Abstract
The Mediterranean basin is considered a hotspot of biological diversity with a long history of modification of natural ecosystems by human activities, and is one of the regions that will face extensive changes in climate. For 181 terrestrial mammals (68% of all Mediterranean mammals), we used an ensemble forecasting approach to model the future (approx. 2100) potential distribution under climate change considering five climate change model outputs for two climate scenarios. Overall, a substantial number of Mediterranean mammals will be severely threatened by future climate change, particularly endemic species. Moreover, we found important changes in potential species richness owing to climate change, with some areas (e.g. montane region in central Italy) gaining species, while most of the region will be losing species (mainly Spain and North Africa). Existing protected areas (PAs) will probably be strongly influenced by climate change, with most PAs in Africa, the Middle East and Spain losing a substantial number of species, and those PAs gaining species (e.g. central Italy and southern France) will experience a substantial shift in species composition.
Keywords: climate change, ensemble modelling, extinction risk, species distribution models, protected areas
1. Introduction
The Mediterranean basin is one of the richest and most complex regions on the Earth from a geological, biological and cultural point of view [1]. Widely considered as one of the global hotspots of biodiversity [2,3], the region hosts about 25 000 plant species (50% of which are endemic), more than 150 000 insect species (on average 15–20% of endemics; up to 90% of endemics in cave systems) and more than 1100 terrestrial vertebrates (endemism rates range from 17% for breeding birds up to 64% for amphibians; [1]). A number of biogeographic, geological and historical factors contribute to this exceptional biological diversity [1,4–8], but the tight integration between traditional human activities and Mediterranean ecosystems may lead to coevolution as an additional important driver [9].
In fact, landscapes in the Mediterranean have been (re)shaped by humans for at least the last 10 000 years [10,11], significantly longer than in any other global biodiversity hotspot. The interaction of natural ecosystems with traditional human activities is one of the reasons for the high environmental diversity that characterizes the region, but it is also the reason why the Mediterranean basin is considered one of the four most significantly altered hotspots on Earth [3,12].
Currently, the high biodiversity that characterizes the Mediterranean basin is highly endangered. Changes in land-use and human population density in the last 50 years have been dramatic [13,14], with both positive and negative consequences for biodiversity [15–17]. At the same time, the Mediterranean region is already home to some 455 million human inhabitants and it is the first vacation destination in the world with 31 per cent of all tourists worldwide visiting the region in 2006 [18]. Moreover, future projections predict a more homogeneous and more densely populated landscape, with tourism expected to double by 2025 [19] with associated overall negative consequences for biodiversity, especially in North Africa and in the Middle East.
The Mediterranean basin is also considered to be one of the regions that will face the largest changes in climate worldwide [20]. The region has already experienced large climate shifts in the past [21]. However, by the end of the twenty-first century, Gao & Giorgi [22] have projected a substantial northward increase of dry and arid lands, with the frequency of hot extremes increasing by 200–500% through the region [23], with most of the changes occurring in summer and spring [24], and with an observed trend of recent warming that is much higher than was simulated by the state-of-the-art global circulation models over the recent past [25].
All of these factors support an evaluation of the available conservation options that include projections of ongoing global change. A number of studies have clearly demonstrated the negative effects that climate change will have and is already having on biodiversity [26,27], and some species have already gone extinct owing to recent climate change [28]. However, only limited attention has been given to the effects of future climate changes on mammals [29–33], and to our knowledge no one has considered the entire Mediterranean basin specifically.
Recently, predictive species distribution models (SDMs) have become increasingly important to address a number of issues in ecology, conservation biology and climate change research [34]. We applied the state-of-the-art bioclimatic SDMs [35] to evaluate the potential effects of climate change on Mediterranean mammals. In particular, we calculated changes in climatically suitable areas for all species, classifying the species into different threat categories depending on the amount of (positive or negative) changes. Furthermore, we calculated species turnover for each pixel in the Mediterranean basin, considering also the existing protected areas (PAs). All results were analysed considering averages across climate change projections originating from five general circulation model (GCM) outputs using two emission scenarios (A2 and B1) and considering two contrasting assumptions on dispersal ability (species unable to disperse versus species dispersing with no constraint [36,37]).
2. Material and methods
(a). Study area and protected areas
We defined our study area (approx. 3 500 000 km2; figure 1) following the boundaries of the Mediterranean biogeographic area [1]. The region, stretching over approximately 5300 km west–east and 2200 km north–south, touches three continents (Europe, Asia and Africa) and 24 different countries.
Figure 1.
Study area (grey regions) and protected areas (black regions).
From the World Database on Protected Areas (http://protectedplanet.net/), we extracted 1390 national and international PAs occurring inside our study area. Moreover, for the European part of the Mediterranean basin, we also obtained a database on the Natura 2000 network (1260 areas inside our study area), a network of areas whose aim is to conserve an extensive range of habitat types and wildlife species throughout Europe under the framework of the European Union Birds and Habitat Directives (http://www.eea.europa.eu/data-and-maps/data/natura). Overall, PAs plus the Natura 2000 cover 8 per cent of the study area, with the large majority of the areas being in Europe (only 26 areas in Africa, corresponding to 0.25% of the total extensions of PAs). PAs and Natura 2000 were overlaid to the final maps (see below) to identify the most critical areas for mammal species conservation.
(b). Species data
To avoid including in the analyses species only marginally present in the Mediterranean basin, we initially considered only the 212 species (electronic supplementary material, table S1) that according to Schipper et al. [38] have at least 5 per cent of their distribution range inside the region (79.8% of all species occurring in the Mediterranean). On average, more than 47 per cent of the distribution range for these 212 species fall inside the Mediterranean basin, with 18 species being sub-endemic of the study area (i.e. more than 90% of the range is included in the study area) and 35 being endemic.
Rodentia represents the order with the highest number of species (92), and Muridae is the most represented family (43 species). Considering the most recent IUCN Red List available [39], three species (one of which is endemic) are classified as Critically Endangered (CE), seven as Endangered (EN) (three endemics), 19 as Vulnerable (VU) (eight endemics or sub-endemics) and one endemic species is considered Extinct in the Wild (electronic supplementary material, table S1).
For each species, we obtained the current distribution range from Schipper et al. [38] that was used later in the analysis to define different dispersal scenarios. Moreover, we collected all the freely and readily available points of presence from a number of published data sources (main references [40–43]; complete list available from L.M.) and online databases (http://data.gbif.org), and, when available, we also obtained the positional error associated with each point (from 300 m to 50 km). To obtain a more general representation of each species' climatic niche, presence points were collected considering an area larger than the final study area, corresponding roughly to the western Palearctic. We excluded 30 species (6 endemics or sub-endemics) from further analysis for which we found very few points of presence to cover most of the distribution range included in the study area. We also excluded Oryx dammah, which is extinct in the wild, obtaining a final set of 181 species (85.4% of the initial set of 212 species; table 1).
Table 1.
