Abstract
We have demonstrated that calbindin D9k can be converted into a calcium-sensing switch (calbindin-AFF) by duplicating the C-terminal half of the protein (residues 44–75) and appending it to the N-terminus (creating residues 44′–75′). This re-engineering results in a ligand-driven interconversion between two native folds: the wild-type structure (N) and a circularly permuted form (N′). The switch between N and N′ is predicted to involve exchange of the 44–75 and 44′–75′ segments, possibly linked to their respective folding and unfolding. Here we present direct structural evidence supporting the existence of N and N′. To isolate the N′ and N conformations, we introduced the knockdown Ca2+ binding mutation Glu → Gln at position 65 (E65Q mutant) or at the analogous position 65′ (E65′Q mutant). E65Q and E65′Q are therefore expected to adopt conformations N′ and N, respectively, in the presence of calcium. Though the amino acid sequences of E65Q and E65′Q differ at only these two positions, nuclear magnetic resonance resonance assignments, chemical shifts, and paramagnetic relaxation enhancement data reveal that they take on separate structures when bound to calcium. Both proteins are comprised of a well-folded domain and a disordered region. However, the segment that is disordered in E65Q (residues 44–75) is folded in E65′Q, and the region that is disordered in E65′Q (residues 44′–75′) is structured in E65Q. The results demonstrate that the N ⇆ N′ conformational change is mediated by a mutually exclusive folding reaction in which folding of one segment of the protein is coupled to unfolding of another segment, and vice versa.
In an earlier study, we created a fluorescent calcium sensor (calbindin-AFF) by applying the alternate frame folding (AFF) strategy to calbindin D9k.1 Wild-type (WT) calbindin (75 amino acids) is composed to two EF-hands, EF1 (residues 1–43) and EF2 (residues 44–75), each of which binds a single calcium ion. To generate calbindin-AFF, we duplicated EF2 and joined it to the amino terminus of calbindin (Figure 1). The duplicate residues are indicated by primes (44′–75′) but are otherwise numbered identically. Residues 44′–75′ constitute EF2′. The extra information encoded by the additional sequence allows calbindin-AFF to adopt two different folds. The first is the wild-type structure (N) in which EF1 and EF2 are folded and EF2′ is unfolded. The second is a circularly permuted conformation (N′) in which EF1 and EF2′ are folded and EF2 is unfolded. N and N′ fold in a mutually exclusive fashion because they compete for a shared stretch of amino acids (EF1). As a result, the protein switches between the two folds on a time scale of seconds.1 The equilibrium ratio of N and N′, as well as the rate at which they interconvert, can be manipulated using established principles of protein stability and binding energetics.2
Figure 1.
Sequence and predicted structure of calbindin-AFF. (A) Linear amino acid sequence and numbering scheme for E65′Q (top) and E65Q (bottom). Red, green, and blue regions correspond to residues 44′–75′ (EF2′), 1–43 (EF1), and 44–75 (EF2), respectively. The permutant linker sequence is colored black and labeled with an L. For each mutant sequence, boxes and wavy lines indicate EF-hands that are expected to be folded and unfolded, respectively, in the presence of calcium. (B) Predicted structures of E65Q in N and N′ conformations. The side chain of E65′ is colored light pink, and the α-carbon of Q65 is colored pale cyan. Calcium ions are shown as gray spheres. Calcium binding induces the red region to fold and the blue region to unfold. For the E65Q variant shown, conformation N′ is expected to be fully populated in the presence of a saturating level of calcium. The placement of Cys residues (C1* and C2*) is indicated by stars.
The aspect of the AFF-engineered conformational change that makes it broadly applicable to biosensor design is that it potentially couples binding-induced folding of one region of the molecule to unfolding of a corresponding region, as discussed below. This order–disorder transition can be harnessed to a fluorescent1,2 or functional output3 to report on ligand binding or regulate the function of an enzyme, respectively. The AFF mechanism is significant because the nature of the N to N′ conformational change is expected to be similar for other proteins to which it is applied. It may therefore be feasible to employ AFF as a general method for creating optical biosensors.
