Abstract
The von Economo neurons (VENs) are large bipolar neurons located in fronto-insular cortex (FI) and anterior limbic area (LA) in great apes and humans but not in other primates. Our stereological counts of VENs in FI and LA show them to be more numerous in humans than in apes. In humans, small numbers of VENs appear the 36th week post conception, with numbers increasing during the first eight months after birth. There are significantly more VENs in the right hemisphere in postnatal brains; this may be related to asymmetries in the autonomic nervous system. VENs are also present in elephants and whales and may be a specialization related to very large brain size. The large size and simple dendritic structure of these projection neurons suggest that they rapidly send basic information from FI and LA to other parts of the brain, while slower neighboring pyramids send more detailed information. Selective destruction of VENs in early stages of fronto-temporal dementia implies that they are involved in empathy, social awareness, and self-control, consistent with evidence from functional imaging.
Keywords: fronto-temporal dementia, autism, schizophrenia, empathy, disgust, self-awareness, hemispheric specialization
Introduction
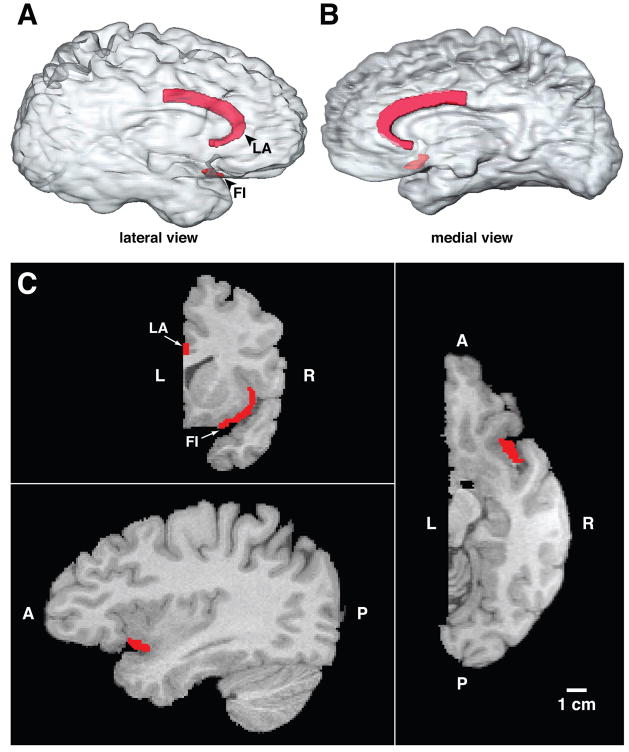
In their comprehensive study of the cytoarchitecture of the human cerebral cortex, von Economo and Koskinas1,2 described large bipolar neurons in fronto-insular (FI) cortex and in the limbic anterior (LA) area, which wraps around the genu of the corpus callosum and extends posteriorly to the midcingulate (see Figure 1). Von Economo3,4 called these specialized neurons the rod and corkscrew cells, referring to the straight and twisted variants of this distinct class of neurons. These unusual cells had previously been observed by many classical neuroanatomists including Betz5 and Ramón y Cajal,6 but von Economo3,4 made a more complete description of their morphology and mapped their specific locations in human cortex. They have often been termed “spindle cells,”7,8 but because of possible confusion with other uses of this term, we have opted to call then von Economo neurons, or VENs.
Figure 1.
The VENs are projection neurons and are substantially larger than their pyramidal cell neighbors.8,9 They possess a single large basal dendrite, distinguishing them from pyramidal neurons, which have an array of smaller basal dendrites.10 This single large basal dendrite may have resulted from a transformation during evolution of the genetic programs for pyramidal neuron development to modify the basal dendrite to concentrate its growth in the primary component and suppress the secondary and tertiary branching. The VENs have a narrow dendritic arborization that spans the layers of the cortex and may be able to sample and rapidly relay the output from a columnar array of neurons.10 The apical and basal dendrites of the VENs are remarkably symmetrical; this architecture suggests that the VENs may be comparing inputs to these two symmetrical dendrites.10 The VENs in LA are filled retrogradely with the carbocyanin tracer DiI after it is deposited in the cingulum bundle, so the VENS are likely to be projection neurons with axonal connections extending through the underlying white mater to other parts of the brain.7 The VENs’ large size and preferential staining with the antibody for non-phosphorylated neurofilament are also characteristic features of cortical projection neurons.7,11 The VENs are thus a significant output from FI and LA, and a consideration of the functions of these areas provides clues as to the kinds of information relayed by the VENs to other parts of the brain.
In anthropoid primates, the posterior insula and anterior cingulate cortex receive differentiated inputs subserving pain, itch, warmth, cooling and sensual touch, which are central components of highly evolved mechanisms for physiological homeostasis.12,13 The VEN-containing areas, FI, which is located in inferior anterior insula, and LA, may be a further elaboration of these mechanisms, which, while retaining some aspects of their basic regulatory functions, have extended to include aspects of the awareness of self and others and decision making under uncertain conditions. In an exhaustive meta-analysis of the imaging data, the inferior anterior insula has been found to be consistently activated by peripheral autonomic changes.14 One such connection between autonomic arousal and decision making is suggested by the findings of Critchley and colleagues,15 who found that anterior insula was activated when subjects had increased galvanic skin responses. The activity of anterior insula also varied as a function of uncertainty during the anticipatory period in a gambling task.16 Preuschoff et al.17 found that the region of anterior insula closely matching the location of FI was specifically activated during “risk prediction error” when a loss as the consequence of a gambling decision was revealed to the subject. The anterior inferior insula, also in a location closely corresponding to FI, is strongly activated by negative feedback in the form of frowning faces in a decision task involving a high degree of uncertainty.18 Frowning faces also activated a site corresponding to the posterior part of LA.18 The region corresponding to FI on the right side is activated when subjects scrutinize facial expressions to discern intentions.19 The integrative functioning of the lateral part of FI in response to negative feedback is illustrated in a series of experiments by Jabbi et al.,20 who elicited activity in this VEN-rich region through experiences involving disgust mediated by taste, by the observation of someone else responding to a disgusting taste, and by imagining a disgusting taste. These data, together with many other experiments, suggest that anterior insula and cingulate cortex are involved in the recognition of error and the initiation of adaptive responses to error and negative feedback.21–24 Anterior insula and cingulate cortex are major components of the system for the flexible control of goal-directed behavior.25 Area LA also includes portions located near the genu of the corpus callosum which are profoundly involved in emotional states; there is substantial functional heterogeneity within the cortex comprising LA, with VENs occurring throughout this structure (see Williamson and Allman26 for a review). The following sections are an attempt to describe some of the complexity of this system.
