujita Y, Cox IM, Wilson C, Pichaud F 2006. Regulated and polarized PtdIns(3,4,5)P3 accumulation is essential for apical membrane morphogenesis in photoreceptor epithelial cells. Curr Biol 16: 140–149 [DOI] [PubMed] [Google Scholar]
- Pouille PA, Ahmadi P, Brunet AC, Farge E 2009. Mechanical signals trigger Myosin II redistribution and mesoderm invagination in Drosophila embryos. Sci Signal 2: ra16. [DOI] [PubMed] [Google Scholar]
- Pramanik MK, Iijima M, Iwadate Y, Yumura S 2009. PTEN is a mechanosensing signal transducer for myosin II localization in Dictyostelium cells. Genes Cells 14: 821–834 [DOI] [PubMed] [Google Scholar]
- Prehoda KE, Scott JA, Mullins RD, Lim WA 2000. Integration of multiple signals through cooperative regulation of the N-WASP-Arp2/3 complex. Science 290: 801–806 [DOI] [PubMed] [Google Scholar]
- Qin Y, Meisen WH, Hao Y, Macara IG 2010. Tuba, a Cdc42 GEF, is required for polarized spindle orientation during epithelial cyst formation. J Cell Biol 189: 661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintin S, Gally C, Labouesse M 2008. Epithelial morphogenesis in embryos: asymmetries, motors and brakes. Trends Genet 24: 221–230 [DOI] [PubMed] [Google Scholar]
- Reiter JF, Mostov K 2006. Vesicle transport, cilium formation, and membrane specialization: The origins of a sensory organelle. Proc Natl Acad Sci 103: 18383–18384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescher U, Ruhe D, Ludwig C, Zobiack N, Gerke V 2004. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J Cell Sci 117: 3473–3480 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Fraticelli AE, Vergarajauregui S, Eastburn DJ, Datta A, Alonso MA, Mostov K, Martin-Belmonte F 2010. The Cdc42 GEF Intersectin 2 controls mitotic spindle orientation to form the lumen during epithelial morphogenesis. J Cell Biol 189: 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Ho HY, Kirschner MW 2000. Mechanism of N-WASP activation by CDC42 and phosphatidylinositol 4, 5-bisphosphate. J Cell Biol 150: 1299–1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas R, Ruiz WG, Leung SM, Jou TS, Apodaca G 2001. Cdc42-dependent modulation of tight junctions and membrane protein traffic in polarized Madin–Darby canine kidney cells. Mol Biol Cell 12: 2257–2274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario M, Birchmeier W 2004. Making tubes: Step by step. Dev Cell 7: 3–5 [DOI] [PubMed] [Google Scholar]
- Saarikangas J, Zhao H, Lappalainen P 2010. Regulation of the actin cytoskeleton–plasma membrane interplay by phosphoinositides. Physiol Rev 90: 259–289 [DOI] [PubMed] [Google Scholar]
- Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B 2010. Apical constriction: A cell shape change that can drive morphogenesis. Dev Biol 341: 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluter MA, Pfarr CS, Pieczynski J, Whiteman EL, Hurd TW, Fan S, Liu CJ, Margolis B 2009. Trafficking of Crumbs3 during cytokinesis is crucial for lumen formation. Mol Biol Cell 20: 4652–4663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, DeSimone DW 2008. Cell adhesion receptors in mechanotransduction. Curr Opin Cell Biol 20: 551–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Turner JR 2008. Intercellular junctions: Actin the PARt. Curr Biol 18: R1014–1017 [DOI] [PubMed] [Google Scholar]
- Sherrard K, Robin F, Lemaire P, Munro E 2010. Sequential activation of apical and basolateral contractility drives ascidian endoderm invagination. Curr Biol 20: 1499–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan AM, Maddugoda M, Kraemer A, Stehbens SJ, Verma S, Kovacs EM, Yap AS 2005. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell–cell contacts. Mol Biol Cell 16: 4531–4542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SH, Jan LY, Jan YN 2003. Hippocampal neuronal polarity specified by spatially localized mPar3/mPar6 and PI 3-kinase activity. Cell 112: 63–75 [DOI] [PubMed] [Google Scholar]
- Simoes Sde M, Blankenship JT, Weitz O, Farrell DL, Tamada M, Fernandez-Gonzalez R, Zallen JA 2010. Rho-kinase directs Bazooka/Par-3 planar polarity during Drosophila axis elongation. Dev Cell 19: 377–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin WC, Chen XQ, Leung T, Lim L 1998. RhoA-binding kinase α translocation is facilitated by the collapse of the vimentin intermediate filament network. Mol Cell Biol 18: 6325–6339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, Hamilton NA, Parton RG, Adelstein RS, Yap AS 2010. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol 12: 696–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solon J, Kaya-Copur A, Colombelli J, Brunner D 2009. Pulsed forces timed by a ratchet-like mechanism drive directed tissue movement during dorsal closure. Cell 137: 1331–1342 [DOI] [PubMed] [Google Scholar]