The goal of this study is to determine the structural basis for the AFF-mediated conformational change by characterizing calbindin-AFF in its two conformational states. The hypothesis is as follows. The general expectation is that, because of the cooperative nature of protein folding, one of the duplicate segments will be disordered in N and the other will be disordered in N′. For example, when calbindin-AFF adopts structure N, EF1 and EF2 are folded. Unable to form native interactions with EF1, the orphaned EF2′ extends from the amino terminus as an unstructured tail (Figure 1). When calbindin-AFF occupies conformation N′, EF2′ displaces EF2 and EF2 exists as a disordered C-terminal tail. It is this coupled order–disorder transition that forms the basis for fluorescence detection.1 The second aspect of the hypothesis is that the switch can be driven, in either direction, by ligand binding. Previous data suggest that, in the absence of calcium, calbindin-AFF exists in an approximately equal ratio of N and N′.2 To isolate the N′ and N conformations, we introduced the Glu → Gln mutation at position 65 in EF2 (E65Q) and position 65′ in EF2′ (E65′Q). This mutation reduces the calcium affinity of EF2 of WT calbindin by 105-fold.4 Thus, calcium binding is expected to trigger the N → N′ shift in E65Q and induce the opposite transition in E65′Q.
Here, we provide direct structural evidence of the model described above. We previously demonstrated that fluorescently labeled calbindin-AFF reports on Ca2+ binding by pyrene excimer formation as well as BODIPY resonance energy transfer.1,2 Other support for the N ⇆ N′ conformational change was proffered by circular dichroism (CD) and thiol–disulfide exchange experiments.1 In this work, we employ nuclear magnetic resonance (NMR) resonance assignment, chemical shift, and paramagnetic relaxation enhancement (PRE) analysis to compare the structures of E65Q and E65′Q in their calcium-bound states (E65Q and E65′Q refer to the calcium-bound forms of the proteins unless specified by the prefix apo, which indicates that the protein is calcium-free). The high degree of sequence duplication, coupled with potentially extensive regions of intrinsic disorder, poses a challenge for obtaining resonance assignments of AFF-modified proteins. Nevertheless, by relying on unique sequences such as the linker peptide in the circular permutant, we were able to assign ~80% of the 13Cα, 13Cβ, and backbone 15N resonances of E65Q and E65′Q.
EXPERIMENTAL PROCEDURES
Protein Preparation
The E65Q and E65′Q constructs used for NMR assignments are the same double-Cys variants (C1* + C2*) employed in our previous study.1 C1* was inserted at the extreme N-terminus of the molecule preceding S44′, and C2* was inserted between M43 and S44. E65′Q PRE experiments employed the single-Cys variant (C1*). E65Q PRE experiments were performed using the I73C/I73′S construct that was used in our earlier study (this protein does not contain C1* or C2*).1 The I73′S mutation was introduced to balance the potentially destabilizing effect of the I73C mutation. Proteins were grown in M9 minimal medium containing 99% [13C]-d-glucose (Spectra Stable Isotopes) and/or 99% 15NH4Cl (Cambridge Isotope Laboratories). Expression and purification were conducted as described previously.1 Proteins were lyophilized, stored at −20 °C, and dissolved in buffer immediately prior to NMR experiments.
Resonance Assignments
NMR samples were prepared by dissolving lyophilized protein in 10 mM Tris (pH 7.5), 2–4 mM CaCl2, 2 mM dithiothreitol (DTT), and 10% 2H2O. Samples were filtered over Millipore centrifugation filters. The protein concentration was 1–2 mM, and the temperature was set to 20 °C. Resonances were assigned using standard three-dimensional HNCACB, CBCACONH, HNCA, and HNCACO experiments. Data were collected on either a Bruker AVANCE 700 MHz instrument at the New York Structural Biology Center or a Varian INOVA 600 MHz spectrometer. Both instruments were equipped with cryogenic probes. Data were processed using NMRPipe5 and analyzed using NMRViewJ.6 Chemical shift indices were calculated according to the values published by Wishart and Sykes.7
PRE Experiments
Calbindin-AFF was labeled either at the extreme N-terminus (C1*) (E65′Q) or at position 73 (E65Q) with 1-oxy-2,2,5,5-tetramethyl-d-pyroline-3-methyl methanethiosulfonate (MTSL, Toronto Research Chemicals). Labeling was performed by dissolving lyophilized protein in 10 mM Tris (pH 7.5) and 10 mM CaCl2. A 10-fold excess of MTSL was added, and the solution was incubated for 15 min at room temperature. Free MTSL was removed using a Sephadex G25 spin column equilibrated in 50 mM Tris (pH 7.5), 0.1 M NaCl, and 2 mM CaCl2. The desalted protein was split into two samples, one containing 0.5 mM DTT and one containing an equivalent volume of buffer. PRE data were acquired on a Varian INOVA 600 MHz instrument equipped with a cryogenic probe.