Anterior insula and anterior cingulated cortex are activated by social error signals
Anterior insula and anterior cingulate cortex are activated by situations that involve social error, a defect in the social network in which the individual is participating, or a change in state of one of the participants. For example, these structures are activated by resentment,27 deception,28 embarrassment,29 and guilt.30 They also are activated by feelings of empathy for the suffering of others, another type of social error signal.31 A meta-analysis of nine brain imaging studies involving empathy revealed consistent activation in FI and the adjacent superior anterior insula as well as in the posterior part of LA in approximately the same region as was activated by frowning faces.32 In mothers, FI in the right hemisphere responds to the cries of distressed infants,33 which are powerful social error signals. The anterior insula (including both superior and inferior components) was activated when partners in the prisoner’s dilemma game failed to reciprocate cooperative moves made by the subject, another type of social error signal.34 Anterior insula and anterior cingulate cortex are activated by prosocial signals such as love and trust,35,36 which suggests that these structures register both negative and positive aspects of the states of social networks. The responses of FI and LA are parametrically related to how humorous subjects judge cartoons to be; the humorous content of the cartoons typically involved social errors.37
VENs in neuropsychiatric disorders
The VENs are implicated in several neuropsychiatric illnesses. In a stereological study, Seeley et al.38–40 found that the VENs are specifically and selectively attacked in the early stages of the behavioral variant of fronto-temporal dementia (FTD), in which empathy, social awareness, and self-control are severely diminished. The VEN population of ACC is reduced by an average of 74% in these patients, and many of the surviving VENs are severely dysmorphic. The destruction of the VENs in FTD results from two distinct molecular mechanisms in different patients. One mechanism is related to abnormal isoforms of the tau protein, and the other is related to abnormal expression in the cytoplasm of VENs of the DNA-binding protein TDP-43.38,40 The VENs are also reduced in FI.39 In contrast, Seeley et al.38,39 found that the VENs were not significantly reduced in Alzheimer’s dementia (AD), although a reduction had been reported in an earlier study that did not use stereological methods.7
Agenesis of the corpus callosum is another condition in which abnormal social behavior may be linked to reduced VEN populations. Patients with agenesis of the corpus callosum often have impoverished and superficial relationships, suffer from social isolation, and have interpersonal conflicts both at home and at work due to misinterpretation of social cues.41 Kaufman et al.42 found that the VENs were reduced by 50% in a subject with partial agenesis of the corpus callosum and by 90% in a subject with complete agenesis when compared to adult controls. The VEN loss could not be attributed to the reduction or absence of the corpus callosum itself, because the VEN concentration in FI was normal in another subject whose corpus callosum was destroyed as the result of a stroke 15 years before her death. Because the surgical sectioning of the corpus callosum does not disrupt social behavior,43 the social deficit in agenesis of the corpus callosum may be related to VEN loss.
There are many features of autism that suggest that the VENs may be involved in this disorder.44,45 An initial stereological study of the number of VENs in area FI in four autistic subjects plus controls did not confirm this conjecture.46 However, a second stereological study of VENs in dorsal ACC in nine autistic subjects plus controls found that the autistic subjects fell into two groups, one with significantly higher numbers of VENs than controls, and the other with significantly fewer VENs than controls.47 Thus the controls occupied a middle zone with little overlap with the high or low VEN autism groups. The results of Simms et al.47 suggest that two different mechanisms influence the number of VENs in autism, possibly through different effects on migration and survival. A very recent study done in FI found a significantly higher ratio of VENs to pyramids in autistic subjects as compared to controls.48 As in fronto-temporal dementia, in autism the VENs may be vulnerable to more than one pathological process contributing to the disorder as it manifests in different individuals. The higher ratio of VENs to pyramids may paradoxically be associated with a reduction in activity in FI. A strong linkage between reduced activity in the right anterior insula in autistic subjects versus controls in social tasks was revealed in a meta-analysis of 24 functional imaging studies.49
Finally, the VENs have been implicated in schizophrenia. The VENs in the right hemisphere in ACC are reduced in number in early onset schizophrenia when compared with later-onset schizophrenia, bipolar disorder, and normal controls.50
VEN locations and stereological counts
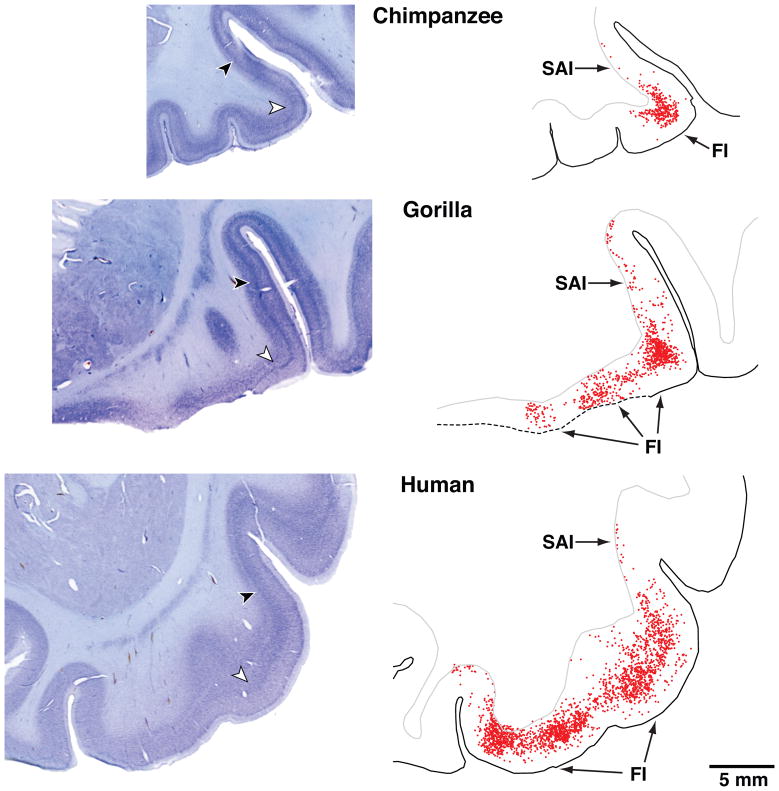
The locations of the VEN-containing areas FI and LA are illustrated in three-dimensional transparent reconstructions of the right hemisphere of a human brain in Figure 1. In Figure 2, every VEN is plotted for a section through FI and adjacent cortex from a chimpanzee, a gorilla, and a human. To the left of each section is a corresponding low-power photomicrograph of the section.