Species considered in the analysis. IUCN red listing considers only Critically Endangered, Endangered and Vulnerable species. Endemic species have their distribution range included completely inside the study area; sub-endemic species have at least 90% of their distribution range inside the study area. Red listing categories and distribution ranges are from Schipper et al. [38]. The columns labelled ‘% change’ give the median change in distribution projected for each family under the different scenario considered (A2 and B1 represent climate scenarios; UnlD, unlimited dispersal scenario; NoD, no-dispersal scenario; see the text for more details).
| order | family | number of species | number of species in IUCN Red List | number of endemic and sub-endemic | % change (B1; UnlD) | % change (A2; UnlD) | % change (B1; NoD) | % change (A2; NoD) |
|---|---|---|---|---|---|---|---|---|
| Carnivora | Canidae | 2 | 0 | 0 | +0.21 | +0.07 | +0.19 | +0.06 |
| Felidae | 4 | 1 | 2 | +0.04 | +0.00 | −0.07 | −0.35 | |
| Hyaenidae | 1 | 0 | 0 | +0.51 | +0.62 | +0.50 | +0.45 | |
| Mustelidae | 6 | 0 | 0 | −0.23 | −0.50 | −0.24 | −0.52 | |
| Cetartiodactyla | Bovidae | 11 | 7 | 0 | −0.07 | −0.17 | −0.14 | −0.35 |
| Cervidae | 4 | 1 | 0 | −0.34 | −0.66 | −0.35 | −0.67 | |
| Suidae | 1 | 0 | 0 | −0.21 | −0.44 | −0.40 | −0.63 | |
| Chiroptera | Hipposideridae | 1 | 0 | 0 | +0.28 | +0.69 | +0.03 | −0.14 |
| Molossidae | 1 | 0 | 0 | −0.05 | −0.21 | −0.05 | −0.21 | |
| Rhinolophidae | 5 | 1 | 0 | −0.26 | −0.54 | −0.26 | −0.54 | |
| Rhinopomatidae | 2 | 0 | 0 | +0.97 | +1.66 | +0.30 | +0.13 | |
| Vespertilionidae | 26 | 2 | 3 | −0.32 | −0.63 | −0.33 | −0.63 | |
| Eulipotyphla | Erinaceidae | 4 | 0 | 1 | −0.10 | −0.29 | −0.25 | −0.49 |
| Soricidae | 18 | 2 | 8 | −0.25 | −0.52 | −0.26 | −0.58 | |
| Talpidae | 6 | 1 | 3 | −0.21 | −0.68 | −0.41 | −0.70 | |
| Lagomorpha | Leporidae | 6 | 2 | 3 | −0.28 | −0.63 | −0.28 | −0.62 |
| Macroscelidea | Macroscelididae | 1 | 0 | 1 | −0.05 | +0.10 | −0.07 | −0.15 |
| Primates | Cercopithecidae | 1 | 1 | 1 | −0.43 | −0.66 | −0.43 | −0.66 |
| Rodentia | Cricetidae | 19 | 2 | 6 | −0.32 | −0.55 | −0.32 | −0.55 |
| Ctenodactylidae | 1 | 0 | 0 | −0.02 | −0.05 | −0.49 | −0.83 | |
| Dipodidae | 5 | 1 | 2 | −0.18 | −0.32 | −0.19 | −0.35 | |
| Gliridae | 7 | 1 | 1 | −0.28 | −0.53 | −0.29 | −0.53 | |
| Hystricidae | 2 | 0 | 0 | +0.02 | −0.03 | +0.01 | −0.05 | |
| Muridae | 38 | 2 | 13 | −0.08 | −0.32 | −0.09 | −0.35 | |
| Sciuridae | 6 | 1 | 2 | −0.31 | −0.53 | −0.34 | −0.66 | |
| Spalacidae | 3 | 0 | 0 | −0.23 | −0.52 | −0.25 | −0.52 | |
| total | 181 | 25 | 46 | −0.21 | −0.48 | −0.24 | −0.51 |
(c). Current climate and climate projections
We considered a set of five bioclimatic variables that are important to shape the climate of the study area, and which are at the same time not highly correlated (i.e. |Pearson r| ≤ 0.8). The Mediterranean climate is transitional between cold temperate and dry tropical, with a unique combination of hot and dry summers, and cool (or cold) and humid winters. Given the limited availability of surface water during the months of maximal solar insolation, the long and dry summer periods (two months in the west and five or six months in the east with basically no precipitation) represent the most unfavourable period of the year for both plants and animals, contrary to the regions further north where the unfavourable period is winter owing to low temperatures. Accordingly, the weather during the short spring and autumn seasons is extremely important, representing a ‘climatic buffer’ against the extreme summer climate.
In a recent review on climate change projections for the Mediterranean region, Giorgi & Lionello [24] considered the most recent and comprehensive ensembles of global and regional climate change simulations and obtained a robust and consistent picture for the future Mediterranean climate. The main features consist of an important decrease in precipitation, especially in summer, and of a pronounced warming, also to occur mostly during the summer season. Moreover, inter-annual variability is projected to increase with a greater occurrence of extremely high temperature events.
Given these characteristics, we considered the following variables: the average moisture index of summer months (MS); the mean temperature of the coldest quarter (MTC); the mean temperature of the driest quarter (MTD); annual precipitation (ANNP); and precipitation of the driest quarter (PDQ). We obtained all climate variables at 1 km resolution from WORLDCLIM [44], with the exception of the moisture index that was calculated as the difference between precipitation and potential evapotranspiration each summed over the three months June, July and August. Potential evapotranspiration was calculated following the empirical equation of Turc [45], which considers monthly average temperature and potential global radiation. The latter was derived in a geographic information system using the method by Kumar et al. [46]. More information is given in Zimmermann et al. [47].
Future projections were derived using climate model outputs made available through the Intergovernmental Panel on Climate Change (IPCC) Data Distribution Centre (http://ipcc-ddc.cru.uea.ac.uk). In particular, we used five climate change model outputs that form part of the fourth assessment report [48] (CGCM31 run by the CCMA, CSIRO's MK35, CM21 run by the GFDL, PCM1 operated by NCAR and UKMO's HADCM3) for two of the IPCC's climate scenarios, the B1 scenario, describing a world with reduced use of natural resources and the use of clean and resource-efficient technologies, and the A2 scenario, where the greenhouse gas emission rate continues to increase [49]. Climate variables were averaged over the period 2071–2100.
The original climate change models came with varying resolutions of roughly 2 × 2°, roughly corresponding in the study area to 180 × 200 km. The Mediterranean basin is characterized by very high topographic complexity, which renders such a resolution too coarse to adequately depict the orographically induced fine-scale structure of the local climate. Thus, we downscaled the available climatic layers to 1 km2 by calculating anomalies of the future monthly temperature and precipitation values against the 1950–2000 means of each GCM model output, where the latter represents the WORLDCLIM base period. Anomalies represent absolute temperature difference (Δ°C) and relative precipitation differences (% change) per coarse resolution pixel measured directly at the model output. Second, we down-scaled these anomalies in two interpolation steps to 1 km2 of spatial resolution, using bilinear resampling. Third, we added temperature anomalies to WORLDCLIM and multiplied precipitation with the respective anomalies to obtain monthly maps of future temperature and precipitation for each model and scenario at a 1 km2 spatial resolution. Finally, we re-calculated all derived climate maps mentioned above. The applied procedure has the advantage that a possible model offset under current climate is not added to the projected climate trends.
(d). Bioclimatic distribution models
We predicted mammal distributions using an ensemble forecasting approach, as implemented in BIOMOD, a bioclimatic niche modelling package [35], within the R environment. We used the following eight models: (i) generalized linear models, (ii) generalized additive models, (iii) classification tree analysis, (iv) artificial neural networks, (v) generalized boosted models, (vi) random forests, (vii) flexible discriminant analysis, and (viii) multivariate adaptive regression spline. See Thuiller et al. [35] for more details.