RESULTS
Previous studies suggested that N and N′ are populated approximately equally in the absence of calcium.1,2 The NMR HSQC spectrum of apo E65Q provides further support for this conclusion in that many resonances appear as doublets (Figure S1 of the Supporting Information), suggesting a slow (on the NMR time scale) interconversion between two conformations. The apo spectrum is inherently complex because each (identical) residue in EF2 and EF2′ can in principle sample folded and unfolded conformations, resulting in up to four resonances. This phenomenon is clearly illustrated in the glycine region of the apo E65Q spectrum (Figure S1 of the Supporting Information and its figure legend). Because of this complexity, and the relatively poor spectral quality and dispersion, further attempts to analyze the apoprotein spectra were unsuccessful.
A critical test of the proposed AFF mechanism is provided by comparing the HSQC spectra of E65Q and E65′Q (Figure 2). Each holo spectrum contains a set of highly resolved, well-dispersed peaks with positions close to those observed in the spectrum of WT calbindin, suggesting that both constructs form a calbindin-like fold. A second set of broadened and poorly resolved peaks is apparent in the central region of the two spectra, indicating that both constructs also contain disordered regions, which would be expected if EF2 or EF2′ is substantially unstructured in the N′ or N state, respectively. The two protein sequences are identical except for the transposition of the Glu → Gln mutation. Therefore, if calcium binding does not induce the predicted fold shift, the cross-peaks in the two spectra should be essentially superposable except around the sites of mutation. Instead, small but clear chemical shift differences are apparent for the resolved resonances that reflect the well-folded calbindin-like domain (Figure S2 of the Supporting Information). This result strongly suggests that E65Q and E65′Q adopt structurally similar, yet topologically distinct, conformations in the presence of calcium.
Figure 2.
HSQC spectra of (A) E65Q and (B) E65′Q showing resonance assignments. The amino acid numbering follows that described in the text, with EF2′ residues designated by primes. EF2′, EF1, and EF2 labels are colored red, green, and blue, respectively. The permutant linker residues GGAAA are indicated by a superscript L and are colored black. C2* is colored purple. Several of the resonances from EF2′ of E65Q are downfield-shifted and well-resolved, signifying that these residues are folded (e.g., G59′, F50′, V61′, S62′, F63′, and F66′). In E65′Q, all of these resonances are replaced with those of the corresponding residues from EF2, indicating that EF2 has folded and EF2′ has unfolded.
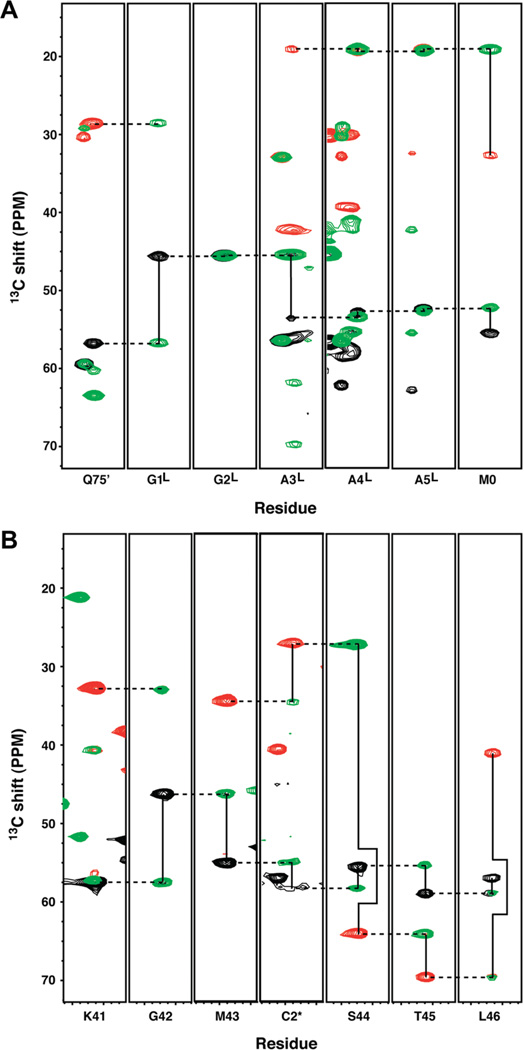
We assigned the backbone NMR resonances of E65Q and E65′Q to investigate the conformational similarities and differences between N and N′. These data can establish which hand (EF2 or EF2′) produces well-dispersed resonances (indicating that it is folded) and which hand gives rise to degenerate or missing peaks (suggesting that it is substantially disordered). Because the sequences of EF2 and EF2′ are nearly identical, differing at only positions 65 and 65′, it is challenging to distinguish whether the well-dispersed resonances in E65Q (Figure 2A) and E65′Q (Figure 2B) originate from EF2 or EF2′. The amino acid sequences linking EF1 to EF2 in E65Q and EF2′ to EF1 in E65′Q serve as