Abstract
Many biological systems use extensive networks for the transport of resources and information. Ants are no exception. How do biological systems achieve efficient transportation networks in the absence of centralized control and without global knowledge of the environment? Here, we address this question by studying the formation and properties of inter-nest transportation networks in the Argentine ant (Linepithema humile). We find that the formation of inter-nest networks depends on the number of ants involved in the construction process. When the number of ants is sufficient and networks do form, they tend to have short total length but a low level of robustness. These networks are topologically similar to either minimum spanning trees or Steiner networks. The process of network formation involves an initial construction of multiple links followed by a pruning process that reduces the number of trails. Our study thus illuminates the conditions under and the process by which minimal biological transport networks can be constructed.
Keywords: ants, self-organized, transportation networks, decentralized
1. Introduction
Biological transport systems are created in the absence of central control, with the final network topology being an emergent result of the interactions between many components, be they cells (as in vascular networks), mycelia (in fungal networks) or individual insects (in social insect foraging networks). There is evidence that several such systems balance construction or maintenance costs, network efficiency and robustness against damage. Fungal mycelial networks achieve efficient transportation and robustness against damage [1], slime moulds create networks that balance robustness and transport efficiency [2], and wood ants create foraging trail networks that provide short travel time for foragers with low maintenance and construction costs [3]. These examples point at the impressive ability of self-organized biological systems to generate networks that satisfy specific goals.
Argentine ants (Linepithima humile) form supercolonies that consist of many nests connected by a network of pheromone trails. These inter-nest trails are used to distribute brood, workers and food between nests [4]. Having multiple, interconnected nests probably increases a colony's foraging efficiency and competitive ability [4]. Little is known about their structural properties or how they form. What is known is that workers deposit pheromone droplets continuously as they move around their surroundings [5], and that, under specific conditions, this process can result in networks that avoid redundant links. For example, Aron et al. [6] arranged nests on triangles and squares and found that the ants would remove one link, resulting in a minimally connected network. These observations suggest that the networks result from a positive feedback loop whereby certain trails are reinforced, while others cease being used.
Here, we examine the topology and construction process underlying the formation of inter-nest transportation networks in the Argentine ant. Because the number of individual components in a system can influence the system's behaviour [7,8], we examined how the number of ants present during the network formation process influences the emergent network topology. We presented ant colonies of three sizes (500, 1000 or 2000 workers) with the task of connecting three or four nests in an open arena. The nests were arranged into either a triangular or a square configuration. We used simple geometrical configurations because the optimal solutions to related network problems and measures on them are well known and relatively easy to calculate. Figure 1 shows these idealized networks for connecting points in a plane.
Figure 1.
Ideal networks for (a–c) three and (d–f) four nests (black points). (a,d) Complete graphs, (b,e) minimum spanning trees and (c,f) Steiner minimum trees of the respective nest distribution. The complete graph minimizes the mean travel distance (MD) and has higher robustness (fault tolerance (FT)) and total length (TL) than the Steiner minimum tree and the minimum spanning tree. The Steiner minimum tree minimizes the TL while minimum spanning trees minimize TL if no additional vertices are allowed. Both the Steiner minimum tree and the minimum spanning tree have zero FT and higher MD than the complete graph.
2. Methods
We studied inter-nest networks using 1 m diameter circular experimental arenas. To start an experiment, we placed nest-boxes containing 500, 1000 or 2000 workers, 3–10 queens, brood in all stages (eggs, pupae and larvae), and males underneath the arena. We used still images to directly count the number of ants present in the arena; this gave us a more accurate estimate of the number of ants in the arena during network construction. We determined that the majority of network formation occurred in the first 2 h; we therefore counted the number of ants in the arena after 120 min. The continuous variable ‘number of ants in the arena’ was used instead of ‘colony size’ in all analyses of the effect of ant numbers on network construction.
Ants entered the arena from one of three or four nests. Each nest was connected to the arena via a stick that passed through a hole drilled through the bottom of the arena. In trials with three nests, the entrances were located at the vertices of an equilateral triangle of edge length 33 cm. In trials with four nests, the entrances were placed at the vertices of a square of edge length 27 cm. Pilot experiments run for 48 h found that network topology changed very little after 4 h. Based on these pilot experiments, we allowed networks to form for 6 h. Pictures of the entire arena were taken every 3 s using Logitech 9000 webcams mounted 1 m above the arena. To visualize final trail networks and extract network data, we created composite pictures by combining 60 pictures from the last minute of the experiment and traced the trail using the image processing software ImageJ (National Institutes of Health). To visualize the formation process, we videotaped a subset of six networks (all squares) as they formed. We then extracted still images every 10 s and created composites consisting of 60 photos (corresponding to 10 min video time). We analysed the effect of the number of ants in the arena on network measures using linear regression models. All statistical analyses were done using JMP v. 7.0 (SAS).