The location error associated with the available points of presence was often larger than the resolution adopted in our analysis, especially for points collected through the Global Biodiversity Information Facility. Thus, around each point of presence, we considered a circular buffer with a radius corresponding to the location error. We then generated a random point inside each buffer to which we associated the value of the climatic variables. We repeated the same procedure 10 times for each species, obtaining 10 sets of points representing species' presence and accounting for positional errors. We also generated 10 sets of 20 000 random background points (drawn from the western Palearctic, i.e. the same area over which we collected presences) that we used to characterize the climate of the study area and to represent pseudo-absences. We combined the 10 sets of points representing species presence with the 10 sets of background points, obtaining 100 sets of points for each species to be used for the calibration of each of the eight niche models. For each of the 100 sets of points, we calibrated the eight models using a 70 per cent random sample, and we evaluated model performance using the remaining 30 per cent of the data. In particular, we measured the area under the receiver-operating characteristic curve (AUC) [50]. Only models with AUC values greater than 0.7 [50] were projected onto the available future climate scenarios.
Probability of occurrence for each species and for each model was transformed into presence–absence values using a threshold maximizing the sum of sensitivity (the percentage of presence correctly predicted) and specificity (the percentage of absence correctly predicted) [51]. Then, a final consensus model for each species was obtained defining as presences all those cells where more than 50 per cent of the models (i.e. five out of eight) predict species' presence. Using the same thresholds, we obtained binary models also for climate change projections, for a total of 160 models for each species (five climate change models, by two IPCC scenarios, by eight modelling techniques, by two dispersal scenarios). We produced four consensus forecasts for each species, one for each possible combination of two IPCC emission scenarios (A2 and B1) and of two dispersal scenarios, i.e. no-dispersal (assuming that species will persist only in suitable areas inside their current range) versus unlimited dispersal (assuming that a species could disperse to all new suitable areas inside the Mediterranean basin). These assumptions on dispersal are commonly used in the literature (e.g. [36]), and represent two extremes, while the realized patterns at the end of the twenty-first century will necessarily be somewhere in between depending on the species' capacity to track climate change. For each ensemble forecast we defined as presences all those cells where more than 50 per cent of the models (i.e. 21 out of 40 available for each scenario) predict species' presence.
(e). Changes in climatically suitable areas and threat categories
We calculated the percentage of cells gained or lost by each species under the B1 and A2 scenarios with respect to the initial climatically suitable range, corresponding to the current distribution range under the no-dispersal hypothesis, and to the full study area under the unlimited dispersal hypothesis. Moreover, following Thuiller et al. [37], we defined the following threat categories based on the IUCN Red List Criteria ([52], but see [53] for a critique of this approach): threat-level 1 (Threat-1; species with a projected range loss of 100%), threat-level 2 (Threat-2; species with a projected range loss >80%), threat-level 3 (Threat-3; species with a projected range loss between 50 and 80%) and threat-level 4 (Threat-4; species with a projected range loss between 30% and 50%). All other species were classified either as threat-level 5 (Threat-5; species losing less than 30%) or as gain (species actually gaining climatic suitability).
(f). Species richness, species turnover and protected areas
Considering all species, we calculated both the current and the future potential species richness and species turnover (measured as percentage of species gained or lost at each cell over the corresponding potential species richness). All calculations were performed following recommendations in Thuiller et al. [35].
To assess the sensitivity of PAs to climate change, we tested if PAs will experience a higher or lower species turnover if compared with a random set of areas sharing the same characteristics as PAs. In particular, we generated 1000 sets of random areas occupying for each country the same area as the existing PAs. For each set of random areas, we calculated the average turnover, obtaining the distribution that would be expected for our study area under the null hypothesis stated above. We calculated the same metric for existing PAs and we rejected the null hypothesis when the metric measured inside PAs was in the top 5 per cent of the null distribution. Always considering PAs, we also compared the current species richness with the richness projected under the different emission and dispersal scenarios. All calculations were performed for both the B1 and the A2 scenarios, and for both dispersal scenarios.
3. Results
(a). Model evaluation
All models showed a good predictive power with a mean AUC of 0.96 (±0.042). The minimum AUC value was 0.79 for the Tarabul's Gerbil (Gerbillus tarabuli), while the maximum AUC value was 0.99 for the James's Gerbil (Gerbillus jamesi). The average AUC value for endemic and sub-endemic species was 0.98, with a maximum of 0.99 and a minimum of 0.94. The same values were obtained for species classified as Critically Endangered, Endangered or Vulnerable, with a mean AUC of 0.98, a minimum of 0.94 and a maximum of 0.99, respectively.
(b). Changes in climatically suitable areas and threat categories: unlimited dispersal
Assuming unlimited dispersal, both positive and negative changes in climatically suitable area are possible (table 1), with Rhinopomatidae being the family with highest gains in climatically suitable areas, and Talpidae and Cervidae showing the highest losses. The comparably mild B1 climate scenario projects that changes in the extent of climatically suitable habitats would vary on average between −91 and +880 per cent (mean = −11%; s.d. = 76%) among all mammals, while under the more severe A2 scenario the projected changes are more extreme, ranging from −100 to +990 per cent (mean = −25%; s.d. = 96%). With few exceptions (e.g. Lepus capensis and Mus cypriacus), all species gaining climatically suitable areas are strongly associated with desert and/or steppe environments.
Considering the overall average across all climate change models, one species only (Sorex arunchi) was predicted to lose completely its climatically suitable range in the future (Threat-1) under the A2 climate scenario. Considering the single climate change models, another 15 species were predicted to become extinct in one or more climate change models under the A2 scenario, while S. arunchi was classified as extinct under the MK35 climate model for the B1 scenario.
Under the A2 scenario (figure 2 and electronic supplementary material, table S2), 8.3 per cent of species were classified in Threat-2, 37.2 per cent in Threat-3 and 20.6 per cent in Threat-4, percentages much higher than the comparable classes in the current red listing (1.0% CE; 3.3% EN; 9.4% VU). Considering endemic and sub-endemic species, 73.9 per cent were classified as Threat-2, Threat-3 or Threat-4 as opposed to 21.7 per cent currently in the comparable IUCN categories (i.e. CE, EN or VU).
Figure 2.
Predicted threat categories according to changes in climatically suitable range. (a) unlimited dispersal under the B1 climate scenario; (b) unlimited dispersal under the A2 climate scenario; (c) no-dispersal under the B1 climate scenario; (d) no-dispersal under the A2 climate scenario. Threat-1, threat-level 1 (species with a projected range loss of 100%, red); Threat-2, threat-level 2 (species with a projected range loss >80%, orange); Threat-3, threat-level 3 (species with a projected range loss between 50% and 80%, dark yellow); Threat-4, threat-level 4 (species with a projected range loss between 30% and 50%, light yellow); Threat-5, threat-level 5 (species with a projected range loss smaller than 30%, light green); gain, species that will gain climatic suitability (dark green).
Projections for the B1 scenario (figure 2 and electronic supplementary material, table S2) produced only 0.5 per cent of species predicted in Threat-2, 8.8 per cent in Threat-3 and 23.8 per cent in Threat-4, percentages that in any case are bigger than the current red list classifications. Considering endemic and sub-endemic species, 30.4 per cent were classified as Threat-2, Threat-3 or Threat-4. For both A1 and B2, some species (respectively, 19.9 and 22.7%) are predicted to gain climatically suitable areas.
