Abstract
Human West Nile virus (WNV) infection was first detected in Cuyahoga County, Ohio in 2002. During that year's extensive epidemic/epizootic among non-immune human and bird populations, the county experienced 155 cases of severe human WNV neuro-invasive disease (WNND, incidence: 11.1 cases/100,000), with 11 fatalities. Structured serosurveys indicated that 1.9%, or ~ 26,000 of county residents (pop. = 1,372,303) were infected that year. In early 2003, in order to better focus monitoring and control efforts, we used a Geographic Information System (GIS) approach and spatial statistical analysis to identify the association of environmental factors and human population structure with the observed local risk for WNV transmission. Within the varied range of urban/suburban/rural habitats across the 458 mi2 (1186 km2) county, exploratory analysis indicated significant clustering of WNND risk in inner-ring suburbs. Subsequent discriminant factor analysis based on inputs of census and land-use/ land cover data was found to effectively classify sub-areas of the county having low, medium, and high WNV risk. On a 4 mi2 (1036 ha.) quadrat scale of resolution, higher risk of human infection was significantly associated with higher-income areas, increased fractionation of habitat, and older housing, while it was negatively associated with areas of agricultural land, wetland, or forest. The areal classification of WNV transmission risk has been validated over time through detection of increased local Culex spp. mosquito density (2002–2006), and increased frequency of WNV positive mosquito pools within the medium- and high-risk quadrats. This timely working identification of the transmission scale effectively focused control interventions against newly invasive WNV in a complex North American habitat.
Keywords: Encephalitis/arbovirus, West Nile virus, epidemiologic factors, cluster analysis
INTRODUCTION
Since 1999, West Nile virus (WNV) has become an established seasonal cause of illness and mortality across North America (Hayes and Gubler 2006). Both the rapid establishment of West Nile virus (WNV) in the eastern United States (CDC, 1999) and its extensive spread across North America (CDC, 2002) indicate the high susceptibility of regional ecosystems to introduction of an 'exotic' pathogen such as WNV. During each of the last seven summers, WNV-related morbidity and mortality have been observed in increasing numbers among avian, human, equine and reptilian hosts, with extensive impact in terms of health costs. This experience with WNV represents a natural model for the introduction of other lethal species (e.g., Dengue or Rift Valley Fever virus (Peters et al. 2002)), and in consequence, an extensive debate has developed over the optimal means of preventing and controlling arbovirus transmission within North America (Roche 2002). Beyond the immediate impact of WNV on its most susceptible vertebrate hosts, there are concerns that WNV will have an important impact on keystone species (avian raptors) with further, significant long-term effects on local ecosystems, and greater risk for transmission of rodent-borne diseases such as LaCrosse virus, hantaviruses, Ehrlichia, and Lyme disease (Weiss 2002). For these reasons, it is now essential to better define the ecology of WNV transmission in different landscape habitats.
As an emerging pathogen, WNV spread rapidly across the United States landscape, adapting to local habitats, mosquito and bird populations in Louisiana, Illinois, Michigan, and Ohio in 2002, Colorado in 2003, and Arizona and California in 2004 (O'Leary et al. 2004; Petersen and Hayes 2004). Both WNV transmission and the observed incidence of WNV-related disease are thought to be due to many factors, including the local susceptibility of human and animal host populations (Mandalakas et al. 2005), the temporal variation of mosquito vector abundance, the impact of mosquito control efforts, local ecology, and other environmental factors (Lord and Day 2001; Turell et al. 2001; Mans et al. 2004; Ruiz et al. 2004). Prominent among the latter are anthropogenic factors, such as housing quality and construction, local drainage, and the risk-related behaviors of susceptible human populations (Shaman et al. 2003; Ruiz et al. 2004; LaBeaud et al. 2007).
The present analysis was conducted using data for Cuyahoga County (2002 mid-year population: 1,372,303), located in northeast Ohio. The County includes the city of Cleveland, its older inner-ring suburbs, and many of its newer second-ring suburbs, as well as several large regional parks, and industrial and agricultural areas. During the first-year 'virgin field' WNV epidemic in the summer and fall months of 2002, we observed and geocoded 221 laboratory-confirmed cases of WNV illness in county residents, including 155 West Nile Neurological Disease (WNND) cases (11.1 cases/100,000 population) with 11 fatalities ((Mandalakas et al. 2005), see Fig. 1). The objective of the present analysis was to identify significant spatial and temporal clustering of cases within the county environment, and to link these observations to underlying environmental features that could likely contribute to transmission risk. Following risk stratification of neighborhoods by discriminant analysis, we are able to identify a link between local GIS-predicted environmental levels of risk and both the independently measured levels of mosquito vector (Culex spp.) abundance and WNV mosquito pool postivity within the same areas.
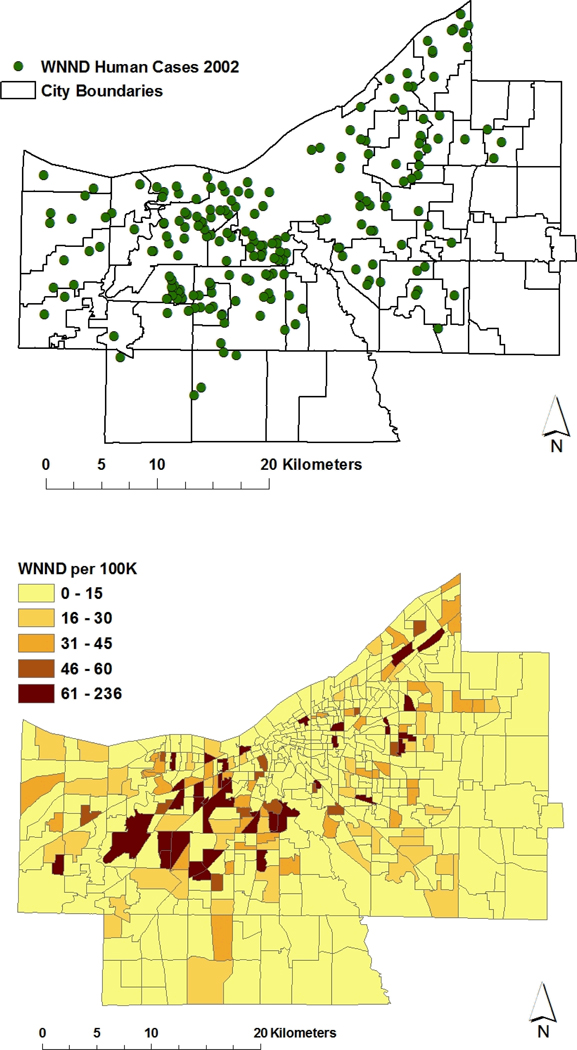
Figure 1.