unique reference points for sequential assignments. Strip plots of HNCACB and CBCACONH spectra of E65Q establish a clear connectivity from EF2′ to the permutant linker GGAAA and from the permutant linker to EF1 (Figure 3A). Similarly, strip plots of E65′Q (Figure 3B) show a clear connectivity from EF1 to the inserted Cys residue (C2*) to EF2. Panels A and B of Figure 2 unambiguously reveal that EF2′ is folded in E65Q and EF2 is folded in E65′ Q. The well-dispersed resonances that are positioned like those of EF2 in WT calbindin belong to EF2′ in E65Q, as indicated by the assignment annotations in Figure 2A. Conversely, the corresponding set of well-resolved peaks in E65′Q belong to EF2 (Figure 2B). We note that resonances from EF1 are well-dispersed in E65Q as well as E65′Q, signifying that EF1 is folded in both N and N′ conformations.
Figure 3.
Strip plots of sequential assignments linking EF2′ to EF1 for E65Q and EF1 to EF2 for E65′Q. (A) HNCACB/CBCACONH overlaid strips showing unambiguous connectivity from Q75′ in EF2′ through the permutant linker (designated by superscript L) to M0 of EF1. (B) HNCACB/CBCACONH overlaid strips showing unambiguous connectivity from the C-terminal residues of EF1 (M43) through C2* to the N-terminal residues of EF2′ (S44-T45-L46). Black (Cα) and red (Cβ) peaks are from the HNCACB experiment, and green peaks are from the corresponding CBCACONH experiment. Solid lines link intra-and inter-residue resonances at the same NH pair of chemical shifts. Dashed lines link inter-residue resonances with intraresidue resonances of the i – 1 NH pair of chemical shifts.
The central broad resonances in the HSQC spectra suggest the presence of a disordered region in each construct. The disordered segments are predicted to be EF2 in E65Q and EF2′ in E65′Q (Figure 1). For E65Q, resonance assignments confirmed that many of these peaks originate from EF2, but in the case of E65′Q, more severe broadening largely precluded assignment of EF2′. We therefore turned to PRE experiments to help resolve the identity of the disordered segments as well as to assess whether they interact with the folded domain of calbindin-AFF. Paramagnetic relaxation severely broadens resonances of residues within ~15 Å of the MTSL spin-label. These residues include those that are adjacent in sequence to the labeled amino acid and any that come into the proximity of the spin-label by virtue of three-dimensional structure. We took advantage of the single-Cys (C1*) construct to conjugate MTSL to the extreme N-terminus of E65′Q. If the protein occupies conformation N′, then EF2′ will be folded, many residues from the neighboring helices will be within 15 Å of C1*, and these cross-peaks will dramatically broaden or disappear from the well-resolved regions of the HSQC spectrum. If E65′Q adopts conformation N and EF2′ is disordered, the MTSL group is expected to severely broaden only other residues in EF2′. The results support the latter scenario. Comparing the HSQC spectrum of MTSL-labeled E65′Q to that of an identical sample in which the MTSL group was cleaved off by dithiothreitol shows that the only resonances that are significantly broadened by MTSL are the already broad, unassignable signals in the central region (Figure 4B) as well as a few side chain resonances. The resolved backbone resonances arising from EF1 and EF2 are unaffected, signifying that the N-terminal portion of EF2′ does not make long-lived contact with the structured helices.