(c). Changes in climatically suitable areas and threat categories: no dispersal
Assuming no dispersal, even if some species would still gain climatically suitable areas, the results predict stronger negative trends (table 1), with Hyaenidae showing the highest gains in climate suitability and Ctenodactylidae and Talpidae showing the highest losses. Under the B1 climate scenario, changes in the extent of climatically suitable areas would vary on average between −100 and +110 per cent (mean = −22%; s.d. = 28%), while under the A2 changes would range from −100 to +90 per cent (mean = −45%; s.d. = 33%). Also in this case, species predicted to gain climatically suitable areas are mostly associated with deserts and/or steppes, while S. arunchi is the only species projected as extinct under A2 (i.e. Threat-1; figure 2 and electronic supplementary material, table S2). Moreover, 17 species were predicted to lose all their climatically suitable range (corresponding to Threat-1) under one or more climate change models for the A2 scenario, and four showed the same results under the B1 scenario.
Considering the A2 scenario (figure 2 and electronic supplementary material, table S2), the projected losses are much stronger if compared with the unlimited dispersal hypothesis, with 14.9 per cent of Threat-2 species, 37.2 per cent of Threat-3 and 22.2 per cent of Threat-4. Comparable results were obtained for the B1 scenario (figure 2 and electronic supplementary material, table S2), with 2.8 per cent of species in Threat-2, 11.1 per cent in Threat-3 and 18.2 per cent in Threat-4. The same trend towards stronger negative changes was measured for endemic and sub-endemic species, with 34.8 per cent of species in Threat-2, Threat-3 or Threat-4 under the B1 scenario, and 91.3 per cent under the A2 scenario.
(d). Species richness, species turnover and protected areas
Under the current climate, the highest values of potential species richness were located in the northern part of the Mediterranean (electronic supplementary material, figure S1a), and particularly in the Italian peninsula, in northern Spain, in the internal part of Greece and in northern Turkey. Other areas of high species richness were predicted in the main islands (Sardinia, Sicily and Corsica) and along the coasts of Morocco, Algeria and Tunisia in Africa. The pattern of species richness is similar under both future climate scenarios (electronic supplementary material, figures S1b,c), with a strong decrease in richness that is predicted for Africa and Spain, and with Italy that should be hosting most of the areas with the highest species richness, representing by far the main centre for mammal diversity in the future.
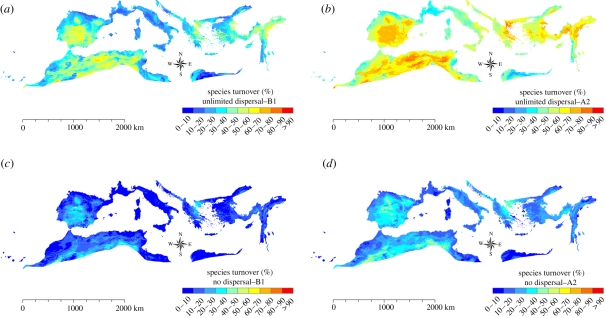
Considering the unlimited dispersal scenario, our models predict that the highest species turnover, corresponding to a net species lost, will be localized especially in Spain and in northern Africa (B1 climate scenario; figure 3a), while areas with high species turnover in the Middle East, in Turkey, in Greece and in central Italy represent mainly areas of positive changes in species richness (figure 3a). Under the A2 climate scenario (figure 3b), the turnover of species richness is predicted to be more extensive, but it should correspond to a positive change in species richness only for a few small areas in central Italy, France, Turkey and in the Middle East. Species turnover under the no-dispersal scenario (figure 3c,d) would basically show the same pattern described for the unlimited dispersal scenario, but with lower intensities as was expected given the assumption on lack of dispersal.
Figure 3.
Species turnover under the B1 climate scenario: (a) unlimited dispersal, (c) no-dispersal; and under the A2 climate scenario (b) unlimited dispersal; (d) no-dispersal.
Considering the average species turnover measured inside PAs, we found no statistically significant difference and thus we could not reject the null hypothesis that PAs are significantly different from a set of random areas. The same general pattern can be inferred considering species richness (figure 4), even with the obvious differences existing throughout the study area. PAs located in drier parts of the Mediterranean basin were most sensitive to climate change, including mainly PAs in northern Africa and the Middle East, where a very high number of species may be lost (on average from 37% to 25% decrease in species richness depending on the scenario), but also in southern Europe, mainly Spain (with an average decrease ranging from 43% to 29%). PAs located in central and northern Italy (along the Apennines), as well as in France (excluding the PAs along the coasts) were projected to gain a substantial number of species (average increase in species richness from 10% to 20%).
Figure 4.
Current and future species richness under the (a) B1 and (b) A2 climate change scenarios assuming full migration within protected areas. Lines indicate the steady state (no gain or loss in species richness).
4. Discussion
In this study, we presented a comprehensive analysis of the potential effects of climate change on Mediterranean mammals. Our results should be considered with particular attention to three main limitations of the approach we adopted [34,54–56]. First, we focused on climatic variables only, even though climate is only one of the many determinants of species distribution [34,57–59]. However, considering the large spatial scale of our analysis, species distribution, even for mammals, is likely to be dominantly determined by climate [29,60,61]. Moreover, the direct effect of climate, especially rainfall, on mammal distribution and biology has been widely documented for a number of species and study areas [31,62,63], and Kearney et al. [64] clearly demonstrated, although for a single species limited to Australia, that physiologically based mechanistic models and correlative distribution models may provide congruent forecasts under climate change, further supporting the approach we adopted. Finally, the Mediterranean basin is going to be an important hotspot for climate change [20], with significant climate shifts being projected by 2100 [22–24]. In addition, human pressure is already stressing many mammal species, possibly enhancing the already negative influence that climate change may have.
Second, we did not consider any evolutionary and/or phenological responses to climate change [27]. Réale et al. [31] demonstrated that the changes in breeding dates as a function of climate change for a population of Red Squirrel in the Arctic was for 62 per cent a result of phenotypic plasticity and for 13 per cent a result of genetic change. More examples are reviewed in Parmesan [27], but despite the existence of data supporting the idea of local adaptations to climate change evolving at specific sites, the fossil record shows only little evidence for an evolutionary response. During Pleistocene glaciations, for example, flora and fauna experienced climate changes 5–10 times stronger than the magnitude of the twentieth century global warming, and the main response was the shift of geographical distributions to track the changing climate [27].
A final limitation is related to the assumptions about the dispersal capacity of species through the Mediterranean basin, an assumption that will strongly influence the possibility to track climate change. The possible consequences for ecological communities are likely to be unpredictable [65] but the current knowledge is so poor that it is difficult to get a reliable estimate of potential dispersal distance for the species we considered. Assuming two extreme scenarios (no dispersal versus full dispersal) is debatable but at least covers the full range of possible outcomes. The available literature, however, leans more towards a limited-dispersal scenario for the Mediterranean basin. In fact, all projection of future land use and human pressure on the Mediterranean basin (e.g. [18,19]) predict a landscape with less and less natural areas and with an increased human pressure, making future habitat connectivity an important issue and questioning the effective possibility of species to track the changing climate.
We considered in our analysis only species whose distribution model gave an AUC score greater than 0.7, and we actually obtained AUC scores greater than 0.79 (average of 0.96) for our models. The lower AUC values were obtained for species only marginally present in the Mediterranean, such as Gerbillus tarabuli (Tarabul's Gerbil) with only 10 per cent of the distribution range inside the study area, or for species with particular habitat requirements, such as Lutra lutra (European Otter).
