Abstract
Aims. To establish the prevalence and the effect of thyroid dysfunction on pregnancy outcomes in Asian-Indian population. Subjects and Methods. The study cohort comprised of 483 consecutive pregnant women in the first trimester attending the antenatal clinic of a tertiary center in Mumbai, India. Thyroid hormone levels and thyroid peroxidase antibody were estimated. Patients with thyroid dysfunction were assessed periodically or treated depending on the severity. Subjects were followed until delivery. Results. The prevalence of hypothyroidism, Graves' disease, gestational transient thyrotoxicosis, and thyroid autoimmunity (TAI) was 4.8% (n = 24), 0.6% (n = 3), 6.4 % (n = 31), and 12.4% (n = 60), respectively. Forty percent of the hypothyroid patients did not have any high-risk characteristics. Hypothyroidism and TAI were associated with miscarriage (P = 0.02 and P = 0.001, resp.). Conclusions. The prevalence of hypothyroidism (4.8%) and TAI (12.4%) is high. TAI and hypothyroidism were significantly associated with miscarriage.
1. Introduction
Pregnancy can be viewed as a state in which a combination of events concurs to modify the thyroidal economy. There is change in the level of thyroxine-binding globulin, total thyroid-hormone level and change in the level of thyroid stimulating hormone (TSH) during normal pregnancy [1]. Thyroid dysfunction (TD) may be overlooked in pregnancy because of the nonspecific symptoms and hypermetabolic state of normal pregnancy.
Thyroid dysfunction has varied impact on pregnancy outcome. The risk of miscarriage is increased in autoimmune thyroid disease. Severe maternal hypothyroidism can result in irreversible neurological deficit in the offspring. Graves' disease (GD) can lead to pregnancy loss as well as fetal thyroid dysfunction.
The prevalence of hypothyroidism in pregnancy is around 2.5% according to the Western literature [2]. The prevalence of GD is around 0.1–0.4% and that of thyroid autoimmunity (TAI) is around 5–10% [3]. Data on the prevalence of TD during pregnancy is lacking in Asian-Indian population. Hence, this study was planned to establish the prevalence of TD and to evaluate maternal outcome in patients with TD.
2. Material and Methods
Study cohort was selected prospectively from consecutive pregnant females in the first trimester of pregnancy who attended the antenatal clinic of a tertiary referral center, in Mumbai, India, between January and April 2007. The patients with documented history of hypothyroidism or thyrotoxicosis were excluded. The females were included irrespective of their gravida status (primigravida/multigravida), and multiple pregnancies were also included.
Institutional ethics committee permission was obtained, and subjects were recruited for the study after obtaining written informed consent. They were subjected to clinical evaluation with emphasis on the family history of thyroid disorder and the obstetric history.
Serum TSH and thyroid peroxidase antibody (TPOAb) were done as initial hormonal investigations, and the subjects were grouped based on the system proposed by Glinoer [4]. The division into different groups and their followup are shown in Figure 1. Subjects with TSH <2 μIU/mL and TPOAb negative (group 1) were considered as normal. Patients with TSH 2–4 μIU/mL, TPOAb positive (group 4) and TSH > 4 μIU/mL (group 5) were treated with thyroxine. The aim of the treatment was to maintain TSH in the range of 0.2–2 μIU/mL. Once treatment was initiated or changed, TSH was repeated at 6 weeks. Once TSH became normal, it was repeated 2 monthly. TPOAb titres were repeated every trimester in those with baseline positivity.
Figure 1.
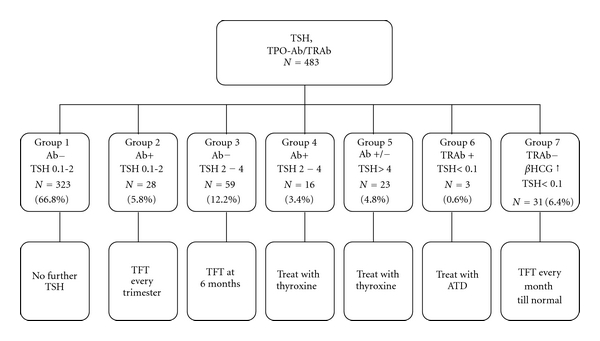
Classification into various groups based on TSH and antibody status. TSH: Thyroid stimulating hormone in μIU/mL, TPOAb: thyroid peroxidase antibody. TRAb: TSH receptor antibody, hCG: human chorionic gonadotropin. TFT: thyroid function tests (FT3, FT4, TSH, and TPO titres in those with positive TPOAb). ATD: antithyroid drugs.