Figure 4.
HSQC spectra of MTSL-conjugated (A) E65Q and (B) E65′Q in the presence (black) and absence (red) of DTT. The single-Cys (C1*) variant of E65′Q and the I73C/I73′S variant of E65Q were used for these measurements. The spectra of these constructs differ slightly from the spectra in Figure 2 because of minor sequence differences. Spectra in the absence of DTT exhibit PRE due the presence of the cysteine-conjugated MTSL spin-label. For both constructs, severe resonance broadening is confined to the central random coil region of the spectrum and the well-resolved resonances [arising from EF1 and EF2 (E65′) or EF1 and EF2′ (E65Q)] are essentially unaffected.
To perform the analogous PRE experiment on E65Q, we employed the E65Q/I73C/I73′S mutant that was used in our previous study.1 Derivatizing I73C with MTSL places the spin-label in EF2 two residues from the C-terminus. Figure 4A shows again that the only signals severely broadened by MTSL are those in the central “random coil” section of the HSQC spectrum, along with a subset of side chain resonances. As was the case with E65′Q, the well-resolved peaks from the structured region of the protein (EF1 and EF2′) are unaffected. Together, these data suggest that (i) EF2 and EF2′ form largely disordered tails in E65Q and E65′Q, respectively, and (ii) these orphaned hands do not make long-lived interactions with the folded calbindin-like domains of either construct.
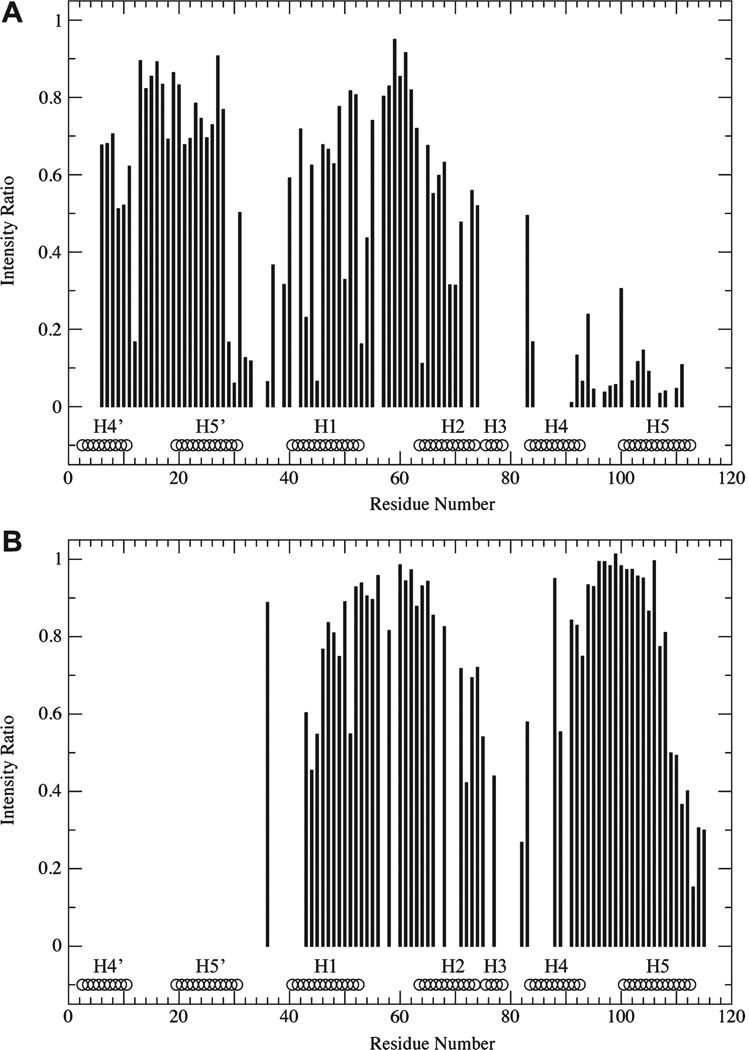
We note that a residue-by-residue analysis of the PRE data is complicated by the fact that the single-cysteine variants used for these measurements exhibit chemical shift changes that make it difficult to reliably transfer many resonance assignments in the less well resolved regions of the E65Q and E65′Q spectra (compare panels A and B of Figure 4 with panels A and B of Figure 2). Our best effort at such an analysis (Figure 5) indicates that there is minor broadening in some of the structured regions of both E65Q and E65′Q. For E65′Q (Figure 5B), the affected regions include the N-terminus of helix 1, the C-terminus of helix 2, the N-terminus of helix 4, and the C-terminus of helix 5. These regions are all located at the “bottom” of the calbindin structure as depicted in Figure 1. Because EF2′ is situated immediately N-terminal to helix 1 and the resonance broadening observed in our spectra suggests that EF2′ is not fully disordered, it seems reasonable that potential interactions of EF2′ will be largely restricted to this side of the structured region of E65′Q. For E65Q (Figure 5A), the affected regions include helix 2, the N-terminus of helix 1, helix 4′, and the C-terminus of helix 5′. As for E65′Q, these regions seem to comprise the face of the calbindin molecule that is closest to the covalent attachment point for EF2 (which follows helix 2), suggesting that in E65Q, EF2 is largely restricted to occupying this region of space. The magnitudes of the PRE effects for residues in the structured region of either construct are too small to indicate long-lived contacts between the structured and disordered domains but could possibly indicate transient contacts between them. However, further evaluation and characterization of such potential contacts is beyond the scope of this work.
