Abstract
Terrestrial mammals are a key component of tropical forest communities as indicators of ecosystem health and providers of important ecosystem services. However, there is little quantitative information about how they change with local, regional and global threats. In this paper, the first standardized pantropical forest terrestrial mammal community study, we examine several aspects of terrestrial mammal species and community diversity (species richness, species diversity, evenness, dominance, functional diversity and community structure) at seven sites around the globe using a single standardized camera trapping methodology approach. The sites—located in Uganda, Tanzania, Indonesia, Lao PDR, Suriname, Brazil and Costa Rica—are surrounded by different landscape configurations, from continuous forests to highly fragmented forests. We obtained more than 51 000 images and detected 105 species of mammals with a total sampling effort of 12 687 camera trap days. We find that mammal communities from highly fragmented sites have lower species richness, species diversity, functional diversity and higher dominance when compared with sites in partially fragmented and continuous forest. We emphasize the importance of standardized camera trapping approaches for obtaining baselines for monitoring forest mammal communities so as to adequately understand the effect of global, regional and local threats and appropriately inform conservation actions.
Keywords: camera traps, global network, occupancy, public data, terrestrial mammals, tropical forests
1. Introduction
Most analyses of biodiversity loss, including analyses of mammal communities, rely on species distributional data and inferences based on habitat association [1–6]. Seldom is information available on the status or trends of mammal communities, particularly in tropical forests. In these forests, terrestrial mammals comprise rich communities of species from a variety of diverse trophic groups and a wide range of body sizes [7]. This diversity plays a significant role in the functioning of these ecosystems [8,9]. Aside from their direct roles in seed dispersal, herbivore control and nutrient cycling, some propose that removal of large-bodied tropical terrestrial mammals through intensive hunting can reduce the capacity of tropical forests to store carbon, either through a reduction in large seeded/high carbon density species mostly dispersed by frugivorous vertebrates [10], or a shift in the dominant dispersal syndrome from animal to wind-dispersed species, mostly lianas, as forest terrestrial herbivores are removed [11].
To test these ideas, and to better understand how tropical mammal communities change in response to anthropogenic factors, such as overexploitation, land-use change and climate change, we need information collected globally on their diversity and community composition, using standardized methods. Only by examining such patterns and relationships at a global scale can we start to disentangle the effects of global, regional and local threats on these communities. Comparing the terrestrial ground-dwelling mammal community structure between sites on different continents can be problematic because the species themselves differ from site to site. In such cases, it is appropriate to classify species into functional groups (e.g. by trophic category, life-history characteristics, social structure and body size).
Here we present data from the first pantropical, standardized monitoring network for tracking the changing state of tropical mammal communities and the drivers of those changes (www.teamnetwork.org). The network spans representative environmental and anthropogenic gradients and currently comprises 17 sites in Africa, Asia and Latin America, with the goal of expanding to 40 sites by 2013. We use these data to assess the community structure, species and functional diversity of seven tropical forest mammal communities spanning continental tropical forests in Africa, Asia and Latin America. We use data from extensive (approx. 120 km2 per site), camera trap arrays to explore patterns of diversity among forests and the relationship with landscape context and forest area.
2. Methods
(a). Data
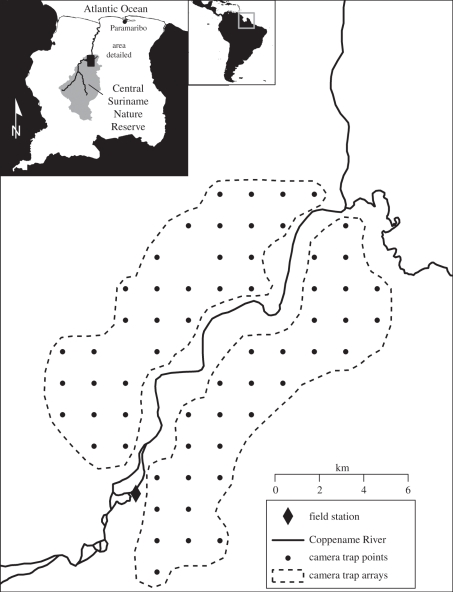
The data presented in this paper come from a global tropical forest monitoring network, the Tropical Ecology Assessment and Monitoring Network (TEAM, http://www.teamnetwork.org). TEAM monitors tropical mammal and bird communities using extensive camera trap arrays, following a standardized protocol [12]. At each site, camera trap arrays cover a minimum of 120 km2, with 60 camera trap points (camera trap model RM45, Reconyx Inc.) deployed at a density of one camera per 2 km2 for at least 30 consecutive days during the dry season (months with less than 100 mm average rainfall). Camera trap points are laid out in a regular grid in sites that are close to animal trails to capture as many species as possible and without using bait to minimize attraction of particular species (e.g. big cats). To minimize costs, at each site, camera traps are deployed sequentially in arrays of 20–30, rather than simultaneously (figure 1). At the end of the sampling period, memory cards are recovered and data from images are extracted using specialized software—DeskTEAM [13] (see also http://www.teamnetwork.org/en/help-deskteam). On average, we obtain 8319 images per site per year (range 1974–14 017). A single taxonomic authority is used for naming all mammal species photographed [14]. All TEAM data are publicly available at http://www.teamnetwork.org/en/data/query.