In patients with TSH <0.1 μIU/mL, TSH receptor antibody (TRAb) was estimated. If TRAb is elevated, diagnosis of GD (group 6) was made. If TRAb was negative and β-human chorionic gonadotropin (hCG) level is elevated, it was diagnosed as gestational transient thyrotoxicosis (GTT) (group 7). In GD, maternal thyroid functions were monitored by free T3 (FT3)/free T4 (FT4) at monthly intervals. The aim of the treatment was to maintain FT3/FT4 in the upper quartile of normal nonpregnant range. Fetal monitoring of the patient with GD was done using ultrasound monthly from the 5th month of gestation focusing on fetal heart rate, goiter, growth, and movements. For patients with GTT, FT3, FT4, and TSH were done at 4 weekly intervals. All subjects were followed till and attended to at the time of delivery.
TSH was estimated by the third generation chemiluminescent immunometric assay (CLIA) (Immulite, analytical sensitivity = 0.004 μIU/mL, reference range = 0.4–4). FT3 (analytical sensitivity = 0.15 pmol/L, reference range = 0.23–0.63) and FT4 (analytical sensitivity = 11.58 pmol/L, reference range = 10.3–24.45) were done by competitive analogue-based immunoassay (Immulite). TPOAb (reference range < 35 IU/mL, analytical sensitivity = 5 IU/mL) and β hCG were done by CLIA (Immulite) (reference ranges vary according to gestational age). TRAb was done by ELISA (Medizyme TRA, reference ranges: negative < 1 IU/L, grey zone = 1–1.5 IU/L, positive > 1.5 IU/L, analytical sensitivity = 0.5 IU/L).
Statistical analysis was done using SPSS Version 17 software. The statistical significance between means was calculated by Student's t-test or Mann-Whitney U test whenever appropriate. P value <0.05 was considered to be significant.
3. Results
Four hundred and eighty-three subjects were recruited for the study. The mean age of the subjects was 25.19 (±4.17) years. The mean gestational age at presentation was 10.03 (±1.87) weeks. The prevalence of hypothyroidism was 4.8% and that of GD was 0.6% (Figure 1). Goiter was present in 78 subjects (16.1%). Family history of thyroid disease was present in 12 subjects (2.5%), and TPOAb positivity was seen in 60 subjects (12.4%). Of the 483 subjects, follow-up data is available for 379 while the rest (21.5%) were lost to follow up. The groupwise followup is as follows.
Group 1 (TSH 0.1–2 μIU/mL, without TAI) —
The baseline characteristics and pregnancy outcome are as given in Tables 1 and 2, respectively. This group was taken as reference for comparison for other groups.
Table 1.
Baseline clinical characteristics in different groups and comparison to subjects with group 1*.
| Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 7 | |
|---|---|---|---|---|---|---|
| N = 323 | N = 28 | N = 59 | N = 16 | N = 23 | N = 31 | |
| Age in years (mean ± SD) | 25.09 ± 4.21 | 25 ± 4.65 | 24.42 ± 3.4 | 25.57 ± 3.3 | 29.37 ± 3.7 | 26.41 ± 4.9 |
| P | 1.00 | 1.00 | 0.622 | 0.022 | 0.544 | |
| H/O miscarriage N (%) | 65 (20.1) | 6 (21.4) | 13 (21.6.) | 2 (12.5) | 5 (21.4) | 7 (22.5) |
| P | 0.620 | 0.728 | 0.513 | 0.712 | 0.726 | |
| H/O stillbirth N (%) | 15 (4.6) | 4 (14.3) | 6 (10) | 0 (0) | 1 (4.2) | 0 (0) |
| P | 0.042 | 0.087 | 0.551 | 0.823 | 0.231 | |
| Infertility treatment N (%) | 8 (2.4) | 1 (3.6) | 1 (1.7) | 0 (0) | 1 (4.2) | 0 (0) |
| P | 0.582 | 0.793 | 0.592 | 0.761 | 0.548 | |
| Family history N (%) | 6 (1.9) | 0 (0) | 1 (1.7) | 0 (0) | 2 (8.9) | 1 (3.2) |
| P | 0.631 | 0.704 | 0.791 | 0.324 | 0.435 | |
| Goiter N (%) | 52 (16.1) | 5 (17.9) | 5 (8.3) | 4 (25) | 5 (21.4) | 4 (12.9) |
| P | 0.587 | 0.130 | 0.155 | 0.241 | 0.451 |
*Patients with GD (Group 6) not included as the number is small (N = 3).
