Abstract
Objective
Most cost and cost-effectiveness studies of substance abuse treatments focus on the costs to the provider/payer. Although this perspective is important, the costs incurred by patients should also be considered when evaluating treatment. This paper presents estimates of patients’ costs associated with the COMBINE alcohol treatments and evaluates the treatments’ cost-effectiveness from the patient perspective.
Study Design
A prospective cost-effectiveness study of patients in COMBINE, a randomized controlled clinical trial of 9 alternative alcohol treatment regimens involving 1,383 patients with diagnoses of primary alcohol dependence across 11 U.S. clinic sites. We followed a micro-costing approach that allowed estimation of patients’ costs for specific COMBINE treatment activities. The primary clinical outcomes from COMBINE are used as indicators of treatment effectiveness.
Results
The average total patient time devoted to treatment ranged from about 30 hours to 46 hours. Time spent traveling to and from treatment sessions and participation in self-help meetings accounted for the largest portion of patient time costs. The cost-effectiveness results indicate that 6 of the 9 treatments were economically dominated and only 3 treatments are potentially cost-effective depending on patient’s willingness to pay for the considered outcomes: medical management + placebo, medical management + naltrexone, and medical management + naltrexone + acamprosate.
Conclusions
Few studies consider the patient’s perspective in estimating costs and cost-effectiveness even though these costs may have a substantial impact on a patient’s treatment choice, ability to access treatment, or treatment adherence. For this study, the choice of the most cost-effective treatment depends on the value placed on the outcomes by the patient, and the conclusions drawn by the patient may differ from that of the provider/payer.
INTRODUCTION
Most cost and cost-effectiveness studies of substance use treatment focus on the provider’s/payer’s costs. Although this perspective is important (especially for government policy makers and third-party payers such as private health insurance), the costs incurred by patients should also be considered when evaluating alternative treatments to ensure that these options are cost-effective for patients as well as for providers. Treatment costs may be substantial to the patient and may affect a patient’s ability to seek treatment or to fully complete a treatment regimen. Several studies have examined the factors associated with an individual’s decision to seek alcohol treatment (e.g., 1–3), and among these factors are the perceived costs and benefits of the treatment.1, 4
Unfortunately, collecting patient cost data can be challenging, and to date few economic evaluations of health care have done so. A small body of literature examining cancer care and screening have found that patient costs significantly contribute to the overall costs of care (e.g., 5–8). To our knowledge, no study has examined the cost-effectiveness of substance use treatment from the patient perspective, and only one study examines patients’ costs for substance use treatment. Salome and colleagues9 found that patient time costs for substance abuse treatment were substantial (greater than 75% of the total patient costs), with out-of-pocket expenses such as travel costs and out-of-pocket fees accounting for the remainder.
The Combined Pharmacotherapies and Behavioral Interventions (COMBINE) study of 9 alcohol treatments provides a unique opportunity to estimate patient costs. The COMBINE study not only collected data on provider/payer costs but also economic data for patients including wage data and time spent participating in treatment. We use these data combined with other information collected as part of the larger study to estimate patient costs and to evaluate the cost-effectiveness of the 9 COMBINE treatments from the patient perspective.
COMBINE Study Design
The COMBINE study design has been described previously (e.g., 10, 11, 12). Briefly, COMBINE randomly assigned 1,383 eligible alcohol-dependent individuals to 1 of 9 treatments for 16 weeks of outpatient treatment. Participants in 8 of these treatments received up to 9 sessions of medical management (MM) delivered by medically trained providers that focused on improving medication adherence and alcohol abstinence. Patients in these treatments received either naltrexone (active or placebo), acamprosate (active or placebo), or both naltrexone and acamprosate. Participants in 4 of the 8 treatments also received up to 20 sessions of more intensive cognitive behavioral therapy (combined behavioral intervention [CBI]) delivered by alcoholism treatment specialists. A ninth treatment group received CBI only with no medication or MM sessions. In addition, participants across all treatments were encouraged to attend local self-help recovery meetings (e.g., Alcoholics Anonymous).
Participants underwent assessments at baseline, at each MM session, and at weeks 8 and 16. MM sessions consisted primarily of biological assessments and discussions of alcohol effects on the body and information about how the medications work. Assessments at baseline and at weeks 8 and 16 included more in-depth data collection including patient surveys that collected data on patient’s time spent traveling to/from treatment sessions, wage information, and information about self-help meeting attendance.
METHODS
Cost Analysis
To compute patients’ costs associated with the COMBINE treatments, we followed the micro-costing approach used by Zarkin et al.13 in estimating COMBINE providers’/payers’ costs. We estimated each patient’s treatment cost as the value of the patient’s time spent participating in treatment and self-help recovery programs, including travel to/from the session/meeting site. We also estimated the patient’s out-of-pocket expenses for medication costs (expected prescription co-payments) and out-of-pocket expenses for session visits (expected office visit co-payments). Our approach presents a lower bound of patient costs because it does not include other potential costs such as out-of-pocket transportation expenses (e.g., bus fare) or other expenses (e.g., child care). These additional expenses and their frequency of occurrence were not collected as part of the COMBINE study; however, time typically accounts for the majority of patient costs (e.g., 8, 9), so our time cost estimates should represent the largest share of patient costs.
Patient Time Costs
Because COMBINE was a research study, we wanted to estimate treatment costs that would be incurred in real-world settings (as opposed to those required to implement a clinical trial research protocol). Zarkin et al.14 identified COMBINE activities that would be needed to implement the therapies in clinical practice, and we used this classification in calculating the patient time.