Figure 1.
Map of a typical TEAM camera trap array at the Central Suriname Nature Reserve, Suriname. Each point represents a camera trap location. Camera traps are placed at a density of one camera trap every 2 km2 and distributed in two sampling arrays of 30 camera traps each (North and South of the Coppename River).
(b). Sites
We analyse data on mammal species occurrence and functional diversity collected in 2008–2010 (table 1), from seven TEAM sites in Africa, Asia and Latin America: Bwindi National Park (Uganda), Udzungwa Mountains National Park (Tanzania), Bukit Barisan Selatan National Park (Indonesia), Nam Kading National Protected Area (Lao PDR), Central Suriname Nature Reserve (Suriname), Manaus (Brazil) and Volcan Barva Transect (Costa Rica). (Detailed information on each site is available at http://www.teamnetwork.org.) We classified each site according to its landscape structure into ‘Continuous habitat’, ‘Partially fragmented habitat’ and ‘Highly fragmented habitat’ (table 1) using the TEAM Human–Ecosystem Interaction Protocol [15,16]. Sites were classified as continuous, partially fragmented or highly fragmented if the area around the protected area was 0–20%, 20–50% or 50–100% fragmented, respectively. Area estimates of the protected area where the TEAM sampling occurs were calculated using shapefiles extracted directly from the World Database of Protected Areas [17].
Table 1.
Site metadata and sampling information for the data analysed in this paper. For more information on each camera trapping array and each site see http://www.teamnetwork.org/en/sites.
| site name, country | site code | latitude, longitude | sampling efforta (no. of images) | sampling dates (yyyy-mm-dd) | area (km2) | landscape typeb | data package IDc |
|---|---|---|---|---|---|---|---|
| Volcan Barva Transect, Costa Rica | VB | 10.3028, −84.0338 | 1619 (6052) | 2010-01-12 to 2010-05-12 | 476 | HF | 20101105141351_1831 |
| Bukit Barisan Selatan National Park, Indonesia | BBS | −5.2500, 104.1666 | 1886 (4715) | 2010-04-08 to 2010-07-19 | 3650 | PF | 20101101065419_1591 |
| Udzungwa Mountains National Park, Tanzania | UDZ | −7.8425, 36.8914 | 1799 (9778) | 2009-07-24 to 2009-12-04 | 1900 | PF | 20101105104207_1582 |
| Central Suriname Nature Reserve, Suriname | CSN | 4.7375, −56.1877 | 1905 (10 217) | 2008-11-21 to 2009-02-28 | 16 183 | CF | 20101105184636_1199 |
| Nam Kading National Protected Area, Lao PDR | NAK | 18.3248, 104.1424 | 1736 (1536) | 2009-11-12 to 2010-02-22 | 1690 | HF | 20101106074110_1014 |
| Bwindi Impenetrable National Park, Uganda | BIF | −1.0805, 29.6613 | 1773 (10 682) | 2010-06-16 to 2010-09-22 | 322 | HF | 20101129153510_5172 |
| Manaus, Brazild | MAS | −2.9290, −59.9764 | 1969 (8969) | 2010-07-06 to 2010-10-06 | 3854 | PF | 20101124113409_4578 |
aSampling effort is the number of sampling days for each camera summed for all the cameras at the site.
bClassification is based on criteria outlined in Defries et al. [15]. HF, highly fragmented area; PF, partially fragmented; and CF, continuous forest, remote area.
cThe Data Package Identification Number uniquely identifies the query made on the TEAM database and allows data users to recreate the exact same dataset used in the current analysis.
dIn Manaus, the sampling activities are not within a protected area. We report here the size of the rectangular region that encloses all sampling units.
(c). Statistical methods and occupancy estimation
Data for mammal species were analysed using R [18]. R code transformed the data into a list of species occupancy matrices (see electronic supplementary material for code). We used occupancy as our state variable [19–21]. Occupancy is defined as the proportion of points in the site where a species is expected to occur and, unlike many methods for population metrics, it does not require individual recognition and identification of animals [19]. Occupancy is often a useful surrogate for abundance [22].