Table 2.
Pregnancy outcome in various groups*.
| Group | Number | Miscarriage n (%) | Stillbirth n (%) | Premature delivery (<37 weeks) n (%) | Full-term delivery n (%) |
|---|---|---|---|---|---|
| Group 1 | 272 | 20 (7.35) | 4 (1.47) | 14 (5.14) | 234 (86.02.) |
| Group 2 | 19 | 5 (26.31) | 1 (5.3) | 2 (10.6) | 11 (57.9) |
| P | 0.001 | $NS | NS | 0.034 | |
| Group 3 | 40 | 3 (7.5.) | 2 (5.0) | 4 (10.0) | 31 (77.5) |
| P | NS | NS | NS | NS | |
| Group 4 | 7 | 1 (14.3) | 0 (0) | 1 (14.3) | 5 (71..4) |
| P | NS | NS | NS | NS | |
| Group 5 | 17 | 4 (23.5) | 0 (0) | 1 (5.9) | 12 (70. 6) |
| P | 0.02 | NS | NS | NS | |
| Group 7 | 22 | 1 (4.5) | 0 (0) | 2 (9.1) | 19 (86.36) |
| P | NS | NS | NS | NS |
$NS: nonsignificant.
*Patients with GD (Group 6) not included as the number is small (N = 2).
Group 2 (TSH 0.1–2 μIU/mL with TAI) —
The baseline characteristics were similar to group 1 except for the significant association with previous history of stillbirth (P = 0.042). Followup revealed a significant rise in TSH by 0.78 μIU/mL as pregnancy advanced. (P = 0.002). The titers of anti-TPO antibodies decreased progressively towards the last trimester by 85% (242 to 34 IU/mL) (P = 0.043). Miscarriage was 3 times more common (26.3% versus 7.35%) in this group of patients. There was no significant association with other pregnancy outcomes.
Group 3 (TSH 2–4 μIU/mL without TAI) —
There was significant increase (by 0.25 μIU/mL) in TSH (P = 0.029) and significant decrease in FT3/FT4 (P = 0.025 and 0.033, resp.) at 6 months of pregnancy. Two patients (5.1%) had TSH >4 μIU/mL at 6 month followup. One of these patients had stillbirth for which no specific cause was found.
Group 4 (TSH 2–4 μIU/mL with TAI) —
These patients were treated with thyroxine and followed up. There was no significant difference in baseline parameters or pregnancy outcome in these patients.
Group 5 (TSH > 4 μIU/mL) —
The women in this group were older as compared to other groups (P = 0.02). Forty percent of these women did not have the high risk characteristics required for targeted case finding as laid down by the Endocrine Society guidelines [5].
The rate of miscarriage was 3 times higher in patients with hypothyroidism (P = 0.02) (Table 2). Four patients with hypothyroidism had miscarriage. Three patients were overtly hypothyroid (TSH > 10 μIU/mL). One patient (with TSH 69 μIU/mL) had abortion at 12 weeks of gestation, and the other two proceeded to term without any significant complications. There was no significant difference in stillbirth or premature delivery.
Group 6 (GD) —
All patients (n = 3) with GD presented with classical features of thyrotoxicosis (palpitation, weight loss, sweating, and tremor). One patient had abortion soon after starting treatment, and another was lost to follow up. One patient regularly followed up and required Propylthiouracil throughout pregnancy in tapering doses. TRAb titres reduced at third trimester (27 IU/L to 14 IU/L). She had normal delivery. Thyroid hormonal evaluation of the baby was normal.