The actual time spent by patients receiving a treatment session (MM and CBI) and the number of sessions received over the 16-week period were collected in the study’s Coordinating Center Data Management System (DMS).14 Time spent in assessments was reported by the project coordinator on the Project Coordinator’s Resource Allocation Worksheet (PC-RAW).14 The PC-RAW captured an estimate of the clinical staff and patient time spent on clinically relevant assessment activities. Because this estimate was not patient-specific we used the median time calculated across clinic sites as the patient’s time receiving the assessment. The number of assessments received by the patient was recorded in the DMS. We used patients’ self-reported data on time spent traveling to/from the clinic site and patient wage data collected in the Form 90 Economic Data (Form 90 ED). Self-reported patient time for self-help program participation (travel and session time) also was collected in the Form 90 ED. The Form 90 ED is a modified version of the Form 90 family of instruments that collects self-reported alcohol use and economic outcome data for alcohol treatment studies (15–18). For the COMBINE study, Form 90 ED was administered to patients at intake and at 8 and 16 weeks post-intake.
We standardized patients’ time costs by using the average hourly wage calculated across all patients in all treatments as the unit price for time. This standardization helped eliminate geographic variation in hourly wages due to cost-of-living differences and it mitigated the effect of wage outliers across treatments. As part of our sensitivity analysis, we estimated costs using each patient’s actual wage as the unit price for time faced by that patient.
For each patient, the patient time cost associated with an activity (e.g., treatment sessions, clinical assessments, self-help meetings) is the product of the time spent in that activity and the average hourly wage rate, which is then summed over all activities. The patient’s travel time cost is the product of the average time reported traveling for each session/meeting across the 3 data collection points (intake, 8 and 16 weeks post-intake) and the average hourly wage. This cost is then multiplied by the number of sessions/meetings that the patient attended. If an MM and CBI session occurred on the same day, travel costs were only applied once for that day. Summing the total activity costs and the total travel costs yields the total patient time cost associated with treatment.
Patient Costs for Medication
For our main analysis, we assumed that patients had prescription drug coverage through private health insurance. We examined the formularies of several large health insurance companies in the United States and we found that, if covered, naltrexone is typically covered in its generic form (lower co-pay) and acamprosate is typically covered as a preferred drug in its brand form of Campral (higher co-pay). Therefore, we assumed a generic co-pay of $11 for a 30-day prescription of naltrexone and a preferred-brand co-pay of $25 for a 30-day prescription of acamprosate. These co-pay amounts were based on average co-pay amounts from the 2005 Kaiser/HRET Employer Health Benefit Survey19 and updated to 2007 dollars using the medical Consumer Price Index (CPI).
As part of our sensitivity analysis, we estimated a scenario in which we assumed that naltrexone and acamprosate have the same generic brand co-pay of $11 per 30-day prescription.
Patient Costs for Session Visits
For our main analysis, we assumed that patients had health insurance coverage for treatment sessions. According to the Insurance Component of the 2005 Medical Expenditure Panel Survey, the average dollar co-pay for a doctor visit is $20 (adjusted to 2007 dollars),20 and we used this amount for each session visit. The total out-of-pocket expense for each patient is the product of the co-payment amount and the number of sessions attended by the patient.
For our sensitivity analysis, we estimated patients’ costs under the assumption that patients had no insurance coverage and must bear the full cost of their treatment. For this scenario, we used the average wholesale price published in Red Book21 for pharmaceutical costs for acamprosate and naltrexone. The average wholesale price is in most cases the manufacturer’s suggested average wholesale price, and it is often higher than the price that large purchasers normally pay.13 The average wholesale price is also the most commonly used price index in pharmaceutical transactions.
The average wholesale prices of acamprosate and naltrexone are $0.74 per 333 mg tab and $4.29 per 50 mg tab, respectively, which for this study translates into a daily cost of $6.67 for acamprosate ($200 for a 30-day supply) and $8.58 for naltrexone ($257 for a 30-day supply). We assumed a charge of $71 per MM session and $105 per CBI session. Our estimates were derived from data on MM and behavioral therapy sessions presented in Dewan22 and updated to 2007 dollars using the medical CPI. These estimates represent the full charge that the patient is billed by the doctor’s office and is usually greater than the actual costs of the care received because it includes fee income.
Effectiveness Measures
Following the COMBINE efficacy study,10 the effectiveness outcomes selected for this analysis are proportion of patients with good clinical outcomesa at 16 weeks,23 proportion of patients that do not return to heavy drinking (defined as ≥ 5 standard drinks per day for men and ≥ 4 standard drinks per day for women) within the 16-week treatment period, and percent days abstinent from alcohol within the 16-week period. The good clinical outcome measure combines alcohol use and alcohol-related problems. Patients may have moderate drinking episodes but still have a good clinical outcome, provided the episodes did not lead to other physical, social, or psychological problems. This measure may be valued by many patients as the best overall reflection of alcohol problem resolution. The dichotomous measure that assesses whether any heavy drinking occurred during the follow-up identifies high severity drinking episodes but does not indicate how often they may have occurred and does not pick up episodes of moderate drinking. This measure may be important to patients who do not have a goal of abstinence but rather want to limit the amount of alcohol they consume at any one sitting. Finally, percent days abstinent provides a continuous measure of drinking frequency during the follow-up but does not differentiate between days with light or moderate drinking and days on which large quantities of alcohol are consumed (i.e., “binges”). Percent days abstinent may be of interest to patients who have a goal of reducing the frequency of drinking.
These outcomes are the same as those analyzed in Zarkin et al.13 and similar to the primary outcomes from Anton et al.10 As in the main findings paper10 and the provider/payer-perspective cost-effectiveness study,13 all outcomes were adjusted for baseline percent days abstinent and clinic site.
Cost-Effectiveness Analysis
The first step in the cost-effectiveness analysis was to rank the treatments in increasing order of mean per-patient cost for each of the effectiveness measures. Incremental cost-effectiveness ratios (ICERij), defined as (Cj-Ci)/(Ej-Ei) where condition j is the next most costly treatment compared to i, were then computed for each treatment relative to the next most costly option after eliminating treatments that are economically dominated by other treatments.25
A treatment is eliminated from the cost-effective choice set through strict dominance if another treatment is less costly and more effective than the eliminated treatment. A treatment is eliminated through extended dominance if it has a greater ICER than a more costly treatment.26 In that case, the cost of achieving a given level of the outcome is lower if the dominated treatment is eliminated. The nondominated treatments that remain comprise the cost-effectiveness frontier (CEF), which represents the maximum attainable effectiveness for a given cost level. ICERs are computed and reported for each treatment on the CEF.