Each site spanned a certain number of sampling days, D (73–132), and a given number of sample points, P, typically 56–60. The species-specific occupancy matrix has a resolution of 1 day and dimensions P rows × D columns, and contains only three possible values for a cell (pi, dj): 1 if the species was observed at point pi on day dj; 0 if the species was not observed at point pi on day dj; and NA if point pi was not sampled on day dj. From these matrices, species-specific occupancy and detection probabilities were estimated based on the statistical framework outlined here [19,20] and using the package Unmarked for R [23]. An initial model for species-specific occupancy and detection probability was fitted to the dataset for each site. After examining the resulting detection probabilities, species with a detection probability <0.02 were grouped together and a second model with species-specific occupancy and common detection probabilities (detection probability was kept unchanged between species) was fitted to improve occupancy estimation for this set of species [20].
(d). Community structure, functional diversity and species diversity
To broadly characterize community structure and functional diversity from the camera trap data, we used two functional traits: body size and trophic category. For each species, we obtained estimates of body size from a global mammal database [24] and assigned general trophic categories, i.e. carnivore, herbivore, insectivore and omnivore, for each species from the literature. Functional diversity was estimated as the functional dispersion index (FDis), a mean of the distance between species in a multivariate functional space to the community centroid, weighted by occupancy for each species [25] using package FD for R [26]. Species richness, Shannon diversity, evenness and dominance (Berger–Parker index, D) were calculated using standard methods [27–29] with package BiodiversityR [30].
3. Results
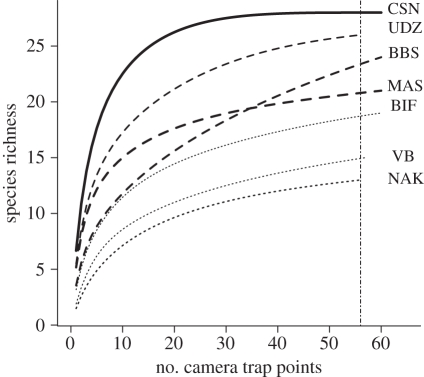
Combining all seven sites, a total of 105 mammal species were identified from 51 949 images (mean number of species = 20.6, range, 13–28 species per site), with a mean sampling effort of 1812.4 camera trap days (s.d. = 118) and a total sampling effort of 12 687 camera trap days (table 1). Nam Kading in Lao PDR and the Central Suriname Nature Reserve in Suriname had the lowest and highest observed species richness (13 and 28 species), respectively. Species accumulation curves for all sites show that sites placed in highly fragmented forests yielded fewer species per unit effort than did sites in partially fragmented or continuous forests (figure 2).
Figure 2.
Species accumulation curves for the seven sites using the exact method described in Colwell et al. [28]. The vertical line is the cut-off point used to estimate species richness for all sites. CSN, Central Suriname Nature Reserve, Suriname; UDZ, Udzungwa, Tanzania; BBS, Bukit Barisan, Indonesia; MAS, Manaus, Brazil; BIF, Bwindi, Uganda; VB, Volcan Barva, Costa Rica; NAK, Nam Kading, Lao PDR. Solid line, continuous forest; dashed lines, partially fragmented forest; dotted lines, highly fragmented forest.
Most of the species photographed were omnivorous and herbivorous (35 in each category), followed by carnivorous (20) and insectivorous (15). Body size of species ranged from 26 g (Linnaeus Mouse Opossum, Marmosa murina) to 3940 kg (African Elephant, Loxodonta africana). Estimated mean occupancy was 0.28 with 48 per cent of species falling below 0.2 (s.d. = 0.236, n = 105). Species detection probabilities were also low (mean = 0.058, s.d. = 0.0317) with a highly skewed distribution (71% of species with detection probabilities below 0.04). Occupancies and detection probabilities for each species and standard errors for both parameters are provided in the electronic supplementary materials.