Group 7 (GTT) —
The prevalence of GTT was 6.4%. Thyroid functions normalized in majority of the patients (70%) by the first followup (average gestational age = 14.4 weeks). In the rest, it normalized by 18 weeks of gestation.
The comparison of baseline characteristics and pregnancy outcome between TPOAb-positive and -negative women is given in Table 3. These women belong to groups 1, 2, 3, 4, and 5.
Table 3.
Comparison between TPOAb-positive and -negative women#.
| Variables | TPOAb + (n = 33) | TPOAb − (n = 322) | P value |
|---|---|---|---|
| Age (yrs) | 25.77 ± 4.24 | 25.09 ± 2.54 | 0.372 |
| Goiter (%) | 23.8 | 15.2 | 0.301 |
| Family history (%) | 3.03 | 4.23 | 0.897 |
| Past H/O miscarriages (%) | 19.93 | 20.6 | 0.892 |
| Infertility treatment (%) | 2.56 | 2.46 | 0.580 |
| TSH (μIU/mL) | 3.88 ± 1.2 | 1.24 ± 1.2 | <0.001 |
| Mean TPO titers (IU/mL) | 352.92 ± 335.6 | 14.71 ± 5.2 | <0.001 |
| Any complication* (%) | 19.04 | 12.8 | 0.463 |
| Miscarriage (%) | 24.24 | 7.76 | 0.005 |
| Preterm delivery (%) | 9.09 | 5.9 | 0.730 |
| Stillbirth (%) | 3.03 | 1.42 | 0.124 |
| Full term delivery (%) | 66.7 | 84.16 | 0.023 |
| Birth weight (kg) | 2.7 ± 0.63 | 2.6 ± 0.52 | 0.590 |
Complication* includes miscarriages, preterm delivery, pregnancy-induced hypertension, gestational diabetes mellitus, and intrauterine death.
#Baseline data of patients who are lost to follow up is not shown. Patients in Group 6 and 7 are excluded.
4. Discussion
The association between TD and adverse pregnancy outcomes has been studied earlier in western countries [6–9]. This has scarcely been studied in Indian population except two studies which looked at the prevalence of hypothyroidism in pregnant females [10, 11].
The mean age at presentation is lower (25.19 ± 4.17 years) compared to Western studies, namely, 27 ± 6 years [12], 29 ± 5 years [9] reflecting early marriage and early conception prevalent in India. The mean gestational age at presentation was 10.03 (±1.87) weeks indicating that most of the pregnant women in India do not visit the antenatal clinic during the first 8 weeks of gestation.
Our study demonstrates a higher incidence of hypothyroidism and TAI. The prevalence of hypothyroidism in this cohort is 4.8% which is higher than that in the western literature (2.5% [13], 2.6% [12]) and a previous Indian study (3.69% [11]). The higher prevalence in our study could be due to the higher prevalence of TAI in our cohort (12.4% versus 6.5% [13] and 8% [12]). Studies systematically assessing the prevalence of TAI during pregnancy, however, have not been reported from India. Iodine deficiency could be a contributory cause, but this information cannot be generated from our study as urinary iodine estimation was not done. The percentage of households consuming iodised salt in India as per the Iodine Network Global score card 2010 is 51% [14].
In the present study, the probable reason for higher miscarriage in patients with hypothyroidism was that they might have had undetected hypothyroidism at conception, and the treatment might have been insufficient to restore euthyroidism. The higher age (mean = 29 years) could also have contributed to miscarriage. Abalovich et al. [9] showed that untreated hypothyroidism, subclinical, or overt, at the time of conception is associated with miscarriage rate of 31.4% compared with 4% in euthyroid subjects at conception. The prevalence of stillbirth and premature delivery was not significantly higher than that in our hypothyroid patient population probably due to the adequate treatment given to the patients to maintain euthyroid state.