Upper panel, distribution of severe human WNV-associated neurological disease (WNND) cases by residence location in Cuyahoga County, 2002. For this map, black outlines indicate boundaries of individual cities and municipal townships included within the county limits; Lower panel, local age-adjusted attack rates for WNND within established US Government census tracts of Cuyahoga County. Increased age-adjusted risk is indicated by darker shades of color. For the county at-large, the 2002 rate of WNND was 16 per 100,000 population.
Cuyahoga County is an urban, suburban, and partly rural county with unique complexity of geology and habitat for birds and mosquitoes. This habitat diversity provides a good prospect for the general study of WNV transmission in an urban and suburban setting.
MATERIALS AND METHODS
Data geocoding and integration for case locations, underlying population structure, and local environmental features, along with quadrat generation, was performed using ArcView 3.1 and ArcGIS 9 (ESRI, Redlands CA). Census data were obtained from US Census Bureau internet resources (www.census.gov and available GIS files at http://arcdata.esri.com/data/tiger2000/tiger_download.cfm), and elevation and land use/land cover information from databases supplied by US Geological Survey (http://data.geocomm.com/catalog/index.html).
Statistical Analysis- Initial exploratory data analysis for spatial clustering of WNV infection was performed using Clusterseer 2 software (BioMedWare, Ann Arbor, MI). Clustering of WNND cases by patient’s home location (adjusted for underlying population density) was assessed using Besag & Newell’s scan statistic.
Quadrat analysis of WNV transmission environment and scale: In this analysis, new areal grid-based subunits were developed on scales of 1, 4 and 10 mile2 using ArcGIS. After joining available demographic, income, housing, road density, and land use data to the component county grid squares, clustered factor analysis and discriminant factor analysis (Minitab 14, State College, PA) were used to examine significant associations between WNV human case density and these grid-referenced values.
Multivariable analysis of mosquito abundance and logistic analysis of WNV mosquito pool positivity were performed using SPSS (SPSS, Inc. Chicago IL) and R software. Variables were selected for multivariate logistic analysis if they were significant at the p<0.25 level on univariate analysis, or considered biologically relevant. Model variables were trimmed in a step-wise fashion based on the Wald statistic, and eliminated if they did not significantly contribute to the model. Model variables were then evaluated for colinearity, and possible interaction with other variables. Variables that contradicted the linearity assumption were further characterized and either transformed, or dichotomized. The final model was then interpreted, including interaction terms and main effects, and checked for goodness of fit for the data.
Mosquito trapping and identification: Standard CDC miniature light traps and gravid traps were placed overnight at geocoded locations across the county during each transmission season (May-October) from 2002 through 2006. In 2002, 660 traps were placed on a non-systematic basis in areas of suspected WNV transmission (based on reports of suspected cases of human WNND, of observed bird mortality, or of high mosquito abundance). From 2003 onward, repeated systematic trapping was performed using rotating coverage over a grid of 119 defined geographic locations distributed across the county at ~ 2 mile intervals. The number of traps sampled in 2003, 2004, 2005, and 2006, were 457, 520, 396 and 410, respectively. Mosquito species identification was performed with the aid of binary keys, and frozen specimens batched for pooled testing for WNV using RT-PCR technique (State of Ohio Department of Health Laboratories, Columbus, Ohio).
RESULTS
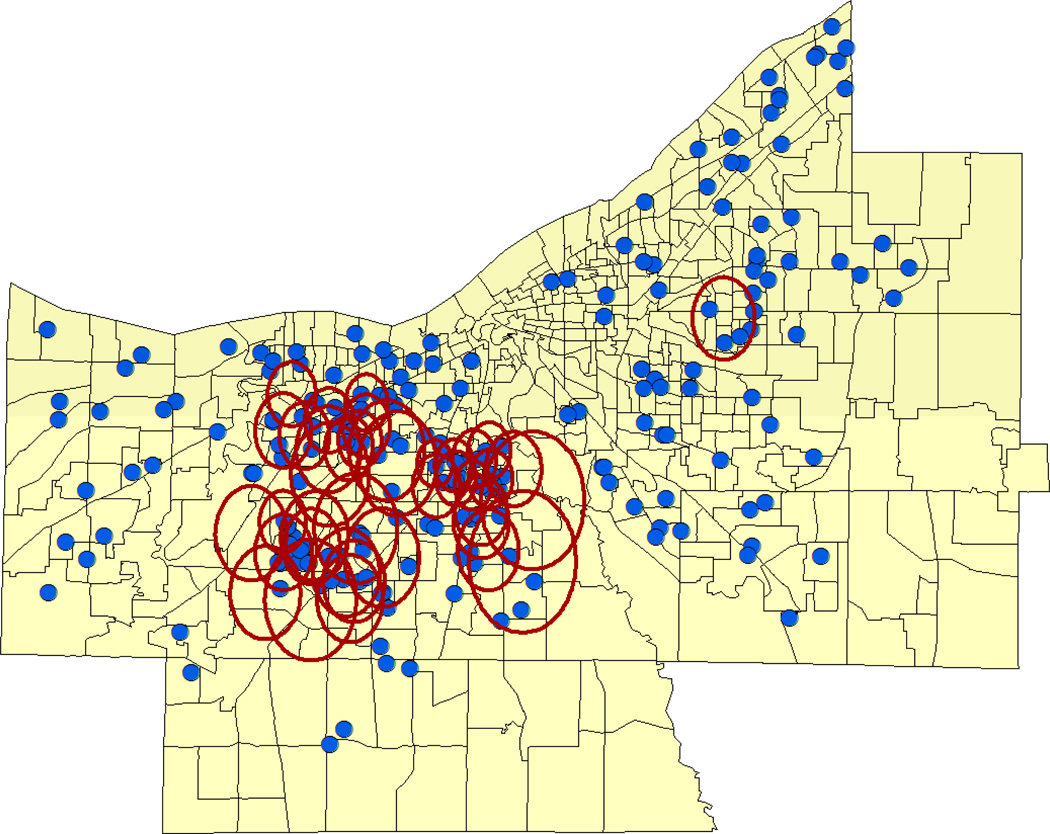
The Problem: Summer 2002, an extensive outbreak of WNV-associated Neurological Disease (WNND) in Cuyahoga County, Ohio. The first human cases of WNV infection in Northeast Ohio were recorded in mid-August 2002, presaging an extensive epidemic of WNND among county residents (Mandalakas et al. 2005), which lasted through mid October, resulting in 221 confirmed cases of severe infection (Fig. 1a). [As the syndrome of West Nile Fever was not readily diagnosable in this period, and was not a reportable illness in 2002, for purposes of the current analysis, only cases of WNND (median age 61 years, range 11–98 years), geocoded to home residence, were used for analysis.] This cohort was comprised of patients admitted to the 18 different hospitals within Cuyahoga County and/or reported by physicians through the established public health reporting system. (CDC ArboNET Surveillance Network). For the county at large, the severe WNV case rate was 16 per 100,000 population. Using available US census data (year 2000) to derive age-adjusted WNV attack rates within census tract locations of the county, we observed an apparent aggregation of high standardized attack rates (to over 61 per 100,000) along the eastern and western borders of the city of Cleveland, and among its immediately neighboring (first ring) suburbs (Fig 1b). Significant clustering of very high case locations, adjusted for underlying population, was noted for the same city border and suburban areas using Besag and Newell’s scan statistic (Fig 2).