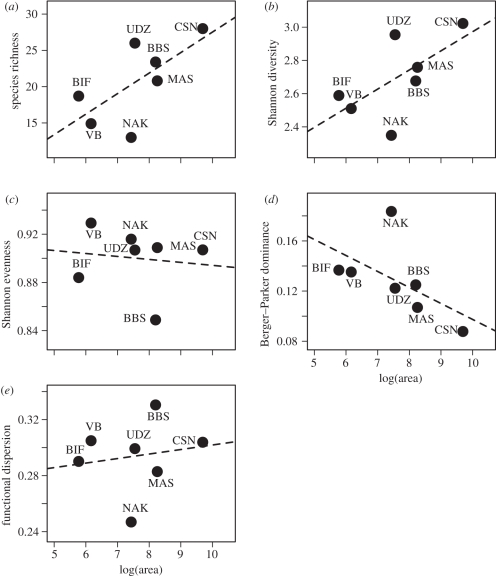
The relationship between protected area size and various measurements of mammal species communities and functional diversity is shown in figure 3. With these comparisons, we examine how the regional landscape surrounding a camera trap array at a site correlates with various measures of diversity, while keeping the sampling intensity and layout of camera traps the same between sites. Relative to the area sampled by the camera trap array, smaller protected areas are surrounded by anthropogenic and agricultural landscapes to a greater extent than larger protected areas. Arrays placed in smaller protected areas harbour fewer terrestrial mammal species, have less diverse terrestrial mammal communities and lower functional diversity than arrays placed in larger protected areas (figure 3a,b,e). Camera trap arrays placed in smaller protected areas have terrestrial communities with higher dominance (figure 3d), but show only a weak relationship with community evenness (figure 3c). A major exception to most of these patterns is the Nam Kading site, which has an unusually low richness, low species and functional diversity, and high dominance relative to other sites of similar size (labelled NAK in figure 3). This may be owing to a history of hunting pressure at this site relative to other sites of similar area (e.g. Udzungwa).
Figure 3.
Relationship between surrounding landscape structure at a site (estimated as the size of the protected area enclosing the camera trap array) and measures of community diversity. (a) Species richness (from figure 2 at cut-off sampling intensity), (b) Shannon diversity (H), (c) evenness (EH), (d) dominance (Berger–Parker D), (e) functional diversity (FDis, functional dispersion). Site abbreviations are shown next to each point (see caption of figure 2). Broken lines are least-square regression models.
In most sites, the dominant species is a herbivore (CSN and VB: Cuniculus paca; UDZ: Cephalophus harveyi; NAK: Capricornis milneedwardsii; BIF: Civettictis civetta; MAS: Dasyprocta leporina) with the exception of Bukit Barisan were it was a carnivore (Catopuma temminckii).
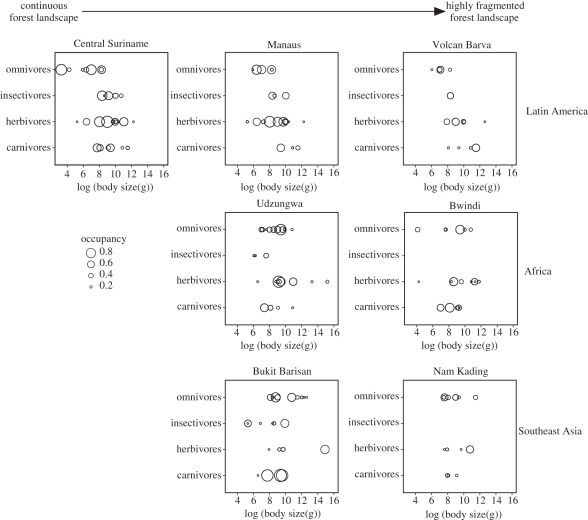
Terrestrial mammal community structure at all seven sites is shown in figure 4 separated by continent, and ordered along a gradient of landscape contexts from continuous forest to highly fragmented forest. We observe that species richness (the number of dots), the functional diversity of the community (the area of two-dimensional trait space occupied by the community) and the occupancy of species (size of the dots) decrease as the landscape becomes more fragmented. Insectivores and omnivores seem to be more sensitive to these changes than carnivores and herbivores, but all functional groups show consistent declines in diversity and occupancy in highly fragmented sites. In most sites, herbivores and omnivores dominate these communities, with the exception of Bukit Barisan, were three carnivores seem to be the most dominant species (Cuon alpinus, Catopuma temminckii and Arctogalidia trivirgata). The fragmented sites had usually one or more functional groups missing (e.g. insectivores in Nam Kading and Bwindi, large ungulates in Bwindi).
Figure 4.
Forest mammal community structure for all seven sites. Sites are arranged by continent (rows) and surrounding landscape structure (columns). Individual figures show the distribution of species along two functional traits: body size (expressed in a log scale) and trophic category. Each circle in the figure represents a species in functional space, with the size of the circle proportional to the estimated occupancy.
4. Discussion
Even though terrestrial mammals are an important component of tropical forest communities, we have quantitative information on the structure of these communities for only a handful of sites. We have reasonably good knowledge of the global distribution of most tropical forest terrestrial mammals, but we lack consistent and comparable population and community-level information on what is happening to most of these species using standardized population and community-level metrics [21]. The most recent Global Mammal Assessment showed that most tropical forest species remain data-deficient [31].