We calculated cost-effectiveness acceptability curves (CEACs) to reflect sampling variability in our analysis. CEACs quantify and graphically represent uncertainty in economic evaluations of health care (e.g., 27–29). CEACs incorporate the inherent variability of the cost and effectiveness estimates and show the probability that a treatment is the cost-effective option compared to the alternatives as a function of the patient’s willingness to pay for the outcome. Willingness to pay refers to the value that a person is willing to pay to achieve a given health outcome.30 We used nonparametric bootstrap methods to calculate CEACs.
RESULTS
As shown in Table 1, the average total patient time ranged from about 30 hours to 46 hours for the 16-week trial. Time spent traveling to/from treatment sessions and self-help program meetings accounted for the largest portion of patient time.
TABLE 1.
Mean Patient Time (in Hours) for 16-Week Treatment (standard errors in parentheses)
| Treatment Arm |
N | Total Patient Time*** |
Total Time in Assessmentsa*** |
Total Time in MM/CBI Sessions |
Total Time in Self-Help Meetings |
Total Travel Timeb*** |
|
|---|---|---|---|---|---|---|---|
| MM | CBI | ||||||
| MM + placebo | 153 | 30.34 (2.60) |
4.47 (0.02) |
3.14 (0.08) |
--- | 8.07 (1.75) |
14.65 (1.12) |
| MM + naltrexone | 154 | 32.60 (3.31) |
4.44 (0.02) |
3.06 (0.10) |
--- | 10.72 (2.25) |
14.39 (1.22) |
| MM + naltrexone + acamprosate | 148 | 31.30 (3.02) |
4.42 (0.02) |
3.00 (0.09) |
--- | 9.31 (1.85) |
14.56 (1.31) |
| CBI only | 155 | 34.85 (3.05) |
3.91 (0.01) |
--- | 8.69 (0.36) |
5.97 (1.71) |
16.28 (1.52) |
| MM + acamprosate | 151 | 33.73 (4.29) |
4.43 (0.02) |
3.07 (0.09) |
--- | 9.38 (2.30) |
16.84 (2.44) |
| MM + placebo + CBI | 156 | 44.64 (2.52) |
4.65 (0.03) |
3.09 (0.08) |
9.02 (0.33) |
7.41 (1.44) |
20.46 (1.36) |
| MM + naltrexone + CBI | 154 | 44.88 (2.82) |
4.65 (0.02) |
3.10 (0.08) |
8.90 (0.30) |
9.15 (1.82) |
19.08 (1.13) |
| MM + naltrexone + acamprosate + CBI | 157 | 43.07 (2.96) |
4.57 (0.03) |
2.90 (0.09) |
8.18 (0.35) |
9.06 (1.84) |
18.35 (1.42) |
| MM + acamprosate + CBI | 151 | 46.38 (3.60) |
4.63 (0.03) |
3.06 (0.09) |
8.56 (0.35) |
9.30 (1.79) |
20.82 (2.07) |
| All Treatmentsc | 1,379 | 38.02 (1.08) |
4.47 (0.01) |
3.05 (0.03) |
8.67 (0.15) |
8.70 (0.62) |
17.28 (0.53) |
Note: MM = medical management; CBI = combined behavioral intervention.
Assessments include assessments at baseline and those vitals/blood alcohol content readings taken at treatment sessions.
Total travel time includes travel to and from MM sessions, CBI sessions, and self-help program meetings.
Mean session times for “All Treatments” are conditional on treatment arms that provided the relevant service.
Mean patient times are statistically significantly different across all treatment arms at the 0.01 level by analysis of variance across treatment arms.
MM + placebob is the least costly treatment from the patient’s perspective ($904 per patient), and MM + acamprosate + CBI is the most costly ($1,592 per patient) (see Table 2). Travel time costs accounted for the largest component of costs across all treatments, and medication prescription co-payments contributed the least to total patient costs.
Table 2.
Mean Patient Costs by Treatment Arm (standard errors in parentheses)
| Treatment Arm | N | Total Cost of Treatment*** |
Cost of Medication (co-pays)a*** |
Cost of Office Visits (co-pays)b*** |
Assessment Time Costc |
Session Time Cost | Self-Help Program Time Cost |
Total Travel Costd*** |
|
|---|---|---|---|---|---|---|---|---|---|
| MM | CBI | ||||||||
| MM + placebo | 153 | $904.47 (64.97) |
— | $151.18 (3.35) |
$110.97 (0.49) |
$78.07 (2.09) |
— | $200.43 (43.34) |
$363.81 (27.82) |
| MM + naltrexone | 154 | $996.50 (83.45) |
$40.29 (1.43) |
$146.73 (3.99) |
$110.28 (0.58) |
$75.85 (2.50) |
— | $266.16 (55.83) |
$357.19 (30.21) |
| MM + naltrexone + acamprosate | 148 | $1,046.21 (75.82) |
$125.30 (4.63) |
$143.87 (4.02) |
$109.82 (0.59) |
$74.39 (2.20) |
— | $231.22 (45.92) |
$361.60 (32.46) |
| CBI only | 155 | $1,060.11 (78.37) |
— | $194.77 (7.77) |
$97.06 (0.33) |
— | $215.83 (9.03) |
$148.32 (42.46) |
$404.13 (37.68) |
| MM + acamprosate | 151 | $1,067.34 (107.93) |
$84.93 (3.04) |
$145.07 (3.96) |
$110.04 (0.58) |
$76.27 (2.28) |
— | $232.83 (57.05) |
$418.20 (60.56) |
| MM + placebo + CBI | 156 | $1,468.32 (66.38) |
— | $359.92 (9.64) |
$115.48 (0.63) |
$76.78 (2.06) |
$224.04 (8.24) |
$184.08 (35.83) |
$508.02 (33.85) |
| MM + naltrexone + CBI | 154 | $1,512.36 (73.67) |
$43.07 (1.36) |
$354.96 (8.74) |
$115.48 (0.62) |
$76.99 (1.91) |
$221.02 (7.46) |
$227.25 (45.23) |
$473.59 (28.04) |
| MM + naltrexone + acamprosate + CBI | 157 | $1,524.79 (79.05) |
$125.37 (4.86) |
$330.17 (10.76) |
$113.56 (0.74) |
$72.02 (2.17) |
$203.08 (8.59) |
$224.94 (45.73) |
$455.65 (35.32) |
| MM + acamprosate + CBI | 151 | $1,592.46 (94.55) |
$95.03 (3.07) |
$346.04 (9.98) |
$115.03 (0.66) |
$75.97 (1.97) |
$212.41 (8.67) |
$230.98 (44.41) |
$517.00 (51.47) |
| All Treatmentse | 1,379 | $1,242.92 (28.32) |
$85.51 (1.80) |
$242.21 (3.60) |
$110.86 (0.24) |
$75.79 (0.76) |
$215.26 (3.76) |
$216.05 (15.49) |
$429.12 (13.21) |
Note: MM = medical management; CBI = combined behavioral intervention.