Figure 2.
Photomicrographs of frontal sections through area FI in a 39-year-old male chimpanzee, a 27-year-old male gorilla, and a 1.6-year-old male human. On the right side are outlines of the corresponding sections in which the location of each VEN has been plotted. There are 354 VENs plotted in the chimpanzee, 919 in the gorilla, and 2415 in the human plotted by scanning through the different depth planes with a 40X oil immersion lens with the aid of Stereoinvestigator software. The sections were 100 micra thick. All images are represented at the same scale. The sections are from FI on the right side except for the gorilla, in which FI was damaged during histological processing and instead the left FI has been used and the image reversed for ease of comparison with the other cases. The locations of the higher magnification photomicrographs shown in Figure 4 are indicated by arrows in the low power photomicrographs. The section illustrated for the gorilla corresponds approximately to Figure 12B, which shows the location of the seed voxels for the tractography done in the same individual. FI, fronto-insular cortex; SAI, superior insular cortex.
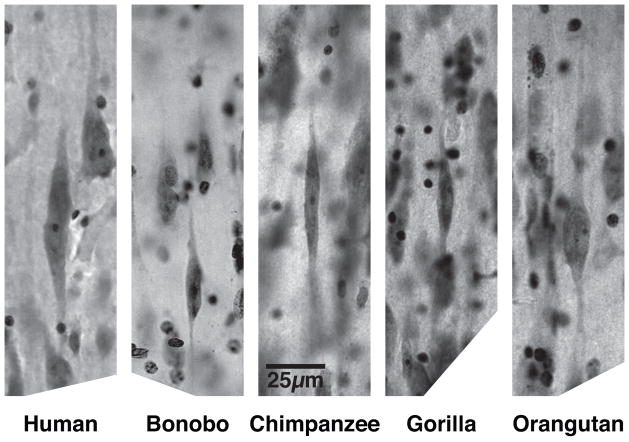
The VEN-containing area is largely confined to the region of high flexure in the chimpanzee, but extends medially in the gorilla and even farther in the medial direction in the human. The transition between FI and superior anterior insula (SAI) corresponds to a gradient in VEN density rather than a sharp border. The cytoarchitecture of area LA and the location of the VENs in this part of anterior cingulate cortex are illustrated in von Economo2 and Nimchinsky et al.7,8 The VENs are illustrated at higher magnification in Figure 3, which shows that they have very similar morphology in the great apes and humans. In primates, the VENs are present in FI only in great apes and humans. This is the same taxonomic distribution as was found for the VENs in LA,8 which suggests that the VENs emerged as a specialized neuron type in the common ancestor of great apes and humans. However, in orangutans we found only one out of seven individuals examined to have a substantial VEN population in FI and LA.
Figure 3.
VENs in area FI of humans and great apes. Photomicrographs are of Nissl-stained sections. All panels share the scale indicated in the central panel.
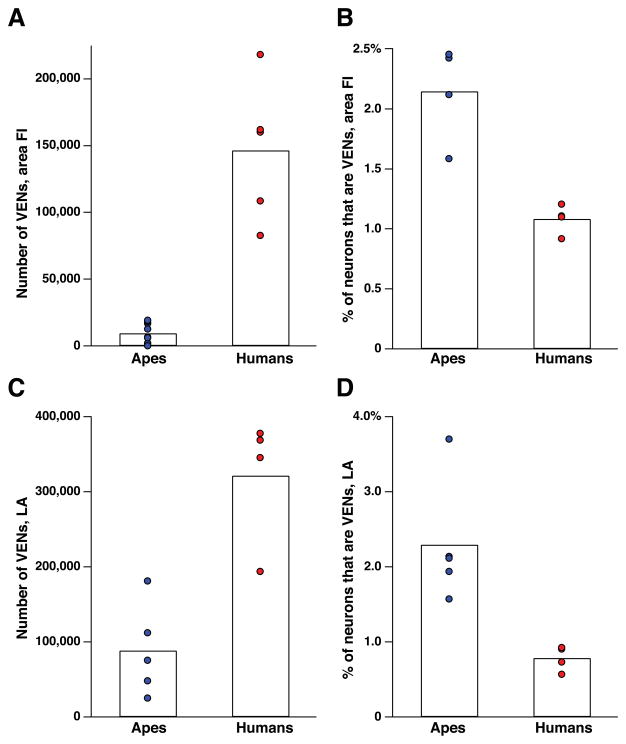
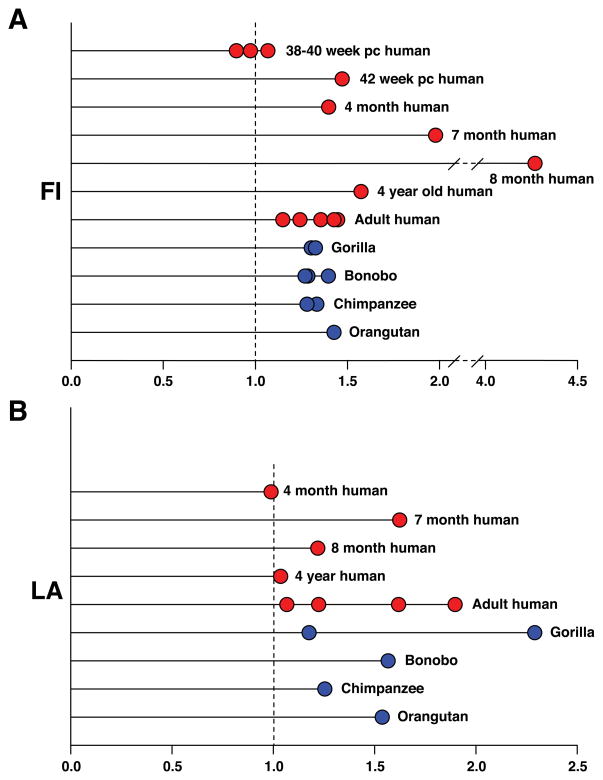
Our key stereological findings are represented in the form of graphs (Figs. 4–6); a more extensive account of the stereological findings together with the methods employed is given in Allman et al.51 Figure 4 shows that the VENs are more numerous in humans than in apes, but that the VENs constitute a higher percentage of the total neurons in the regions of interest in apes than in humans. In LA, one of the individual apes (a gorilla) stands out as having considerably more VENs than the others, approaching the lower end of the human range. The VEN count was similarly elevated relative to the other apes in this same gorilla in FI on the left side and also approached the lower end of the human range for this structure; unfortunately, postmortem damage to right FI in this individual made it impossible to make a stereological count for this structure in the right hemisphere. The relative abundance of VENs in this gorilla is also illustrated in comparison to a chimpanzee in Figure 2. This individual gorilla had an exceptionally enriched environment.52 Although we can conclude nothing definitive from this isolated observation, it does raise the possibility that VEN abundance may be related to environmental influences.