This paper assesses the community structure, species and functional diversity of seven tropical forest terrestrial mammal communities spanning three continental regions and includes forests embedded in different landscape configurations. Our results suggest that a number of important relationships need to be further tested and refined by incorporating further sites and replications. In particular, our data show that landscape context leaves a strong signature in the structure of the mammal community; forest areas surrounded by a larger proportion of anthropogenic habitats (highly fragmented sites) harbour terrestrial mammal communities with a lower species richness, lower species diversity, lower functional diversity and higher dominance. One site in particular (Nam Kading, abbreviated NAK) seems to have lower diversity (functional and species) and higher dominance compared to other sites with similar landscape configurations. It remains to be investigated whether this is because of a smaller regional species pool, higher levels of hunting, a combination of both or some other unmeasured factor.
The fact that common relationships seem to hold for several dimensions of diversity and community structure in the absence of within-site replication is a strong indication that fragmentation and landscape structure may override inherent differences in the terrestrial mammal community structure between regions. Additional sites and information are required to test the generality and robustness of these patterns.
Our general results are unsurprising given the well-documented effects of habitat fragmentation and habitat loss on populations and entire ecological communities in tropical forests [32–34]. Our data, however, suggest that some functional groups are more sensitive to these changes than others. In our study, insectivores and omnivores showed larger reductions in both species' richness and occupancy compared with carnivores and herbivores as a site becomes more fragmented (figure 4). It is well known that fragmentation affects vertebrate functional guilds in different ways [35], with the more sensitive feeding guilds, in at least some regions of the tropics, being insectivores, carnivores and omnivores, while many herbivores and nectivores tend to respond neutrally or positively to fragmentation [36]. Most of the literature on the effects of fragmentation on vertebrate functional groups in tropical forests is biased towards small- to medium-sized birds and bats, with little information for larger and harder-to-detect terrestrial ground-dwelling mammals [36]. However, our preliminary results seem to confirm the higher sensitivity of omnivores and insectivores observed in meta-analyses of wider groups of tropical vertebrates [36]. Whether this is owing to loss of habitat, food resources, hunting or a combination of these factors remains unknown for our sites. This suggests that species loss in fragments might be precipitated when local food abundance, habitat cover and connectivity between patches for these species reach a ‘tipping point’ [37]. Perhaps insectivorous and omnivorous mammals reach this point sooner than herbivores and carnivores because changes in forest cover affect abundance of their food resources or habitat preferences more strongly (see [38,39] for tropical bird examples). Hunting pressure is another potentially important variable for mammals, but has not yet been assessed in a standardized way across sites. Hunting pressure protocols are relatively simple to implement [15] and could provide useful information to disentangle several confounding effects affecting influencing tropical mammal community structure.
Body size is a functional trait that might predispose some mammal species to respond differentially to changes in landscape structure as it is positively correlated with home range and density [7,35,40]. Although we did not attempt to test how body size changes with landscape structure across sites, differences are not consistent across feeding guilds or landscape structure types. The relevant literature for terrestrial mammals is scarce (but see [41]), but in other groups of terrestrial vertebrates, the effects of landscape structure and fragmentation on body size distribution seem less strong than the effects on specific feeding functional groups [36].
(a). Camera traps—a global tool for monitoring terrestrial mammals
Camera traps are a useful, efficient, cost-effective, easily replicable tool to study and monitor terrestrial mammals [21,22,42–44]. In comparison with other field-sampling methods, they are well suited to standardization, as human influence and error are reduced to placement and maintenance of the traps and identification of the photographs. There are currently some technical limitations on the metrics that can be extracted from them in relation to population abundance and density for species that cannot be identified to individuals from photographs, which are the majority (but see [22,45]). However, occupancy estimation is a useful surrogate for population abundance despite limitations for specific applications that require density (e.g. biomass estimation). An important limitation of occupancy analysis is the difficulty of estimating it reliably for species that are very rare and/or possess very low detection probabilities [21]. This is an important concern when evaluating changes through time as occupancy estimates tend to be biased when detection probabilities are low (<0.02) [19,21]. This is certainly a characteristic of the terrestrial mammal communities analysed in this paper, where approximately 60 per cent of the species have detection probabilities below 0.05. Several solutions to this problem have been proposed including increasing the number of sampling points and combining rare species to estimate common occupancy and detection probability parameters [46]. However, for monitoring purposes and to detect changes in community structure, occupancy-based community indexes such as the Wildlife Picture Index (WPI [21]) represent a promising metric for camera trap data [47].