For our baseline cost estimates, we assumed a co-pay of $11 for a 30-day prescription of naltrexone and $25 for a 30-day prescription of acamprosate.
For our baseline cost estimates, we assumed a co-pay of $19.77 for MM and CBI office visits.
Assessments include assessments at baseline and vitals/blood alcohol content readings taken at relevant treatment visits.
Total travel cost includes time spent in travel to and from MM sessions, CBI sessions, and self-help program meetings. It excludes out-of-pocket expenses (e.g., fuel expense, bus fare) associated with travel.
Mean costs for “All Treatments” are conditional on treatment arms that provided the relevant service.
Mean patient costs are statistically significantly different across all treatment arms at the 0.01 level by analysis of variance across treatment arms.
Table 3 reports the mean total costs and effectiveness for each outcome followed by the resulting ICER. For all outcomes, MM + placebo is the least costly treatment from the patient perspective and therefore remains on the CEF; however, MM + placebo also has the smallest mean effectiveness for all outcomes. MM + naltrexone (MM+N) and MM + naltrexone + acamprosate (MM+N+A) are more costly but yield greater mean effectiveness and are also on the CEF. All the remaining treatments are strictly economically dominated because they are more costly and have smaller mean effectiveness compared to MM+N and MM+N+A.
Table 3.
Incremental Cost-Effectiveness Ratios
| Proportion with Good Clinical Outcomes | Proportion that Avoid Heavy Drinking | Percent Days Abstinent | |||||
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | (5) | (6) | ||
| Treatment Arm | Mean Cost | Mean Effectiveness |
Incremental CE Ratio (ΔC/ΔE, $)a |
Mean Effectiveness |
Incremental CE Ratio (ΔC/ΔE, $)b |
Mean Effectiveness |
Incremental CE Ratio (ΔC/ΔE, $)c |
| MM + placebo | $904.47 (64.97) |
0.58 (0.04) |
— | 0.26 (0.03) |
— | 73.8 (2.32) |
— |
| MM + naltrexone | $996.50 (83.45) |
0.74 (0.04) |
$575.19 | 0.35 (0.04) |
$1022.56 | 80.0 (2.01) |
$14.84 |
| MM + naltrexone + acamprosate | $1,046.21 (75.82) |
0.78 (0.04) |
$1242.75 | 0.39 (0.04) |
$1242.75 | 80.5 (1.90) |
$99.42 |
| CBI only | $1,060.11 (78.37) |
0.61 (0.04) |
Dominated | 0.24 (0.04) |
Dominated | 66.7 (2.55) |
Dominated |
| MM + acamprosate | $1,067.34 (107.93) |
0.61 (0.04) |
Dominated | 0.33 (0.04) |
Dominated | 75.6 (2.20) |
Dominated |
| MM + placebo + CBI | $1,468.32 (66.38) |
0.71 (0.04) |
Dominated | 0.31 (0.04) |
Dominated | 79.8 (2.03) |
Dominated |
| MM + naltrexone + CBI | $1,512.36 (73.67) |
0.75 (0.04) |
Dominated | 0.34 (0.04) |
Dominated | 75.9 (2.26) |
Dominated |
| MM + naltrexone + acamprosate + CBI | $1,524.79 (79.05) |
0.74 (0.04) |
Dominated | 0.28 (0.04) |
Dominated | 77.6 (2.26) |
Dominated |
| MM + acamprosate + CBI | $1,592.46 (94.55) |
0.75 (0.04) |
Dominated | 0.35 (0.04) |
Dominated | 78.3 (2.05) |
Dominated |
Note: MM = medical management; CBI = combined behavioral intervention.
ICER is dollars ($) per patient achieving a good clinical outcome.
ICER is dollars ($) per patient avoiding a return to heavy drinking.
ICER is dollars ($) per patient for a percentage point increase in percent days abstinent.
The cost-effectiveness results for all 3 outcomes indicate that only MM + placebo, MM+N, and MM+N+A are potentially cost-effective depending on patient’s willingness to pay for the outcomes. The ICER moving from MM + placebo to MM+N is $575 per patient achieving a good clinical outcome, $1,023 per patient avoiding a return to heavy drinking, and $15 per patient for a percentage point increase in percent days abstinent.c The ICER moving from MM+N to MM+N+A is $1,243 per patient achieving a good clinical outcome, $1,243 per patient avoiding a return to heavy drinking, and $99 per patient for a percentage point increase in percent days abstinent.