Figure 4.
A comparison of the number and proportion of VENs in area FI and ACC of adult humans and great apes. (See Tables 2 and 3 for data.) Bars indicate the average of all data points in a given column. (A) The number of VENs in area FI (both hemispheres combined). FI contains many more VENs in humans than in great apes (P = 0.001). (B) The percentage of neurons in area FI that are VENs. Although the great apes have a smaller total number of VENs in FI, the have a higher proportion of VENs to non-VEN neurons in FI (P = 0.029). (C) The number of VENs in ACC (both hemispheres combined). As in area FI, humans have more VENs than the great apes (P = 0.016), although this difference is less great in ACC. (D) The percentage of neurons in ACC that are VENs. Again, as in FI, the great apes have a higher percentage of neurons that are VENs (P = 0.016). All comparisons are Mann–Whitney U tests.
Figure 6.
The ratio of the number of VENs in the right hemisphere to the number of VENs in the left hemisphere. (A) In postnatal humans and great apes, there are consistently more VENs in FI on the right side. This ratio develops after birth. In neonates, the numbers in each hemisphere are almost even, while in infants, juveniles, and adults there are many more VENs in the right hemisphere. When the numbers of VENs in the right and left hemispheres were compared for FI in the postnatal cases, the difference was statistically significant both with and without the eight-month-old outlier (P = 0.0039 for all postnatal humans and P = 0.0078 without the eight-month-old case). For postnatal apes and humans combined, the hemispheric difference for FI was significant at P < 0.0001. (B) The ratio of VENs in right and left LA. This ratio is less consistent than in area FI, but in almost all cases there are more VENs on the right side. When the number of VENs in the right and left hemispheres in postnatal humans was compared for LA, the result was statistically significant (P = 0.03). When postnatal apes and humans were combined, the difference was significant at P = 0.001. Significance was determined using the Mann–Whitney U test.
VENs emerge mostly postnatally and are more abundant in the right hemisphere
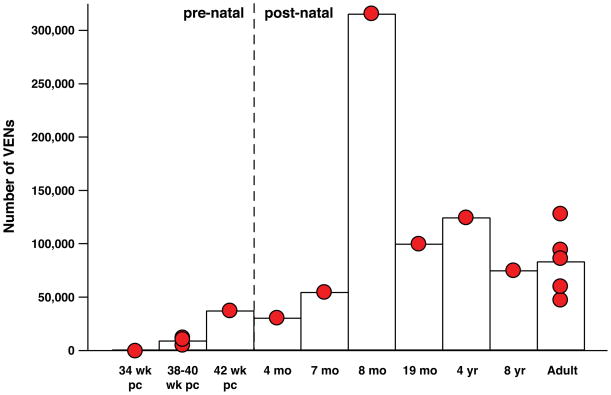
We examined FI and LA in fetal brains at post-conception ages of 32 weeks (n = 1), 33 weeks (n = 1), 34 weeks (n = 3), and 35 weeks (n = 2), and no VENs were found. In one 36-week post-conception brain, small numbers of VENs were present in FI and LA. Figure 5 shows that the VENs exist in relatively low numbers in FI at birth; in LA, the VENs were so rare that we were not able to make stereological estimates in the brains of neonates. In the late-term neonate (42 weeks post-conception), the number of VENs in FI was considerably higher than in the normal-term neonates (38 to 40 weeks post-conception), suggesting that the number of VENs in FI increases immediately after the normal time of birth. The number of VENs is significantly greater in the postnatal brains relative to the neonatal brains. The percentage of total neurons that are VENs is relatively stable in adulthood and is similar to the percentages observed by Seeley et al.38
Figure 5.
The number of VENs increases after birth. The number of VENs in right hemisphere FI in humans of different ages. VEN numbers are low in neonates and increase after birth. The eight-month-old individual examined had markedly more VENs in the right hemisphere than any other subject in this study; this might possibly be due to individual variation. The right hemisphere VEN measurement in this individual was repeated with similar results (see Table 2). The difference between the number of VENs in right FI for pre- and post-natal subjects was statistically significant (P = 0.0029), and this significance remained when the eight-month-old individual was removed from the comparison (P = 0.0040). The number of VENS in left FI and in both hemispheres together was also significantly different for pre- and post-natal individuals (P = 0.0056 for both). Significance was determined using the Mann–Whitney U test.
Figure 6 illustrates the ratio between the number of VENs in the right hemisphere and that in the left. In newborns, there is no clear hemispheric preference, but nearly all of the post-natal humans and all of the apes show a clear predominance of VENs in the right hemisphere in both FI and LA. In FI, nearly all the postnatal cases have 20–40% more VENs in the right hemisphere, except for the eight-month-old infant, which is an extreme outlier. In ACC, nearly all the cases show a rightward predominance, but the numbers are much more variable than in FI. It is interesting in this context that the VENs are vulnerable in the right hemisphere in ACC in early onset schizophrenia.50
The VENs mostly emerge postnatally, which can be seen in their numbers, concentrations, and the formation of the hemispheric predominance of VENs on the right side in the first few months after birth. This emergence could come about by the transformation of another cell type into the VENs or by postnatal neurogenesis. The long, thin spindle shape of the VENs with their sometimes undulating apical and basal dendrites closely resembles that of migrating neurons with undulating leading and trailing processes, and this is particularly evident in infant brains.11 Although there are many technical difficulties in experimentally resolving whether the VENs arise by transformation or postnatal neurogenesis, future research should reveal whether either of these possibilities is correct.
An important finding in our study is the larger number of VENs in the right hemisphere than the left except in very young subjects, and thus that the rightward asymmetry emerges during the first few months of postnatal life. Stereological evidence for hemispheric differences in neuron number in primates (including humans) is very limited. Uylings et al.53 found a trend toward a larger number of total neurons in the left hemisphere of Broca’s area for a population of five female brains. Sherwood et al.54 found that right–left differences in the density of parvalbumin-positive interneurons in layers 2 and 3 of primary motor cortex in chimpanzees is linked to hand preference.