Figure 5.
Paramagnetic relaxation enhancement as a function of residue number for (A) E65Q (I73C/I73′S variant) and (B) E65′Q. The ratio of peak intensities in spectra from samples containing conjugated MTSL to those in spectra where MTSL has been cleaved from the protein by DTT is used as the measure for PRE-induced broadening. Values of 1 indicate no effect. Values near zero indicate severe broadening reflecting long-lived proximity to the MTSL spin-label. The absence of bars indicates no data due to missing assignment and/or broadening in the absence of MTSL. The positions of helices H1–H5 and H4′ and H5′ are indicated at the bottom of each plot (taken from Protein Data Bank entry 3ICB).
To further characterize the N and N′ conformations, we examined the Cα secondary chemical shifts of E65′Q and E65Q (Figure 6). α-Helical regions are determined by stretches of at least four residues whose Cα shifts are displaced by ≥1 ppm from the consensus random coil values. The locations of helices in E65Q, as indicated by the Cα secondary shifts, are completely consistent with the predicted structure of N′, with helices 1 and 2 evident in EF1 and helix 5′ apparent in EF2′. Resonances from the short five-residue helix 3 could not be observed or assigned, suggesting that this region is partially destabilized in the N′ fold. Similarly, resonances from the N-terminal portion of helix 4′, which is at the N-terminus of the permuted protein, were not observed, possibly indicating that this helix is frayed in N′. Interestingly, resonances originating from EF2 also exhibit generally positive secondary shifts (though much smaller in amplitude), hinting that despite the evident loss of tertiary structure in EF2, it retains a weak preference for helical secondary structure.
Figure 6.
Cα chemical shift deviations from random coil values (secondary shifts) for (A) E65Q and (B) E65′Q (black bars). Secondary shifts from the corresponding residues of WT calbindin are shown as green bars for comparison and are generally in excellent agreement with those observed for E65Q and E65′Q. Short regions where significant differences occur are located between helices H3 and H4 for E65′Q, where the amino acid sequences of the two constructs differ at two positions, and at the end of H5′ for E65Q, which is immediately adjacent to the non-native linker region. Positions of helices are indicated at the bottom of each plot.
The secondary chemical shifts for E65′Q are likewise consistent with the expected structure of N. EF1 and EF2 possess substantial helix content at locations corresponding to all five of the WT calbindin helices. As mentioned previously, residues 44′–75′ of E65′Q exhibit extensive line broadening that precludes chemical shift assignment for this region. This observation suggests that EF2′ exchanges between multiple conformations on the intermediate NMR time scale. For both E65Q and E65′Q, the observed Cα secondary shifts are also in excellent agreement with those calculated for WT calbindin,8 confirming that the expected WT calbindin fold is adopted in both N′ and N.