Figure 2.
Cuyahoga County census tract locations showing significant local clustering of high WNND incidence for 2002 within southern and western Cleveland and its inner ring suburbs in the west, southwest, and east. Rings indicate significant population-adjusted case clustering by Besag-Newell scan statistic.
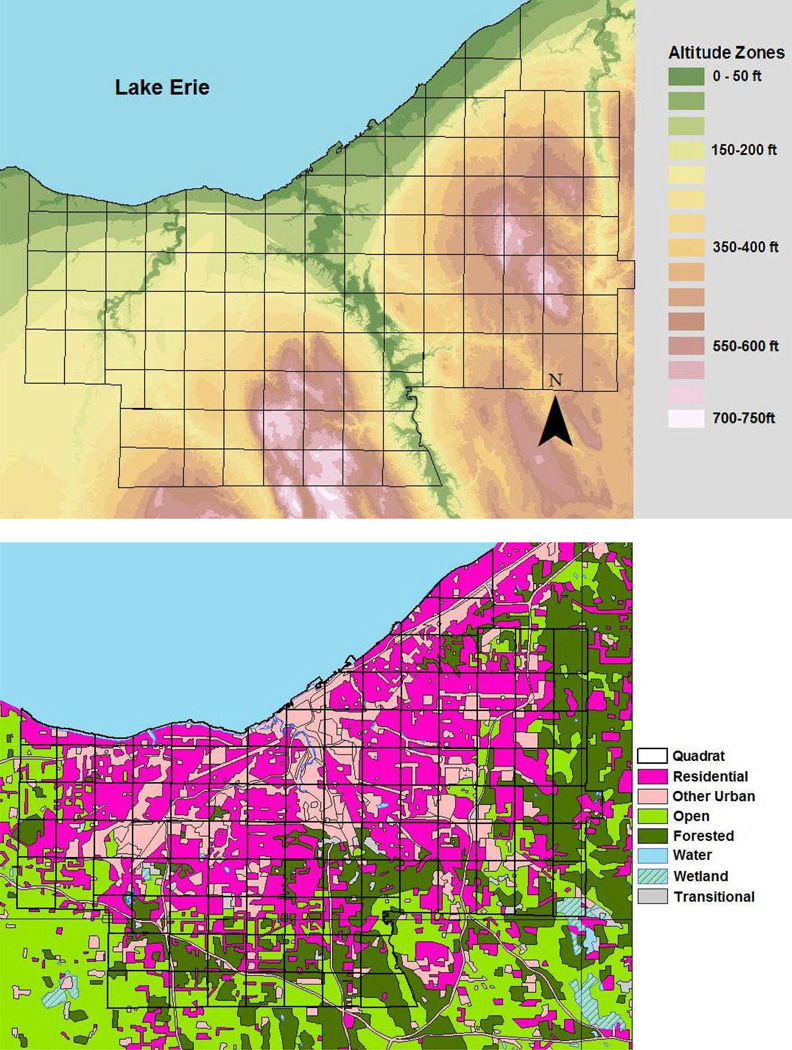
Landscape Setting of WNV Transmission: Cuyahoga County and the city of Cleveland straddle the junction of two major land forms of North America, the glaciated Allegheny Plateau, which gradually rises eastward to the Appalachian Crest, and the Central Lowlands, which occupy the prairie areas westward into Illinois and the trans-Mississippi plains (Fig 3a). Its entire northern border is formed by the freshwater Lake Erie, part of the Great Lakes watershed that borders between the U.S. and Canada. Based on Landsat Thematic Mapper Data from October 1994, Cuyahoga County has a heterogeneous land cover with 92% of the area in three major categories (54% wooded suburban, 12% urban, and 26% open urban or agricultural areas) (Fig 3b). Since 1948, the developed land in the county has risen from 26% to >90% of the available 458-sq. mi (1186 km2).
Figure 3.
Topographic and Land use/ Land cover characteristics of Cuyahoga County. Upper panel, elevation map of the county, showing altitude above mean Lake Erie shoreline level. Higher altitudes in the east and south drain toward the lake to the north and west. Black outlines indicate the chosen analytic quadrat grid used in data aggregation for discriminant analysis of WNV risk; Lower panel, Land use classification of Cuyahoga County and surrounding areas. The central county is predominantly urban (magenta and pink) surrounded at the periphery by open agricultural and parkland, including forested and wetland areas.