However, there is excellent evidence that the anterior cingulate cortex is larger on the right side from a structural MRI study of 100 young adult subjects. One study found that ACC is 13% larger on the right side, while the size of posterior cingulate cortex is the same in both hemispheres.55 There is also structural MRI data for 142 young adults that suggests that FI is enlarged by about the same amount on the right side.56 The significantly increased number of VENs in the right hemisphere in FI and ACC is among the few demonstrations of hemispheric differences in neuron number based on stereological techniques, and these rightward predominances correspond to size differences in FI and ACC observed in structural MRI studies done in large populations of adult human subjects. The fact that these hemispheric differences are present in both humans and great apes suggests that they may have existed in the common ancestor of both groups. There is recent evidence from a developmental MRI study based on 316 right-handed subjects that the anterior insular and posterior orbitofrontal cortex is thinner on the right side than the left at age four in normal subjects but progresses by age 20 to be significantly thicker, by about 0.3 mm, on the right side than the left.57 The rightward asymmetry in cortical thickness for the region containing FI in the adult brain is consistent with previous MRI studies done in adults and with the rightward asymmetry in VEN numbers in our study; however, in our study we found a rightward asymmetry in VEN numbers throughout postnatal life. Thus, the rightward predominance of VEN numbers develops before the predominance in cortical thickness in this cortical region.
What is the biological significance of the rightward hemispheric asymmetry of the VENs in FI and ACC? The VEN asymmetry may be related to asymmetry in the autonomic nervous system in which the right hemisphere is preferentially involved in sympathetic activation, as would result from negative feedback and subsequent error-correcting behavior; the left hemisphere is preferentially involved in parasympathetic activity associated with reduced tension or calming responses.58 Following this reasoning, there may be more VENs on the right side, because the responses to negative feedback require more complex and more urgent behavioral responses than do situations that are calming and involve reduced tension. Many of these experiments probably have in common a right FI response similar to that which has been specifically linked to sympathetic arousal as measured by the galvanic skin response.15 A meta-analysis of coactivation of amygdala and insula involving 955 responses in 86 papers reported coactivation between the amygdala and inferior anterior insula on both sides, but found it to be more pronounced on the right.14 In a meta-analysis of 23 functional imaging studies conducted in children and adolescents performing various executive functioning tasks such as go vs. nogo, which typically involve intense focus and self-control, Houdé et al.59 found that in children the most consistent site of activation was in anterior insula on the left side, while in adolescents the most consistent site of activation was the inferior anterior insula corresponding to FI on the right side. Thus, the right FI becomes strongly engaged in executive functioning and self-control in adolescents. Houdé et al.59 say that this change “is consistent with the fact that adolescents are often psychologically embedded in a period of great emotional reactivity and sensitivity with negative feelings. Recognizing the necessity of being wrong is necessary to achieve high levels of adult adaptation and maturity in cognitive control. Our result might reflect a key transition around the time of adolescence toward increased influence of negative feedback (i.e. error detection and/or anticipation) on cognitive control.”
There is also some evidence of preferential leftward activation in FI and ACC involving positive and affiliative emotions.13,35,60 The left anterior cingulate cortex was preferentially activated when subjects relaxed and reduced their sympathetic arousal through biofeedback.61 There is evidence that the right hemisphere of the brain is related to sympathetic arousal and the left hemisphere to parasympathetic quietude.58,62,65 This autonomic asymmetry is consistent with the proposal that the right hemisphere responds to the unexpected and the left hemisphere to more routine stimuli.64 There is also evidence for this autonomic asymmetry from electrical stimulation of the insular cortex on the right and left sides in human subjects.65 Craig58 suggests that sympathetic activation on the right side and consequent energy expenditure by the organism, and parasympathetic activation on the left and energy conservation together, function to serve as a balancing mechanism for managing the organism’s energy resources. These mechanisms involve in part highly conserved circuits in the vagal complex that regulate respiration and the production of vocalizations throughout vertebrates.66
VENS in evolution
In immunocytochemical studies, many VENS in FI and LA express gastrin-releasing peptide (GRP) and neuromedin B (NMB), which are bombesin hormones involved in gastric function and peristalsis in the gut and satiety in the brain.46,67 GRP and NMB are expressed in very specific populations of neurons in anterior insula and anterior cingulate cortex in mice in the in situ hybridization maps in the Allen Brain Atlas (http://www.brain-map.org/). These findings, together with the presence of VENs in the apparent homologs of FI and ACC in such phylogenetically diverse mammals such as apes, humans, elephants and whales, suggests that they are derived from common populations of neurons in anterior insula and anterior cingulate cortex that were present in primitive mammals.68–70 The existence of VENs in primates is not related to relative brain size or encephalization.51 Instead, it appears to be related to absolute brain size. The VENs are present in primates with adult brain sizes greater than about 300 grams and in elephants and whales, which also have very large brains. We suggest that large brain size and complex social behavior both favor specialized neural systems for rapid communication within brain circuits. Large brains may be inherently slower because of the greater distances over which messages must be sent. Large brains also suffer from the limitations associated with packing large myelinated axons into a restricted space. The corpus callosum in large brains has small numbers of very large axons.71 These very large axons may relay the gist of the information between the hemispheres, which is then followed by more detailed information communicated by smaller, slower-conducting axons. These very large axons would thus enable the fast communication between the hemispheres that would otherwise be limited by size and distance in large brains while conforming with the packing constraint that would not allow the scaling up of all axons because of spatial limitations. We suggest that there is an analogous temporal division of labor between the VENs and the neighboring pyramids, which may serve as a compromise between the needs for rapid communication and the inability to enlarge all axons.71 We believe that the large size and simple dendritic architecture of the VENs supports the conjecture that they are built for speed. We predict that the axon calibers of the VENs are larger than in the neighboring pyramids and that the VEN axons are faster conducting than their neighbors. The evolution of the VENs may be an adaptation related to large brain size allowing the gist of the information processed within a cortical column to be relayed rapidly to other brain structures. This putative rapid relay may be particularly important for the relay of social decisions, although not restricted to the social domain.26,41 Complex social behavior is often fast-paced, and this puts a premium on the capacity to respond quickly to changing conditions. A basic function of FI may be to register feedback crucial for initiating fast adaptive responses to changes, which would be consistent with the activity of FI preceding linked activity in ACC and other cortical areas.72
We found two interesting differences between the distribution of VENs in humans and in apes. The first difference between humans and apes is the relationship between VEN location and agranular insular cortex, i.e., insular cortex lacking a layer 4. In humans, area FI, which is defined by the presence of VENs, appears to correspond to most of agranular insular cortex as delinated by Rose.73 However, in apes, area FI appears to correspond to a smaller part of the total agranular insular cortex. This difference may explain why there are typically considerably more VENs in humans than in apes. The second difference between humans and apes is that the density of VENs relative to other neurons is significantly higher for apes than humans in FI and ACC. One possible explanation for this surprising finding is that there may be other specialized neuronal populations that are differentially expanded in humans relative to apes.