We produced our models at a fine spatial resolution (1 km2) to avoid the problem of hidden local refugia, one of the most important issues in climate change projections based on correlative distribution models [66]. Moreover, we developed our models considering an ensemble modelling framework, and accounted for uncertainty in both statistical models and climate changes models [67]. However, it should be recognized that the location errors associated with the available points of presence were often larger than the 1 km2 resolution that we adopted. Although we explicitly accounted for this factor, we cannot avoid possible negative effects in the analyses originating from these positional errors, which possibly result in smoothing the response to climate that we had measured for all species using SDMs.
Of the 181 species that we considered, only the Lowland Shrew (S. arunchi; but see [68] for the taxonomic validity of the species) was committed to extinction considering the final ensemble forecasts, whatever scenario was considered for dispersal (no-dispersal versus full dispersal). However, the variability between the different climate change models was large (see electronic supplementary material, figure S1d,e for a representation of the uncertainty in estimates of species richness), with the number of species predicted to become extinct (i.e. Threat-1) under one or more climate change model, but not under the final ensemble forecast. Overall, the uncertainty linked to the different climate change models that we considered is important for all scenarios, with the majority of species being projected into more than one threat level (90.6% under A2 no-dispersal; 85.6% under A2 unlimited dispersal; 74.4% under B1 no-dispersal; 71.1% under B1 unlimited dispersal) and many species spanning at least three different threat levels (43.3% under A2 no-dispersal; 22.8% under A2 unlimited dispersal; 5.6% under B1 no-dispersal; 2.8% under B1 unlimited dispersal). The species Mesocricetus auratus provides an extreme example of this variability: under the B1, unlimited dispersal scenario, the species was predicted to lose more than 80 per cent of its climatically suitable area according to the MK35 climate change model, more than 50 per cent under the PCM1 model, while under the CM21 model its climatically suitable range was predicted to increase. These results confirm what has already been found in previous analyses (e.g. [67,69]) and further stress the importance of adopting a full ensemble forecasting approach in studying the effects of climate change on species distribution [70].
Overall, a substantial number of Mediterranean mammals will be severely threatened by future climate change (electronic supplementary material, table S2), particularly endemic species. Moreover, additional drivers could enhance the risk of species extinction or at least could increase further the uncertainty linked to our results (e.g. parasites, fire frequency, diseases; [71]). Particularly important is the lack of consideration for the effects of land use and other types of human pressure. Currently, only 5 per cent of the original natural vegetation is remaining and, while in the northern part of the basin land use changes are going towards a more natural landscape (e.g. [14]), in the Middle East, as well as across North Africa, land use change and human pressure are intensifying, with potentially important and negative effects on biodiversity. This is obviously going to exacerbate the effects of climate change, at least for some species, particularly considering the almost complete absence of PAs in the African part of the Mediterranean basin.
Our models also predict that a number of species will gain climatically suitable areas as a result of climate change (electronic supplementary material, table S2). Although this is possible for many species, a number of factors may actually influence these results. For example, a species may not be able to colonize all climatically suitable areas because they may be limited by dispersal capabilities, by behavioural patterns or biotic interactions, by habitat fragmentation and natural barriers, or by the absence of environmentally suitable habitats. This may be the case of species inhabiting the Mediterranean peninsulas, who will have difficulty moving northwards owing to east–west-oriented mountain ranges such as the Pyrenees and the Alps. The same is true for species inhabiting Mediterranean islands (among which there are many endemics), which will face even greater barriers, making it basically impossible for them to benefit from gains in climatic suitability.
We found important changes in potential species richness owing to climate change (but see Bartolino et al. [72] for a discussion on the threshold approach that we used to identify hotspots). As a result of shifts in species ranges, mostly towards higher elevations but also northward, the areas that will gain species are predicted to concentrate around mountainous regions (e.g. central Italy), as already predicted by other studies (e.g. [33]; but see Thuiller et al. [37] for the opposite result). In the European part of the study area, our models predict species losses from peninsulas and islands, with the main hotspots of species loss being concentrated in the western Mediterranean basin (mostly Spain and North Africa). Species turnover will be higher under the unlimited dispersal assumption (and especially for the A2 climate scenario), a result that was clearly expected given the complete lack of movement constraints. The areas with the highest turnover rates (figure 3) basically correspond to the hotspots of species loss (European peninsulas and islands, and northern African mountain ranges). Obviously, these estimates should be considered carefully as it is possible that species from regions around the Mediterranean might be able to immigrate to these newly available areas.
The existing PAs would be strongly influenced by climate change according to our results. Basically all PAs in Africa, in the Middle East and in Spain would lose a substantial amount of their current mammalian species diversity, with significant losses that are not compensated by species influxes. Overall, also those PAs that would gain species or remain stable in central Italy and southern France should experience a substantial shift in species composition of a magnitude unprecedented in recent geological times [32], with potentially important consequences because of changes in the pattern of interspecific interactions [73]. These conclusions are based on the assumption that species will be able to track shifts in habitats/climate with no lagged response. These assumptions are debatable in general [74], although rapid range adjustments are not entirely improbable for mammals [32]. Moreover, climate warming is likely to result in changes in species phenology, which can further disrupt species associations [27]. The outcome of such new species assemblages may be particularly difficult to predict, for example because of nonlinearities that may emerge.
Managing natural systems in the face of such changes is a challenging task, especially as decisions need to be taken using limited and highly uncertain projections of future climate impacts [75]. However, a few indications are possible given the results of our analyses for Mediterranean mammals. In particular, it is clear that the adopted conservation strategy should follow different paths in the European and in the African/Asian parts of the Mediterranean. In the Mediterranean Europe, the number and extent of PAs make it almost impossible to propose expanding the existing networks. However, new strategies could be considered. First of all, it seems important to avoid non-climate-related threats, like invasive species, further habitat loss and increased fragmentation, overharvest, etc. [75]. Moreover, we suggest using tools such as the Natura 2000 network to increase habitat connectivity between PAs [40]. Finally, we also propose considering adaptive management strategies both inside and outside existing reserves as a critical step forward [76].
The PA system in northern Africa is completely different. Here, the PA network is limited to few small and mostly spatial disconnected areas. Establishing new PAs is the first and most important need. Particular attention should be given to the inclusion of high habitat heterogeneity and, possibly, on large reserves across biotic transition zones that would allow for the continued protection under ongoing climate change [75]. Furthermore, a focus on the socio-economic components (i.e. increasing socio-economic conditions over a simple subsistence level, introducing eco-tourism, enforcing conservation measures, etc.) would represent a clear step forward to ensure long-term conservation success.
5. Conclusion
Our results suggest that the effects of climate change on mammal species distributions and communities they form may not be a drastic loss of species from their current distribution, but a change in community structure. The results of this study should be considered as a first approximation but the main pattern that we obtained should remain stable even considering possible improvements (e.g. better presence/absence data [77]; mechanistic models [57]). Species richness is predicted to shift and the extinction risk for many species is going to increase. In this context, the current network of PAs is not expected to effectively conserve existing species, as most PAs will potentially lose species according to our analyses. Expanding the network (e.g. more PAs are urgently needed in northern Africa) and complementing it with other strategies should be urgently considered. In particular, we suggest a stronger emphasis on off-reserve conservation, explicitly considering connectivity among PAs, as well as management and mitigation actions, among which going back to the traditional agricultural and husbandry practices may represent an important first step.