Grid-based environmental risk assessment: After the severe 2002 WNV transmission season, a coordinated effort was made to predict sub-regions of the county likely to have persistent high-level risk for WNV transmission in 2003 and in later years. In the risk assessment, in order to eliminate the influence of data aggregation by arbitrary political boundaries and to integrate available data on land use and habitat as well as human population, housing, and economic data into the analysis, we used GIS software to develop a new quadrat system for the county that integrated area information on grid-specified locations on scales of either one, four, or ten square mile grid areas. Clustered factor analysis of the association between environmental variables and grid-based WNV case density indicated a significant relationship between land-use factors (developed areas, high-intensity residential areas) as well as measures of habitat fractionation (effective land-use mesh size, or road density as a proxy of habitat fractionation) and the observed WNV case density (Table 1), particularly on the 4 mi2 scale. WNV case density was greater in areas with higher income, more residents over age 65, greater road density (Fig 4a) and older housing stock (Fig 4b). Risk was observed to be higher in grids having a greater percentage of urban/suburban land use, and lower in areas with more parkland, agricultural use, open water or non-forested wetland.
Table 1.
Unadjusted non-parametric correlation of local West Nile virus case density with local environmental landscape features, housing age, and other human population factors within 4 mile2 grid cells of Cuyahoga County
| Variable | Rank Correlation | P value |
|---|---|---|
| Total population | 0.042 | NS |
| Population over 65 yr | 0.265 | < 0.001 |
| Median income | 0.182 | 0.002 |
| Median age of housing | 0.131 | 0.03 |
| Road density | 0.390 | < 0.001 |
| Parkland density | − 0.203 | 0.001 |
| Percent open/agricultural area | − 0.394 | < 0.001 |
| Percent urban/suburban areas | 0.513 | < 0.001 |
| Percent open water | − 0.218 | < 0.001 |
| Percent shrub/scrub areas | − 0.224 | < 0.001 |
| Percent forest/wooded | − 0.005 | NS |
| Percent non-forested wetland | − 0.390 | < 0.001 |
| Percent barren land | 0.017 | NS |
| Case density | 1.00 | Comparison |
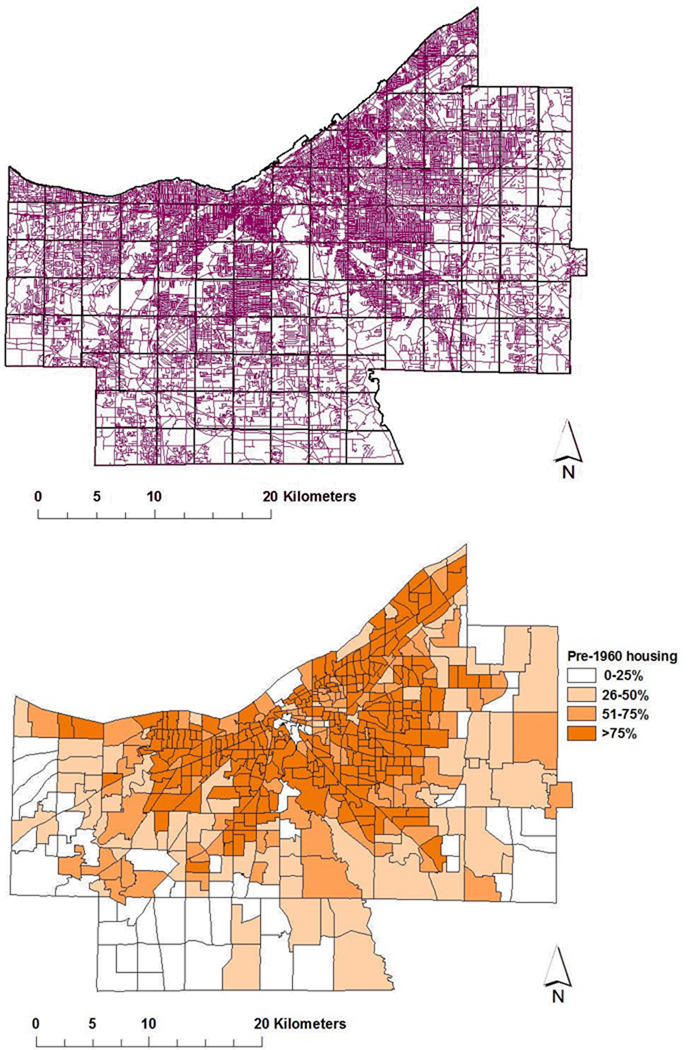
Figure 4.
Examples of GIS data inputs for risk modeling. Upper panel, All Cuyahoga County roads in purple, with analytic quadrat grid overlaid in black; Lower panel, location of older housing stock as determined by census tract for year 2000 US census data on age of individual domiciliary structures.
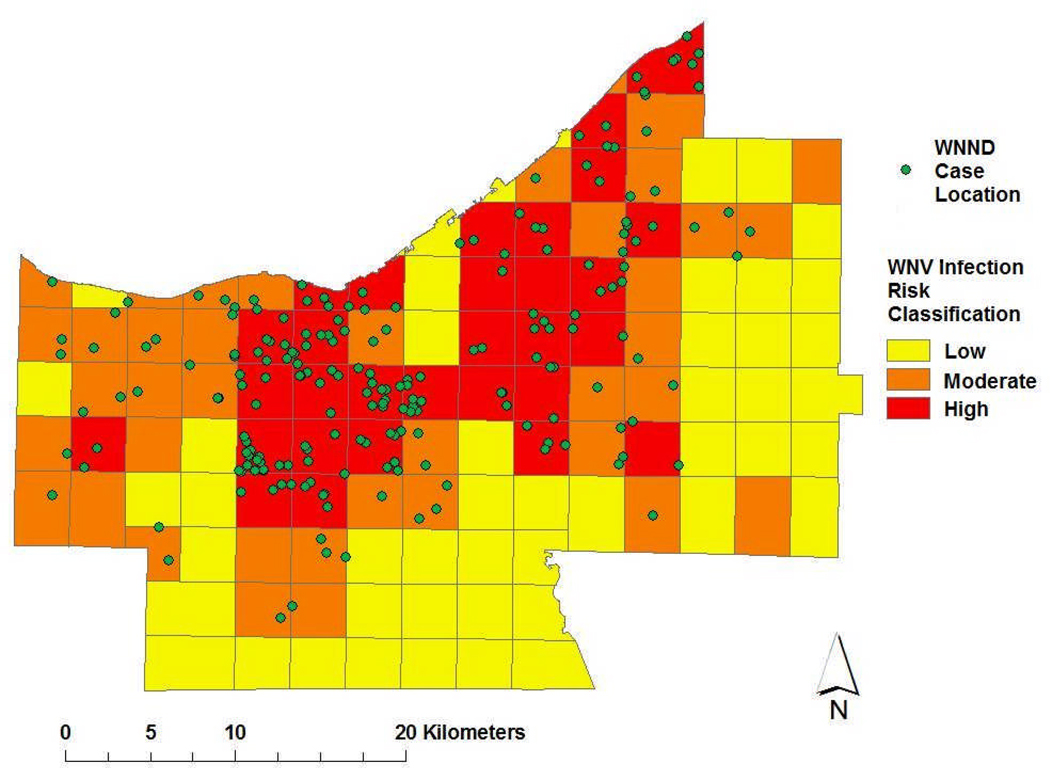
The Cuyahoga County Board of Health had noted that in parts of the county with homes built before 1960, catch basins located within storm sewer systems provide effective habitat for breeding and overwintering of Culex spp. mosquitoes, unless these basins were treated with larvicides. Therefore, in our next discriminant factor assessment, using three level of predicted grid risk (i.e., high, medium, or low transmission risk/grid square) we examined whether WNV infection risk was associated with median housing age, median local income, and grid-contained road length, road density, park area, and park density, while adjusting for human population density and population over 65 years old. Land cover was also assessed in terms of percentage of land used for agriculture, or left as barren land, wooded vs. non-forested areas, open water, shrub and scrubland, or developed as urban environment. Best classification results (82% accuracy) were obtained using 4 sq.mi.-gridding to define high-, intermediate- and low-risk areas within the overall region (Fig 5). Of note, on this optimal scale of resolution, only 3 high- or medium-risk areas were misclassified as low-risk, and only 3 low-risk areas were misclassified as medium or high risk.