The miscarriage rate was 3 times more common in subjects with TAI (7.35 versus 26.5%) in our cohort (Table 3). The association has previously been established by various studies [6–8, 13]. TAI may be viewed as a marker of generalized immune imbalance that will explain the rejection of fetal graft [15]. Presence of TAI could be associated with a subtle thyroid hormone deficiency, due to the reduced functional reserve characteristic of chronic thyroiditis [15]. Women with thyroid antibodies tend to become pregnant at an average 3-4 years later and are, therefore, more prone to pregnancy loss. In our cohort, the relatively higher age in the patients with miscarriage might also have contributed to pregnancy loss.
In subjects with TSH <2 μIU/mL with TAI, history of stillbirth was significantly higher suggesting the association between thyroid autoimmunity and pregnancy loss. Some of the previous studies showed higher number of premature deliveries in women with TAI compared to normal women [9, 13]. Our study did not reveal such association.
Thyroid function in subjects with TSH 2–4 μIU/mL without TAI showed significant increase in TSH and decrease in FT3 and FT4 at 6 months compared to baseline. The significant decline in thyroid functions for this subgroup at the latter half of pregnancy may justify thyroxine supplementation and regular monitoring (though presently not recommended) in this subgroup in the second half of gestation.
Patients with TSH 2–4 μIU/mL with TPOAb positivity were treated with thyroxine. The rationale for opting for treatment in these patients is the fact that despite the TSH downregulation in the first trimester by hCG, TSH level is in the upper half of normal, and there would be a tendency for progressive decline in thyroid function since they have TAI [16]. Though it is recommended to perform FT4 estimation before initiating treatment (in low-normal or low FT4) in this subset of individuals, the lack of trimester-specific normal values for FT4 and the inherent problems with FT4 assay made us focus on serum TSH as the marker for initiating and monitoring treatment. There was no significant difference in the pregnancy outcome in this group of patients compared to normal. This may be due to the smaller number of patients in this subset and the treatment given to these patients.
The prevalence of GTT in the cohort was 6.4%. In India, the prevalence of GTT has not been assessed previously. The prevalence of GTT varies from 2-3% in the Western literature [17]. The prevalence of GD in this cohort is 0.6%, higher than that published in the Western literature (0.2–0.4%) [18]. Further conclusions could not be derived since the sample size was small.
Our data gives a prevalence of thyroid dysfunction in subjects attending a tertiary care centre in Western India which can be generalized to population in the same setting in other parts of India. One limitation of our study was that 21% of subjects were lost to follow up. The Endocrine Society guidelines suggest that universal thyroid screening during pregnancy cannot be recommended, and aggressive case finding is recommended in specific subsets of subjects [5]. But recent studies have shown that targeted case finding will miss around 30–50% cases of hypothyroidism and/or TAI [12, 19]. This is similar to the present study in which 40% of the hypothyroid and 45% of TPOAb positive patients did not have high-risk characteristics. Approximately 60% of the hypothyroid or TPOAb positive pregnant women could have been missed by targeted case finding.
5. Conclusions
The prevalence of hypothyroidism (4.8%) and TAI (12.4%) was found to be high in the present study. TAI and hypothyroidism were found to be significantly associated with miscarriage.