The anterior insula and VENs may be linked to awareness
Recent work suggests that the anterior insula is involved in awareness.74–76 This connection with awareness was initially suggested in an fMRI experiment by Kikyo and Ohki,77 in which they observed activity in anterior insula when subjects reported the subjective sense of knowing a word before recalling it in a memory task, which these authors called the “feeling of knowing.” More recently, in an experiment employing a behavioral paradigm in which objects gradually emerge from noise, Ploran et al.74 found that the activity of anterior insula was strongly linked to the moment when the subjects became aware of the identity of the object.74 More recent work from the same group has identified several components of this activity, including one in inferior anterior insula, particularly on the right side.75 Devue et al.78 found foci of activity in FI and LA in subjects when viewing their own faces as compared with the familiar faces of colleagues, suggesting that these foci may be involved in discriminating self from other. We have proposed44 that the VENs and related circuitry are involved in rapid intuition, which, like perceptual recognition, involves immediate effortless awareness rather than the engagement of deliberative processes. Recently, Aziz-Zadeh et al.79 found that when subjects were solving anagrams and arrived at rapid insightful solutions (“aha” moments), both anterior insula and anterior cingulate cortex were activated. These aspects of awareness are not limited to body states, but involve visual and linguistic experiences as well, suggesting the hypothesis that the role of anterior insula in awareness may include most or all aspects of perception and cognition. An extension of this hypothesis is that the VENs in FI serve as a fast relay of this information to other parts of the brain.
Acknowledgments
The authors would like to thank Dr. Barbara Wold, Dr. Chet Sherwood, Dr. Bill Seeley, and Dr. A. D. Craig for their invaluable comments and discussion. We thank Dr. Micheal Tyszka and Dr. Jason Kaufman for the MRI imaging of the ape brains. We thank Dr. Kristen Tillisch and Dr. Emeran Mayer for the MR images of the young adult human subject. We thank Dr. Heidi Griffith for her help in collecting some of the human stereological data. We thank Archibald Fobbs, curator of the Yakovlev and Welker Brain Collections, and Dr. Adrianne Noe, Director of the National Museum of Health and Medicine, for their crucial role in preserving these collections and making them available to us and to the broader scientific community. In the Hof lab, technical help was provided by B. Wicinski and S. Harry. Several of the great ape brains involved in this study were on loan to the “Great Ape Aging Project” from zoological gardens that are accredited by the Association of Zoos and Aquariums (AZA) and that participate in the Ape Taxon Advisory Group (Ape-TAG). We especially appreciate the contribution of zoo veterinarians and staff in collecting and providing specimens.
Additional human tissue was obtained from the National Institute of Child Health and Human Development Brain and Tissue Bank for Developmental Disorders. Some comparative specimens were collected under the “Comparative Neurobiology of Aging Resource,” NIH/NIA grant AG14308, J. Erwin, PI. This research was supported by the James S. McDonnell Foundation, the David and Lucille Packard Foundation, the Simons Foundation, and the National Institute of Mental Health.
References
- 1.von Economo C, Koskinas G. Die cytoarchitectonik der hirnrinde des erwachsenen menschen. Berlin: Springer; 1925. [Google Scholar]
- 2.von Economo C. In: Cellular structure of the human cerebral cortex. Triarhou LC, translator. Basel: Karger; 2009. [Google Scholar]
- 3.von Economo C. Eine neue Art Spezialzellen des Lobus cinguli und Lobus insulae. Zschr ges Neurol Psychiat. 1926;100:706–712. [Google Scholar]
- 4.Seeley W, Craig A, Hof P, Merkle F, Gaus S, Allman J. Distinctive neurons in anterior cingulated and frontoinsular cortex: a historical perspective. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr005. to be published in April 2011. [DOI] [PubMed] [Google Scholar]
- 5.Betz W. Ueber die feinere Structur der Gehirnrinde des Menschen. Zentralbl Med Wiss. 1881;19:193–195. 209–213, 231–234. [Google Scholar]
- 6.Ramón y, Cajal S. Textura del Sistema Nervioso del Hombre y de los Vertebrados, Tomo II. Madrid: Nicolás Moya; 1899. [Google Scholar]
- 7.Nimchinsky EA, Vogt BA, Morrison JH, Hof PR. Spindle neurons of the human anterior cingulate cortex. J Comp Neurol. 1995;355:27–37. doi: 10.1002/cne.903550106. [DOI] [PubMed] [Google Scholar]
- 8.Nimchinsky E, Gilissen E, Allman J, Perl D, Erwin J, Hof P. A neuronal morphologic type unique to humans and great apes. Proc Nat Acad Sci U S A. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. Anterior cingulate cortex: The evolution of an interface between emotion and cognition. Ann NY Acad Sci. 2001;935:107–117. [PubMed] [Google Scholar]
- 10.Watson KK, Jones TK, Allman JM. Dendritic architecture of the von Economo neurons. Neuroscience. 2006;141:1107–1112. doi: 10.1016/j.neuroscience.2006.04.084. [DOI] [PubMed] [Google Scholar]
- 11.Allman J, Hakeem A, Watson K. Two phylogenetic specializations in the human brain. Neuroscientist. 2002;8:335–346. doi: 10.1177/107385840200800409. [DOI] [PubMed] [Google Scholar]
- 12.Craig AD. A new view of pain as a homeostasis emotion. Trends in Neuroscience. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. [DOI] [PubMed] [Google Scholar]
- 13.Craig AD. How do you feel--now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 14.Mutschler I, Wieckhorst B, Kowalevski S, Derix J, Wentlandt J, Schulze-Bonhage A, Ball T. Functional organization of the human inferior insular cortex. Neurosci Lett. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. [DOI] [PubMed] [Google Scholar]