Acknowledgements
L.M., A.G., N.E.Z., A.P. and J.P. were funded under the FP6 EU-funded ECOCHANGE project (Challenges in assessing and forecasting biodiversity and ecosystem changes in Europe; number 066866 GOCE). David M. Theobald and three anonymous referees provided helpful comments on a previous version of the manuscript.
References
- 1.Blondel J., Aronson J., Bodiou J. Y., Boeuf G. 2010. The Mediterranean region: biological diversity in space and time, 2nd edn. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Médail F., Quézel P. 1999. Biodiversity hotspots in the Mediterranean basin: setting global conservation priorities. Conserv. Biol. 13, 1510–1513 10.1046/j.1523-1739.1999.98467.x (doi:10.1046/j.1523-1739.1999.98467.x) [DOI] [Google Scholar]
- 3.Myers N., Mittermeier R. A., Mittermeier C. G., da Fonseca G. A. B., Kents J. 2000. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 10.1038/35002501 (doi:10.1038/35002501) [DOI] [PubMed] [Google Scholar]
- 4.Blondel J., Catzefilis F., Perret P. 1996. Molecular phylogeny and historical biogeography of the warblers of the genus Sylvia (Aves). J. Evol. Biol. 9, 871–891 10.1046/j.1420-9101.1996.9060871.x (doi:10.1046/j.1420-9101.1996.9060871.x) [DOI] [Google Scholar]
- 5.Taberlet P., Cheddadi R. 2002. Quaternary refugia and persistence of biodiversity. Science 297, 2009–2010 10.1126/science.297.5589.2009 (doi:10.1126/science.297.5589.2009) [DOI] [PubMed] [Google Scholar]
- 6.Thompson J. D. 2005. Plant evolution in the Mediterranean. Oxford, UK: Oxford University Press [Google Scholar]
- 7.Blondel J. 2007. Copying with habitat heterogeneity: the story of the Mediterranean blue tits. J. Ornithol. 148, S3–S15 10.1007/s10336-007-0161-1 (doi:10.1007/s10336-007-0161-1) [DOI] [Google Scholar]
- 8.Médail F., Diadema K. 2009. Glacial refugia influence plant diversity patterns in the Mediterranean basin. J. Biogeogr. 36, 1333–1345 10.1111/j.1365-2699.2008.02051.x (doi:10.1111/j.1365-2699.2008.02051.x) [DOI] [Google Scholar]
- 9.di Castri F., Goodall W., Specht R. L. (eds) 1981. Ecosystems of the world II: Mediterranean-type shrublands. Amsterdam, The Netherlands: Elsevier Scientific Publishing [Google Scholar]
- 10.Butzer K. W. 2005. Environmental history in the Mediterranean world: cross-disciplinary investigation of cause-and-effect for degradation and soil history. J. Archaeol. Sci. 32, 1773–1800 10.1016/j.jas.2005.06.001 (doi:10.1016/j.jas.2005.06.001) [DOI] [Google Scholar]
- 11.Blondel J. 2006. Man as designer of Mediterranean landscapes: a millennium story of humans and ecological systems during the historic period. Hum. Ecol. 34, 713–729 10.1007/s10745-006-9030-4 (doi:10.1007/s10745-006-9030-4) [DOI] [Google Scholar]
- 12.Hoekstra J. M., Boucher T. M., Ricketts T. H., Roberts C. 2005. Confronting a biome crisis: global disparities of habitat loss and protection. Ecol. Lett. 8, 23–29 10.1111/j.1461-0248.2004.00686.x (doi:10.1111/j.1461-0248.2004.00686.x) [DOI] [Google Scholar]
- 13.Debussche M., Lepart J., Dervieux A. 1999. Mediterranean landscape changes: evidence from old postcards. Glob. Ecol. Biogeogr. 8, 3–15 10.1046/j.1365-2699.1999.00316.x (doi:10.1046/j.1365-2699.1999.00316.x) [DOI] [Google Scholar]
- 14.Falcucci A., Maiorano L., Boitani L. 2007. Changes in land-use/land-cover patterns in Italy and their implications for biodiversity conservation. Landscape Ecol. 22, 617–631 10.1007/s10980-006-9056-4 (doi:10.1007/s10980-006-9056-4) [DOI] [Google Scholar]
- 15.Sala O. E., et al. 2000. Global biodiversity scenarios for the year 2100. Science 287, 1770–1774 10.1126/science.287.5459.1770 (doi:10.1126/science.287.5459.1770) [DOI] [PubMed] [Google Scholar]
- 16.Falcucci A., Maiorano L., Ciucci P., Garton E. O., Boitani L. 2008. Land-cover change and the future of the Apennine brown bear: a perspective from the past. J. Mammal. 89, 1502–1511 10.1644/07-MAMM-A-229.1 (doi:10.1644/07-MAMM-A-229.1) [DOI] [Google Scholar]
- 17.Maiorano L., Falcucci A., Boitani L. 2008. Size-dependent resistance of protected areas to land-use change. Proc. R. Soc. B 275, 1297–1304 10.1098/rspb.2007.1756 (doi:10.1098/rspb.2007.1756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blue Plan 2008. The Blue Plan's sustainable development outlook for the Mediterranean. Sophia Antipolis, France: UNEP Blue Plan Activity Centre [Google Scholar]
- 19.De Stefano D. 2004. Freshwater and tourism in the Mediterranean. Rome, Italy: WWF Mediterranean Programme [Google Scholar]
- 20.Giorgi F. 2006. Climate change hot-spots. Geophys. Res. Lett. 33, L08707. 10.1029/2006GL025734 (doi:10.1029/2006GL025734) [DOI] [Google Scholar]
- 21.Luterbacher J., Dietrich D., Xoplaki E., Grosjean M., Wanner H. 2004. European seasonal and annual temperature variability, trends, and extremes since 1500. Science 303, 1499–1503 10.1126/science.1093877 (doi:10.1126/science.1093877) [DOI] [PubMed] [Google Scholar]
- 22.Gao X., Giorgi F. 2008. Increased aridity in the Mediterranean region under greenhouse gas forcing estimated from high resolution simulations with a regional climate model. Global Planet. Change 62, 195–209 10.1016/j.gloplacha.2008.02.002 (doi:10.1016/j.gloplacha.2008.02.002) [DOI] [Google Scholar]
- 23.Diffenbaugh N. S., Pal J. S., Giorgi F., Gao X. 2007. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 34, L11706. 10.1029/2007GL030000 (doi:10.1029/2007GL030000) [DOI] [Google Scholar]
- 24.Giorgi F., Lionello P. 2008. Climate change projections for the Mediterranean region. Global Planet. Change 63, 90–104 10.1016/j.gloplacha.2007.09.005 (doi:10.1016/j.gloplacha.2007.09.005) [DOI] [Google Scholar]
- 25.van Oldenborgh G. J., Drijfhout S., van Ulden A., Haarsma R., Sterl A., Severijns C., Hazeleger W., Dijkstra H. 2009. Western Europe is warming much faster than expected. Clim. Past 5, 1–12 10.5194/cp-5-1-2009 (doi:10.5194/cp-5-1-2009) [DOI] [Google Scholar]
- 26.Walther G. R., Post E., Convey P., Menzel A., Parmesan C., Beebee T. J. C., Fromentin J. M., Hoegh-Guldberg O., Bairlein F. 2002. Ecological responses to recent climate change. Nature 416, 389–395 10.1038/416389a (doi:10.1038/416389a) [DOI] [PubMed] [Google Scholar]