DISCUSSION
This study establishes a structural basis for the conformational change that underlies the AFF mechanism. Apo calbindin-AFF adopts two structures that interconvert slowly on the NMR time scale (Figure S1 of the Supporting Information) in agreement with fluorescent studies that find the calcium-induced conformational switching rates to be 6.85 and 1.29 s−1 for E65Q and E65′Q, respectively.2 Upon addition of calcium, E65Q and E65′Q each exhibit two-dimensional NMR spectra that display a single set of resonances that are distinct from one another. Chemical shift analysis confirms that the folded cores of E65Q and E65′Q are comprised of the nonpermuted (EF1 and EF2) and circularly permuted (EF2′ and EF1) forms of calbindin, respectively.
Chemical shift data also indicate that the orphan hand in each protein is substantially disordered. PRE experiments find no indication that the orphan EF2 interacts with the folded region of E65Q or that the orphan EF2′ interacts with the folded region of E65′Q. The available data cannot rule out the presence of a minor population of N′ in E65Q or of N in E65′Q. These alternate conformations, if present, are likely populated at very low levels. The folded and disordered regions of calbindin-AFF therefore appear to act as independent entities. Other lines of evidence also support these views. First, the isolated EF2 fragment exhibits a weak helical CD signal ([θ]222 = −4000 deg cm2 dmol−1).9 Our CD experiments also established that calbindin-AFF contains less helical structure, on a percentage basis, than either WT or permuted calbindin.1 Second, thiol–disulfide exchange experiments with the E65Q/I73C/I73′S variant show that C73 (which is partially buried between EF1 and EF2) is protected from exchange in the absence of calcium and exposed in its presence.1 This result suggests that the EF1–EF2 packing interface is disrupted in N′. Finally, when the F66W hydrophobic core mutation is introduced into EF2, it strongly destabilizes calcium-bound E65′Q.2 The F66′W mutation in EF2′ has no effect, likely because EF2′ is already at least partially unfolded.2
The extent to which residual structure is present in the orphaned regions has implications for biosensor design. Commonly used strategies for detecting binding employ distance-sensitive fluorophores such as FRET pairs or donor-quencher groups. If fluorophores are placed at the starred locations in Figure 1B, they will always be in the proximity of each other in conformation N′ regardless of the structure of the carboxy-terminal tail. In conformation N, one might predict a priori that they will be farthest apart (on average) when the amino-terminal tail is completely unstructured and can thus sweep out a large radius. Clearly, the length and composition of the duplicated sequence will dictate whether it can take on residual structure. In the case of calbindin-AFF, the data suggest that the orphan hands are not well folded, but neither are they completely unstructured. The Cα chemical shift deviations of EF2 in E65Q are much less pronounced than those of EF2′ and EF1 but are nonetheless indicative of residual helical structure. We previously found that the calcium-induced fluorescence change of BODIPY-labeled E65′Q is increased in the presence of subdenaturing amounts of urea.2 This effect was attributed to unfolding of residual structure in EF2′.
Extensive line broadening in the orphaned segments (especially EF2′ of E65′Q) argues that they exist as an ensemble of partially ordered forms that exchange on the microsecond to millisecond time scale. They are not fully unfolded, as unfolded polypeptides produce sharp, intense lines with random coil chemical shifts.10,11 In this regard, calbindin-AFF is similar to intrinsically disordered proteins that are found in increasing numbers in nature.12–15 Intrinsically disordered proteins couple binding to folding. The AFF mechanism incorporates this thermodynamic linkage, but it additionally couples folding of one region of the molecule with unfolding of another. These two reactions are balanced so that no net folding or unfolding occurs; rather, the native structure is remodeled. Our results provide the first direct evidence of this model and reveal that folding and unfolding of the duplicated segment form the basis for the AFF switching mechanism. This finding provides a structural explanation of the calcium-dependent fluorescence change that we reported previously and suggests that similar binding-induced fluorescence changes can be elicited from other AFF-modified proteins.
Supplementary Material
Acknowledgments
Funding Sources
This work was supported by National Institutes of Health Grants R01 GM069755 (S.N.L.) and R01 AG019391 and R01 AG025440 (D.E.), a gift from Herbert and Ann Siegel (D.E.), and the Irma T. Hirschl Foundation (D.E.). D.E. is a member of the New York Structural Biology Center, a STAR center supported by the New York State Office of Science, Technology, and Academic Research.