- St Johnston D, Ahringer J 2010. Cell polarity in eggs and epithelia: Parallels and diversity. Cell 141: 757–774 [DOI] [PubMed] [Google Scholar]
- Subauste MC, Nalbant P, Adamson ED, Hahn KM 2005. Vinculin controls PTEN protein level by maintaining the interaction of the adherens junction protein β-catenin with the scaffolding protein MAGI-2. J Biol Chem 280: 5676–5681 [DOI] [PubMed] [Google Scholar]
- Suetsugu S, Kurisu S, Oikawa T, Yamazaki D, Oda A, Takenawa T 2006. Optimization of WAVE2 complex-induced actin polymerization by membrane-bound IRSp53, PIP(3), and Rac. J Cell Biol 173: 571–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Ohno S 2006. The PAR-aPKC system: Lessons in polarity. J Cell Sci 119: 979–987 [DOI] [PubMed] [Google Scholar]
- Tahirovic S, Bradke F 2009. Neuronal polarity. Cold Spring Harb Perspect Biol 1: a001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama S, Hirose T, Ohno S 2008. aPKC restricts the basolateral determinant PtdIns(3,4,5)P3 to the basal region. Biochem Biophys Res Commun 368: 249–255 [DOI] [PubMed] [Google Scholar]
- ten Klooster JP, Hordijk PL 2007. Targeting and localized signalling by small GTPases. Biol Cell 99: 1–12 [DOI] [PubMed] [Google Scholar]
- Thumkeo D, Keel J, Ishizaki T, Hirose M, Nonomura K, Oshima H, Oshima M, Taketo MM, Narumiya S 2003. Targeted disruption of the mouse Rho-associated kinase 2 gene results in intrauterine growth retardation and fetal death. Mol Cell Biol 23: 5043–5055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemula S, Shi J, Hanneman P, Wei L, Kapur R 2010. ROCK1 functions as a suppressor of inflammatory cell migration by regulating PTEN phosphorylation and stability. Blood 115: 1785–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicente-Manzanares M, Ma X, Adelstein RS, Horwitz AR 2009. Non-muscle myosin II takes centre stage in cell adhesion and migration. Nat Rev Mol Cell Biol 10: 778–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Stein W, Ramrath A, Grimm A, Muller-Borg M, Wodarz A 2005. Direct association of Bazooka/PAR-3 with the lipid phosphatase PTEN reveals a link between the PAR/aPKC complex and phosphoinositide signaling. Development 132: 1675–1686 [DOI] [PubMed] [Google Scholar]
- Wang Q, Margolis B 2007. Apical junctional complexes and cell polarity. Kidney Int 72: 1448–1458 [DOI] [PubMed] [Google Scholar]
- Wang F, Kovacs M, Hu A, Limouze J, Harvey EV, Sellers JR 2003. Kinetic mechanism of non-muscle myosin IIB: functional adaptations for tension generation and maintenance. J Biol Chem 278: 27439–27448 [DOI] [PubMed] [Google Scholar]
- Wang A, Ma X, Conti MA, Liu C, Kawamoto S, Adelstein RS 2010. Nonmuscle myosin II isoform and domain specificity during early mouse development. Proc Natl Acad Sci 107: 14645–14650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Rossi G, Brennwald P 2008. The ghost in the machine: Small GTPases as spatial regulators of exocytosis. Trends Cell Biol 18: 397–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M 2007. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell 28: 886–898 [DOI] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ 2007. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell–cell adhesion. J Cell Biol 178: 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki D, Oikawa T, Takenawa T 2007. Rac-WAVE-mediated actin reorganization is required for organization and maintenance of cell–cell adhesion. J Cell Sci 120: 86–100 [DOI] [PubMed] [Google Scholar]
- Yoneda A, Multhaupt HA, Couchman JR 2005. The Rho kinases I and II regulate different aspects of myosin II activity. J Cell Biol 170: 443–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda A, Ushakov D, Multhaupt HA, Couchman JR 2007. Fibronectin matrix assembly requires distinct contributions from Rho kinases I and -II. Mol Biol Cell 18: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura S, Wada Y, Watanabe T, Nagafuchi A, Shibata M 2010. α-Catenin as a tension transducer that induces adherens junction development. Nat Cell Biol 12: 533–542 [DOI] [PubMed] [Google Scholar]
- Yu W, Datta A, Leroy P, O’Brien LE, Mak G, Jou TS, Matlin KS, Mostov KE, Zegers MM 2005. β1-integrin orients epithelial polarity via Rac1 and laminin. Mol Biol Cell 16: 433–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Macara IG 2006. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol 8: 227–237 [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG 2008. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell 14: 216–226 [DOI] [PMC free article] [PubMed] [Google Scholar]