Figure 5.
Identified high-, medium-, and low-risk areas for human WNV infection within Cuyahoga County in 2002, based on discriminant analysis of environmental and population features derived from US census and land-use/ land-cover databases (see Figures 3 and 4). Green circles indicate approximate residence locations of 2002 human WNND cases.
Validating grid-based environmental risk assessment against observed mosquito outcomes: To test whether the initial 2002 grid risk assessment, as described above, was significantly associated with other important biological factors linked to transmission, we tested whether Culex spp. mosquito abundance and WNV mosquito pool positivity were significantly different in the identified low-, medium- and high-risk grids. In order to do this, multivariable analysis was performed on mosquito trap outcomes per grid square for the period 2002–2006. Potential predictors tested in the model included individual grid risk score (Low/Medium/High), as possibly modified by environmental effect modifiers including trap easting (projected longitude), northing (projected latitude), shortest distance from Lake Erie, altitude, year, and month of trapping.
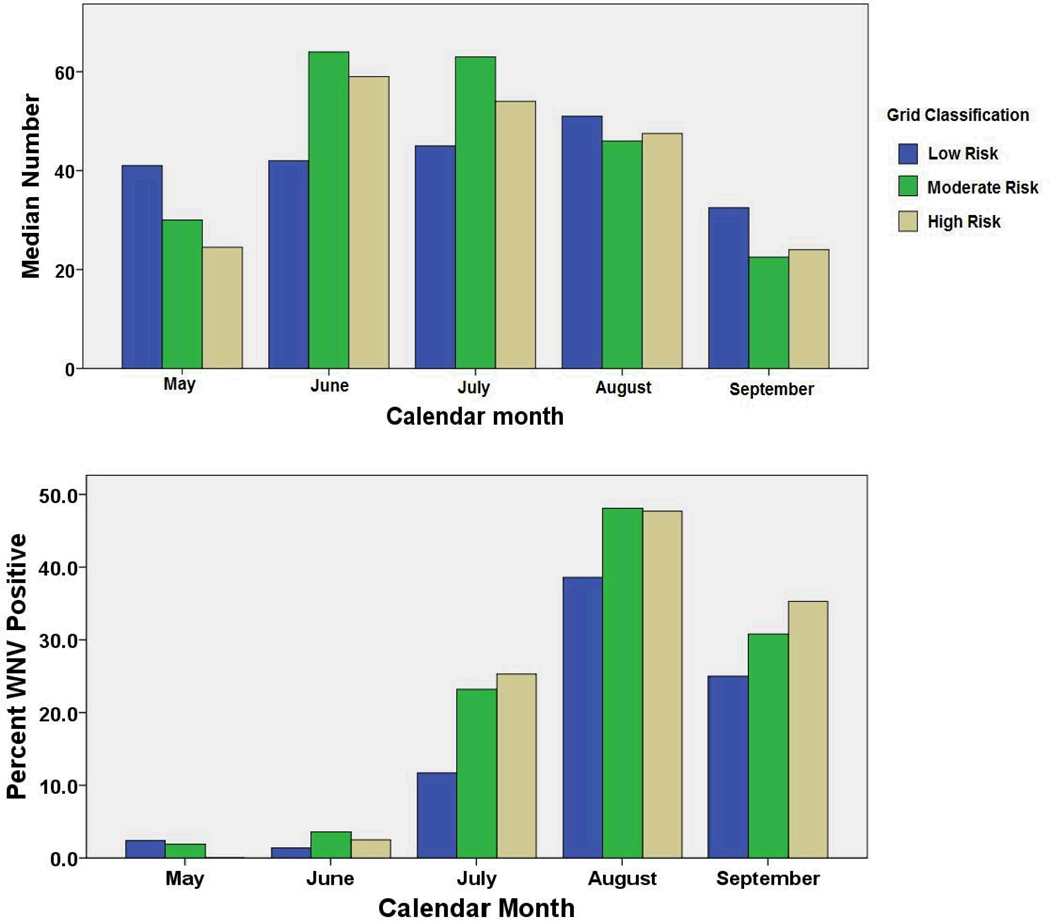
Our analysis of 2002–2006 mosquito data indicated a significantly higher abundance of Culex spp. mosquitoes early in the season (June and July, (Fig 6a)) in the high- and medium-risk areas. This phenomenon is followed in July-Sept by more frequent detection of WNV-positive mosquito pools in these same areas. Not shown, analysis of mosquito trap numbers by month and year revealed that the severe year for WNND, 2002, was different in that peak mosquito numbers were present for all grid squares in May of that year. In multivariable logistic analysis of gravid trap WNV positivity, there was a significant variation by month and year of collection, but even with adjustment, the grid-based risk level assessment remained a significant predictor, with medium-and high-risk grid areas having respectively, 53% and 67%, greater odds of producing a WNV positive mosquito trap result. Easting, northing, altitude, and distance from water did not prove to be significant predictors in the multivariable models, and were not included in the final model presented in Table 2.