3. Analysis and results
Our first goal was to quantify transport efficiency, maintenance costs and robustness. These correspond to the network metrics mean travel distance (MD), total length (TL) and fault tolerance (FT). Figures 2 and 3 show typical examples of inter-nest networks observed during the experiment. An inter-nest network can be defined as an undirected graph G = (V,E,T,w) embedded in the Euclidean plane ℜ2 with a set of vertices V ⊆ ℜ2, edges E ⊆ V2 between vertices, which are plane curves that intersect only at their end points, a set of terminals T ⊆ V and the lengths of the curves as the weights on the edges w : E → ℜ. In the terminology of ant trail networks: the edges correspond to sections of trails; the vertices to branching points, end points and nests; the terminals to nests; and the weights represent the lengths of trails. In our analysis of measures applied to inter-nest networks, we only consider those networks that connect all nests. A network was considered to be disconnected if one or more nests had no trails connecting them with the other nests in the network.
Figure 2.
Composite pictures showing representative ant inter-nest trail networks with three nests arranged at the corners of an equilateral triangle. (a) Minimum spanning tree, (b) minimum spanning tree with one extra edge, (c) Steiner minimum tree, and (d) Steiner minimum tree with one extra edge.
Figure 3.
Composite pictures showing representative ant inter-nest trail networks with four nests arranged at the corners of a square. (a) Minimum spanning tree, (b) network of class ‘other networks’ without extra edges, (c) network of class ‘other networks’ with extra edges, (d) Steiner minimum tree, and (e) Steiner minimum tree with one extra edge.
Three network measures are of central interest to our study: MD, TL and FT. The MD is the average minimum distance an ant has to travel from one arbitrary nest to another assuming it always walks on trails. It is defined as
 |
3.1 |
where |T| is the number of terminals and w′(i,j) is the length of the edges of a shortest path from i to j in G. A path from i to j is a sequence of neighboured vertices (a1, a2, … , an), where each vertex is connected to its successor, i.e. for each k ∈ {1, … , n − 1}, ak is connected to ak+1 by an edge and the sequence starts at i (a1 = i) and ends at j (an = j). In case there is no path from i to j, w′(i,j) is set infinite.
The maintenance costs of an inter-nest network reflect the resources the colony has to spend in order to sustain the trails, e.g. the amount of energy required by ants travelling on the trail, the amount of pheromone ants need to deposit to maintain the trail, and the number of ants necessary to maintain the trails. Larger trail networks imply more maintenance costs and we assume the maintenance costs to be proportional to the total network length. Therefore, we define the total network length as
| 3.2 |
TL is the sum of all trail connections between branching points, nests and points where trails end.
FT gives the probability that the network stays connected if a randomly chosen point on the trail is blocked. It is defined as
 |
3.3 |
where dc(e) = 0 if the terminals of the graph G are disconnected when the edge e is removed from G and dc(e) = 1 otherwise. In a robust network, FT should be close to one and a FT of zero means the network is not robust against the random destruction of a trail.
To compare our measures to idealized networks, we consider the complete graph (figure 1a,d), the Steiner minimum tree (figure 1c) and the minimum spanning tree (figure 1b,e). The complete graph minimizes the MD, Steiner minimum trees minimize TL and minimum spanning trees minimize TL if no additional vertices are allowed. We use the word ‘relative’ throughout the paper to refer to metrics that have been scaled in relation to the appropriate idealized network.
The complete graph CG(T) = (T, EC, T, d) contains the edges (EC) between all pairs of different nests i and j having the Euclidean distance d(i,j) as their weight function. For a given set of terminals, the complete graph minimizes the mean distance and therefore the relative MD for an observed network is MD*(G) = MD(G)/MD(CG(T)). The Steiner minimum tree SMT(T) = (T ∪ S, ES, T, d) describes a network that minimizes the TL while simultaneously connecting all nests. Note that, to achieve minimality, in both idealized networks it is allowed to introduce additional vertices S ⊆ ℜ2, called ‘Steiner points’. Those vertices correspond to branching points or points in the Euclidean plane where edges cross. The relative TL of an observed network is then TL*(G) = TL(G)/TL(SMT(T)). Both measures, MD* and TL*, have a minimal value of 1.