- 15.Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses. Journal of Neuroscience. 2000;20:3033–3040. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Critchley HD, Mathias C, Dolan R. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29:537–545. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- 17.Preuschhoff K, Quartz S, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28:2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- 19.Baron-Cohen S, Ring HA, Wheelwright S, Bullmore ET, Brammer MJ, Simmons A, Williams SC. Social intelligence in the normal and autistic brain: an fMRI study. Eur J Neurosci. 1999;11:1891–1898. doi: 10.1046/j.1460-9568.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 20.Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLos One. 2008;8:e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gehring WJ, Goss B, Coles MGH, Meyer DE, Donchin E. A neural system of error detection and compensation. Psychol Sci. 1993;4:385–390. [Google Scholar]
- 22.Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–305. [Google Scholar]
- 23.Klein TA, Endrass T, Kathmann N, Neumann J, von Cramon DY, Ullsperger M. Neural correlates of error awareness. NeuroImage. 2007;34:1774–1781. doi: 10.1016/j.neuroimage.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Lamm C, Singer T. Role of anterior insular cortex in social emotions. Brain Struct Funct. 2010;214:579–591. doi: 10.1007/s00429-010-0251-3. [DOI] [PubMed] [Google Scholar]
- 25.Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Fox MD, Snyder AZ, Vincent JL, Raichle ME, Schlaggar BL, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci USA. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Williamson PC, Allman JM. The Human Illnesses: Neuropsyciatric Disorders and the Nature of the Human Brain. New York: Oxford University Press; 2010. [Google Scholar]
- 27.Sanfey AG, Rilling RJ, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the ultimatum game. Science. 2003;300:1755–1758. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 28.Spence SA, Farrow TF, Herford AE, Wilkinson ID, Zheng Y, Woodruff PW. Behavioural and functional anatomical correlates of deception in humans. Neuroreport. 2001;12:2849–2853. doi: 10.1097/00001756-200109170-00019. [DOI] [PubMed] [Google Scholar]
- 29.Berthoz S, Armony JL, Blair RJR, Dolan RJ. An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain. 2002;125:1696–1708. doi: 10.1093/brain/awf190. [DOI] [PubMed] [Google Scholar]
- 30.Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML, Alpert NM, Fischman AJ, Rauch SL. Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry. 2000;48:43–50. doi: 10.1016/s0006-3223(00)00251-1. [DOI] [PubMed] [Google Scholar]
- 31.Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004a;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 32.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. Neuroimage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51:431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 34.Rilling J, Goldsmith D, Glenn A, Jairam M, Elfenbein H, Dagenais J, Murdock C, Pagnoni Neural correlates of the affective response to unreciprocated cooperation. Neuropsychologia. 2008;46:1265–1266. doi: 10.1016/j.neuropsychologia.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 35.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 36.Singer T, Kiebel SJ, Winston JS, Dolan RJ, Frith CD. Brain responses to the acquired moral status of faces. Neuron. 2004;41:653–662. doi: 10.1016/s0896-6273(04)00014-5. [DOI] [PubMed] [Google Scholar]
- 37.Watson KK, Matthews BJ, Allman JM. Brain activation during sight gags and language-dependent humor. Cereb Cortex. 2007;17:314–324. doi: 10.1093/cercor/bhj149. [DOI] [PubMed] [Google Scholar]
- 38.Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL, Dearmond SJ. Early fronto-temporal dementia targets neurons unique to apes and humans. Ann Neurol. 2006;60:660–667. doi: 10.1002/ana.21055. [DOI] [PubMed] [Google Scholar]
- 39.Seeley WW, Allman JM, Carlin DA, Crawford RK, Macedo MN, Greicius MD, Dearmond SJ, Miller BL. Divergent social functioning in behavioral variant fronto-temporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord. 2007;21:S50–57. doi: 10.1097/WAD.0b013e31815c0f14. [DOI] [PubMed] [Google Scholar]
- 40.Seeley WW. Selective functional, regional, and neuronal vulnerability in fronto-temporal dementia. Curr Opin Neurol. 2008;21:701–707. doi: 10.1097/WCO.0b013e3283168e2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P, Sherr EH. Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci. 2007;8:287–299. doi: 10.1038/nrn2107. [DOI] [PubMed] [Google Scholar]
- 42.Kaufman JA, Paul LK, Manaye KF, Granstedt AE, Hof PR, Hakeem AY, Allman JM. Selective reduction of Von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathol. 2008;116:479–489. doi: 10.1007/s00401-008-0434-7. [DOI] [PubMed] [Google Scholar]
- 43.Shorvon S. Handbook of Epilepsy Treatment. New York: Wiley; 2005. [Google Scholar]
- 44.Allman J, Watson K, Tetreault N, Hakeem A. Intuition and autism: a possible role for von Economo neurons. Trends Cognit Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 45.van Kooten . Dissertation. Maastricht University; 2008. Autism counts. [Google Scholar]
- 46.Kennedy DP, Semendeferi K, Courchesne E. No reduction of spindle neuron number in frontoinsular cortex in autism. Brain Cogn. 2007;64:124–129. doi: 10.1016/j.bandc.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Simms ML, Kemper TL, Timbie CM, Bauman ML, Blatt GJ. The anterior cingulate cortex in autism: heterogeneity of qualitative and quantitative cytoarchitectonic features suggests possible subgroups. Acta Neuropathol. 2009;118:673–684. doi: 10.1007/s00401-009-0568-2. [DOI] [PubMed] [Google Scholar]
- 48.Santos M, Uppal N, Butti C, Wicinski B, Schmeidler J, Giannakopoulos P, Heinsen H, Schmitz C, Hof P. Von Economo neurons in autism: a stereological study of the frontoinsular cortex in children. Brain Res. 2011;1380:206–217. doi: 10.1016/j.brainres.2010.08.067. [DOI] [PubMed] [Google Scholar]
- 49.Di Martino A, Ross K, Uddin L, Sklar A, Castellanos F, Milham M. Processes in autism spectrum disorders: an activation likelihood estimation meta-analysis. Biological Psychiatry. 2009;65:63–74. doi: 10.1016/j.biopsych.2008.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Brune M, Schobel A, Karau R, Benali A, Faustmann P, Juckel G, Petrasch-Parwez E. Von Economo neuro density in anterior cingulate cortex is reduced in early onset schizophrenia. Acta Neuropathol. 2010;119:771–778. doi: 10.1007/s00401-010-0673-2. [DOI] [PubMed] [Google Scholar]