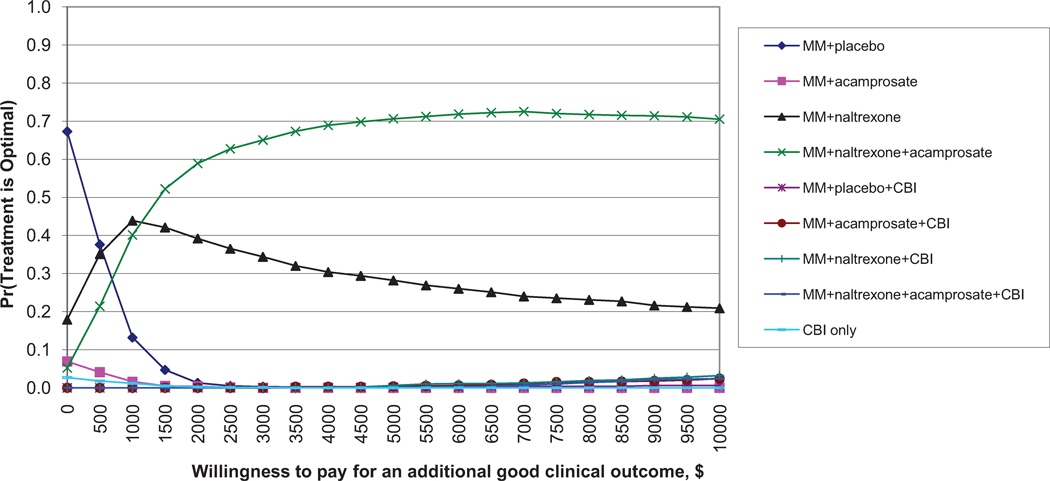
For good clinical outcomes (Figure 1a), MM + placebo has the highest probability of being the most cost-effective for small WTP values (< $500). MM+N has the highest probability of being the most cost-effective for WTP values between about $500 and $1,000. MM+N+A has the highest probability of being the most cost-effective for WTP values greater than $1,000, although this probability does not exceed 75%. All other treatments have extremely low probabilities of being optimal.
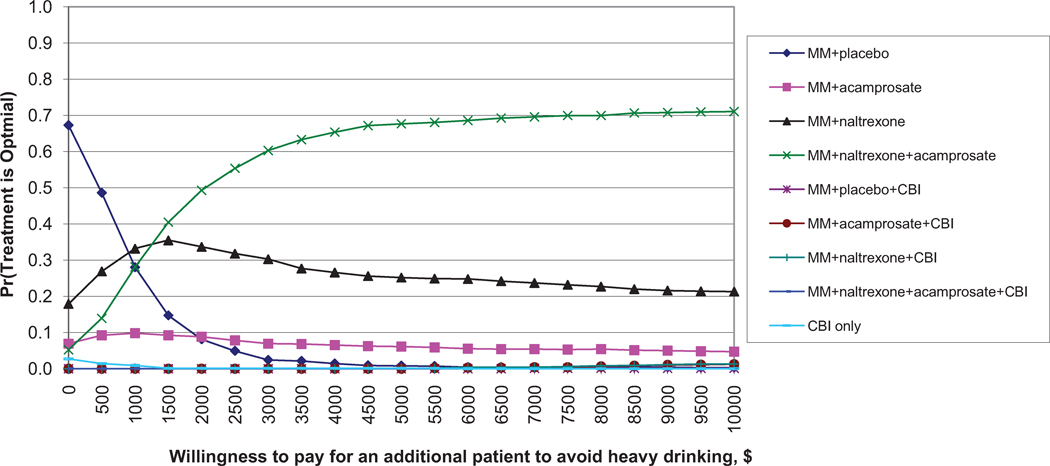
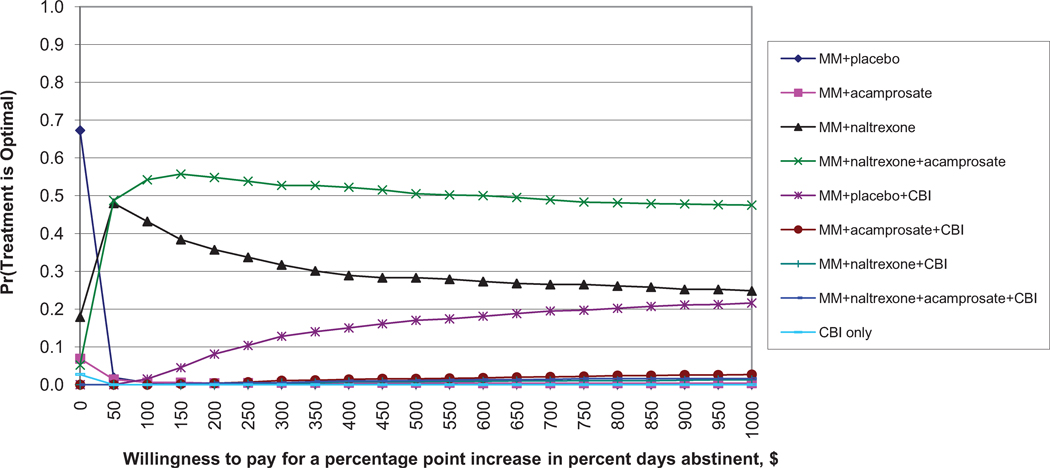
Figure 1.
Similar results are found for avoiding return to heavy drinking (Figure 1b). Again, MM + placebo has the highest probability of being the most cost-effective for small WTP values. MM+N+A has the highest probability of being the most cost-effective for WTP values greater than $1,000. For percent days abstinent (Figure 1c), MM+N+A has the highest probability of being the most cost-effective for WTP values above $50, although this probability never exceeds 60%.