References
- 1.Burrow GN. Thyroid function and hyperfunction during gestation. Endocrine Reviews. 1993;14(2):194–202. doi: 10.1210/edrv-14-2-194. [DOI] [PubMed] [Google Scholar]
- 2.LeBeau SO, Mandel SJ. Thyroid disorders during pregnancy. Endocrinology and Metabolism Clinics of North America. 2006;35(1):117–136. doi: 10.1016/j.ecl.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 3.Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III) The Journal of Clinical Endocrinology and Metabolism. 2002;87(2):489–499. doi: 10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- 4.Glinoer D. The systematic screening and management of hypothyroidism and hyperthyroidism during pregnancy. Trends in Endocrinology and Metabolism. 1998;9(10):403–411. doi: 10.1016/s1043-2760(98)00095-2. [DOI] [PubMed] [Google Scholar]
- 5.Abalovich M, Amino N, Barbour LA, et al. Management of thyroid dysfunction during pregnancy and postpartum: an endocrine society clinical practice guideline. The Journal of Clinical Endocrinology and Metabolism. 2007;92(8):S1–47. doi: 10.1210/jc.2007-0141. [DOI] [PubMed] [Google Scholar]
- 6.Glinoer D, Soto MF, Bourdoux P, et al. Pregnancy in patients with mild thyroid abnormalities: maternal and neonatal repercussions. The Journal of Clinical Endocrinology and Metabolism. 1991;73(2):421–427. doi: 10.1210/jcem-73-2-421. [DOI] [PubMed] [Google Scholar]
- 7.Stagnaro-Green A, Roman SH, Cobin RH, El-Harazy E, Alvarez-Marfany M, Davies TF. Detection of at-risk pregnancy by means of highly sensitive assays for thyroid autoantibodies. Journal of the American Medical Association. 1990;264(11):1422–1425. [PubMed] [Google Scholar]
- 8.Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: effects on obstetrical complications. The Journal of Clinical Endocrinology and Metabolism. 2006;91(7):2587–2591. doi: 10.1210/jc.2005-1603. [DOI] [PubMed] [Google Scholar]
- 9.Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O. Overt and subclinical hypothyroidism complicating pregnancy. Thyroid. 2002;12(1):63–68. doi: 10.1089/105072502753451986. [DOI] [PubMed] [Google Scholar]
- 10.Sahu MT, Das V, Mittal S, Agarwal A, Sahu M. Overt and subclinical thyroid dysfunction among Indian pregnant women and its effect on maternal and fetal outcome. Archives of Gynecology and Obstetrics. 2010;281(2):215–220. doi: 10.1007/s00404-009-1105-1. [DOI] [PubMed] [Google Scholar]
- 11.Mukhopadhyay A, Pati S, Mukherjee S, Das N, Mukhopadhyay P, Saumandal B. Autoimmune thyroid disorders and pregnancy outcome: a prospective observational study. Thyroid Research and Practice. 2007;4(1):50–52. [Google Scholar]
- 12.Vaidya B, Anthony S, Bilous M, et al. Detection of thyroid dysfunction in early pregnancy: universal screening or targeted high-risk case finding? The Journal of Clinical Endocrinology and Metabolism. 2007;92(1):203–207. doi: 10.1210/jc.2006-1748. [DOI] [PubMed] [Google Scholar]
- 13.Glinoer D, Riahi M, Grun JP, Kinthaert J. Risk of subclinical hypothyroidism in pregnant women with asymptomatic autoimmune thyroid disorders. The Journal of Clinical Endocrinology and Metabolism. 1994;79(1):197–204. doi: 10.1210/jcem.79.1.8027226. [DOI] [PubMed] [Google Scholar]
- 14.Global Scorecard 2010. June 2010, http://www.iodinenetwork.net/documents/scorecard-2010.pdf.
- 15.Glinoer D, de Nayer P, Bourdoux P, et al. Regulation of maternal thyroid during pregnancy. The Journal of Clinical Endocrinology and Metabolism. 1990;71(2):276–287. doi: 10.1210/jcem-71-2-276. [DOI] [PubMed] [Google Scholar]
- 16.Poppe K, Glinoer D. Thyroid autoimmunity and hypothyroidism before and during pregnancy. Human Reproduction Update. 2003;9(2):149–161. doi: 10.1093/humupd/dmg012. [DOI] [PubMed] [Google Scholar]
- 17.Glinoer D. The regulation of thyroid function in pregnancy: pathways of endocrine adaptation from physiology to pathology. Endocrine Reviews. 1997;18(11):404–433. doi: 10.1210/edrv.18.3.0300. [DOI] [PubMed] [Google Scholar]
- 18.Luton D, Le Gac I, Vuillard E, et al. Management of Graves’ disease during pregnancy: the key role of fetal thyroid gland monitoring. The Journal of Clinical Endocrinology and Metabolism. 2005;90(11):6093–6098. doi: 10.1210/jc.2004-2555. [DOI] [PubMed] [Google Scholar]
- 19.Horacek J, Spitalnikova S, Dlabalova B, et al. Universal screening detects two-times more thyroid disorders in early pregnancy than targeted high-risk case finding. European Journal of Endocrinology. 2010;163(4):645–650. doi: 10.1530/EJE-10-0516. [DOI] [PubMed] [Google Scholar]