4. Experimental results
Figure 4 shows relative MD, relative TL and FT of the ant inter-nest networks. For both the triangle and square configurations, the MD ranged from almost minimal to two times as long as the complete graph (figure 4). The TL ranged from almost minimal to three times as long as the Steiner minimum tree. The mean probability of disrupting a triangle configuration network with a single point failure was 66 per cent; for a square configuration network it was 64 per cent. Furthermore, 10 of the 15 measured triangle networks had FT zero, and seven of the 15 square networks had FT zero. Network configuration (square or triangle) had no effect on relative MD (t-test: p = 0.22, t-ratio = 1.25, n = 30), relative TL (t-test: p = 0.8, t-ratio = 0.24, n = 30) or FT (t-test: p = 0.34, t-ratio = 0.93, n = 30).
Figure 4.
Properties of inter-nest networks. Frequency distribution of (a) relative MD, triangle configuration, (b) relative MD, square configuration, (c) relative TL, triangle configuration, (d) relative TL, square configuration, (e) FT, triangle configuration and (f) FT, square configuration. Boxplots show the spread of data (the line in the centre of each box indicates the median). n = 15 for all metrics.
To better characterize the topology of the networks we again compared them with idealized networks (figure 1). First, we noted whether the final network was connected or disconnected. If removing any edge of a connected network would result in it becoming disconnected, it was classified as a minimal network, otherwise we classified it as an extra edge network. We also assessed if the networks were topologically similar to the Steiner minimum tree or the minimum spanning tree, which is by definition the shortest sub-network of the complete graph connecting all nests. Networks containing both topologies, the minimum spanning tree and the Steiner minimum tree, were never observed in our experiments. The class Steiner minimum trees + X (SMT + X) included all networks that contained a Steiner minimum tree and X additional edges. Similarly, we defined networks as minimum spanning trees + X (MST + X), including all networks that contained a minimum spanning tree and X additional edges. For example, figure 2b shows a three nest network containing a minimum spanning tree with 1 additional edge (MST + 1), and figure 2d shows a Steiner minimum tree with 1 additional edge (SMT + 1). All connected networks in the square arrangement, which were neither MST nor SMT were classified as ‘other networks’.
In both the triangle and the square nest configuration, we frequently observed topologies that were either Steiner minimum trees or minimum spanning trees; these were sometimes extended by a few additional edges, which introduced alternative point to point routes or dead ends. In the triangle case, the 15 connected networks consisted of 10 minimum networks and five ‘extra-edge networks’ (table 1). We observed eight networks with a topology similar to the Steiner minimum tree (i.e. having less than or equal to one extra edge) and seven with a topology similar to minimum spanning tree (less than or equal to one extra edge; table 1). The 15 connected networks with square configuration (seven minimum networks and eight extra-edge networks) included five Steiner minimum trees (less than or equal to two extra edges) and two minimum spanning trees (less than or equal to one extra edge; table 1). In eight cases, the network topology was a combination of a Steiner minimum tree connecting three nests, with a trail connecting the fourth nest and sometimes extra edges (other networks, less than or equal to two extra edges; table 1).
Table 1.
Number of networks in each topology class. The ‘general classification’ classifies each network into one of three broad categories: DC (disconnected networks), MN (minimal networks) and EE (extra edges). ‘Specific classification’ divides networks into classes by comparing their topology with Steiner minimum trees and minimum spanning trees.
| general topology | class | triangle configuration | square configuration |
|---|---|---|---|
| general classification | DC | 2 | 6 |
| MN | 10 | 7 | |
| EE | 5 | 8 | |
| specific classification | SMT +0 | 7 | 2 |
| MST + 0 | 3 | 2 | |
| SMT + 1 | 1 | 2 | |
| SMT + 2 | 0 | 1 | |
| MST + 1 | 4 | 0 | |
| other | — | 8 |
We used a multiple logistic regression model to examine the effect of number of ants in the arena on final network classification, with configuration and number of ants as independent variables, and network classification (disconnected, minimal or extra edge) as the dependent variable. The number of ants in the arena had a significant effect on the classification of the final network such that networks classified as extra-edge networks tended to form more often than disconnected networks when there were a greater mean number of ants (p = 0.05, n = 30, χ2 = 3.70, figure 5). Configuration did not have a significant effect on classification (p = 0.9, n = 30, χ2 = 0.01).