Sensitivity Analysis
For our sensitivity analyses, we considered 2 alternative scenarios regarding patients’ insurance coverage (full results available on request). First, we allowed a generic drug option for acamprosate and naltrexone so that the prescription co-pay is the same for both drugs. Next, we estimated a scenario in which patients do not have any insurance coverage and must bear the full treatment costs. For each of these scenarios, we only examined the effect on costs and the subsequent cost-related effect on the cost-effectiveness ratios. Because we did not actually observe patients under these scenarios, we were unable to collect effectiveness data within each scenario, and therefore we assumed no change in treatment effectiveness.
Under these scenarios, MM + placebo, MM+N, and MM+N+A remain on the CEF as viable options. Compared to the main analysis, our first scenario (generic drug option for both drugs) yields slightly lower costs for treatments that include acamprosate. Because of these lower costs, MM+N+A has the highest probability of being the most cost-effective except at small WTP values. Thus, if patients face similar costs for naltrexone and acamprosate, then it appears that most patients would opt for MM+N+A as the preferred treatment.
Our second scenario (no insurance coverage) yields greater costs for patients compared to the main analysis. MM + placebo (the least costly treatment) has the highest probability of being the most cost-effective treatment at much greater WTP values compared to the main analysis. MM+N and MM+N+A do not have the highest probability of being the most cost-effective treatment except at much greater WTP values. This scenario demonstrates that the costs faced by the patient can greatly affect their conclusions regarding the optimal treatment option.
Finally, we also conducted a sensitivity analysis on our use of the average wage as the unit price for patient time. We estimated each patient’s cost using their reported wage rather than the average wage calculated over all patients. This analysis shows that patient costs and the cost-effectiveness analysis are sensitive to the observed geographic differences and outliers in our patient-reported wages. MM+N replaces MM + placebo as the least costly treatment, and therefore MM + placebo is strictly dominated. MM+N and MM+N+A are the only 2 treatments on the CEF. Given that MM + placebo is not a practical clinical option, the relevant choice set in our analysis does not change for patients.
DISCUSSION
This paper presents the first cost and cost-effectiveness study from the patient perspective of pharmaceutical and behavioral treatments for alcohol dependence. No previous studies of alcohol treatment costs have specifically examined the costs incurred by patients and thus are limited in their ability to inform policy makers about how these treatments may impact patients. Patient costs should not be ignored or considered as trivial because they may affect a patient’s ability to undergo treatment or to fully follow a prescribed treatment plan.
The cost analysis reveals that patients incur significant costs to participate in clinical care and also devote significant time and resources to access care (e.g., travel time) and participate in self-help programs. The cost analysis offers a striking view of the cost associated with self-help program attendance, one that runs counter to conventional wisdom that mutual-help participation is free and readily accessible.
The results of the cost-effectiveness analysis show that only 3 of the 9 COMBINE treatments are cost-effective choices for patients: MM + placebo, MM+N, and MM+N+A. As noted in Zarkin et al.,13 the statistical tests in Anton et al.10 were the clinical study’s prespecified ANOVA-type tests of main effects and interactions. These tests did not find a benefit for acamprosate either as a main effect or in 2- or 3-way interactions. Pairwise comparisons, such as between MM+N and MM+N+A were not examined. In contrast, Zarkin et al.13 and the study presented here performed prespecified pairwise comparisons for the cost-effectiveness analysis to examine each treatment relative to every other treatment. The pairwise comparisons presented in Zarkin et al.13 and here are not formal statistical tests; on efficacy alone, MM+N+A is not significantly better than MM+N.10 However, based on the joint distribution of patient cost and effectiveness, MM+N+A is a potentially cost-effective choice that may be selected by patients under certain circumstances. The patient’s choice between MM+N and the more effective and more costly MM+N+A depends on whether the cost of the incremental increase in effectiveness is worth it to the patient.
Our results show that the provider/payer perspective and the patient perspective may differ. As presented in Zarkin et al.,13 the provider/payer perspective indicates that MM+N has the highest probability of being the most cost-effective treatment for WTP values up to about $7,000 to $7,500 for good clinical outcomes and avoiding heavy drinking. From the patient perspective, MM+N has the highest probability of being the most cost-effective treatment for these 2 outcomes only up to a WTP value just below $1,500. Beyond this WTP value, MM+N+A has the highest probability of being the optimal choice. Between WTP values of $1,500 and $7,000, providers/payers and patients may draw different conclusions regarding the optimal treatment. Thus, this study highlights 2 key issues: (1) provider/payer and patient may have different WTP for a given outcome; and (2) for the same WTP value, the provider/payer and patient may draw different conclusions. As shown here, completely different conclusions about the optimal treatment may be drawn depending on one’s perspective. This finding raises questions as to whether this difference between patient and provider/payer preferences might lead to a less than optimal choice set for patients; that is, would a provider/payer offer a treatment that they conclude is not an optimal choice even if it might be the preferred choice for patients? However, patients do not provide treatment so this difference may always exist. The challenge remains for policy makers to determine policies that help align the preferences of these 2 key decision makers.
Our study has a few limitations that should be noted. Because MM + placebo is not available clinically and MM was not evaluated in the absence of a placebo, we cannot determine the impact of MM alone. Our patient cost analysis relies on patient self-reported data regarding average time spent traveling to and from sessions. As with most self-reported data, these data may suffer from recall bias leading to an under- or overestimation of travel time.31 Furthermore, because this was a research study, patients were required to receive sessions from 1 of 11 clinic sites. In a real-world setting, patients may be able to access treatment providers that are closer to them.
Another limitation is that we only have data on patient time and wages, and we estimated coinsurance co-payments based on national averages. COMBINE trial participants received prescription drugs and treatment sessions free-of-charge, and data were not collected on out-of-pocket expenses that might be incurred in a real-world setting (e.g., coinsurance, travel expenses). Because patient time represents the majority of cost, any estimation error due to our assumptions about insurance coverage should have a small effect on the results.