Figure 6.
Observed variation in mosquito trap outcomes according to grid area classification for WNV risk. Upper panel, Median mosquito numbers recovered per gravid trap night in high-, moderate-, and low-risk grid areas; Lower panel, percentage of per trap mosquito pools that tested positive for WNV each month in the stratified grid risk areas between May 2002 and September 2006.
Table 2.
Link between gridded landscape/population-based risk classification and observed West Nile virus RT-PCR positivity among mosquito pools in Cuyahoga County, 2002–2006
| Mosquito Pool WNV positivity: | |||
|---|---|---|---|
| Model Variable | Multiply-Adjusted Odds Ratio |
95% CI | P value |
| Calendar Month | |||
| May | Reference | -- | -- |
| June | 1.74 | 0.38,7.89 | NS |
| July | 16.4 | 3.96,68.1 | < 0.001 |
| August | 56.9 | 13.7,235 | < 0.001 |
| September | 16.4 | 3.74, 72 | < 0.001 |
| Year | |||
| 2002 | 4.2 | 2.9,6.1 | < 0.001 |
| 2004 | 0.69 | 0.46, 1.03 | NS |
| 2005 | 1.04 | 0.69, 1.6 | NS |
| 2006 | Reference | -- | -- |
| Grid Risk Category | |||
| Low | Reference | -- | -- |
| Medium | 1.53 | 1.08,2.2 | 0.02 |
| High | 1.67 | 1.18,2.4 | 0.004 |
DISCUSSION
Our results indicate that, over a 458 mi2 county control area, West Nile virus transmission risk effectively resolves into high, medium and low risk areas on the scale of a ~ 4 mi2 grid. As in many complex systems (Kitron et al. 2006), the ecology of WNV transmission is a function of interactions between mosquito, bird and human hosts, which at first glance, might seem quite difficult to predict based on limited data inputs. In the case of our Cuyahoga County analysis, the strength of the GIS analysis was its ability to integrate large amounts of readily available environmental data (land use classification, human population data, as well as proxies for drainage features and habitat fractionation) in an empirical formulation of risk areas across the greater county area. This early environment-based classification proved to capture important variation of Culex spp. abundance and mosquito pool WNV positivity across the county. Furthermore, over the past 5 years, the designated areas have provided the basis for more systematic seasonal mosquito sampling, and also serve as a useful ‘implementation unit’ for countywide mosquito abatement planning.
Over the past five decades, the geographic range of WNV has been increasing dramatically, reaching into many new habitats. Epidemics have been reported in Israel and Egypt since the 1950s (Weinberger et al. 2001), France and Italy since the 1960s, Romania in 1996–1997 (Tsai et al. 1998), and in the North America in 1999–2006 (1999). There is significant local variation in WNV transmissibility within a given enzootic area, as well as significant variation in transmission over time. In temperate northern climates, epidemics of WNV encephalitis typically occur only in the late summer and early fall. Although over 130 species of birds and 36 species of insects have been found infected with WNV, it is clear that different bird and mosquito species pose widely varying risks for transmission to humans (Goddard et al. 2002; Turell et al. 2002).
Observed WNV infection rates can be affected by a population’s background immunity, practice of personal protection, mosquito vectors and their density, vector control efforts, poor housing quality, inadequate screening, and local ecology (Shaman et al. 2002; Hayes and O'Leary 2004; Ruiz et al. 2004). Greater WNND incidence should result from outbreaks in older populations, increased virus virulence, and higher WNV infection rates. In the first wave of transmission, Cuyahoga County reported ~3 times more WNND cases in 2002 than New York City (NYC) reported in 1999, although NYC population is nearly 14 times larger in size. The populations of both Cuyahoga County and NYC were immunologically naïve and exposed to higher than normal levels of urban Culex mosquitoes, Cx. pipiens and Cx. restuans (Mans et al. 2004). Although the proportion of residents aged 65 years and older differed between Cuyahoga County (15.6%, 2002 US census data) and NYC (11.7%, 2000 US census data), this small difference is unlikely to account for the degree of difference in WNND incidence. The Cuyahoga County WNND:infection ratio was remarkably similar to the 1999 NYC (Mostashari et al. 2001) and 1996 Romania outbreaks (Tsai et al. 1998), suggesting that virus virulence did not change. In addition, recent genetic studies of U.S. WNV isolates have suggested that the genome remains remarkably stable (Lanciotti et al. 2002). Similar infection rates, no change in virulence, and a very small difference in the age structure of the two populations suggest that another explanation is needed for differences in local transmission patterns.