Figure 5.
The effect of ant number on inter-nest networks in the four nest configuration. Figures show the effect of ants on (a) FT, (b) relative TL and (c) final network topology. Error bars show standard errors. Dissimilar letters indicate those categories which are significantly different from one another (Tukey's post hoc test). Note the axes have been reversed for ease of presentation in (c).
The difference in topology in relation to the number of ants coincides with a difference in how relative TL and FT change with number of ants for the square case, but not in the triangle case (table 2). In the square configuration, FT and relative TL increased as the number of ants increased (table 2 and figure 5a). Relative MD was not influenced by the number of ants in either the square or the triangle configuration (table 2).
Table 2.
The effect of number of ants on network measures (linear regression). The relative MD is scaled to the complete graph and the relative TL is scaled to the Steiner minimum tree. Relative MD and relative TL have a minimum value of 1. FT close to one indicates a robust network, and a FT of zero means the network is not robust against the random destruction of a trail. Bold type indicates a statistically significant effect of number of ants (p<0.05).
| n | mean ± s.e. | test statistic | coefficient estimate | intercept | p | |
|---|---|---|---|---|---|---|
| relative mean travel distance (MD*) | ||||||
| three nests | 15 | 1.59 ± 0.06 | F = 1.24 | 0.0004 ± 0.0003 | 1.50 ± 0.115 | 0.283 |
| four nests | 15 | 1.67 ± 0.07 | F = 0.66 | −0.0003 ± 0.0003 | 1.77 ± 0.153 | 0.429 |
| relative total length (TL*) | ||||||
| three nests | 15 | 1.79 ± 0.17 | F = 1.9 | 0.0016 ± 0.001 | 1.59 ± 0.35 | 0.187 |
| four nests | 15 | 1.65 ± 0.09 | F = 5.21 | 0.0009 ± 0.0004 | 1.34 ± 0.159 | 0.04 |
| fault tolerance (FT) | ||||||
| three nests | 15 | 0.24 ± 0.08 | F = 0.38 | 0.0003 ± 0.0005 | 0.27 ± 0.17 | 0.54 |
| four nests | 15 | 0.22 ± 0.69 | F = 7.13 | 0.001 ± 0.00041 | −0.021 ± 0.165 | 0.02 |
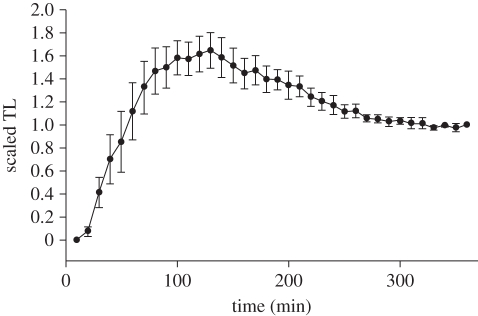
We propose three models for the process of network development. In the additive construction model, total trail length increases linearly as trails between nests develop and trails never disappear once formed. In the pruning model, an initially complex network develops and then trails are gradually removed until the final network is reached. In the refinement model, trail length follows a similar pattern to the pruning model, but decreasing path length is achieved through a continuous process of trail ‘straightening’ rather than through pruning. The additive model can be distinguished from the others by the lack of decreasing path length (figure 6), while pruning and refinement can be distinguished by examining the stages of network formation. In our experiments, the mean scaled TL, i.e. the TL at a given time divided by the TL after 6 h, increased steadily, reaching a peak after approximately 120 min. We call the network that occurred at this peak the ‘climax network’. The peak was followed by a gradual decrease in scaled TL, reaching a stable plateau after 200 min (figure 7). This pattern was clearly seen in overlay pictures, where in five out of six cases, the final network was present at the time of maximum trail length (climax network; figure 8 and the electronic supplementary material). In the sixth network, a phase of parallel pruning and creation of new trails was observed with a peak in scaled TL at 130 min, followed at 200 min by the characteristic pruning process found in the other five networks. The scaled total number of edges followed the same pattern over time.
Figure 6.
Graph illustrating three possible network formation processes. The pruning and refinement processes result in identical graphs but can be distinguished from one another by examining the formation process. In pruning, the decrease in scaled TL occurs because trails are removed, while in a refinement process, the reduction in relative TL is caused by straightening of trails. Solid line, additive process; dashed line, pruning or refinement process.