The data we present here provide a baseline of mammal community composition in seven tropical forests, spanning three continents, against which future changes can be measured. The data already indicate strong effects of fragmentation on species and functional diversity and community structure. Future analysis of multi-year data will allow for temporal comparison within sites, and, therefore, deciphering whether trends detected reflect local and/or global patterns of change. As TEAM is an ongoing and expanding network, the freely available data will track trends in mammal communities, and will complement other systems, such as the IUCN Red List, which can be used to monitor changes in the status of individual mammal species. Relationships between landscape structure, hunting intensity, climate variability, etc., and tropical terrestrial mammal species and functional diversity will become clearer as more sites are analysed. Our study shows that in the face of the pervasive influence of landscape degradation and fragmentation, conserving large forested areas is critical to maintain the integrity of mammal community structure and maximize species diversity. We hope that these data contribute to a better management and conservation of these populations and species and encourage a more widespread use of standardized camera-trapping methods to monitor tropical terrestrial mammals.
Acknowledgements
All data in this paper were provided by the TEAM Network, a partnership between Conservation International, The Missouri Botanical Garden, The Smithsonian Institution and The Wildlife Conservation Society, and partially funded by these institutions and the Gordon and Betty Moore Foundation. We thank all the participating institutional partners: Instituto Nacional de Pesquisas da Amazônia (INPA), Conservation International Suriname, Organization for Tropical Studies, Uganda Wildlife Authority, Museo Tridentino di Scienze Naturali and Institute of Tropical Forest Conservation. We also thank all the field staff at the following TEAM sites for their hard and invaluable work: Bwindi, Bukit Barisan Selatan, Manaus, Nam Kading, Suriname, Udzungwa and Volcan Barva. S.J.A. was also supported by NSF award no. DEB-0443453.
References
- 1.Ceballos G., Ehrlich P., Soberon J., Salazar I., Fay J. 2005. Global mammal conservation: what must we manage? Science 309, 603–607 10.1126/science.1114015 (doi:10.1126/science.1114015) [DOI] [PubMed] [Google Scholar]
- 2.Ceballos G., Ehrlich P. R. 2006. Global mammal distributions, biodiversity hotspots, and conservation. Proc. Natl Acad. Sci. USA 103, 19 374–19 379 10.1073/pnas.0609334103 (doi:10.1073/pnas.0609334103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., et al. 2010. The impact of conservation on the status of the world's vertebrates. Science 330, 1503–1509 10.1126/science.1194442 (doi:10.1126/science.1194442) [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann M., Belant J. L., Chanson J. S., Cox N. A., Lamoreux J., Rodrigues A. S. L., Schipper J., Stuart S. N. 2011. The changing fates of the world's mammals. Phil. Trans. R. Soc. B 366, 2598–2610 10.1098/rstb.2011.0116 (10.1098/rstb.2011.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rondinini C., Rodrigues A. S. L., Boitani L. 2011. The key elements of a comprehensive global mammal conservation strategy. Phil. Trans. R. Soc. B 366, 2591–2597 10.1098/rstb.2011.0111 (doi:10.1098/rstb.2011.0111) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Visconti P., et al. 2011. Future hotspots of terrestrial mammal loss. Phil. Trans. R. Soc. B 366, 2693–2702 10.1098/rstb.2011.0105 (doi:10.1098/rstb.2011.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robinson J., Redford K. 1986. Body size, diet, and population density of neotropical forest mammals. Am. Nat. 128, 665–680 10.1086/284596 (doi:10.1086/284596) [DOI] [Google Scholar]
- 8.Struhsaker T. T. 1997. Ecology of an African rain forest: logging in Kibale and the conflict between conservation and exploitation. Gainesville, FL: University Press of Florida [Google Scholar]
- 9.Weber W. 2001. African rain forest ecology and conservation: an interdisciplinary perspective. New Haven, CT: Yale University Press [Google Scholar]
- 10.Brodie J. F., Gibbs H. 2009. Bushmeat hunting as climate threat. Science 326, 364–365 10.1126/science.326_364b (doi:10.1126/science.326_364b) [DOI] [PubMed] [Google Scholar]
- 11.Jansen P., Muller-Landau H. C., Wright S. 2010. Bushmeat hunting and climate: an indirect link. Science 327, 30. 10.1126/science.327.5961.30-a (doi:10.1126/science.327.5961.30-a) [DOI] [PubMed] [Google Scholar]
- 12.TEAM Network 2008. Terrestrial vertebrate monitoring protocol, v. 3.0. TEAM Standardized Monitoring Protocols. Andelman S. Conservation International; See http://www.teamnetwork.org/en/bio/protocols/terrestrial-vertebrate [Google Scholar]
- 13.Fegraus E., Lin K., Ahumada J. A., Baru C., Chandra S., Yoon C. In press Data acquisition and management software for camera trap data: a case study from the team network. Ecological Informatics.