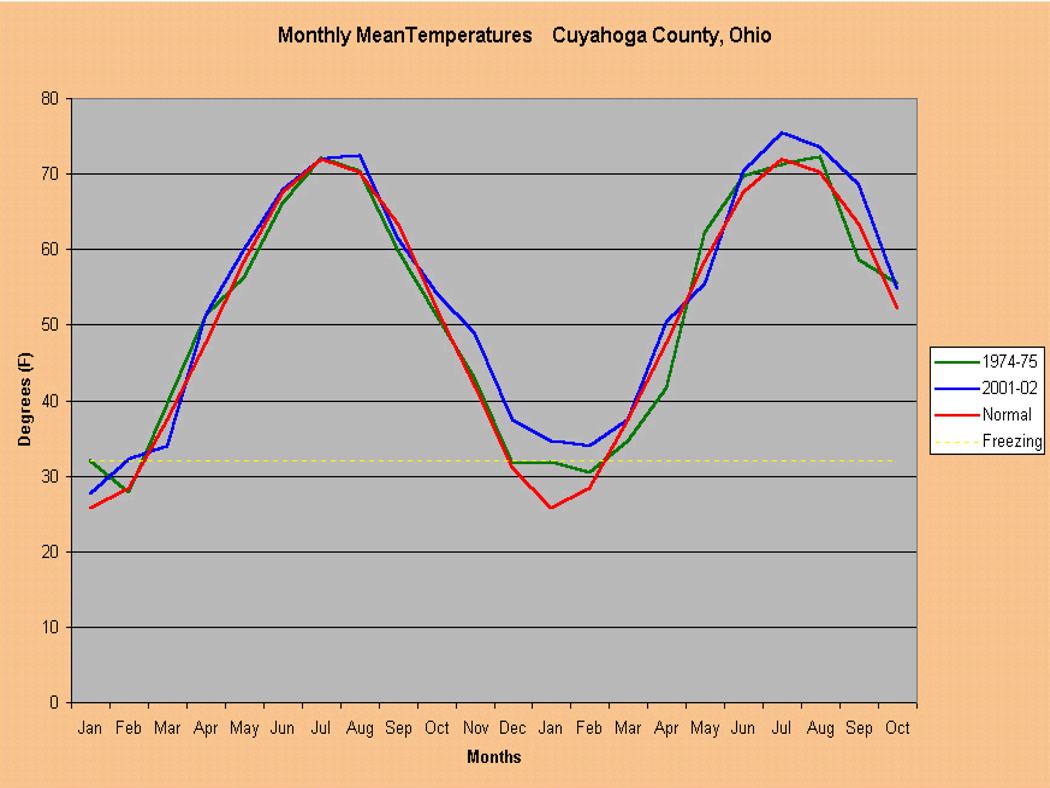
Ohio has been the site of numerous mosquito-borne disease outbreaks, including SLE, malaria, Eastern Equine Encephalitis and La Crosse Encephalitis, suggesting that our local ecology and environment may foster such outbreaks. Most notable, the 1975 SLE outbreak resulted in 84 cases and 8 fatalities within Cuyahoga County. Both the 1975 SLE outbreak and the 2002 WNV outbreak were preceded by mild winters, which probably allowed vector Culex mosquitoes to overwinter with lower mortality, and then propagate earlier in the season (Fig. 7). The 2002 WNV outbreak may also have been enhanced by a spring season with high precipitation, followed by a relative period of drought (Shaman et al. 2002; Shaman et al. 2003).
Figure 7.
Mean monthly temperatures in Cuyahoga County, Ohio (red line), showing two periods of anomalous warm winter temperatures in 1974–75 (green line), and in 2001–2002 (blue line). The 1974–75 event was associated with an outbreak of St. Louis Encephalitis (SLE) virus-related disease. The 2001–2002 event was associated with the County’s first WNV epidemic.
Analysis of our study area, Cuyahoga County, Ohio, confirms that WNV transmission was quite uneven within the irregular borders of the 59 various component municipalities, which vary from 0.1 to 78 sq. mi. in size. This variance within political administrative boundaries suggested the need for a more systematic assessment of the role played by focal habitat and reservoir factors in facilitating or retarding transmission. Dynamic modeling of vector-borne diseases indicates that some features of the insect-bird-human transmission cycle are likely to be more significant than others in determining risk for infection (Garrett-Jones 1964; Dye 1994). In particular, the vector’s biting rate, its abundance relative to birds and humans, and its preference for feeding on a single versus several hosts, all have a significant impact on the observed rates of local WNV transmission. Bird diversity and abundance can increase across a gradient of rural-suburban habitats due to increase environmental fractionation (and possibly limitations on predator abundance) (Blair 1996). Our discriminant analysis suggests that greater fractionation of habitat strongly coincides with increases in WNV transmission risk.
Not all ecological factors can be measured in sufficient detail to fully define an individual area's WNV transmission risk (Dye 1986). Unfortunately, a recent survey of 25 different agencies responsible for directing WNV and mosquito abatement programs (Stein and Claborn 2005) indicates that there is currently no consistency in monitoring or implementing WNV vector control across the Eastern US. However, as we have demonstrated, within a given region it appears that dynamics of transmission self-organize on a meso-scale (1 to 5 square miles), such that risk is linked to measurable areal landscape attributes likely to be affecting local mosquito and bird populations. From the mosquito management standpoint, information on such scales can offer a useful support framework to focus intervention (Kitron et al. 2006). On this basis, we believe that it is possible to develop accurate predictions of meso-scale WNV transmission threats, providing the opportunity to design more efficient interventions for prevention of human infection and disease.
WNND case clustering and pockets of high risk identified within the County offer strong evidence that WNV risk varies on small definable scales. Because human and land use factors were able to accurately define areas in the county at high risk for WNV transmission, it is probable that these same areas will be at risk for other mosquito-borne infections. These identified transmission “hotspots” can be closely monitored for the transmission of known, endemic pathogens, such as WNV, SLE, or LaCrosse virus, as well as any additional emerging mosquito-borne diseases of concern. Available public health resources can be focused on these hotspots during outbreaks. In this fashion, effective inputs on transmission heterogeneity can leverage control efforts to more effectively halt the spread of disease within regions of this size and complexity.
Acknowledgements
The authors would like to give thanks to the residents of Cuyahoga County who participated in this study and the agencies who supported this effort including the Cuyahoga County Board of Health, the Centers for Disease Control and Prevention, the Ohio Department of Health, the City of Cleveland Health Department, the City of Lakewood Health Department, the St. Luke’s Foundation, the Mt. Sinai Health Care Foundation, the George Gund Foundation, and the Sisters of Charity/St. Ann’s Foundation. The authors would like to give special thanks to Mr. Terry Allan, Health Commissioner for the Cuyahoga County Board of Health, and Dr. Grant “Roy” Campbell, Centers for Disease Control and Prevention (CDC) for their invaluable insight and critical review.
This work was supported in part by NIH grants K23 HD40982 and TW/ES01543.