Figure 7.
Change in mean scaled TL of inter-nest networks over time. Error bars are standard error. Note that dividing by the TL of the final network yields standard errors that decrease to 0 as the network reaches its final state. n = 6.
Figure 8.
Example of overlay technique: (a) network at the peak of trail formation; (b) final network shape after 6 h; (c) final network overlain on the initial network. The overlay picture shows that the final network was already present at the peak of network construction. Additional examples are available in the electronic supplementary material.
A large fraction of the climax networks contained ‘dead end’ trails, where one end of the trail failed to link to nests or other trails. Of the nine dead ends observed, all but one was pruned away in the final networks. The remaining dead end resulted from a formerly connected trail that subsequently became disconnected.
5. Discussion
In our experiments, ants created networks with relatively short relative TL thus minimizing maintenance costs. Short pheromone trails require fewer ants to maintain and thus have greater stability [7]. Comparative data on network minimization are scarce; however, our ant networks achieved shorter relative TL than slime mould networks (TL*=2.35 ± 0.101; T. Nakagaki 2004, personal communication). In this context it is important to note the degree to which the initial network created by the ants contracts through the process of pruning to create a near minimal network. Our Argentine ant networks contain less than two-thirds of the trail structure seen in the ‘climax networks’ and rarely contained loops, redundant edges or dead ends. However, the fact that these networks are near minimal does not imply a complex mechanism for their construction. Several authors have modelled trail formation, mainly in the context of foraging for food [9–14]. The active walker model of Helbing and co-workers is particularly relevant in this context since it predicts a contracted square shape for walkers, which have both global navigation and local reinforcement of trails [15]. However, the Steiner-like network for four points does not arise in their model. Development of a mechanistic model of our experimental results will require more detailed studies on the navigational abilities of Argentine ants.
Having a short relative MD could decrease the distance individual ants need to travel between nests, and could therefore minimize the risk of predation and reduce individual energy costs. Our Argentine ant networks, however, had relatively long individual travel distances (compared with ideal networks). This is in contrast to wood ant foraging networks where networks balance short TL and low travel time [3]. However, these networks were far larger (with edges up to 300 m long) and more complex than our experimental networks. We cannot rule out the possibility that larger Argentine ant networks, such as those found under natural conditions, might be able to balance competing network measures. Further, we do not take into account the movement of individual ants. For example, ants could minimize individual travel distance by travelling primarily to nests directly connected to their home nest via a direct trail. Understanding how ants actually move through the network will help clarify the overall efficiency of ant inter-nest networks.
In our experiments, Argentine ant inter-nest networks tended to have low FT, i.e. low robustness against damage. Networks constructed by both slime moulds and fungi have a high FT that minimizes the probability that the destruction of a part of the network results in the network becoming disconnected [1,2,16]. The FT of Argentine ant networks was much lower than in the networks of slime moulds (0.89 ± 0.0387, three nodes [2]); moreover, a high number of Argentine ant inter-nest networks had FT zero. We suggest that the lack of FT in Argentine ant networks is due to the ants' ability to rapidly repair damage to trails [17]. In both the slime mould and fungi, the network is part of the organism, and separate pieces may have difficulty re-establishing connections following an accidental disconnection. In ants, however, pheromone trails are maintained by moving ants that individually have the capacity to navigate and make decisions. A high FT may not be necessary because ants can quickly establish new routes to disconnected parts of the network. This hypothesis is further supported by the rapidity with which the ants generated the networks in our experiment.
For triangle configurations, all connected networks were topologically similar to Steiner minimum trees and minimum spanning trees, sometimes with additional edges. Full Steiner minimum trees and minimum spanning trees were relatively uncommon for the square configuration, but it was common to find three of the four nests connected by Steiner networks, with extra links to the fourth. This result is analogous to the composition of networks from a set of basic topologies reported by Nakagaki et al. [2] for the slime mould Physarum polycephalum. Because the relative MD, relative TL and FT were approximately the same for triangle (three nests) and square (four nests) configurations, this construction by local composition of solutions might provide a way of efficiently connecting nests as the number of nests increases.