- 14.Wilson D. E., Reeder D. M. (eds) 2005. Mammals species of the world. A taxonomic and geographic reference. Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 15.Defries R., et al. 2010. From plot to landscape scale: linking tropical biodiversity measurements across spatial scales. Front. Ecol. Environ. 8, 153–160 10.1890/080104 (doi:10.1890/080104) [DOI] [Google Scholar]
- 16.TEAM Network 2010. Zone of human dynamics and ecosystem change (ZoHDEC) protocol implementation manual, v. 0.3a. TEAM Standardized Monitoring Protocols. Andelman S. Conservation International; See http://www.teamnetwork.org/protocols/bio/zohdec [Google Scholar]
- 17.IUCN and UNEP 2010. The World Database on Protected Areas (WDPA). Cambridge, UK: UNEP-WCMC.
- 18.R Development Core Team 2010. R: a language and environment for statistical computing. R Foundation for Statistical Computing; See http://www.R-project.org [Google Scholar]
- 19.Mackenzie D., Nichols J., Lachman G., Droege S., Andrew Royle J., Langtimm C. 2002. Estimating site occupancy rates when detection probabilities are less than one. Ecology 83, 2248–2255 10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2 (doi:10.1890/0012-9658(2002)083[2248:ESORWD]2.0.CO;2) [DOI] [Google Scholar]
- 20.MacKenzie D. I., Nichols J. D., Royle J. A., Pollock K. H., Bailey L. L., Hines J. E. 2006. Occupancy estimation and modeling. Amsterdam, The Netherlands: Elsevier [Google Scholar]
- 21.O'Brien T. G., Baillie J., Krueger L., Cuke M. 2010. The wildlife picture index: monitoring top trophic levels. Anim. Conserv. 13, 335–343 10.1111/j.1469-1795.2010.00357.x (doi:10.1111/j.1469-1795.2010.00357.x) [DOI] [Google Scholar]
- 22.Rovero F., Marshall A. 2009. Camera trapping photographic rate as an index of density in forest ungulates. J. Appl. Ecol. 46, 1011–1017 10.1111/j.1365-2664.2009.01705.x (doi:10.1111/j.1365-2664.2009.01705.x) [DOI] [Google Scholar]
- 23.Fiske I., Chandler R. 2010. Unmarked: models for data from unmarked animals. R package version 0.8-7. See http://cran.r-project.org/web/packages/unmarked/
- 24.Smith F., Lyons S., Ernest S., Jones K., Kauffman D., Dayan T., Marquet P., Brown J., Haskell J. 2003. Body mass of late quaternary mammals. Ecology 84, 3403. 10.1890/02-9003 (doi:10.1890/02-9003) [DOI] [Google Scholar]
- 25.Etienne L., Pierre L. 2010. A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 10.1890/08-2244.1 (doi:10.1890/08-2244.1) [DOI] [PubMed] [Google Scholar]
- 26.Etienne L., Bill S. 2010. Fd: measuring functional diversity from multiple traits, and other tools for functional ecology. R package version 1.0-9. See http://cran.r-project.org/web/packages/FD/
- 27.Magurran A. E. 1988. Ecological diversity and its measurement. Princeton, NJ: Princeton University Press [Google Scholar]
- 28.Colwell R., Mao C., Chang J. 2004. Interpolating, extrapolating, and comparing incidence-based species accumulation curves. Ecology 85, 2717–2727 10.1890/03-0557 (doi:10.1890/03-0557) [DOI] [Google Scholar]
- 29.Pielou E. 1966. The measurement of diversity in different types of biological collections. J. Theor. Biol. 13, 131–144 10.1016/0022-5193(66)90013-0 (doi:10.1016/0022-5193(66)90013-0) [DOI] [Google Scholar]
- 30.Kindt R., Coe R. 2005. Tree diversity analysis: a manual and software for common statistical methods for ecological and biodiversity studies. See http://www.worldagroforestry.org/treesandmarkets/tree_diversity_analysis.asp
- 31.Schipper J., et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 10.1126/science.1165115 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 32.Fahrig L. 2003. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34, 487–515 10.1146/annurev.ecolsys.34.011802.132419 (doi:10.1146/annurev.ecolsys.34.011802.132419) [DOI] [Google Scholar]
- 33.Harcourt A. H., Doherty D. A. 2005. Species–area relationships of primates in tropical forest fragments: a global analysis. J. Appl. Ecol. 42, 630–637 10.1111/j.1365-2664.2005.01037.x (doi:10.1111/j.1365-2664.2005.01037.x) [DOI] [Google Scholar]