- 51.Allman JM, Tetreault NA, Hakeem AY, Manaye KF, Semendeferi K, Erwin JM, Park S, Goubert V, Hof PR. The von Economo neurons in frontoinsular and anterior cingulate cortex in great apes and humans. Brain Struct Funct. 2010;214:495–517. doi: 10.1007/s00429-010-0254-0. [DOI] [PubMed] [Google Scholar]
- 52.Patterson F, Gordon W. Twenty-seven years of Project Koko and Michael. In: Galdikas B, Briggs N, Sheeran L, Shapiro G, Goodall J, editors. All apes great and small. I. New York: Kluwer Press; 2002. pp. 165–176. [Google Scholar]
- 53.Uylings H, Jacosen A, Zilles K, Amunts K. Left-right asymmetry in volume and number of neurons in adult Broca’s area. Cortex. 2006;42:652–658. doi: 10.1016/s0010-9452(08)70401-5. [DOI] [PubMed] [Google Scholar]
- 54.Sherwood CC, Wahl E, Erwin JM, Hof PR, Hopkins WD. Histological asymmetries of primary motor cortex predict handedness in chimpanzees. J Comp Neurol. 2007;503:525–537. doi: 10.1002/cne.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gündel H, López-Sala A, Ceballos-Baumann AO, Deus J, Cardoner N, Marten-Mittag B, Soriano-Mas C, Pujol J. Alexithymia correlates with the size of right anterior cingulate. Psychosom Med. 2004;66:132–140. doi: 10.1097/01.psy.0000097348.45087.96. [DOI] [PubMed] [Google Scholar]
- 56.Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, Taylor J, Worsley KJ, Evans AC. Structural asymmetries in human brain: a voxel-based statistical analysis of 142 brains. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 57.Shaw P, Lalonde F, Lepage C, Rabin C, Eckstrand K, Sharp W, Greenstein D, Evans A, Giedd JM, Rapoport J. Development of cortical asymmetry in typically developing children and its disruption in attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2009;66:888–896. doi: 10.1001/archgenpsychiatry.2009.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craig AD. Forebrain emotional asymmetry: a neuroanatomical basis? Trends Cognit Sci. 2005;912:566–571. doi: 10.1016/j.tics.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 59.Houdé O, Rossi S, Lubin A, Joliot M. Mapping numerical processing, reading, and executive functions in the developing brain: an fMRI meta-analysis of 52 studies including 842 children. Developmental Science. 2010;1:1–10. doi: 10.1111/j.1467-7687.2009.00938.x. [DOI] [PubMed] [Google Scholar]
- 60.Ortigue S, Grafton S, Bianchi-Demicheli F. Correlation between insula activation in self-reported quality of orgasm in women. Neuroimage. 2007;37:551–560. doi: 10.1016/j.neuroimage.2007.05.026. [DOI] [PubMed] [Google Scholar]
- 61.Critchley HD, Melmed RN, Featherstone E, Mathias CJ, Dolan RJ. Brain activity during biofeedback relaxation: a functional neuroimaging investigation. Brain. 2001;124:1003–1012. doi: 10.1093/brain/124.5.1003. [DOI] [PubMed] [Google Scholar]
- 62.Wittling W. Brain Asymmetry in the control of autonomic-physiological activity. In: Davidson RJ, Hugdahl K, editors. Brain asymmetry. Cambridge: MIT Press; 1995. pp. 305–356. [Google Scholar]
- 63.Rogers L, Andrew R. Comparative Vertebrate Lateralization. Cambridge: Cambridge University Press; 2002. [Google Scholar]
- 64.MacNeilage PF, Rogers LJ, Vallortigara G. Origins of the left & right brain. Sci Am. 2009;301:60–67. doi: 10.1038/scientificamerican0709-60. [DOI] [PubMed] [Google Scholar]
- 65.Oppenheimer SM, Gelb A, Girvin JP, Hachinski VC. Cardiovascular effects of human insular cortex stimulation. Neurology. 1992;42:1727–32. doi: 10.1212/wnl.42.9.1727. [DOI] [PubMed] [Google Scholar]
- 66.Bass AH, Gilland EH, Baker R. Evolutionary origins for social vocalization in a vertebrate hindbrain-spinal compartment. Science. 2008;321:417–421. doi: 10.1126/science.1157632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stimpson C, Tetreault N, Allman J, Jacobs B, Butti C, Hof P, Sherwood C. Biochemical specificity of von Economo neurons in hominoids. American Journal of Human Biology. 2010;23:22–28. doi: 10.1002/ajhb.21135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hof PR, Van der Gucht E. Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae) Anat Rec. 2007;290:1–31. doi: 10.1002/ar.20407. [DOI] [PubMed] [Google Scholar]
- 69.Butti C, Sherwood CC, Hakeem AY, Allman JM, Hof PR. Total number and volume of Von Economo neurons in the cerebral cortex of cetaceans. J Comp Neurol. 2009;515:243–259. doi: 10.1002/cne.22055. [DOI] [PubMed] [Google Scholar]
- 70.Hakeem AY, Sherwood CC, Bonar CJ, Butti C, Hof PR, Allman JM. Von Economo neurons in the elephant brain. Anat Rec (Hoboken) 2009;292:242–248. doi: 10.1002/ar.20829. [DOI] [PubMed] [Google Scholar]
- 71.Wang SS, Shultz JR, Burish MJ, Harrison KH, Hof PR, Towns LC, Wagers MW, Wyatt KD. Functional trade-offs in white matter axonal scaling. J Neurosci. 2008;28:4047–4056. doi: 10.1523/JNEUROSCI.5559-05.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rose M. Die Inselrinde des Menschen und der Tiere. J Psychol Neurol. 1928;37:467–624. [Google Scholar]
- 74.Ploran E, Nelson S, Velanova K, Petersem S, Wheeler M. Evidence accumulation and moment of recognition: Dissociating perceptual recognition processes using fMRI. Journal of Neuroscience. 2007;27:11012–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nelson S, Dosenbach N, Cohen A, Schlaggar B, Petersen S. Role of the anterior insula in task-level control and focal attention. Brain Struct Funct. 2010;214:669–680. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Craig AD. The sentient self. Brain Struct Funct. 2010;214:563–577. doi: 10.1007/s00429-010-0248-y. [DOI] [PubMed] [Google Scholar]
- 77.Kikyo H, Ohki K, Miyashita Y. Neural correlates for feeling-of-knowing: an fMRI parametric analysis. Neuron. 2002;36:177–186. doi: 10.1016/s0896-6273(02)00939-x. [DOI] [PubMed] [Google Scholar]
- 78.Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Bredart S. Here I am: the cortical correlates of visual self-recognition. Brain Res. 2007;143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 79.Aziz-Zadeh L, Kaplan J, Iocoboni M. “Aha”: The neural correlates of verbal insight solutions. Human Brain Mapping. 2009;30:908–916. doi: 10.1002/hbm.20554. [DOI] [PMC free article] [PubMed] [Google Scholar]