Furthermore, we do not take into account full patient preferences and the benefits and costs associated with other treatment attributes beyond patients’ time and selected out-of-pocket treatment expenses. For example, patients’ attitudes and preferences may differ regarding medication side effects, aversion to taking medication, or participation in behavioral therapy.
Finally, although we use activities identified as best clinical practice14, the treatment regimen we use in our costing algorithm follows the COMBINE protocol. As noted by Zarkin et al.,13 patients probably are seen more frequently in a clinical trial compared to best clinical practice so in this case we expect our patient cost estimates to represent upper bounds of the actual best practice treatment costs for patients. Furthermore, if patients are required to partially or fully pay for medication and session visits, these costs may inadvertently create an economic barrier to treatment and adversely affect session attendance or adherence to medication regimens. We do not fully observe the relationship between patient behavior and expected out-of-pocket costs within the COMBINE trial, and therefore we cannot test how this relationship might affect patient adherence and overall costs.
Notwithstanding these limitations, our study makes an important contribution to the literature because it provides an economic analysis of the COMBINE therapies from the patient perspective and it provides quantitative evidence of the impact that perspective can have on conclusions about alternative treatment options. For this study, the choice of the economically optimal treatment depends on the value placed on the outcomes by the patient rather than the provider/payer, and as we have shown the conclusions drawn by the patient may differ significantly from that of the provider/payer.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
A good clinical outcome is defined as abstinent or moderate drinking without problems with moderate drinking defined as a maximum of 11 (women) and 14 (men) drinks per week with no more than 2 days on which more than 3 drinks (women) or 4 drinks (men) were consumed; and problems defined as endorsing 3 or more items on a standardized questionnaire24 assessing physical, social, and psychological consequences of drinking.
Although no cost was attributed to the placebo, MM sessions involve an active intervention.
A percentage point increase in percent days abstinent may not be clinically meaningful because 1% represents less than 1 day. Instead, if we consider days as our unit, 1 additional day of abstinence per month (3.3 percentage point increase) would be associated with an ICER moving from MM + placebo to MM+N of approximately $50 ($15 × 3.3).
Contributor Information
Laura J. Dunlap, RTI International, 6110 Executive Blvd., Suite 902, Rockville, MD 20852, Voice: (301) 816–4616, Fax: (301) 230–4647, ljd@rti.org
Gary A. Zarkin, RTI International, 3040 East Cornwallis Road, Post Office Box 12194, Research Triangle Park, NC 27709–2194, Voice: (919) 541–5858, gaz@rti.org
Jeremy W. Bray, RTI International, 3040 East Cornwallis Road, Post Office Box 12194, Research Triangle Park, NC 27709–2194, Voice: (919) 541–7003, brray@rti.org
Michael Mills, RTI International, 3040 East Cornwallis Road, Post Office Box 12194, Research Triangle Park, NC 27709–2194, Voice: (919) 316–3427, mmills@rti.org
Daniel R. Kivlahan, Department of Veterans Affairs, HSR&D Center of Excellence, 1100 Olive Way, Suite 1400, Seattle, WA 98101, Box 358280 (S-152), Voice: (206) 277–1914, Daniel.Kivlahan@va.gov
James R. McKay, University of Pennsylvania, Department of Psychiatry, Center on the Continuum of Care, 3440 Market Street, Suite 370, Philadelphia, PA 19104, Voice: (215) 746–7704, mckay_j@mail.trc.upenn.edu
Patricia Latham, Medical University of South Carolina, Department of Psychiatry and Behavioral Sciences, 67 President Street, Room PH563, Post Office Box 250861, Charleston, SC 29425, Voice: (843) 792–1230, lathampk@musc.edu
J. Scott Tonigan, University of New Mexico, Center on Alcoholism, Substance Abuse, and Addictions, 2650 Yale SE. Suite 243, Albuquerque, NM 87106, Voice: (505) 925–2384, jtonigan@unm.edu
REFERENCES
- 1.Tucker JA, Vuchinich RE, Rippens PD. A factor analytic study of influences on patterns of help-seeking among treated and untreated alcohol dependent persons. J Subst Abuse Treat. 2004;26:237–242. doi: 10.1016/S0740-5472(03)00209-5. [DOI] [PubMed] [Google Scholar]
- 2.Weisner C, Matzger H, Tam T, et al. Who goes to alcohol and drug treatment? Understanding utilization within the context of insurance. J Stud Alcohol. 2002;63:673–682. doi: 10.15288/jsa.2002.63.673. [DOI] [PubMed] [Google Scholar]
- 3.Hasin DS, Grant BF. AA and other helpseeking for alcohol problems: former drinkers in the U.S. general population. J Subst Abuse Treat. 1995;7:281–292. doi: 10.1016/0899-3289(95)90022-5. [DOI] [PubMed] [Google Scholar]
- 4.Hser Y, Maglione M, Polinsky ML, et al. Predicting drug treatment entry among treatment-seeking individuals. J Subst Abuse Treat. 1998;15(3):213–220. doi: 10.1016/s0740-5472(97)00190-6. [DOI] [PubMed] [Google Scholar]
- 5.Yarbroff KR, Warren JL, Knopf K, et al. Estimating patient time costs associated with colorectal cancer care. Med Care. 2005;43(7):640–648. doi: 10.1097/01.mlr.0000167177.45020.4a. [DOI] [PubMed] [Google Scholar]
- 6.Shireman TI, Tsevat J, Goldie SJ. Time costs associated with cervical cancer screening. Int J Technol Assess Health Care. 2001;17(1):146–152. doi: 10.1017/s0266462301104137. [DOI] [PubMed] [Google Scholar]