- 27.Parmesan C. 2006. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 37, 637–669 10.1146/annurev.ecolsys.37.091305.110100 (doi:10.1146/annurev.ecolsys.37.091305.110100) [DOI] [Google Scholar]
- 28.Pounds J. A., et al. 2006. Widespread amphibian extinctions from epidemic disease driven by global warming. Nature 439, 161–167 10.1038/nature04246 (doi:10.1038/nature04246) [DOI] [PubMed] [Google Scholar]
- 29.Humpries M. M., Thomas D. W., Speakman J. R. 2002. Climate-mediated energetic constraints on the distribution of hibernating mammals. Nature 418, 313–316 10.1038/nature00828 (doi:10.1038/nature00828) [DOI] [PubMed] [Google Scholar]
- 30.Burns C. E., Johnston K. M., Schmitz O. J. 2003. Global climate change and mammalian species diversity in U.S. national parks. Proc. Natl Acad. Sci. USA 100, 11 474–11 477 10.1073/pnas.1635115100 (doi:10.1073/pnas.1635115100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Réale D., McAdam A. G., Boutin S., Berteaux D. 2003. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. Lond. B 270, 591–596 10.1098/rspb.2002.2224 (doi:10.1098/rspb.2002.2224) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thuiller W., Broennimann O., Hughes G., Alkemade J. R. M., Midgley G. F., Corsi F. 2006. Vulnerability of African mammals to anthropogenic climate change under conservative land transformation assumptions. Glob. Change Biol. 12, 424–440 10.1111/j.1365-2486.2006.01115.x (doi:10.1111/j.1365-2486.2006.01115.x) [DOI] [Google Scholar]
- 33.Levinsky I., Skov F., Svenning J. C., Rahbek C. 2007. Potential impacts of climate change on the distribution and diversity patterns of European mammals. Biodivers. Conserv. 16, 3803–3816 10.1007/s10531-007-9181-7 (doi:10.1007/s10531-007-9181-7) [DOI] [Google Scholar]
- 34.Guisan A., Thuiller W. 2005. Predicting species distribution: offering more than simple habitat models. Ecol. Lett. 8, 993–1009 10.1111/j.1461-0248.2005.00792.x (doi:10.1111/j.1461-0248.2005.00792.x) [DOI] [PubMed] [Google Scholar]
- 35.Thuiller W., Lafourcade B., Engler R., Araujo M. B. 2009. BIOMOD: a platform for ensemble forecasting of species distributions. Ecography 32, 369–373 10.1111/j.1600-0587.2008.05742.x (doi:10.1111/j.1600-0587.2008.05742.x) [DOI] [Google Scholar]
- 36.Thomas C. D., et al. 2004. Extinction risk from climate change. Nature 427, 145–148 10.1038/nature02121 (doi:10.1038/nature02121) [DOI] [PubMed] [Google Scholar]
- 37.Thuiller W., Lavorel S., Araùjo M. B., Sykes M. T., Prentice I. C. 2005. Climate change threats to plant diversity in Europe. Proc. Natl Acad. Sci. USA 102, 8245–8250 10.1073/pnas.0409902102 (doi:10.1073/pnas.0409902102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schipper J., et al. 2008. The status of the world's land and marine mammals: diversity, threat, and knowledge. Science 322, 225–230 10.1126/science.1165115 (doi:10.1126/science.1165115) [DOI] [PubMed] [Google Scholar]
- 39.IUCN 2010. The IUCN Red List of threatened species, version 2010.4. See www.iucnredlist.org
- 40.Maiorano L., Falcucci A., Garton E. O., Boitani L. 2007. Contribution of the Natura 2000 network to biodiversity conservation in Italy. Conserv. Biol. 21, 1433–1444 10.1111/j.1523-1739.2007.00831.x (doi:10.1111/j.1523-1739.2007.00831.x) [DOI] [PubMed] [Google Scholar]
- 41.Boitani L., Sinibaldi I., Corsi F., De Biase A., D'Inzillo Carranza I., Ravagli M., Reggiani G., Rondinini C., Trapanese P. 2008. Distribution of medium- to large-sized African mammals based on habitat suitability models. Biodivers. Conserv. 17, 605–621 10.1007/s10531-007-9285-0 (doi:10.1007/s10531-007-9285-0) [DOI] [Google Scholar]
- 42.Puddu G., Maiorano L., Falcucci A., Corsi F., Boitani L. 2009. Spatial explicit assessment of current and future conservation options for the endangered Corsican Red Deer (Cervus elaphus corsicanus) in Sardinia. Biodivers. Conserv. 18, 2001–2016 10.1007/s10531-008-9569-z (doi:10.1007/s10531-008-9569-z) [DOI] [Google Scholar]
- 43.Maiorano L., et al. In preparation Fine scale diversity and endemism hotspots for terrestrial vertebrates in Europe.
- 44.Hijmans R. J., Cameron S. E., Parra J. L., Jones P. G., Jarvis A. 2005. Very high resolution interpolated climate surfaces for global land areas. Int. J. Climatol. 25, 1965–1978 10.1002/joc.1276 (doi:10.1002/joc.1276) [DOI] [Google Scholar]
- 45.Turc L. 1963. Evaluation des besoins en eau d'irrigation, évapotranspiration potentielle, formulation simplifié et mise à jour. Ann. Agron. 12, 13–49 [Google Scholar]
- 46.Kumar L., Skidmore A. K., Knowles E. 1997. Modelling topographic variation in solar radiation in a GIS environment. Int. J. Geogr. Inf. Sci. 11, 475–497 10.1080/136588197242266 (doi:10.1080/136588197242266) [DOI] [Google Scholar]
- 47.Zimmermann N. E., Yoccoz N. G., Edwards T. C., Meier E. S., Thuiller W., Guisan A., Schmatz D. R., Pearman P. B. 2009. Climatic extremes improve predictions of spatial patterns of tree species. Proc. Natl Acad. Sci. USA 106, 19 723–19 728 10.1073/pnas.0901643106 (doi:10.1073/pnas.0901643106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.IPCC 2007. Climate change 2007: synthesis report. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Geneva: IPCC [Google Scholar]
- 49.Anonymous 2001. Climate change 2001. The intergovernmental panel on climate change. Cambridge, UK: Cambridge University Press [Google Scholar]
- 50.Swets J. A. 1988. Measuring the accuracy of diagnostic systems. Science 240, 1285–1293 10.1126/science.3287615 (doi:10.1126/science.3287615) [DOI] [PubMed] [Google Scholar]
- 51.Fielding A. H., Bell J. F. 1997. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 24, 38–49 10.1017/S0376892997000088 (doi:10.1017/S0376892997000088) [DOI] [Google Scholar]
- 52.IUCN 2001. IUCN Red List Categories and Criteria, version 3.1. Gland, Switzerland: IUCN [Google Scholar]
- 53.Akçakaya H. B., Butchart S. H. M., Mace G. M., Stuart S. N., Hilton-Taylor C. 2006. Use and misuse of the IUCN Red List Criteria in projecting climate change impacts on biodiversity. Glob. Change Biol. 12, 2037–2043 10.1111/j.1365-2486.2006.01253.x (doi:10.1111/j.1365-2486.2006.01253.x) [DOI] [Google Scholar]