ABBREVIATIONS
- AFF
alternate frame folding
- calbindin
calbindinD9k
- calbindin-AFF
calbindin D9k containing the amino acid sequence duplication as described
- DTT
dithiothreitol
- MTSL
1-oxy-2,2,5,5-tetramethyl-d-pyroline-3-methyl methanethiosulfonate
- PRE
paramagnetic relaxation enhancement
- HSQC
heteronuclear single-quantum correlation
Footnotes
Supporting Information. HSQC spectra of apo E65Q (Figure S1) and E65Q/E65′Q superposed on each other (Figure S2). This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
M.M.S. and S.M. contributed equally to this work.
REFERENCES
- 1.Stratton MM, Mitrea DM, Loh SN. A Ca2+-sensing molecular switch based on alternate frame protein folding. ACS Chem. Biol. 2008;3:723–732. doi: 10.1021/cb800177f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stratton MM, Loh SN. On the mechanism of protein fold-switching by a molecular sensor. Proteins: Struct., Funct., Bioinf. 2010;78:3260–3269. doi: 10.1002/prot.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitrea DM, Parsons L, Loh SN. Engineering an artificial zymogen by alternate frame protein folding. Proc. Natl. Acad. Sci. U.S.A. 2010;107:2824–2829. doi: 10.1073/pnas.0907668107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlstrom G, Chazin WJ. Two-dimensional 1H nuclear magnetic resonance studies of the half-saturated (Ca2+)1 state of calbindin D9k: Further implications for the molecular basis of cooperative Ca2+ binding. J. Mol. Biol. 1993;231:415–430. doi: 10.1006/jmbi.1993.1291. [DOI] [PubMed] [Google Scholar]
- 5.Delaglio F, Grzesiek S, Vuister GW, Zhu G, Pfeifer J, Bax A. NMRPipe: A multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- 6.Johnson B, Blevins R. NMRView: A computer program for the visualization and analysis of NMR data. J. Biomol. NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- 7.Wishart DS, Sykes BD. Chemical shifts as a tool for structure determination. Methods Enzymol. 1994;239:363–392. doi: 10.1016/s0076-6879(94)39014-2. [DOI] [PubMed] [Google Scholar]
- 8.Oktaviani NA, Otten R, Dijkstra K, Scheek RM, Thulin E, Akke M, Mulder FA. 100% complete assignment of non-labile 1H, 13C, and 15N signals for calcium-loaded calbindin D9k P43G. Biomol. NMR Assignments. 2011;5:79–84. doi: 10.1007/s12104-010-9272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Julenius K, Robblee J, Thulin E, Finn BE, Fairman R, Linse S. Coupling of ligand binding and dimerization of helix-loop-helix peptides: Spectroscopic and sedimentation analyses of calbindin D9k EF-hands. Proteins. 2002;47:323–333. doi: 10.1002/prot.10080. [DOI] [PubMed] [Google Scholar]
- 10.Eliezer D. Characterizing residual structure in disordered protein states using NMR. Methods Mol. Biol. 2006;350:49–68. doi: 10.1385/1-59745-189-4:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eliezer D. Biophysical characterization of intrinsically disordered proteins. Curr. Opin. Struct. Biol. 2009;19:23–30. doi: 10.1016/j.sbi.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunker AK, Silman I, Uversky VN, Sussman JL. Function and structure of inherently disordered proteins. Curr. Opin. Struct. Biol. 2008;18:756–764. doi: 10.1016/j.sbi.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 13.Wright PE, Dyson HJ. Linking folding and binding. Curr. Opin. Struct. Biol. 2009;19:31–38. doi: 10.1016/j.sbi.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galea CA, Pagala VR, Obenauer JC, Park CG, Slaughter CA, Kriwacki RW. Proteomic studies of the intrinsically unstructured mammalian proteome. J. Proteome Res. 2006;5:2839–2848. doi: 10.1021/pr060328c. [DOI] [PubMed] [Google Scholar]
- 15.Uversky VN, Dunker AK. Understanding protein non-folding. Biochim. Biophys. Acta. 2010;1804:1231–1264. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.