- 7.Schulper M, Palmer MK, Heyes A. Costs incurred by patients undergoing advanced colorectal cancer therapy. Pharmacoeconomics. 2000;17(4):361–370. doi: 10.2165/00019053-200017040-00006. [DOI] [PubMed] [Google Scholar]
- 8.Secker-Walker RH, Vacek PM, Hooper GJ, et al. Screening for breast cancer: time, travel and out-of-pocket expenses. J Natl Cancer Inst. 1999;91:702–708. doi: 10.1093/jnci/91.8.702. [DOI] [PubMed] [Google Scholar]
- 9.Salome H, French MT, Miller M, et al. Estimating the client costs of addiction treatment: first findings from the client drug abuse treatment cost analysis program (Client DATCAP) Drug Alcohol Depend. 2003;71(2):195–206. doi: 10.1016/s0376-8716(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 10.Anton RF, O’Malley SS, Ciraulo DA, et al. the COMBINE Study Research Group. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295(17):2003–2217. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 11.Pettinati HM, Zweben A, Mattson ME The COMBINE Study. Conceptual, Methodological and Practical Issues in a Clinical Trial That Combined Medication and Behavioral Treatments. Piscataway, NJ: Center of Alcohol Studies, Rutgers University; 2005. [Google Scholar]
- 12.The COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res. 2003;27(7):1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Zarkin GA, Bray JW, Aldridge A, et al. Cost and cost-effectiveness of the COMBINE study for alcohol-dependent patients. Arch Gen Psychiatry. 2008;65(10):1214–1221. doi: 10.1001/archpsyc.65.10.1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarkin GA, Bray JW, Mitra D, et al. Cost methodology of COMBINE. J Stud Alcohol. 2005 Supplement No. 15:50–55. doi: 10.15288/jsas.2005.s15.50. [DOI] [PubMed] [Google Scholar]
- 15.Bray JW, Zarkin GA, Miller WR, et al. Measuring economic outcomes of alcohol treatment using the Economic Form 90. J Stud Alcohol Drugs. 2007;68(2):248–255. doi: 10.15288/jsad.2007.68.248. [DOI] [PubMed] [Google Scholar]
- 16.Miller WR, Del Boca FK. Measurement of drinking behavior using the Form 90 family of instruments. J Stud Alcohol Suppl. 1994;12:112–118. doi: 10.15288/jsas.1994.s12.112. [DOI] [PubMed] [Google Scholar]
- 17.Tonigan JS, Miller WR, Brown JM. The reliability of Form 90: an instrument for assessing alcohol treatment outcome. J Stud Alcohol. 1997;58:358–364. doi: 10.15288/jsa.1997.58.358. [DOI] [PubMed] [Google Scholar]
- 18.Scheurich A, Müller MJ, Anghelescu I, et al. Reliability and validity of the Form 90 interview. Eur Addict Res. 2005;11(1):50–56. doi: 10.1159/000081417. [DOI] [PubMed] [Google Scholar]
- 19.Gabel J, Claxton G, Gil I, et al. Health benefits in 2005: premium increases slow down, coverage continues to erode. Health Aff. 2005;24(5):1273–1280. doi: 10.1377/hlthaff.24.5.1273. [DOI] [PubMed] [Google Scholar]
- 20.Sommers JP. Agency for Healthcare Research and Quality; 2007. Aug, [Accessed July 17, 2008]. Medical Expenditure Panel Survey: co-pays, deductibles, and coinsurance percentages for employer-sponsored health insurance in the non-federal workforce, by industry classification, 2005. Statistical brief 182. Available at: www.meps.ahrq.gov/mepsweb/data_files/publications/st182/stat182.pdf. [Google Scholar]
- 21.Thomson MicroMedex. Version 61127. Volume 41. Montvale, NJ: Thomson PDR; 2006. Jul, RedBook™ for Windows®. [Google Scholar]
- 22.Dewan M. Are psychiatrists cost-effective? An analysis of integrated versus split treatment. Am J Psychiatry. 1999;156(2):324–326. doi: 10.1176/ajp.156.2.324. [DOI] [PubMed] [Google Scholar]
- 23.Cisler R, Zweben A. Development of a composite measure for assessing alcohol treatment outcome. Alcohol Clin Exp Res. 1999;23:263–271. [PubMed] [Google Scholar]
- 24.Miller WR, Tonigan JS, Longabaugh R. Test Manual. Volume 4. Rockville MD: National Institute on Alcohol Abuse and Alcoholism; 1995. The Drinker Inventory of Consequences (DrInC): An instrument for assessing adverse consequences of alcohol abuse. Project MATCH Monograph series. [Google Scholar]
- 25.Siegel JE, Weinstein MC, Torrance GW. Reporting cost-effectiveness studies and results. In: Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost Effectiveness of Health and Medicine. New York, NY: Oxford University Press; 1996. pp. 276–303. [Google Scholar]
- 26.Drummond MF, Sculpher MJ, Torrance GW, et al. Methods for the Economic Evaluation of Health Care Programmes. 3rd ed. New York: Oxford University Press; 2005. [Google Scholar]
- 27.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves—facts, fallacies and frequently asked questions. Health Econ. 2004;13:405–414. doi: 10.1002/hec.903. [DOI] [PubMed] [Google Scholar]
- 28.Fenwick E, Claxton K, Sculpher M. Representing uncertainty: the role of costeffectiveness acceptability curves. Health Econ. 2001;10(8):779–787. doi: 10.1002/hec.635. [DOI] [PubMed] [Google Scholar]
- 29.Fenwick E, Marshall DA, Levy AR, et al. Using and interpreting cost-effectiveness acceptability curves: an example using data from a trial of management strategies for atrial fibrillation. BMC Health Serv Res. 2006;6(1):52–59. doi: 10.1186/1472-6963-6-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tolley G, Kenkel D, Fabian R. Valuing Health for Policy, An Economic Approach. Chicago: The University of Chicago Press; 1994. [Google Scholar]
- 31.Juster FT, Stafford FP. The allocation of time: empirical findings, behavioral models, and problems of measurement. J Econ Lit. 1991;29(2):471–522. [Google Scholar]