References
- Blair RB. Land use and avian species diversity along an urban gradient. Ecological Applications. 1996;6:506–519. [Google Scholar]
- CDC. Outbreak of West Nile-like viral encephalitis--New York, 1999. MMWR Morb Mortal Wkly Rep. 1999;48:845–849. [PubMed] [Google Scholar]
- CDC. West Nile virus activity--United States, September 12–18, 2002, and Ohio, January 1-September 12, 2002. MMWR Morb Mortal Wkly Rep. 2002;51:836–837. [PubMed] [Google Scholar]
- Dye C. Vectorial Capacity: Must we measure all its components? Parasitology Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Dye C. Approaches to vector control: The epidemiological context of vector control. Trans R Soc Trop Med Hyg. 1994;88:147–149. doi: 10.1016/0035-9203(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Garrett-Jones C. Prognosis for interruption of malaria transmission through the assessment of the mosquito's vectorial capacity. Nature. 1964;204:1173–1175. doi: 10.1038/2041173a0. [DOI] [PubMed] [Google Scholar]
- Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385–1391. doi: 10.3201/eid0812.020536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes EB, Gubler DJ. West Nile virus: epidemiology and clinical features of an emerging epidemic in the United States. Annu Rev Med. 2006;57:181–194. doi: 10.1146/annurev.med.57.121304.131418. [DOI] [PubMed] [Google Scholar]
- Hayes EB, O'Leary DR. West Nile virus infection: a pediatric perspective. Pediatrics. 2004;113:1375–1381. doi: 10.1542/peds.113.5.1375. [DOI] [PubMed] [Google Scholar]
- Kitron U, Clennon JA, Cecere MC, Gürtler RE, King CH, Vazquez-Prokopec G. Upscale or downscale: applications of fine scale remotely sensed data to Chagas disease in Argentina and schistosomiasis in Kenya. Geospatial Health. 2006;1:49–58. doi: 10.4081/gh.2006.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBeaud AD, Kile JR, Kippes C, King CH, Mandalakas AM. Exposure to West Nile virus during the 2002 epidemic in Cuyahoga County, Ohio: A comparison of pediatric and adult behaviors. Public Health Reports. 2007;122:356–361. doi: 10.1177/003335490712200309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, Bowen M, McKinney N, Morrill WE, Crabtree MB, Kramer LD, Roehrig JT. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96–105. doi: 10.1006/viro.2002.1449. [DOI] [PubMed] [Google Scholar]
- Lord CC, Day JF. Simulation studies of St. Louis encephalitis and West Nile viruses: the impact of bird mortality. Vector Borne Zoonotic Dis. 2001;1:317–329. doi: 10.1089/15303660160025930. [DOI] [PubMed] [Google Scholar]
- Mandalakas AM, Kippes C, Sedransk J, Kile JR, Garg A, McLeod J, Berry RL, Marfin AA. West Nile virus epidemic, northeast Ohio, 2002. Emerg Infect Dis. 2005;11:1774–1777. doi: 10.3201/eid1111.040933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mans NZ, Yurgionas SE, Garvin MC, Gary RE, Bresky JD, Galaitsis AC, Ohajuruka OA. West Nile virus in mosquitoes of Northern Ohio, 2001–2002. Am J Trop Med Hyg. 2004;70:562–565. [PubMed] [Google Scholar]
- Mostashari F, Bunning ML, Kitsutani PT, Singer DA, Nash D, Cooper MJ, Katz N, Liljebjelke KA, Biggerstaff BJ, Fine AD, Layton MC, Mullin SM, Johnson AJ, Martin DA, Hayes EB, Campbell GL. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet. 2001;358:261–264. doi: 10.1016/S0140-6736(01)05480-0. [DOI] [PubMed] [Google Scholar]
- O'Leary DR, Marfin AA, Montgomery SP, Kipp AM, Lehman JA, Biggerstaff BJ, Elko VL, Collins PD, Jones JE, Campbell GL. The epidemic of West Nile virus in the United States, 2002. Vector Borne Zoonotic Dis. 2004;4:61–70. doi: 10.1089/153036604773083004. [DOI] [PubMed] [Google Scholar]
- Peters CJ, Spertzel R, Patrick W. Aerosol Technology and Biological Weapons. In: Knobler SL, Mahmoud AAF, Pray LA, editors. Biological Threats and Terrorism: Assessing the Science and Response Capabilities. National Academy Press; 2002. pp. 66–77. [PubMed] [Google Scholar]
- Petersen LR, Hayes EB. Westward ho?--The spread of West Nile virus. N Engl J Med. 2004;351:2257–2259. doi: 10.1056/NEJMp048261. [DOI] [PubMed] [Google Scholar]
- Roche JP. Print media coverage of risk-risk tradeoffs associated with West Nile encephalitis and pesticide spraying. J Urban Health. 2002;79:482–490. doi: 10.1093/jurban/79.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz MO, Tedesco C, McTighe TJ, Austin C, Kitron U. Environmental and social determinants of human risk during a West Nile virus outbreak in the greater Chicago area, 2002. Int J Health Geogr. 2004;3:8. doi: 10.1186/1476-072X-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. Drought-induced amplification of Saint Louis encephalitis virus, Florida. Emerg Infect Dis. 2002;8:575–580. doi: 10.3201/eid0806.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaman J, Day JF, Stieglitz M. St. Louis encephalitis virus in wild birds during the 1990 south Florida epidemic: the importance of drought, wetting conditions, and the emergence of Culex nigripalpus (Diptera: Culicidae) to arboviral amplification and transmission. J Med Entomol. 2003;40:547–554. doi: 10.1603/0022-2585-40.4.547. [DOI] [PubMed] [Google Scholar]
- Stein KJ, Claborn DM. Telephonic survey of surveillance and control procedures for the mosquito vectors of West Nile virus near naval installations in the Eastern United States Military Medicine. 2005;170:658–662. doi: 10.7205/milmed.170.8.658. [DOI] [PubMed] [Google Scholar]
- Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767–771. doi: 10.1016/s0140-6736(98)03538-7. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O'Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Turell MJ, Sardelis MR, O'Guinn ML, Dohm DJ. Potential vectors of West Nile virus in North America. Curr Top Microbiol Immunol. 2002;267:241–252. doi: 10.1007/978-3-642-59403-8_12. [DOI] [PubMed] [Google Scholar]
- Weinberger M, Pitlik SD, Gandacu D, Lang R, Nassar F, Ben David D, Rubinstein E, Izthaki A, Mishal J, Kitzes R, Siegman-Igra Y, Giladi M, Pick N, Mendelson E, Bin H, Shohat T. West Nile fever outbreak, Israel, 2000: epidemiologic aspects. Emerg Infect Dis. 2001;7:686–691. doi: 10.3201/eid0704.010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R. Ecological Impact of West Nile Virus. Washington DC: Washington Post; 2002. [Google Scholar]