The number of ants affects both network type and relative TL. There is a threshold below which stable trails connecting all nests do not occur. This is similar to the vascular network assembly of human endothelial cells, where connected networks only appeared when the cell density in the experiments was above a critical value [8]. With an increased number of ants, the topology changes from networks with minimal topology to networks with extra edges, which yield a slightly higher FT and longer relative TL. Our analysis implies a density-dependent continuous change from disconnected to connected networks, with minimal networks occurring at intermediate ant densities. This is in contrast to the more rapid transitions observed in the foraging of pharaoh ants [7], the nest digging of Lasius niger ants [18], and traffic flow [19].
The inter-nest networks form through a pruning process. Ants initially build up a complex network by adding trails until the TL reaches a maximal value. They then prune away extra edges resulting in a shorter network. This deletion from a formerly established set of sub-networks is also observed in the slime mould [2,16], the mycelial networks of saprophytic fungi [1] and in the development of mammalian vascular networks [20] and has been predicted for the galleries networks of termites [21]. The finding of similar construction processes in vastly different taxa, representing several levels of biological organization suggests that such network assembly mechanisms are widespread in the natural world.
The inter-nest trail networks of Argentine ants were remarkably consistent, with most topologies closely resembling minimal topologies. In addition to transportation networks, ants also use trophalaxis networks to distribute foods [22], exploration networks to explore new environments [23], and tunnel networks to link chambers of the nest [24]. Understanding how natural systems form networks as an emergent phenomenon can inform the design of artificial networks in situations lacking central control or global knowledge of the environment.
Acknowledgements
We thank members of the Behaviour and Genetics of Social Insects Lab for helpful comments and discussion. This work was funded by the Human Frontier Science Programme grant RGP51/2007. Further financial support was provided by the Australian Research Council (to M.B.) and the University of Sydney (to M.B.).
References
- 1.Bebber D. P., Hynes J., Darrah P. R., Boddy L., Fricker M. D. 2007. Biological solutions to transport network design. Proc. R. Soc. B 274, 2307–2315 10.1098/rspb.2007.0459 (doi:10.1098/rspb.2007.0459) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakagaki T., Kobayashi R., Nishiura Y. 2004. Obtaining multiple seperate food sources: behavioural intelligence in the Physarum plasmodium. Proc. R. Soc. Lond. B 271, 2305–2310 10.1098/rspb.2004.2856 (doi:10.1098/rspb.2004.2856) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buhl J., Hicks K., Miller E., Persey S., Alinvi O., Sumpter D. 2009. Shape and efficiency of wood ant foraging networks. Behav. Ecol. Sociobiol. 63, 451–460 10.1007/s00265-008-0680-7 (doi:10.1007/s00265-008-0680-7) [DOI] [Google Scholar]
- 4.Holway D. A., Case T. J. 2000. Mechanisms of dispersed central-place foraging in polydomous colonies of the Argentine ant. Anim. Behav. 59, 433–441 10.1006/anbe.1999.1329 (doi:10.1006/anbe.1999.1329) [DOI] [PubMed] [Google Scholar]
- 5.Aron S., Pasteels J. M., Deneubourg J. L. 1989. Trail-laying behaviour during exploratory recruitment in the Argentine ant, Iridomyrmex humilis (Mayr). Biol. Behav. 14, 207–217 [Google Scholar]
- 6.Aron S., Deneubourg J. L., Goss S., Pasteels J. M. 1990. Functional self-organization illustrated by inter-nest traffic in ants: the case of the argentine ant. In Biological motion (eds Alt W., Hoffman G.), pp. 533–547 Berlin, Germany: Springer [Google Scholar]