- 54.Pearson R. G., Dawson T. P. 2003. Predicting the impacts of climate change on the distribution of species: are bioclimatic envelope models useful? Global Ecol. Biogeogr. 12, 361–372 10.1046/j.1466-822X.2003.00042.x (doi:10.1046/j.1466-822X.2003.00042.x) [DOI] [Google Scholar]
- 55.Hampe A. 2004. Bioclimate envelope models: what they detect and what they hide. Global Ecol. Biogeogr. 13, 469–476 10.1111/j.1466-822X.2004.00090.x (doi:10.1111/j.1466-822X.2004.00090.x) [DOI] [Google Scholar]
- 56.Thuiller W. 2004. Patterns and uncertainties of species' range shifts under global change. Glob. Change Biol. 10, 2020–2027 10.1111/j.1365-2486.2004.00859.x (doi:10.1111/j.1365-2486.2004.00859.x) [DOI] [Google Scholar]
- 57.Kearney M. R., Porter W. P. 2009. Mechanistic niche modelling: combining physiological and spatial data to predict species' ranges. Ecol. Lett. 12, 334–350 10.1111/j.1461-0248.2008.01277.x (doi:10.1111/j.1461-0248.2008.01277.x) [DOI] [PubMed] [Google Scholar]
- 58.Visconti P., et al. 2011. Future hotspots of terrestrial mammal loss. Phil. Trans. R. Soc. B 366, 2693–2702 10.1098/rstb.2011.0105 (doi:10.1098/rstb.2011.0105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rondinini C., et al. 2011. Global habitat suitability models of terrestrial mammals. Phil. Trans. R. Soc. B 366, 2633–2641 10.1098/rstb.2011.0113 (doi:10.1098/rstb.2011.0113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fischer J., Lindenmayer D. B., Nix H. A., Stein J. L., Stein J. A. 2001. Climate and animal distribution: a climatic analysis of the Australian marsupial, Trichosurus caninus. J. Biogeogr. 28, 293–304 10.1046/j.1365-2699.2001.00554.x (doi:10.1046/j.1365-2699.2001.00554.x) [DOI] [Google Scholar]
- 61.Thiam M., Bâ K., Duplantier J. M. 2008. Impacts of climatic changes on small mammal communities in the Sahel (West Africa) as evidenced by owl pellet analysis. Afr. Zool. 43, 135–143 10.3377/1562-7020-43.2.135 (doi:10.3377/1562-7020-43.2.135) [DOI] [Google Scholar]
- 62.Owen-Smith N. 1990. Demography of a large herbivore, the greater kudu, in relation to rainfall. J. Anim. Ecol. 59, 893–913 10.2307/5021 (doi:10.2307/5021) [DOI] [Google Scholar]
- 63.Mills M. G. L., Biggs H. C., Whyte I. J. 1995. The relationship between rainfall, lion predation and population trends in African herbivores. Wildlife Res. 22, 75–88 10.1071/WR9950075 (doi:10.1071/WR9950075) [DOI] [Google Scholar]
- 64.Kearney M. R., Wintle B. A., Porter W. P. 2010. Correlative and mechanistic models of species distribution provide congruent forecasts under climate change. Conserv. Lett. 3, 203–213 10.1111/j.1755-263X.2010.00097.x (doi:10.1111/j.1755-263X.2010.00097.x) [DOI] [Google Scholar]
- 65.Higgins S. I., Clark J. S., Nathan R., Hovestadt T., Schurr F., Fragoso J. M. V., Aguiar M. R., Ribbens E., Lavorel S. 2003. Forecasting plant migration rates: managing uncertainty for risk assessment. J. Ecol. 91, 341–347 10.1046/j.1365-2745.2003.00781.x (doi:10.1046/j.1365-2745.2003.00781.x) [DOI] [Google Scholar]
- 66.Randin C. F., Engler R., Normand S., Zappa M., Zimmermann N. E., Pearman P. B., Vittoz P., Thuiller W., Guisan A. 2009. Climate change and plant distribution: local models predict high-elevation persistence. Glob. Change Biol. 15, 1557–1569 10.1111/j.1365-2486.2008.01766.x (doi:10.1111/j.1365-2486.2008.01766.x) [DOI] [Google Scholar]
- 67.Buisson L., Thuiller W., Casajus N., Lek S., Grenouillet G. 2010. Uncertainty in ensemble forecasting of species distribution. Glob. Change Biol. 16, 114–1157 10.1111/j.1365-2486.2009.02000.x (doi:10.1111/j.1365-2486.2009.02000.x) [DOI] [Google Scholar]
- 68.Nappi A., Aloise G. 2008. Sorex arunchi Lapini & Testone, 1998. In Fauna d'Italia: Mammalia II. Erinaceomorpha, Soricomorpha, Lagomorpha, Rodentia (eds Amori G., Contoli L., Nappi A.), pp. 153–156 Milano, Italy: Edizioni Calderini [Google Scholar]
- 69.Thuiller W., Araújo M. B., Pearson R. G., Whittaker R. J., Brotons L., Lavorel S. 2004. Uncertainty in predictions of extinction risk. Nature 430, 34. 10.1038/nature02716 (doi:10.1038/nature02716) [DOI] [PubMed] [Google Scholar]
- 70.Araújo M. B., New M. 2006. Ensemble forecasting of species distribution. Trends Ecol. Evol. 22, 42–47 10.1016/j.tree.2006.09.010 (doi:10.1016/j.tree.2006.09.010) [DOI] [PubMed] [Google Scholar]
- 71.Hoffmann M., Belant J. L., Chanson J. S., Cox N. A., Lamoreux J., Rodrigues A. S. L., Schipper J., Stuart S. N. 2011. The changing fates of the world's mammals. Phil. Trans. R. Soc. B 366, 2598–2610 10.1098/rstb.2011.0116 (doi:10.1098/rstb.2011.0116) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bartolino V., Maiorano L., Colloca F. 2010. A frequency distribution approach to hotspots identification. Popul. Ecol. 53, 351–359 10.1007/s10144-010-0229-2 (doi:10.1007/s10144-010-0229-2) [DOI] [Google Scholar]
- 73.Stenseth N. C., Mysterud A., Ottersen G., Hurrell J. W., Chan K. S., Lima M. 2002. Ecological effects of climate fluctuations. Science 297, 1292–1296 10.1126/science.1071281 (doi:10.1126/science.1071281) [DOI] [PubMed] [Google Scholar]
- 74.Pimm S. L. 2001. Entrepreneurial insects. Nature 411, 531–532 10.1038/35079206 (doi:10.1038/35079206) [DOI] [PubMed] [Google Scholar]
- 75.Lawler J. J. 2009. Climate change adaptation strategies for resource management and conservation planning. Ann. NY Acad. Sci. 1162, 78–98 10.1111/j.1749-6632.2009.04147.x (doi:10.1111/j.1749-6632.2009.04147.x) [DOI] [PubMed] [Google Scholar]
- 76.Millar C. I., Stephenson N. L., Stephens S. L. 2007. Climate change and forests of the future: managing on the face of uncertainty. Ecol. Appl. 17, 2145–2151 10.1890/06-1715.1 (doi:10.1890/06-1715.1) [DOI] [PubMed] [Google Scholar]
- 77.Boitani L., Maiorano L., Baisero D., Falcucci A., Visconti P., Rondinini C. 2011. What spatial data do we need to develop global mammal conservation strategies? Phil. Trans. R. Soc. B 366, 2623–2632 10.1098/rstb.2011.0117 (doi:10.1098/rstb.2011.0117) [DOI] [PMC free article] [PubMed] [Google Scholar]