- 34.Turner I. M. 1996. Species loss in fragments of tropical rain forest: a review of the evidence. J. Appl. Ecol. 33, 200–209 [Google Scholar]
- 35.Henle K., Davies K. F., Kleyer M., Margules C., Settele J. 2004. Predictors of species sensitivity to fragmentation. Biodivers. Conserv. 13, 207–251 10.1023/B:BIOC.0000004319.91643.9e (doi:10.1023/B:BIOC.0000004319.91643.9e) [DOI] [Google Scholar]
- 36.Vetter D., Hansbauer M. M., Vegvari Z., Storch I. 2011. Predictors of forest fragmentation sensitivity in neotropical vertebrates: a quantitative review. Ecography 34, 1–8 10.1111/j.1600-0587.2010.06453.x (doi:10.1111/j.1600-0587.2010.06453.x) [DOI] [Google Scholar]
- 37.Pardini R., Bueno A. D. A., Gardner T. A., Prado P. I., Metzger J. P. 2010. Beyond the fragmentation threshold hypothesis: regime shifts in biodiversity across fragmented landscapes. PLoS ONE 5, e13666. 10.1371/journal.pone.0013666 (doi:10.1371/journal.pone.0013666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferraz G., Russell G. J., Stouffer P. C., Bierregaard R. O., Pimm S. L., Lovejoy T. E. 2003. Rates of species loss from Amazonian forest fragments. Proc. Natl Acad. Sci. USA 100, 14 069–14 073 10.1073/pnas.2336195100 (doi:10.1073/pnas.2336195100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratford J. A., Stouffer P. C. 1999. Local extinctions of terrestrial insectivorous birds in a fragmented landscape near Manaus, Brazil. Conserv. Biol. 13, 1416–1423 10.1046/j.1523-1739.1999.98494.x (doi:10.1046/j.1523-1739.1999.98494.x) [DOI] [Google Scholar]
- 40.Lindstedt S. L., Miller B. J., Buskirk S. W. 1986. Home range, time, and body size in mammals. Ecology 67, 413–418 10.2307/1938584 (doi:10.2307/1938584) [DOI] [Google Scholar]
- 41.Sampaio R., Lima A. P., Magnusson W. E., Peres C. A. 2010. Long-term persistence of midsized to large-bodied mammals in Amazonian landscapes under varying contexts of forest cover. Biodivers. Conserv. 19, 2421–2439 10.1007/s10531-010-9848-3 (doi:10.1007/s10531-010-9848-3) [DOI] [Google Scholar]
- 42.Kinnaird M., Sanderson E., O'Brien T. G., Wibisono H., Woolmer G. 2003. Deforestation trends in a tropical landscape and implications for endangered large mammals. Conserv. Biol. 17, 245–257 10.1046/j.1523-1739.2003.02040.x (doi:10.1046/j.1523-1739.2003.02040.x) [DOI] [Google Scholar]
- 43.Rovero F., Tobler M., Sanderson J. 2010. Camera-trapping for inventorying terrestrial vertebrates. In Manual on field recording techniques and protocols for All Taxa Biodiversity Inventories and Monitoring (eds Eymann J., Degreef J., Häuser C., Monje J. C., Samyn Y., VandenSpiegel D.), pp. 100–128 The Belgian National Focal Point to the Global Taxonomy Initiative. [Google Scholar]
- 44.Tobler M. W., Carrillo-Percastegui S. E., Leite Pitman R., Mares R., Powell G. 2008. An evaluation of camera traps for inventorying large- and medium-sized terrestrial rainforest mammals. Anim. Conserv. 11, 169–178 10.1111/j.1469-1795.2008.00169.x (doi:10.1111/j.1469-1795.2008.00169.x) [DOI] [Google Scholar]
- 45.Rowcliffe J., Field J., Turvey S., Carbone C. 2008. Estimating animal density using camera traps without the need for individual recognition. J. Appl. Ecol. 45, 1228–1236 10.1111/j.1365-2664.2008.01473.x (doi:10.1111/j.1365-2664.2008.01473.x) [DOI] [Google Scholar]
- 46.Bailey L. L., Hines J., Nichols J., Mackenzie D. 2007. Sampling design trade-offs in occupancy studies with imperfect detection: examples and software. Ecol. Appl. 17, 281–290 10.1890/1051-0761(2007)017[0281:SDTIOS]2.0.CO;2 (doi:10.1890/1051-0761(2007)017[0281:SDTIOS]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 47.Nichols J. 2010. The wildlife picture index, monitoring and conservation. Anim. Conserv. 13, 344–346 10.1111/j.1469-1795.2010.00382.x (doi:10.1111/j.1469-1795.2010.00382.x) [DOI] [Google Scholar]