- 7.Beekman M., Sumpter D. J. T., Ratnieks F. L. W. 2001. Phase transition between disordered and ordered foraging in Pharaoh's ants. Proc. Natl Acad. Sci. USA 98, 9703–9706 10.1073/pnas.161285298 (doi:10.1073/pnas.161285298) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Serini G., Ambrosi D., Giraudo E., Gamba A., Preziosi L., Bussolino F. 2003. Modeling the early stages of vascular network assembly. EMBO J. 22, 1771–1779 10.1093/emboj/cdg176 (doi:10.1093/emboj/cdg176) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deneubourg J. L., Aron S., Goss S., Pasteels J. M. 1990. The self-organizing exploratory pattern of the argentine ant. J. Insect Behav. 3, 159–168 10.1007/BF01417909 (doi:10.1007/BF01417909) [DOI] [Google Scholar]
- 10.Schweitzer F., Lao K., Family F. 1997. Active random walkers simulate trunk trail formation by ants. BioSystems 41, 153–166 10.1016/S0303-2647(96)01670-X (doi:10.1016/S0303-2647(96)01670-X) [DOI] [PubMed] [Google Scholar]
- 11.Ngenkaew W., Ono S., Nakayama S. 2007. Multiple pheromone deposition in ant-based clustering as an ant foraging concept. In Proc. Int. Conf. on Advances in Computer Science and Technology, pp. 432–436 Calgary, Canada: ACTA Press [Google Scholar]
- 12.Tao T., Nakagawa H., Yamasaki M., Nishimori H. 2004. Flexible foraging of ants under unsteadily varying environment. J. Phys. Soc. Jpn 73, 2333–2341 10.1143/JPSJ.73.2333 (doi:10.1143/JPSJ.73.2333) [DOI] [Google Scholar]
- 13.Panait L., Luke S. 2004. A pheromone-based utility model for collaborative foraging. In Proc. 3rd Int. Joint Conf. on Autonomous Agents and Multiagent Systems, vol. 1, pp. 36–43. Washington, DC: IEEE Computer Society [Google Scholar]
- 14.Solé R., Bonabeau E., Delgado J., Fernández P., Marín J. 2000. Pattern formation and optimization in army ant raids. Artif. Life 6, 219–226 10.1162/106454600568843 (doi:10.1162/106454600568843) [DOI] [PubMed] [Google Scholar]
- 15.Helbing D., Schweitzer F., Keltsch J., Molnar P. 1997. Active walker model for the formation of human and animal trail systems. Phys. Rev. E 56, 2527–2539 10.1103/PhysRevE.56.2527 (doi:10.1103/PhysRevE.56.2527) [DOI] [Google Scholar]
- 16.Nakagaki T., Yamada H., Hara M. 2004. Smart network solutions in an amoeboid organism. Biophys. Chem. 107, 1–5 10.1016/S0301-4622(03)00189-3 (doi:10.1016/S0301-4622(03)00189-3) [DOI] [PubMed] [Google Scholar]
- 17.Goss S., Aron S., Deneubourg J., Pasteels J. 1989. Self-organized shortcuts in the Argentine ant. Naturwissenschaften 76, 579–581 10.1007/BF00462870 (doi:10.1007/BF00462870) [DOI] [Google Scholar]
- 18.Toffin E., Di Paolo D., Campo A., Detrain C., Deneubourg J. 2009. Shape transition during nest digging in ants. Proc. Natl Acad. Sci. USA 106, 18 616–18 620 10.1073/pnas.0902685106 (doi:10.1073/pnas.0902685106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kerner B., Rehborn H. 1997. Experimental properties of phase transitions in traffic flow. Phys. Rev. Lett. 79, 4030–4033 10.1103/PhysRevLett.79.4030 (doi:10.1103/PhysRevLett.79.4030) [DOI] [Google Scholar]
- 20.Risau W. 1997. Mechanisms of angiogenesis. Nature 386, 671–674 10.1038/386671a0 (doi:10.1038/386671a0) [DOI] [PubMed] [Google Scholar]
- 21.Perna A., Valverde S., Gautrais J., Jost C., Solé R., Kuntz P., Theraulaz G. 2008. Topological efficiency in three-dimensional gallery networks of termite nests. Physica A 387, 6235–6244 10.1016/j.physa.2008.07.019 (doi:10.1016/j.physa.2008.07.019) [DOI] [Google Scholar]
- 22.Sendova-Franks A. B., Hayward R. K., Wulf B., Klimek T., James R., Planqué R., Britton N. F., Franks N. R. 2010. Emergency networking: famine relief in ant colonies. Anim. Behav. 79, 473–485 10.1016/j.anbehav.2009.11.035 (doi:10.1016/j.anbehav.2009.11.035) [DOI] [Google Scholar]
- 23.Gordon D. M. 1995. The expandable network of ant exploration. Anim. Behav. 50, 995–1007 10.1016/0003-3472(95)80100-6 (doi:10.1016/0003-3472(95)80100-6) [DOI] [Google Scholar]
- 24.Buhl J., Gautrais J., Solé R. V., Kuntz P., Valverde S., Deneubourg J. L., Theraulaz G. 2004. Efficiency and robustness in ant networks of galleries. Eur. Phys. J. B 42, 123–129 10.1140/epjb/e2004-00364-9 (doi:10.1140/epjb/e2004-00364-9) [DOI] [Google Scholar]